Study on the Combined Behaviour of Montmorillonite and Carbonate Mineralizing Bacteria on Lead Retention and Fixation
Abstract
1. Introduction
2. Materials and Methods
2.1. Montmorillonite and Its Purification
2.2. Experimental Strains and Cultures
2.3. Pb2+ Retention and Fixation by Montmorillonite and Carbonate Mineralizing Bacteria
2.4. Analysis of Data
3. Results
3.1. Analysis of pH Changes
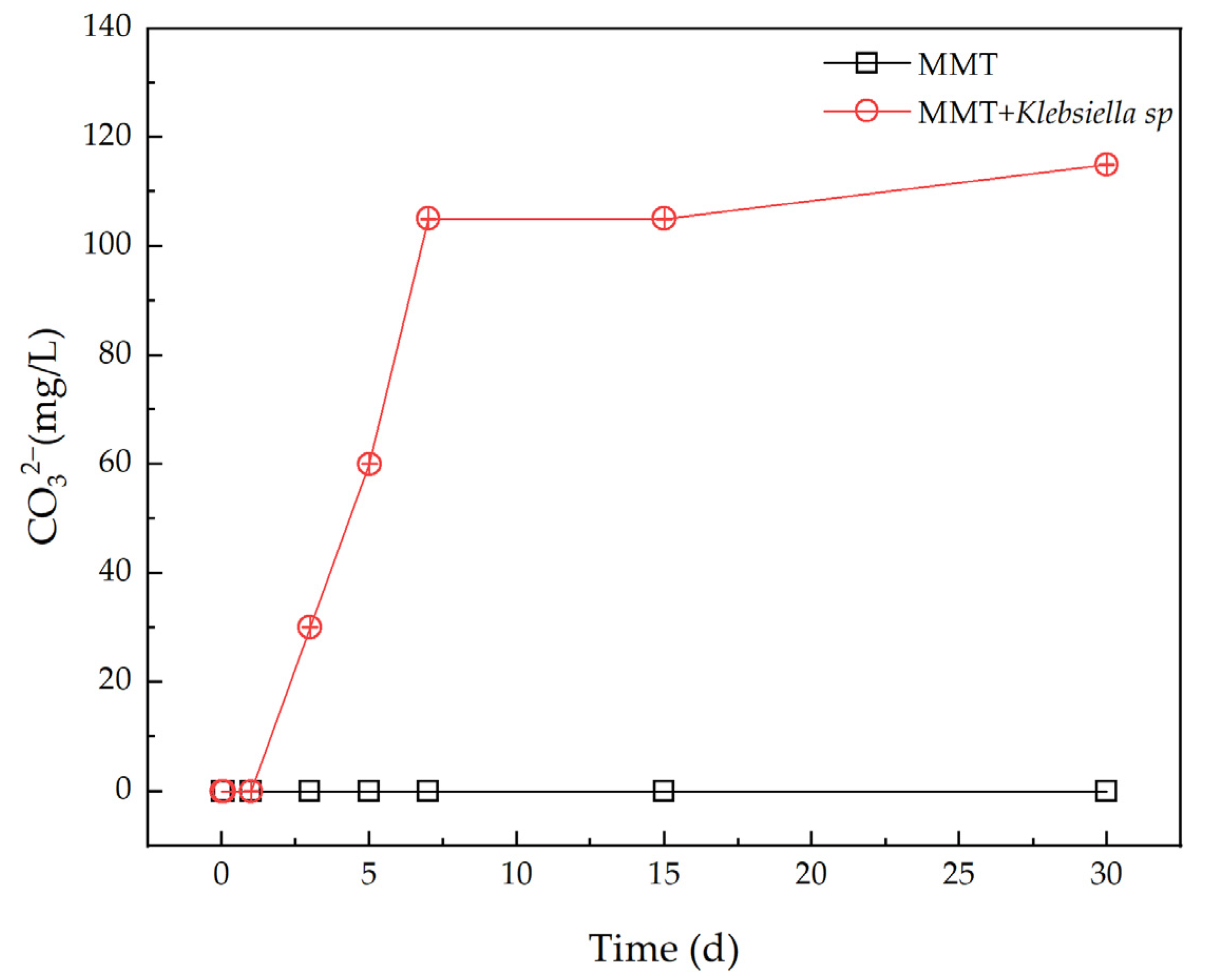
3.2. Analysis of the Variation of CO32− Concentration
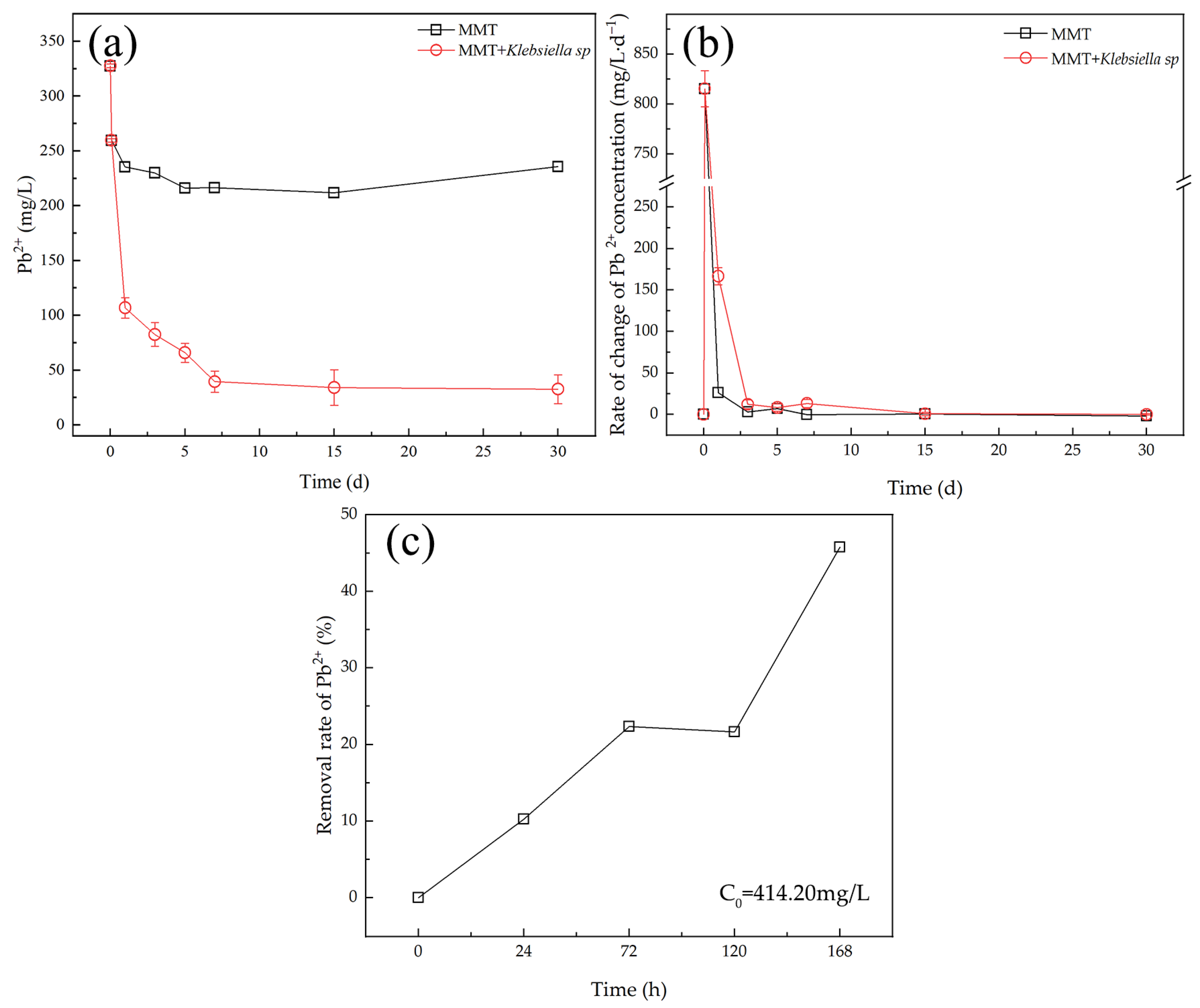
3.3. Analysis of the Variation of Pb2+ Concentration
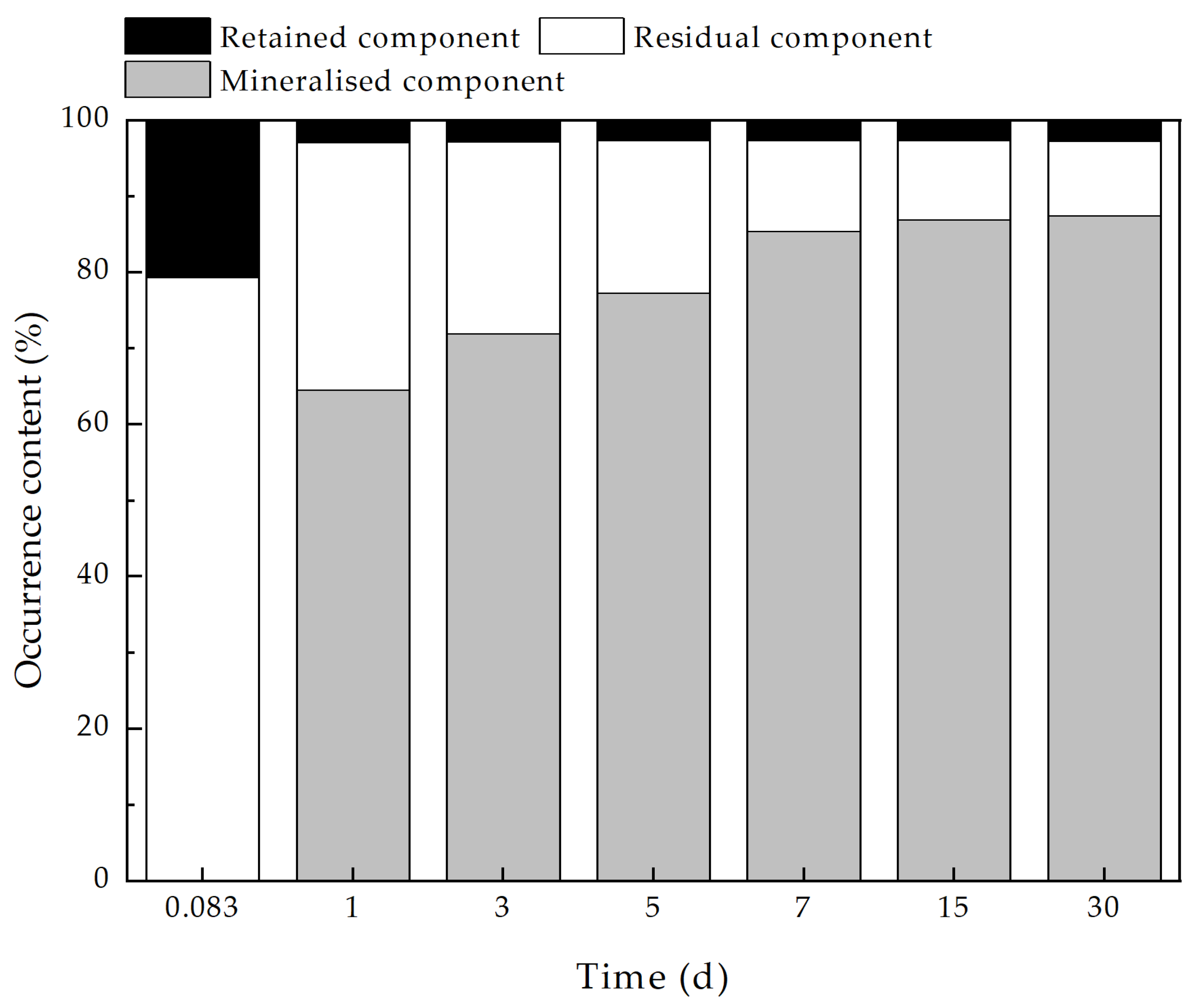
3.4. Analysis of the Variation of Pb2+ Fugitive Content in MMT-CMB Systems
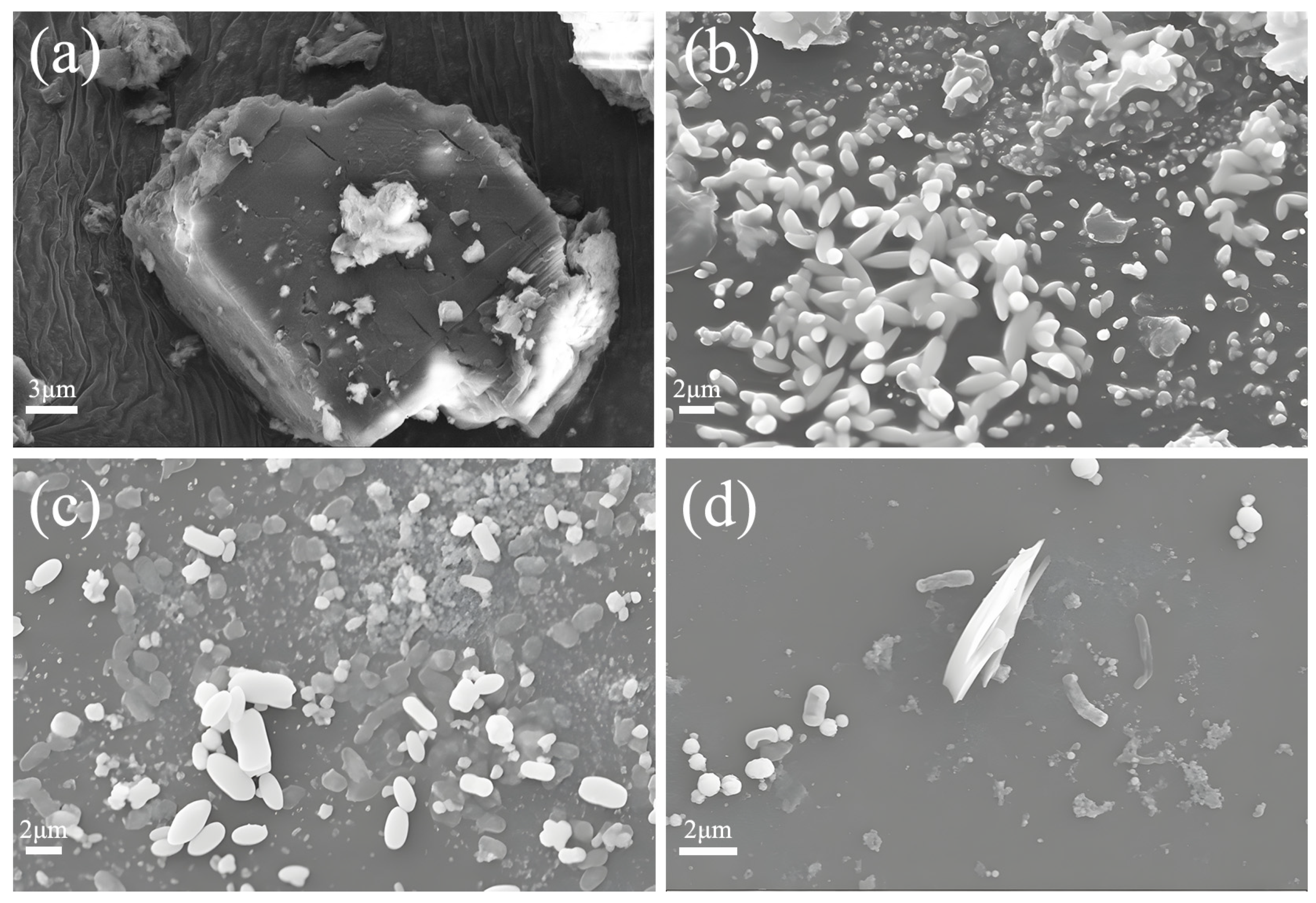
3.5. Analysis of Sediment SEM in the MMT-CMB System
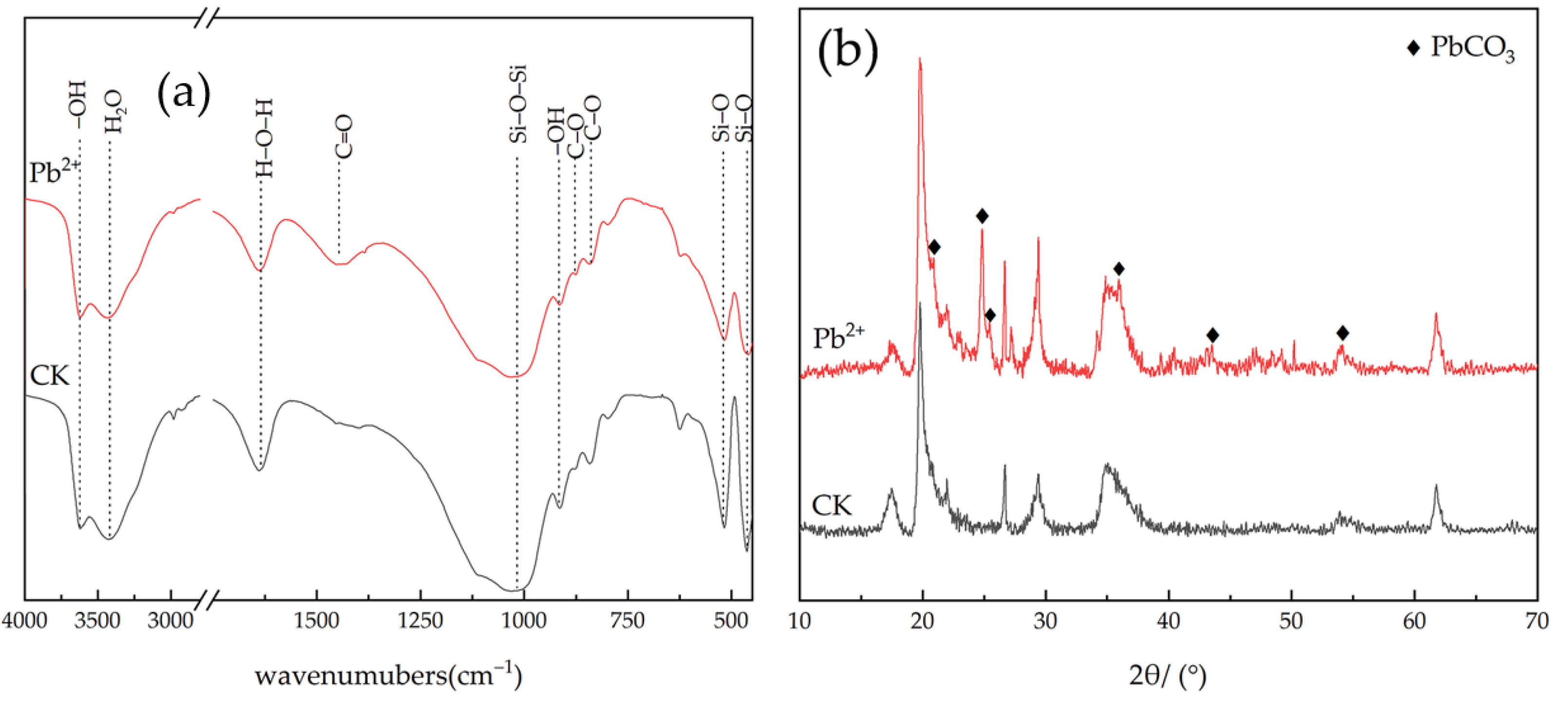
3.6. Analysis of FTIR and XRD Profiles of Sedimentary Minerals
3.6.1. FTIR Analysis
3.6.2. XRD Analysis
4. Discussion
4.1. Montmorillonite Structure and Adsorption Properties
4.2. Mechanism of Combined Removal by Montmorillonite-Carbonate Mineralizing Bacteria
4.3. The Influence of Montmorillonite on Carbonate-Mineralizing Bacterial Growth
4.4. Performance Evaluation of MMT-CMB System for Heavy Metal Pollution Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, X.; Fang, C.; Huang, M.; Achal, V. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. J. Clean. Prod. 2017, 164, 198–208. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Sevak, P.I.; Pushkar, B.K.; Kapadne, P.N. Lead pollution and bacterial bioremediation: A review. Environ. Chem. Lett. 2021, 19, 4463–4488. [Google Scholar] [CrossRef]
