Study of 405 nm Laser-Induced Time-Resolved Photoluminescence Spectroscopy on Spinel and Alexandrite
Abstract
1. Introduction
2. Materials and Methods
3. Results
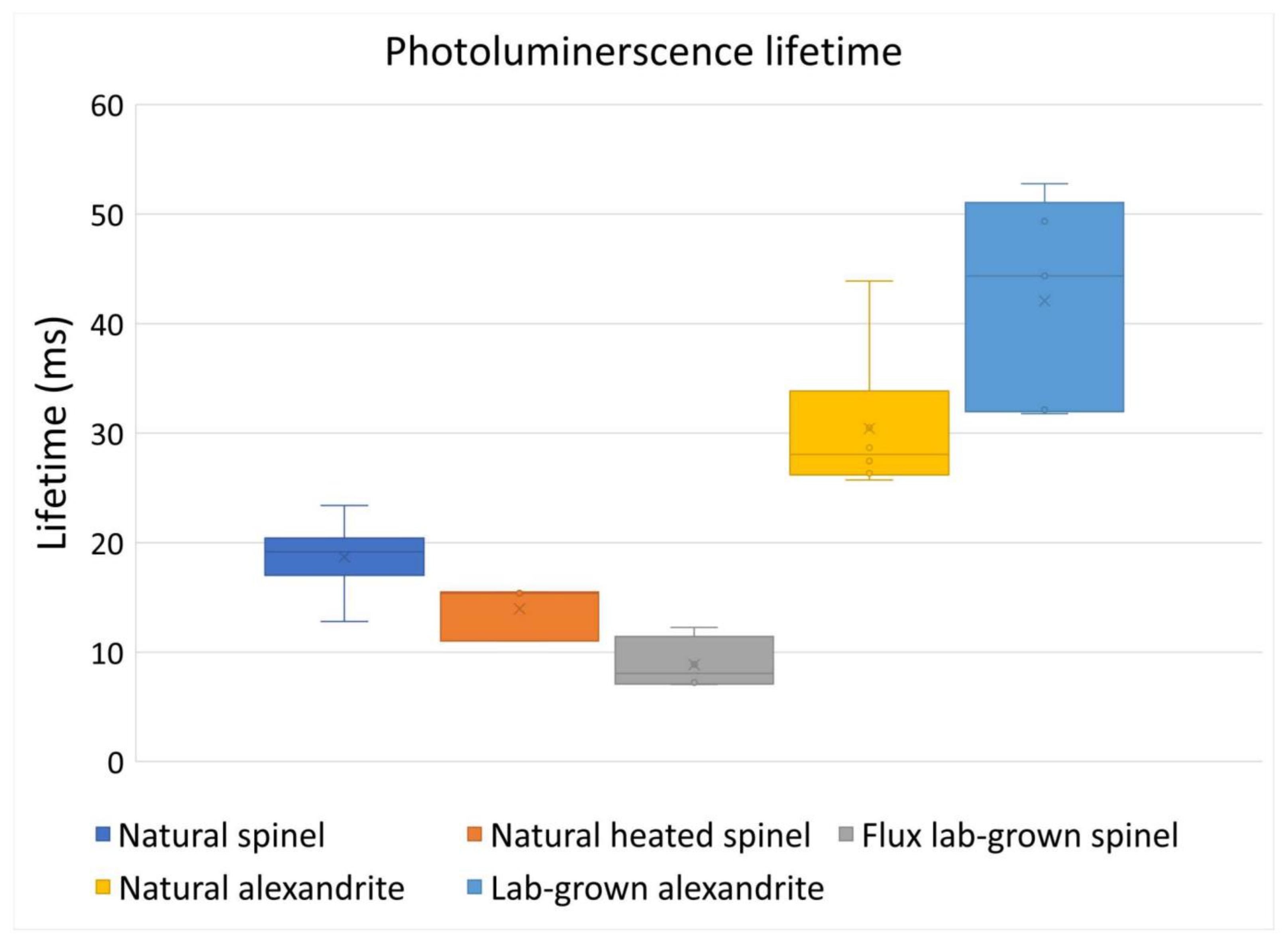
3.1. Photoluminescence Decay Spectra and Duration of Decay
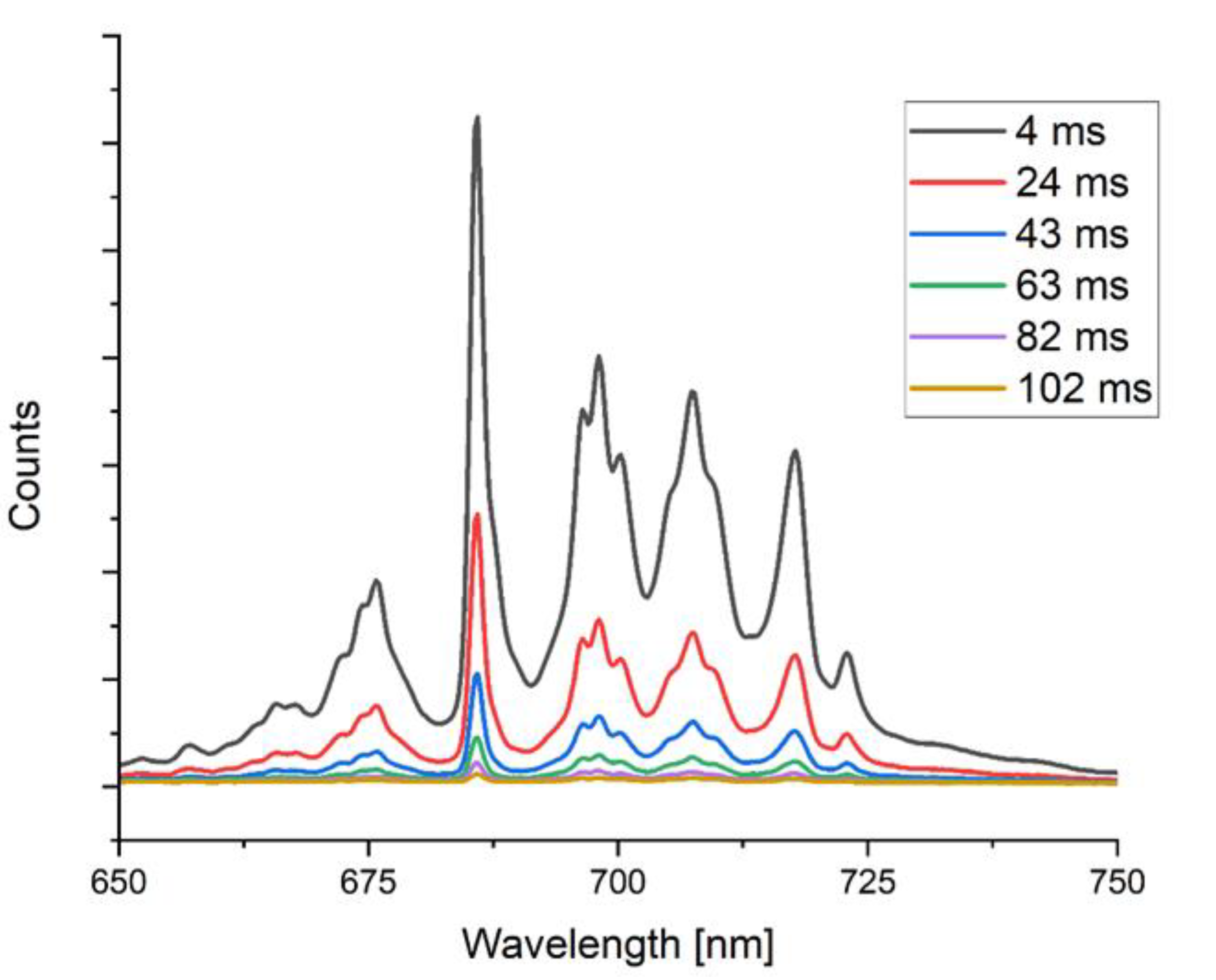
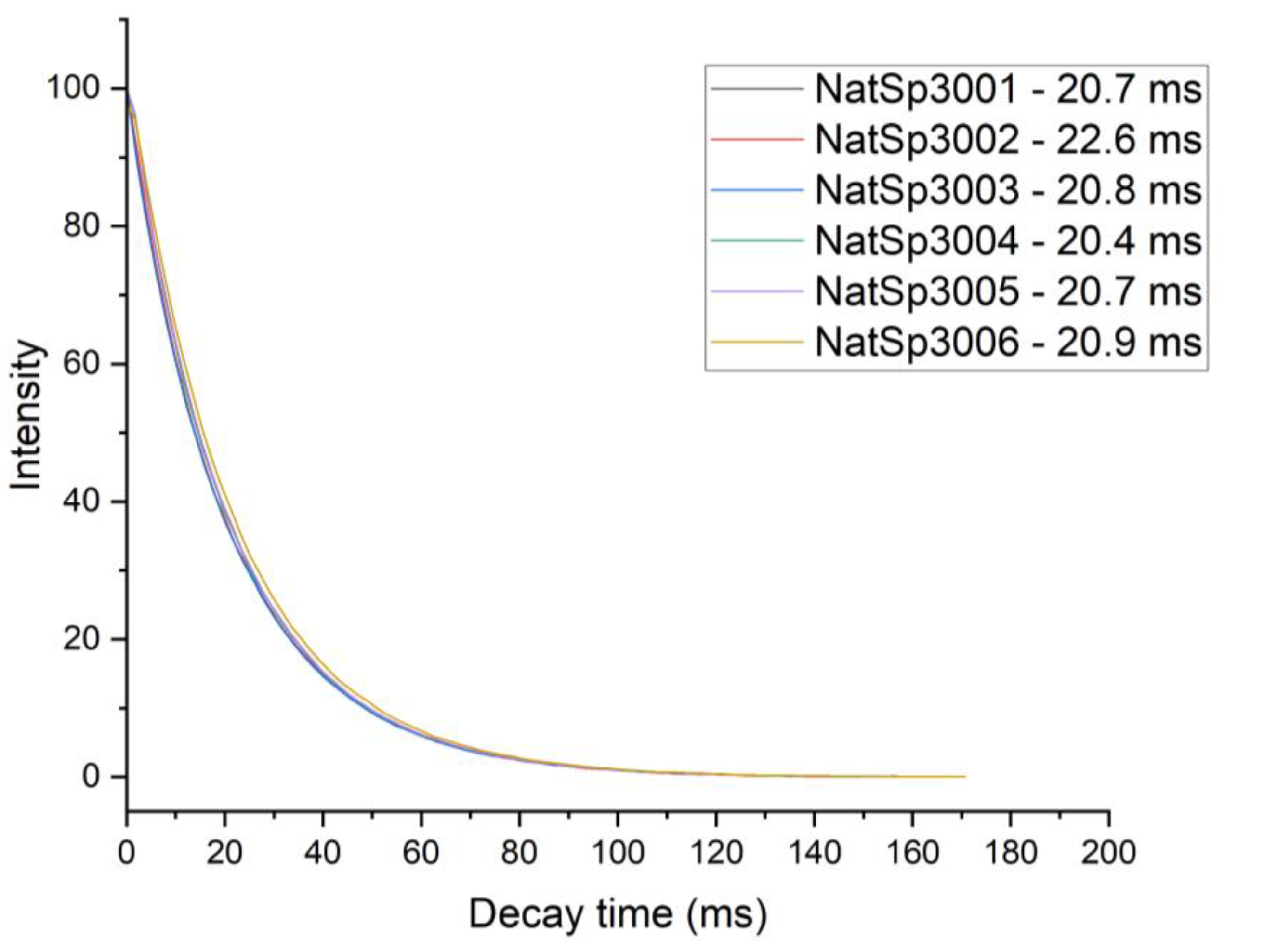
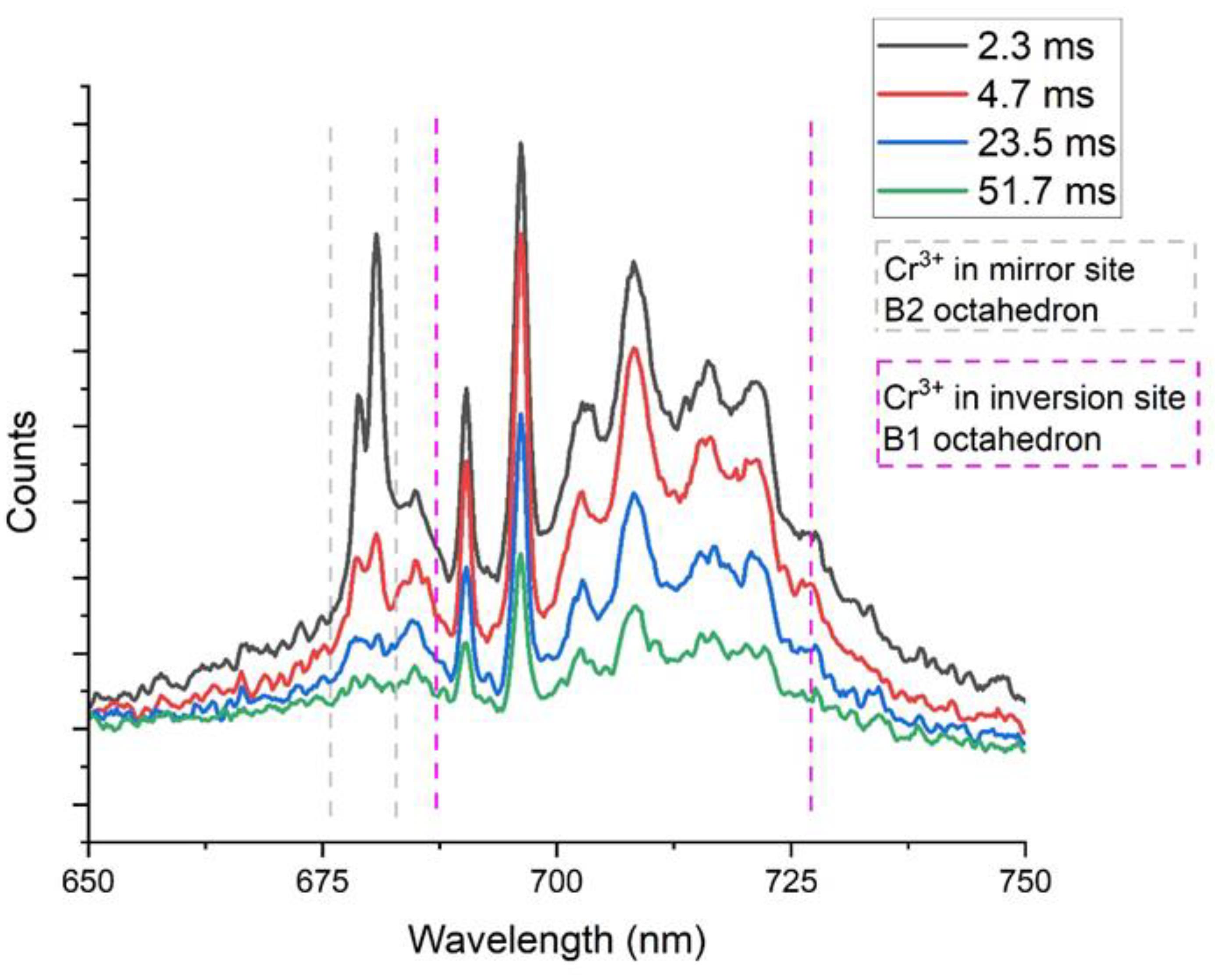
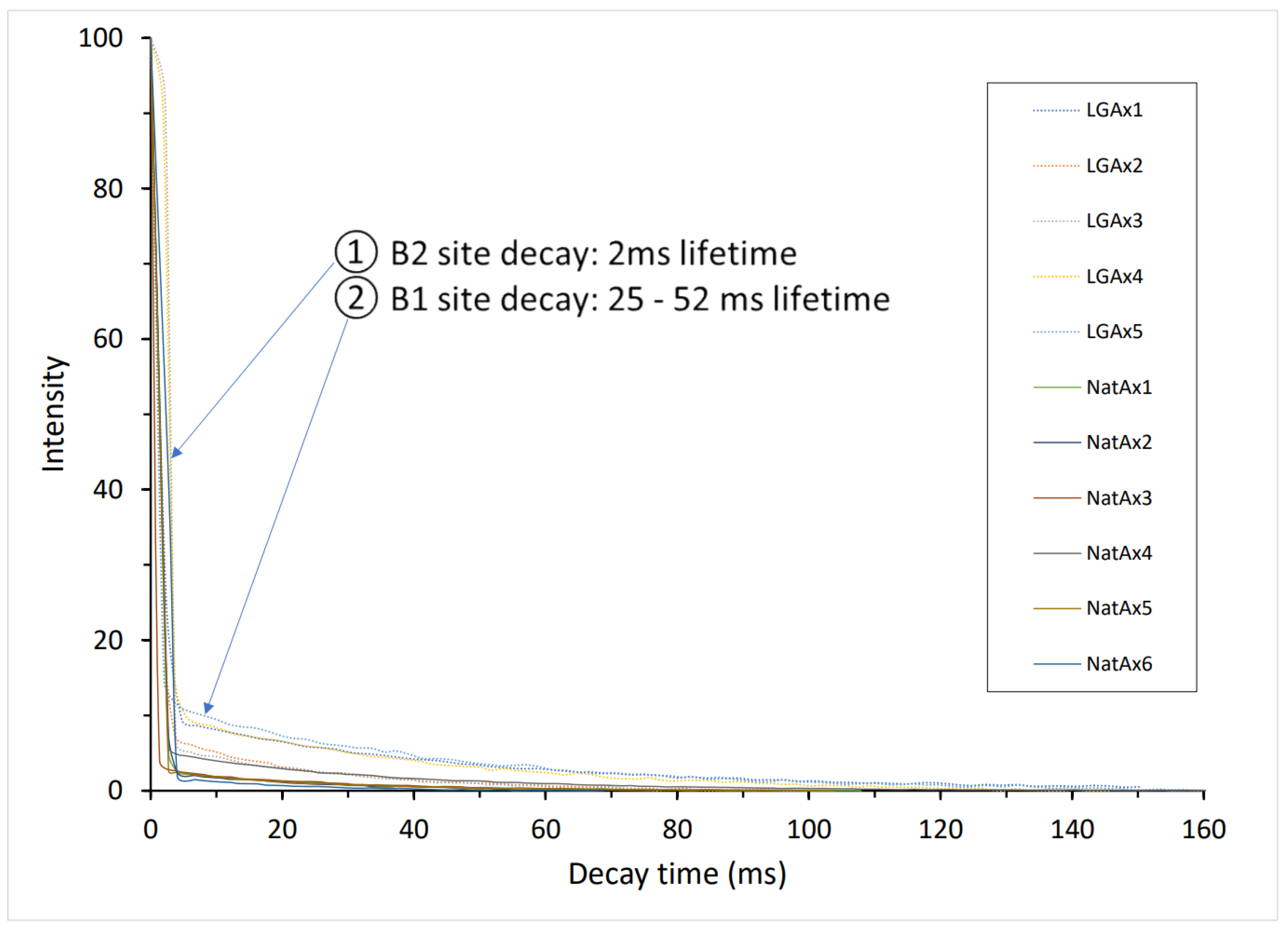
3.1.1. Orientational Homogeneity
