Clumped Isotope Reordering and Kinetic Differences in Co-Hosted Calcite and Dolomite Minerals throughout Burial Diagenesis and Exhumation
Abstract
:1. Introduction
2. Geological Settings of the Locations
3. Materials and Methods
3.1. Materials
3.2. Petrography
3.3. FTIR Mineralogy and X-ray Diffraction Analysis of Dolomite
3.4. Clumped Isotopes
3.5. Modelling
4. Results
4.1. Petrography Observations
4.2. δ18O and δ13C Results
4.3. Clumped Isotope (Δ47)
5. Discussion
5.1. Evidence of Recrystallisation
5.2. Water–Rock Ratio during Recrystallisation Process
5.3. Calcite vs. Dolomite Temperature
5.4. Temperature and Dolomite Mineralogy Relationship
5.5. Modelling Applications
5.5.1. Offshore Arabian Gulf
5.5.2. Jabal Akhdar, Central Oman Mountains
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, P.; Adkins, J.; Affek, H.; Balta, B.; Guo, W.; Schauble, E.A.; Schrag, D.; Eiler, J.M. 13C–18O bonds in carbonate minerals: A new kind of paleothermometer. Geochim. Cosmochim. Acta 2006, 70, 1439–1456. [Google Scholar] [CrossRef]
- Eiler, J.M. “Clumped-isotope” geochemistry—The study of naturally-occurring, multiply-substituted isotopologues. Earth Planet. Sci. Lett. 2007, 262, 309–327. [Google Scholar] [CrossRef]
- Lechler, A.R.; Niemi, N.A.; Hren, M.T.; Lohmann, K.C. Paleoelevation estimates for the northern and central proto—Basin and Range from carbonate clumped isotope thermometry. Tectonics 2013, 32, 295–316. [Google Scholar] [CrossRef]
- Snell, K.E.; Thrasher, B.L.; Eiler, J.M.; Koch, P.L.; Sloan, L.C.; Tabor, N.J. Hot summers in the Bighorn Basin during the early Paleogene. Geology 2013, 41, 55–58. [Google Scholar] [CrossRef]
- Garzione, C.N.; Auerbach, D.J.; Smith, J.J.-S.; Rosario, J.J.; Passey, B.H.; Jordan, T.E.; Eiler, J.M. Clumped isotope evidence for diachronous surface cooling of the Altiplano and pulsed surface uplift of the Central Andes. Earth Planet. Sci. Lett. 2014, 393, 173–181. [Google Scholar] [CrossRef]
- Fan, M.; Heller, P.; Allen, S.D.; Hough, B.G. Middle Cenozoic uplift and concomitant drying in the central Rocky Mountains and adjacent Great Plains. Geology 2014, 42, 547–550. [Google Scholar] [CrossRef]
- Stolper, D.A.; Eiler, J.M. The Kinetics of Solid-State Isotope-Exchange Reactions for Clumped Isotopes: A Study of Inorganic Calcites and Apatites from Natural and Experimental Samples. Am. J. Sci. 2015, 315, 363–411. [Google Scholar] [CrossRef]
- Passey, B.H.; Henkes, G.A. Carbonate clumped isotope bond reordering and geospeedometry. Earth Planet. Sci. Lett. 2012, 351–352, 223–236. [Google Scholar] [CrossRef]
- John, C.M. Burial estimates constrained by clumped isotope thermometry: Example of the Lower Cretaceous Qishn Formation (Haushi-Huqf High, Oman). Geol. Soc. Lond. Spec. Publ. 2018, 435, 107–121. [Google Scholar] [CrossRef]
- Lacroix, B.; Niemi, N.A. Investigating the effect of burial histories on the clumped isotope thermometer: An example from the Green River and Washakie Basins, Wyoming. Geochim. Cosmochim. Acta 2019, 247, 40–58. [Google Scholar] [CrossRef]
- Ryb, U.; Lloyd, M.K.; Eiler, J.M. Carbonate clumped isotope constraints on burial, uplift and exhumation histories of the Colorado Plateau. Earth Planet. Sci. Lett. 2021, 566, 116964. [Google Scholar] [CrossRef]
- Winkelstern, I.Z.; Lohmann, K.C. Shallow burial alteration of dolomite and limestone clumped isotope geochemistry. Geology 2016, 44, 467–470. [Google Scholar] [CrossRef]
- Gallagher, T.M.; Sheldon, N.D.; Mauk, J.L.; Petersen, S.V.; Gueneli, N.; Brocks, J.J. Constraining the thermal history of the North American Midcontinent Rift System using carbonate clumped isotopes and organic thermal maturity indices. Precambr. Res. 2017, 294, 53–66. [Google Scholar] [CrossRef]
