Study the Mechanical Properties of Geopolymer under Different Curing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Orthogonal Test Design
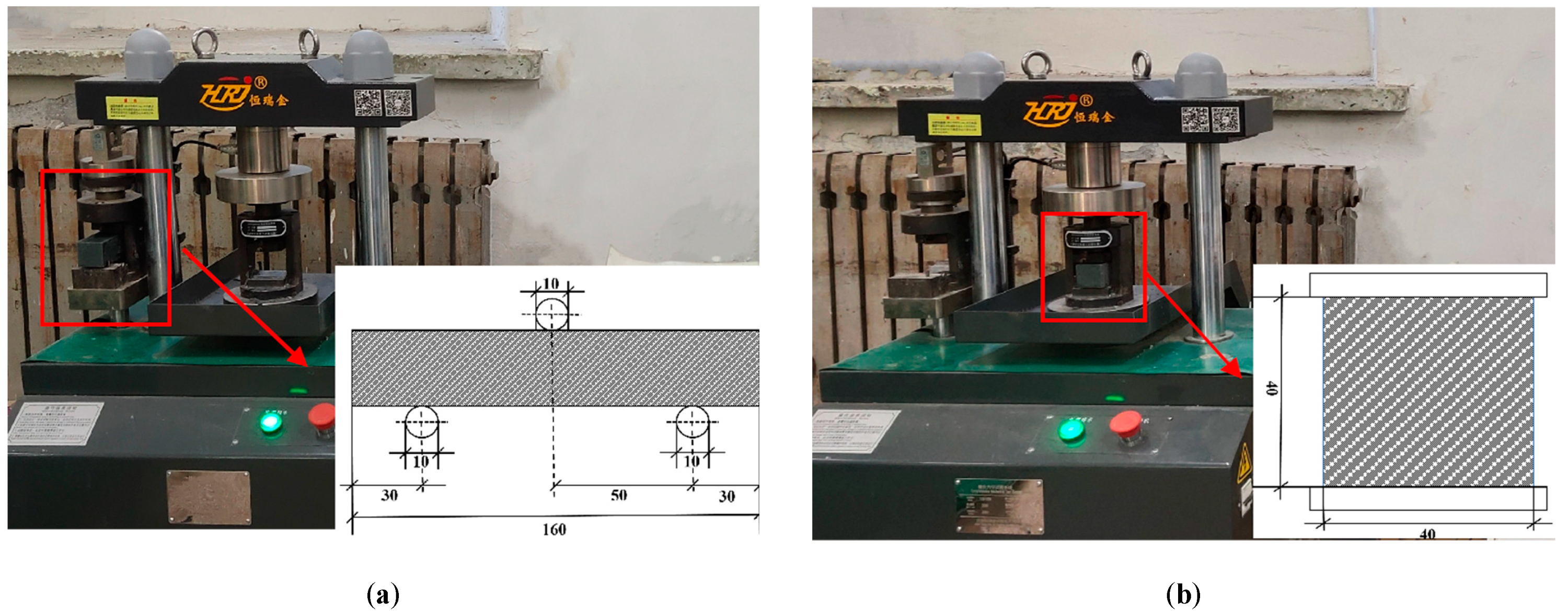
2.3. Sample Preparation and Curing
2.4. Mechanical Properties Test
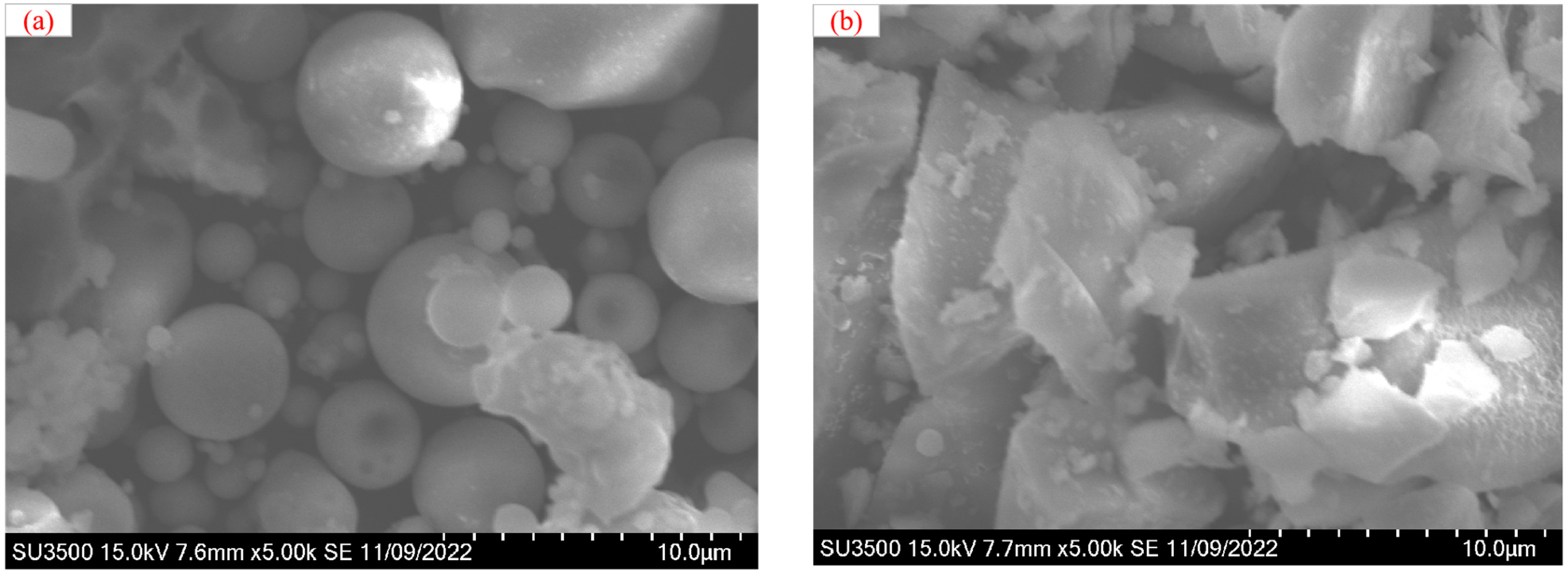
2.5. Microstructure Test
3. Results and Discussion
3.1. The Optimum Ratio under Standard Curing
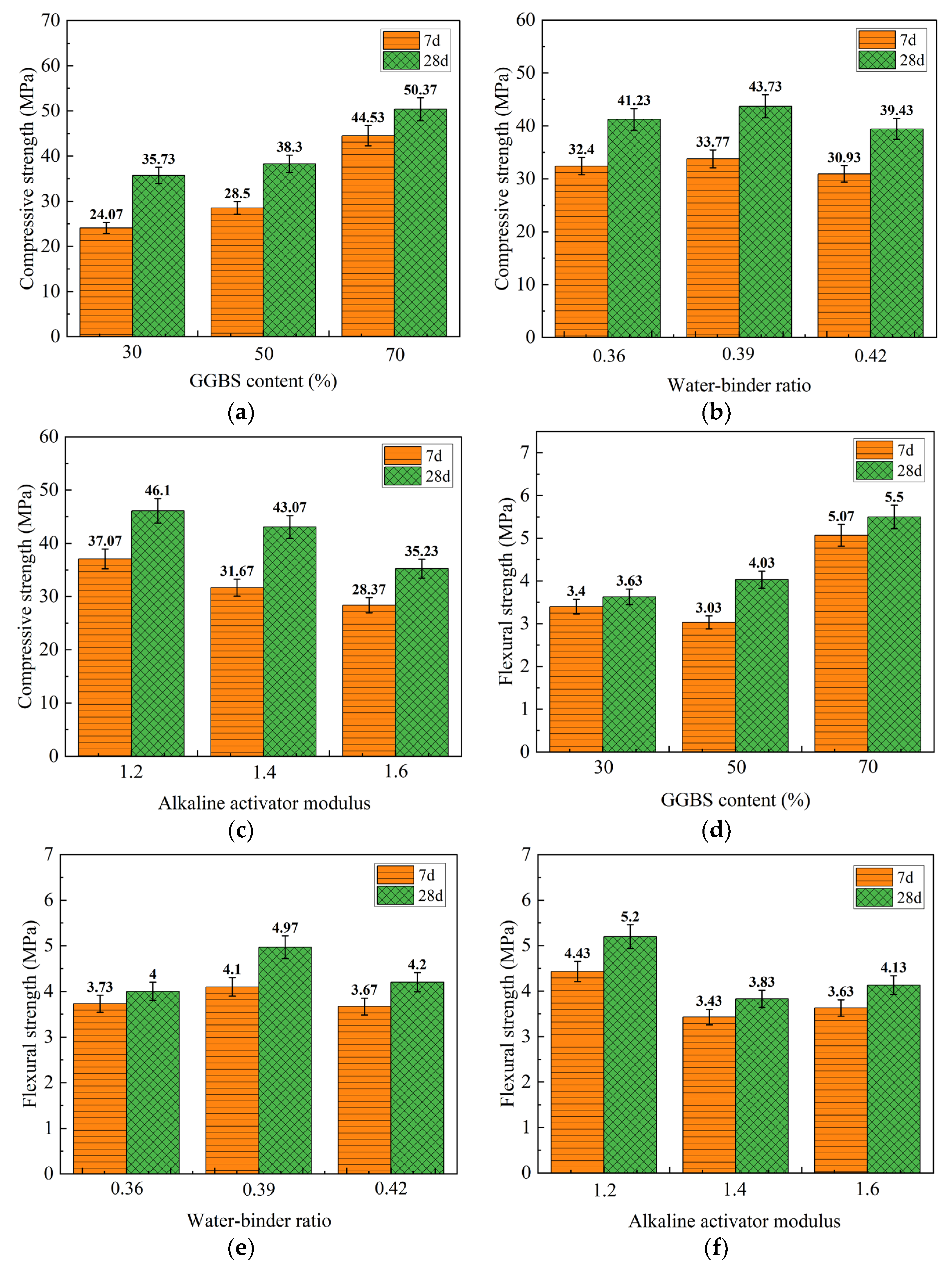
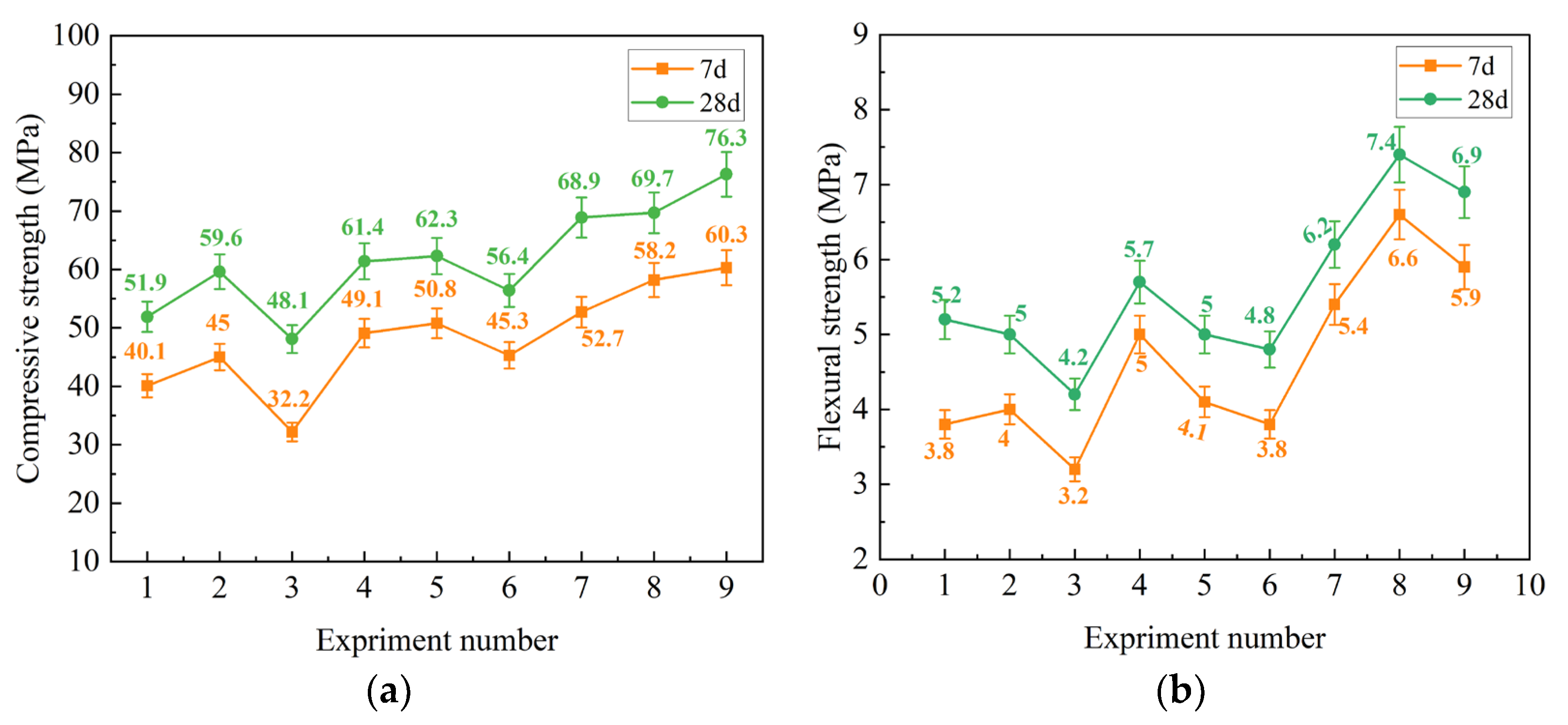
3.1.1. Visual Analysis of Standard Curing Mechanical Property Test Results
3.1.2. Results of Mechanical Property Testing under Standard Curing by Range Analysis
3.1.3. Results of Mechanical Property Testing under Standard Curing by Variance Analysis
3.2. The Optimum Ratio under 40 °C Water Curing
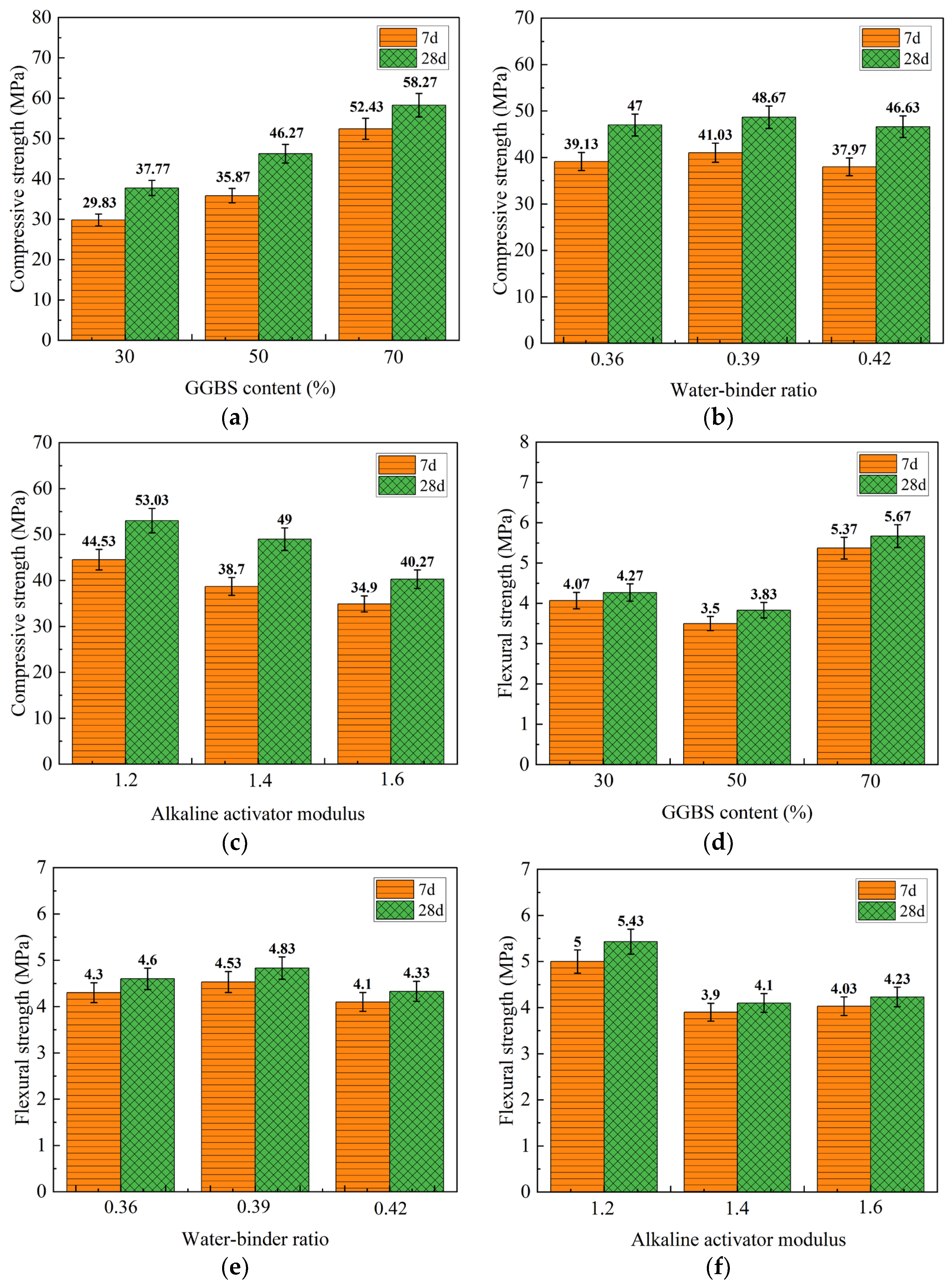
3.2.1. Visual Analysis of 40 °C Water Curing Mechanical Property Test Results
3.2.2. Results of Mechanical Property Testing under 40 °C Water Curing by Range Analysis
3.2.3. Results of Mechanical Property Testing under 40 °C Water Curing by Variance Analysis
3.3. The Optimum Ratio under 40 °C Heat Curing
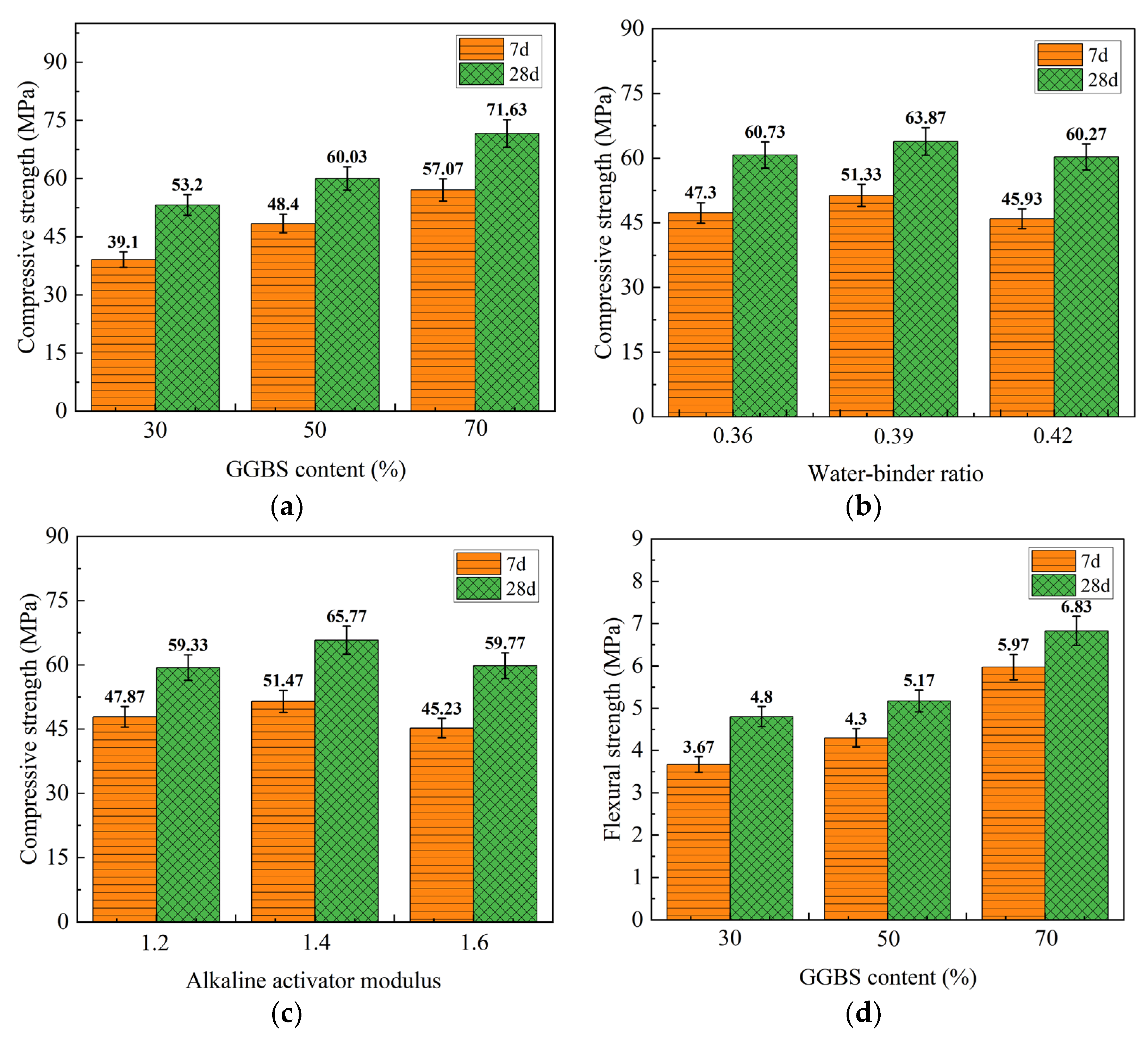
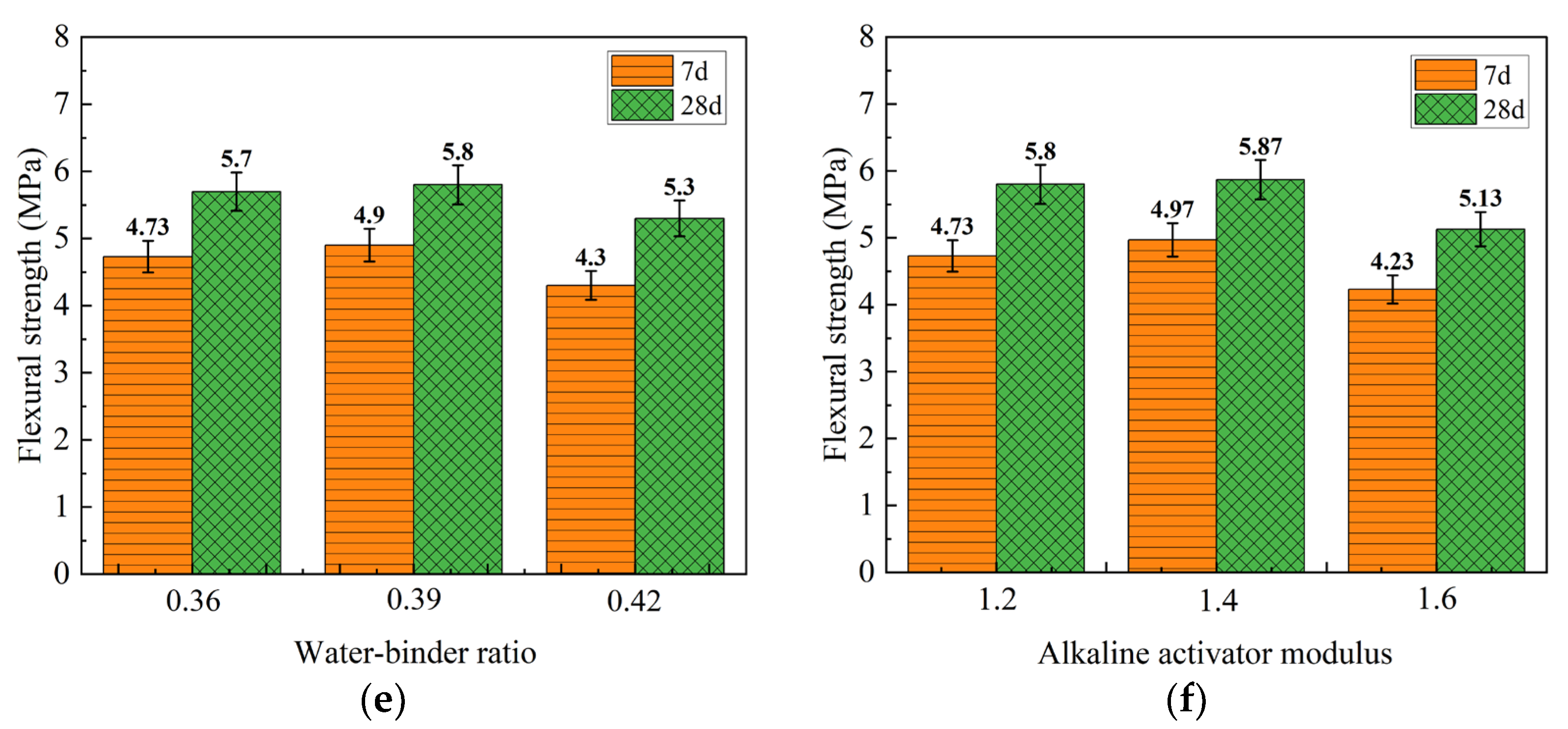
3.3.1. Visual Analysis of 40 °C Heat Curing Mechanical Property Test Results
3.3.2. Results of Mechanical Property Testing under 40 °C Heat Curing by Range Analysis
3.3.3. Results of Mechanical Property Testing under 40 °C Heat Curing by Variance Analysis
4. Strength Prediction Model
5. Microscopic Mechanism Analysis
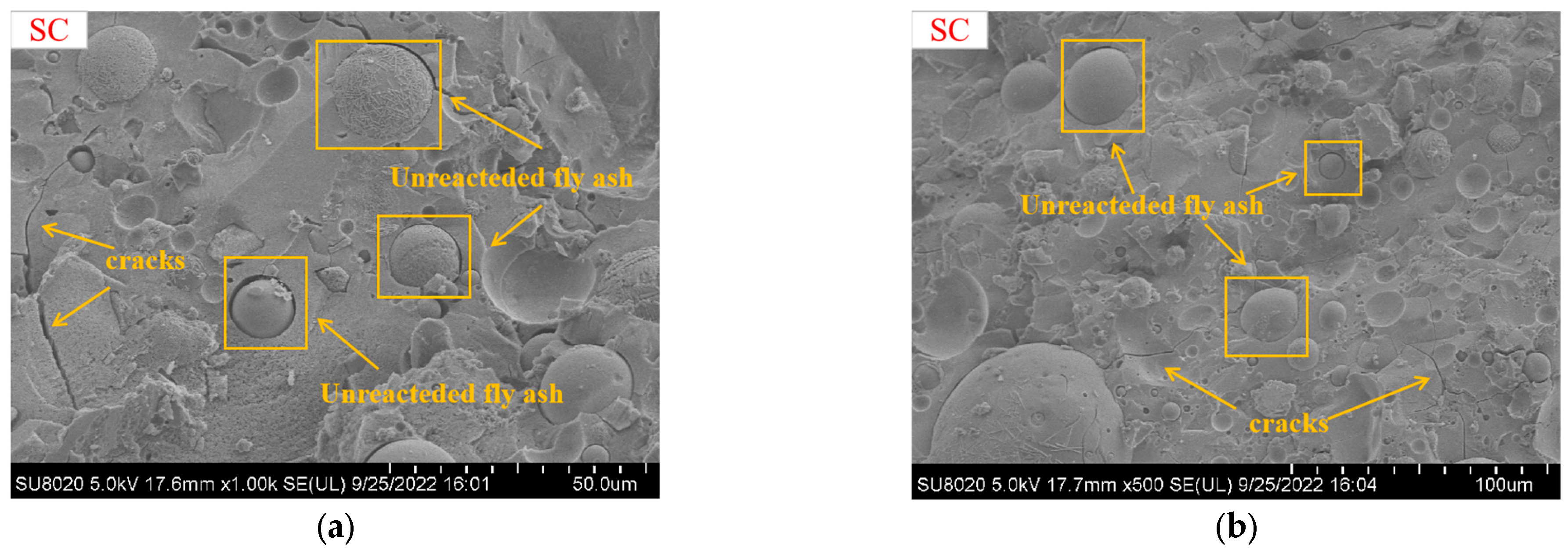
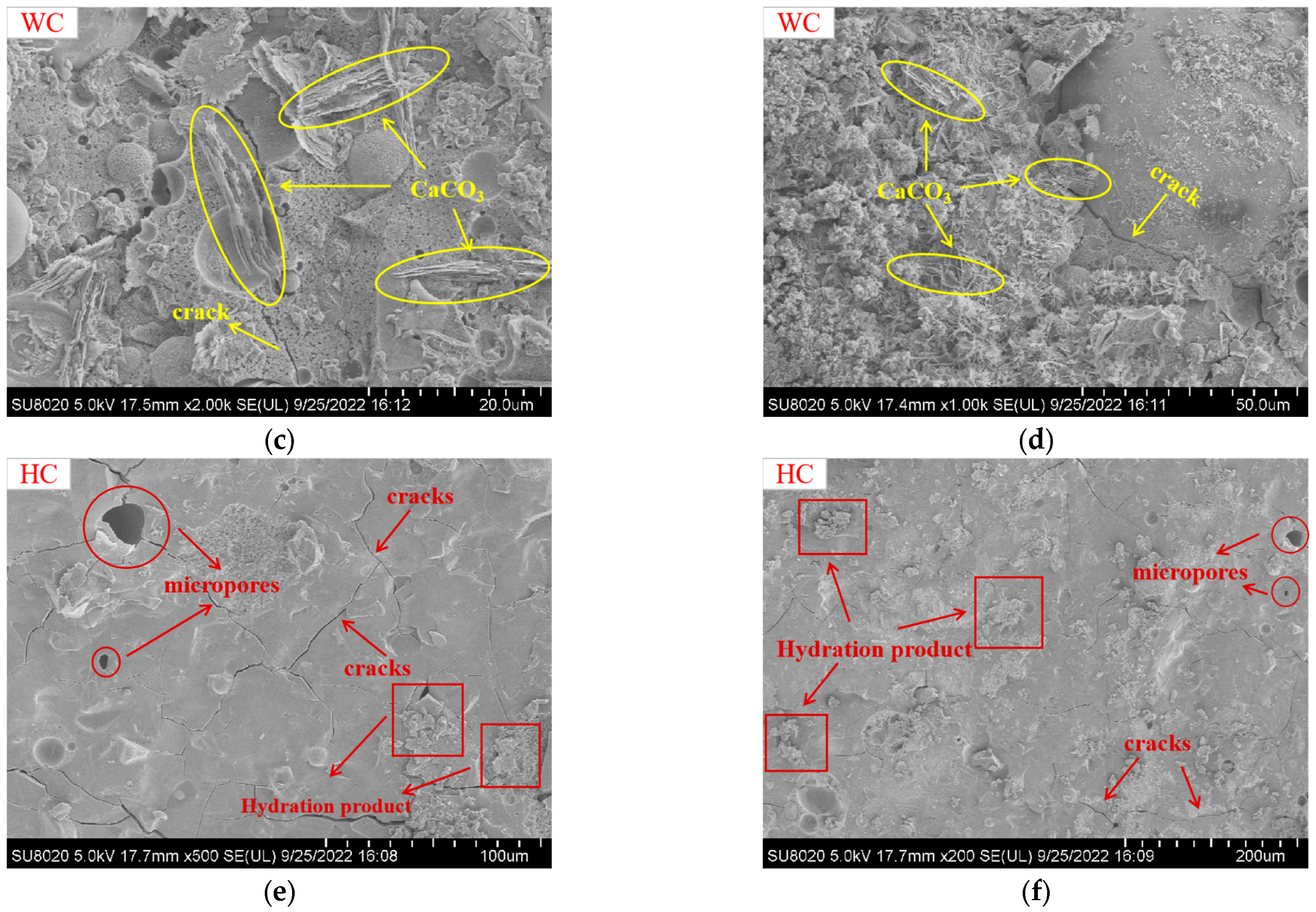
5.1. SEM Analysis
5.2. XRD Analysis
6. Conclusions
- (1)
- Under the standard curing conditions, the compressive strength and flexural strength of 28 days were higher than 7 days, which were attributed to the continuation of the geopolymerization reaction. The contribution of the three influencing factors to the compressive strength and flexural strength of the geopolymers was ranked as follows: GGBS content > alkaline activator modulus > water/binder ratio. Taking the 7 days and 28 days compressive strength and flexural strength of the geopolymer specimens as the evaluation criteria, the optimum ratio of preparing the geopolymer was about a GGBS content of 70%, a water/binder ratio of 0.39, and an alkaline activator modulus of 1.2.
- (2)
- Compared with standard curing, the mechanical properties of the geopolymer prepared by 40 °C water curing were improved, which had a positive effect on the development of geopolymer strength. The contribution of the three influencing factors to the compressive strength and flexural strength of the geopolymers was ranked as follows: GGBS content > alkaline activator modulus > water/binder ratio. Taking the 7 days and 28 days compressive strength and flexural strength of the geopolymer specimens as the evaluation criteria, the optimum ratio of the prepared geopolymer was a GGBS content of 70%, a water/binder ratio of 0.39, and an alkaline activator modulus of 1.2.
- (3)