- Pal, A.; Bhattacharjee, S.; Saha, J.; Sarkar, M.; Mandal, P. Bacterial survival strategies and responses under heavy metal stress: A comprehensive overview. Crit. Rev. Microbiol. 2022, 48, 327–355. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, P.; Ashraf, M.A.; Aqma, W.S. Microbial stress response to heavy metals in the environment. RSC Adv. 2016, 6, 109862–109877. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food Chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, J.E.; Islam, M.S.; Parvez, S.M.; Raqib, R.; Rahman, M.S.; Muehe, E.M.; Fendorf, S.; Luby, S.P. Prevalence of elevated blood lead levels among pregnant women and sources of lead exposure in rural Bangladesh: A case control study. Environ. Res. 2018, 166, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Zawadzki, M.; Poreba, R.; Gać, P. Mechanisms and toxic effects of lead on the cardiovascular system. Med. Pr. 2006, 57, 543–550. [Google Scholar]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Staessen, J.; Bruaux, P.; Claeys-Thoreau, F.; DePlaen, P.; Ducoffre, G.; Lauwerys, R.; Roels, H.; Rondia, D.; Sartor, F.; Amery, A. The relationship between blood pressure and environmental exposure to lead and cadmium in Belgium. Environ. Health Perspect. 1988, 78, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.V.; Agovino, P.; Niu, X.; Roché, L. Linkage study of cancer risk among lead-exposed workers in New Jersey. Sci. Total Environ. 