Calcium Sulfate Nanoparticles in Surface Sediments of Lingding Bay of the Pearl River Estuary, China: Implications for the Nonclassical Crystallization Pathway of Gypsum in the Natural Estuary Environment
Abstract
1. Introduction
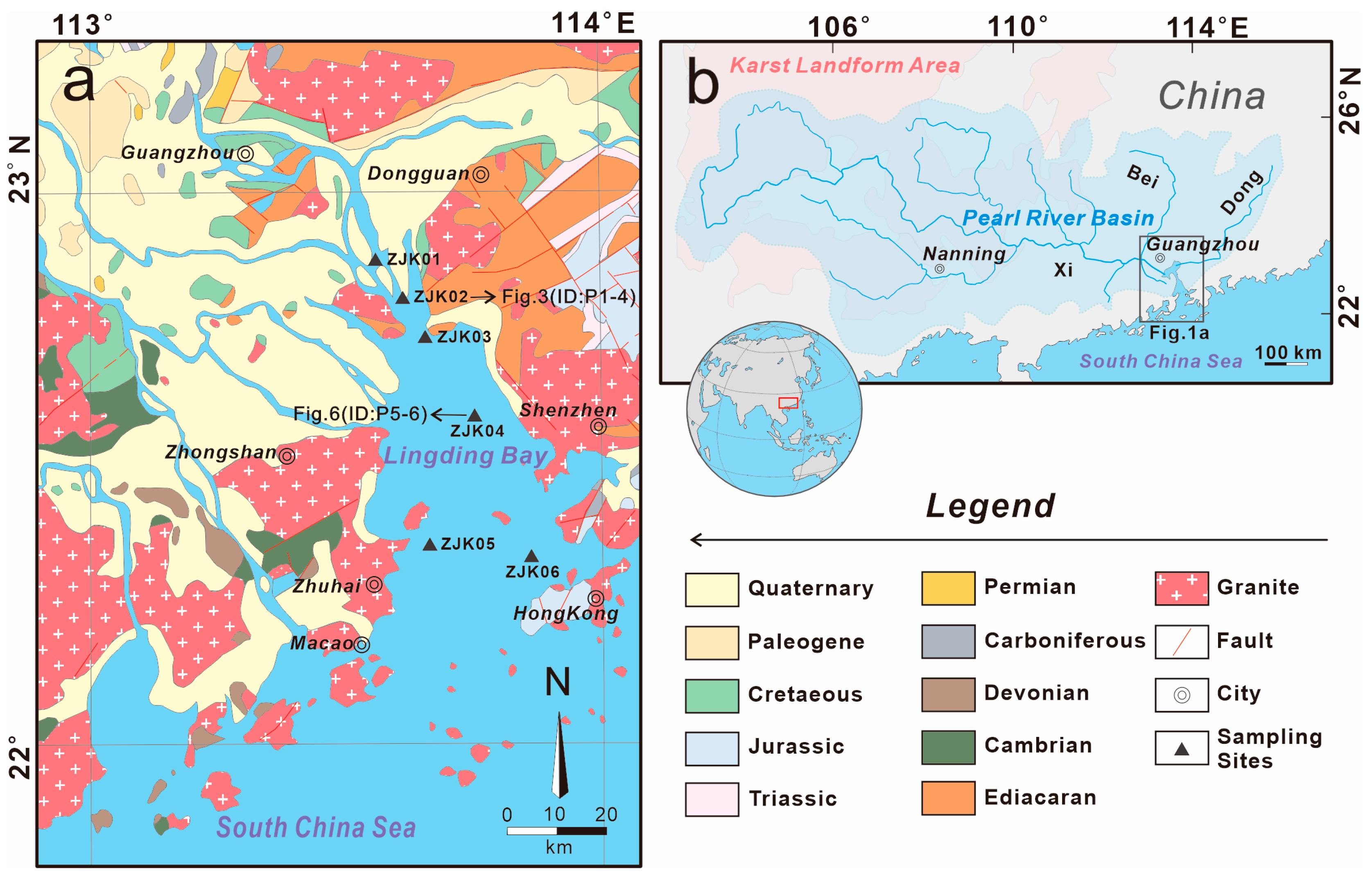
2. Geological Setting
3. Materials and Methods
3.1. Sampling Location and Methods
3.2. Analytical Methods
4. Results