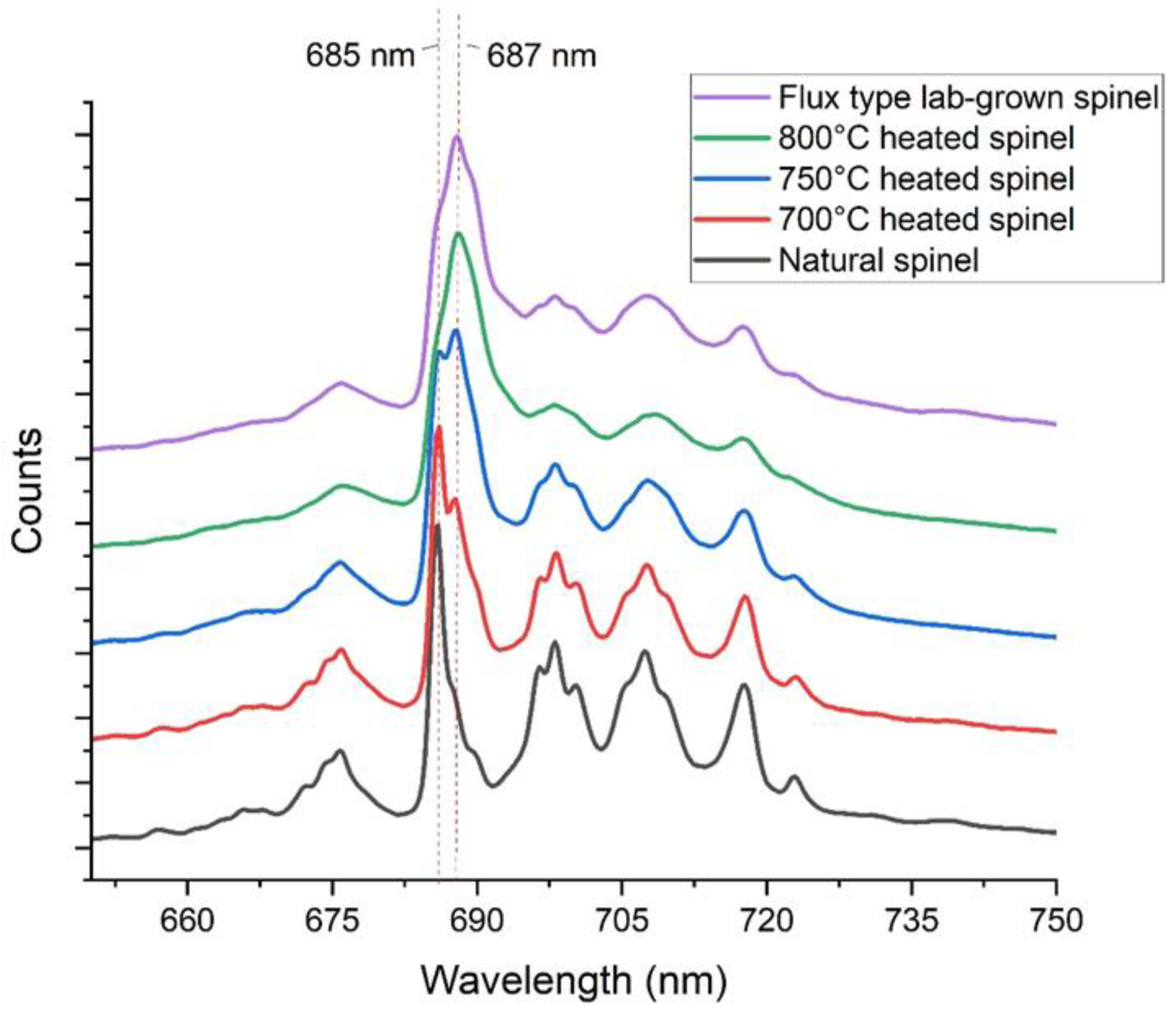
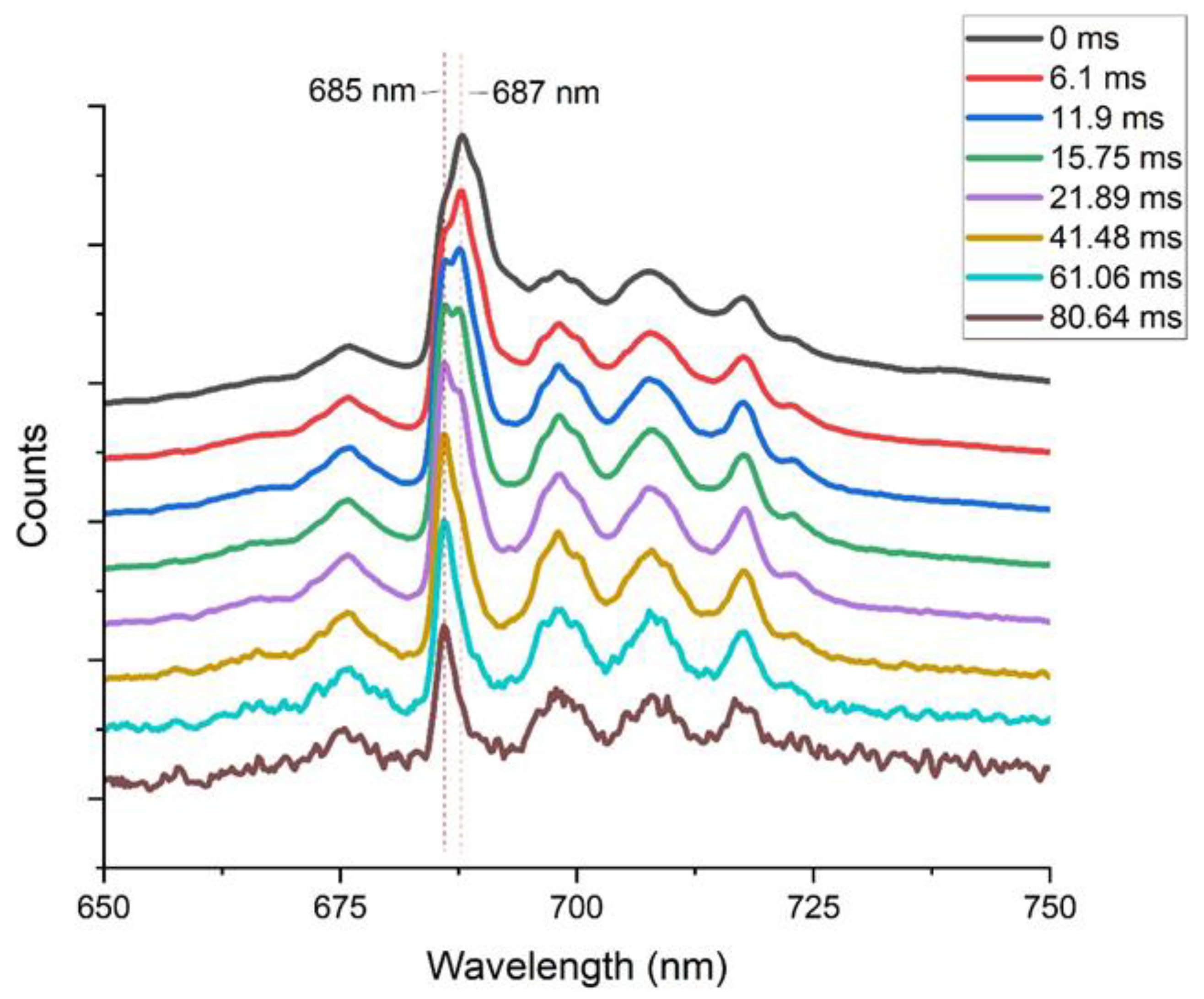
3.1.2. Comparison Natural Spinel, Heated Spinel, and Flux Lab-Grown Spinel
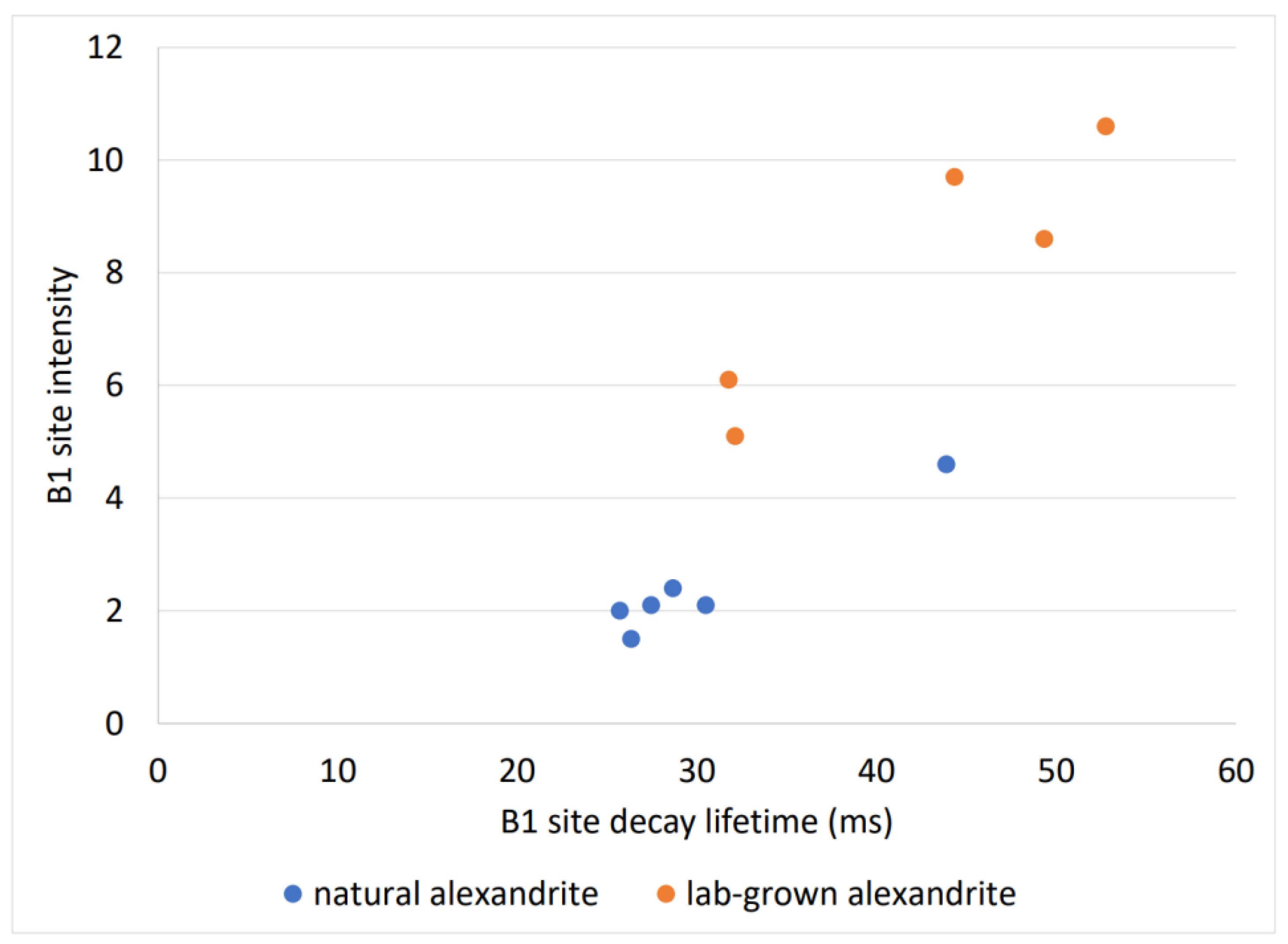
3.1.3. Alexandrite’s Decay Spectra and Duration of Decay
3.2. Decay Curve Fitting with Exponential Function
3.2.1. Spinel Decay Curve Fitting
3.2.2. Alexandrite Decay Curve Fitting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsai, T.H.; D’Haenens-Johansson, U.F.S. Rapid