2007, 372, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Boffetta, P. Lead and Cancer in Humans: Where Are We Now? Am. J. Ind. Med. 2000, 38, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E.K. Facilitative mechanisms of lead as a carcinogen. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2003, 533, 121–133. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Waldherr, M.; Setayesh, T.; Mišík, M.; Wultsch, G.; Filipic, M.; Barcelos, G.R.M.; Knasmueller, S. Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat. Res./Rev. Mutat. Res. 2016, 770, 119–139. [Google Scholar] [CrossRef]
- Rhee, J.; Graubard, B.I.; Purdue, M.P. Blood lead levels and lung cancer mortality: An updated analysis of NHANES II and III. Cancer Med. 2021, 10, 4066–4074. [Google Scholar] [CrossRef]
- Anttila, A.; Heikkilä, P.; Pukkala, E.; Nykyri, E.; Kauppinen, T.; Hernberg, S.; Hemminki, K. Excess lung cancer among workers exposed to lead. Scand. J. Work. Environ. Health 1995, 21, 460–469. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, Y.; Zhao, L.; Chen, Y.; Li, H.; Liu, Y.; Li, L.; Xu, F.; Li, M. Functional wastepaper-montmorillonite composite aerogel for Cd2+ adsorption. Environ. Sci. Pollut. Res. 2020, 27, 38644–38653. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Singh, B.K.; Um, W. Application of Clay Materials for Sorption of Radionuclides from Waste Solutions. Minerals 2023, 13, 239. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Kahkha, M.R.R.; Kaykhaii, M.; Kahkha, B.R.; Khosravi, H.; Tohidlou, E. Simultaneous Removal of Heavy Metals from Wastewater Using Modified Sodium Montmorillonite Nanoclay. Anal. Sci. 2020, 36, 1039–1043. [Google Scholar] [CrossRef]