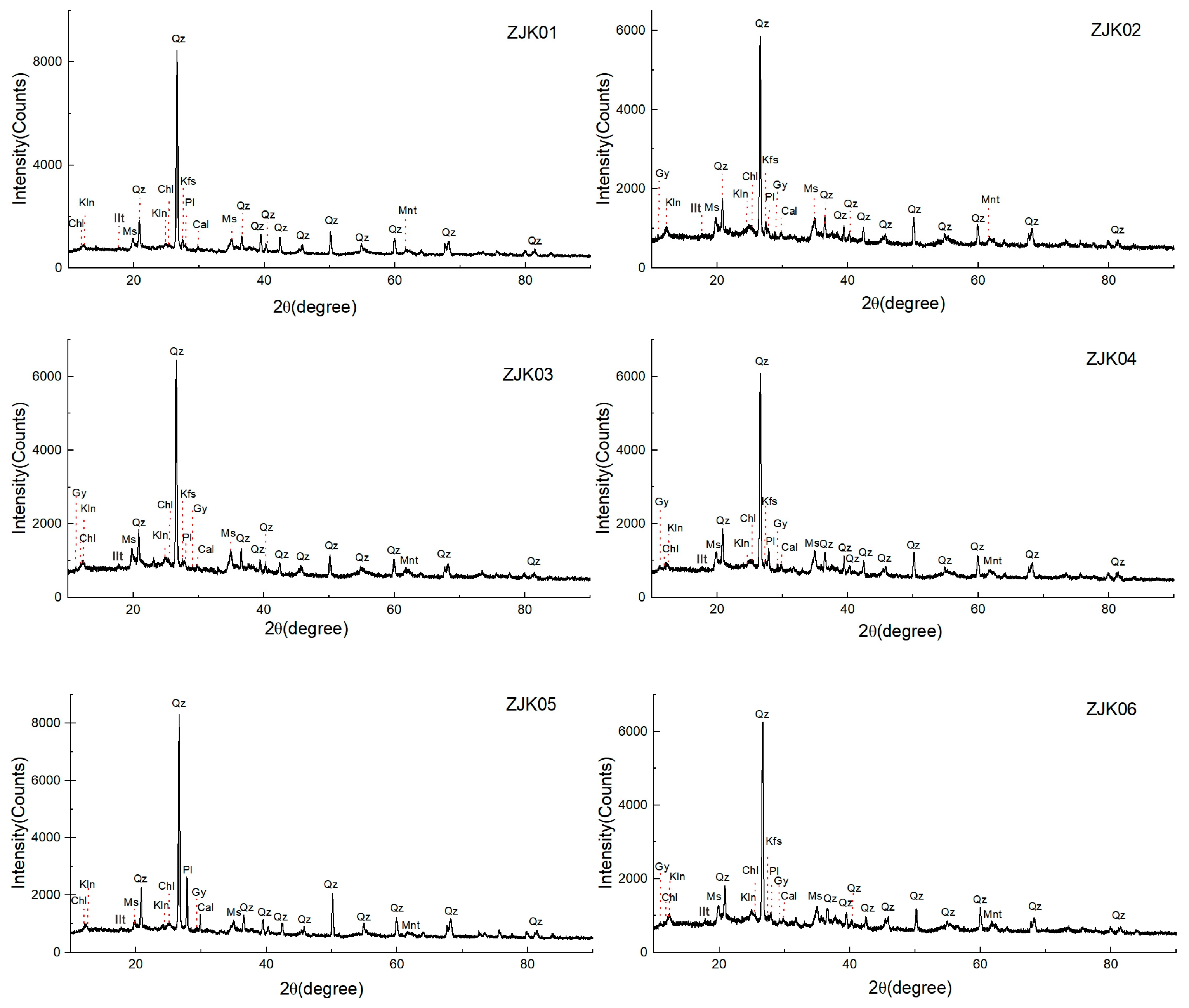
4.1. Mineral Composition of the Surface Sediments
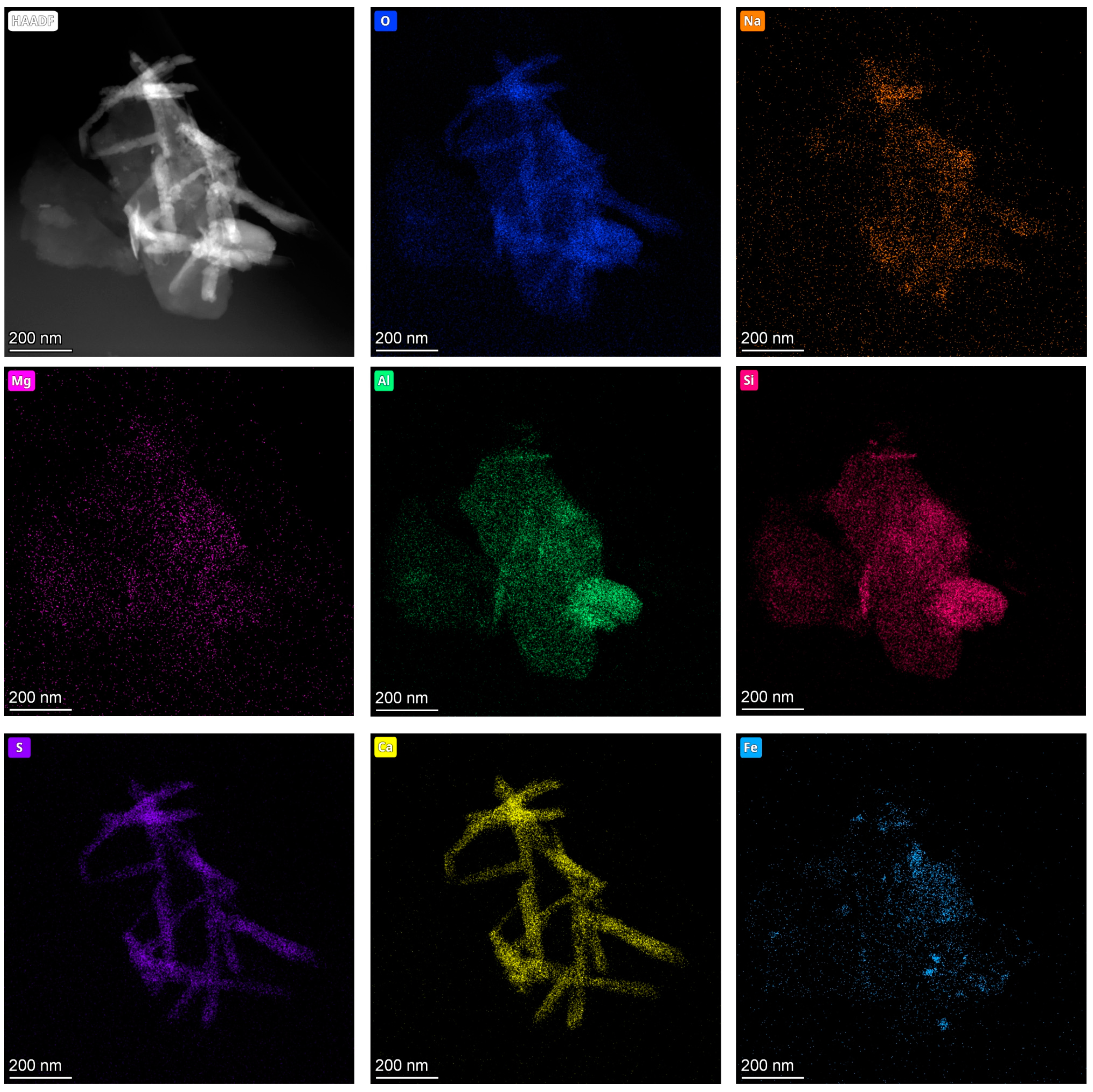
4.2. Characteristics of Calcium Sulfate Nanoparticles in the Surface Sediments
5. Discussion
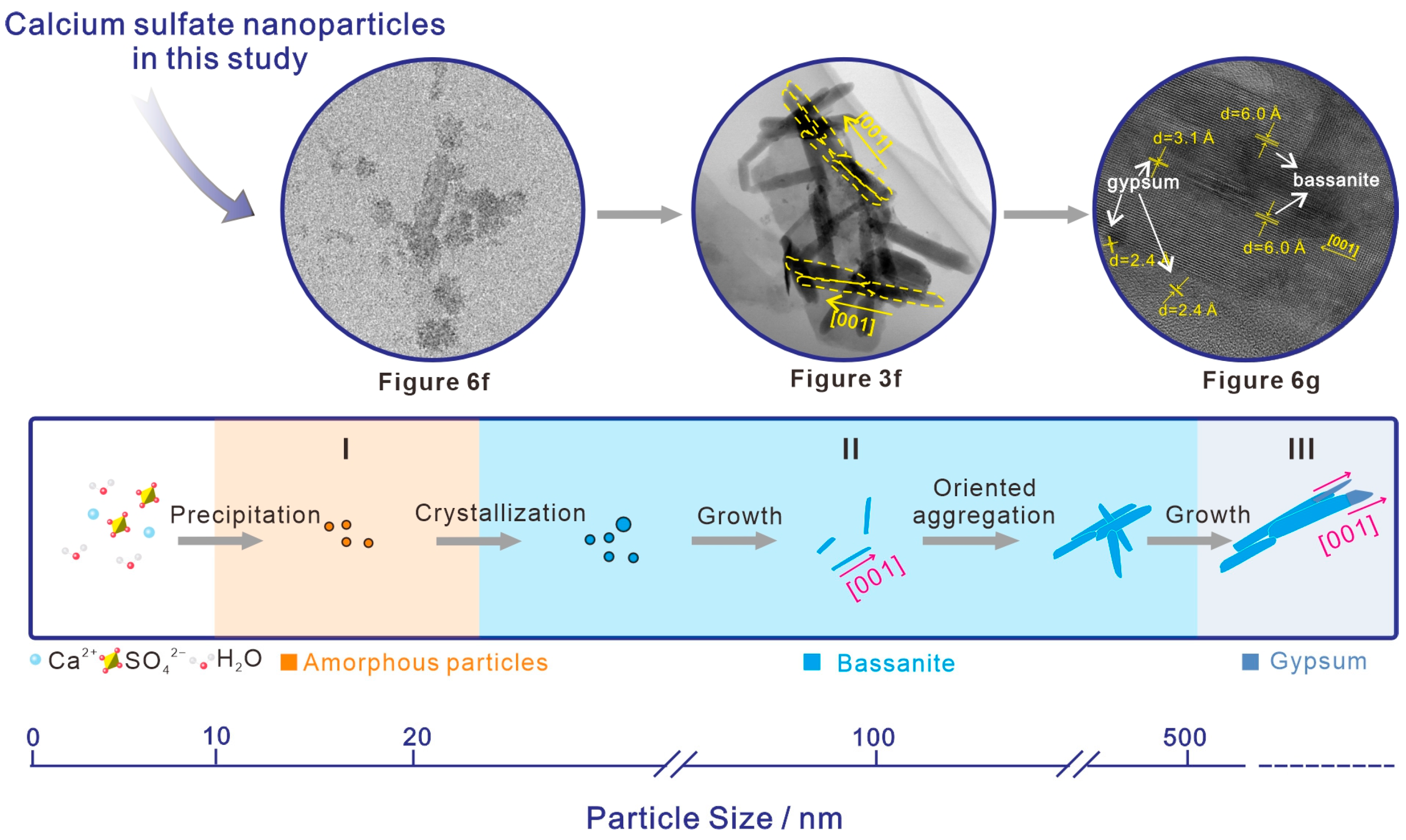
5.1. Implication for the Nonclassical Crystallization Pathway of Calcium Sulfate Dihydrate (Gypsum) in the Natural Estuary Environment
5.2. Implications for Interactions between Calcium Sulfate Nanoparticles and the Estuary Environment
6. Conclusions
- The mineral components of the surface sediments of Lingding Bay were similar, mainly including quartz, muscovite, plagioclase, potassium feldspar, clay minerals and calcareous minerals. Among them, calcium sulfate mainly existed in the form of gypsum.