gemstone screening and identification using fluorescence spectroscopy. Appl. Opt. 2021, 60, 3412–3421. [Google Scholar] [CrossRef]
- Nevin, A.; Cesaratto, A.; Bellei, S.; D’Andrea, C.; Toniolo, L.; Valentini, G.; Comelli, D. Time-resolved photoluminescence spectroscopy and imaging: New approaches to the analysis of cultural heritage and its degradation. Sensors 2014, 14, 6338–6355. [Google Scholar] [CrossRef]
- Gupta, A.; Hacquebard, L.; Childress, L. Efficient signal processing for time-resolved fluorescence detection of nitrogen-vacancy spins in diamond. J. Opt. Soc. Am. 2016, 33, B28–B34. [Google Scholar] [CrossRef]
- Jones, D.C.; Alexandrov, Y.; Curry, N.; Kumar, S.; Lanigan, P.M.P.; McGuiness, C.D.; Dale, M.W.; Twitchen, D.J.; Fisher, D.; Neil, M.A.A. Multidimensional spectroscopy and imaging of defects in synthetic diamond: Excitation-emission-lifetime luminescence measurements with multiexponential fitting and phasor analysis. J. Phys. D Appl. Phys. 2021, 54, 045303. [Google Scholar] [CrossRef]
- Saeseaw, S.; Wang, W.; Scarratt, K.; Emmett, J.L.; Douthit, T.R. Distinguishing Heated Spinels from Unheated Natural Spinels and from Synthetic Spinels: A Short Review of Ongoing Research. 2009. Available online: http://www.giathai.net/pdf/Heated_spinel_Identification_at_May_25_2009.pdf (accessed on 7 January 2014).
