Activation of Dolomite Flotation by Ferrous Hydroxide and Carbonate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Micro-Flotation Tests
2.4. Contact Angle Measurements
2.5. Thermodynamic Analysis
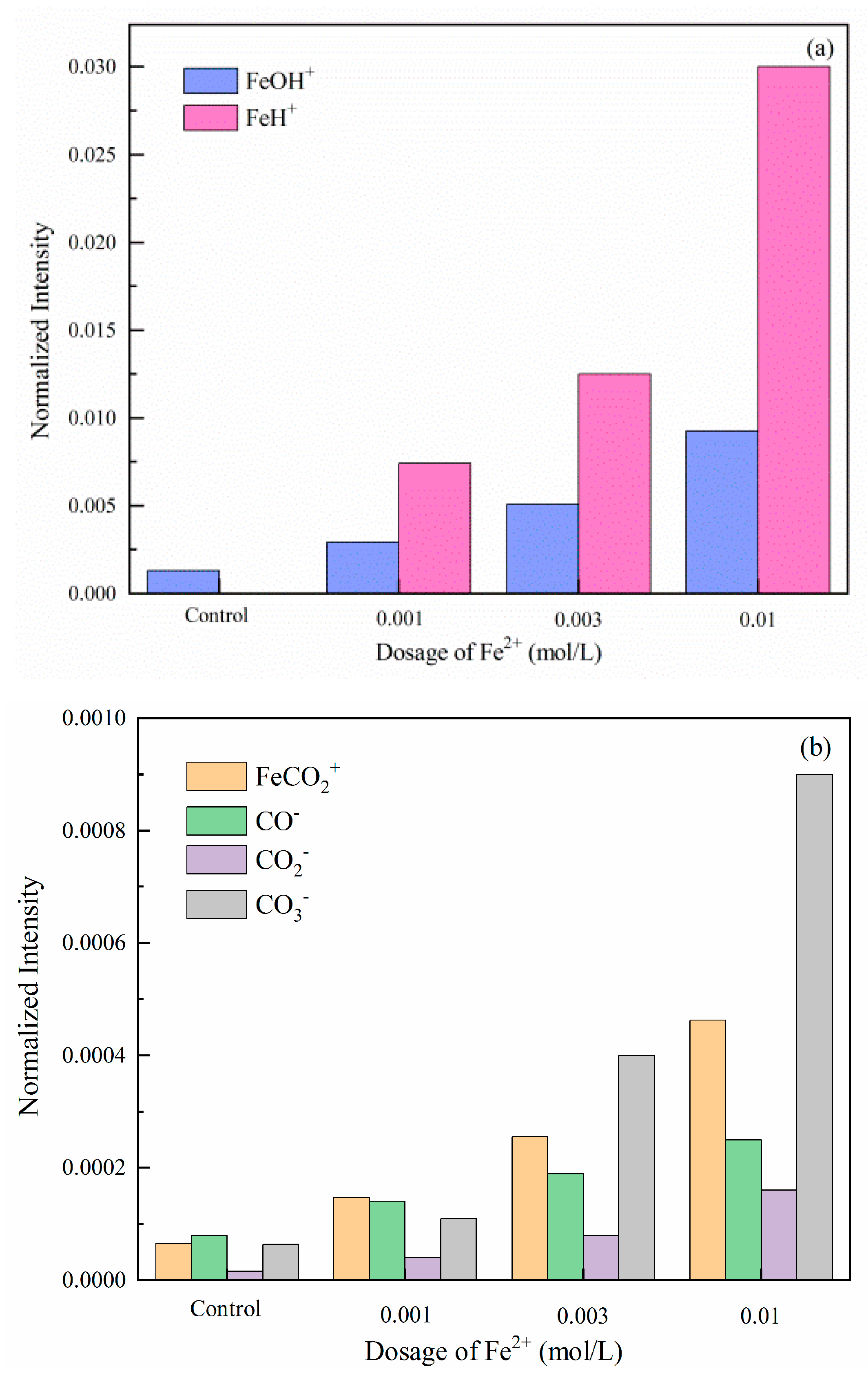
2.6. XPS Analysis
2.7. Time-of-Flight Secondary Ion Mass Spectrometry (Tof-SIMS) Tests
2.8. Computational Methods
3. Results and Discussion
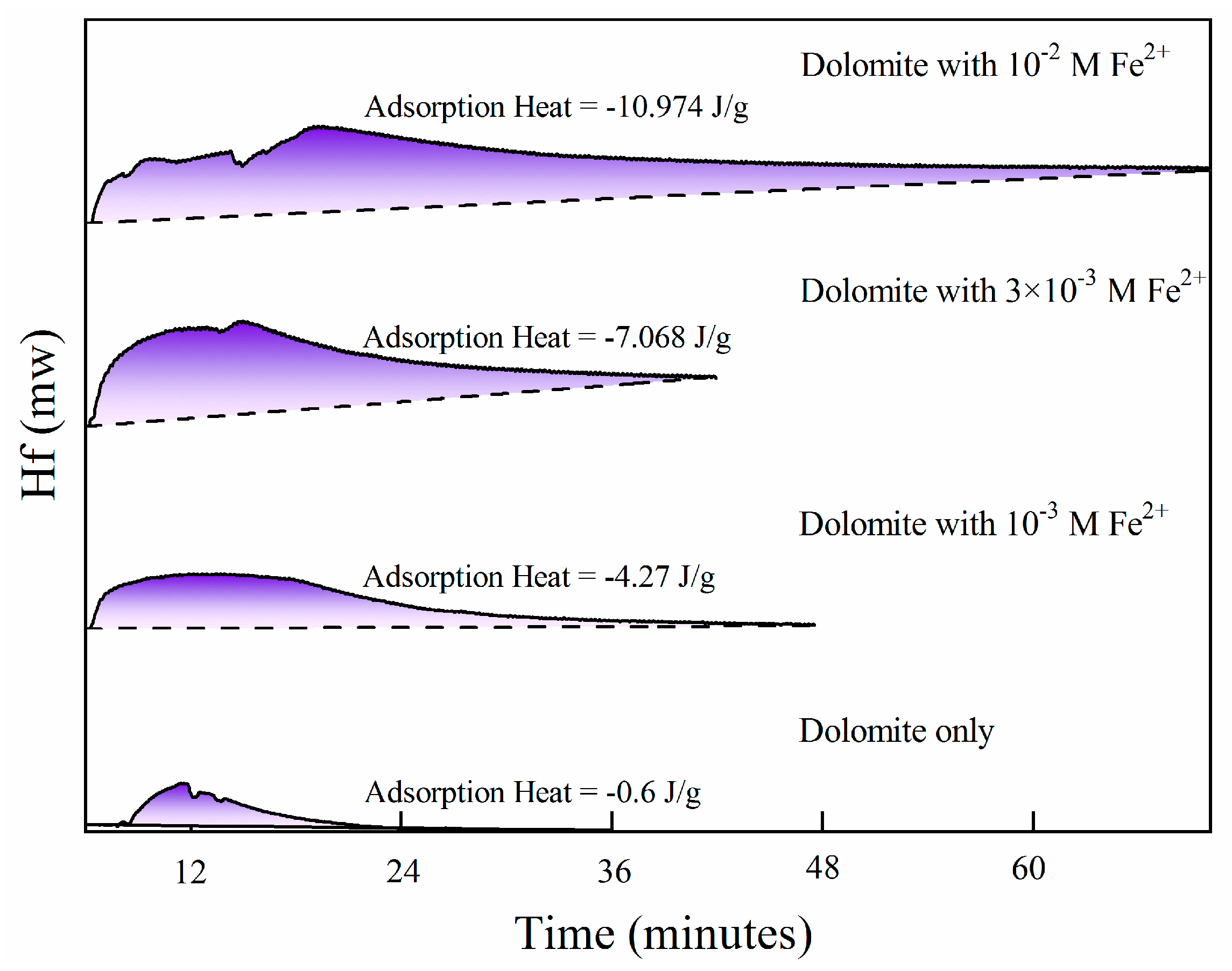
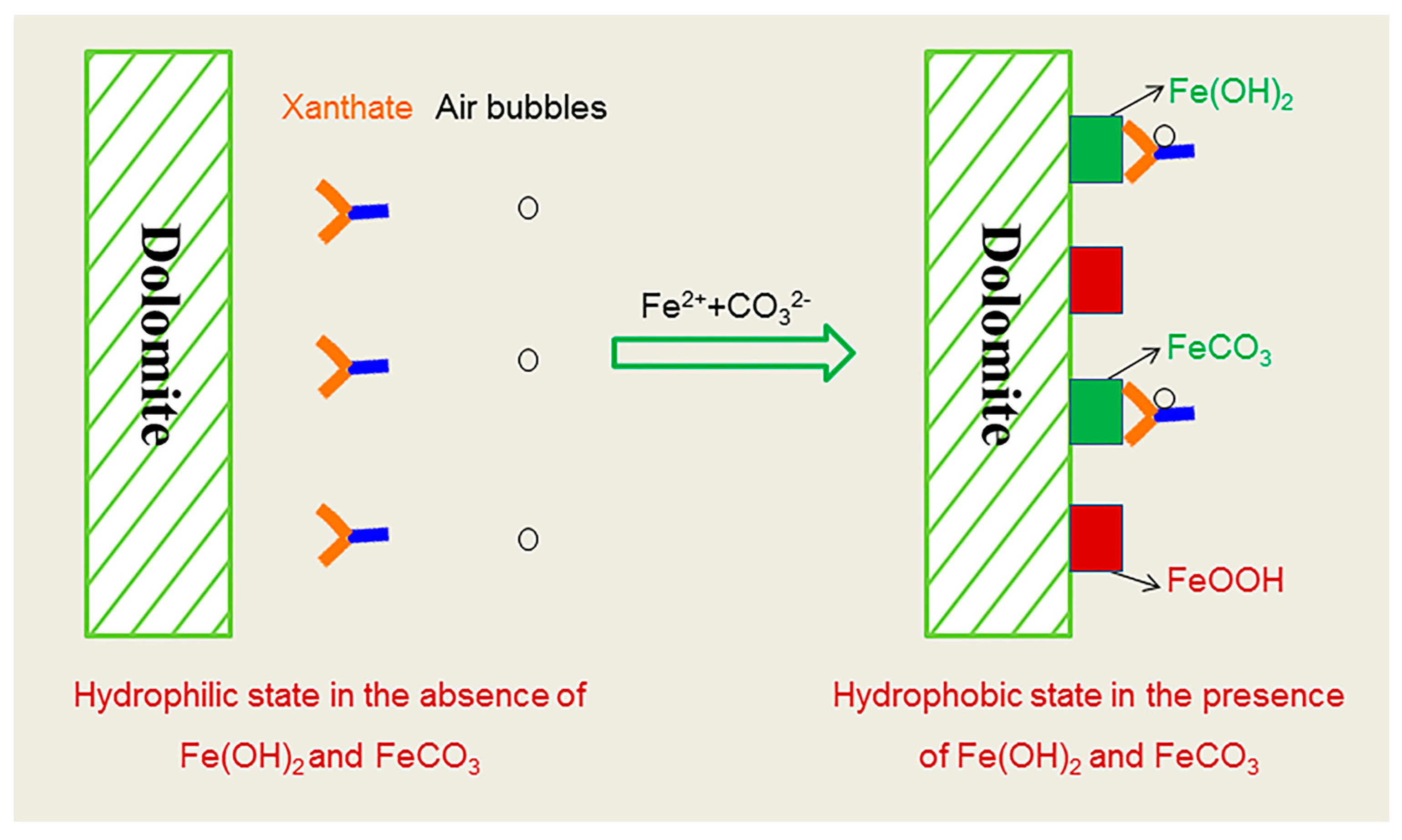
3.1. Floatability of Fe2+-Modified Dolomite
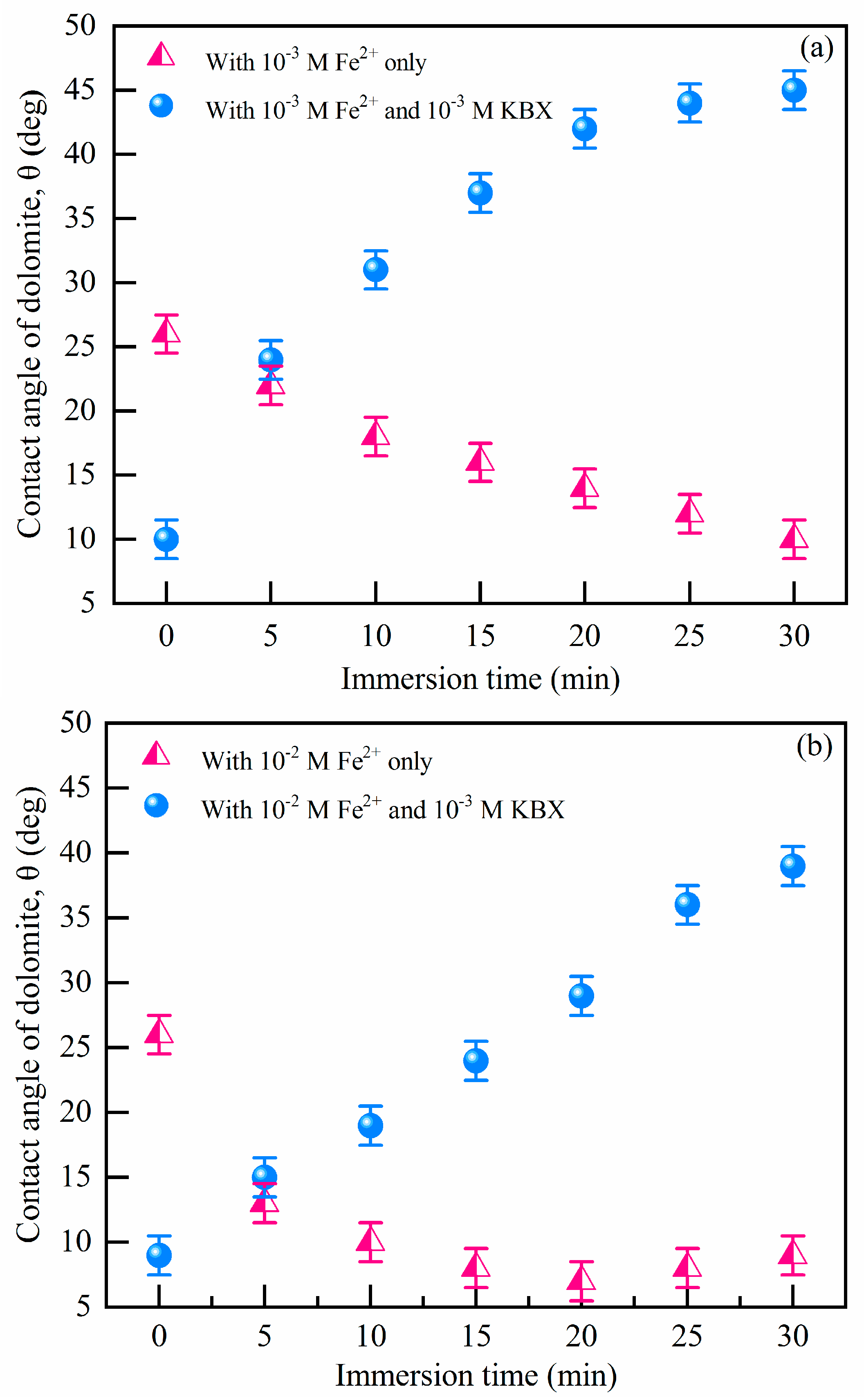
3.2. Assessment of Hydrophobicity
3.3. Identification of Xanthate Chemisorption
3.4. Characterization of Modified Surface Profile
3.4.1. Depth Profile
3.4.2. Species Profile
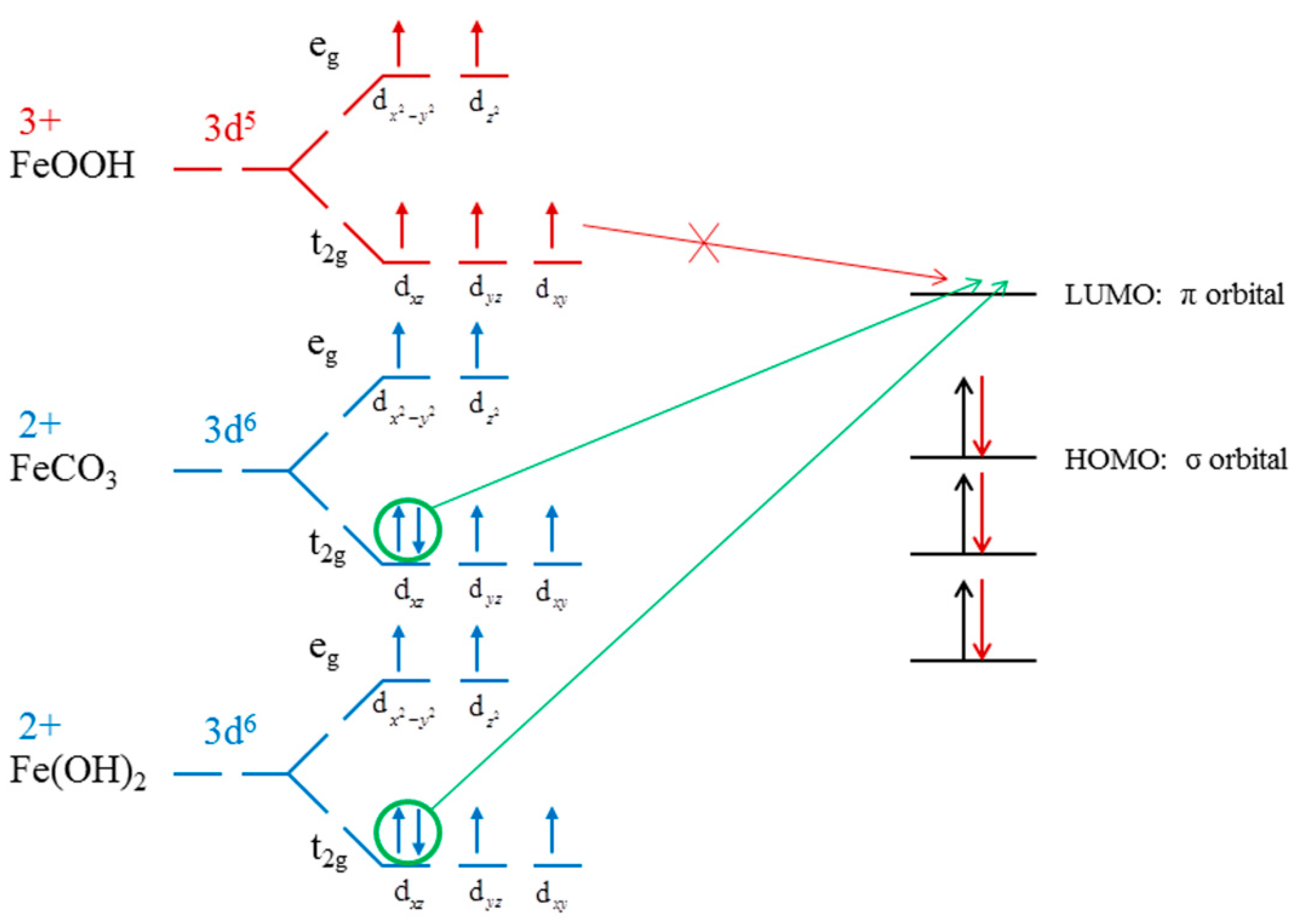
3.5. Xanthate Molecule Adsorption on the Dolomite (101) Surface (DFT Simulation)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassas, B.; Kappes, R.; Miller, J. Fundamental Surface Chemistry Aspects of Auriferous Pyrite Flotation with Carbon Dioxide and Nitrogen. Ph.D Thesis, The University of Utah, Salt Lake City, UT, USA, 2018. [Google Scholar]
- Luo, X.; Yin, W.; Ma, Y.; Sun, C.; Yao, J. New Flotation Technology Research on Carbonate-containing Hematite. Adv. Mater. Res. 2012, 454, 210–215. [Google Scholar] [CrossRef]
- Simmons, G.; Gathje, J. Method for processing gold-bearing sulfide ores involving preparation of a sulfide concentrate. Miner. Eng. 1998, 11, 307. [Google Scholar] [CrossRef]
- Berninger, U.N.; Saldi, G.D.; Jordan, G.; Schott, J.; Oelkers, E.H. Assessing Dolomite Surface Reactivity at TemperatuRes. from 40 to 120 °C by Hydrothermal Atomic Force Microscopy. Geochim. Cosmochim. Acta 2016, 199, 130–142. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J. Dissolution kinetics of calcite, dolomite and magnesite at 25 °C and 0 to 50 atm pCO2. Chem. Geol. 2005, 217, 3. [Google Scholar] [CrossRef]
