Pyrite Sulfur Isotope Systematics Suggest Low Marine Sulfate Levels across the Ediacaran–Cambrian Transition
Abstract
1. Introduction
2. Geologic Setting and Samples
3. Methods
4. Results
5. Discussion
5.1. Syndepositional Early Diagenetic Origin of Pyrite in the Dengying Formation
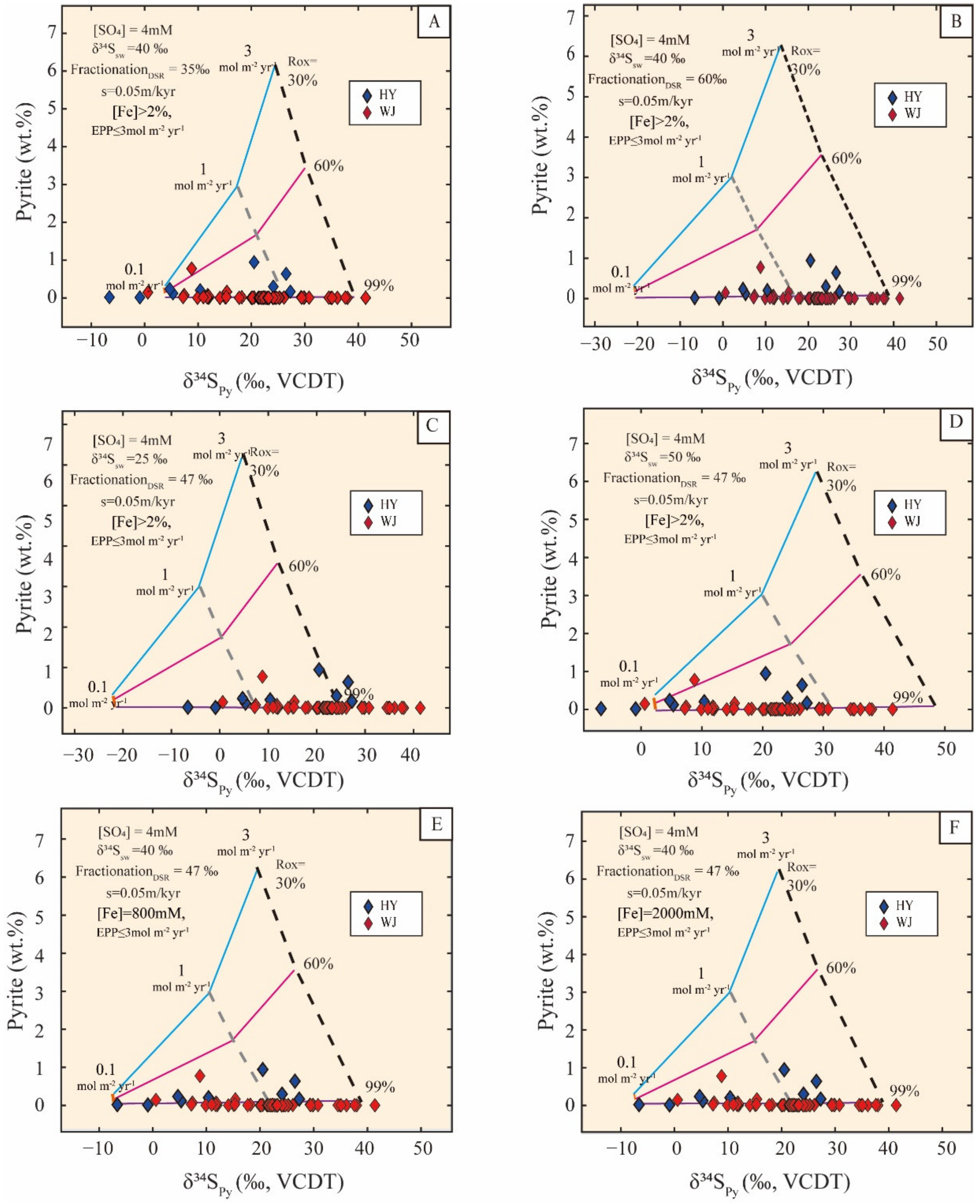
5.2. Modeling of the Bulk-Sample δ34Spy and Pyrite Content of the Yangtze Block
5.2.1. Model Description
5.2.2. Parameter Setup and Boundary Constraints
5.2.3. Modeling Results
5.2.4. Sensitivity Tests
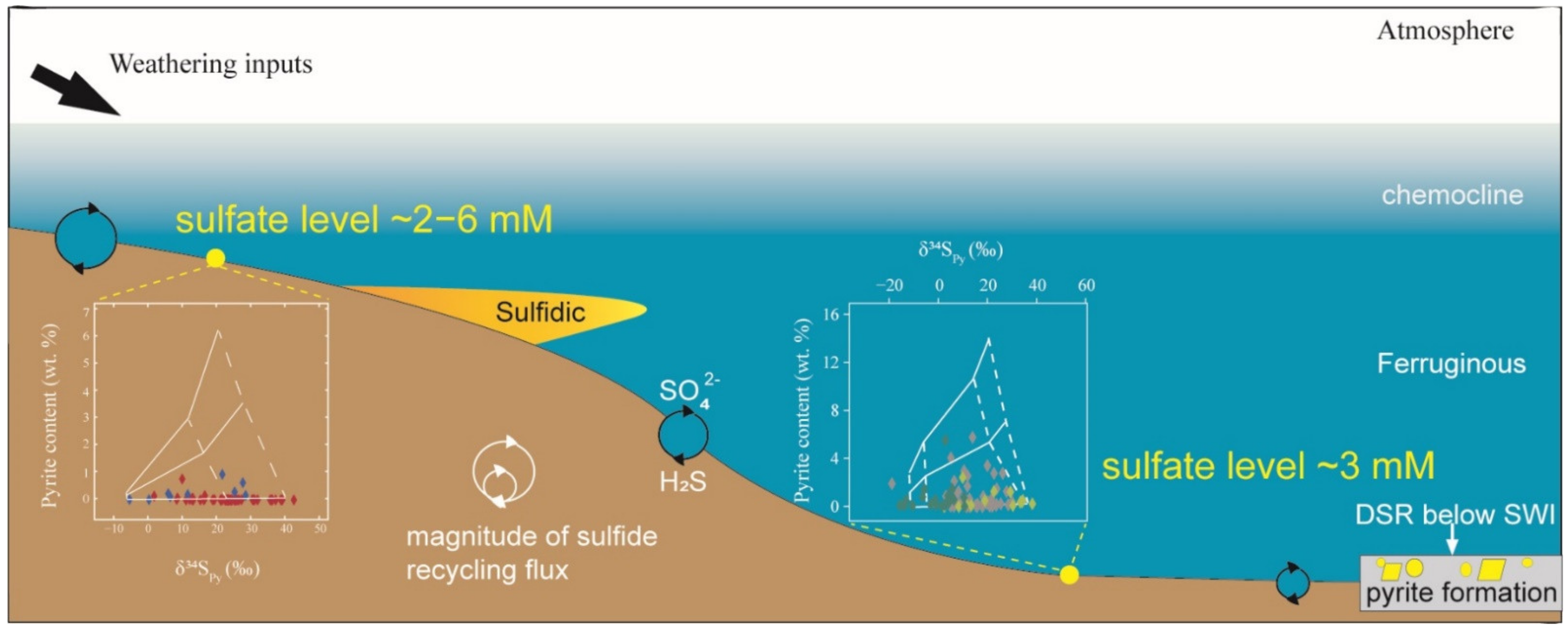
5.3. Implications for Marine Sulfate Levels in the Terminal Ediacaran Ocean
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canfield, D.E.; Poulton, S.W.; Narbonne, G.M. Late-Neoproterozoic Deep-Ocean Oxygenation and the Rise of Animal Life. Science 2007, 315, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.U.; Gaines, R.R.; Prokopenko, M.G.; Qi, C.; Hou, X.-G.; Canfield, D.E. Early Cambrian oxygen minimum zone-like conditions at Chengjiang. Earth Planet. Sci. Lett. 2017, 475, 160–168. [Google Scholar] [CrossRef]
- He, T.; Zhu, M.; Mills, B.J.W.; Wynn, P.M.; Zhuravlev, A.Y.; Tostevin, R.; von Strandmann, P.A.E.P.; Yang, A.; Poulton, S.W.; Shields, G.A. Possible links between extreme oxygen perturbations and the Cambrian radiation of animals. Nat. Geosci. 2019, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, C.; Algeo, T.J.; Planavsky, N.J.; Cui, H.; Yang, X.; Zhao, Y.; Zhang, X.; Xie, S. A highly redox-heterogeneous ocean in South China during the early Cambrian (∼529–514 Ma): Implications for biota-environment co-evolution. Earth Planet. Sci. Lett. 2016, 441, 38–51. [Google Scholar] [CrossRef]
- Kimura, H.; Watanabe, Y. Oceanic anoxia at the Precambrian-Cambrian boundary. Geology 2001, 29, 995–998. [Google Scholar] [CrossRef]
- Li, C.; Love, G.D.; Lyons, T.W.; Fike, D.A.; Sessions, A.L.; Chu, X. A Stratified Redox Model for the Ediacaran Ocean. Science 2010, 328, 80–83. [Google Scholar] [CrossRef]
- Mills, D.B.; Ward, L.M.; Jones, C.A.; Sweeten, B.; Forth, M.; Treusch, A.H.; Canfield, D.E. Oxygen requirements of the earliest animals. Proc. Natl. Acad. Sci. USA 2014, 111, 4168–4172. [Google Scholar] [CrossRef]
- Li, D.; Ling, H.-F.; Shields-Zhou, G.A.; Chen, X.; Cremonese, L.; Och, L.; Thirlwall, M.; Manning, C.J. Carbon and strontium isotope evolution of seawater across the Ediacaran–Cambrian transition: Evidence from the Xiaotan section, NE Yunnan, South China. Precambrian Res. 2013, 225, 128–147. [Google Scholar] [CrossRef]
- Sperling, E.A.; Wolock, C.J.; Morgan, A.S.; Gill, B.C.; Kunzmann, M.; Halverson, G.P.; Macdonald, F.A.; Knoll, A.H.; Johnston, D.T. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 2015, 523, 451–454. [Google Scholar] [CrossRef]
- Wood, R.; Liu, A.G.; Bowyer, F.; Wilby, P.R.; Dunn, F.S.; Kenchington, C.G.; Cuthill, J.F.H.; Mitchell, E.G.; Penny, A. Integrated records of environmental change and evolution challenge the Cambrian Explosion. Nat. Ecol. Evol. 2019, 3, 528–538. [Google Scholar] [CrossRef]
- Cheng, M.; Li, C.; Zhou, L.; Feng, L.; Algeo, T.J.; Zhang, F.; Romaniello, S.; Jin, C.; Ling, H.; Jiang, S. Transient deep-water oxygenation in the early Cambrian Nanhua Basin, South China. Geochim. Cosmochim. Acta 2017, 210, 42–58. [Google Scholar] [CrossRef]
- Cui, H.; Kaufman, A.J.; Xiao, S.; Peek, S.; Cao, H.; Min, X.; Cai, Y.; Siegel, Z.; Liu, X.-M.; Peng, Y.; et al. Environmental context for the terminal Ediacaran biomineralization of animals. Geobiology 2016, 14, 344–363. [Google Scholar] [CrossRef]
