Ceramic Aggregate Material Formulated with MSWI Fly Ash and Fuel Ash for Use as Filter Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
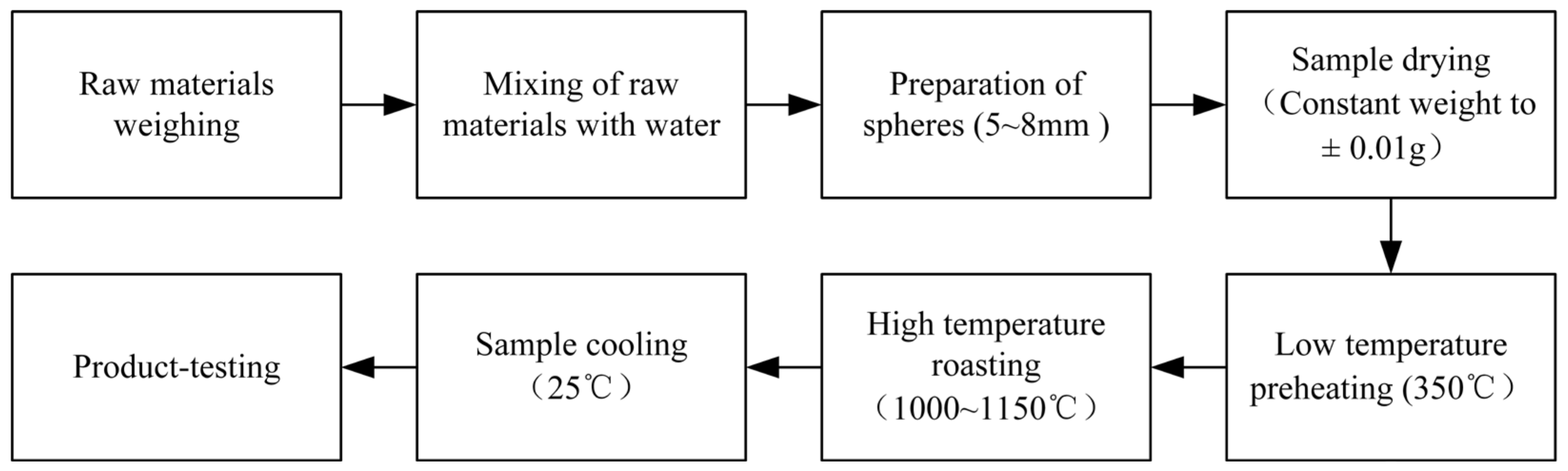
2.2. Preparation Method of the FMC
2.3. Method for Measuring the Performance of the FMC
- P—adsorbate partial pressure, PA;
- P0—the saturated vapor pressure of the adsorbent, PA;
- V—actual adsorption volume, cm3;
- VM—the saturated adsorption volume of a single layer, cm3;
- C—the BET constant.
- Cp—porosity;
- m0—the weight of the ceramic aggregate sample before immersion, g;
- m1—the weight of the ceramic particle sample after soaking in water for 2 h, g.