- Essebaai, H.; Lgaz, H.; Alrashdi, A.A.; Habsaoui, A.; Lebkiri, A.; Marzak, S.; Rifi, E.H. Green and eco-friendly montmorillonite clay for the removal of Cr(III) metal ion from aqueous environment. Int. J. Environ. Sci. Technol. 2022, 19, 2443–2454. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, M.; Li, B.; Dong, Y. Mechanisms, application advances and future perspectives of microbial-induced heavy metal precipitation: A review. Int. Biodeterior. Biodegrad. 2023, 178, 105544. [Google Scholar] [CrossRef]
- Wang, S.; Liu, T.; Xiao, X.; Luo, S. Advances in microbial remediation for heavy metal treatment: A mini review. J. Leather Sci. Eng. 2021, 3, 1. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhong, M.; Jing, C.; Guo, B. Research status and development of microbial induced calcium carbonate mineralization technology. PLoS ONE 2022, 17, e0271761. [Google Scholar] [CrossRef]
- Dauphin, Y. A Brief History of Biomineralization Studies. ACS Biomater. Sci. Eng. 2023, 9, 1774–1790. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of Heavy Metals Zinc, Lead, and Cadmium by Biomineralization of Urease-Producing Bacteria Isolated from Iranian Mine Calcareous Soils. J. Soil Sci. Plant Nutr. 2020, 20, 206–219. [Google Scholar] [CrossRef]
- Dhami, N.K.; Quirin, M.E.C.; Mukherjee, A. Carbonate biomineralization and heavy metal remediation by calcifying fungi isolated from karstic caves. Ecol. Eng. 2017, 103, 106–117. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-Y. An optimum condition of MICP indigenous bacteria with contaminated wastes of heavy metal. J. Mater. Cycles Waste Manag. 