- Halverson, G.P.; Hurtgen, M.T. Ediacaran growth of the marine sulfate reservoir. Earth Planet. Sci. Lett. 2007, 263, 32–44. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Jin, C.; Cheng, M.; Wang, H.; Huang, J.; Algeo, T.J. Spatiotemporal evolution and causes of marine euxinia in the early Cambrian Nanhua Basin (South China). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 546, 109676. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Yan, D.; Wei, H.; Xiang, L. Evolution from an anoxic to oxic deep ocean during the Ediacaran–Cambrian transition and implications for bioradiation. Chem. Geol. 2012, 306, 129–138. [Google Scholar] [CrossRef]
- Wen, H.; Carignan, J.; Chu, X.; Fan, H.; Cloquet, C.; Huang, J.; Zhang, Y.; Chang, H. Selenium isotopes trace anoxic and ferruginous seawater conditions in the Early Cambrian. Chem. Geol. 2014, 390, 164–172. [Google Scholar] [CrossRef]
- Canfield, D.E.; Poulton, S.W.; Knoll, A.H.; Narbonne, G.M.; Ross, G.; Goldberg, T.; Strauss, H. Ferruginous Conditions Dominated Later Neoproterozoic Deep-Water Chemistry. Science 2008, 321, 949–952. [Google Scholar] [CrossRef]
- Cremonese, L.; Shields-Zhou, G.; Struck, U.; Ling, H.-F.; Och, L.; Chen, X.; Li, D. Marine biogeochemical cycling during the early Cambrian constrained by a nitrogen and organic carbon isotope study of the Xiaotan section, South China. Precambrian Res. 2013, 225, 148–165. [Google Scholar] [CrossRef]
- Qin, Z.; Xu, D.; Kendall, B.; Zhang, X.; Ou, Q.; Wang, X.; Li, J.; Liu, J. Molybdenum isotope-based redox deviation driven by continental margin euxinia during the early Cambrian. Geochim. Cosmochim. Acta 2022, 325, 152–169. [Google Scholar] [CrossRef]
- Och, L.M.; Shields-Zhou, G.A.; Poulton, S.W.; Manning, C.; Thirlwall, M.F.; Li, D.; Chen, X.; Ling, H.; Osborn, T.; Cremonese, L. Redox changes in Early Cambrian black shales at Xiaotan section, Yunnan Province, South China. Precambrian Res. 2013, 225, 166–189. [Google Scholar] [CrossRef]
- Fan, H.; Wen, H.; Han, T.; Zhu, X.; Feng, L.; Chang, H. Oceanic redox condition during the late Ediacaran (551–541 Ma), South China. Geochim. Cosmochim. Acta 2018, 238, 343–356. [Google Scholar] [CrossRef]
- Chang, H.; Chu, X.; Feng, L.; Huang, J. Iron speciation in cherts from the Laobao Formation, South China: Implications for anoxic and ferruginous deep-water conditions. Chin. Sci. Bull. 2010, 55, 3189–3196. [Google Scholar] [CrossRef]
- Chang, H.-J.; Chu, X.-L.; Feng, L.; Huang, J. Progressive oxidation of anoxic and ferruginous deep-water during deposition of the terminal Ediacaran Laobao Formation in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 321, 80–87. [Google Scholar] [CrossRef]
- Blättler, C.L.; Bergmann, K.D.; Kah, L.C.; Gómez-Pérez, I.; Higgins, J.A. Constraints on Meso- to Neoproterozoic seawater from ancient evaporite deposits. Earth Planet. Sci. Lett. 2020, 532, 115951. [Google Scholar] [CrossRef]
- Brennan, S.T.; Lowenstein, T.K.; Horita, J. Seawater chemistry and the advent of biocalcification. Geology 2004, 32, 473–476. [Google Scholar] [CrossRef]
- Gill, B.C.; Lyons, T.W.; Saltzman, M.R. Parallel, high-resolution carbon and sulfur isotope records of the evolving Paleozoic marine sulfur reservoir. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 256, 156–173. [Google Scholar] [CrossRef]