2.4. Characterization of Microstructure
2.5. Determination of the Ability of the FMC to Remove Ammonia and Nitrogen
3. Test Results and Analysis
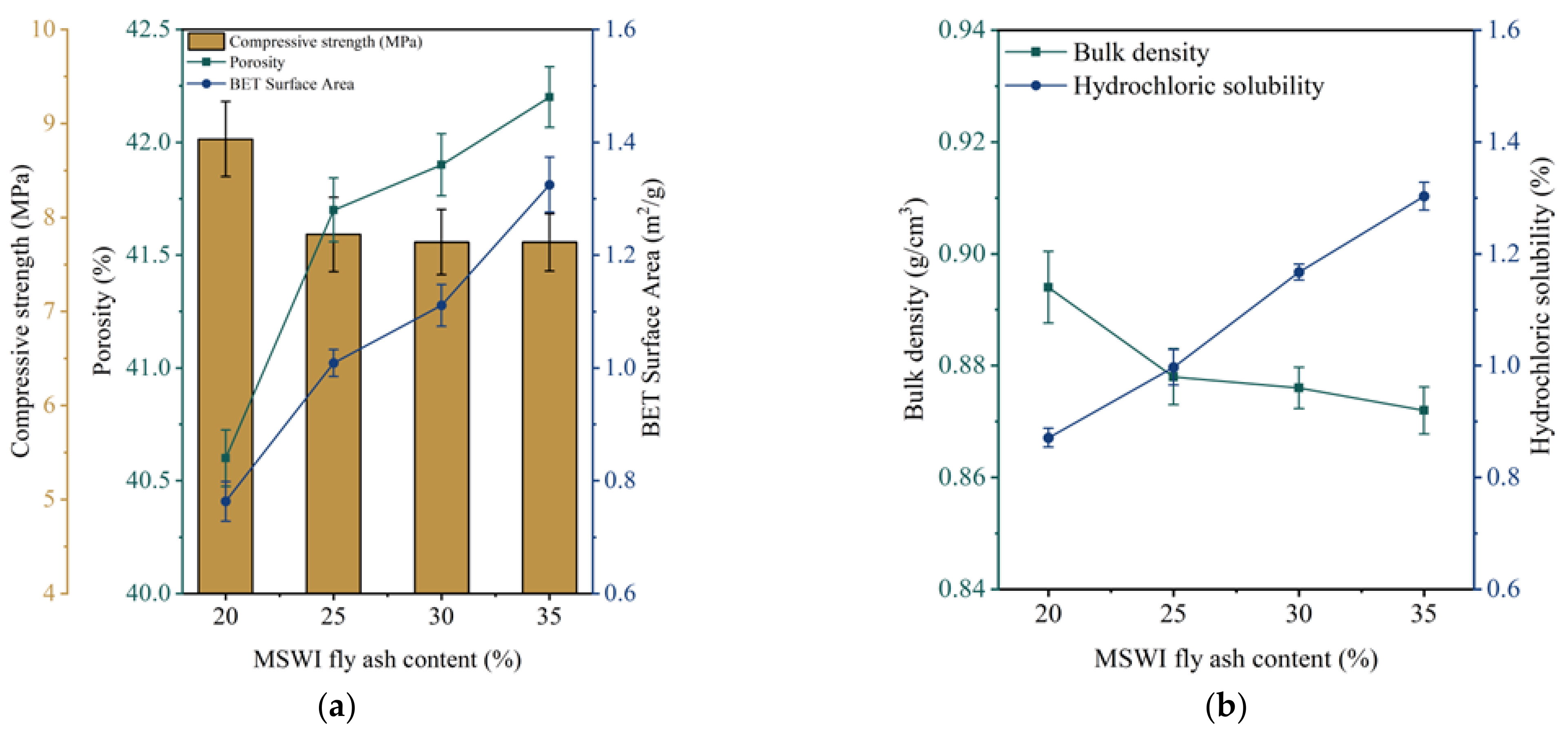
3.1. The Optimization of Fly Ash Content
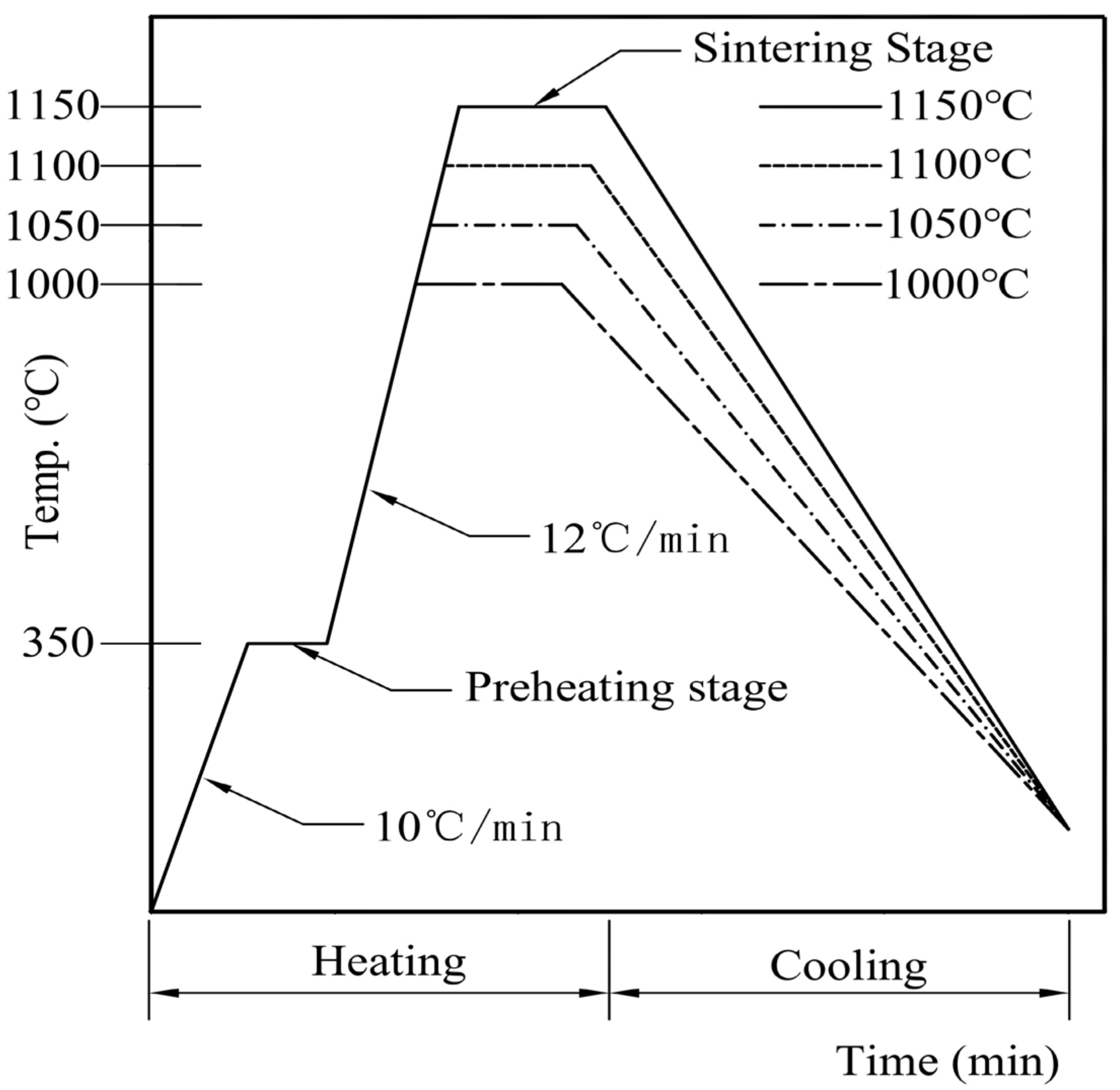
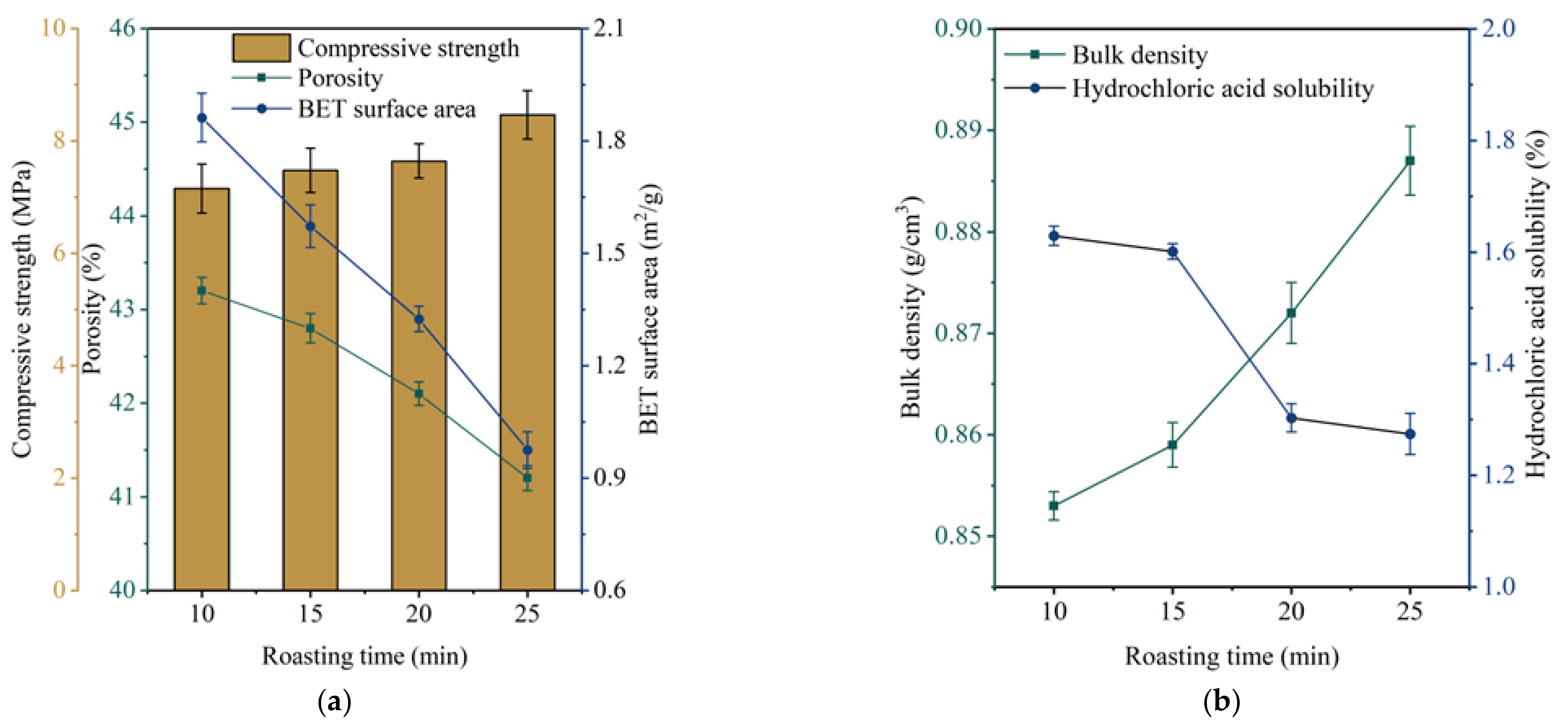
3.2. The Influence and Determination of Roasting Time
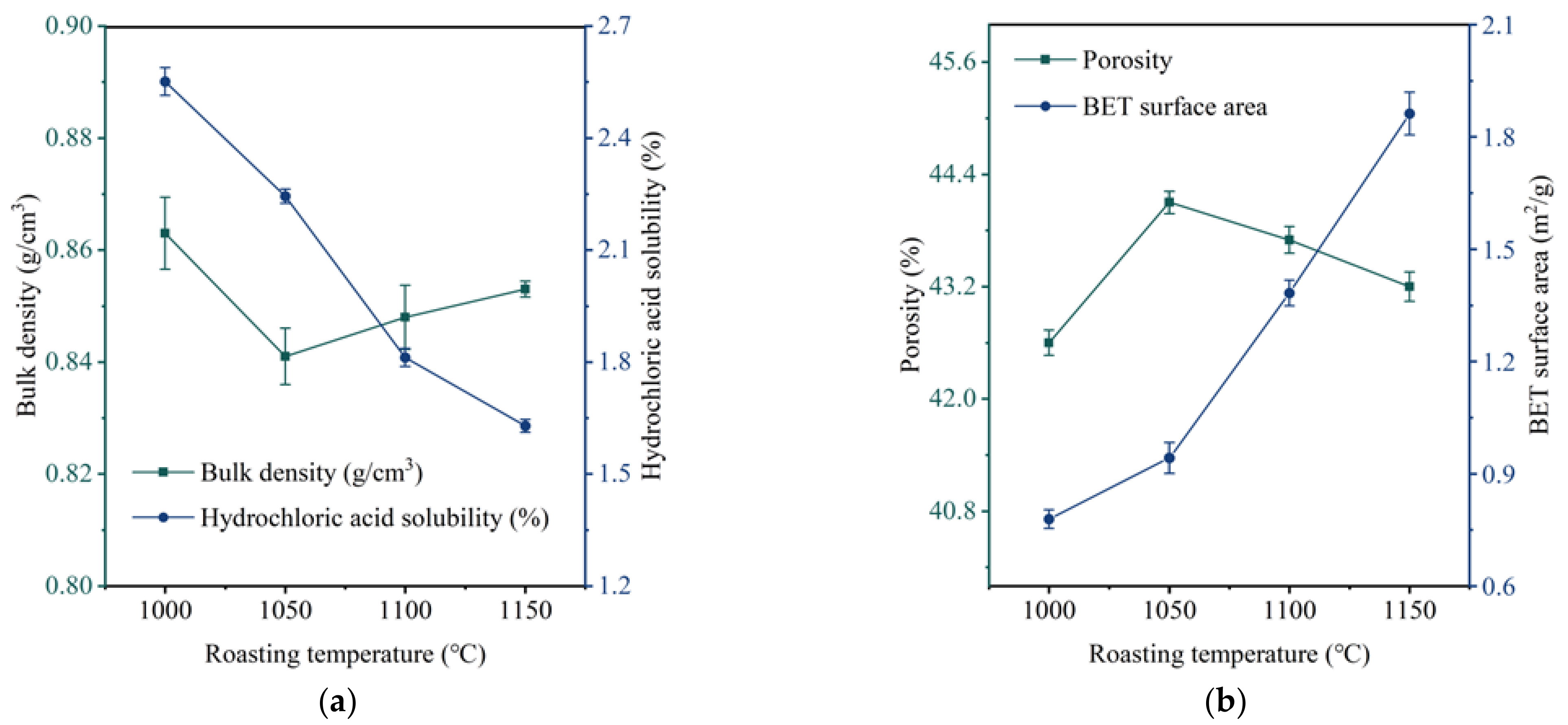
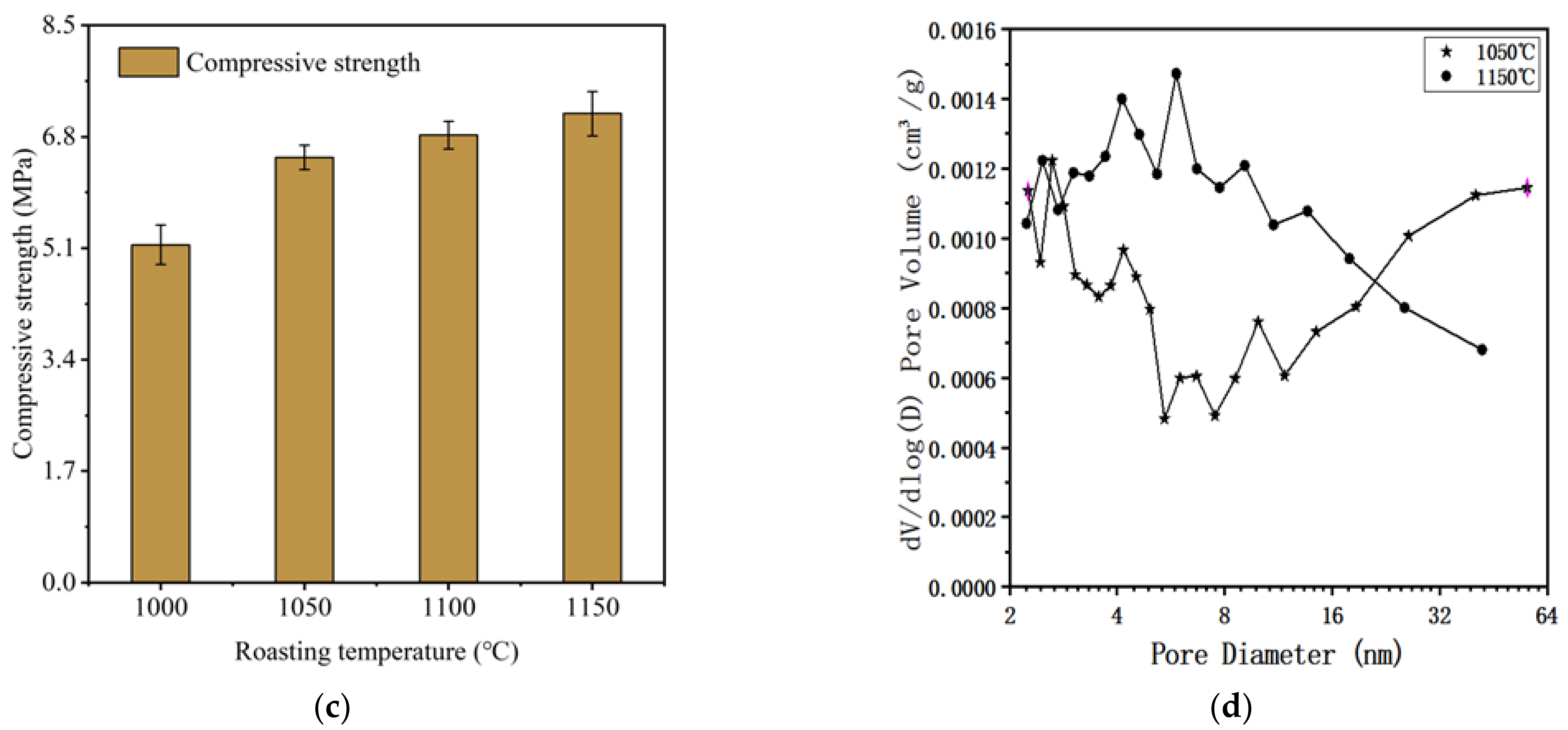
3.3. The Influence and Determination of Roasting Temperature
3.4. Analysis of the FMC Morphology
3.5. Analysis of Ammonia Nitrogen Removal Effect