- Schott, J.; Pokrovsky, O.; Oelkers, E. The Link Between Mineral Dissolution/Precipitation Kinetics and Solution Chemistry. Rev. Miner. Geochem. 2009, 70, 1. [Google Scholar] [CrossRef]
- Nunes, A.; Peres, A.; De Araujo, A.; Valadao, G. Electrokinetic properties of wavellite and its floatability with cationic and anionic collectors. J. Colloid Interface Sci. 2011, 361, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, G.; Shi, Q.; Yang, S.; Wang, M. Utilization of trisodium phosphate to eliminate the adverse effect of Mg2+ on the flotation of pyrite. Miner. Eng. 2020, 150, 106281. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R. The depression of pyrite in selective flotation by different reagent systems—A review. Miner. Eng. 2016, 96–97, 143–156. [Google Scholar] [CrossRef]
- Li, R.; Li, Q.; Sun, X.; Li, J.; Wang, L. Removal of lead complexes by ferrous phosphate and iron phosphate: Unexpected favorable role of ferrous ions. J. Hazard Mater. 2020, 392, 122509. [Google Scholar] [CrossRef] [PubMed]
- Azizi, D.; Larachi, F. Surface interactions and flotation behavior of calcite, dolomite and ankerite with alkyl hydroxamic acid bearing collector and sodium silicate. Colloid Surf. A 2017, 537, 126–138. [Google Scholar] [CrossRef]
- Niu, X.; Ruan, R.; Xia, L.; Li, L.; Sun, H.; Jia, Y.; Tan, Q. Correlation of Surface Adsorption and Oxidation with a Floatability Difference of Galena and Pyrite in High-Alkaline Lime Systems. Langmuir 2018, 34, 2716–2724. [Google Scholar] [CrossRef]
- Zeng, M.; Yang, B.; Guan, Z.; Zeng, L.; Luo, H.; Deng, B. The selective adsorption of xanthan gum on dolomite and its implication in the flotation separation of dolomite from apatite. Appl. Surf. Sci. 2021, 551, 149301. [Google Scholar] [CrossRef]
