1. Introduction
Traditional drug delivery methods include oral and injection methods; however, these drug delivery methods often have problems such as high drug dosage, frequent use of drugs, strong irritation to the gastrointestinal tract, non-specific drug release, and unsustainable drug delivery. Therefore, enhancing drugs’ sustained and controlled release is critical for current clinical drug therapy. Drug carrier materials usually require drug carriers to be non-toxic, low-cost, capable of loading an appropriate dose of the drug, and able to release the drug at a stable and effective concentration for a certain period and to the correct target site [
1]. However, in many drug-controlled release systems, the initial explosive effect of drugs often occurs, causing toxic side effects and harm to the body’s organs. Therefore, investigating suitable drug delivery carriers significantly reduces the biological toxicity and adverse effects of therapeutic drugs [
2].
The controlled release carrier of therapeutic drugs refers to a class of carrier preparations that can release the loaded drugs to the target point or target organ in a controlled form at a constant speed (at a low or near-zero-order rate) to play a therapeutic role. Compared with traditional large-size drug carriers (such as collagen, gum Arabic, and alginate), inorganic carrier materials such as calcium carbonate, calcium phosphate, and silicon dioxide are characterized by hollow or porous structure, micro-nano particle size, high hydrophilicity, and specific surface area, which can not only significantly improve the loading rate and utilization rate of ligands or functional, active substances, it can also achieve efficient delivery of water-insoluble drugs [
3]. However, the alginic acid gel is fragile, soluble in water, and has poor tensile properties and traditional manufacturing methods cannot guarantee the consistency of its structure [
4]. The slow degradation rate of silicon dioxide and refractory properties in the body also limit its use [
5]. As a drug carrier, calcium phosphate is more difficult to dissolve. It requires gastric acid dissociation to be absorbed and utilized by the human body, which can easily affect the acidic environment in the stomach. When the level of gastric acid is lacking, low (or non-existent), the dissociation is incomplete, its bioavailability is reduced, and it is more likely to cause gastrointestinal irritation symptoms [