- Widmer, R.; Malsy, A.K.; Armbruster, T. Effects of heat treatment on red gemstone spinel: Single-crystal X-ray, Raman, and photoluminescence study. Phys. Chem. Miner. 2015, 42, 251–260. [Google Scholar] [CrossRef]
- Okrusch, M.; Matthes, S. Oxide und Hydroxide. In Mineralogie; Springer-Lehrbuch; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Huminicki, D.M.C. The Crystal Chemistry of Beryllium. Rev. Mineral. Geochem. 2002, 50, 333–403. [Google Scholar] [CrossRef]
- Weber, S.U.; Grodzicki, M.; Lottermoser, W.; Redhammer, G.J.; Topa, D.; Tippelt, G.; Amthauer, G. 57Fe Mössbauer Spectroscopy, X–Ray Single Crystal Diffractometry and Electronic Structure Calculations on Natural Alexandrite. Phys. Chem. Miner. 2007, 34, 507–515. [Google Scholar] [CrossRef]
- Gaft, M.; Reisfeld, R.; Panczer, G. Modern Luminescence Spectroscopy of Minerals and Materials; Springer: Berlin/Heidelberg, Germany, 2015; 606p. [Google Scholar]
- Mikenda, W.; Preisinger, A. N–Lines in the luminescence spectra of Cr3+–doped spinels (i) identification of n–lines. J. Lumin. 1981, 26, 53–66. [Google Scholar] [CrossRef]
- Mikenda, W.; Preisinger, A. N–Lines in the luminescence spectra of Cr3+–doped spinels (ii) origins of n–lines. J. Lumin. 1981, 26, 67–68. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takéuchi, Y. Order-disorder transition in MgAl2O4 spinel at high temperatures up to 1700 °C. Z Kristallogr. 1983, 165, 65–78. [Google Scholar] [CrossRef]
- Peterson, R.C.; Lager, G.A.; Hitterman, R.L. A time-of-flight powder diffraction study of MgAl2O4 at temperatures up to 1273 k. Am. Miner. 1991, 76, 1455–1458. [Google Scholar]
- Redfern, S.A.T.; Harrison, R.J.; O’Neill, H.; Wood, D.R.R. Thermodynamics and kinetics of cation ordering in MgAl2O4 spinel up to 1600 °C from in situ neutron diffraction. Am. Miner. 1999, 84, 299–310. [Google Scholar] [CrossRef]
- Andreozzi, G.B.; Princivalle, F.; Skogby, H.; Della Giusta, A. Cation ordering and structural variations with temperature in MgAl2O4 spinel: An X-ray single crystal study. Am. Miner. 2000, 85, 1164–1171. [Google Scholar] [CrossRef]
- Andreozzi, G.B.; Princivalle, F. Kinetics of cation ordering in synthetic MgAl2O4 spinel. Am. Miner. 2002, 87, 838–844. [Google Scholar] [CrossRef]
- Méducin, F.; Redfern, S.A.T. Study of cation order-disorder in spinel by in situ neutron diffraction up to 1600 K and 3.2 GPa. Am. Miner. 2004, 89, 981–986. [Google Scholar] [CrossRef]
- Princivalle, F.; Martignago, F.; Dal Negro, A. Kinetics of cation ordering in natural Mg(Al, Cr3+)2O4 spinels. Am. Miner. 2006, 91, 313–318. [Google Scholar] [CrossRef]
- Barpanda, P.; Behera, S.K.; Gupta, P.K.; Pratihar, S.K.; Bhattacharya, S. Chemically induced disorder order transition in magnesium aluminium spinel. J. Eur. Ceram. Soc. 2006, 26, 2603–2609. [Google Scholar] [CrossRef]
- Cynn, H.; Sharma, S.K.; Cooney, T.F.; Nicol, M. High-temperature Raman investigation of order-disorder behavior in the MgAl2O4 spinel. Phys. Rev. 1992, B45, 500–502. [Google Scholar] [CrossRef]
- Slotznick, S.P.; Shim, S.H. In situ Raman spectroscopy measurements of MgAl2O4 spinel up to 1400 °C. Am. Miner. 2008, 93, 470–476. [Google Scholar] [CrossRef]
- Malsy, A.K.; Karampelas, S.; Schwarz, D.; Klemm, L.; Armbruster, T.; Tuan, D.A. Orange–red to orange–pink gem spinels from a new deposit at Lang Chap (Tan Huong-Truc Lau). Vietnam. J. Gemmol. 2012, 33, 19–27. [Google Scholar] [CrossRef]
- Wood, D.L.; Imbusch, G.F.; Macfarlane, R.M.; Kisliuk, P.; Larkin, D.M. Optical spectrum of Cr3+ ions in spinels. J. Chem. Phys. 1968, 48, 5255–5263. [Google Scholar] [CrossRef]
- Derkosch, J.; Mikenda, W. N-lines in the luminescence spectra of Cr3+-doped spinels: (IV) excitation spectra. J. Lumines 1983, 28, 431–441. [Google Scholar] [CrossRef]
- Strek, W.; Derén, P.; Jezowska-Trzebiatowska, B. Optical properties of Cr3+ in MgAl2O4 spinel. Physica 1988, B152, 379–384. [Google Scholar] [CrossRef]
- Phan, T.L.; Yu, S.C.; Phan, M.H.; Han, T.P.J. Photoluminescence properties Cr3+-doped MgAl2O4 natural spinel. J. Korean Phys. Soc. 2004, 45, 63–66. [Google Scholar]