- Yin, W.; Sun, H.; Hong, J.; Cao, S.; Yang, B.; Won, C.; Song, M. Effect of Ca selective chelator BAPTA as depressant on flotation separation of magnesite from dolomite. Miner. Eng. 2019, 144, 106050. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Y.; Guo, X.; Guo, W. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Jin, Y.; By, A.; Kc, A.; Hs, A.; Zz, A.; Wya, C.; Ns, A.; Qs, A. Sodium tripolyphosphate as a selective depressant for separating magnesite from dolomite and its depression mechanism. Powder Technol. 2021, 382, 244–253. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New insights into selective-depression mechanism of novel depressant EDTMPS on magnesite and quartz surfaces: Adsorption mechanism, DFT calculations, and adsorption model. Miner. Eng. 2020, 160, 106660. [Google Scholar] [CrossRef]
- Yaoyang, R.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz. Minerals 2018, 8, 141. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y. Transformation of heavy metals and dewaterability of waste activated sludge during the conditioning by Fe2+-activated peroxymonosulfate oxidation combined with rice straw biochar as skeleton builder. Chemosphere 2020, 238, 124628. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Yan, P.; Zhang, J.; Wang, Z.; Zhao, S.; Chen, Z. Catalytic ozonation of 4-chloronitrobenzene by goethite and Fe2+-modified goethite with low defects: A comparative study. J. Hazard Mater. 2019, 365, 744–750. [Google Scholar] [CrossRef]
- Breitner, D.; Osán, J.; Fábián, M.; Zagyvai, P.; Szabó, C.; Dähn, R.; Marques Fernandes, M.; Sajó, I.E.; Máthé, Z.; Török, S. Characteristics of uranium uptake of Boda Claystone Formation as the candidate host rock of high level radioactive waste repository in Hungary. Environ. Earth Sci. 2015, 73, 1. [Google Scholar] [CrossRef]