- Horita, J.; Zimmermann, H.; Holland, H.D. Chemical evolution of seawater during the Phanerozoic: Implications from the record of marine evaporites. Geochim. Cosmochim. Acta 2002, 66, 3733–3756. [Google Scholar] [CrossRef]
- Kah, L.C.; Lyons, T.W.; Frank, T.D. Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature 2004, 431, 834–838. [Google Scholar] [CrossRef]
- Kovalevych, V.M.; Marshall, T.; Peryt, T.M.; Petrychenko, O.Y.; Zhukova, S.A. Chemical composition of seawater in Neoproterozoic: Results of fluid inclusion study of halite from Salt Range (Pakistan) and Amadeus Basin (Australia). Precambrian Res. 2006, 144, 39–51. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Hardie, L.A.; Timofeeff, M.N.; Demicco, R.V. Secular variation in seawater chemistry and the origin of calcium chloride basinal brines. Geology 2003, 31, 857–860. [Google Scholar] [CrossRef]
- Loyd, S.J.; Marenco, P.J.; Hagadorn, J.W.; Lyons, T.W.; Kaufman, A.J.; Sour-Tovar, F.; Corsetti, F.A. Sustained low marine sulfate concentrations from the Neoproterozoic to the Cambrian: Insights from carbonates of northwestern Mexico and eastern California. Earth Planet. Sci. Lett. 2012, 339, 79–94. [Google Scholar] [CrossRef]
- Staudt, W.J.; Schoonen, M.A.A. Sulfate Incorporation into Sedimentary Carbonates. ACS Symp. Ser. 1995, 612, 332–345. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Y.; Muscente, A.D.; Cui, H.; Guan, C.; Hao, J.; Zhou, C. Revisiting Ediacaran sulfur isotope chemostratigraphy with in situ nanoSIMS analysis of sedimentary pyrite. Geology 2021, 49, 611–616. [Google Scholar] [CrossRef]
- Fike, D.A.; Bradley, A.S.; Rose, C.V. Rethinking the Ancient Sulfur Cycle. Annu. Rev. Earth Planet. Sci. 2015, 43, 593–622. [Google Scholar] [CrossRef]
- Ries, J.B.; Fike, D.A.; Pratt, L.M.; Lyons, T.W.; Grotzinger, J.P. Superheavy pyrite (δ34Spyr > δ34SCAS) in the terminal Proterozoic Nama Group, southern Namibia: A consequence of low seawater sulfate at the dawn of animal life. Geology 2009, 37, 743–746. [Google Scholar] [CrossRef]
- Johnson, D.L.; Present, T.M.; Li, M.; Shen, Y.; Adkins, J.F. Carbonate associated sulfate (CAS) δ34S heterogeneity across the End-Permian Mass Extinction in South China. Earth Planet. Sci. Lett. 2021, 574, 117172. [Google Scholar] [CrossRef]
- Rose, C.V.; Webb, S.M.; Newville, M.; Lanzirotti, A.; Richardson, J.A.; Tosca, N.J.; Catalano, J.G.; Bradley, A.S.; Fike, D.A. Insights into past ocean proxies from micron-scale mapping of sulfur species in carbonates. Geology 2019, 47, 833–837. [Google Scholar] [CrossRef]
- Cui, H.; Kitajima, K.; Orland, I.J.; Xiao, S.; Baele, J.-M.; Kaufman, A.J.; Denny, A.; Zhou, C.; Spicuzza, M.J.; Fournelle, J.H.; et al. Deposition or diagenesis? Probing the Ediacaran Shuram excursion in South China by SIMS. Glob. Planet. Chang. 2021, 206, 103591. [Google Scholar] [CrossRef]
- Berg, P.; Risgaard-Petersen, N.; Rysgaard, S. Interpretation of measured concentration profiles in sediment pore water. Limnol. Oceanogr. 1998, 43, 1500–1510. [Google Scholar] [CrossRef]
- Boudreau, B.P. Diagenetic Models and Their Implementation: Modelling Transport and Reactions in Aquatic Sediments; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Lang, X.; Shen, B.; Peng, Y.; Xiao, S.; Zhou, C.; Bao, H.; Kaufman, A.J.; Huang, K.; Crockford, P.W.; Liu, Y.; et al. Transient marine euxinia at the end of the terminal Cryogenian glaciation. Nat. Commun. 2018, 9, 3019. [Google Scholar] [CrossRef]