6]. Among them, calcium carbonate is widely present in the teeth, shells, pearls, and gallstones of living organisms. It has excellent biocompatibility and bioavailability, conferring outstanding advantages as a drug carrier. Connecting specific calcium carbonate materials with targeting ligands or functional, active substances to prepare targeted carriers with loaded active substances has enormous potential in targeted drug delivery and controlled release [
7]. The synthesis and structural characteristics of calcium carbonate carrier materials and the research and application progress of calcium carbonate as a drug-controlled release carrier are introduced herein.
2. Synthesis and Structural Characteristics of Calcium Carbonate Carrier Materials
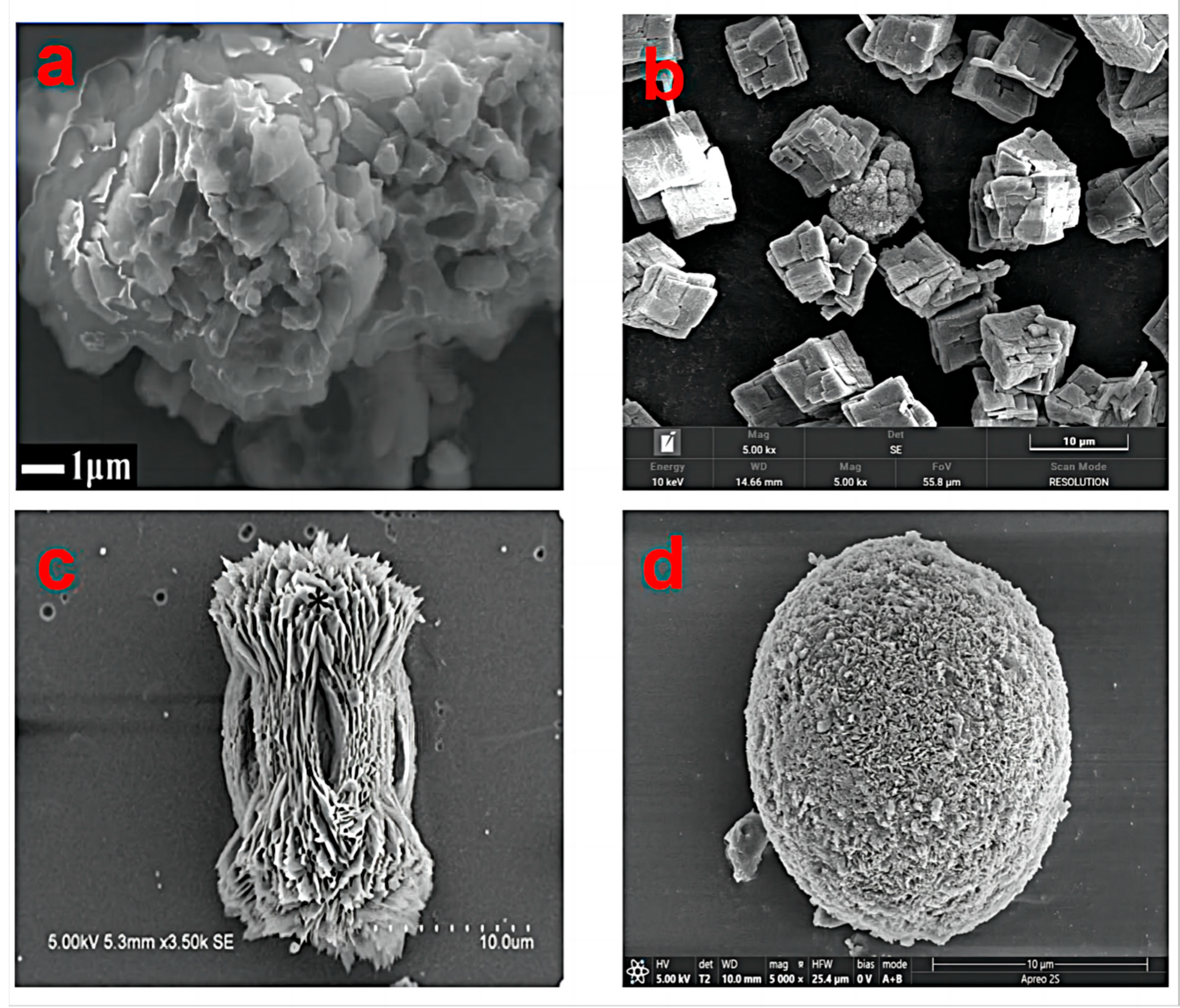
The common forms of calcium carbonate as one of the common inorganic substances on the earth include amorphous calcium carbonate, calcite, aragonite and vaterite (
Figure 1). It exists in chalk, limestone, marble, and other rocks and is the main component of animal bones or shells. In addition, there are several forms of calcium carbonate in the form of hydrate, of which calcite is the most thermodynamic stable crystal phase (other forms are thermodynamically [
8]).
Although calcium carbonate materials are widely distributed, the components of carbonate rocks formed in different geological deposition environments and geological ages vary greatly, especially the post-deposition alteration, which can affect the cementation, secondary pore formation and morphological changes in carbonate particles. In addition, naturally formed carbonate rocks will also contain some amounts of associated minerals—such as silicates, phosphates, glauconite and hydrogenic rock minerals, and it is difficult to ensure a low cost of production and the quality of carrier materials with such raw materials for making drug carriers. Therefore, it is necessary to investigate the effective synthetic methods of calcium carbonate for drug carrier materials to reduce the cost and improve the loading efficiency and controlled release performance of calcium carbonate carriers. At present, related scholars pay much attention to the preparation of porous calcium carbonate materials. Based on the research reports, the main methods for the preparation of porous calcium carbonate can be summarized as shown in
Table 1.