- Doi, A.; Ejtemaei, M.; Nguyen, A.V. Effects of ion specificity on the surface electrical properties of kaolinite and montmorillonite. Miner. Eng. 2019, 143, 105929. [Google Scholar] [CrossRef]
- Tournassat, C.; Vinsot, A.; Gaucher, E.C.; Altmann, S. Chemical Conditions in Clay-Rocks. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 6. [Google Scholar] [CrossRef]
- Herman, J.S.; White, W.B. Dissolution kinetics of dolomite: Effects of lithology and fluid flow velocity. Geochim. Cosmochim. Acta 1985, 49, 10. [Google Scholar] [CrossRef]
- Feng, Q.M.; Liu, G.S.; Zheng-Jun, Y.U.; Yi-Ping, L.U.; Le-Ming, O.U.; Zhang, G.F. Influence and mechanism of ferric and ferrous ions on flotation of talc. J. Cent. South Univ. 2006, 37, 3. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, X.; Tong, X.; Feng, D.; Song, Q. The activation mechanism of Fe(II) ion-modified cassiterite surface to promote salicylhydroxamic acid adsorption. Miner. Eng. 2021, 160, 106707. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Chen, J. The interaction of flotation reagents with metal ions in mineral surfaces: A perspective from coordination chemistry. Miner. Eng. 2021, 171, 107067. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Erratum. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Perdew, P.J.; Chevary, A.J.; Vosko, H.S.; Jackson, A.K.; Pederson, R.M.; Singh, J.D.; Fiolhais, C. Erratum: Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1993, 48, 7. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244, Erratum in Phys. Rev. B 2018, 98, 079904. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Yin, W.Z.; Wang, Y.F.; Sun, C.Y.; Ma, Y.Q.; Liu, J. Effect and mechanism of dolomite with different size fractions on hematite flotation using sodium oleate as collector. J. Cent. South Univ. 2016, 23, 3. [Google Scholar] [CrossRef]