- In total, 40 °C heat curing accelerated the dissolution of silicate and promoted the formation of more gels. Under the three curing conditions, at 40 °C heat curing compressive strength and flexural strength were the largest. The contribution of the three influencing factors to the compressive strength and flexural strength of the geopolymers was ranked as follows: GGBS content > alkaline activator modulus > water/binder ratio. Taking the 7 days and 28 days compressive strength and flexural strength of the geopolymer specimens as the evaluation criteria, the optimum ratio of the prepared geopolymer was a GGBS content of 70%, a water/binder ratio of 0.39, and an alkaline activator modulus of 1.4.
- (4)
- The prediction model of compressive strength under various curing conditions had been developed. The model demonstrated high accuracy in predicting results and could serve as an important reference tool for engineering applications.
- (5)
- The mechanical properties and microstructures indicated that 40 °C heat curing was the best curing condition, which exhibited a dense internal microstructure and the presence of many C-S-H gels, C-A-S-H gels, and N-A-S-H gels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiapei, D.; Yuhuan, B.; Xuechao, C.; Zhonghou, S.; Baojiang, S. Utilization of alkali-activated slag based composite in deepwater oil well cementing. Constr. Build. Mater. 2018, 186, 114–122. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S. Study on processability, compressive strength, drying shrinkage and evolution mechanisms of microstructures of alkali-activated slag-glass powder cementitious material. Constr. Build. Mater. 2022, 344, 128196. [Google Scholar] [CrossRef]
- Kroehong, W.; Sinsiri, T.; Jaturapitakkul, C.; Chindaprasirt, P. Effect of palm oil fuel ash fineness on the microstructure of blended cement paste. Constr. Build. Mater. 2011, 25, 4095–4104. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-Ngernkham, T.; Li, L.-Y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Yıldırım, G.; Kul, A.; Özçelikci, E.; Şahmaran, M.; Aldemir, A.; Figueira, D.; Ashour, A. Development of alkali-activated binders from recycled mixed masonry-originated waste. J. Build. Eng. 2021, 33, 101690. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Soutsos, M.; Boyle, A.P.; Vinai, R.; Hadjierakleous, A.; Barnett, S.J. Factors influencing the compressive strength of fly ash based geopolymers. Constr. Build. Mater. 2016, 110, 355–368. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Phoo-Ngernkham, T.; Hanjitsuwan, S.; Horpibulsuk, S.; Poowancum, A.; Injorhor, B. Effect of calcium-rich compounds on setting time and strength development of alkali-activated fly ash cured at ambient temperature. Case Stud. Constr. Mater. 2018, 9, e00198. [Google Scholar] [CrossRef]
- Xiang, J.; He, Y.; Liu, L.; Zheng, H.; Cui, X. Exothermic behavior and drying shrinkage of alkali-activated slag concrete by low temperature-preparation method. Constr. Build. Mater. 2020, 262, 120056. [Google Scholar] [CrossRef]
- Suttiprapa, P.; Tangchirapat, W.; Jaturapitakkul, C.; Rattanasak, U.; Jitsangiam, P. Strength behavior and autogenous shrinkage of alkali-activated mortar made from low-calcium fly ash and calcium carbide residue mixture. Constr. Build. Mater. 2021, 312, 125438. [Google Scholar] [CrossRef]