- The calcium sulfate nanoparticles in the surface sediments of Lingding Bay were mainly spherical calcium sulfate nanoparticles (with a diameter ranging from 10 to 50 nm) and bassanite nanorod clusters (sizes ranging from 50 nm × 150 nm to 100 nm × 650 nm), and their main elements included O, S and Ca, with small amounts of N, Si, Na and Mg. The spherical calcium sulfate nanoparticles were mainly amorphous (diameter ranging from 10–50 nm), and a few of them appeared to be crystallized into bassanite. The bassanite nanorods self-assembled into aggregates primarily co-oriented along the c axis [001]. During epitaxial growth into larger bassanite nanorods (100 nm × 650 nm), the crystal form of gypsum could be observed.
- Based on the observations and analyses above, we propose that the crystallization of gypsum in surface sediments of the natural estuary environment could occur through the nonclassical crystallization pathway. In this pathway, amorphous calcium sulfate nanoparticles precipitate from the solution saturated with Ca2+/SO42−, and they then crystallize into bassanite nanoparticles as the nanoscale precursor emerges. Afterward, bassanite nanoparticles grow into nanorods, and the bassanite nanorods self-assembled along the c axis grow into larger nanorods, which are finally transformed into gypsum.
- Calcium sulfate nanoparticles could interact with materials and factors in the natural estuarine bay environment, and participate in geological and ecological processes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomis-Yagües, V.; Boluda-Botella, N.; Ruiz-Beviá, F. Gypsum precipitation/dissolution as an explanation of the decrease of sulphate concentration during seawater intrusion. J. Hydrol. 2000, 228, 48–55. [Google Scholar] [CrossRef]
- He, K.; Nie, A.; Yuan, Y.; Ghodsi, S.M.; Song, B.; Firlar, E.; Lu, J.; Lu, Y.P.; Shokuhfar, T.; Megaridis, C.M. In Situ Transmission Electron Microscopy Explores a New Nanoscale Pathway for Direct Gypsum Formation in Aqueous Solution. ACS Appl. Nano Mater. 2018, 1, 5430–5440. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, Y.; Christenson, H.K.; Meldrum, F.C. A new precipitation pathway for calcium sulfate dihydrate (gypsum) via amorphous and hemihydrate intermediates. Chem. Commun. 2012, 48, 504–506. [Google Scholar] [CrossRef]
- Saha, A.; Lee, J.; Pancera, S.M.; Bräeu, M.F.; Kempter, A.; Tripathi, A.; Bose, A. New insights into the transformation of calcium sulfate hemihydrate to gypsum using time-resolved cryogenic transmission electron microscopy. Langmuir 2012, 28, 11182–11187. [Google Scholar] [CrossRef]
- Fu, H.; Jia, C.; Chen, Q.; Jiang, G. Calcium sulfate polymorph evolution dominated by competitive nucleation in gypsum metastable zone. J. Cryst. Growth 2017, 470, 143–148. [Google Scholar] [CrossRef]