- Jiang, L.C.; Wang, H.X.; Parekh, K.B.; Leonard, J.W. The surface and solution chemistry of pyrite flotation with xanthate in the presence of iron ions. Colloid Surf. A 1998, 136, 51–62. [Google Scholar] [CrossRef]
- Sun, W.; Sun, C.; Liu, Q.R.; Cao, F.X.; Tao, H.B. Electrochemical behavior of galena and jamesonite flotation in high alkaline pulp. Trans. Nonferr. Met. Soc. 2016, 26, 2. [Google Scholar] [CrossRef]
- Zhong, W.; Yin, W.; Wang, Y.; Yao, J. Selective flotation of magnesite from dolomite using α-chloro-oleate acid as collector. Powder Technol. 2020, 373, 147–151. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Yin, Z.W.; Cao, H.S.; Yang, B.; Sun, R.H.; Tang, Y.; Wang, H.D.; Yao, J. Adsorption kinetics and thermodynamics of sodium butyl xanthate onto bornite in flotation. J. Cent. South Univ. 2019, 26, 11. [Google Scholar] [CrossRef]
- Yuasa, K.; Kumasaki, M.; Mizutani, T.; Arai, M. Thermal hazard evaluation procedure for detoxifying system of hazardous gases by using of reaction calorimeter. J. Therm. Anal. Calorim. 2008, 93, 41–45. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Gao, P.; Gu, X.; Li, Y. An Investigation into the Effects of Grinding Media on Grinding Products Characteristics and Flotation Performance of Pyrite. Miner. Process. Extr. Metall. Rev. 2020, 42, 367–373. [Google Scholar] [CrossRef]
- Carbone, M.; Castle, E.J.; Ciriello, R.; Salvi, M.A.; Treacy, J.; Zhdan, P. In Situ Electrochemical–AFM and Cluster-Ion-Profiled XPS Characterization of an Insulating Polymeric Membrane as a Substrate for Immobilizing Biomolecules. Langmuir 2017, 33, 10. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, J.; Zhang, S.; Wang, J.; Song, Y. Cationic etching of ZIF-67 derived LaCoO3/Co3O4 as high-efficiency electromagnetic absorbents. Chem. Eng. J. 2020, 421, 127829. [Google Scholar] [CrossRef]