- Jokar, Z.; Mokhtar, A. Policy making in the cement industry for CO2 mitigation on the pathway of sustainable development- A system dynamics approach. J. Clean. Prod. 2018, 201, 142–155. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Jang, J.; Lee, H. Effect of fly ash characteristics on delayed high-strength development of geopolymers. Constr. Build. Mater. 2016, 102, 260–269. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Li, X.; Li, O.X.; Rao, F.; Song, S.; Ortiz-Lara, N.; Aguilar-Reyes, E.A. Microstructural evolution in sulfate solutions of alkali-activated binders synthesized at various calcium contents. J. Mater. Res. Technol. 2020, 9, 10377–10385. [Google Scholar] [CrossRef]
- Xu, F.; Gu, G.; Zhang, W.; Wang, H.; Huang, X.; Zhu, J. Pore structure analysis and properties evaluations of fly ash-based geopolymer foams by chemical foaming method. Ceram. Int. 2018, 44, 19989–19997. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Karim, R.; Hossain, M.; A Elahi, M.; Mohd Zain, M.F. Effects of source materials, fineness and curing methods on the strength development of alkali-activated binder. J. Build. Eng. 2020, 29, 101147. [Google Scholar] [CrossRef]
- Al-Otaibi, S. Durability of concrete incorporating GGBS activated by water-glass. Constr. Build. Mater. 2008, 22, 2059–2067. [Google Scholar] [CrossRef]
- Buchwald, A.; Hilbig, H.; Kaps, C. Alkali-activated metakaolin-slag blends—Performance and structure in dependence of their composition. J. Mater. Sci. 2007, 42, 3024–3032. [Google Scholar] [CrossRef]
- Alanazi, H.; Hu, J.; Kim, Y.-R. Effect of slag, silica fume, and metakaolin on properties and performance of alkali-activated fly ash cured at ambient temperature. Constr. Build. Mater. 2019, 197, 747–756. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Effects of slag substitution on physical and mechanical properties of fly ash-based alkali activated binders (AABs). Cem. Concr. Res. 2019, 122, 118–135. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Chloride binding of alkali-activated slag/fly ash cements. Constr. Build. Mater. 2019, 226, 21–31. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Saha, S.; Rajasekaran, C. Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr. Build. Mater. 2017, 146, 615–620. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shehab, E.; Al-Sallamin, A. Influence of Different Curing Regimes on the Performance and Microstructure of Alkali-Activated Slag Concrete. J. Mater. Civ. Eng. 2018, 30, 04018230. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A. Curing Conditions of Alkali-Activated Fly Ash and Slag Mortar. J. Mater. Civ. Eng. 2020, 32, 04020122. [Google Scholar] [CrossRef]
- Mohamed, O.A. Effect of immersing geopolymer slag-fly ash mortar in sulfuric acid on strength development and stability of mass. Constr. Build. Mater. 2022, 341, 127786. [Google Scholar] [CrossRef]
- Maghsoodloorad, H.; Khalili, H.; Allahverdi, A. Alkali-activated phosphorous slag performance under different curing conditions: Compressive strength, hydration products, and microstructure. J. Mater. Civ. Eng. 2018, 30, 04017253. [Google Scholar] [CrossRef]
- Dehghani, A.; Aslani, F.; Panah, N.G. Effects of initial SiO2/Al2O3 molar ratio and slag on fly ash-based ambient cured geopolymer properties. Constr. Build. Mater. 2021, 293, 123527. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Mechanical and microstructural evolutions of fly ash/slag-based geopolymer at high temperatures: Effect of curing conditions. Ceram. Int. 2023, 49, 2091–2101. [Google Scholar] [CrossRef]
- El-Feky, M.; Kohail, M.; El-Tair, A.; Serag, M. Effect of microwave curing as compared with conventional regimes on the performance of alkali activated slag pastes. Constr. Build. Mater. 2020, 233, 117268. [Google Scholar] [CrossRef]