- Lawson, M.; Shenton, B.J.; Stolper, D.A.; Eiler, J.M.; Rasbury, E.T.; Becker, T.P.; Phillips-Lander, C.M.; Buono, A.S.; Becker, S.P.; Pottorf, R. Deciphering the diagenetic history of the El Abra Formation of eastern Mexico using reordered clumped isotope temperatures and U-Pb dating. GSA Bull. 2018, 130, 617–629. [Google Scholar] [CrossRef]
- Naylor, H.N.; Defliese, W.F.; Grossman, E.L.; Maupin, C.R. Investigation of the thermal history of the Delaware Basin (West Texas, USA) using carbonate clumped isotope thermometry. Basin Res. 2020, 32, 1140–1155. [Google Scholar] [CrossRef]
- Lloyd, M.K.; Eiler, J.M.; Nabelek, P.I. Clumped isotope thermometry of calcite and dolomite in a contact metamorphic environment. Geochim. Cosmochim. Acta 2017, 197, 323–344. [Google Scholar] [CrossRef]
- Hemingway, J.D.; Henkes, G.A. A disordered kinetic model for clumped isotope bond reordering in carbonates. Earth Planet. Sci. Lett. 2021, 566, 116962. [Google Scholar] [CrossRef]
- Dennis, K.J.; Schrag, D.P. Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration. Geochim. Cosmochim. Acta 2010, 74, 4110–4122. [Google Scholar] [CrossRef]
- Mangenot, X.; Gasparrini, M.; Rouchon, V.; Bonifacie, M. Basin-scale thermal and fluid flow histories revealed by carbonate clumped isotopes (Δ47)—Middle Jurassic carbonates of the Paris Basin depocentre. Sedimentology 2018, 65, 123–150. [Google Scholar] [CrossRef]
- Brenner, D.C.; Passey, B.H.; Stolper, D.A. Influence of water on clumped-isotope bond reordering kinetics in calcite. Geochim. Cosmochim. Acta 2018, 224, 42–63. [Google Scholar] [CrossRef]
- Lloyd, M.K.; Ryb, U.; Eiler, J.M. Experimental calibration of clumped isotope reordering in dolomite. Geochim. Cosmochim. Acta 2018, 242, 1–20. [Google Scholar] [CrossRef]
- Ryb, U.; Lloyd, M.K.; Stolper, D.A.; Eiler, J.M. The clumped-isotope geochemistry of exhumed marbles from Naxos, Greece. Earth Planet. Sci. Lett. 2017, 470, 1–12. [Google Scholar] [CrossRef]
- Dodson, M.H. Closure temperature in cooling geochronological and petrological systems. Contrib. Mineral. Petrol. 1973, 40, 259–274. [Google Scholar] [CrossRef]
- Henkes, G.A.; Passey, B.H.; Grossman, E.L.; Shenton, B.J.; Perez-Huerta, A.; Yancey, T.E. Temperature limits for preservation of primary calcite clumped isotope paleotemperatures. Geochim. Cosmochim. Acta 2014, 139, 362–382. [Google Scholar] [CrossRef]
- Chen, S.; Ryb, U.; Piasecki, A.M.; Lloyd, M.K.; Baker, M.B.; Eiler, J.M. Mechanism of solid-state clumped isotope reordering in carbonate minerals from aragonite heating experiments. Geochim. Cosmochim. Acta 2019, 258, 156–173. [Google Scholar] [CrossRef]
- Staudigel, P.T.; Swart, P.K. Isotopic behavior during the aragonite-calcite transition: Implications for sample preparation and proxy interpretation. Chem. Geol. 2016, 442, 130–138. [Google Scholar] [CrossRef]
- Shenton, B.J.; Grossman, E.L.; Passey, B.H.; Henkes, G.A.; Becker, T.P.; Laya, J.C.; Perez-Huerta, A.; Becker, S.P.; Lawson, M. Clumped isotope thermometry in deeply buried sedimentary carbonates: The effects of bond reordering and recrystallization. Geol. Soc. Am. Bull. 2015, 127, 1036–1051. [Google Scholar] [CrossRef]
- Ingalls, M. Reconstructing carbonate alteration histories in orogenic sedimentary basins: Xigaze forearc, southern Tibet. Geochim. Cosmochim. Acta 2019, 251, 284–300. [Google Scholar] [CrossRef]
- Fernandez, A.; Korte, C.; Ullmann, C.V.; Looser, N.; Wohlwend, S.; Bernasconi, S.M. Reconstructing the magnitude of Early Toarcian (Jurassic) warming using the reordered clumped isotope compositions of belemnites. Geochim. Cosmochim. Acta 2021, 293, 308–327. [Google Scholar] [CrossRef]
- Boni, M.; Parente, G.; Bechstaedt, T.; De Vivo, B.; Iannace, A. Hydrothermal dolomites in SW Sardinia (Italy): Evidence for a widespread late-Variscan fluid flow event. Sediment. Geol. 2000, 131, 181–200. [Google Scholar] [CrossRef]
- Al-Aasm, I. Origin and characterization of hydrothermal dolomite in the Western Canada Sedimentary Basin. J. Geochem. Explor. 2003, 78, 9–15. [Google Scholar] [CrossRef]