- Bai, S.; Yu, P.; Li, C.; Wen, S.; Ding, Z. Depression of pyrite in a low-alkaline medium with added calcium hypochlorite: Experiment, visual MINTEQ models, XPS, and ToF–SIMS studies. Miner. Eng. 2019, 141, 105853. [Google Scholar] [CrossRef]
- Lai, H.; Liu, Q.; Deng, J.; Wen, S.; Liu, Z. Surface chemistry study of Cu-Pb sulfide ore using ToF-SIMS and multivariate analysis—ScienceDirect. Appl. Surf. Sci. 2020, 518, 146270. [Google Scholar] [CrossRef]
- An, B.A.; Deland, E.; Sobol, O.; Yao, J.; Skovhus, T.L.; Koerdt, A. The differences in the corrosion product compositions of Methanogen-induced microbiologically influenced corrosion (Mi-MIC) between static and dynamic growth conditions. Corros. Sci. 2021, 180, 109179. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 8. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Ojumu, T.V.; Petersen, J.; Hansford, G.S. The effect of dissolved cations on microbial ferrous-iron oxidation by Leptospirillum ferriphilum in continuous culture. Hydrometallurgy 2008, 94, 69–76. [Google Scholar] [CrossRef]
- Wang, L.; Daub, K.; Qin, Z.; Wren, J. Effect of dissolved ferrous iron on oxide film formation on carbon steel. Electrochim. Acta 2012, 76, 208–217. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S. Inferring the distribution of iron oxidation species on mineral surfaces during grinding of base metal sulphides. Electrochim. Acta 2010, 55, 19. [Google Scholar] [CrossRef]