- Lang, X.; Tang, W.; Ma, H.; Shen, B. Local environmental variation obscures the interpretation of pyrite sulfur isotope records. Earth Planet. Sci. Lett. 2020, 533, 116056. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, Z.; Ma, H.; Huang, K.; Li, S.; Zhou, C.; Xiao, S.; Peng, Y.; Liu, Y.; Tang, W.; et al. Cracking the superheavy pyrite enigma: Possible roles of volatile organosulfur compound emission. Natl. Sci. Rev. 2021, 8, nwab034. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Wang, R.; Huang, T.; Lang, X.; Ma, H.; Shen, B. Constraining the redox landscape of Mesoproterozoic mat grounds: A possible oxygen oasis in the ‘Boring Billion’ seafloor. Precambrian Res. 2022, 376, 106681. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.-X. History of Neoproterozoic rift basins in South China: Implications for Rodinia break-up. Precambrian Res. 2003, 122, 141–158. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, X.; Zhang, S.; Wang, Y.; Xiao, S. Stratigraphy and paleogeography of the Ediacaran Doushantuo Formation (ca. 635–551Ma) in South China. Gondwana Res. 2011, 19, 831–849. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Zhao, J. Zircon U/Pb dating and Hf-O isotopes of the Zhouan ultramafic intrusion in the northern margin of the Yangtze Block, SW China: Constraints on the nature of mantle source and timing of the supercontinent Rodinia breakup. Chin. Sci. Bull. 2013, 58, 777–787. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Liu, S.; Ran, B.; Song, J.; Li, J.; Ye, Y.; Li, N. Ediacaran extension along the northern margin of the Yangtze Platform, South China: Constraints from the lithofacies and geochemistry of the Doushantuo Formation. Mar. Pet. Geol. 2020, 112, 104056. [Google Scholar] [CrossRef]
- Vernhet, E.; Reijmer, J.J.G. Sedimentary evolution of the Ediacaran Yangtze platform shelf (Hubei and Hunan provinces, Central China). Sediment. Geol. 2010, 225, 99–115. [Google Scholar] [CrossRef]
- Wang, H.; Wu, W.; Liu, S.; Zhang, X.; Song, J.; Li, S.; Ran, B.; Wang, Z.; Han, Y.; Wang, W.; et al. Initial separation of the South Qinling Terrane from the Yangtze Block during the Ediacaran: Insights from sequence correlation and zircon Hf isotope of tuff. Mar. Pet. Geol. 2020, 122, 104613. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.-F.; Li, L. Neoproterozoic tectonic transition in the South Qinling Belt: New constraints from geochemistry and zircon U–Pb–Hf isotopes of diorites from the Douling Complex. Precambrian Res. 2018, 306, 112–128. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, X.; Fu, Y.; Wang, J.; Yan, D. New U-Pb zircon ages of the Ediacaran-Cambrian boundary strata in South China. Terra Nova 2015, 27, 62–68. [Google Scholar] [CrossRef]
- Canfield, D.E.; Thamdrup, B. Towards a consistent classification scheme for geochemical environments, or, why we wish the term ‘suboxic’ would go away. Geobiology 2009, 7, 385–392. [Google Scholar] [CrossRef]
- Pasquier, V.; Sansjofre, P.; Rabineau, M.; Revillon, S.; Houghton, J.; Fike, D.A. Pyrite sulfur isotopes reveal glacial−interglacial environmental changes. Proc. Natl. Acad. Sci. USA 2017, 114, 5941–5945. [Google Scholar] [CrossRef]
- Pasquier, V.; Fike, D.A.; Halevy, I. Sedimentary pyrite sulfur isotopes track the local dynamics of the Peruvian oxygen minimum zone. Nat. Commun. 2021, 12, 4403. [Google Scholar] [CrossRef]
- Strauss, H. The isotopic composition of sedimentary sulfur through time. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 132, 97–118. [Google Scholar] [CrossRef]