Calcium carbonate is a white fine crystalline powder that is odorless, low-density, and acid-soluble. Crystalline calcium carbonate can also be divided into a rhombic crystal system and a hexagonal crystal system (e.g., anhydrous calcium carbonate is an orthorhombic crystal, while hexahydrate calcium carbonate is a monoclinic crystal), which typically appears as prismatic or rhombic in shape. Aragonite commonly occurs in prismatic, tabular, bipyramidal, and rhombic forms [
15]. Aragonite belongs to the orthorhombic system, the crystal form thereof is columnar or spear-like, and there are often pseudo-hexagonal triple crystals. Vaterite-type calcium carbonate is a polycrystal formed by the aggregation of nanoscale spherical particles belonging to the hexagonal crystal system. The CO
32− triangular plane is oblique to the third axis, resulting in the carbonate group of aragonites being parallel to the C-axis, while the carbonate group of calcites is perpendicular to the C-axis, which also leads to the instability and looseness of vaterite [
16]. Vaterite has a polycrystalline form, so it is difficult to study its crystal structure and characteristics [
3]. The generally recognized structural model of vaterite is the hexagonal symmetric structure proposed by Mugnaioli et al. [
17]. This model is a hypothetical structure model of vaterite proposed by molecular dynamics simulation and optimization geometric calculation methods. Among the three common crystalline calcium carbonate, vaterite type calcium carbonate has more hollow or porous structures, uniform particle size distribution, micro-nano-size, high hydrophilicity, large specific surface area, good dispersion, better biological compatibility and safety, good degradation, strong phase changeability, good mechanical performance and thermal stability, and spherical distribution [
3]. Therefore, vaterite-type calcium carbonate has more application prospects in biomedicine, new materials, and other related disciplines.
As an essential phase in carbonate, vaterite has been found in many geological environments, such as marine and freshwater crustaceans, bird eggs, salmon inner ears, meteorites, rocks in caves, etc. Vaterite is generally considered to be the most thermodynamically unstable. A phase change will occur when it exists in an aqueous solution for 20 to 25 h at room temperature [
18], and it will generally be converted into the stable phase of calcite [
19]. However, compared with vaterite found in the natural environment, vaterite induced by microorganisms under experimental conditions seems more stable. In research by our group, Liu et al. [
20] found that vaterite induced by
Bacillus subtilis can be stored in the air for at least one year, and the crystal form of vaterite in deionized water for one year does not change, which shows that the vaterite induced by bacteria is stable. According to the FTIR analysis results of the vaterite, these biogenic calcium carbonates contain some organic functional groups (such as -OH, -CH
3, -CH
2, -CO-NH, C=O, -COOH, and -NH). It can be inferred that in the process of bio-induced vaterite mineralization, carbonate and calcium ions combined with some organic matter to form vaterite containing organic matter, and these organic substances combined with minerals can prevent the transformation of unstable phase vaterite into stable phase calcite.
Research has shown that vaterite is rich in protein and plays an important role in mineral-induced formation [
13,
21]. Protein can not only serve as a template for the formation of minerals but also plays an important role in the stability of minerals. In vaterite, protein can not only bind to the mineral surface through functional groups such as amino, carboxyl, and hydroxyl groups but also enhance its hydrophobicity and improve its stability through the interaction with those electrons on the mineral surface. Many proteins adsorbed on the surface of the mineral add many active sites to its surface. The increased number of active sites can not only adsorb more organic molecules to cover the surface of the mineral but also change the charge density of the mineral surface, endowing it with a wider range of pH-adaptability [
13,
21]. This enables the biogenic vaterite to maintain good heavy metal adsorption characteristics in an environment with a pH greater than 3.