- Chen, J.; Yang, X.; Li, Y.; Liu, Y. Influences of electronic spin structures on the magnetic properties of Fe, Co and Ni ions and the adsorption of collectors. Miner. Eng. 2020, 154, 6. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Li, Y.; Xia, L.; Li, L.; Sun, H.; Ruan, R. Correlation of surface oxidation with xanthate adsorption and pyrite flotation. Appl. Surf. Sci. 2019, 495, 143411. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapor and air. Geochim. Cosmochim. Acta 1994, 58, 21. [Google Scholar] [CrossRef]
- Yang, J.; Liang, C. Investigation of changes in surface properties of bituminous coal during natural weathering processes by XPS and SEM. Appl. Surf. Sci. 2014, 293, 293–298. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci. 1999, 41, 7. [Google Scholar] [CrossRef]
- Christie, A.B.; Sutherland, I.; Walls, J.M. An XPS study of ion-induced dissociation on metal carbonate surfaces. Vacuum 1981, 31, 513–517. [Google Scholar] [CrossRef]
- Rao, S.R.; Finch, J.A. Base metal oxide flotation using long chain xanthates. Int J. Miner. Process. 2003, 69, 251–258. [Google Scholar] [CrossRef]
- Escamilla-Roa, E.; Sainz-Díaz, C.I.; Huertas, F.J.; Hernandez-Laguna, A. Adsorption of molecules onto (1014) dolomite surface: An application of computational studies for microcalorimetry. J. Phys. Chem. C 2016, 117, 34. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, Y.; Zhang, J.; Kang, D. First-principles study on the adsorption structure of water molecules on a pyrite (100) surface. Physicochem. Probl. Miner. Process. 2021, 57, 121–130. [Google Scholar] [CrossRef]
- Zhang, H.; Han, C.; Liu, W.; Hou, D.; Wei, D. The chain length and isomeric effects of monohydric alcohols on the flotation of magnesite and dolomite by sodium oleate. J. Mol. Liq. 2019, 276, 471–479. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Yuan, Z.; Hao, H.; Zhao, C. Density Functional Theory and Atomic Force Microscopy Study of Oleate Functioned on Siderite Surface. Minerals 2018, 8, 33. [Google Scholar] [CrossRef]
