Antarctic Bioconstructional Bryozoans from Terra Nova Bay (Ross Sea): Morphology, Skeletal Structures and Biomineralization
Abstract
1. Introduction
2. Materials and Methods
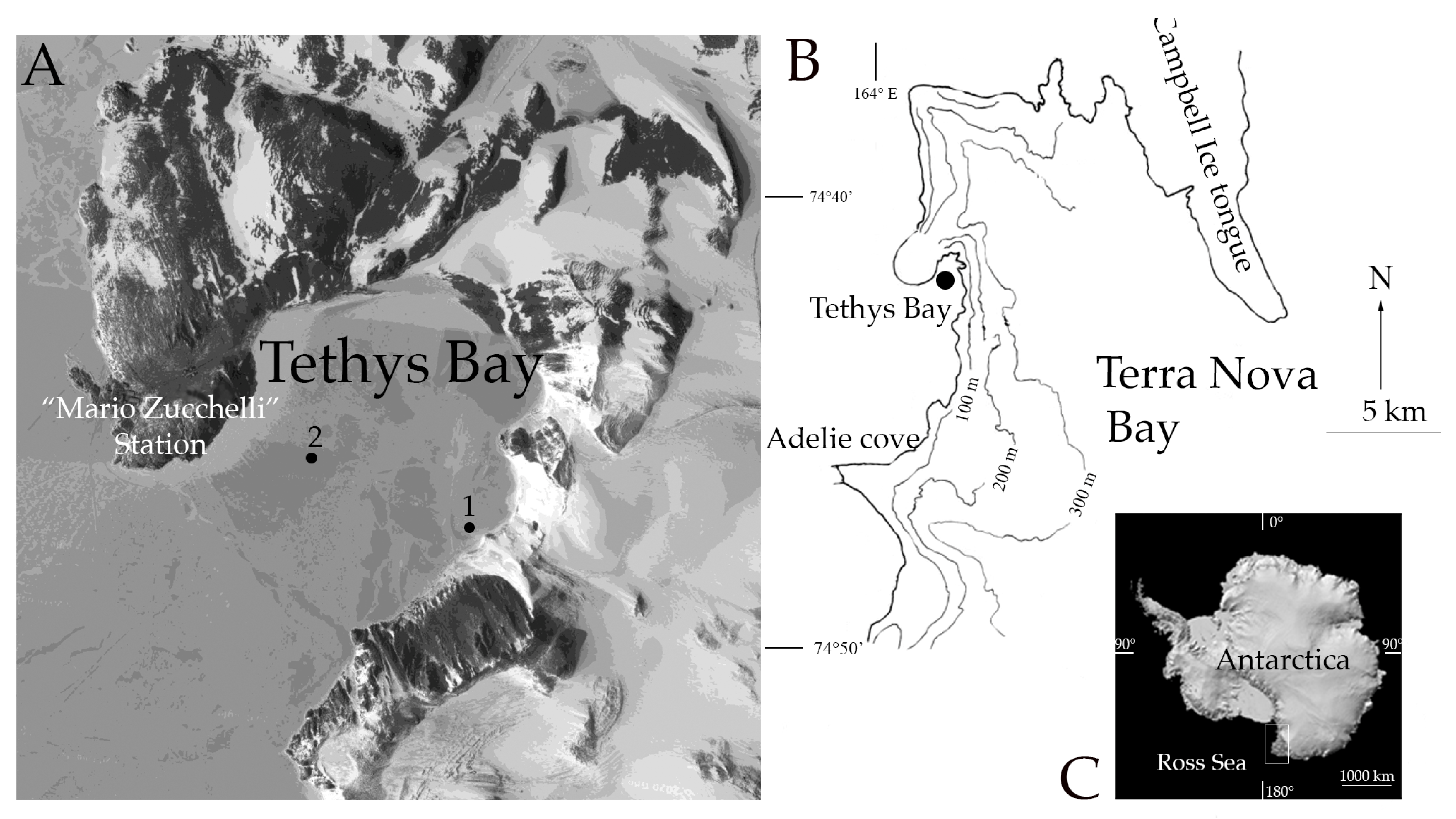
2.1. Study Area, Biological and Seawater Collection
2.2. Morphological and Skeletal Investigations
2.3. Biomineralization—The Skeletal Organic Matrix
2.3.1. Cleaning and Extraction
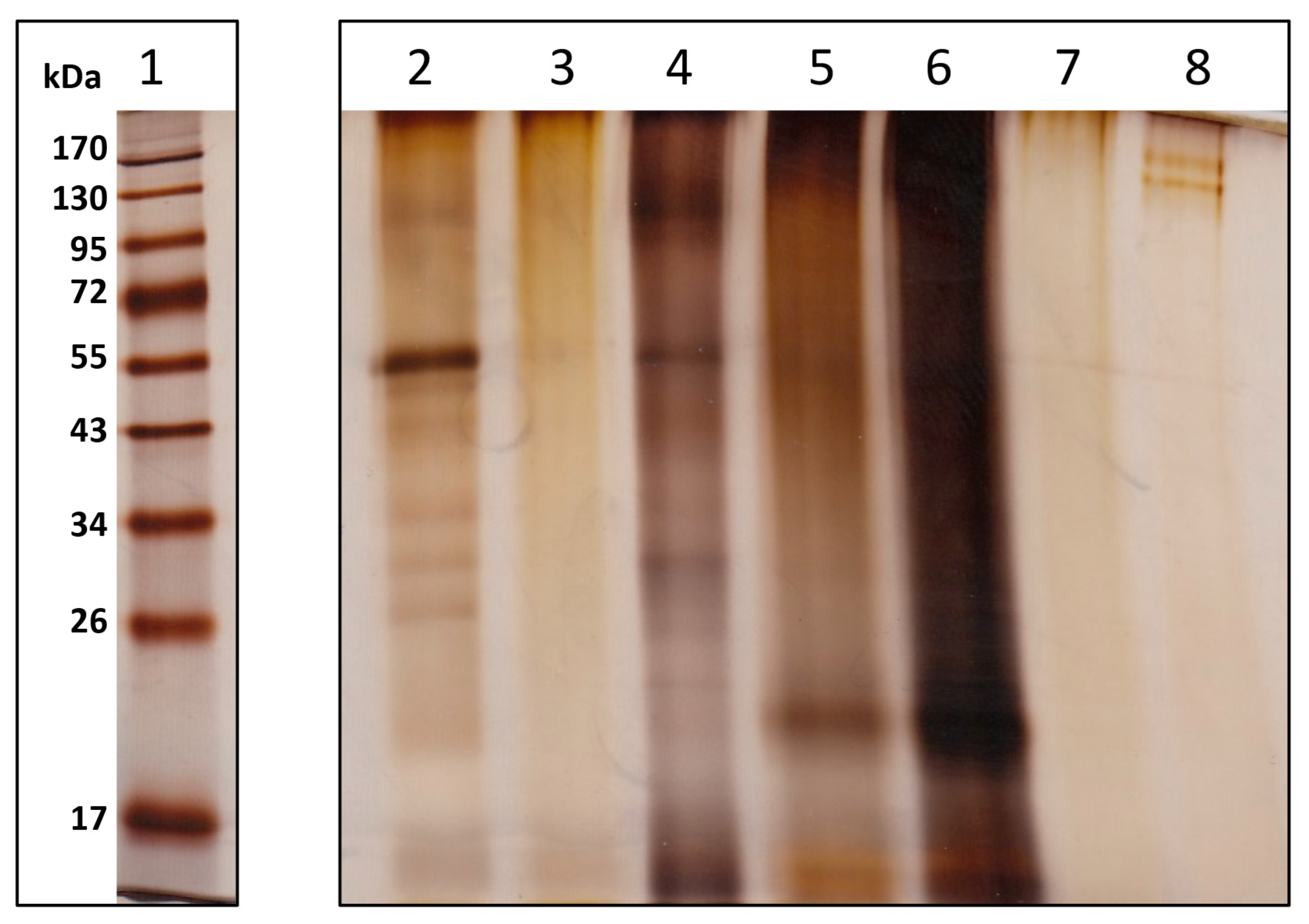
2.3.2. SDS-PAGE
2.3.3. Fourier Transform—Infra Red Spectroscopy (FT-IR)
2.3.4. Enzyme-Linked Lectin Assay (ELLA)
2.4. Seawater Isotopic Composition and DIC at Sampling Site
2.4.1. Sea-Water Isotopy of δ18O and δ2H
2.4.2. Carbon Stable Isotope (δ13CDIC) in Seawater—Gasbench-IRMS
2.4.3. DIC Concentration—Gasbench-IRMS
3. Results
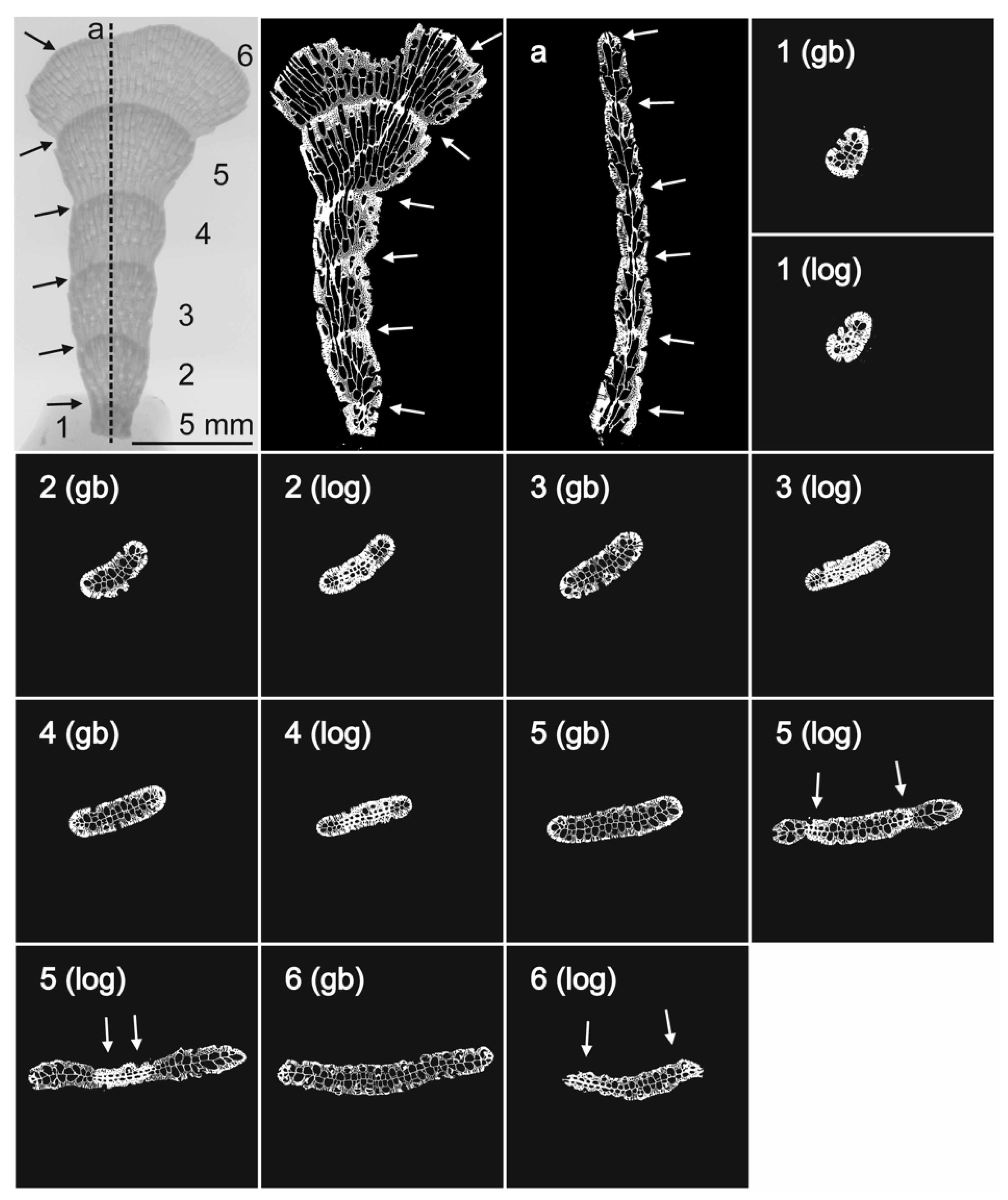
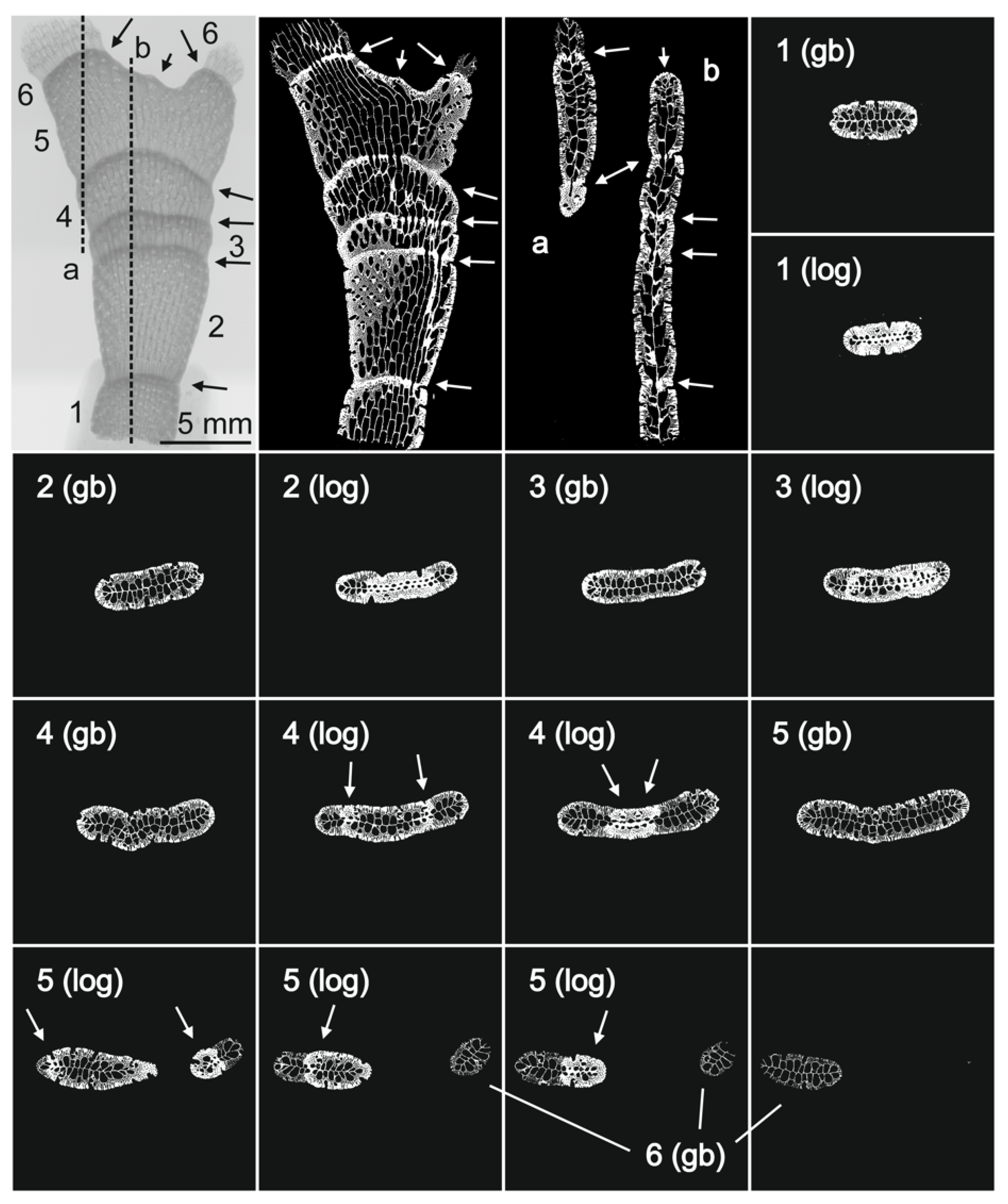
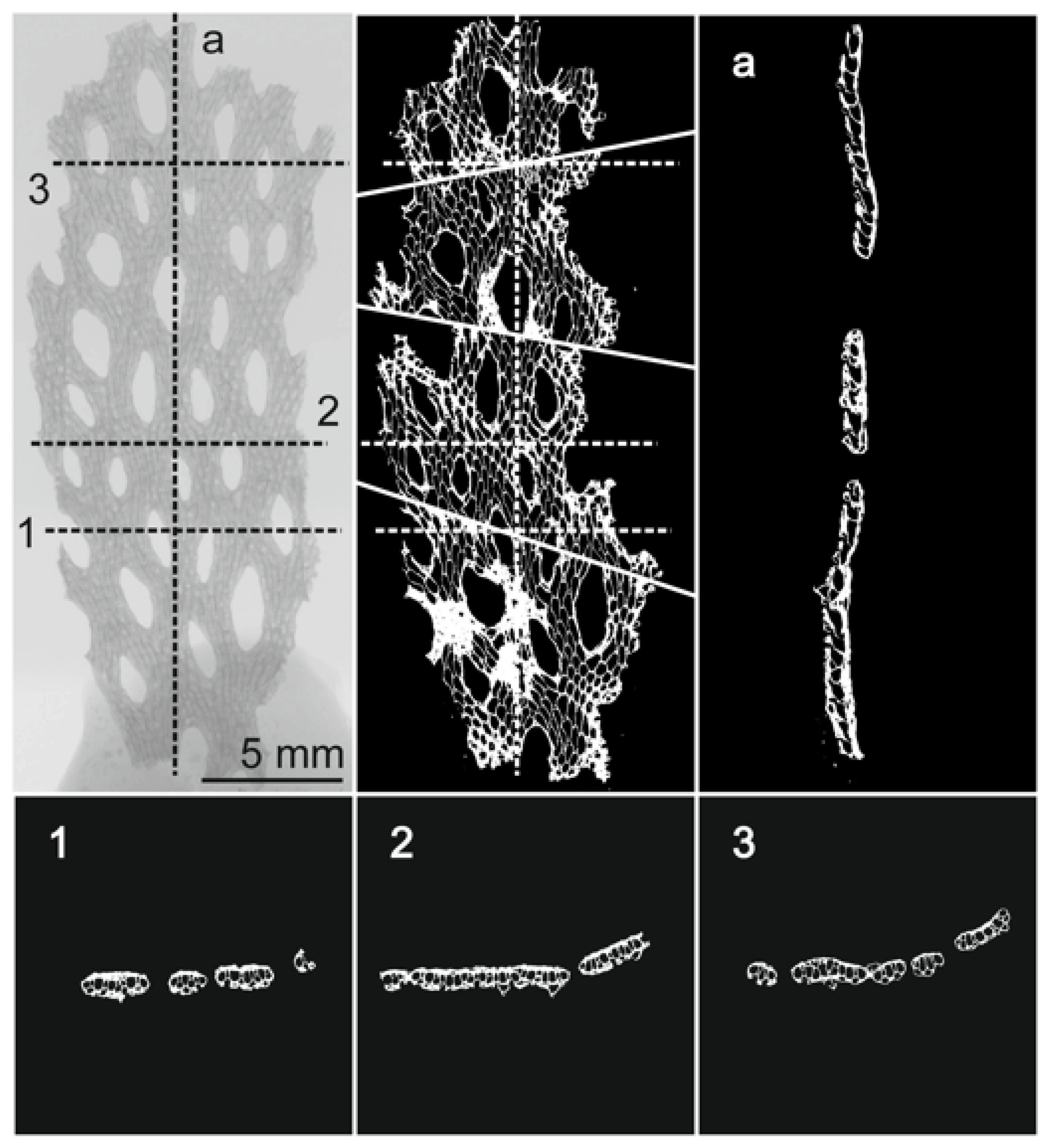
3.1. Morphological Identification and Skeletal Structures
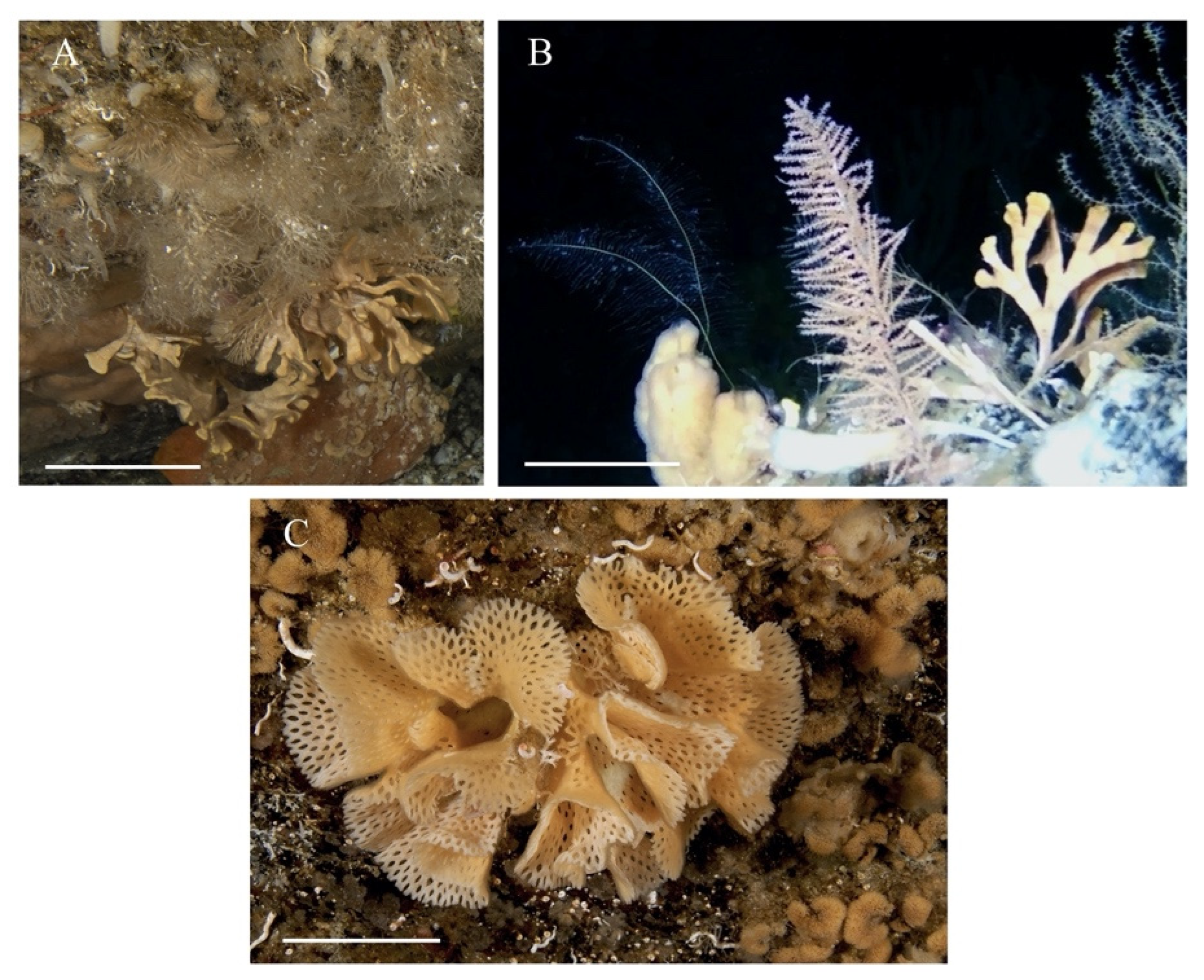
3.1.1. Species Identity
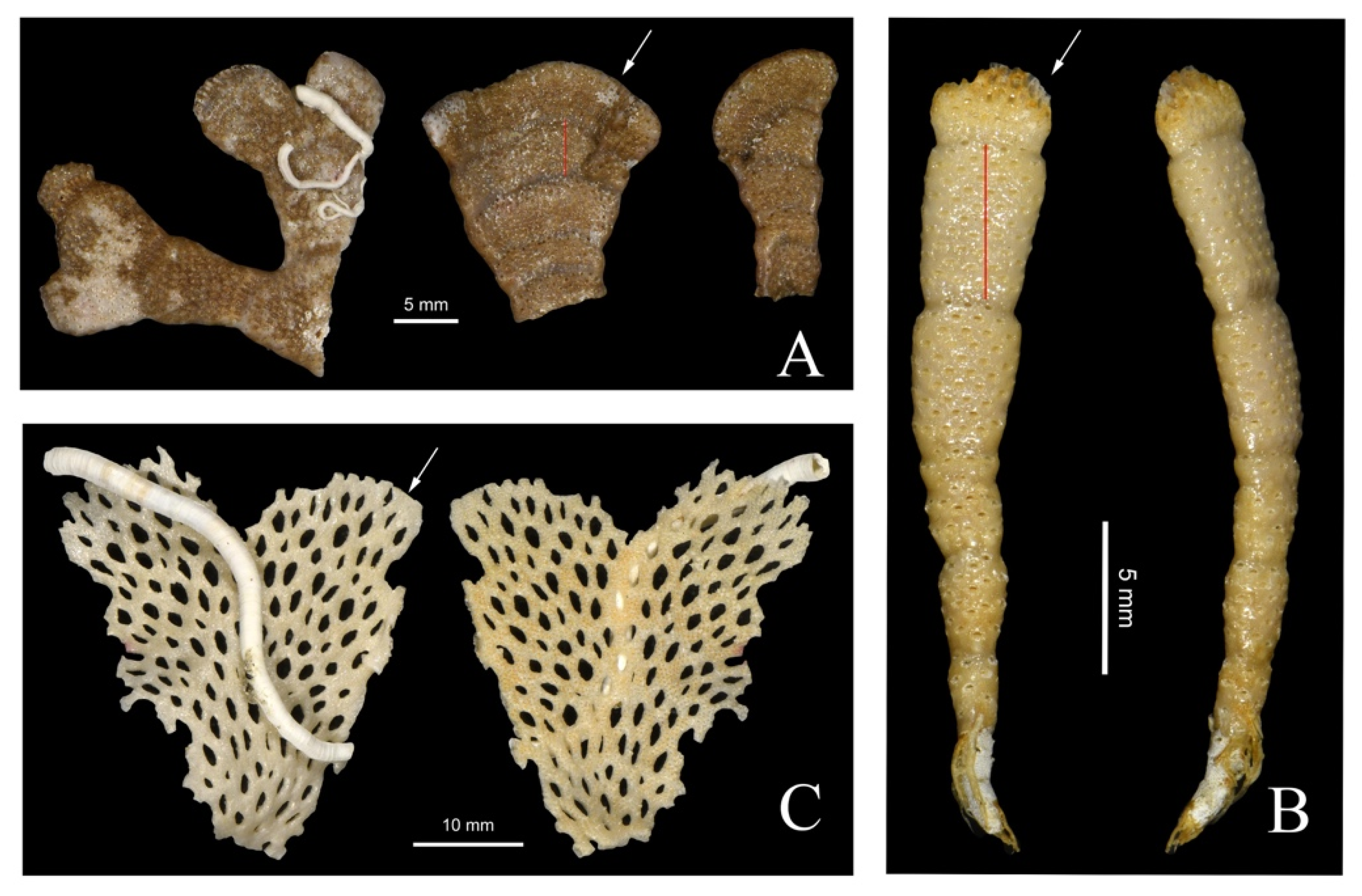
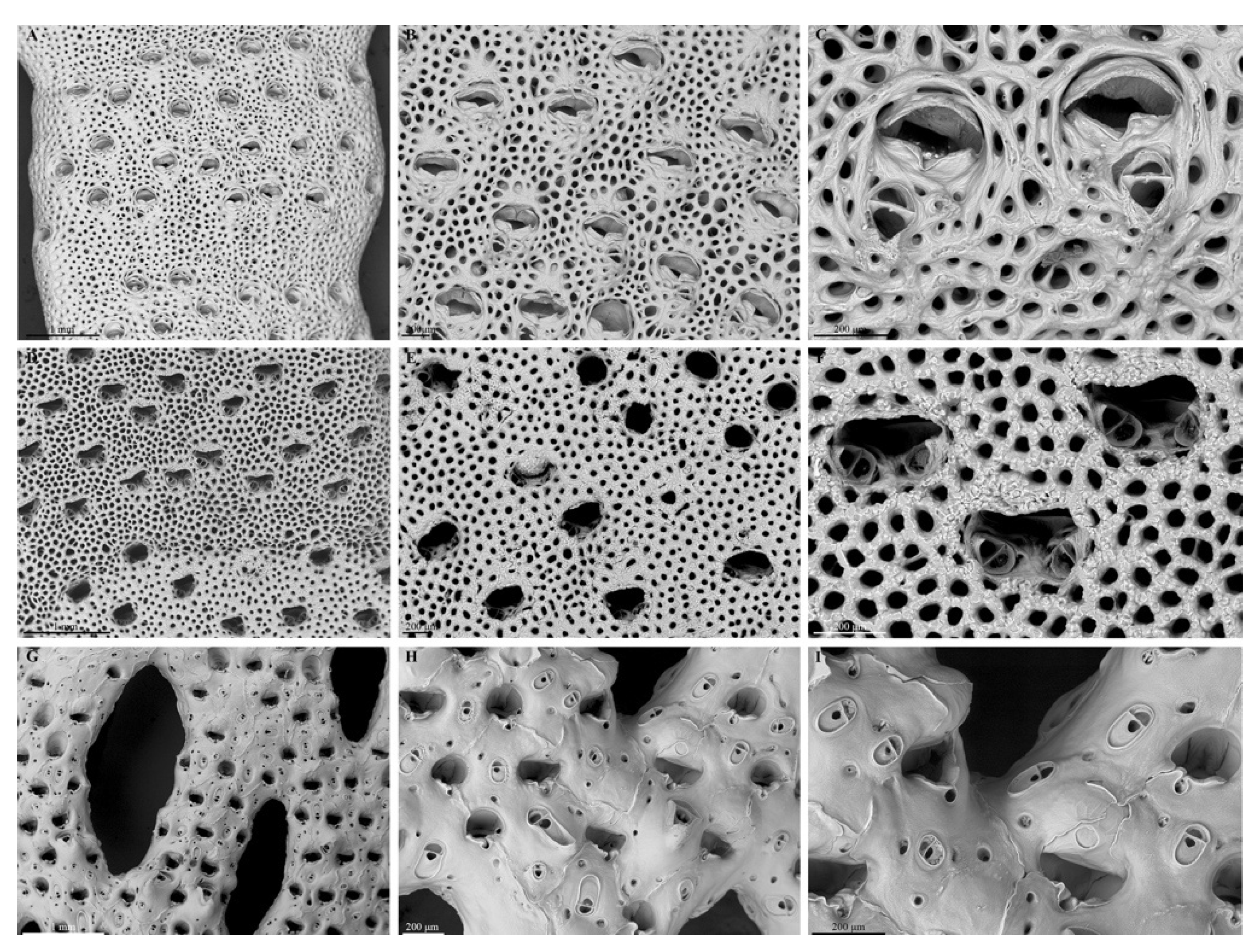
3.1.2. Skeletal Characteristics
3.2. Biomineralization—The Skeletal Organic Matrix
3.2.1. Quantification of the Matrix and SDS PAGE
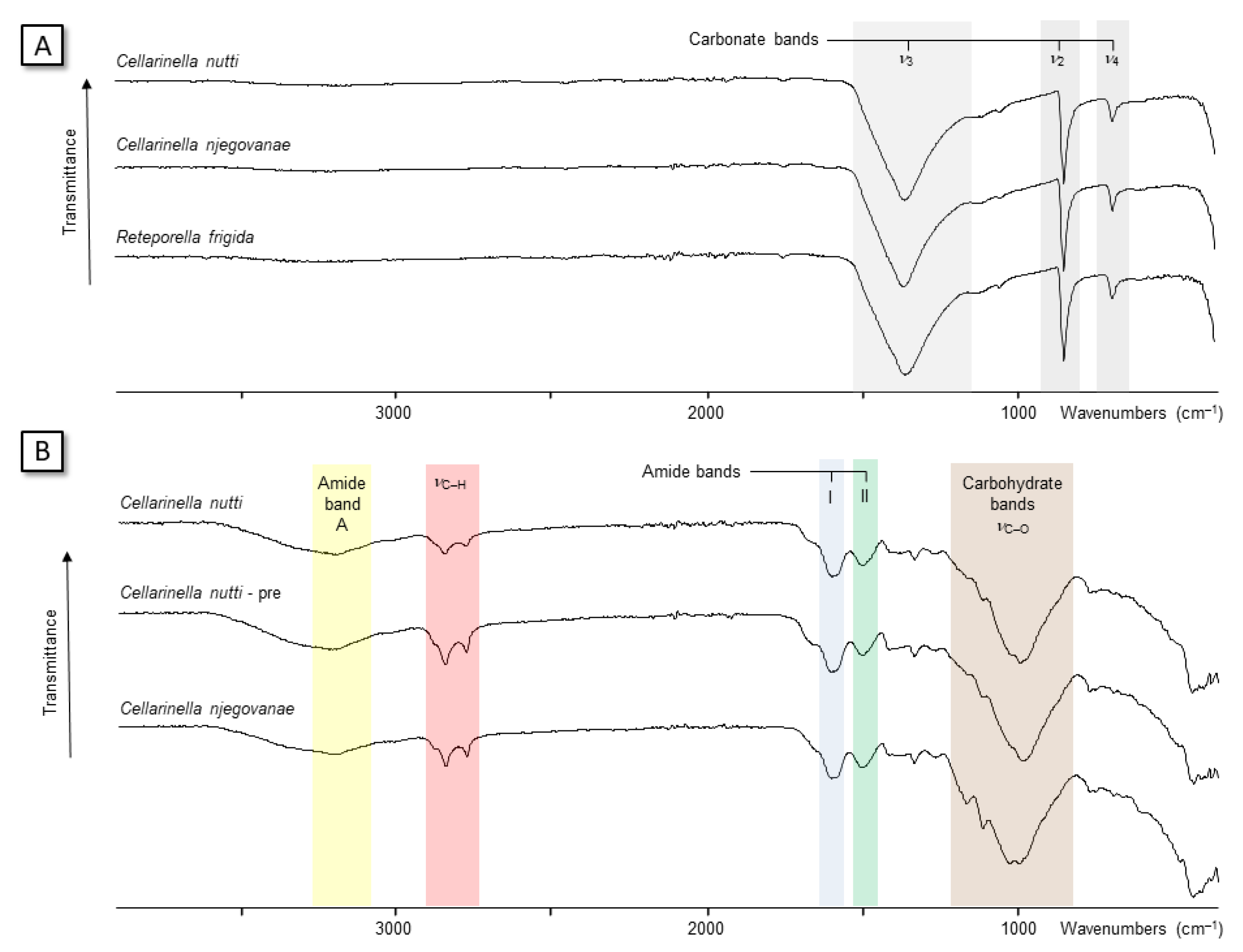
3.2.2. FT-IR Spectroscopy
3.2.3. ELLA Test
3.3. Sea-Water Characteristics and Isotopy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, P.D. Bryozoan Paleobiology; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Zhang, Z.; Zhang, Z.; Ma, J.; Taylor, P.D.; Strotz, L.C.; Jacquet, S.M.; Skovsted, C.B.; Chen, F.; Han, J.; Brock, G.A. Fossil evidence unveils an early Cambrian origin for Bryozoa. Nature 2022, 599, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N. Lessons in modularity: The evolutionary ecology of colonial invertebrates. Sci. Mar. 2005, 69, 169–179. [Google Scholar] [CrossRef]
- Carroll, S.B. Chance and necessity: The evolution of morphological complexity and diversity. Nature 2001, 409, 1102–1109. [Google Scholar] [CrossRef]
- Ma, J.; Taylor, P.D.; Xia, F.; Zhan, R. The oldest known bryozoan: Prophyllodictya (Cryptostomata) from the lower Tremadocian (Lower Ordovician) of Liujiachang, south-western Hubei, central China. Palaeontology 2015, 58, 925–934. [Google Scholar] [CrossRef]
- Taylor, P.D.; Lombardi, C.; Cocito, S. Biomineralization in bryozoans: Present, past and future: Bryozoan biomineralization. Biol. Rev. 2015, 90, 1118–1150. [Google Scholar] [CrossRef] [PubMed]
- Wray, G.A. Molecular clocks and the early evolution of metazoan nervous systems. Philos. Trans. 2015, 370, 20150046. [Google Scholar] [CrossRef]
- Wood, A.C.L.; Probert, P.K.; Rowden, A.; Smith, A. Complex habitat generated by marine bryozoans: A review of its distribution, structure, diversity, threats and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 547–563. [Google Scholar] [CrossRef]
- Taylor, P.D.; Kudryavtsev, A.B.; Schopf, J.W. Calcite and aragonite distributions in the skeletons of bimineralic bryozoans as revealed by Raman spectroscopy. Invertebr. Biol. 2008, 127, 87–97. [Google Scholar] [CrossRef]
- Hiscock, K. Marine Biodiversity Conservation, a Practical Approach; Earthscan from Routledge (Taylor and Francis Group): London, UK; New York, NY, USA, 2014; p. 298. [Google Scholar]