At present, the commonly used methods for preparing vaterite mainly include the carbonization method and double decomposition method [
3]. The carbonization method refers to the Ca (OH)
2-H
2O-CO
2 reaction system, that is, the alkaline solution containing calcium salt is used as the calcium source to prepare vaterite by introducing CO
2 into the solution. The double decomposition method refers to the Ca
2+-H
2O-CO
32− reaction system, that is, the calcium salt solution and carbonate solution were mixed under certain conditions to prepare vaterite. Several studies have shown that vaterite’s crystal form and morphology can be controlled by using these two methods. Due to the fact that the carbonization method utilizes the reaction between CO
32− obtained from CO
2 dissolution and Ca
2+ to generate calcium carbonate, the rate of calcium carbonate generation depends on the rate of dissolution of CO
2. Under normal temperature and pressure, the dissolution rate of CO
2 is generally low, so the yield of vaterite prepared by the carbonization method is low. The double decomposition method entails direct mixing of the two salt solutions, and CO
32− reacts rapidly with Ca
2+, producing a high yield, endowing the method with good prospects for large-scale application; however, it should be pointed out that the use of this method often requires the addition of surfactants to stabilize the crystal form and morphology of the vaterite. Although vaterite is the most unstable anhydrous form of calcium carbonate, its appearance in nature is rare, and it is difficult to synthesize by spontaneous precipitation in the laboratory [
22]. However, since there are many applications of vaterite particles, exploring the optimal experimental conditions under which to prepare vaterite remains the focus of scholars around the world. Some scholars have studied the synthesis of vaterite from a statistical point of view to determine the optimal experimental conditions for obtaining vaterite [
23]. By creating some if-then decision rules, using oven drying temperature, pH, and salt concentration to control the polycrystallinity, they finally obtained the optimal polycrystalline abundance of vaterite of 93.6 ± 0.3%.
3. Calcium Carbonate as a Controlled-Release Drug Carrier
The history of research on calcium carbonate as a drug carrier can be traced back to the 1960s [
24]. At that time, researchers [
25] used the double oil in a water emulsion system as a biomimetic environment to prepare calcium carbonate particles. They proposed that calcium carbonate can be used as a drug carrier according to the synthesized particle morphology. Since then, the research on calcium carbonate as a drug carrier has gradually gained widespread attention. Currently, the application of calcium carbonate as a drug carrier is mainly used in oral drugs, cancer treatment, topical drugs, bone repair materials, gene delivery and other drug carriers.
(1) Oral drugs: calcium carbonate can be used as a slow-release agent to control the rate of release of the drug, thus enhancing the efficacy of oral drugs. In addition, calcium carbonate can also act as a protective agent to protect the drug from damage by gastric acid. CaCO
3-based composite nanocarriers have the potential for pulmonary aerosol delivery of peptides and proteins and oral delivery of insulin. Liu et al. [
26] demonstrated that in a rat model, better hypoglycemic effects in vivo can be obtained by oral medicine using a calcium carbonate carrier compared to subcutaneous insulin injections. To overcome the insulin absorption barrier in oral delivery, insulin-loaded calcium carbonate/hyaluronic acid composite particles were prepared using reversed-phase microemulsion precipitation and surface coating. The morphology and particle size were characterized by transmission electron microscopy (TEM) and dynamic light scattering. The results showed the composite particles exhibited low cytotoxicity and good resistance to protein degradation. In addition, the endocytosis behavior of the composite particles by HT-29 cells was studied by laser confocal microscopy, and further oral administration study in diabetic rats showed they have a prolonged hypoglycemic effect. Nie et al. [
27] used sodium polystyrene sulphonate as a dispersant to prepare calcite-type CaCO
3 microspheres and loaded them with ibuprofen. The prepared core-shell drug carrier can delay the rate of drug release. Kulsoom et al. [
28] reported on the development of a composite system prepared by biomineralization of calcium carbonate in mucilage obtained from seeds of naturally occurring quince plants. The developed composite showed restricted release of paracetamol in the acidic media, while a controlled fashion of drug release was observed in the basic media over a period of 12 h. Study outcomes suggested that the developed composite can be considered a novel drug carrier system for oral colon-targeted delivery of hydrophilic/hydrophobic drugs. The restricted drug release in an acidic medium and the controlled release fashion of the drug in a basic medium make this composite an attractive model to mitigate risk factors associated with non-steroidal anti-inflammatory drug-associated gastric ulcers. Song et al. [
29] used egg yolk lecithin as a template to prepare CaCO
3 microspheres and selected curcumin (Cur) as a model drug to obtain curcumin calcium carbonate microspheres using a solvent method. The results showed that the curcumin calcium carbonate microspheres prepared when the ratio of Cur to CaCO
3 microspheres was 1:4 could significantly improve the dissolution rate and dissolution of Cur.