2019, 21, 239–247. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.P.; Chen, G.-S.; Huang, Y.-H.; Sun, A.-C.; Chen, C.-Y. Ecofriendly Heavy Metal Stabilization: Microbial Induced Mineral Precipitation (MIMP) and Biomineralization for Heavy Metals within the Contaminated Soil by Indigenous Bacteria. Geomicrobiol. J. 2019, 36, 612–623. [Google Scholar] [CrossRef]
- Kumar, A.; Song, H.-W.; Mishra, S.; Zhang, W.; Zhang, Y.-L.; Zhang, Q.-R.; Yu, Z.-G. Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: A critical review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zeng, G.; Wang, X.; Dai, C.; Sheng, M.; Chen, Q.; Xu, F.; Xu, H. Multiple heavy metals immobilization based on microbially induced carbonate precipitation by ureolytic bacteria and the precipitation patterns exploration. Chemosphere 2021, 274, 129661. [Google Scholar] [CrossRef]
- Khadim, H.J.; Ammar, S.H.; Ebrahim, S.E. Biomineralization based remediation of cadmium and nickel contaminated wastewater by ureolytic bacteria isolated from barn horses soil. Environ. Technol. Innov. 2019, 14, 100315. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, F.; Dai, Q.; Li, G.; Ma, J. Variation of preserving organic matter bound in interlayer of montmorillonite induced by microbial metabolic process. Environ. Sci. Pollut. Res. 2018, 25, 22348–22355. [Google Scholar] [CrossRef]