- Cocito, S.; Novosel, M.; Novosel, A. Carbonate bioformations around underwater freshwater springs in the north-eastern Adriatic Sea. Facies 2004, 50, 13–17. [Google Scholar] [CrossRef]
- Lombardi, C.; Cocito, S.; Taylor, P.D. Bryozoan Bioconstructions in a Changing Mediterranean Sea. In The Mediterranean Sea—Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: New York, NY, USA, 2013; pp. 373–384. [Google Scholar]
- Lombardi, C.; Taylor, P.D.; Cocito, S. Bryozoans: The Forgotten Bioconstructors. In Perspective on the Marine Animal Forest; Rossi, S., Bramanti, L., Eds.; Springer Nature: Berlin, Germany, 2021; Volume 11, pp. 193–217. [Google Scholar]
- Meredith, M.P.; Schofield, O.; Newman, L.; Urban, E.; Sparrow, M. The vision for a Southern Ocean Observing System. Curr. Opin. Environ. Sustain. 2013, 5, 306–313. [Google Scholar] [CrossRef]
- Meredith, M.P.; Sommerkorn, M.; Cassotta, S.; Derksen, C.; Ekaykin, A.; Hollowed, A.; Kofinas, G.; Mackintosh, A.; Mel-bourne-Thomas, J.; Muelbert, M.M.C.; et al. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O.D.C., Roberts, V., Masson-Delmotte, P., Zhai, M., Tignor, E., Poloczanska, K., Mintenbeck, A., Alegría, M., Nicolai, A., Okem, J., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019. [Google Scholar]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef]
- Caldeira, K.; Duffy, P.B. The Role of the Southern Ocean in Uptake and Storage of Anthropogenic Carbon Dioxide. Science 2000, 287, 620–622. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The Oceanic Sink for Anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Convey, P.; Peck, L.S. Antarctic environmental change and biological responses. Sci. Adv. 2019, 5, eaaz0888. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Iceberg killing fields limit huge potential for benthic blue carbon in Antarctic shallows. Glob. Chang. Biol. 2016, 23, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A. Polar zoobenthos blue carbon storage increases with sea ice losses, because across-shelf growth gains from longer algal blooms outweigh ice scour mortality in the shallows. Glob. Chang. Biol. 2017, 23, 5083–5091. [Google Scholar] [CrossRef]
- Gutt, J.; Isla, E.; Bertler, A.; Bodeker, G.; Bracegirdle, T.; Cavanagh, R.; Comiso, J.; Convey, P.; Cummings, V.; De Conto, R.; et al. Cross-disciplinarity in the advance of Antarctic ecosystem research. Mar. Genom. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Henley, S.F.; Schofield, O.M.; Hendry, K.R.; Schloss, I.R.; Steinberg, D.K.; Moffat, C.; Peck, L.S.; Costa, D.P.; Bakker, D.C.; Hughes, C.; et al. Variability and change in the west Antarctic Peninsula marine system: Research priorities and opportunities. Prog. Oceanogr. 2019, 173, 208–237. [Google Scholar] [CrossRef]
- Gutt, J.; Starmans, A. Structure and biodiversity of megabenthos in the Weddell and Lazarev Seas (Antarctica): Ecological role of physical parameters and biological interactions. Polar Biol. 1998, 20, 229–247. [Google Scholar] [CrossRef]
- Parker, S.J.; Bowden, D.A. Identifying taxonomic groups vulnerable to bottom longline fishing gear in the Ross Sea region. CCAMLR Sci. 2010, 17, 105–127. [Google Scholar]
- Barnes, D.K.A.; Downey, R.V. Bryozoa. In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi., P., Griffiths, H.J., Raymond, B., Udekem d’Acoz, C.d., Eds.; SCAAR: Cambridge, UK, 2014; pp. 195–199. [Google Scholar]