(2) Cancer therapy: taking advantage of the sensitivity of drug-loaded calcium carbonate to acidic conditions, the drug can be released slowly after being transported to specific organs or tissues with pH slightly acidic in the body, which can improve the drug utilization rate [
30]. Li et al. [
31] prepared hollow calcium carbonate microspheres with sodium dodecyl sulphate as the surfactant and CaCl
2 and Na
2CO
3 as the substrate and loaded ibuprofen. The results showed that the microspheres had both a good loading rate and rate of drug release; because calcium carbonate is very stable in alkaline body fluid (pH is about 7.4), the loaded drug will barely be released, which can greatly reduce the damage to normal cells. In the acidic environment of cancer cells, calcium carbonate loaded with anticancer drugs such as doxorubicin hydrochloride (DOX) can be released slowly. Wei et al. [
32] prepared hollow calcium carbonate particles using starch as a template and used it as a carrier for the anticancer drug DOX. The results showed that the drug released from the carrier was pH-dependent, which could make the drug gather around the target cancer cell and enter the nucleus to enhance its toxicity to cancer cells. Currently, most cancer chemotherapy drugs, such as DOX, interact with nuclear DNA, resulting in DNA damage and inhibition of topoisomerase activity, thus stopping DNA replication of cancer cells, and leading to cancer cell apoptosis. However, research by Yang et al. [
33] shows that cancer cells have developed several possible resistance mechanisms, such as loss of T cell function β
2 microglobulin gene mutation, and changes in tumor target antigens, Apelin receptor (APLNR) gene mutation, intestinal flora imbalance, and so on. In order to obtain a better anti-cancer effect, the drug carrier prepared by Wei et al. [
32] can directly invade the nucleus of cancer cells, but the process and mechanism of its entry into the nucleus are unclear. In addition to increasing uptake, these behaviors significantly improve the nuclear delivery efficiency of anticancer drugs and obtain better tumor cytotoxicity. Svenskaya et al. [
34] simply adsorbed photosensitizer drugs on calcium carbonate carriers to explore the drug release characteristics of calcium carbonate carriers under different environments. The results showed that the drug release process also showed a dependence on environmental pH value. Photodynamic therapy is a new method for treating tumors, precancerous lesions, proliferative skin diseases, and vascular diseases with photosensitive drugs and laser activation. Irradiation of the focus with a specific wavelength can activate the photosensitive drugs selectively gathered in the focal tissue and cause the destruction of the focus by a luminescent chemical reaction [
35]. The combination of photosensitizers with poor selectivity to aragonite carriers may become a new cancer treatment tool. Qiu et al. [
36] synthesized porous aragonite calcium carbonate microspheres with bovine serum albumin as an additive. The application of aragonite calcium carbonate microspheres loaded with camptothecin showed pH-dependent and sustained cell growth inhibition, both of which are associated with controlled drug release. Han et al. [
37] prepared calcium carbonate microspheres by chemical precipitation and used them as gene carriers with polyethyleneimine (PEI) surface modification to investigate the potential of PEI-modified calcium carbonate microspheres as gene delivery carriers and the cancer inhibitory effects of the recombinant plasmid pEGFP-C1-p53. In vitro experiments confirmed that surface-modified microspheres as a gene delivery vehicle could successfully deliver the gene into cells and express the fusion protein GFP-p53, inhibiting the proliferation of cancer cells. In vivo, animal experiments showed that SCa-PEI-pEGFP-C1-p53 could effectively inhibit tumor growth.