- Gong, Z.; Liao, L.; Lv, G.; Wang, X. A simple method for physical purification of bentonite. Appl. Clay Sci. 2016, 119, 294–300. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Yao, Q.-Z.; Li, H.; Zhou, G.-T.; Sheng, Y.-M. Formation of Vaterite Mesocrystals in Biomineral-like Structures and Implication for Biomineralization. Cryst. Growth Des. 2015, 15, 1714–1725. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Zhao, H.; Tucker, M.E.; Zhao, Y.; Wu, G.; Zhou, J.; Yin, J.; Zhang, H.; Yan, H. Mechanism of Biomineralization Induced by Bacillus subtilis J2 and Characteristics of the Biominerals. Minerals 2019, 9, 218. [Google Scholar] [CrossRef]
- Al Kausor, M.; Gupta, S.S.; Bhattacharyya, K.G.; Chakrabortty, D. Montmorillonite and modified montmorillonite as adsorbents for removal of water soluble organic dyes: A review on current status of the art. Inorg. Chem. Commun. 2022, 143, 109686. [Google Scholar] [CrossRef]
- Maged, A.; Iqbal, J.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Tuning tetracycline removal from aqueous solution onto activated 2:1 layered clay mineral: Characterization, sorption and mechanistic studies. J. Hazard. Mater. 2020, 384, 121320. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Li, P.; Pan, D. Chapter 1–Radionuclides Sorption on Typical Clay Minerals: Modeling and Spectroscopies. In Interface Science and Technology; Chen, C., Ed.; Emerging Natural and Tailored Nanomaterials for Radioactive Waste Treatment and Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2019; Volume 29, pp. 1–38. [Google Scholar]
- Grančič, P.; Böhm, L.; Gerzabek, M.H.; Tunega, D. On the Nature of Hydrophobic Organic Compound Adsorption to Smectite Minerals Using the Example of Hexachlorobenzene-Montmorillonite Interactions. Minerals 2023, 13, 280. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, Q.; Dong, F.; Han, L.; Yan, W. Strontium Removal by Montmorillonite–Pseudomonas Fluorescens System. Res. Rev. J. Microbiol. Biotechnol. 2016, 5, 39–45. [Google Scholar]