- De Broyer, C.; Danis, B. How many species in the Southern Ocean? Towards a dynamic inventory of the Antarctic marine species. Deep. Sea Res. Part II 2011, 58, 5–17. [Google Scholar] [CrossRef]
- Figuerola, B.; Gordon, D.P.; Polonio, V.; Cristobo, J.; Avila, C. Cheilostome bryozoan diversity from the Southwest Atlantic region: Is Antarctica really isolated? J. Sea Res. 2014, 85, 1–17. [Google Scholar] [CrossRef]
- Rosso, A. Bryozoa from Terra Nova Bay (Ross Sea, Antarctica). In Oceanografia in Antartide; Gallardo, V.A., Ferretti, O., Moyano, H.I., Eds.; Universidad de Concepcion: Centro Eula, Chile, 1992; pp. 359–369. [Google Scholar]
- Pabis, K.; Hara, U.; Presler, P.; Sicinski, J. Structure of bryozoan communities in an Antarctic glacial fjord (Admiralty Bay, South Shetlands). Polar Biol. 2014, 37, 737–751. [Google Scholar] [CrossRef]
- Figuerola, B.; Hancock, A.M.; Bax, N.; Cummings, V.J.; Downey, R.; Griffiths, H.J.; Smith, J.; Stark, J.S. A Review and Meta-Analysis of Potential Impacts of Ocean Acidification on Marine Calcifiers from the Southern Ocean. Front. Mar. Sci. 2021, 8, 584445. [Google Scholar] [CrossRef]
- Eggleston, D. The Marine Polyzoa of the Isle of Man. Ph.D. Thesis, University of Liverpool, Liverpool, UK, 1963; pp. 1–297. [Google Scholar]
- Bader, B.; Schäfer, P. Skeletal morphogenesis and growth check lines in the Antarctic bryozoan Melicerita obliqua. J. Nat. Hist. 2004, 38, 2901–2922. [Google Scholar] [CrossRef]
- Smith, A.M.; Stewart, B.; Key, M.M., Jr.; Jamet, C.M. Growth and carbonate production by Adeonellopsis (Bryozoa: Cheilostomata) in Doubtful Sound, New Zealand. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 175, 201–210. [Google Scholar] [CrossRef]
- Barnes, D.K. Seasonal and annual growth in erect species of Antarctic bryozoans. J. Exp. Mar. Biol. Ecol. 1995, 188, 181–198. [Google Scholar] [CrossRef]
- Barnes, D.; Webb, K.; Linse, K. Slow growth of Antarctic bryozoans increases over 20 years and is anomalously high in 2003. Mar. Ecol. Prog. Ser. 2006, 314, 187–195. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Webb, K.E.; Linse, K. Growth rate and its variability in erect Antarctic bryozoans. Polar Biol. 2007, 30, 1069–1081. [Google Scholar] [CrossRef]
- Tavener-Smith, R.; Williams, A. The secretion and structure of the skeleton of living and fossil Bryozoa. Philos. Trans. R. Soc. London B Biol. Sci. 1972, 264, 97–160. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University: Oxford, UK, 1989. [Google Scholar]
- Dove, P.M.; deYoreo, J.J.; Weiner, S. Biomineralization. Rev. Min. Geochem. 2003, 54, 1–381. [Google Scholar]
- Marin, F.; Bundeleva, I.; Takeuchi, T.; Immel, F.; Medakovic, D. Organic matrices in metazoan calcium carbonate skeletons: Composition, functions, evolution. J. Struct. Biol. 2016, 196, 98–106. [Google Scholar] [CrossRef]
- Barnes, D. Antarctic sea ice losses drive gains in benthic carbon drawdown. Curr. Biol. 2015, 25, R789–R790. [Google Scholar] [CrossRef] [PubMed]
- Peck, L.S.; Barnes, D.K.A.; Coock, A.J.; Fleming, A.H.; Clarke, A. Negative feedback in the cold: Ice retreat produces new carbin sink in Antarctica. Glob. Chang. Biol. 2010, 16, 2614–2623. [Google Scholar] [CrossRef]