3.6. Adsorption Mechanism of the FMC
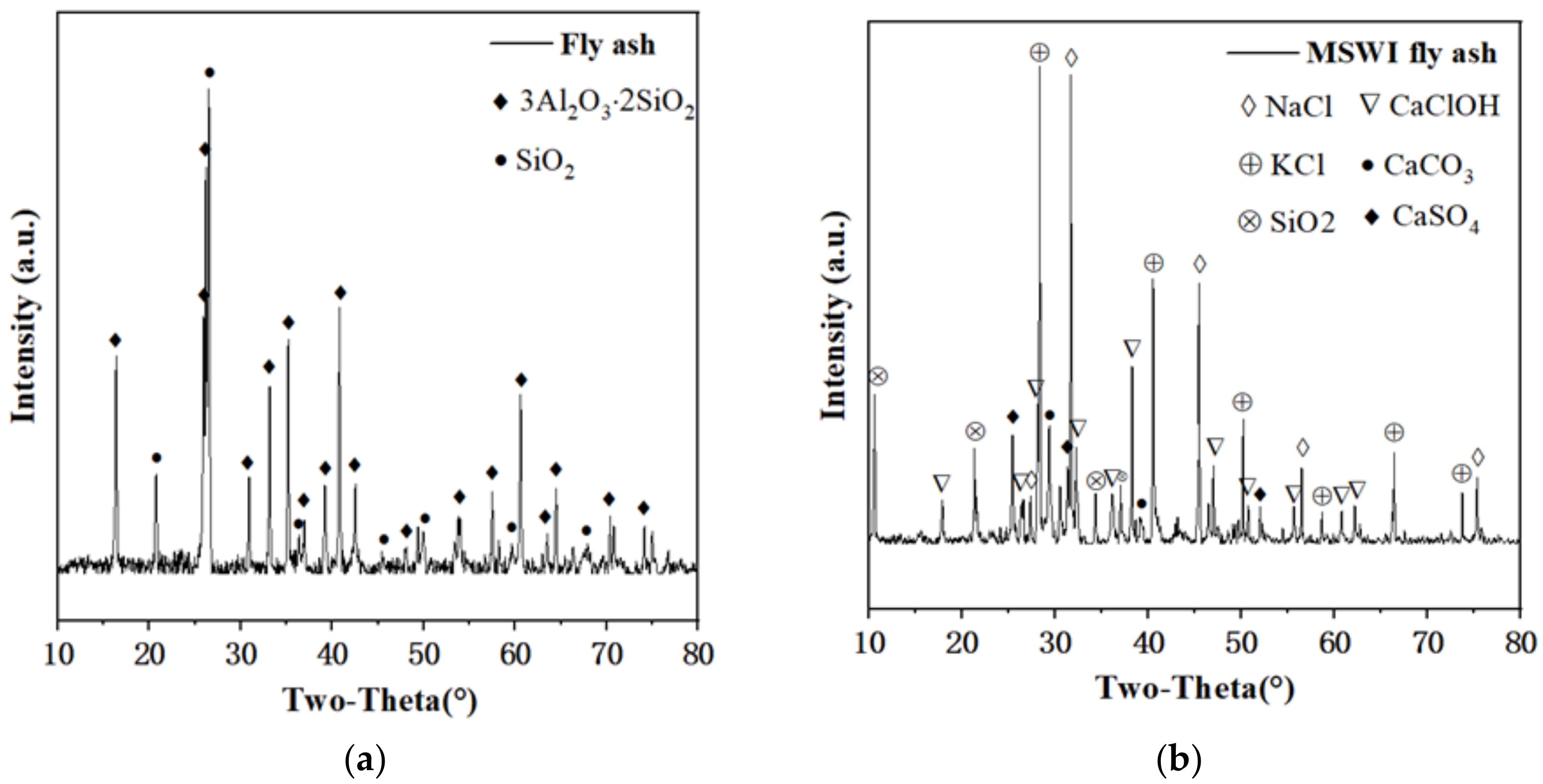
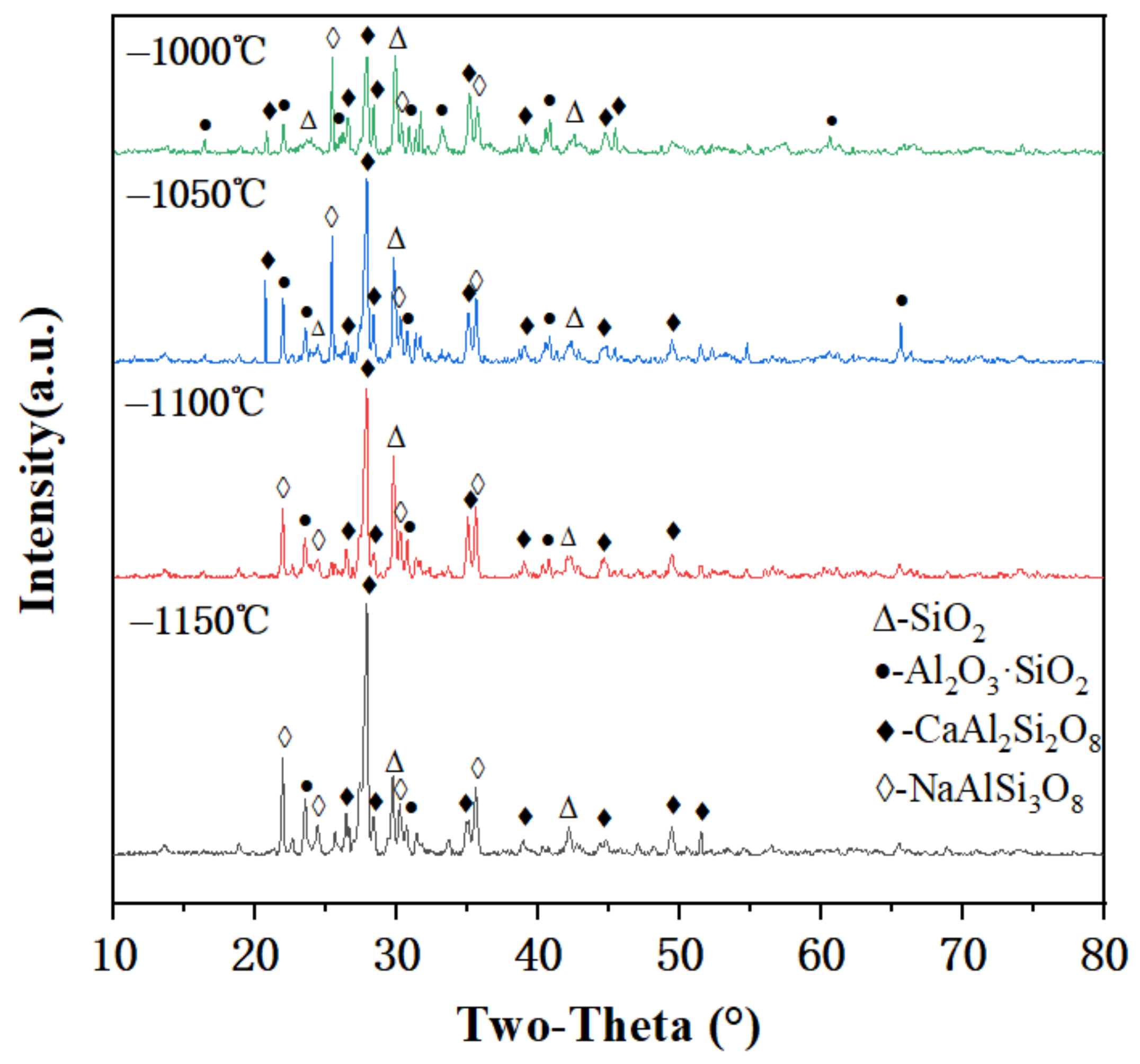
3.6.1. Mineral Composition
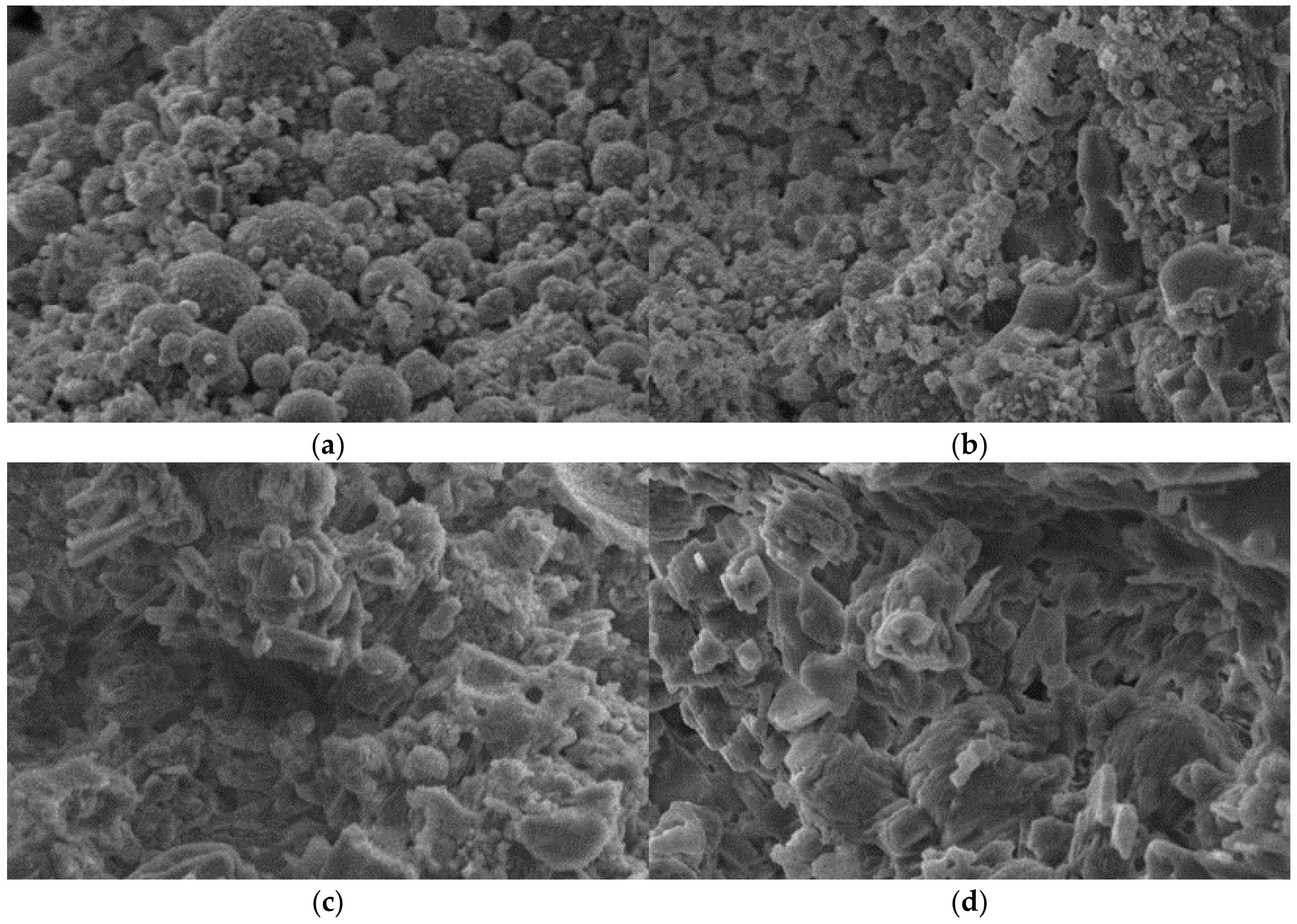
3.6.2. Microstructure
3.7. Analysis of the Leaching Effect of Heavy Metals
4. Conclusions and Perspectives
4.1. Conclusions
- (1)
- The optimal formulation for preparing FMCs is MSWI fly ash:fuel ash:silicon carbide:magnesium oxide = 35:63:1:1. Under the optimal sintering conditions determined with the single-factor experiments, an FMC with high ammonium nitrogen adsorption and high porosity can be obtained. The sintering conditions are as follows: preheating temperature, 350°C; preheating time, 20 min; sintering temperature, 1150 °C; and sintering time, 10 min.
- (2)
- XRD analysis indicated that the main minerals in the FMC were sodium feldspar, calcium feldspar, and mullite. The Ca2+ in the feldspar crystals of the FMC provided a large number of active sites for ion exchange. SEM analysis showed that a structure dominated by small pores was formed inside the FMC during sintering; this could encapsulate heavy metal ions for heavy metal fixation and immobilize through ion embedding. Ion exchange plays a dominant role in the adsorption process.
- (3)
- The ammonium nitrogen adsorption rate of the FMC prepared in this study reached up to 61.0%. Ammonium nitrate can be used as an FMC material, and the ammonium nitrogen removal capacity of the resulting FMC will be superior to that of zeolite and comparable to that of commercial FMC; moreover, the compressive strength of the FMC in our study was 7.7 MPa, the porosity was 43.2%, the specific surface area was 1.9 m2/g, the hydrochloric acid solubility rate was 1.6%, and the bulk density was 853.0 kg/m3, all of which meet the Chinese national standards (CJ/T299-2008).