- Kürklü, G. The effect of high temperature on the design of blast furnace slag and coarse fly ash-based geopolymer mortar. Compos. Part B Eng. 2016, 92, 9–18. [Google Scholar] [CrossRef]
- Gholampour, A.; Ho, V.D.; Ozbakkaloglu, T. Ambient-cured geopolymer mortars prepared with waste-based sands: Mechanical and durability-related properties and microstructure. Compos. Part B Eng. 2019, 160, 519–534. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, M.; Ye, Y. Formulation and properties of a newly developed powder geopolymer grouting material. Constr. Build. Mater. 2020, 258, 120304. [Google Scholar] [CrossRef]
- Ma, H.; Wu, C. Mechanical and microstructural properties of alkali-activated fly ash-slag material under sustained moderate temperature effect. Cem. Concr. Compos. 2022, 134, 104744. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Li, W.; Shi, G.; Lu, P. Effect of slag on the mechanical properties and bond strength of fly ash-based engineered geopolymer composites. Compos. Part B Eng. 2019, 164, 747–757. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-ash based geopolymer mortar for high-temperature application—Effect of slag addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, W.; Su, X.; Xie, X. Assessment and prediction of the mechanical properties of ternary geopolymer concrete. Front. Struct. Civ. Eng. 2022, 16, 1436–1452. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Jiao, Z. Influence of Curing on the Strength Development of Calcium-Containing Geopolymer Mortar. Materials 2013, 6, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Li, X.; Yu, Q. Effect of curing conditions on freeze-thaw resistance of geopolymer mortars containing various calcium resources. Constr. Build. Mater. 2021, 313, 125507. [Google Scholar] [CrossRef]
- State Bureau of Quality and Technical Supervision. Cement Mortar Strength Test Method (ISO Method); State Bureau of Quality and Technical Supervision: Beijing, China, 1999. [Google Scholar]
- Cheng, H.; Lin, K.-L.; Cui, R.; Hwang, C.-L.; Cheng, T.-W.; Chang, Y.-M. Effect of solid-to-liquid ratios on the properties of waste catalyst–metakaolin based geopolymers. Constr. Build. Mater. 2015, 88, 74–83. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Han, Z.; Song, S.; Li, J. Study on the preparation and combustion performance of Al F composites prepared by a solvent/non-solvent method. Surf. Coat. Technol. 2022, 440, 128455. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Xing, J.; Sun, X. Fly Ash/Blast Furnace Slag-Based Geopolymer as a Potential Binder for Mine Backfilling: Effect of Binder Type and Activator Concentration. Adv. Mater. Sci. Eng. 2019, 2019, 060803. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; van Deventer, J.S. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Prusty, J.K.; Pradhan, B. Investigation on effect of precursor materials and sand-to-binder ratio on strength development, acid resistance and microstructure evolution of geopolymer mortar. Constr. Build. Mater. 2022, 346, 128501. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Brouwers, H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Liu, J.; Su, X.; Yan, F. Experimental investigation on the effect of geopolymer adhesive on the bond behavior between CFRP and concretes. Polym. Compos. 2022, 43, 3259–3275. [Google Scholar] [CrossRef]