- Wang, G.; Cao, W.; Liang, G.; Xiang, J.; Chen, Y.; Liu, H. Leaching Behavior of Heavy Metals from Pb–Zn Tailings and Remediation by Ca- or Na-Montmorillonite. Water Air Soil Pollut. 2023, 234, 101. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, P.; Cai, M.; Hu, H.; Fu, Q. Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: Kinetic, thermodynamic and equilibrium studies. Appl. Clay Sci. 2017, 143, 320–326. [Google Scholar] [CrossRef]
- Hu, L.; Wang, H.; Xu, P.; Zhang, Y. Biomineralization of hypersaline produced water using microbially induced calcite precipitation. Water Res. 2021, 190, 116753. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Liu, R.; Du, Y.-J.; Bi, Y.-Z. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Naik, S.; Khare, S. Harnessing the bio-mineralization ability of urease producing Serratia marcescens and Enterobacter cloacae EMB19 for remediation of heavy metal cadmium (II). J. Environ. Manag. 2018, 215, 143–152. [Google Scholar] [CrossRef]
- Priya, A.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between microorganisms and clay minerals: New insights and broader applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Ma, J.; Khan, M.A.; Xia, M.; Fu, C.; Zhu, S.; Chu, Y.; Lei, W.; Wang, F. Effective adsorption of heavy metal ions by sodium lignosulfonate reformed montmorillonite. Int. J. Biol. Macromol. 2019, 138, 188–197. [Google Scholar] [CrossRef]
