Conversion of Aluminosilicate Residue Generated from Lithium Extraction Process to NaX Zeolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Aluminosilicate Residue and Commercial Zeolite
2.2. Zeolite Synthesis Testing
2.3. Evaluation of Operational Parameters
2.3.1. Effect of Crystallization Time as well as Aging Time and Temperature
2.3.2. Effect of Solid/Liquid Ratio
2.4. Determination of Ion-Exchange Capacity
2.5. Analytical Methods
3. Results and Discussion
3.1. Physico-Chemical and Mineralogical Characterization of Aluminosilicate Residue
3.2. Characterization of the Zeolite Produced
3.2.1. Physico-Chemical Characterization of Synthesized Zeolite
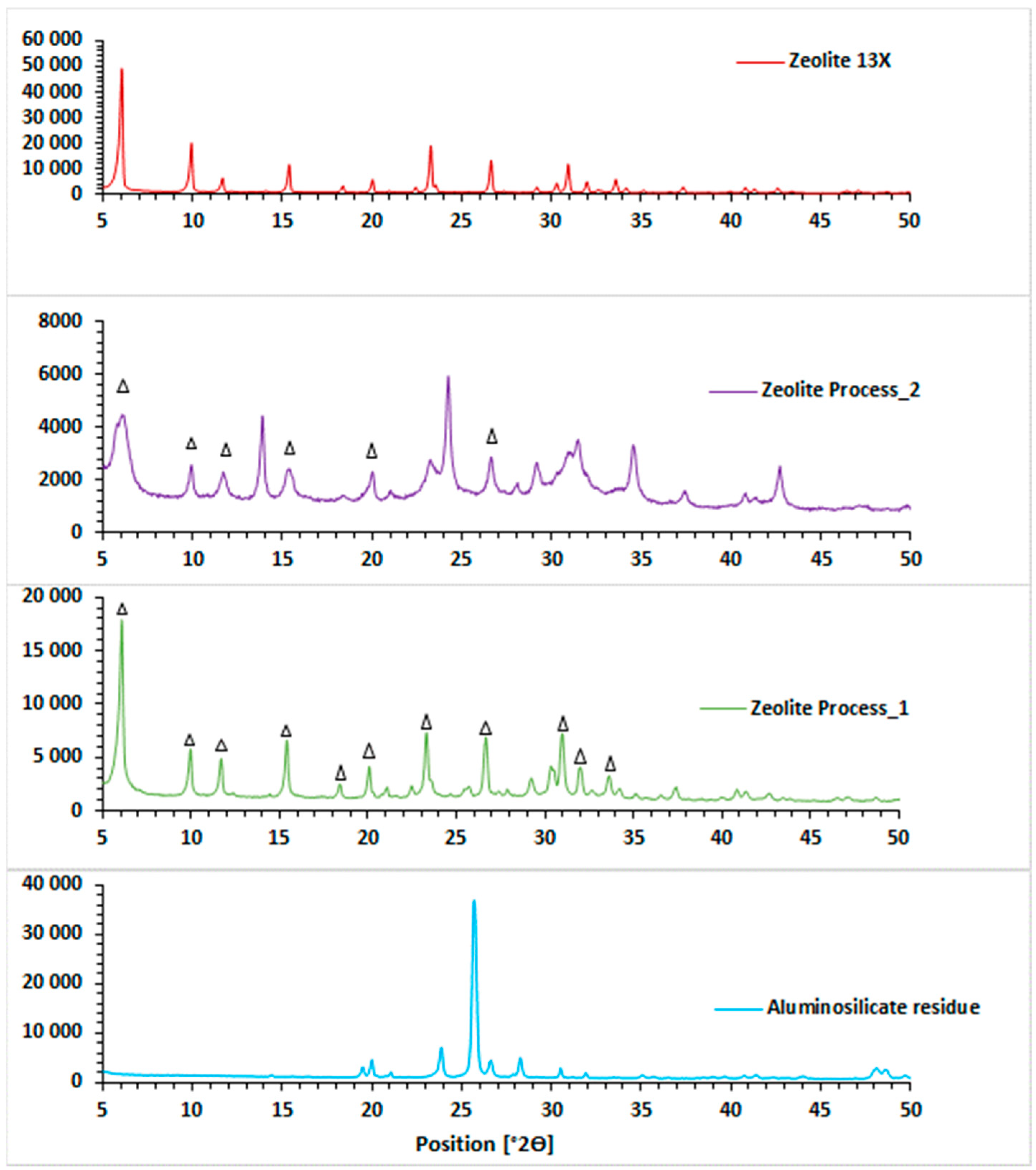
3.2.2. Mineralogical Characterization of Synthesized Zeolite
3.3. Ion-Exchange Capacity of Synthesized and Commercial Zeolites
3.4. Evaluation of Operating Parameters on the Performances of the Conventional Hydrothermal Process to Produce Zeolite
3.4.1. Effect of Aging Time
3.4.2. Effect of Aging Temperature
3.4.3. Effect of Crystallization Time
3.4.4. Effect of Solid/Liquid Ratio on Zeolite Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.F. Spodumene: The lithium market, resources and processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Azizi, D.; Ibsaine, F.; Dionne, J.; Pasquier, L.C.; Coudert, L.; Blais, J.F. Microporous and macroporous materials state-of-the-art of the technologies in zeolitization of aluminosilicate bearing residues from mining and metallurgical industries: A comprehensive review. Microporous Mesoporous Mater. 2021, 318, 111029. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and characterization of zeolite X from lithium slag. Appl. Clay Sci. 2012, 59–60, 148–151. [Google Scholar] [CrossRef]
- Dewangan, B.J.P.; Yenkie, M.N. Novel Applications in Polymers and Waste Management; CRC Press: Boca Raton, FL, USA; Apple Academic Press: New York, NY, USA, 2018; 364p. [Google Scholar]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Zeolite Science and Technology; CRC Press: Boca Raton, FL, USA; Marcel Dekker, Inc.: New York, NY, USA, 2003; 1184p. [Google Scholar]
- Qiang, Z.; Shen, X.; Guo, M.; Cheng, F.; Zhang, M. A simple hydrothermal synthesis of zeolite X from bauxite tailings for highly efficient adsorbing CO2 at room temperature. Microporous Mesoporous Mater. 2019, 287, 77–84. [Google Scholar] [CrossRef]
- Lin, G.; Zhuang, Q.; Cui, Q.; Wang, H.; Yao, H. Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid. Chin. J. Chem. Eng. 2015, 23, 1768–1773. [Google Scholar] [CrossRef]