- Jia, C.Y.; Wu, L.C.; Fulton, J.L.; Liang, X.R.; De Yoreo, J.J.; Guan, B.H. Structural characteristics of amorphous calcium sulfate: Evidence to the role of water molecules. J. Phys. Chem. C 2021, 125, 3415–3420. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Tlili, M.; Amor, M.B.; Bacha, H.B.; Elleuch, B. Calcium sulphate scale prevention in a desalinaton unit using the SMCEC technique. Desalination 2004, 167, 311–318. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Gypsum scaling and cleaning in forward osmosis: Measurements and mechanisms. Environ. Sci. Technol. 2010, 44, 2022–2028. [Google Scholar] [CrossRef]
- Lu, H.; Kan, A.T.; Zhang, P.; Yu, J.; Fan, C.; Work, S.; Tomson, M.B. Phase stability and inhibition of calcium sulfate in the system NaCl/monoethylene glycol/H2O. SPE J. 2011, 17, 187–197. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Calcium sulphate hemihydrate hydration leading to gypsum crystallization. Prog. Cryst. Growth Charact. Mater. 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Benning, L.G.; Rodriguez-Blanco, J.D.; Ossorio, M.; Bots, P.; García-Ruiz, J.M. The role and implications of bassanite as a stable precursor phase to gypsum precipitation. Science 2012, 336, 69–72. [Google Scholar] [CrossRef]
- Gendrin, A.; Mangold, N.; Bibring, J.P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in Martian layered terrains: The Omega/Mars express view. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef]
- Wray, J.J.; Squyres, S.W.; Roach, L.H.; Bishop, J.L.; Mustard, J.F.; Noe Dobrea, E.Z. Identification of the Ca-sulfate bassanite in Mawrth Vallis, Mars. Icarus 2010, 209, 416–421. [Google Scholar] [CrossRef]
- Rapin, W.; Meslin, P.Y.; Maurice, S.; Vaniman, D.; Nachon, M.; Mangold, N.; Schröder, S.; Gasnault, O.; Forni, O.; Wiens, R.C.; et al. Hydration state of calcium sulfates in Gale Crater, Mars: Identification of bassanite veins. Earth Planet. Sci. Lett. 2016, 452, 197–205. [Google Scholar] [CrossRef]
- Guan, B.; Yang, L.; Wu, Z.; Shen, Z.; Ma, X.; Ye, Q. Preparation of α-calcium sulfate hemihydrate from FGD gypsum in K, Mg-containing concentrated CaCl2 solution under mild conditions. Fuel 2009, 88, 1286–1293. [Google Scholar] [CrossRef]
- Fu, H.; Guan, B.; Jiang, G.; Yates, M.Z.; Wu, Z. Effect of supersaturation on competitive nucleation of CaSO4 phases in a concentrated CaCl2 solution. Cryst. Growth Des. 2012, 12, 1388–1394. [Google Scholar] [CrossRef]
- Jiang, G.; Fu, H.; Savino, K.; Qian, J.; Wu, Z.; Guan, B. Nonlattice cation-SO42− ionpairs in calcium sulfate hemihydrate nucleation. Cryst. Growth Des. 2013, 13, 5128–5134. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Stawski, T.M.; Kellermeier, M. Calcium sulfate precipitation pathways in natural and engineering environments. Chem. Geol. 2019, 530, 119274. [Google Scholar] [CrossRef]