- Zhang, L.; Suleiman, A.; Nehdi, M. Self-healing in fiber-reinforced alkali-activated slag composites incorporating different additives. Constr. Build. Mater. 2020, 262, 120059. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Abdullah, M.M.A.B.; Cheng-Yong, H.; Hussin, K. Effect of Sodium Hydroxide Molarity on Physical, Mechanical and Thermal Conductivity of Metakaolin Geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 343, 012015. [Google Scholar] [CrossRef]
- Hu, X.; Shi, C.; Shi, Z.; Zhang, L. Compressive strength, pore structure and chloride transport properties of alkali-activated slag/fly ash mortars. Cem. Concr. Compos. 2019, 104, 103392. [Google Scholar] [CrossRef]
- Jittin, V.; Madhuri, P.; Santhanam, M.; Bahurudeen, A. Influence of preconditioning and curing methods on the durability performance of alkali-activated binder composites. Constr. Build. Mater. 2021, 311, 125346. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Du, Z.; Zhou, S.; Zhang, Y.; Li, F. Recycling and utilization assessment of steel slag in metakaolin based geopolymer from steel slag by-product to green geopolymer. Constr. Build. Mater. 2021, 305, 124654. [Google Scholar] [CrossRef]
- Nie, W.; Yi, S.; Xu, C.; Zhang, S.; Peng, H.; Ma, Q.; Guo, C.; Cha, X.; Jiang, C. Numerical simulation analysis of a combined wind-fog dust removal device in return air roadways based on an orthogonal test. Powder Technol. 2023, 413, 117890. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Effect of admixtures on properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Tian, X.; Xu, W.; Song, S.; Rao, F.; Xia, L. Effects of curing temperature on the compressive strength and microstructure of copper tailing-based geopolymers. Chemosphere 2020, 253, 126754. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Chen, B.; Oderji, S.Y.; Haque, M.A.; Ahmad, M.R. Improvement of early strength of fly ash-slag based one-part alkali activated mortar. Constr. Build. Mater. 2020, 246, 118533. [Google Scholar] [CrossRef]
- El-Hassan, H.; Elkholy, S.A. Performance evaluation and microstructure characterization of steel fiber–reinforced alkali-activated slag concrete incorporating fly ash. J. Mater. Civ. Eng. 2019, 31, 04019223. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Shoaei, P.; Musaeei, H.R.; Mirlohi, F.; Zamanabadi, S.N.; Ameri, F.; Bahrami, N. Waste ceramic powder-based geopolymer mortars: Effect of curing temperature and alkaline solution-to-binder ratio. Constr. Build. Mater. 2019, 227, 116686. [Google Scholar] [CrossRef]
- Viravaidya, C.; Li, M.; Mann, S. Microemulsion-based synthesis of stacked calcium carbonate (calcite) superstructures. Chem. Commun. 2004, 19, 2182–2183. [Google Scholar] [CrossRef]
- Williams, P.; Biernacki, J.J.; Walker, L.R.; Meyer, H.; Rawn, C.J.; Bai, J. Microanalysis of alkali-activated fly ash–CH pastes. Cem. Concr. Res. 2002, 32, 963–972. [Google Scholar] [CrossRef]