- Powell, R.C.; Xi, L.; Gang, X.; Quarles, G.J.; Walling, J.C. Spectroscopic properties of alexandrite crystals. Phys. Rev. B 1985, 32, 2788. [Google Scholar] [CrossRef]
- Malickova, I.; Bacik, P.; Fridrichová, J.; Hanus, R.; Štubňa, J.; Milovská, S.; Škoda, R. Detailed luminescence spectra interpretation of selected oxides: Spinel from Myanmar and chrysoberyl—Var. alexandrite from Tanzania. Acta Geol. Slovaca 2020, 12, 69–74. [Google Scholar]
- Yeom, T.H.; Choh, S.H.; Honhg, K.S.; Yeom, H.Y.; Park, Y.H.; Yu, Y.M. Nuclear Quadrupole Interactions of 27Al in Alexandrite Single Crystal. Z. Nat. 1998, 53, 568–572. [Google Scholar] [CrossRef]
- Yeom, T.H.; Choh, S.H. Cr3+ (I) and Cr3+ (II) Centers in Alexandrite Single Crystal. J. Appl. Phys. 2001, 90, 5946–5950. [Google Scholar] [CrossRef]
- Bordage, A.; Rossano, S.; Horn, A.H.; Fuchs, Y. Site partitioning of Cr3+ in the trichroic alexandrite BeAl2O4:Cr3+ crystal: Contribution from X-ray absorption spectroscopy. J. Phys. 2012, 24, 225401–225409. [Google Scholar] [CrossRef]
- Urusov, V.S.; Gromalova, N.A.; Vyatkin, S.V.; Rusakovn, V.S.; Maltsev, V.V.; Eremin, N.N. Study of structural and valence state of Cr and Fe in chrysoberyl and alexandrite with EPR and Mössbauer spectroscopy. Mosc. Univ. Geol. Bull. 2011, 66, 102–110. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.V.; Yang, I.-S. A Raman study of cation-disorder transition temperature of natural MgAl2O4 spinel. Vibr. Spec. 2004, 35, 93–96. [Google Scholar] [CrossRef]
- Aizawa, H.; Ohishi, N.; Ogawa, S.; Watanabe, E.; Katsumata, T.; Komuro, S.; Morikawa, T.; Toba, E. Characteristics of chromium doped spinel crystals for a fiber-optic thermometer application. Rev. Sci. Instrum. 2002, 73, 3089. [Google Scholar] [CrossRef]
- Ollier, N.; Fuchs, Y.; Cavani, O.; Horn, A.H.; Rossano, S. Influence of impurities on Cr3+ luminescence properties in Brazilian emerald and alexandrite. Eur. J. Mineral. 2015, 27, 783–792. [Google Scholar] [CrossRef]
- Schmetzer, K. Russian Alexandrites; Schweizerbart Science: Stuttgart, Germany, 2010. [Google Scholar]
- Schmetzer, K.; Malsy, A.-K. Natural Alexandrite with an Irregular Growth Pattern: A Case Study. J. Gemmol. Assoc. Hong Kong 2011, 32, 68–75. Available online: http://www.gahk.org/journal/Alexandrite_for%20web_v1.pdf (accessed on 7 January 2014).
- Schmetzer, K.; Malsy, A.-K. Alexandrite and colour-change chrysoberyl from the Lake Manyara alexandrite-emerald deposit in northern Tanzania. J. Gemmol. 2011, 32, 179–209. [Google Scholar] [CrossRef]
- Sun, Z.; Palke, A.C.; Muyal, J.; DeGhionno, D.; McClure, S.F. Geographic Origin Determination of Alexandrite. Gems Gemol. 2019, 55, 4. [Google Scholar] [CrossRef]
- Chandler, D.E.; Majumdar, Z.K.; Heiss, G.J.; Clegg, R.M. Ruby Crystal for Demonstrating Time- and Frequency-Domain Methods of Fluorescence Lifetime Measurements. J. Fluoresc. 2006, 16, 793–807. [Google Scholar] [CrossRef]
- Schwarz, D.; Schmetzer, K. Rubies from the Vatomandry area, eastern Madagascar. J. Gemmol. 2001, 27, 409–416. [Google Scholar] [CrossRef]
- Sorokina, E.S.; Litvinenko, A.K.; Hofmeister, W.; Häger, T.; Jacob, D.E.; Nasriddinov, Z.Z. Rubies and sapphires from Snezhnoe, Tajikistan. G&G 2015, 51, 160–175. [Google Scholar] [CrossRef]
- Stone-Sundberg, J.; Thomas, T.; Sun, Z.; Guan, Y.; Cole, Z.; Equall, R.; Emmett, J.L. Accurate reporting of key trace elements in ruby and sapphire using matrix-matched standards. G&G 2017, 53, 438–451. [Google Scholar] [CrossRef]
- Palke, A.C.; Saeseaw, S.; Renfro, N.D.; Sun, Z.; McClure, S.F. Geographic Origin Determination of Ruby. Gems Gemol. 2019, 55, 4. [Google Scholar] [CrossRef]


| Sample | Type | Shape | Weight (Carats) | Color |
|---|---|---|---|---|
| NatSp1 | Natural spinel | Rough | 0.89 | pink |
| NatSp2 | Natural spinel | Rough | 0.75 | pink |
| NatSp3 | Natural spinel | Rough | 0.92 | pink |
| NatSp4 | Natural spinel | Faceted | 3.56 | Purple red |
| NatSp5 | Natural spinel | Faceted | 5.12 | Red |