- (4)
- The leaching concentrations of Zn, Cd, and Cu were 0.00155 mg/L, 0.00033 mg/L, and 0.00047 mg/L, respectively, while Pb was not detected. The heavy metal concentrations complied with the Chinese national standards (GB8978-1996), indicating that MSWI fly-ash-based FMC is a safe adsorbent material.
4.2. Perspectives
- (1)
- A quantitative study of the relationship between the porosity of MSWI fly ash ceramic particles and their adsorption capacity;
- (2)
- A quantitative study of the relationships between pore characteristics of MSWI fly ash ceramics and sintering temperature;
- (3)
- A quantitative study of the relationships between crystal phase variations and sintering temperature in MSWI fly ash ceramics;
- (4)
- A quantitative study on the relationship between the specific surface area of MSWI fly ash ceramics and sintering temperature;
- (5)
- Quantitative research of the relationship between heavy metal migration and sintering temperature in MSWI fly ash ceramics.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nanda, S.; Berruti, F. Municipal solid waste management and landfilling technologies: A review. Environ. Chem. Lett. 2020, 19, 1433–1456. [Google Scholar] [CrossRef]
- Chang, F.-Y.; Wey, M.-Y. Comparison of the characteristics of bottom and fly ashes generated from various incineration processes. J. Hazard. Mater. 2006, 138, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Quina, M.J.; Bordado, J.M.; Quinta-Ferreira, R.M. Recycling of air pollution control residues from municipal solid waste incineration into lightweight aggregates. Waste Manag. 2014, 34, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Fedje, K.K.; Andersson, S. Zinc recovery from waste-to-energy fly ash—A pilot test study. Waste Manag. 2020, 118, 90–98. [Google Scholar] [CrossRef]
- Sun, H.; Wu, W.; Zhao, Y.; Lin, Y.; Xu, S.; Zhang, T.; Zhang, X.; Xing, F.; Ren, J. Mechanical and durability properties of blended OPC mortar modified by low-carbon belite (C2S) nanoparticles. J. Clean. Prod. 2021, 305, 127087. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, M.; Jiang, Z.; Liu, S.; Yang, L.; Mao, Y.; Pan, C. Microwave preparation and carbonation properties of low-carbon cement. Constr. Build. Mater. 2022, 320, 126239. [Google Scholar] [CrossRef]
- Du, B.; Li, J.; Fang, W.; Liu, Y.; Yu, S.; Li, Y.; Liu, J. Characterization of naturally aged cement-solidified MSWI fly ash. Waste Manag. 2018, 80, 101–111. [Google Scholar] [CrossRef]
- Ministry of Housing and Urban-Rural Development. Bulletin on the Status of Urban Construction in China in 2021. Available online: https://www.mohurd.gov.cn/ess/?ty=a&query=2021%E5%B9%B4%E4%B8%AD%E5%9B%BD%E5%9F%8E%E5%B8%82%E5%BB%BA%E8%AE%BE%E7%8A%B6%E5%86%B5%E5%85%AC%E6%8A%A5&ukl=&uka=&ukf=2021%E5%B9%B4%E4%B8%AD%E5%9B%BD%E5%9F%8E%E5%B8%82%E5%BB%BA%E8%AE%BE%E7%8A%B6%E5%86%B5%E5%85%AC%E6%8A%A5&ukt=&sl=&ts=&te=&upg=1 (accessed on 8 February 2023).
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y.; Li, N. Microstructure and Strength of Alkali-Activated Bricks Containing Municipal Solid Waste Incineration (MSWI) Fly Ash Developed as Construction Materials. Sustainability 2019, 11, 1283. [Google Scholar] [CrossRef]
- Wang, S.; Guo, W.; Bai, Y.; Pan, H.; Qiu, Y.; Xue, C.; Zhao, Q. Preparation and characterization of mortar specimens based on municipal solid waste incineration fly ash-activated slag. J. Build. Eng. 2023, 69, 106254. [Google Scholar] [CrossRef]
- Jin, L.; Chen, M.; Wang, Y.; Peng, Y.; Yao, Q.; Ding, J.; Ma, B.; Lu, S. Utilization of mechanochemically pretreated municipal solid waste incineration fly ash for supplementary cementitious material. J. Environ. Chem. Eng. 2023, 11, 109112. [Google Scholar] [CrossRef]
- Lu, N.; Ran, X.; Pan, Z.; Korayem, A.H. Use of Municipal Solid Waste Incineration Fly Ash in Geopolymer Masonry Mortar Manufacturing. Materials 2022, 15, 8689. [Google Scholar] [CrossRef]
- Voišnienė, V.; Kizinievič, O.; Kizinievič, V. Recycling Municipal Solid Waste Incineration (MSWI) Fly Ash as Addition for Clay Brick. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kazimierz Dolny, Poland, 21–23 November 2019; Volume 660. [Google Scholar] [CrossRef]
- Han, S.; Ju, T.; Meng, F.; Lin, L.; Li, J.; Chen, K.; Jiang, J. Comprehensive study of recycling municipal solid waste incineration fly ash in lightweight aggregate with bloating agent: Effects of water washing and bloating mechanism. Sci. Total Environ. 2023, 881, 163267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Qin, J.; Liu, Y.; Qu, Y.; Liu, J.; Cao, R.; Zhang, Y. Mechanical properties of sintered ceramsite from iron ore tailings affected by two-region structure. Constr. Build. Mater. 2020, 240, 117919. [Google Scholar] [CrossRef]
- Ji, T.; Zheng, D.-D.; Chen, X.-F.; Lin, X.-J.; Wu, H.-C. Effect of prewetting degree of ceramsite on the early-age autogenous shrinkage of lightweight aggregate concrete. Constr. Build. Mater. 2015, 98, 102–111. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, L.; Wang, L.; Gong, J.; Wang, X.; Song, X.; Xu, T. Co-sintering MSWI fly ash with electrolytic manganese residue and coal fly ash for lightweight ceramisite. Chemosphere 2021, 263, 127914. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Shao, Y.; Zhang, W.; Zhu, Y.; Dou, T.; Chu, L.; Liu, Z. Preparation of municipal solid waste incineration fly ash-based ceramsite and its mechanisms of heavy metal immobilization. Waste Manag. 2022, 143, 54–60. [Google Scholar] [CrossRef]