- Sajan, P.; Jiang, T.; Lau, C.; Tan, G.; Ng, K. Combined effect of curing temperature, curing period and alkaline concentration on the mechanical properties of fly ash-based geopolymer. Clean. Mater. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Mo, B.-H.; Zhu, H.; Cui, X.-M.; He, Y.; Gong, S.-Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, Y.; Zhang, J.; Men, J.; Sun, T.; Zhao, H.; Wu, H.; Wang, H.; Dai, S. Orthogonal analysis and mechanism of compressive strength and microstructure of the metakaolin-fly ash geopolymer. Case Stud. Constr. Mater. 2022, 17, e01154. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, H.; Gu, X.L.; Zhang, W.P. Failure mode-based calculation method for bending bearing capacities of normal cross-sections of corroded reinforced concrete beams. Eng. Struct. 2022, 258, 114113. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, H.; Gu, X.L.; Zhang, W.P. Failure mode-based calculation method for bearing capacities of corroded RC columns under eccentric compression. Eng. Struct. 2023, 285, 116038. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, H.; Gu, X.L.; Zhang, W.P. Calibration analysis of calculation formulas for shear capacities of corroded RC beams. Eng. Struct. 2023, 286, 116090. [Google Scholar] [CrossRef]




| Fly Ash (FA) | Ground Granulated Blast Furnace Slag (GGBS) | |
|---|---|---|
| Composition (mass % as oxide) | ||
| Calcium oxide (CaO) | 11.85 | 34.0 |
| Silica (SiO2) | 45.1 | 34.5 |
| Alumina (Al2O3) | 24.2 | 17.7 |
| Iron Oxide (Fe2O3) | 0.85 | 1.03 |
| Magnesium oxide (MgO) | 1.26 | 6.01 |
| Sulfur trioxide (SO3) | 2.1 | 1.64 |
| Physical characteristics | ||
| Loss on ignition | 2.8 | 0.84 |
| Specific surface area (m2/kg) | 450 | 429 |
| Level | Factor A | Factor B | Factor C |
|---|---|---|---|
| GGBS Content (g) | Water/Binder Ratio | Alkaline Activator Modulus | |
| 1 | 30% | 0.36 | 1.2 |
| 2 | 50% | 0.39 | 1.4 |
| 3 | 70% | 0.42 | 1.6 |
| Test Number | Factors | Mass Distribution (g) | Alkaline Activator (g) | |||||
|---|---|---|---|---|---|---|---|---|
| GGBS Content (A) | Water/Binder Ratio (B) | Alkaline Activator Modulus (C) | Fly Ash (FA) | Ground Granulated Blast Furnace Slag (GGBS) | NaOH | Na2SiO3 | Water | |
| 1 | 30% | 0.36 | 1.2 | 840 | 360 | 52.27 | 323.6 | 252.4 |
| 2 | 30% | 0.39 | 1.4 | 840 | 360 | 44.53 | 342.9 | 269.1 |
| 3 | 30% | 0.42 | 1.6 | 840 | 360 | 38.1 | 364.7 | 283.3 |
| 4 | 50% | 0.36 | 1.4 | 600 | 600 | 44.53 | 342.9 | 233.1 |
| 5 | 50% | 0.39 | 1.6 | 600 | 600 | 38.1 | 364.7 | 247.3 |
| 6 | 50% | 0.42 | 1.2 | 600 | 600 | 52.27 | 323.6 | 324.4 |
| 7 | 70% | 0.36 | 1.6 | 840 | 360 | 38.1 | 364.7 | 211.3 |
| 8 | 70% | 0.39 | 1.2 | 840 | 360 | 52.27 | 323.6 | 288.4 |
| 9 | 70% | 0.42 | 1.4 | 840 | 360 | 44.53 | 342.9 | 305.1 |
| Level | 7 Days and 28 Days Compressive Strength | 7 Days and 28 Days Flexural Strength | ||||
|---|---|---|---|---|---|---|
| Factor A | Factor B | Factor C | Factor A | Factor B | Factor C | |
| K1 | 72.21 (107.19) | 97.20 (123.69) | 111.21 (138.30) | 10.20 (10.89) | 11.19 (12.00) | 13.29 (15.60) |
| K2 | 85.50 (114.90) | 101.31 (131.19) | 95.01 (129.21) | 9.09 (12.09) | 12.30 (14.91) | 10.29 (11.49) |
| K3 | 133.59 (151.11) | 92.79 (118.29) | 85.11 (105.69) | 15.21 (16.50) | 11.01 (12.60) | 10.89 (12.39) |
| k1 | 24.07 (35.73) | 32.40 (41.23) | 37.07 (46.10) | 3.40 (3.63) | 3.73 (4.00) | 4.43 (5.20) |
| k2 | 28.50 (38.30) | 33.77 (43.73) | 31.67 (43.07) | 3.03 (4.03) | 4.01 (4.97) | 3.43 (3.83) |
| k3 | 44.53 (50.37) | 30.93 (39.43) | 28.37 (35.23) | 5.07 (5.50) | 3.67 (4.20) | 3.63 (4.13) |
| R | 20.47 (14.63) | 2.84 (4.3) | 8.70 (10.87) | 2.04 (1.87) | 0.43 (0.97) | 1.00 (1.37) |