(3) Topical drugs: calcium carbonate mainly acts as an adsorbent and protective agent in topical drugs, enhancing the permeability and stability of the drugs. As mentioned, calcium carbonate can also be used as a slow-release agent to control the rate of release of the drug, thus enhancing the efficacy of the drug. Volodkin et al. [
38] prepared porous calcium carbonate microspheres for loading lactalbumin and applied them in clinical research. The results showed that the aragonite-type calcium carbonate has good drug-loading capacity. The use of high-molecular-weight polymer improves the encapsulation efficiency because the polymer is only adsorbed on the surface of porous particles and does not come into contact with materials previously adsorbed in the pores. The preparation can be used to encapsulate biomaterials, such as proteins, enzymes, and combinations of various bioactive macromolecules. Liu et al. [
26] successfully synthesized Bi nanodots by a rapid reduction method. Then, they prepared Bi-Met-CaCO
3/PVP microneedles by mixing them with gels obtained from metformin, calcium carbonate, and a PVP matrix. The prepared LA/Bi-Met-CaCO
3/PVP composite microneedles had low cytotoxicity and acute toxicity, and the transdermal administration of metformin, a hypoglycemic agent triggered by near-infrared light, was realized. The results showed that the microneedle had a prolonged hypoglycemic effect. This composite microneedle has potential application in transdermal drug delivery.
(4) Special biotherapeutic materials: Kim’s research team [
39] coated calcium carbonate nano-materials into a flexible biocompatible nano-carrier based on Plannik and used it as an ultrasound contrast agent (UECA). The results showed that this nanoscale UECA showed high colloidal stability in the medium containing serum and greatly improved the intensity of ultrasound imaging contrast signal at tumor sites within 1 h: This indicates that nanoscale UECA can be used as an effective diagnostic method for ultrasound imaging of tumors in vivo. The CaCO
3 nanoparticles in the nano-carrier prepared by the research group are not completely crystalline but form a mixture in a relatively unstable state. After intravenous injection of the nano-UECA into tumor-bearing mice, UECA can preferentially target the tumor site within 1 h without targeting ligands and then be rapidly cleared from the body. Liu et al. [
40] prepared sodium alginate-calcium carbonate hybrid nanoparticles with adjustable size and morphology by biomineralization using the assembled sodium alginate micelles as templates. In this study, the assembled sodium alginate polysaccharide micelles were used as the template for calcium carbonate biomineralization to prepare Alg-CaCO
3, and the polyamine (PDA) and cationic polymer PGED (ethylenediamine functionalized poly (glycidyl methacrylate)) were coupled to the surface of Alg-CaCO
3 nanoparticles. On the one hand, the coupled PGED can be used for gene transfection; on the other hand, PDA gives the synthesized Alg-CaCO
3-PDA-PGED (ACDP) carrier photothermal properties, which can be used for mild hyperthermia and photoacoustic imaging. Low-power density laser irradiation can trigger mild hyperthermia and enhance cell uptake. At the same time, the pH-responsive degradation of the gene carrier will further promote the release of the gene, thus improving the efficiency of gene transfection. Enhanced gene therapy can induce tumor cell apoptosis, thus preventing an inflammatory reaction. Liu et al. [
41] established a novel nanoplatform consisting of calcium carbonate-coated gold nanostars (GNSs) and indocyanine green (ICG). In combined photodynamic/photothermal therapy (PDT/PTT), calcium carbonate acts as a drug retention agent to sandwich ICG in the form of stable aggregates on the surface of GNSs to avoid blood clearance and achieve efficient local release of tumor-triggered drugs. Applying GNS@CaCO
3/ICG to both in vitro and in vivo treatment, a combined anti-tumor effect was achieved under near-infrared(NIR) irradiation, which is much better than single PDT or PTT.