- Chen, J.H.; Lan, L.H.; Chen, Y. Computational simulation of adsorption and thermodynamic study of xanthate, dithiophosphate and dithiocarbamate on galena and pyrite surfaces. Miner. Eng. 2013, 46, 136–143. [Google Scholar] [CrossRef]
- Frolova, L. Investigation of co-precipitation of Fe(II) and Ni(II) hydroxides. Mater. Lett. 2020, 275, 128065. [Google Scholar] [CrossRef]



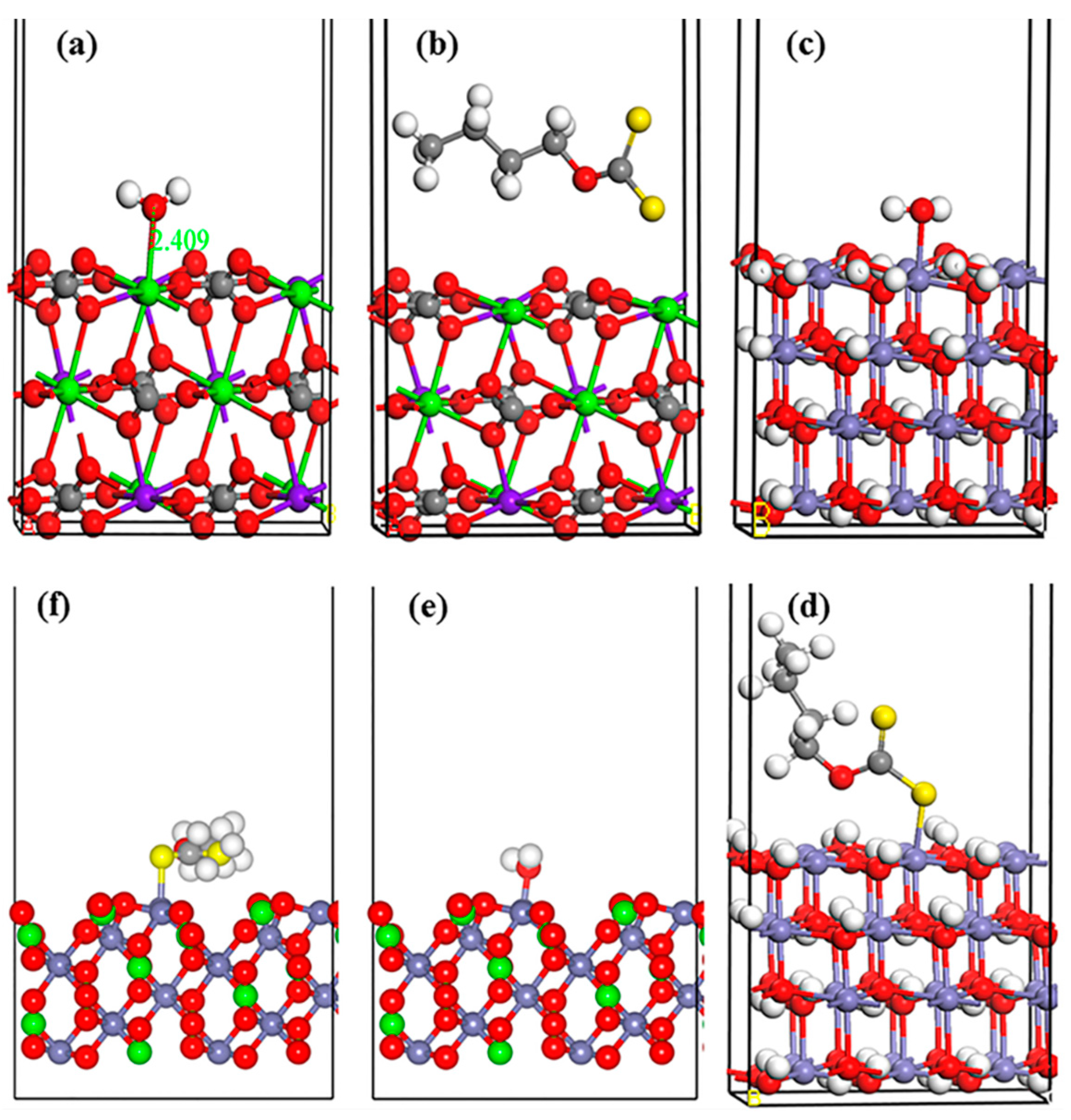


| Surface + Adsorbate | Adsorption Energy (KJ/mol) | Ca-O (Å) | Mg-O (Å) | Fe-O (Å) | Fe-S (Å) |
|---|---|---|---|---|---|
| Dolomite Only | — | 2.412 | 2.146 | — | — |
| Dolomite + H2O | −57.86 | 2.368 | — | — | — |
| Dolomite+ Xanthate | 0.35 | — | — | — | — |
| Fe(OH)2 + H2O Fe(OH)2 + Xanthate FeCO3 +H2O FeCO3 +Xanthate | −124.99 −183.46 −20.35 −113.38 | — — — — | — — — — | 2.136 — 2.151 — | — 2.165 — 2.529 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Niu, X.; Dong, B.; Jia, X.; Ruan, R. Activation of Dolomite Flotation by Ferrous Hydroxide and Carbonate. Minerals 2023, 13, 200. https://doi.org/10.3390/min13020200
Zhao H, Niu X, Dong B, Jia X, Ruan R. Activation of Dolomite Flotation by Ferrous Hydroxide and Carbonate. Minerals. 2023; 13(2):200. https://doi.org/10.3390/min13020200
Chicago/Turabian StyleZhao, Haiping, Xiaopeng Niu, Bingxu Dong, Xianbing Jia, and Renman Ruan. 2023. "Activation of Dolomite Flotation by Ferrous Hydroxide and Carbonate" Minerals 13, no. 2: 200. https://doi.org/10.3390/min13020200
APA StyleZhao, H., Niu, X., Dong, B., Jia, X., & Ruan, R. (2023). Activation of Dolomite Flotation by Ferrous Hydroxide and Carbonate. Minerals, 13(2), 200. https://doi.org/10.3390/min13020200