- Canfield, D.E. The evolution of the Earth surface sulfur reservoir. Am. J. Sci. 2004, 304, 839–861. [Google Scholar] [CrossRef]
- Wu, N.; Farquhar, J.; Strauss, H.; Kim, S.-T.; Canfield, D.E. Evaluating the S-isotope fractionation associated with Phanerozoic pyrite burial. Geochim. Cosmochim. Acta 2010, 74, 2053–2071. [Google Scholar] [CrossRef]
- Planavsky, N.J.; Reinhard, C.T.; Wang, X.; Thomson, D.; McGoldrick, P.; Rainbird, R.H.; Johnson, T.; Fischer, W.W.; Lyons, T.W. Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 2014, 346, 635–638. [Google Scholar] [CrossRef]
- Schieber, J. Marcasite in Black Shales--a Mineral Proxy for Oxygenated Bottom Waters and Intermittent Oxidation of Carbonaceous Muds. J. Sediment. Res. 2011, 81, 447–458. [Google Scholar] [CrossRef]
- Cui, H.; Kitajima, K.; Spicuzza, M.J.; Fournelle, J.H.; Denny, A.; Ishida, A.; Zhang, F.; Valley, J.W. Questioning the biogenicity of Neoproterozoic superheavy pyrite by SIMS. Am. Mineral. J. Earth Planet. Mater. 2018, 103, 1362–1400. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Arthur, M.A. Variations in pyrite texture, sulfur isotope composition, and iron systematics in the Black Sea: Evidence for Late Pleistocene to Holocene excursions of the o2-h2s redox transition. Geochim. Cosmochim. Acta 2001, 65, 1399–1416. [Google Scholar] [CrossRef]
- Seal, I.R.R. Sulfur Isotope Geochemistry of Sulfide Minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Rickard, D. The Evolution of the Sedimentary Sulfur Cycle; Elsevier: Amsterdam, The Netherlands, 2012; Volume 65. [Google Scholar]
- Berner, R.A. Sedimentary pyrite formation: An update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Kampschulte, A.; Strauss, H. The sulfur isotopic evolution of Phanerozoic seawater based on the analysis of structurally substituted sulfate in carbonates. Chem. Geol. 2004, 204, 255–286. [Google Scholar] [CrossRef]
- Wu, N.; Farquhar, J.; Fike, D.A. Ediacaran sulfur cycle: Insights from sulfur isotope measurements (Δ33S and δ34S) on paired sulfate–pyrite in the Huqf Supergroup of Oman. Geochim. Cosmochim. Acta 2015, 164, 352–364. [Google Scholar] [CrossRef]
- Wu, N.; Farquhar, J.; Strauss, H. δ34S and Δ33S records of Paleozoic seawater sulfate based on the analysis of carbonate associated sulfate. Earth Planet. Sci. Lett. 2014, 399, 44–51. [Google Scholar] [CrossRef]
- Tostevin, R.; He, T.; Turchyn, A.V.; Wood, R.A.; Penny, A.M.; Bowyer, F.; Antler, G.; Shields, G.A. Constraints on the late Ediacaran sulfur cycle from carbonate associated sulfate. Precambrian Res. 2017, 290, 113–125. [Google Scholar] [CrossRef]
- Cui, H.; Grazhdankin, D.V.; Xiao, S.; Peek, S.; Rogov, V.I.; Bykova, N.V.; Sievers, N.E.; Liu, X.-M.; Kaufman, A.J. Redox-dependent distribution of early macro-organisms: Evidence from the terminal Ediacaran Khatyspyt Formation in Arctic Siberia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 461, 122–139. [Google Scholar] [CrossRef]
- Fike, D.A.; Grotzinger, J.P.; Pratt, L.M.; Summons, R.E. Oxidation of the Ediacaran Ocean. Nature 2006, 444, 744–747. [Google Scholar] [CrossRef]
- Holser, W.T.; Kaplan, I.R. Isotope geochemistry of sedimentary sulfates. Chem. Geol. 1966, 1, 93–135. [Google Scholar] [CrossRef]
- Strauss, H. The sulfur isotopic record of Precambrian sulfates: New data and a critical evaluation of the existing record. Precambrian Res. 1993, 63, 225–246. [Google Scholar] [CrossRef]