- Santagata, S.; Ade, V.; Mahon, A.R.; Wisocki, P.A.; Halanych, K.M. Compositional Differences in the Habitat-Forming Bryozoan Communities of the Antarctic Shelf. Front. Ecol. Evol. 2018, 6, 116. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Romagnoli, T.; Libani, G.; Antonucci, M.; Scarponi, G.; Totti, C.; Truzzi, C. Distribution of Cd, Pb and Cu between dissolved fraction, inorganic particulate and phytoplankton in seawater of Terra Nova Bay (Ross Sea, Antarctica) during austral summer 2011–12. Chemosphere 2017, 185, 1122–1135. [Google Scholar] [CrossRef]
- Budillon, G.; Pacciaroni, M.; Cozzi, S.; Rivaro, P.; Catalano, G.; Ianni, C.; Cantoni, C. An optimum multiparameter mixing analysis of the shelf waters in the Ross Sea. Antarct. Sci. 2003, 15, 105–118. [Google Scholar] [CrossRef]
- Nihashi, S.; Ohshima, K.I. Circumpolar Mapping of Antarctic Coastal Polynyas and Landfast Sea Ice: Relationship and Variability. J. Clim. 2015, 28, 3650–3670. [Google Scholar] [CrossRef]
- Faranda, F.M.; Guglielmo, L.; Ianora, A. The Italian Oceanographic Cruises in the Ross Sea (1987-95): Strategy, General Consideration Description of the Sampling Sites. In Ross Sea Ecology; Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–14. [Google Scholar]
- Silvano, A.; Foppert, A.; Rintoul, S.R.; Holland, P.R.; Tamura, T.; Kimura, N.; Castagno, P.; Falco, P.; Budillon, G.; Haumann, F.A.; et al. Recent recovery of Antarctic Bottom Water formation in the Ross Sea driven by climate anomalies. Nat. Geosci. 2020, 13, 780–786. [Google Scholar] [CrossRef]
- Lombardi, C.; Kuklinski, P.; Bordone, A.; Spirandelli, E.; Raiteri, G. Assessment of Annual Physico-Chemical Variability via High-Temporal Resolution Monitoring in an Antarctic Shallow Coastal Site (Terra Nova Bay, Ross Sea). Minerals 2021, 11, 374. [Google Scholar] [CrossRef]
- Pusceddu, A.; Dell’Anno, A.; Fabiano, M. Organic matter composition in coastal sediments at Terra Nova Bay (Ross Sea) during summer. Polar Biol. 2000, 23, 288–293. [Google Scholar] [CrossRef]
- Alonso-Saez, L.; Sanchez, O.; Gasol, J.; Balaguè, V.; Pedros-Alio, C. Winter-to-summer changes in the composition and sin-gle-cell activity of near-surface Arctic prokaryotes. Environ. Microbiol. 2008, 10, 2444–2454. [Google Scholar] [CrossRef]
- Caputi, S.S.; Careddu, G.; Calizza, E.; Fiorentino, F.; Maccapan, D.; Rossi, L.; Costantini, M.L. Seasonal Food Web Dynamics in the Antarctic Benthos of Tethys Bay (Ross Sea): Implications for Biodiversity Persistence Under Different Seasonal Sea-Ice Coverage. Front. Mar. Sci. 2020, 7, 594454. [Google Scholar] [CrossRef]
- Cantero, L.P.; Boero, F.; Piraino, S. Shallow-water benthic hydroids from Tethys Bay (Terra Nova Bay, Ross Sea, Antarctica). Polar Biol. 2013, 36, 731–753. [Google Scholar] [CrossRef]
- Calizza, E.; Rossi, L.; Careddu, G.; Caputi, S.S.; Costantini, M.L. Species richness and vulnerability to disturbance propagation in real food webs. Sci. Rep. 2019, 9, 19331. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.N.; Danis, B.; Dubois, P.; Eleaume, M.; Fournier, J.; Gallut, C.; Jane, P.; Lepoint, G. Increased sea ice cover alters food web structure in East Antarctica. Sci. Rep. 2019, 9, 8062. [Google Scholar] [CrossRef]
- Clark, G.F.; Stark, J.S.; Palmer, A.S.; Riddle, M.J.; Johnston, E.L. The Roles of Sea-Ice, Light and Sedimentation in Structuring Shallow Antarctic Benthic Communities. PLoS ONE 2017, 12, e0168391. [Google Scholar] [CrossRef] [PubMed]