- Cejka, J.; van Bekkum, H.; Corma, A.; Schueth, F. Introduction to Zeolite Science and Practice; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Abdullahi, T.; Harun, Z.; Othman, M.H.D. A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv. Powder Technol. 2017, 28, 1827–1840. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Zeng, L.; Ma, B.; Wang, L.; Chen, Y.; Yang, C. Lithium extraction and hydroxysodalite zeolite synthesis by hydrothermal conversion of α-spodumene. ACS Sustain. Chem. Eng. 2019, 7, 9498–9505. [Google Scholar] [CrossRef]
- Alkan, M.; Hopa, Ç.; Yilmaz, Z.; Güler, H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater. 2005, 86, 176–184. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; Morales-Ospino, R.; Finocchio, E.; Busca, G.; Sapag, K.; Villarroel-Rocha, J.; Bastos-Neto, M.; Azevedo, D.C.S.; Rodríguez-Castellón, E. Kaolinite-based zeolites synthesis and their application in CO2 capture processes. Fuel 2022, 320, 123953. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Dere Ozdemir, O.; Piskin, S. A novel synthesis method of zeolite X from coal fly ash: Alkaline fusion followed by ultrasonic-assisted synthesis method. Waste Biomass Valorization 2017, 10, 143–154. [Google Scholar] [CrossRef]
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.F.; et al. Synthesis of high quality zeolites from coal fly ash: Mobility of hazardous elements and environmental applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Yang, T.; Han, C.; Liu, H.; Yang, L.; Liu, D.; Tang, J.; Luo, Y. Synthesis of Na-X zeolite from low aluminum coal fly ash: Characterization and high efficient As(V) removal. Adv. Powder Technol. 2019, 30, 199–206. [Google Scholar] [CrossRef]
- Yang, L.; Qian, X.; Yuan, P.; Bai, H.; Miki, T.; Men, F.; Li, H.; Nagasaka, T. Green synthesis of zeolite 4A using fly ash fused with synergism of NaOH and Na2CO3. J. Clean. Prod. 2019, 212, 250–260. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of pure Na–X and Na–A zeolite from bagasse fly ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Z.; Guo, M.; Zhang, M.; Liu, J. Feasible conversion of solid waste bauxite tailings into highly crystalline 4A zeolite with valuable application. Waste Manag. 2014, 34, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ma, H.; Chuan, X. Hydrothermal synthesis of zeolite A from K-feldspar and its crystallization mechanism. Adv. Powder Technol. 2016, 27, 139–144. [Google Scholar] [CrossRef]
- Outram, J.G.; Collins, F.J.; Millar, G.J.; Couperthwaite, S.J.; Beer, G. Process optimisation of low silica zeolite synthesis from spodumene leachate residue. Chem. Eng. Res. Des. 2023, 189, 358–370. [Google Scholar] [CrossRef]
- Sharma, Y. Synthesis of Zeolites. With International Search Report (Art. 21(3)), Australia. Patent N° WO 2019/068135 A1, 1 April 2019. [Google Scholar]