- Gu, X.; Evans, L.J.; Barabash, S.J. Modeling the adsorption of Cd (II), Cu (II), Ni (II), Pb (II) and Zn (II) onto montmorillonite. Geochim. Cosmochim. Acta 2010, 74, 5718–5728. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S. Bioremediation of lead-contaminated mine waste by Pararhodobacter sp. based on the microbially induced calcium carbonate precipitation technique and its effects on strength of coarse and fine grained sand. Ecol. Eng. 2017, 109, 57–64. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Wang, W.; Xu, F.; Zhao, Y.; Zhou, L.; Wang, L.; Jiang, R. Study on the Combined Behaviour of Montmorillonite and Carbonate Mineralizing Bacteria on Lead Retention and Fixation. Minerals 2023, 13, 763. https://doi.org/10.3390/min13060763
Dai Q, Wang W, Xu F, Zhao Y, Zhou L, Wang L, Jiang R. Study on the Combined Behaviour of Montmorillonite and Carbonate Mineralizing Bacteria on Lead Retention and Fixation. Minerals. 2023; 13(6):763. https://doi.org/10.3390/min13060763
Chicago/Turabian StyleDai, Qunwei, Weifu Wang, Fengqin Xu, Yulian Zhao, Lei Zhou, Lihui Wang, and Ruiyang Jiang. 2023. "Study on the Combined Behaviour of Montmorillonite and Carbonate Mineralizing Bacteria on Lead Retention and Fixation" Minerals 13, no. 6: 763. https://doi.org/10.3390/min13060763
APA StyleDai, Q., Wang, W., Xu, F., Zhao, Y., Zhou, L., Wang, L., & Jiang, R. (2023). Study on the Combined Behaviour of Montmorillonite and Carbonate Mineralizing Bacteria on Lead Retention and Fixation. Minerals, 13(6), 763. https://doi.org/10.3390/min13060763