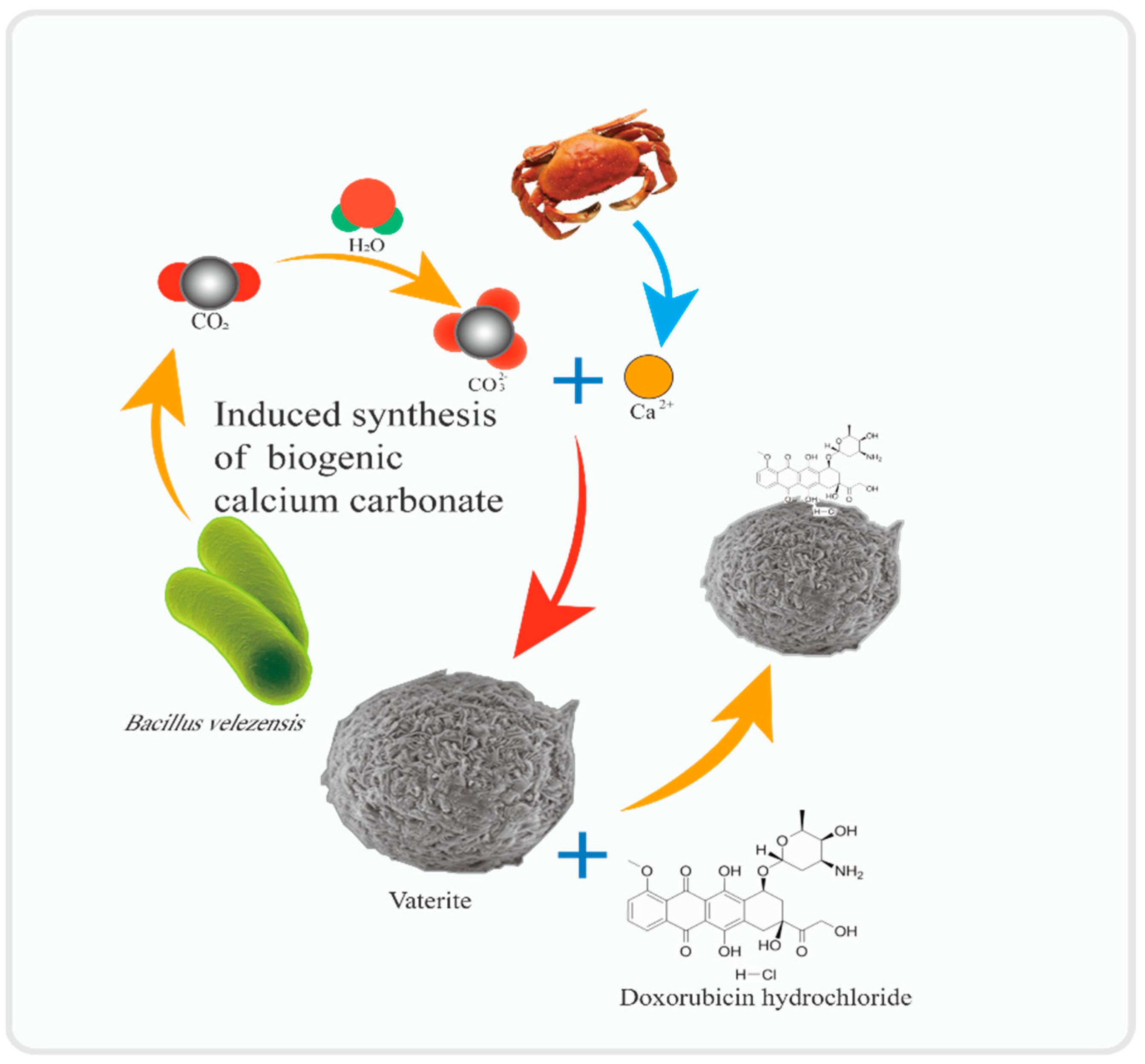
Our research group used wastewater from chitin extraction from crab shells as the medium. It synthesized bio-sourced micro-nano carbonate minerals (BV) using
Bacillus fermentation culture and used BV to load the drug DOX (
Figure 2) [
14]. The results showed that this biogenic BV had good loading performance, with a maximum unit loading capacity of 447.58 mg/g. In addition, BV is shown to have good, sustained release performance for DOX in simulated extracellular tumor fluid pH (6.0) environment. This study provides essential information for the application of BV as a new drug carrier.