- Tritschler, U.; Van Driessche, A.E.S.; Kempter, A.; Kellermeier, M.; Cölfen, H. Controlling the selective formation of calcium sulfate polymorphs at room temperature. Angew. Chem. Int. Ed. 2015, 54, 4083–4086. [Google Scholar] [CrossRef]
- Stawski, T.M.; Van Driessche, A.E.S.; Ossorio, M.; Diego Rodriguez-Blanco, J.; Besselink, R.; Benning, L.G. Formation of calcium sulfate through the aggregation of sub-3 nanometre primary species. Nat. Commun. 2016, 7, 11177. [Google Scholar] [CrossRef]
- Liu, R.; Lin, X.B.; Wang, G.Q.; Liu, X. Natural N-bearing nanoparticles in sediments of a shallow bay of the south China: A new N form in N-cycling. Ecol. Indic. 2021, 122, 107281. [Google Scholar] [CrossRef]
- Yon, J.N.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total. Environ. 2008, 400, 396–414. [Google Scholar]
- Lu, M.Q.; Cao, J.J.; Wang, Z.Y.; Wang, G.Q. Characteristics of Naturally Formed Nanoparticles in Various Media and Their Prospecting Significance in Chaihulanzi Deposit. Minerals 2022, 12, 1289. [Google Scholar] [CrossRef]
- Yücel, M.; Gartman, A.; Chan, C.S.; Luther III, G.W. Hydrothermal vents as a kinetically stable source of iron-sulphide-bearing nanoparticles to the ocean. Nat. Geosci. 2011, 4, 367–371. [Google Scholar] [CrossRef]
- Borm, P.J.; Kreyling, W. Toxicological hazards of inhaled nanoparticles-potential implications for drug delivery. J. Nanosci. Nanotechnol. 2004, 4, 521–531. [Google Scholar] [CrossRef]
- Craft, E.; Abu-Qare, A.; Flaherty, M.; Garofolo, M.; Rincavage, H.; Abou-Donia, M. Depleted and natural uranium: Chemistry and toxicological effects. J. Toxicol. Environ. Health Part B 2004, 7, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Garzanti, E.; Cao, L.C.; Wang, H. The zircon story of the Pearl River (China) from Cretaceous to present. Earth-Sci. Rev. 2020, 201, 103078. [Google Scholar] [CrossRef]
- Yuan, X.Q.; Yang, Q.S.; Luo, X.X.; Yu, F.L.; Liu, F.; Li, J.Y.; Wang, Z.H. Distribution of grain size and organic elemental composition of the surficial sediments in Lingding Bay in the Pearl River Delta, China: A record of recent human activity. Ocean. Coast. Manag. 2019, 178, 104849. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, M.; Singh, V.P.; Li, J. Regionalization and spatial changing properties of droughts across the Pearl River basin, China. J. Hydrol. 2012, 472–473, 355–366. [Google Scholar] [CrossRef]
- He, J.; Garzanti, E.; Dinis, P.; Yang, S.; Wang, H. Provenance versus weathering control on sediment composition in tropical monsoonal climate (South China)-1. Geochemistry and clay mineralogy. Chem. Geol. 2020, 558, 119860. [Google Scholar] [CrossRef]