- Shields, G.A.; Strauss, H.; Howe, S.S.; Siegmund, H. Sulphur isotope compositions of sedimentary phosphorites from the basal Cambrian of China: Implications for Neoproterozoic-Cambrian biogeochemical cycling. J. Geol. Soc. 1999, 156, 943–955. [Google Scholar] [CrossRef]
- SchrÖder, S.; Schreiber, B.C.; Amthor, J.E.; Matter, A. Stratigraphy and environmental conditions of the terminal Neoproterozoic–Cambrian Period in Oman: Evidence from sulphur isotopes. J. Geol. Soc. 2004, 161, 489–499. [Google Scholar] [CrossRef]
- Wing, B.A.; Halevy, I. Intracellular metabolite levels shape sulfur isotope fractionation during microbial sulfate respiration. Proc. Natl. Acad. Sci. USA 2014, 111, 18116–18125. [Google Scholar] [CrossRef]
- Canfield, D.E. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim. Cosmochim. Acta 2001, 65, 1117–1124. [Google Scholar] [CrossRef]
- Chambers, L.A. Sulfur isotope study of a modern intertidal environment, and the interpretation of ancient sulfides. Geochim. Cosmochim. Acta 1982, 46, 721–728. [Google Scholar] [CrossRef]
- Chambers, L.A.; Trudinger, P.A. Microbiological fractionation of stable sulfur isotopes: A review and critique. Geomicrobiol. J. 1979, 1, 249–293. [Google Scholar] [CrossRef]
- Leavitt, W.D.; Bradley, A.S.; Santos, A.A.; Pereira, I.A.C.; Johnston, D.T. Sulfur Isotope Effects of Dissimilatory Sulfite Reductase. Front. Microbiol. 2015, 6, 1392. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Beulig, F.; Egger, M.; Petro, C.; Scholze, C.; Røy, H. Organoclastic sulfate reduction in the sulfate-methane transition of marine sediments. Geochim. Cosmochim. Acta 2019, 254, 231–245. [Google Scholar] [CrossRef]
- Rees, C.E. A steady-state model for sulphur isotope fractionation in bacterial reduction processes. Geochim. Cosmochim. Acta 1973, 37, 1141–1162. [Google Scholar] [CrossRef]
- Sim, M.S.; Ogata, H.; Lubitz, W.; Adkins, J.F.; Sessions, A.L.; Orphan, V.J.; McGlynn, S.E. Role of APS reductase in biogeochemical sulfur isotope fractionation. Nat. Commun. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.S.; Paris, G.; Adkins, J.F.; Orphan, V.J.; Sessions, A.L. Quantification and isotopic analysis of intracellular sulfur metabolites in the dissimilatory sulfate reduction pathway. Geochim. Cosmochim. Acta 2017, 206, 57–72. [Google Scholar] [CrossRef]
- Schulz, H.D. Quantification of Early Diagenesis: Dissolved Constituents in Pore Water and Signals in the Solid Phase. In Marine Geochemistry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 73–124. [Google Scholar] [CrossRef]
- Middelburg, J.J. Marine Carbon Biogeochemistry: A Primer for Earth System Scientists; Springer Nature: London, UK, 2019. [Google Scholar]
- Xiao, S.; Shen, B.; Zhou, C.; Xie, G.; Yuan, X. A uniquely preserved Ediacaran fossil with direct evidence for a quilted bodyplan. Proc. Natl. Acad. Sci. USA 2005, 102, 10227–10232. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, X.; Fang, C.; He, X.; Fang, Z.; Zhang, X. Reconstruction of the Ediacaran sulfur cycle and oceanic redox evolution in shallow-water regions of the Yangtze platform, South China. Precambrian Res. 2021, 353, 106004. [Google Scholar] [CrossRef]
- Fan, H.; Wen, H.; Zhu, X.; Hu, R.; Tian, S. Hydrothermal activity during Ediacaran–Cambrian transition: Silicon isotopic evidence. Precambrian Res. 2013, 224, 23–35. [Google Scholar] [CrossRef]