| NatSp6 | Natural spinel | Rough | 1.18 | Pink |
| NatSp7 | Natural spinel | Rough | 1.32 | Pink |
| NatSp8 | Natural spinel | Rough | 1.15 | Pink |
| FLGSp1 | Lab-grown spinel | Faceted | 2.14 | Red |
| FLGSp2 | Lab-grown spinel | Polished Slab | 0.53 | Red |
| FLGSp3 | Lab-grown spinel | Polished Slab | 0.56 | Red |
| FLGSp4 | Lab-grown spinel | Polished Slab | 0.76 | Red |
| NatAx1 | Natural alexandrite | Faceted | 1.23 | Dark green to purple |
| NatAx2 | Natural alexandrite | Faceted | 1.43 | Dark green to purple |
| NatAx3 | Natural alexandrite | Faceted | 1.35 | Dark green to purple |
| NatAx4 | Natural alexandrite | Faceted | 1.56 | Dark green to purple |
| NatAx5 | Natural alexandrite | Faceted | 1.11 | Dark green to purple |
| NatAx6 | Natural alexandrite | Faceted | 1.89 | Dark green to purple |
| LGAx1 | Lab-grown alexandrite | Faceted | 2.01 | Green to red purple |
| LGAx2 | Lab-grown alexandrite | Faceted | 1.98 | Green to red |
| LGAx3 | Lab-grown alexandrite | Faceted | 2.56 | Green to purple |
| LGAx4 | Lab-grown alexandrite | Faceted | 2.98 | Green to red |
| LGAx5 | Lab-grown alexandrite | Faceted | 1.34 | Green to purple |
| Type | Sample | Lifetime (ms) | Exponential Fitting Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Half-Life A1 (t1/2) (ms) | Standard Error | Half-life A2 (t1/2) (ms) | Standard Error | A1 | A2 | |||
| Natural spinel | NatSp1 | 17.5 | 11.8 | 0.11 | ||||
| NatSp2 | 18.7 | 13.2 | 0.07 | |||||
| NatSp3 | 20.5 | 14.4 | 0.05 | |||||
| NatSp4 | 23.4 | 15.2 | 0.13 | |||||
| NatSp5 | 12.8 | 8.7 | 0.14 | |||||
| NatSp6 | 19.6 | 13.5 | 0.06 | |||||
| NatSp7 | 20.3 | 14.3 | 0.05 | |||||
| NatSp8 | 16.9 | 11.8 | 0.07 | |||||
| Average | 18.7 | 12.9 | ||||||
| Standard error | 3.1 | 2.1 | ||||||
| Natural heated spinel | NatSp6 800 | 11.0 | 14.8 | 1.87 | 5.57 | 0.33 | 0.28 | 0.73 |
| NatSp7 750 | 15.5 | 15.2 | 1.17 | 6.4 | 0.65 | 0.54 | 0.47 | |
| NatSp8 700 | 15.4 | 12.8 | 0.08 | 4.34 | 0.13 | 0.78 | 0.21 | |
| Average | 14.0 | 14.3 | ||||||
| Standard error | 2.6 | 1.2 | ||||||
| Flux lab-grown spinel | FLGSp1 | 7.2 | 8.4 | 0.12 | 2.22 | 0.06 | 0.52 | 0.47 |
| FLGSp2 | 12.3 | 14.5 | 1.61 | 5.89 | 0.41 | 0.36 | 0.65 | |
| FLGSp3 | 8.9 | 10.1 | 0.19 | 3.1 | 0.08 | 0.52 | 0.48 | |
| FLGSp4 | 7.1 | 42.1 | 160.9 | 4.35 | 0.34 | 0.05 | 1.02 | |
| Average | 8.9 | 18.8 | ||||||
| Standard error | 2.4 | 17.4 | ||||||
| Natural alexandrite | NatAx1 | 30.5 | 0.5 | 0 | 19.73 | 0.5 | ||
| NatAx2 | 25.7 | 0.7 | 0.03 | 17.56 | 1.08 | |||
| NatAx3 | 28.7 | 0.2 | 0.01 | 20.75 | 0.89 | |||
| NatAx4 | 43.9 | 0.5 | 0.02 | 24.37 | 0.22 | |||
| NatAx5 | 27.5 | 0.4 | 0.04 | 17.41 | 0.38 | |||
| NatAx6 | 26.3 | 2.1 | 1.63 | 14.03 | 0.72 | |||
| Average | 30.4 | 0.7 | 18.97 | |||||
| Standard error | 6.8 | 0.7 | 3.51 | |||||
| Lab-grown alexandrite | LGAx1 | 49.3 | 0.9 | 0.01 | 34.67 | 1.85 | ||
| LGAx2 | 31.8 | 0.8 | 0.03 | 16.79 | 0.41 | |||
| LGAx3 | 32.1 | 4.2 | 8.05 | 20.05 | 0.83 | |||
| LGAx4 | 44.3 | 2.7 | 2.8 | 31.03 | 0.53 | |||
| LGAx5 | 52.8 | 0.5 | 0.03 | 31.14 | 0.49 | |||
| Average | 42.1 | 1.8 | 26.74 | |||||
| Standard error | 9.7 | 1.6 | 7.82 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Tsai, T.-H.; Palke, A. Study of 405 nm Laser-Induced Time-Resolved Photoluminescence Spectroscopy on Spinel and Alexandrite. Minerals 2023, 13, 419. https://doi.org/10.3390/min13030419
Xu W, Tsai T-H, Palke A. Study of 405 nm Laser-Induced Time-Resolved Photoluminescence Spectroscopy on Spinel and Alexandrite. Minerals. 2023; 13(3):419. https://doi.org/10.3390/min13030419
Chicago/Turabian StyleXu, Wenxing, Tsung-Han Tsai, and Aaron Palke. 2023. "Study of 405 nm Laser-Induced Time-Resolved Photoluminescence Spectroscopy on Spinel and Alexandrite" Minerals 13, no. 3: 419. https://doi.org/10.3390/min13030419
APA StyleXu, W., Tsai, T.-H., & Palke, A. (2023). Study of 405 nm Laser-Induced Time-Resolved Photoluminescence Spectroscopy on Spinel and Alexandrite. Minerals, 13(3), 419. https://doi.org/10.3390/min13030419