- Wang, B.; Li, J.; Zhou, X.; Hao, W.; Zhang, S.; Lan, C.; Wang, X.; Wang, Z.; Xu, J.; Zhang, J.N.; et al. Facile activation of lithium slag for the hydrothermal synthesis of zeolite A with commercial quality and high removal efficiency for the isotope of radioactive 90Sr. Inorg. Chem. Front. 2022, 9, 468–477. [Google Scholar] [CrossRef]
- Tauanov, Z.; Azat, S.; Baibatyrova, A. A mini-review on coal fly ash properties, utilization and synthesis of zeolites. Int. J. Coal Prep. Util. 2020, 42, 1968–1990. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, J.S.; Wang, L.J.; Sun, X.Y. Influence of NaOH concentrations on synthesis of pure-form zeolite A from fly ash using two-stage method. J. Hazard. Mater. 2008, 155, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, D.; Zhang, M.; Yang, R. Synthesis of NaX zeolite: Influence of crystallization time, temperature and batch molar ratio SiO2/Al2O3 on the particulate properties of zeolite crystals. Powder Technol. 2013, 235, 322–328. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y.; Díaz, I. Synthesis of zeolite A from Ethiopian kaolin. Microporous Mesoporous Mater. 2015, 215, 29–36. [Google Scholar] [CrossRef]
- Nazir, L.S.M.; Yeong, Y.F.; Sabdin, S. Formation of pure NaX zeolite: Effect of ageing and hydrothermal synthesis parameters. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Nanjing, China, 17–19 August 2018; IOP Publishing: Bristol, UK, 2018; Volume 458, p. 012002. [Google Scholar]
- Wałek, T.T.; Saito, F.; Zhang, Q. The effect of low solid/liquid ratio on hydrothermal synthesis of zeolites from fly ash. Fuel 2008, 87, 3194–3199. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of lithium—A literature review. Part 2. Extraction from spodumene. Miner. Process. Extr. Metall. Rev. 2019, 42, 268–283. [Google Scholar] [CrossRef]
- Yoldi, M.; Fuentes-Ordoñez, E.G.; Korili, S.A.; Gil, A. Zeolite synthesis from industrial wastes. Microporous Mesoporous Mater. 2019, 287, 183–191. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Ingamells, C. Lithium metaborate flux in silicate analysis. Anal. Chim. Acta 1970, 52, 323–334. [Google Scholar] [CrossRef]
- Rayalu, S.; Meshram, S.; Hasan, M. Highly crystalline faujasitic zeolites from fly ash. J. Hazard. Mater. 2000, 77, 123–131. [Google Scholar] [CrossRef]
- Lee, Y.R.; Soe, J.T.; Zhang, S.; Ahn, J.W.; Park, M.B.; Ahn, W.S. Synthesis of nanoporous materials via recycling coal fly ash and other solid wastes: A mini review. Chem. Eng. J. 2017, 317, 821–843. [Google Scholar] [CrossRef]