- Cremonese, L.; Shields-Zhou, G.A.; Struck, U.; Ling, H.-F.; Och, L.M. Nitrogen and organic carbon isotope stratigraphy of the Yangtze Platform during the Ediacaran–Cambrian transition in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 165–186. [Google Scholar] [CrossRef]
- Guo, Q.; Strauss, H.; Zhu, M.; Zhang, J.; Yang, X.; Lu, M.; Zhao, F. High resolution organic carbon isotope stratigraphy from a slope to basinal setting on the Yangtze Platform, South China: Implications for the Ediacaran–Cambrian transition. Precambrian Res. 2013, 225, 209–217. [Google Scholar] [CrossRef]
- Och, L.M.; Cremonese, L.; Shields-Zhou, G.A.; Poulton, S.W.; Struck, U.; Ling, H.; Li, D.; Chen, X.; Manning, C.; Thirlwall, M.; et al. Palaeoceanographic controls on spatial redox distribution over the Yangtze Platform during the Ediacaran-Cambrian transition. Sedimentology 2016, 63, 378–410. [Google Scholar] [CrossRef]
- Chang, H.; Chu, X.; Feng, L.; Huang, J.; Chen, Y. Marine redox stratification on the earliest Cambrian (ca. 542–529 Ma) Yangtze Platform. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 504, 75–85. [Google Scholar] [CrossRef]
- Cui, H.; Xiao, S.; Cai, Y.; Peek, S.; Plummer, R.E.; Kaufman, A.J. Sedimentology and chemostratigraphy of the terminal Ediacaran Dengying Formation at the Gaojiashan section, South China. Geol. Mag. 2019, 156, 1924–1948. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Guan, C.; Yuan, X.; Chen, Z.; Wan, B. An integrated carbon, oxygen, and strontium isotopic studies of the Lantian Formation in South China with implications for the Shuram anomaly. Chem. Geol. 2014, 373, 10–26. [Google Scholar] [CrossRef]
- Shi, W.; Li, C.; Luo, G.; Huang, J.; Algeo, T.J.; Jin, C.; Zhang, Z.; Cheng, M. Sulfur isotope evidence for transient marine-shelf oxidation during the Ediacaran Shuram Excursion. Geology 2018, 46, 267–270. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Hu, D.; Li, D.; Zhang, G.; Zhang, X.; Ling, H.-F.; Xu, Y.; Shen, Y. Multiple S-isotopic constraints on paleo-redox and sulfate concentrations across the Ediacaran-Cambrian transition in South China. Precambrian Res. 2020, 349, 105500. [Google Scholar] [CrossRef]
- Canfield, D.E.; Farquhar, J. Animal evolution, bioturbation, and the sulfate concentration of the oceans. Proc. Natl. Acad. Sci. USA 2009, 106, 8123–8127. [Google Scholar] [CrossRef]
- Duda, J.-P.; Zhu, M.; Reitner, J. Depositional dynamics of a bituminous carbonate facies in a tectonically induced intra-platform basin: The Shibantan Member (Dengying Formation, Ediacaran Period). Carbonates Evaporites 2016, 31, 87–99. [Google Scholar] [CrossRef]
- Meng, F.; Ni, P.; Schiffbauer, J.D.; Yuan, X.; Zhou, C.; Wang, Y.; Xia, M. Ediacaran seawater temperature: Evidence from inclusions of Sinian halite. Precambrian Res. 2011, 184, 63–69. [Google Scholar] [CrossRef]
- Saitoh, M.; Ueno, Y.; Matsu’Ura, F.; Kawamura, T.; Isozaki, Y.; Yao, J.; Ji, Z.; Yoshida, N. Multiple sulfur isotope records at the end-Guadalupian (Permian) at Chaotian, China: Implications for a role of bioturbation in the Phanerozoic sulfur cycle. J. Asian Earth Sci. 2017, 135, 70–79. [Google Scholar] [CrossRef]
- Zhang, F.; Xiao, S.; Kendall, B.; Romaniello, S.J.; Cui, H.; Meyer, M.; Gilleaudeau, G.J.; Kaufman, A.J.; Anbar, A.D. Extensive marine anoxia during the terminal Ediacaran Period. Sci. Adv. 2018, 4, eaan8983. [Google Scholar] [CrossRef]