- Wu, X.; Gu, F.; Su, C.; Wang, W.; Pu, K.; Shen, D.; Long, Y. Preparing high-strength ceramsite from ferronickel slag and municipal solid waste incineration fly ash. Ceram. Int. 2022, 48, 34265–34272. [Google Scholar] [CrossRef]
- Long, Y.; Pu, K.; Yang, Y.; Huang, H.; Fang, H.; Shen, D.; Geng, H.; Ruan, J.; Gu, F. Preparation of High-strength ceramsite from municipal solid waste incineration fly ash and clay based on CaO-SiO2-Al2O3 system. Constr. Build. Mater. 2023, 368, 130492. [Google Scholar] [CrossRef]
- Ren, Z.; Jia, B.; Zhang, G.; Fu, X.; Wang, Z.; Wang, P.; Lv, L. Study on adsorption of ammonia nitrogen by iron-loaded activated carbon from low temperature wastewater. Chemosphere 2021, 262, 127895. [Google Scholar] [CrossRef]
- Chen, T.-L.; Chen, L.-H.; Lin, Y.J.; Yu, C.-P.; Ma, H.-w.; Chiang, P.-C. Advanced ammonia nitrogen removal and recovery technology using electrokinetic and stripping process towards a sustainable nitrogen cycle: A review. J. Clean. Prod. 2021, 309, 127369. [Google Scholar] [CrossRef]
- Shao, Q.; Lu, M.; Zhou, J.; Zhu, Z.; Song, Y. Preparation of non-sintered fly ash filter (NSFF) for ammonia nitrogen adsorption. Environ. Technol. 2019, 40, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, N.; An, S.; Cai, C.; Peng, J.; Xie, M.; Peng, J.; Song, X. Synthesis of novel hierarchical porous zeolitization ceramsite from industrial waste as efficient adsorbent for separation of ammonia nitrogen. Sep. Purif. Technol. 2022, 297, 121418. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.-F.; George, L.; Wuhrer, R. High-temperature performance of alkali-activated binders of fly ash and calcium aluminate. Ceram. Int. 2023, 49, 14389–14398. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.-H.; Zhang, L.; Wang, Q.; Huang, X.; Zhang, Y.; Liu, X.; Liang, J.; Duan, X. Study on preparation and performance of iron tailings-based porous ceramsite filter materials for water treatment. Sep. Purif. Technol. 2021, 276, 119380. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C. Preparation and characterization of ceramsite from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Tianyi, S.; Xingyu, Q.; Zhu, P. Research Progress on High Temperature Resistance of Geopolymers. Mater. Rep. 2023, 37, 17. [Google Scholar] [CrossRef]
- Shang, S.; Fan, H.; Li, Y.; Li, L.; Li, Z. Preparation of Lightweight Ceramsite from Solid Waste Using SiC as a Foaming Agent. Materials 2022, 15, 325. [Google Scholar] [CrossRef]
- Mi, H.; Yi, L.; Wu, Q.; Xia, J.; Zhang, B. Preparation of high-strength ceramsite from red mud, fly ash, and bentonite. Ceram. Int. 2021, 47, 18218–18229. [Google Scholar] [CrossRef]
- Shao, Q.; Xu, H.; Tong, M.M.; Li, S. The Technical Study on the Preparation of Lightweight Ceramsite Made from Sewage Sludge and Fly Ash. Adv. Mater. Res. 2011, 356–360, 1871–1875. [Google Scholar] [CrossRef]
- Liu, Y.D.; Yang, D.Y.; Wang, L.L. Research on Using Sludge to Manufacture Lightweight Ceramsite and Sintering Mechanism. Adv. Mater. Res. 2013, 689, 358–362. [Google Scholar] [CrossRef]
- Lu, H. New Sludge Ceramsite and Its Application in Sewage Treatment. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2018. [Google Scholar]
- Nie, J.; Wang, Q.; Gao, S.; Poon, C.S.; Zhou, Y.; Li, J.S. Novel recycling of incinerated sewage sludge ash (ISSA) and waste bentonite as ceramsite for Pb-containing wastewater treatment: Performance and mechanism. J. Environ. Manag. 2021, 288, 112382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, D.; Zhang, J.; Liu, C.; Shi, Y. Measurement and statistics of single pellet mechanical strength of differently shaped catalysts. Powder Technol. 2000, 113, 176–184. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, Y.; Sheng, L. Preparation of ceramsite from municipal sludge and its application in water treatment: A review. J. Environ. Manag. 2021, 287, 112374. [Google Scholar] [CrossRef] [PubMed]
- Wei, N. Leachability of heavy metals from lightweight aggregates made with sewage sludge and municipal solid waste incineration fly ash. Int. J. Environ. Res. Public Health 2015, 12, 4992–5005. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Zhang, Y.; Liu, Z.; Long, L.; Liu, Z.; Chen, Y.; Hu, X.M.; Lu, M.; Huang, L.Z. Phosphorus and nitrogen recovery from wastewater by ceramsite: Adsorption mechanism, plant cultivation and sustainability analysis. Sci. Total Environ. 2022, 805, 150288. [Google Scholar] [CrossRef]
- Kockal, N.U.; Ozturan, T. Characteristics of lightweight fly ash aggregates produced with different binders and heat treatments. Cem. Concr. Compos. 2011, 33, 61–67. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Rong, H.; Zhou, X.; Chen, F.; Li, X.; Wang, T.; Hou, H. Adsorption mechanism of lead ions on porous ceramsite prepared by co-combustion ash of sewage sludge and biomass. Sci. Total Environ. 2020, 702, 135017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture 2016, 450, 187–193. [Google Scholar] [CrossRef]