| Factor | Deviation Sum of Squares | Degrees of Freedom | F Value | Fa | Significant Level |
|---|---|---|---|---|---|
| GGBS content | 695.607 (366.327) | 2 | 239.287 (86.255) | F0.05 (2, 2) = 19 | ** (*) |
| Water/binder ratio | 12.047 (27.980) | 2 | 4.144 (6.588) | F0.01 (2, 2) = 99 | / |
| Alkaline activator modulus | 115.740 (188.647) | 2 | 39.814 (44.419) | * (*) | |
| Error | 2.91 (4.25) | 2 |
| Factor | Deviation Sum of Squares | Degrees of Freedom | F Value | Fa | Significant Level |
|---|---|---|---|---|---|
| GGBS content | 7.047 (5.796) | 2 | 81.000 (42.618) | F0.05 (2, 2) = 19 | * (*) |
| Water/binder ratio | 0.327 (1.562) | 2 | 3.759 (11.485) | F0.01 (2, 2) = 99 | / |
| Alkaline activator modulus | 1.680 (3.06) | 2 | 19.310 (22.765) | * (*) | |
| Error | 0.09 (0.14) | 2 |
| Level | 7 Days and 28 Days Compressive Strength | 7 Days and 28 Days Flexural Strength | ||||
|---|---|---|---|---|---|---|
| Factor A | Factor B | Factor C | Factor A | Factor B | Factor C | |
| K1 | 89.49 (113.31) | 117.39 (141.00) | 133.59 (159.09) | 12.21 (12.81) | 12.90 (13.80) | 15.00 (16.29) |
| K2 | 107.61 (138.81) | 123.09 (146.01) | 116.19 (147.00) | 10.50 (11.49) | 13.59 (14.49) | 11.70 (12.30) |
| K3 | 157.29 (174.81) | 113.91 (139.89) | 104.70 (120.81) | 16.11 (17.01) | 12.30 (12.99) | 12.09 (12.69) |
| k1 | 29.83 (37.77) | 39.13 (47.00) | 44.53 (53.03) | 4.07 (4.27) | 4.30 (4.60) | 5.00 (5.43) |
| k2 | 35.87 (46.27) | 41.03 (48.67) | 38.70 (49.00) | 3.50 (3.83) | 4.53 (4.83) | 3.90 (4.10) |
| k3 | 52.43 (58.27) | 37.97 (46.63) | 34.90 (40.27) | 5.37 (5.67) | 4.10 (4.33) | 4.03 (4.23) |
| R | 22.60 (20.50) | 3.06 (2.04) | 9.63 (12.76) | 1.87 (1.84) | 0.43 (0.50) | 1.10 (1.33) |
| Factor | Deviation Sum of Squares | Degrees of Freedom | F Value | Fa | Significant Level |
|---|---|---|---|---|---|
| GGBS content | 821.616 (636.500) | 2 | 219.919 (107.390) | F0.05 (2, 2) = 19 | ** (**) |
| Water/binder ratio | 14.376 (7.047) | 2 | 3.848 (1.189) | F0.01 (2, 2) = 99 | / |
| Alkaline activator modulus | 141.269 (255.527) | 2 | 37.813 (43.112) | * (*) | |
| Error | 3.74 (5.93) | 2 |
| Factor | Deviation Sum of Squares | Degrees of Freedom | F Value | Fa | Significant Level |
|---|---|---|---|---|---|
| GGBS content | 5.496 (5.509) | 2 | 50.422 (189.966) | F0.05 (2, 2) = 19 | * (**) |
| Water/binder ratio | 0.282 (0.376) | 2 | 2.587 (12.966) | F0.01 (2, 2) = 99 | / |
| Alkaline activator modulus | 2.162 (3.236) | 2 | 19.835 (111.586) | * (**) | |
| Error | 0.11 (0.03) | 2 |
| Level | 7 Days and 28 Days Compressive Strength | 7 Days and 28 Days Flexural Strength | ||||
|---|---|---|---|---|---|---|
| Factor A | Factor B | Factor C | Factor A | Factor B | Factor C | |
| K1 | 117.30 (159.60) | 141.90 (182.19) | 143.61 (177.99) | 11.01 (14.40) | 14.19 (17.10) | 14.19 (17.40) |
| K2 | 145.20 (180.09) | 153.99 (191.61) | 154.41 (197.31) | 12.90 (15.51) | 14.70 (17.40) | 14.91 (17.61) |
| K3 | 171.21 (214.89) | 137.79 (180.81) | 135.69 (179.31) | 17.91 (20.49) | 12.90 (15.90) | 12.69 (15.39) |
| k1 | 39.10 (53.20) | 47.30 (60.73) | 47.87 (59.33) | 3.67 (4.80) | 4.73 (5.70) | 4.73 (5.80) |
| k2 | 48.40 (60.03) | 51.33 (63.87) | 51.47 (65.77) | 4.30 (5.17) | 4.90 (5.80) | 4.97 (5.87) |
| k3 | 57.07 (71.63) | 45.93 (60.27) | 45.23 (59.77) | 5.97 (6.83) | 4.30 (5.30) | 4.23 (5.13) |
| R | 17.97 (18.43) | 5.40 (3.60) | 6.24 (6.44) | 2.30 (2.03) | 0.60 (0.50) | 0.74 (0.74) |