- Sawaki, Y.; Ohno, T.; Tahata, M.; Komiya, T.; Hirata, T.; Maruyama, S.; Windley, B.F.; Han, J.; Shu, D.; Li, Y. The Ediacaran radiogenic Sr isotope excursion in the Doushantuo Formation in the Three Gorges area, South China. Precambrian Res. 2010, 176, 46–64. [Google Scholar] [CrossRef]
- Vishnevskaya, I.A.; Letnikova, E.F.; Vetrova, N.I.; Kochnev, B.B.; Dril, S.I. Chemostratigraphy and detrital zircon geochronology of the Neoproterozoic Khorbusuonka Group, Olenek Uplift, Northeastern Siberian platform. Gondwana Res. 2017, 51, 255–271. [Google Scholar] [CrossRef]
- Kaufman, A.J.; Jacobsen, S.B.; Knoll, A.H. The Vendian record of Sr and C isotopic variations in seawater: Implications for tectonics and paleoclimate. Earth Planet. Sci. Lett. 1993, 120, 409–430. [Google Scholar] [CrossRef]
- Sansjofre, P.; Cartigny, P.; Trindade, R.; Nogueira, A.C.R.; Agrinier, P.; Ader, M. Multiple sulfur isotope evidence for massive oceanic sulfate depletion in the aftermath of Snowball Earth. Nat. Commun. 2016, 7, 12192. [Google Scholar] [CrossRef] [PubMed]
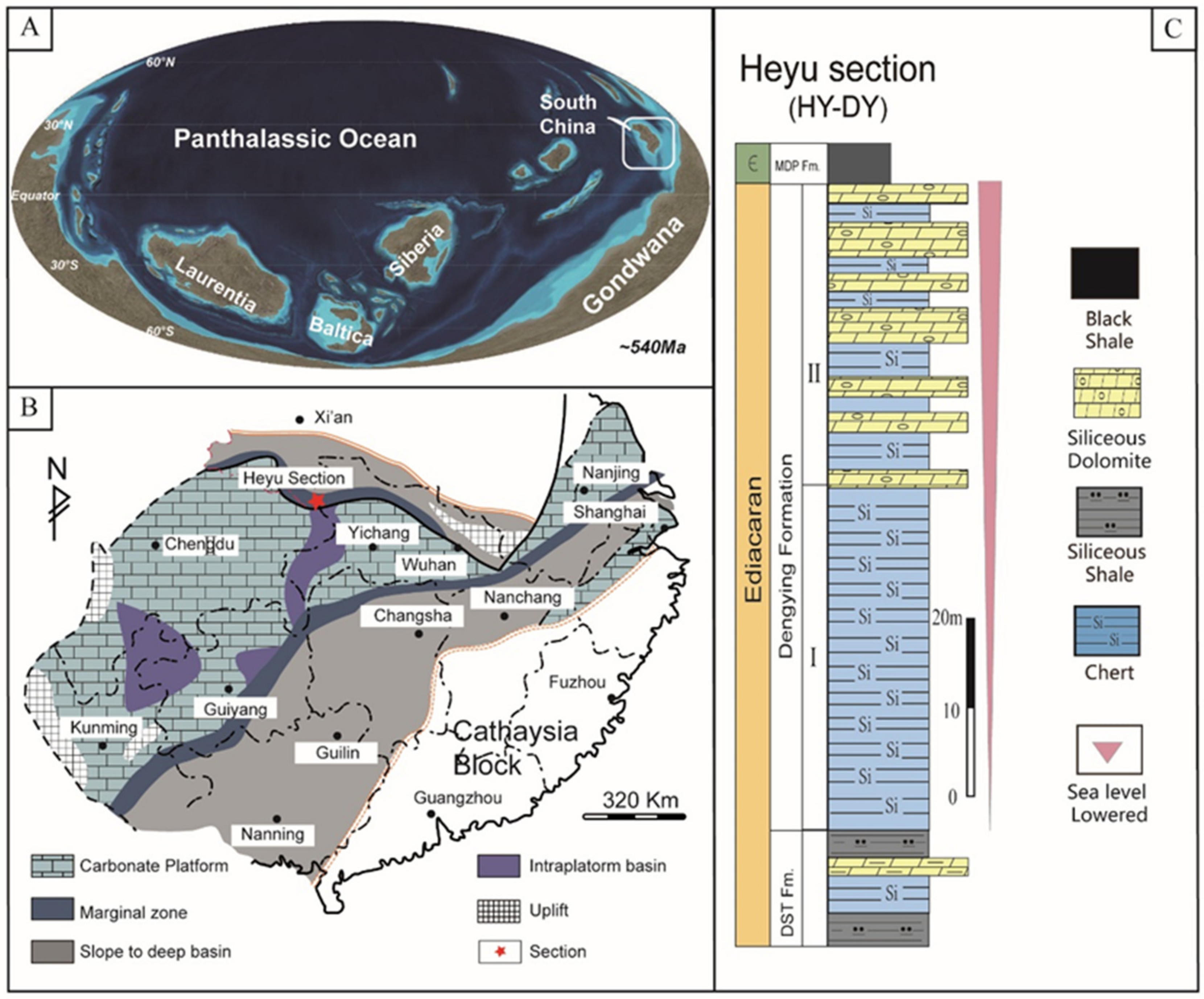
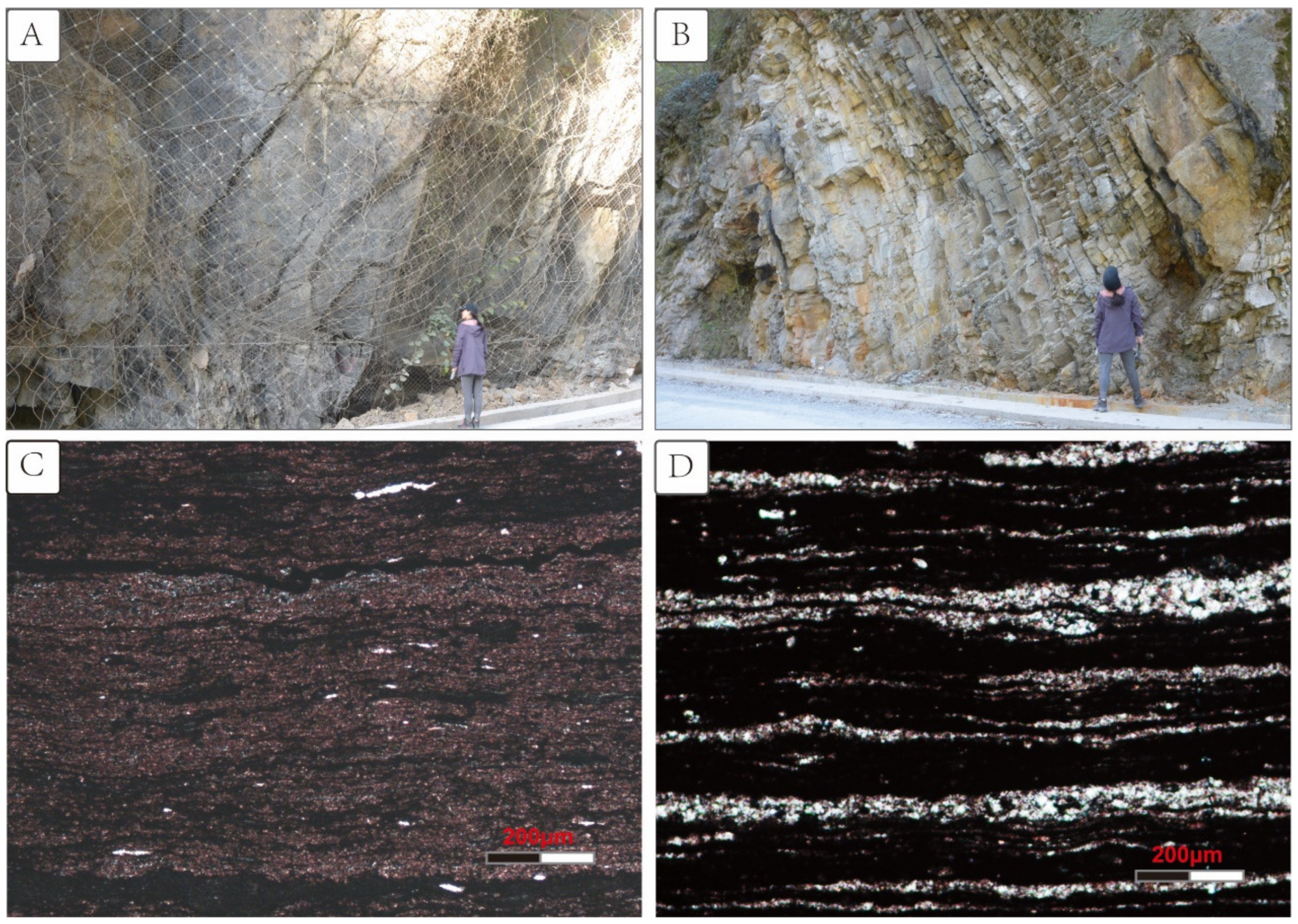
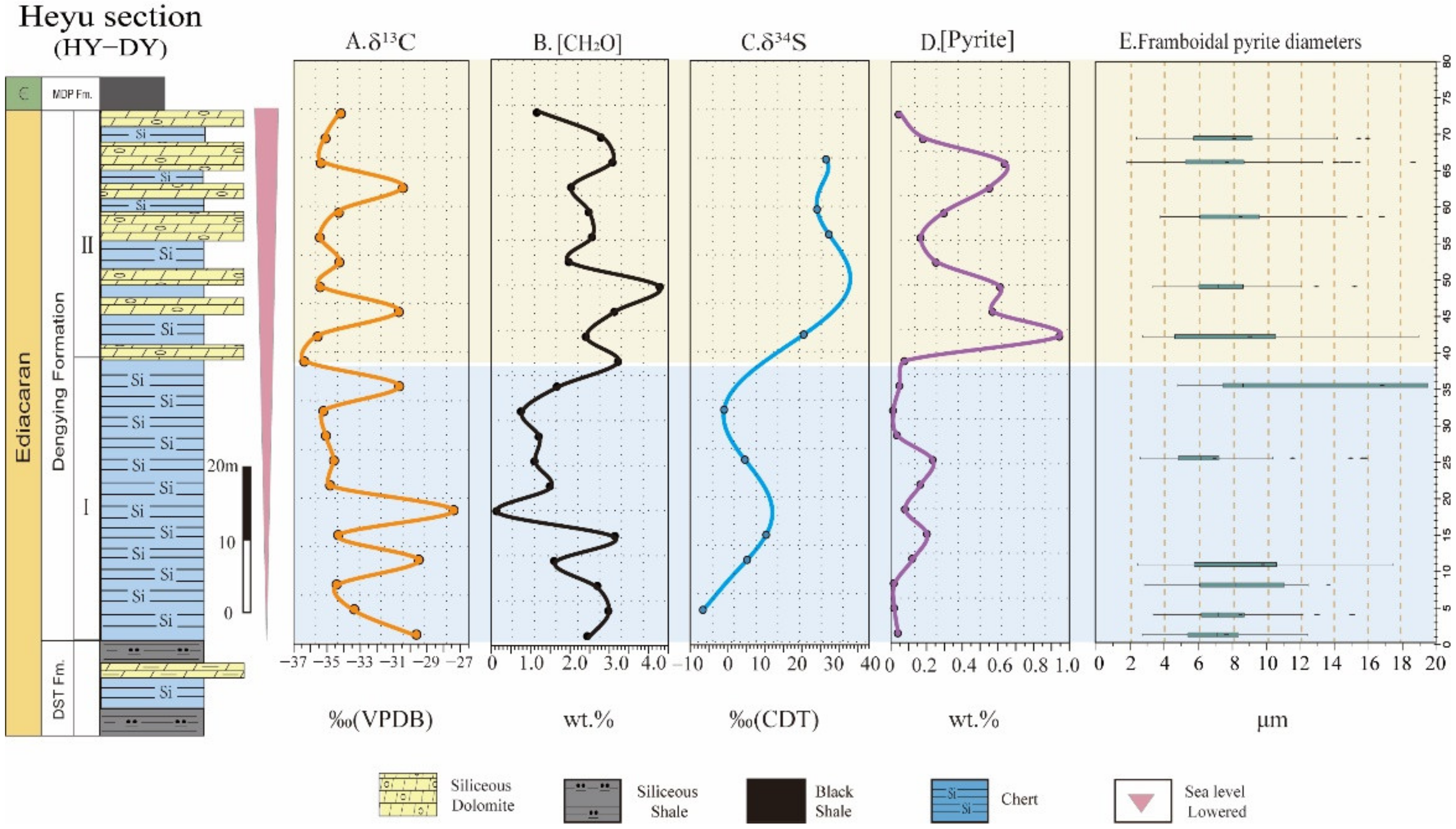

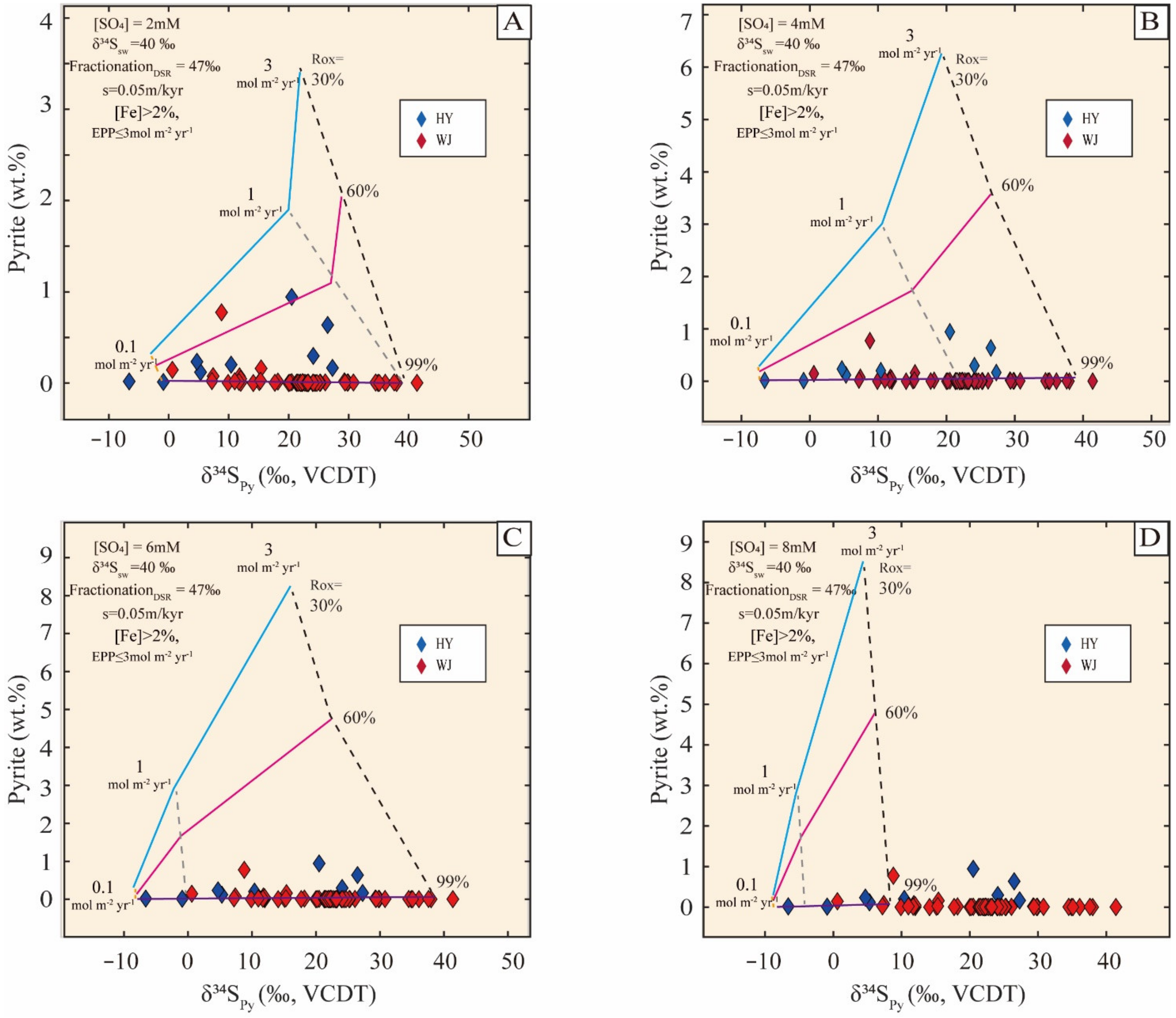

| Sample No. | Height (m) | δ13Corg (‰) | TOC | δ34Spy | Pyrite | Diameter |
|---|---|---|---|---|---|---|
| (%) | (‰) | (%) | (μm) | |||
| HY-LCP-1 | 1.3 | −29.6 | 2.44 | n.a. | 0.04 | 7.64 |
| HY-LCP-2 | 4.8 | −33.4 | 2.98 | −6.6 | 0.02 | 8.46 |
| HY-LCP-3 | 8.2 | −34.4 | 2.69 | n.a. | 0.02 | 13.63 |
| HY-LCP-4 | 11.6 | −29.5 | 1.60 | 5.3 | 0.12 | n.a. |
| HY-LCP-5 | 15.0 | −34.3 | 3.14 | 10.4 | 0.20 | 9.78 |
| HY-LCP-6 | 18.4 | −27.4 | 0.13 | n.a. | 0.08 | n.a. |
| HY-LCP-7 | 21.8 | −34.8 | 1.49 | n.a. | 0.16 | n.a. |
| HY-LCP-8 | 25.2 | −34.6 | 1.10 | 4.7 | 0.23 | 6.95 |
| HY-LCP-9 | 28.6 | −35.1 | 1.20 | n.a. | 0.03 | n.a. |
| HY-LCP-10 | 32.0 | −35.2 | 0.75 | −0.9 | 0.01 | n.a. |
| HY-LCP-11 | 35.4 | −30.7 | 1.67 | n.a. | 0.05 | 16.79 |
| HY-LCP-12 | 38.8 | −36.4 | 3.22 | n.a. | 0.08 | n.a. |
| HY-LCP-13 | 42.2 | −35.6 | 2.40 | 20.5 | 0.94 | 9.03 |
| HY-LCP-14 | 45.6 | −30.7 | 3.13 | n.a. | 0.57 | n.a. |
| HY-LCP-15 | 49.0 | −35.4 | 4.28 | n.a. | 0.61 | 8.31 |
| HY-LCP-16 | 52.4 | −34.3 | 1.97 | n.a. | 0.25 | n.a. |
| HY-LCP-17 | 55.8 | −35.4 | 2.56 | 27.3 | 0.17 | n.a. |
| HY-LCP-18 | 59.2 | −34.3 | 2.47 | 24.1 | 0.30 | 8.48 |
| HY-LCP-19 | 62.6 | −30.4 | 2.03 | n.a. | 0.55 | n.a. |
| HY-LCP-20 | 66.0 | −35.4 | 3.08 | 26.5 | 0.64 | 7.67 |
| HY-LCP-21 | 69.4 | −35.1 | 2.78 | n.a. | 0.18 | 8.08 |
| HY-LCP-22 | 72.8 | −34.2 | 1.16 | n.a. | 0.04 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Lang, X.; Wang, H.; Zhu, S.; Zhao, K.; Hou, M. Pyrite Sulfur Isotope Systematics Suggest Low Marine Sulfate Levels across the Ediacaran–Cambrian Transition. Minerals 2023, 13, 227. https://doi.org/10.3390/min13020227
Li S, Lang X, Wang H, Zhu S, Zhao K, Hou M. Pyrite Sulfur Isotope Systematics Suggest Low Marine Sulfate Levels across the Ediacaran–Cambrian Transition. Minerals. 2023; 13(2):227. https://doi.org/10.3390/min13020227
Chicago/Turabian StyleLi, Songzhuo, Xianguo Lang, Han Wang, Shengxian Zhu, Kun Zhao, and Mingcai Hou. 2023. "Pyrite Sulfur Isotope Systematics Suggest Low Marine Sulfate Levels across the Ediacaran–Cambrian Transition" Minerals 13, no. 2: 227. https://doi.org/10.3390/min13020227
APA StyleLi, S., Lang, X., Wang, H., Zhu, S., Zhao, K., & Hou, M. (2023). Pyrite Sulfur Isotope Systematics Suggest Low Marine Sulfate Levels across the Ediacaran–Cambrian Transition. Minerals, 13(2), 227. https://doi.org/10.3390/min13020227