- Pearse, J.S.; McClintock, J.B.; Bosch, I. Reproduction of Antarctic Benthic Marine Invertebrates: Tempos, Modes, and Timing. Am. Zoöl. 1991, 31, 65–80. [Google Scholar] [CrossRef]
- Hayward, P.J. Antarctic Chilostomatus Bryozoa; Oxford University Press Inc.: New York, NY, USA, 1995; p. 355. [Google Scholar]
- Kuklinski, P. Atlas of Antarctic Bryozoa. Available online: http://www.iopan.gda.pl/ekologia/Antarctica/index.php (accessed on 1 May 2017).
- Morrissey, J.H. Silver stain for proteins in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal. Biochem. 1981, 117, 307–310. [Google Scholar] [CrossRef]
- Mouchi, V.; Lartaud, F.; Guichard, N.; Immel, F.; de Rafélis, M.; Broussard, C.; Crowley, Q.G.; Marin, F. Chalky versus foliated: A discriminant immunogold labelling of shell microstructures in the edible oyster Crassostrea gigas. Mar. Biol. 2016, 163, 256. [Google Scholar] [CrossRef]
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22. [Google Scholar] [CrossRef]
- Dauphin, Y. Comparative studies of skeletal soluble matrices from some Scleractinian corals and Molluscs. Int. J. Biol. Macromol. 2001, 28, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kanold, J.M.; Guichard, N.; Immel, F.; Plasseraud, L.; Corneillat, M.; Alcaraz, G.; Brümmer, F.; Marin, F. Spine and test skeletal matrices of the Mediterranean sea urchin Arbacia lixula—A comparative characterization of their sugar signature. FEBS J. 2015, 282, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Immel, F.; Broussard, C.; Catherinet, B.; Plasseraud, L.; Alcaraz, G.; Bundeleva, I.; Marin, F. The Shell of the Invasive Bivalve Species Dreissena polymorpha: Biochemical, Elemental and Textural Investigations. PLoS ONE 2016, 11, e0154264. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef]
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
- van Geldern, R.; Barth, J.A. Optimization of instrument setup and post-run corrections for oxygen and hydrogen stable isotope measurements of water by isotope ratio infrared spectroscopy (IRIS). Limnol. Oceanogr. Methods 2012, 10, 1024–1036. [Google Scholar] [CrossRef]
- van Geldern, R.; Schulte, P.; Mader, M.; Baier, A.; Barth, J.A.C. Spatial and temporal variations of pCO2, dissolved inorganic carbon and stable isotopes along a temperate karstic watercourse. Hydrol. Process. 2015, 29, 3423–3440. [Google Scholar] [CrossRef]
- Cheng, L.; Normandeau, C.; Bowden, R.; Doucett, R.; Gallagher, B.; Gillikin, D.P.; Kumamoto, Y.; McKay, J.L.; Middlestead, P.; Ninnemann, U.; et al. An international intercomparison of stable carbon isotope composition measurements of dissolved inorganic carbon in seawater. Limnol. Oceanogr. Methods 2019, 17, 200–209. [Google Scholar] [CrossRef]
- Barnes, D.K.; Ireland, L.; Hogg, O.T.; Morely, S.; Enederlein, P.; Sands, C.J. Why is the Southern Orkney Isalnd shelf (the world’s first high seas marine protected area) a carbon immobilization hotspot? Glob. Chang. Biol. 2016, 22, 1110–1120. [Google Scholar] [CrossRef]
- Key, M.M.; Rossi, R.K.; Smith, A.M.; Hageman, S.J.; Patterson, W.P. Stable isotope profiles of skeletal carbonate validate annually-produced growth checks in the bryozoan Melicerita chathamensis from Snares Platform, New Zealand. Bull. Mar. Sci. 2018, 94, 1447–1464. [Google Scholar] [CrossRef]