| Factor | Deviation Sum of Squares | Degrees of Freedom | F Value | Fa | Significant Level |
|---|---|---|---|---|---|
| GGBS content | 484.402 (521.042) | 2 | 20.148 (24.483) | F0.05 (2, 2) = 19 | * (*) |
| Water/binder ratio | 47.296 (22.996) | 2 | 1.967 (1.081) | F0.01 (2, 2) = 99 | / |
| Alkaline activator modulus | 58.749 (77.576) | 2 | 2.444 (3.645) | / | |
| Error | 24.04 (21.28) | 2 |
| Factor | Deviation Sum of Squares | Degrees of Freedom | F Value | Fa | Significant Level |
|---|---|---|---|---|---|
| GGBS content | 8.469 (7.047) | 2 | 19.424 (21.550) | F0.05 (2, 2) = 19 | * (*) |
| Water/binder ratio | 0.576 (0.420) | 2 | 1.321 (1.284) | F0.01 (2, 2) = 99 | / |
| Alkaline activator modulus | 0.842 (0.987) | 2 | 1.931 (3.018) | / | |
| Error | 0.44 (0.33) | 2 |
| 7 Days | 28 Days | |||||
|---|---|---|---|---|---|---|
| Non-Standardized Coefficient | Standardized Coefficient | Non-Standardized Coefficient | Standardized Coefficient | |||
| Regression Coefficient | Standard Error | Regression Coefficient | Standard Error | |||
| Constant | 46.767 | 24.675 | / | 72.908 | 25.097 | / |
| GGBS content | 51.167 | 8.227 | 0.872 | 36.583 | 8.367 | 0.74 |
| Water/binder ratio | −24.444 | 54.844 | −0.062 | −30 | 55.781 | −0.091 |
| Alkaline activator modulus | −21.750 | 8.227 | −0.371 | −27.167 | 8.367 | −0.549 |
| R2 | 0.902 | 0.857 | ||||
| 7 Days | 28 Days | |||||
|---|---|---|---|---|---|---|
| Non-Standardized Coefficient | Standardized Coefficient | Non-Standardized Coefficient | Standardized Coefficient | |||
| Regression Coefficient | Standard Error | Regression Coefficient | Standard Error | |||
| Constant | 52.428 | 23.492 | / | 68.875 | 14.983 | / |
| GGBS content | 56.500 | 7.832 | 0.884 | 51.250 | 4.995 | 0.835 |
| Water/binder ratio | −19.444 | 0.078 | −0.046 | −6.111 | 33.301 | −0.015 |
| Alkaline activator modulus | −24.083 | 7.832 | −0.377 | −31.917 | 4.995 | −0.52 |
| R2 | 0.925 | 0.967 | ||||
| 7 Days | 28 Days | |||||
|---|---|---|---|---|---|---|
| Non-Standardized Coefficient | Standardized Coefficient | Non-Standardized Coefficient | Standardized Coefficient | |||
| Regression Coefficient | Standard Error | Regression Coefficient | Standard Error | |||
| Constant | 43.831 | 29.628 | / | 40.097 | 31.531 | / |
| GGBS content | 44.917 | 9.878 | 0.888 | 46.083 | 10.512 | 0.89 |
| Water/binder ratio | −22.778 | 65.852 | −0.068 | −7.778 | 70.081 | −0.023 |
| Alkaline activator modulus | −6.583 | 9.878 | −0.13 | 1.083 | 10.512 | 0.021 |
| R2 | 0.809 | 0.794 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Shi, X.; Zhang, G.; Li, L. Study the Mechanical Properties of Geopolymer under Different Curing Conditions. Minerals 2023, 13, 690. https://doi.org/10.3390/min13050690
Liu J, Shi X, Zhang G, Li L. Study the Mechanical Properties of Geopolymer under Different Curing Conditions. Minerals. 2023; 13(5):690. https://doi.org/10.3390/min13050690
Chicago/Turabian StyleLiu, Jinliang, Xiaohui Shi, Guanhua Zhang, and Linfei Li. 2023. "Study the Mechanical Properties of Geopolymer under Different Curing Conditions" Minerals 13, no. 5: 690. https://doi.org/10.3390/min13050690
APA StyleLiu, J., Shi, X., Zhang, G., & Li, L. (2023). Study the Mechanical Properties of Geopolymer under Different Curing Conditions. Minerals, 13(5), 690. https://doi.org/10.3390/min13050690