- Shigemoto, N.; Hayashi, H.; Miyaura, K. Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J. Mater. Sci. 1993, 28, 4781–4786. [Google Scholar] [CrossRef]
- Fotovat, F.; Kazemian, H.; Kazemeini, M. Synthesis of Na-A and faujasitic zeolites from high silicon fly ash. Mater. Res. Bull. 2009, 44, 913–917. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; William Andrew: Norwich, NY, USA, 2012. [Google Scholar]
- Oyinade, A.; Kovo, A.S.; Hill, P. Synthesis, characterization and ion exchange isotherm of zeolite Y using Box–Behnken design. Adv. Powder Technol. 2016, 27, 750–755. [Google Scholar] [CrossRef]



| Parameters | Aluminosilicate Residue | Granulometric Fraction (µm) | |||||
|---|---|---|---|---|---|---|---|
| >500 | 250–500 | 106–250 | 75–106 | 53–75 | <53 | ||
| Weight (%) | 100 | 12.3 ± 0.6 | 8.1 ± 0.2 | 11.9 ± 0.8 | 6.0 ± 1.0 | 5.6 ± 0.6 | 56.0 ± 1.0 |
| Major elements (%) | |||||||
| Al2O3 | 24.6 ± 0.6 | 8.9 ± 0.6 | 13.0 ± 0.2 | 19.3 ± 0.4 | 24.1± 0.6 | 27.3 ± 0.1 | 29.0 ± 0.2 |
| SiO2 | 74.0 ± 0.7 | 88.0 ± 0.6 | 83.7 ± 0.2 | 78.4 ± 0.5 | 74.3 ± 0.6 | 71.4 ± 0.1 | 70.4 ± 0.1 |
| Fe2O3 | 0.40 ± 0.10 | 0.83 ± 0.03 | 0.61 ± 0.02 | 0.42 ± 0.05 | 0.44 ± 0.04 | 0.45 ± 0.03 | 0.45 ± 0.02 |
| K2O | 0.30 ± 0.10 | 0.73 ± 0.03 | 0.74 ± 0.03 | 0.39 ± 0.04 | 0.26 ± 0.03 | 0.19 ± 0.02 | 0.05 ± 0.01 |
| MnO | 0.01 ± 0.02 | 0.14 ± 0.01 | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 |
| Na2O | 0.70 ± 0.02 | 0.72 ± 0.03 | 1.56 ± 0.01 | 1.20 ± 0.10 | 0.70 ± 0.10 | 0.45 ± 0.03 | 0.13 ± 0.04 |
| CaO | 0.04 ± 0.01 | 0.40 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.02 | 0.15 ± 0.01 | 0.10 ± 0.01 | 0.01 ± 0.01 |
| MgO | 0.03 ± 0.02 | 0.21 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.01 ± 0.01 |
| Minor elements (mg/kg) | |||||||
| Cr | 15 ± 3 | 273 ± 26 | 282 ± 17 | 24 ± 9 | 24 ± 8 | 23 ± 3 | 20 ± 9 |
| Cu | <5 | <5 | <5 | 24 ± 15 | <5 | 36 ± 17 | 74 ± 50 |
| Li | 5000 | 2900 | 4000 | 8200 | 10,600 | 11,600 | 3400 |
| Sr | <0.2 | 4 ± 1 | 3 ± 1 | 0.9 ± 0.4 | <0.2 | <0.2 | <0.2 |
| Ti | <4 | 129 ± 34 | 15 ± 13 | 27 ± 38 | 32 ± 18 | <4 | <4 |
| Zn | 16 ± 5 | 152 ± 60 | 174 ± 8 | 169 ± 12 | 66 ± 28 | 61 ± 17 | 28 ± 14 |
| Si/Al ratio | 2.55 | 8.37 | 5.46 | 3.45 | 2.61 | 2.22 | 2.06 |
| Zeolite Process_1 | Zeolite Process_2 | Commercial Zeolite 13X | |
|---|---|---|---|
| Major elements (%) | |||
| Al2O3 | 29.8 ± 0.1 | 31.2 ± 0.1 | 32.8 |
| SiO2 | 51.7 ± 0.2 | 44.8 ± 0.2 | 47.2 |
| Fe2O3 | 0.63 ± 0.01 | 0.74 ± 0.01 | 0.01 |
| K2O | 0.07 ± 0.03 | 0.09 ± 0.01 | 0.12 |
| MnO | 0.018 ± 0.001 | 0.019 ± 0.001 | <0.002 |
| Na2O | 17.8 ± 0.1 | 23.1 ± 0.2 | 19.9 |
| CaO | <0.02 | <0.02 | <0.02 |
| MgO | <0.02 | <0.02 | <0.02 |
| Minor elements (mg/kg) | |||