- Shen, W.; Zhu, N.; Xi, Y.; Huang, J.; Li, F.; Wu, P.; Dang, Z. Effects of medical waste incineration fly ash on the promotion of heavy metal chlorination volatilization from incineration residues. J. Hazard. Mater. 2022, 425, 128037. [Google Scholar] [CrossRef]
- Colangelo, F.; Messina, F.; Cioffi, R. Recycling of MSWI fly ash by means of cementitious double step cold bonding pelletization: Technological assessment for the production of lightweight artificial aggregates. J. Hazard. Mater. 2015, 299, 181–191. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.-S.; Chen, Z.; Xue, Q.; Sun, Q.; Zhou, Y.; Chen, X.; Liu, L.; Poon, C.S. Production of lightweight aggregate ceramsite from red mud and municipal solid waste incineration bottom ash: Mechanism and optimization. Constr. Build. Mater. 2021, 287, 122993. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, Y.; Zhang, P.; Cheng, S.; Tan, X.; Shen, Z.; Wen, W.; Wei, C.; Miao, S. Preparation of Highly Bloating Ceramsite from “White Mud” and Oil Shale with Incorporation of Black Cotton Soil. Waste Biomass Valorization 2019, 11, 3609–3619. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, R.; Xu, Y.; Ye, F.; Xu, R.; Han, Y. Effect of SiO2, Al2O3 and CaO on characteristics of lightweight aggregates produced from MSWI bottom ash sludge (MSWI-BAS). Constr. Build. Mater. 2019, 205, 368–376. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, C.; Shen, H.; Du, J.; Li, Q.; Wu, W.; Guo, B.; Liu, G. Green synthesis of ceramsite from industrial wastes and its application in selective adsorption: Performance and mechanism. Environ. Res. 2022, 214, 113786. [Google Scholar] [CrossRef]
- Han, S.; Yue, Q.; Yue, M.; Gao, B.; Li, Q.; Yu, H.; Zhao, Y.; Qi, Y. The characteristics and application of sludge-fly ash ceramic particles (SFCP) as novel filter media. J. Hazard. Mater. 2009, 171, 809–814. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of ammonium from greywater using natural zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]

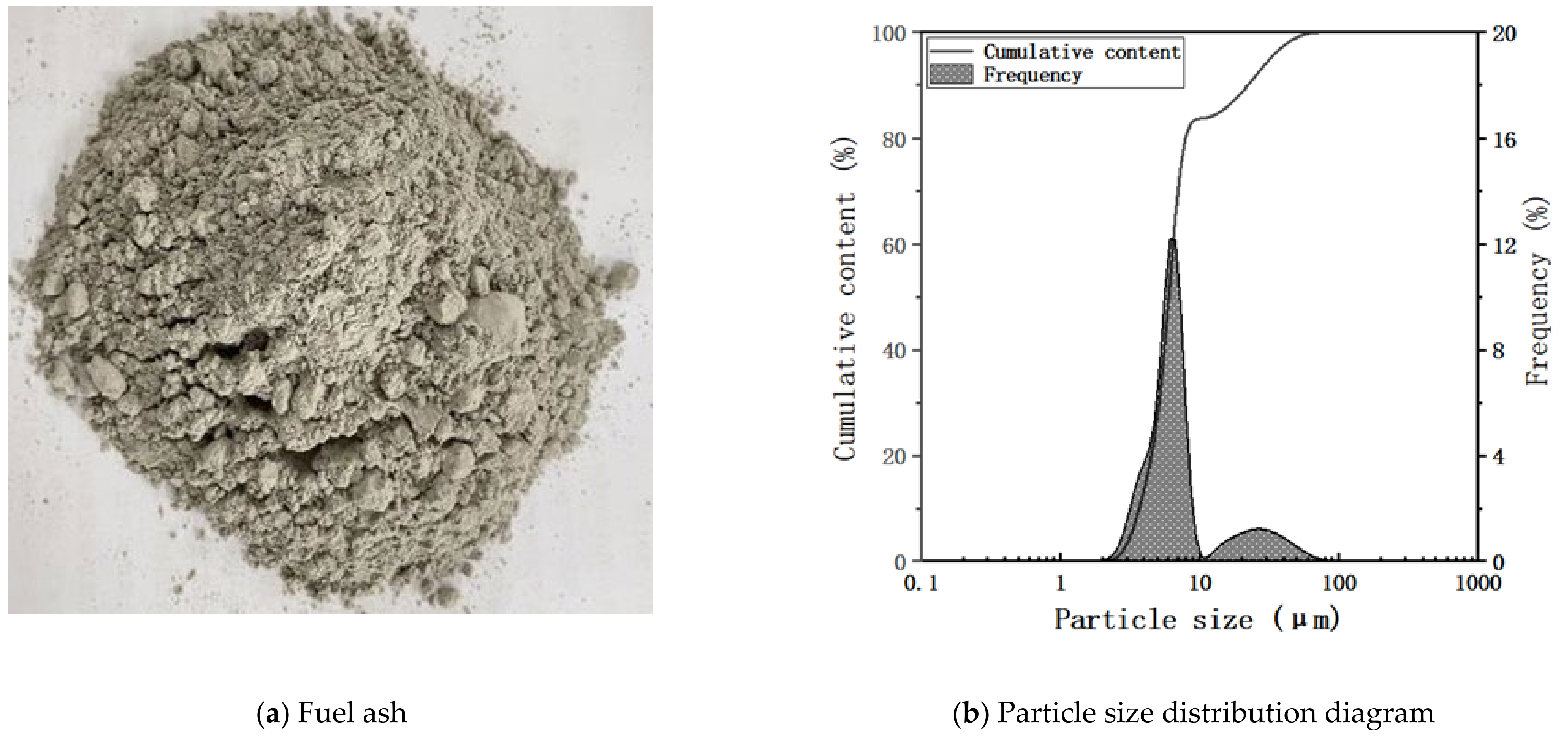




| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Cl | SO3 | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| MSWI Fly Ash | 5.46 | 3.16 | 0.94 | 43.07 | 0.91 | 0.07 | 6.37 | 26.33 | 4.75 | 8.94 a |
| Fuel Ash | 49.8 | 33.6 | 3.73 | 4.8 | 0.67 | 0.26 | 0.31 | ND | 0.37 | 6.46 b |
| Material | Zn | Cd | Pb | Cu | Ba | Ni | As | Se |
|---|---|---|---|---|---|---|---|---|
| MSWI Fly Ash | 353 | 4.32 | 2.06 | 1.68 | 0.796 | 0.02 | 0.0014 | 0.0012 |
| Sample | MSWI FA (g) | FA (g) | Water (g) | SiC (g) | MgO (g) | MSWI FA (wt.%) | FA (wt.%) | Time (min) | Temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|
| M20Ti20Te150 | 200 | 780 | 250 | 10 | 10 | 20% | 78% | 20 | 1150 |
| M25Ti20Te150 | 250 | 730 | 250 | 10 | 10 | 25% | 73% | 20 | 1150 |
| M30Ti20Te150 | 300 | 680 | 250 | 10 | 10 | 30% | 68% | 20 | 1150 |
| M35Ti20Te150 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 20 | 1150 |
| M35Ti10Te150 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 10 | 1150 |
| M35Ti15Te150 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 15 | 1150 |
| M35Ti20Te150 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 20 | 1150 |
| M35Ti25Te150 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 25 | 1150 |
| M35Ti10Te0 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 10 | 1000 |
| M35Ti10Te50 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 10 | 1050 |
| M35Ti10Te100 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 10 | 1100 |
| M35T10Te150 | 350 | 630 | 250 | 10 | 10 | 35% | 63% | 10 | 1150 |
| Roasting Temperature | 1000 °C | 1050 °C | 1100 °C | 1150 °C | GB8978-1996 |
|---|---|---|---|---|---|
| Zn | 0.00276 | 0.00299 | 0.00142 | 0.00155 | 0.2 |
| Cd | 0.00214 | 0.00131 | 0.0008 | 0.00033 | 0.1 |
| Pb | 0.00011 | 0.00011 | ND | ND | 1.0 |
| Cu | 0.00352 | 0.00139 | 0.00073 | 0.00047 | 0.5 |
| Type of Heavy Metal | MSWI Fly Ash | GB8978-1996 |
|---|---|---|
| Zn | 76.6 | 0.2 |
| Cd | 4.2 | 0.1 |
| Pb | 0.48 | 1.0 |
| Cu | 0.11 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Chen, H.; Chen, J.; Cao, Y.-F. Ceramic Aggregate Material Formulated with MSWI Fly Ash and Fuel Ash for Use as Filter Media. Minerals 2023, 13, 845. https://doi.org/10.3390/min13070845
Lu N, Chen H, Chen J, Cao Y-F. Ceramic Aggregate Material Formulated with MSWI Fly Ash and Fuel Ash for Use as Filter Media. Minerals. 2023; 13(7):845. https://doi.org/10.3390/min13070845
Chicago/Turabian StyleLu, Ning, Hougang Chen, Jiao Chen, and Yi-Fang Cao. 2023. "Ceramic Aggregate Material Formulated with MSWI Fly Ash and Fuel Ash for Use as Filter Media" Minerals 13, no. 7: 845. https://doi.org/10.3390/min13070845
APA StyleLu, N., Chen, H., Chen, J., & Cao, Y.-F. (2023). Ceramic Aggregate Material Formulated with MSWI Fly Ash and Fuel Ash for Use as Filter Media. Minerals, 13(7), 845. https://doi.org/10.3390/min13070845