- Yuan, X.Q.; Yang, Q.S.; Meadows, M.E.; Luo, X.X.; Wang, Z.H. Grain size and organic geochemistry of recent sediments in Lingding Bay, Pearl River Delta, China: Implications for sediment dispersal and depositional processes perturbed by human activities. Anthr. Coasts 2021, 4, 147–167. [Google Scholar] [CrossRef]
- Chen, S.Z.; Pei, C.M. Geology and geochemistry of source rocks of the eastern Pearl River mouth basin, South China Sea. J. Asian Earth Sci. 1993, 8, 393–406. [Google Scholar]
- Hu, D.; Clift, P.D.; Böning, P.; Hannigan, R.; Hillier, S.; Blusztajn, J.; Wan, S.M.; Fuller, D.Q. Holocene evolution in weathering and erosion patterns in the Pearl River delta. Geochem. Geophys. Geosyst. 2013, 14, 2349–2368. [Google Scholar] [CrossRef]
- Tang, T. Surface sediment characteristics and tower karst dissolution, Guilin, southern China. Geomorphology 2002, 49, 231–254. [Google Scholar] [CrossRef]
- Han, G.; Liu, C. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem. Geol. 2004, 204, 1–21. [Google Scholar] [CrossRef]
- Jones, F. Infrared investigation of barite and gypsum crystallization: Evidence for an amorphous to crystalline transition. CrystEngComm 2012, 14, 8374–8381. [Google Scholar] [CrossRef]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergstrom, L.; Colfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, a6760. [Google Scholar] [CrossRef]
- Sun, S.; Chevrier, D.M.; Zhang, P.; Gebauer, D.; Cölfen, H. Distinct short-range order is inherent to small amorphous calcium carbonate clusters (<2 nm). Angew. Chem. Int. Ed. 2016, 55, 12206–12209. [Google Scholar]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V.; Loery, G.V.; Bernhardt, E.S.; Dionysiou, D.D.; Wiesner, M.R. Nanoparticle aggregation: Hallenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909. [Google Scholar] [CrossRef] [PubMed]
- Afshinnia, K.; Baalousha, M. Effect of phosphate buffer on aggregation kinetics of citrate-coated silver nanoparticles induced by monovalent and divalent electrolytes. Sci. Total Environ. 2017, 581–582, 268–276. [Google Scholar] [CrossRef]
- Yang, X.Z.; Wang, Q.; Qu, X.L.; Jiang, W. Bound and unbound humic acids perform different roles in the aggregation and deposition of multi-walled carbon nanotubes. Sci. Total Environ. 2017, 586, 738–745. [Google Scholar] [CrossRef]
- Cao, J.J.; Hu, X.Y.; Jiang, Z.T.; Li, H.W.; Zou, X.Z. Simulation of adsorption of gold nanoparticles carried by gas ascending from the earth’s interior in alluvial cover of the middle–lower reaches of the yangtze river. Geofluids 2010, 10, 438–446. [Google Scholar] [CrossRef]

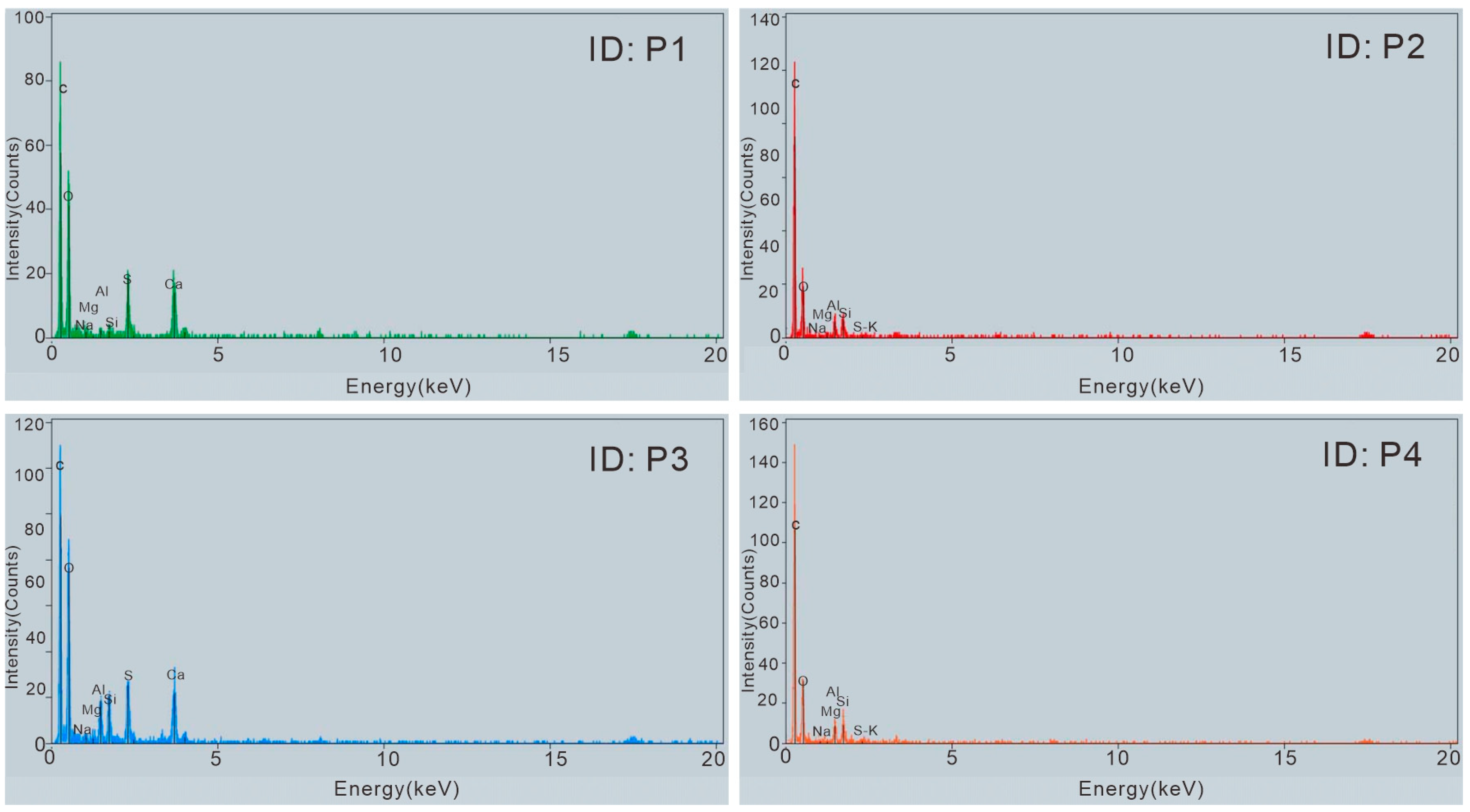
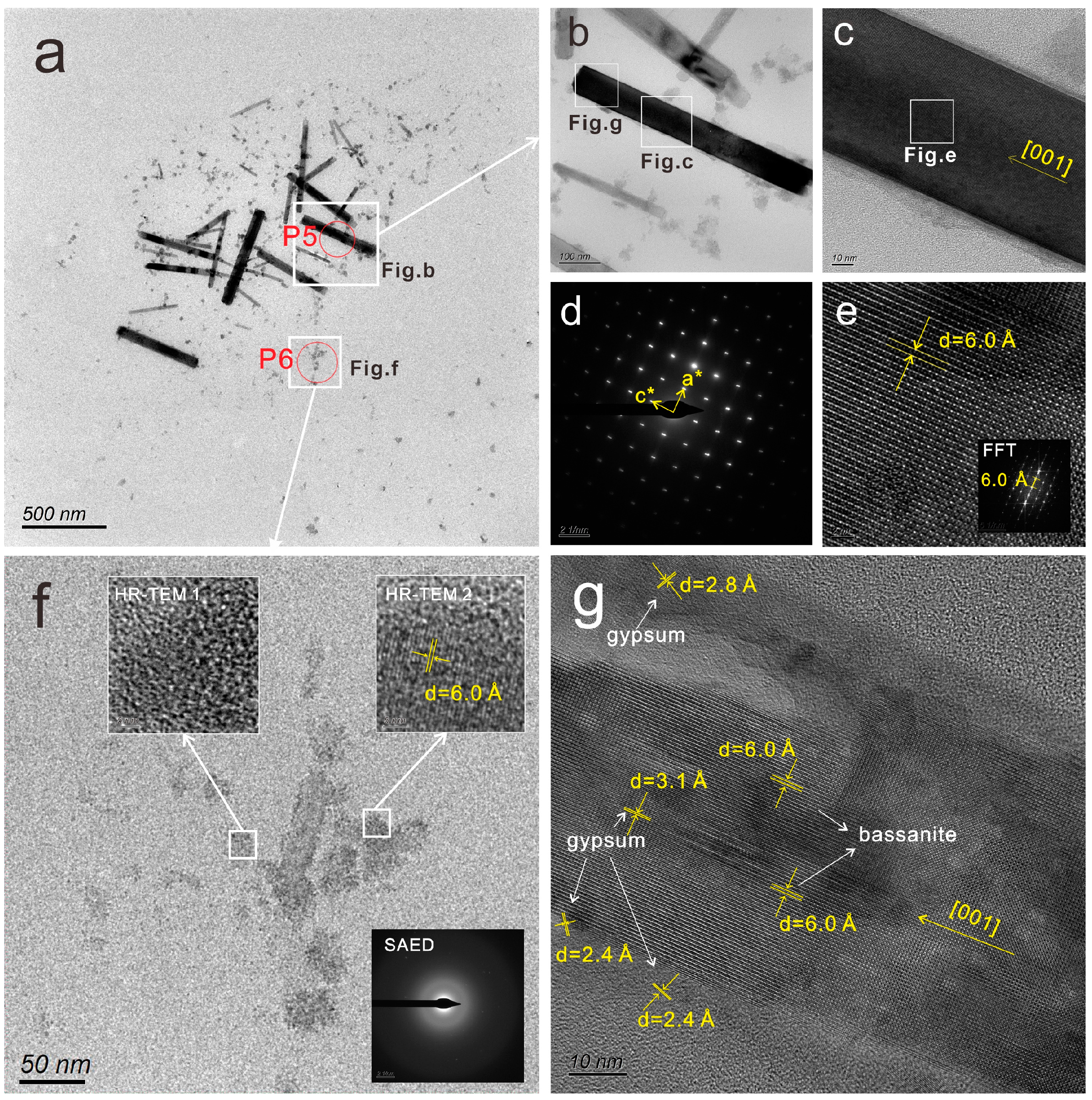