| Cr | 18 ± 17 | 8 ± 4 | 9.7 |
| Cu | 10 ± 4 | 15 ± 2 | <6 |
| Sr | 5.0 ± 0.1 | 6.1 ± 0.1 | <5 |
| Ti | <11 | <11 | 77 |
| Zn | 11 ± 2 | 12 ± 3 | N.D. |
| Si/Al ratio | 1.47 | 1.22 | 1.32 |
| Products | Aluminosilicate Residue | Zeolite Process_1 | Zeolite Process_2 | Zeolite 13X |
|---|---|---|---|---|
| Calcium-exchange capacity (mg Ca/g) | 1.1 ± 0.5 | 60 ± 1 | 65 ± 1 | 76 ± 1 |
| Experiments | Aging Time (h) | Aging Temperature (°C) | Crystallization Time (°C) | Sorption Capacity (mg Ca/g) |
|---|---|---|---|---|
| 1 | 16 | 50 | 16 | 56.54 |
| 2 | 16 | 50 | 16 | 54.67 |
| 3 | 16 | 50 | 16 | 56.56 |
| 4 | 16 | 50 | 16 | 55.51 |
| 5 | 16 | 50 | 16 | 54.94 |
| 6 | 16 | 25 | 8 | 27.00 |
| 7 | 8 | 50 | 8 | 29.26 |
| 8 | 24 | 50 | 8 | 42.67 |
| 9 | 16 | 75 | 8 | 56.04 |
| 10 | 24 | 25 | 16 | 56.03 |
| 11 | 8 | 25 | 16 | 57.41 |
| 12 | 24 | 75 | 16 | 57.37 |
| 13 | 8 | 75 | 16 | 56.21 |
| 14 | 16 | 25 | 24 | 55.72 |
| 15 | 8 | 50 | 24 | 56.21 |
| 16 | 24 | 50 | 24 | 56.66 |
| 17 | 16 | 75 | 24 | 56.15 |
| Experiments | S/L Ratio (%—w/v) | Sorption Capacity (mg Ca/g) |
|---|---|---|
| 3 | 10 | 58.08 |
| 5 | 10 | 58.11 |
| 7 | 15 | 41.18 |
| 2 | 20 | 34.26 |
| 4 | 25 | 28.94 |
| 1 | 30 | 21.13 |
| 6 | 30 | 23.68 |
| Sample | Si/Al Ratio | Sorption Capacity (mg Ca/g) | Medium Particle Size (µm) | SBET (m2/g) |
|---|---|---|---|---|
| Zeolite-efficient conditions | 1.48 ± 0.01 | 58.4 ± 0.4 | 10.2 ± 0.3 | 371 |
| Aluminosilicate residue (<53 µm) | 2.06 | 1.1 ± 0.5 | 21.5 | 5.6 |
| Commercial zeolite 13X | 1.32 | 76 ± 1 | 3.8 | 962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibsaine, F.; Azizi, D.; Dionne, J.; Tran, L.H.; Coudert, L.; Pasquier, L.-C.; Blais, J.-F. Conversion of Aluminosilicate Residue Generated from Lithium Extraction Process to NaX Zeolite. Minerals 2023, 13, 1467. https://doi.org/10.3390/min13121467
Ibsaine F, Azizi D, Dionne J, Tran LH, Coudert L, Pasquier L-C, Blais J-F. Conversion of Aluminosilicate Residue Generated from Lithium Extraction Process to NaX Zeolite. Minerals. 2023; 13(12):1467. https://doi.org/10.3390/min13121467
Chicago/Turabian StyleIbsaine, Fatima, Dariush Azizi, Justine Dionne, Lan Huong Tran, Lucie Coudert, Louis-César Pasquier, and Jean-François Blais. 2023. "Conversion of Aluminosilicate Residue Generated from Lithium Extraction Process to NaX Zeolite" Minerals 13, no. 12: 1467. https://doi.org/10.3390/min13121467
APA StyleIbsaine, F., Azizi, D., Dionne, J., Tran, L. H., Coudert, L., Pasquier, L.-C., & Blais, J.-F. (2023). Conversion of Aluminosilicate Residue Generated from Lithium Extraction Process to NaX Zeolite. Minerals, 13(12), 1467. https://doi.org/10.3390/min13121467