- Lombardi, C.; Gambi, M.C.; Vasapollo, C.; Taylor, P.; Cocito, S. Skeletal alterations and polymorphism in a Mediterranean bryozoan at natural CO2 vents. Zoomorphology 2011, 130, 135–145. [Google Scholar] [CrossRef]
- Lombardi, C.; Cocito, S.; Gambi, M.C.; Taylor, P.D. Morphological plasticity in a calcifying modular organism: Evidence from an in situ transplant experiment in a natural CO2 vent system. R. Soc. Open Sci. 2015, 2, 140413. [Google Scholar] [CrossRef]
- Lombardi, C.; Taylor, P.D.; Cocito, S.; Bertolini, C.; Calosi, P. Low pH conditions impair module capacity to regenerate in a calcified colonial invertebrate, the bryozoan Cryptosula pallasiana. Mar. Environ. Res. 2017, 125, 110–117. [Google Scholar] [CrossRef]
- Kuklinski, P.; Taylor, P.D. Mineralogy of Arctic bryozoan skeletons in a global context. Facies 2009, 55, 489–500. [Google Scholar] [CrossRef]
- Krzeminska, M.; Kuklinski, P.; Najorka, J.; Iglikowska, A. Skeletal Mineralogy Patterns of Antarctic Bryozoa. J. Geol. 2016, 124, 411–422. [Google Scholar] [CrossRef]
- Smith, A.M.; Key, M.M.; Gordon, D.P. Skeletal mineralogy of bryozoans: Taxonomic and temporal patterns. Earth-Sci. Rev. 2006, 78, 287–306. [Google Scholar] [CrossRef]
- Ciavatta Ml Lefranc, F.; Vieira, L.; Kiss, R.; Carbone, M.; van Otterlo, W.A.L.; Lopanik, N.B.; Waeshenback, A. The Phylum Bryozoa: From Biology to Biometical Potentia. Mar. Drugs 2020, 18, 200. [Google Scholar] [CrossRef]
- Clamp, A.; Jayson, G. The clinical development of the bryostatins. Anti-Cancer Drugs 2002, 13, 673–683. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Bharate, S.B. Preclinical and clinical studies on bryostains, a class of marine-derived protein kinase C modulators: A mini review. Curr. Top. Med. Chem. 2020, 20, 1124–1135. [Google Scholar] [CrossRef]
- Ramos-Silva, P.; Marin, F.; Kaandorp, J.; Marie, B. Biomineralization toolkit: The importance of sample cleaning prior to the characterization of biomineral proteomes. Proc. Natl. Acad. Sci. USA 2013, 110, E2144–E2146. [Google Scholar] [CrossRef]
- Weiner, S.; Traub, W. Macromolecules in mollusc shells and their functions in biomineralization. Philos. Trans. R. Soc. London B Biol. Sci. 1984, 304, 425–434. [Google Scholar] [CrossRef]
- Marin, F.; Marie, B.; Hamada, S.B.; Silva, P.; Le Roy, N.; Guichard, N.; Wolf, S.; Montagnani, C.; Joubert, C.; Piquemal, D.; et al. Shellome: Proteins involved in molluscs shell biomineralization—Diversity, functions. In Recent Advances in Pearl Research; Terra Pub: San Francisco, CA, USA, 2013; pp. 149–168. [Google Scholar]
- Osuna-Mascaró, A.; Cruz-Bustos, T.; Benhamada, S.; Guichard, N.; Marie, B.; Plasseraud, L.; Corneillat, M.; Alcaraz, G.; Checa, A.; Marin, F. The shell organic matrix of the crossed lamellar queen conch shell (Strombus gigas). Comp. Biochem. Physiol. Part B (Biochem. Mol. Biol.) 2014, 168, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Luquet, G. Unusually acidic proteins in biomineralization. In Handbook of Biomineralization: Biological Aspects and Structure Formation; Wiley: Hoboken, NJ, USA, 2007; pp. 273–290. [Google Scholar]
- Hunt, S. Scleroprotein and chitin in the exoskeleton of the ectoproct Flustra foliacea. Comp. Biochem. Physiol. 1972, 43, 571–574. [Google Scholar] [CrossRef]