According to the literature, calcium carbonate, as a drug-controlled-release carrier, shows the following advantages: (1) Good biocompatibility: As a natural mineral, calcium carbonate has good biocompatibility and will not cause rejection by the human immune system. Therefore, calcium carbonate can be absorbed by the human digestive system, thus achieving effective drug delivery; (2) High biodegradability: calcium carbonate presents good biodegradability and can be decomposed into carbon dioxide and water in the acidic environment encountered in the human body without causing harm thereto. Therefore, calcium carbonate can be a safe drug carrier for drug delivery and controlled release. (3) Polyporous structures, such as biogenic calcite, have a spherical porous structure, which allows them to load more drugs, some of which have a hollow structure or porous shell, controlling the size of the cavity and the porosity of the shell can regulate the drug loading capacity and release rate; (4) Low cost of synthesis: the synthetic conditions for calcium carbonate are simple and can be controlled. Thus, the reaction cost is low, which is a significant advantage compared to other inorganic drug carriers. Although the research on calcium carbonate drug carriers has made encouraging progress, most experimental results are still obtained in the simulated human drug delivery environment or animal models. Since the results of simulated environments or animal experiments do not represent real clinical manifestations, more clinical research data are needed to verify the effects of various calcium carbonate carrier-loaded drugs [
42]. In addition, since the calcium carbonate carrier is easy to crystallize and aggregate at room temperature, how to ensure its drug-carrying capacity and controlled-release stability is also a scientific problem worthy of research and required to be solved as a matter of urgency [
43].
4. Summary and Prospects
In summary, as an inorganic controlled release carrier, calcium carbonate shows excellent characteristics in drug delivery applications. It can potentially provide new strategies for the long-term treatment of many malignant diseases, especially cancer. However, to make this emerging inorganic controlled release carrier more widely used in clinical drug therapy, the following issues need to be addressed:
- (1)
The application research of calcium carbonate prepared in the laboratory in drug carrier needs to be expanded, such as the loading and slow-release ability and stability of enzymes, proteins, and other active substances, the safety of calcium carbonate carriers from different sources and types in the human body, and how to ensure the new metabolism to ensure that the drug carrier can be rapidly discharged from the body after playing its role, which warrants further research;
- (2)
The conditions in the preparation process, such as temperature, pH value and calcium ion concentration, will be further explored to endow the calcium carbonate microspheres with more effective morphological characteristics and microsphere size distributions, the better to load drugs and improve their utilization;
- (3)
The optimal conditions of the release process, such as drug loading, loading time, release rate, and environmental factors, should be investigated to achieve the goal of accurate, targeted, stable release and delivery of drugs.
Although significant progress has been made in synthesising these calcium carbonate biomaterials, the true biomedical challenge requires further breakthroughs in synthesising complex multi-component carriers with high repeatability and homogeneity to achieve effective treatment at the single-cell level. The application of microfluidic technology is one of the feasible methods to overcome these limitations, opening up new avenues for the synthesis of advanced nanostructured materials [
44]. The emerging interdisciplinary technology based on microfluidic technology is currently at the forefront of laboratory methods and a unique method for producing nanometers and microparticles. Microfluid technology has various advanced characteristics, such as high repeatability, small differences between batches, precise control of particle characteristics, and ease of scaling up. Although predicting the future development of CaCO
3 nanoparticle and microparticle synthesis may be challenging, the overall progress trend driven by state-of-the-art science and technology is evident. In addition, artificial intelligence methods are the next development direction for solving practical problems in science and technology, including the development of new materials. These technologies go far beyond the scope of computer science and provide new insights for professional fields in chemical applications. One highly attractive application is the automated material synthesis laboratory driven by artificial intelligence. The introduction of advanced laboratory automation and artificial intelligence methods into Microfluidics technology will determine the future of new material synthesis and known reaction optimization.
It should be noted that there are many advantages associated with CaCO3 controlled-release drug carriers, but at present, the relevant policy documents regulating the use of drug carriers have not been issued in China, and the biological safety of its clinical application needs to be further confirmed. The application of calcium carbonate as a drug carrier from laboratory to clinical practice warrants more explorations.