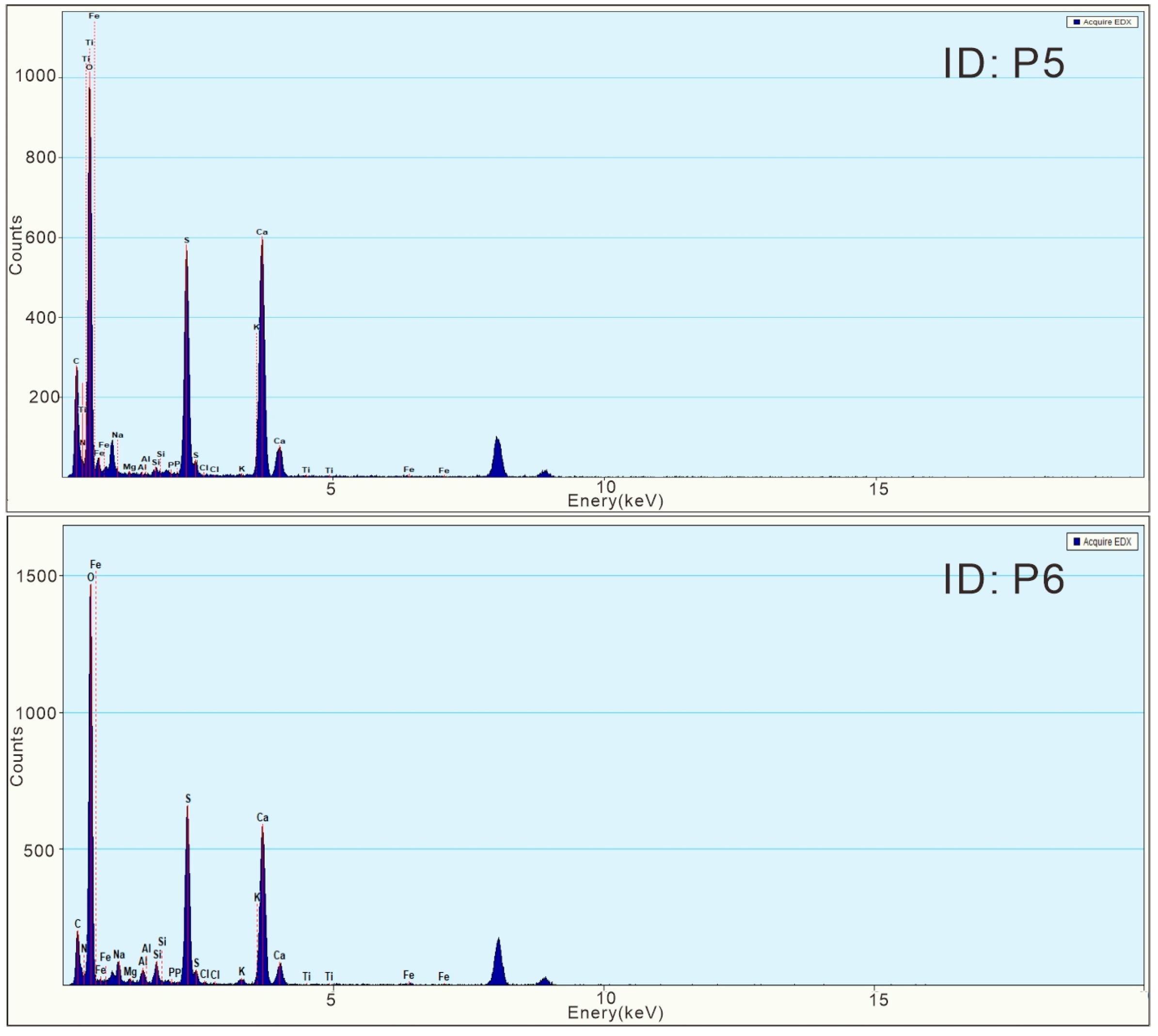
| Sample No. | EDS ID | Particle Type | Element | N | O | Na | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZJK02 | P1 | Calcium sulfate nanorod | Weight % | 46.48 | 1.98 | 0.62 | 2.90 | 2.47 | 20.83 | 24.73 | ||||||
| Atomic % | 64.88 | 1.93 | 0.56 | 2.41 | 1.96 | 14.49 | 13.78 | |||||||||
| ZJK02 | P2 | Calcium sulfate nanorod attached to silicate nanoparticle | Weight % | 41.84 | 1.86 | 1.14 | 9.07 | 10.63 | 17.07 | 18.39 | ||||||
| Atomic % | 58.77 | 1.83 | 1.07 | 7.55 | 8.50 | 11.97 | 10.31 | |||||||||
| ZJK02 | P3 | Layered silicate nanoparticle | Weight % | 53.60 | 1.69 | 3.62 | 17.48 | 20.99 | 2.62 | |||||||
| Atomic % | 66.32 | 1.48 | 2.96 | 12.82 | 14.76 | 1.65 | ||||||||||
| ZJK02 | P4 | Layered silicate nanoparticle | Weight % | 54.38 | 1.93 | 1.65 | 18.94 | 20.43 | 2.67 | |||||||
| Atomic % | 67.17 | 1.63 | 1.34 | 13.85 | 14.38 | 1.63 | ||||||||||
| ZJK04 | P5 | Calcium sulfate nanorods | Weight % | 2.55 | 48.89 | 0.43 | 0.04 | 0.28 | 0.20 | 21.38 | 0.12 | 0.11 | 25.93 | 0.06 | ||
| Atomic % | 3.97 | 66.51 | 0.40 | 0.03 | 0.21 | 0.14 | 14.51 | 0.07 | 0.06 | 14.08 | 0.01 | |||||
| ZJK04 | P6 | Spherical calcium sulfate nanoparticles | Weight % | 2.41 | 50.95 | 2.07 | 0.22 | 1.43 | 2.11 | 0.08 | 18.29 | 0.17 | 0.39 | 21.71 | 0.17 | |
| Atomic % | 3.64 | 67.53 | 1.91 | 0.19 | 1.12 | 1.60 | 0.05 | 12.09 | 0.10 | 0.21 | 11.49 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Yang, T.; Fan, Y.; Bai, S.; Yin, P. Calcium Sulfate Nanoparticles in Surface Sediments of Lingding Bay of the Pearl River Estuary, China: Implications for the Nonclassical Crystallization Pathway of Gypsum in the Natural Estuary Environment. Minerals 2023, 13, 903. https://doi.org/10.3390/min13070903
Wang G, Yang T, Fan Y, Bai S, Yin P. Calcium Sulfate Nanoparticles in Surface Sediments of Lingding Bay of the Pearl River Estuary, China: Implications for the Nonclassical Crystallization Pathway of Gypsum in the Natural Estuary Environment. Minerals. 2023; 13(7):903. https://doi.org/10.3390/min13070903
Chicago/Turabian StyleWang, Guoqiang, Tianjian Yang, Yitong Fan, Shushu Bai, and Peiyuan Yin. 2023. "Calcium Sulfate Nanoparticles in Surface Sediments of Lingding Bay of the Pearl River Estuary, China: Implications for the Nonclassical Crystallization Pathway of Gypsum in the Natural Estuary Environment" Minerals 13, no. 7: 903. https://doi.org/10.3390/min13070903
APA StyleWang, G., Yang, T., Fan, Y., Bai, S., & Yin, P. (2023). Calcium Sulfate Nanoparticles in Surface Sediments of Lingding Bay of the Pearl River Estuary, China: Implications for the Nonclassical Crystallization Pathway of Gypsum in the Natural Estuary Environment. Minerals, 13(7), 903. https://doi.org/10.3390/min13070903