- Kaya, M.; Baublys, V.; Šatkauskienė, I.; Akyuz, B.; Bulut, E.; Tubelytė, V. First chitin extraction from Plumatella repens (Bryozoa) with comparison to chitins of insect and fungal origin. Int. J. Biol. Macromol. 2015, 79, 126–132. [Google Scholar] [CrossRef]
- Goffinet, G.; Jeuniaux, C. Distribution et importance quantitative de la chitine dans les coquilles de mollusques. Cah. Biol. Mar. 1979, 20, 341–349. [Google Scholar]
- Furuhashi, T.; Schwarzinger, C.; Miksik, I.; Smrz, M.; Beran, A. Molluscan shell evolution with review of shell calcification hypothesis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 154, 351–371. [Google Scholar] [CrossRef]
- Oudot, M.; Neige, P.; Ben Shir, I.; Schmidt, A.; Strugnell, J.M.; Plasseraud, L.; Broussard, C.; Hoffmann, R.; Lukeneder, A.; Marin, F. The shell matrix and microstructure of the Ram’s Horn squid: Molecular and structural characterization. J. Struct. Biol. 2020, 211, 107507. [Google Scholar] [CrossRef]
- Agbaje, O.B.A.; Ben Shir, I.; Zax, D.B.; Schmidt, A.; Jacob, D.E. Biomacromolecules within bivalve shells: Is chitin abundant? Acta Biomater. 2018, 80, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Dini, M.; Stenni, B. Oxygen isotope characterization of Terra Nova Bay seawater. In Ross Sea Ecology; Faranda, F., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; Chapter 3; pp. 27–37. [Google Scholar]
- Jacobs, S.S.; Giulivi, C.F.; Mele, P.A. Freshening of the Ross Sea During the Late 20th Century. Science 2002, 297, 386–389. [Google Scholar] [CrossRef] [PubMed]
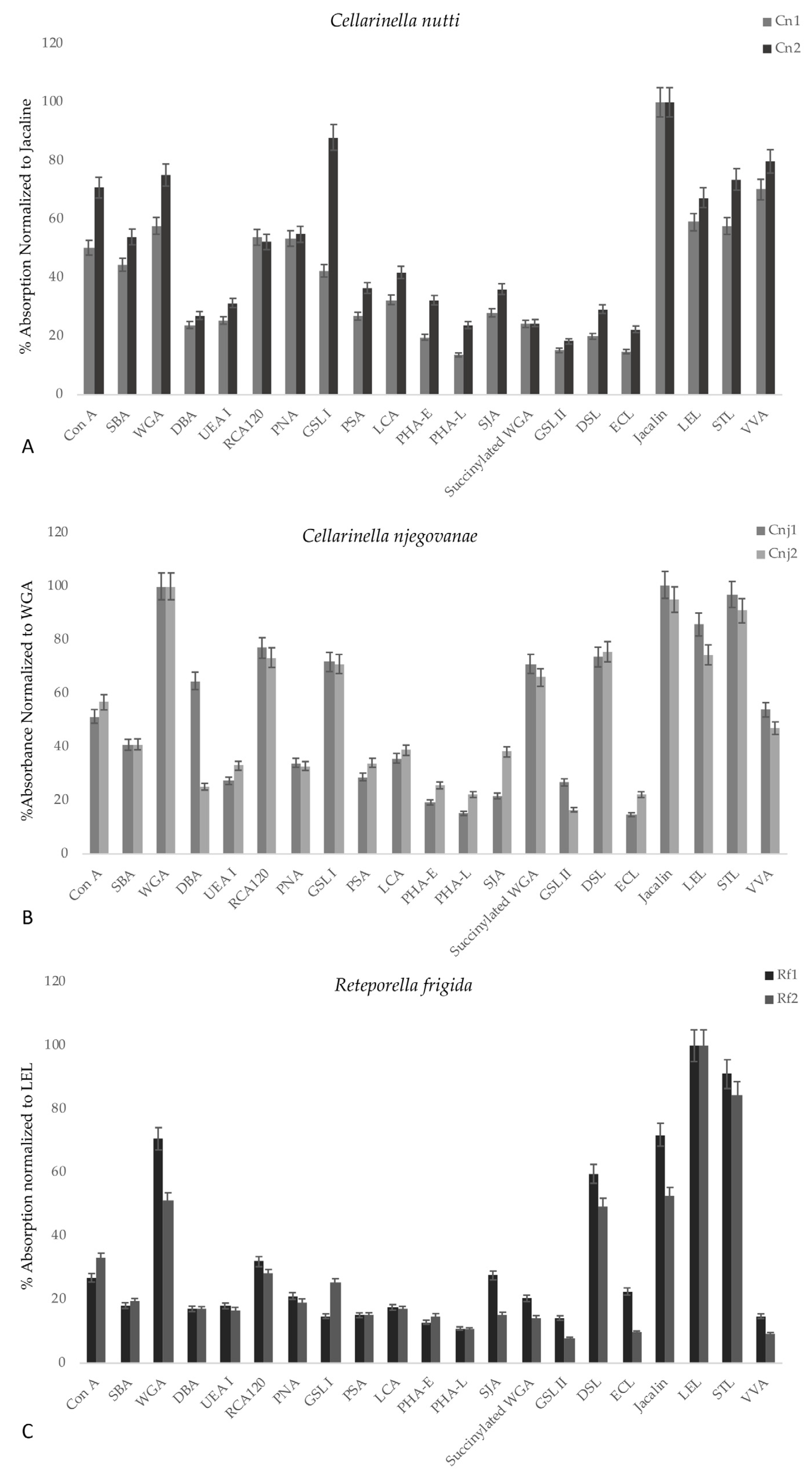
| Code | Abbreviation | Extract Name | Lectin Type |
|---|---|---|---|
| A1 | Con A | Canavalia ensiformis | D-Mannose/D-Glucose/N-acetylglucosamine |
| B1 | SBA | Glycine max | Galactose/N-acetylgalactosamine |
| C1 | WGA | Triticum vulgare | Chitin-binding lectins/N-acetylglucosamine/N-acetyllactosamine |
| D1 | DBA | Dolichos biflorus | N-acetylgalactosamine |
| E1 | UEA I | Ulex europaeus | Fucose |
| F1 | RCA 120 | Ricinus communis | Galactose/N-acetylgalactosamine |
| G1 | PNA | Arachis hypogaea | Galactose/N-acetylgalactosamine |
| A2 | GLS I | Griffonia simplicifolia | Galactose/N-acetylgalactosamine |
| B2 | PSA | Pisum sativum | D-Mannose/D-Glucose/N-acetylglucosamine |
| C2 | LCA | Lens culinaris | D-Mannose/D-Glucose/N-acetylglucosamine |
| D2 | PHA-E | Phaseolus vulgaris | Galactose/N-acetylglucosamine/Mannose |
| E2 | PHA-L | Phaseolus vulgaris | Galactose/N-acetylglucosamine/Mannose |
| F2 | SJA | Sophora japonica | Galactose/N-acetylgalactosamine |
| G2 | Succinylated WGA | Triticum vulgare | Chitin-binding lectins/N-acetylglucosamine/N-acetyllactosamine |
| A3 | GLS II | Griffonia simplicifolia | N-acetylglucosamine |
| B3 | DLS | Datura stramonium | Chitin-binding lectins/N-acetylglucosamine/N-acetyllactosamine |
| C3 | ECL | Erythrina cristagalli | N-acetyllactosamine/N-acetylgalactosamine |
| D3 | Jacalin | Artocarpus integrifolia | Galactose/N-acetylgalactosamine |
| E3 | LEL | Lycopersicon esculentum | Chitin-binding lectins/N-acetylglucosamine/N-acetyllactosamine |
| F3 | STL | Solanum tuberosum | Chitin-binding lectins/N-acetylglucosamine/N-acetyllactosamine |
| G3 | VVA | Vicia villosa | N-acetylgalactosamine |
| Species | n gb | gbL (µm) |
|---|---|---|
| Cellarinella nutti | 6 | 1449 |
| 5 | 3360 | |
| 4 | 3547 | |
| 3 | 3286 | |
| 2 | 4274 | |
| 1 | 4099 | |
| Cellarinella njegovanae | ||
| 6 | 3685 | |
| 5 | 7127 | |
| 4 | 1974 | |
| 3 | 3384 | |
| 2 | 5859 | |
| 1 | 2829 |
| Species | n°gb | bwTgb (µm) | bwTgcl (µm) | RbwT gcl/gb | lwTgb (µm) | lwTgcl (µm) | RlwT gcl/gb | fwT (µm) |
|---|---|---|---|---|---|---|---|---|
| 6 | 45.9 ± 6.8 | 140.0 ± 24.0 | 3.0 | 51.6 ± 9.0 | 115.1 ± 20.3 | 2.2 | 316.6 ± 86.3 | |
| 5 | 33.7 ± 6.8 | 125.1 ± 6.8 | 3.7 | 33.4 ± 9.0 | 94.2 ± 9.0 | 2.8 | ||
| Cellarinella | 4 | 33.0 ± 7.5 | 135.8 ± 21.1 | 4.1 | 31.6 ± 7.2 | 101.2 ± 17.4 | 3.2 | |
| nutti | 3 | 30.6 ± 4.5 | 118.0 ± 20.3 | 3.9 | 36.3 ± 7.7 | 106.5 ± 16.8 | 2.9 | |
| 2 | 32.5 ± 4.3 | 119.9 ± 19.3 | 3.7 | 31.1 ± 7.2 | 108.5 ± 19.8 | 3.5 | ||
| 1 | 26.6 ± 5.6 | 130.4 ± 18.9 | 4.9 | 30.8 ± 9.3 | 105.7 ± 13.3 | 3.4 | ||
| 6 | 56.7 ± 13.5 | 184.7 ± 23.8 | 3.3 | 49.1 ± 16.4 | 142.0 ± 15.7 | 2.9 | 445.0 ± 80.3 | |
| 5 | 42.4 ± 13.5 | 171.0 ± 13.5 | 4.0 | 41.7 ± 16.4 | 142.8 ± 16.4 | 3.4 | ||
| Cellarinella | 4 | 51.6 ± 9.1 | 118.5 ± 20.1 | 2.3 | 46.3 ± 11.5 | 181.1 ± 34.4 | 3.9 | |
| njegovanae | 3 | 37.9 ± 9.8 | 165.5 ± 30.5 | 4.4 | 37.4 ± 8.6 | 143.6 ± 27.9 | 3.8 | |
| 2 | 29.6 ± 7.2 | 173.2 ± 28.6 | 5.9 | 26.8 ± 6.3 | 146.3 ± 31.3 | 5.4 | ||
| 1 | 25.7 ± 7.5 | 29.2 ± 6.9 | ||||||
| Reteporella frigida | 166.9 ± 40 | 31.9 ± 9.1 | 226.0 ± 33.5 |
| Sample | Powder Weight gr | ASM mg (% Weight) | AIM mg (% Weight) | Total Matrix mg (% Weight) | ASM/AIM Ratio |
|---|---|---|---|---|---|
| C. nutti | 9.05 | 2.24 ± 0.024 | 7.25 ± 0.080 | 9.49 ± 0.105 | 0.31 |
| C. nutti PRE | 1.45 | 0.70 ± 0.048 | 0.57 ± 0.039 | 1.27 ± 0.087 | 1.23 |
| C. njegovanae | 1.36 | 0.60 ± 0.044 | 9.55 ± 0.702 | 10.15 ± 0.746 | 0.06 |
| R. frigida | 3.56 | 0.31 ± 0.009 | 14.78 ± 0.415 | 15.09 ± 0.424 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, C.; Kuklinski, P.; Spirandelli, E.; Bruzzone, G.; Raiteri, G.; Bordone, A.; Mazzoli, C.; López Correa, M.; van Geldern, R.; Plasseraud, L.; et al. Antarctic Bioconstructional Bryozoans from Terra Nova Bay (Ross Sea): Morphology, Skeletal Structures and Biomineralization. Minerals 2023, 13, 246. https://doi.org/10.3390/min13020246
Lombardi C, Kuklinski P, Spirandelli E, Bruzzone G, Raiteri G, Bordone A, Mazzoli C, López Correa M, van Geldern R, Plasseraud L, et al. Antarctic Bioconstructional Bryozoans from Terra Nova Bay (Ross Sea): Morphology, Skeletal Structures and Biomineralization. Minerals. 2023; 13(2):246. https://doi.org/10.3390/min13020246
Chicago/Turabian StyleLombardi, Chiara, Piotr Kuklinski, Edoardo Spirandelli, Giorgio Bruzzone, Giancarlo Raiteri, Andrea Bordone, Claudio Mazzoli, Matthias López Correa, Robert van Geldern, Laurent Plasseraud, and et al. 2023. "Antarctic Bioconstructional Bryozoans from Terra Nova Bay (Ross Sea): Morphology, Skeletal Structures and Biomineralization" Minerals 13, no. 2: 246. https://doi.org/10.3390/min13020246
APA StyleLombardi, C., Kuklinski, P., Spirandelli, E., Bruzzone, G., Raiteri, G., Bordone, A., Mazzoli, C., López Correa, M., van Geldern, R., Plasseraud, L., Thomas, J., & Marin, F. (2023). Antarctic Bioconstructional Bryozoans from Terra Nova Bay (Ross Sea): Morphology, Skeletal Structures and Biomineralization. Minerals, 13(2), 246. https://doi.org/10.3390/min13020246