- Luczaj, J.A.; Harrison III, W.B.; Smith Williams, N. Fractured hydrothermal dolomite reservoirs in the Devonian Dundee Formation of the central Michigan Basin. AAPG Bull. 2006, 90, 1787–1801. [Google Scholar] [CrossRef]
- Adlan, Q.; John, C.M. Clumped isotope record of individual limestone fabrics: A potential method to constrain the timing of oil migration. Chem. Geol. 2023, 616, 121245. [Google Scholar] [CrossRef]
- Abd-Allah, A.M.A.; Hashem, W.A.; Abdelghany, O. Post-obduction deformations of the northwestern end of the Hatta Shear Zone, El Rawdah area, Northern Oman Mountains. Math. Comput. Sci. Eng. 2009, 18, 79–84. [Google Scholar]
- Aldega, L.; Carminati, E.; Scharf, A.; Mattern, F. Thermal maturity of the Hawasina units and origin of the Batinah Mélange (Oman Mountains): Insights from clay minerals. Mar. Pet. Geol. 2021, 133, 105316. [Google Scholar] [CrossRef]
- Beckert, J.; Vandeginste, V.; John, C.M. Exploring the geological features and processes that control the shape and internal fabrics of late diagenetic dolomite bodies (Lower Khuff equivalent—Central Oman Mountains). Mar. Pet. Geol. 2015, 68, 325–340. [Google Scholar] [CrossRef]
- Bergmann, K.D.; Al Balushi, S.A.K.; Mackey, T.J.; Grotzinger, J.P.; Eiler, J.M. A 600-Million-Year Carbonate Clumped-Isotope Record from the Sultanate of Oman. J. Sediment. Res. 2018, 88, 960–979. [Google Scholar] [CrossRef]
- Carminati, E.; Aldega, L.; Smeraglia, L.; Scharf, A.; Mattern, F.; Albert, R.; Gerdes, A. Tectonic Evolution of the Northern Oman Mountains, Part of the Strait of Hormuz Syntaxis: New Structural and Paleothermal Analyses and U-Pb Dating of Synkinematic Calcite. Tectonics 2020, 39, e2019TC005936. [Google Scholar] [CrossRef]
- Grobe, A.; von Hagke, C.; Littke, R.; Dunkl, I.; Wübbeler, F.; Muchez, P.; Urai, J.L. Tectono-thermal evolution of Oman’s Mesozoic passive continental margin under the obducting Semail Ophiolite: A case study of Jebel Akhdar, Oman. Solid. Earth 2019, 10, 149–175. [Google Scholar] [CrossRef]
- Immenhauser, A.; Hillgärtner, H.; Sattler, U.; Bertotti, G.; Schoepfer, P.; Homewood, P.; Vahrenkamp, V.; Steuber, T.; Masse, J.-P.; Droste, H.; et al. Barremian-lower Aptian Qishn Formation, Haushi-Huqf area, Oman: A new outcrop analogue for the Kharaib/Shu’aiba reservoirs. Geoarabia 2004, 9, 153–194. [Google Scholar] [CrossRef]
- Ries, A.C.; Shackleton, R.M. Structures in the Huqf-Haushi Uplift, east Central Oman. Geol. Soc. Lond. Spec. Publ. 1990, 49, 653. [Google Scholar] [CrossRef]
- Rousseau, M.; Dromart, G.; Garcia, J.-P.; Atrops, F.; Guillocheau, F. Jurassic evolution of the Arabian carbonate platform edge in the central Oman mountains. J. Geol. Soc. Lond. 2005, 162, 349–362. [Google Scholar] [CrossRef]
- Searle, M.P.; Cherry, A.G.; Ali, M.Y.; Cooper, D.J.W. Tectonics of the Musandam Peninsula and northern Oman Mountains: From ophiolite obduction to continental collision. Geoarabia 2014, 19, 135–174. [Google Scholar] [CrossRef]
- van Buchem, F.S.; Pittet, B.; Hillgärtner, H.; Grötsch, J.; Al Mansouri, A.I.; Billing, I.M.; Droste, H.H.; Oterdoom, W.H.; van Steenwinkel, M. High-resolution sequence stratigraphic architecture of Barremian/Aptian carbonate systems in northern Oman and the United Arab Emirates (Kharaib and Shu’aiba formations). Geoarabia 2002, 7, 461–500. [Google Scholar] [CrossRef]
- Warburton, J.; Burnhill, T.; Graham, R.; Isaac, K. The evolution of the Oman Mountains foreland basin. Geol. Soc. Lond. Spec. Publ. 1990, 49, 419–427. [Google Scholar] [CrossRef]
- Montenat, C.; Barrier, P.; Soudet, H.J. Aptian faulting in the Haushi-Huqf (Oman) and the tectonic evolution of the southeast Arabian platform-margin. Geoarabia 2003, 8, 643–662. [Google Scholar] [CrossRef]
- Noufal, A.; Obaid, K.; Ali, M.Y. Tectonic Map of Abu Dhabi, UAE. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016. [Google Scholar]
- Alsharhan, A.S.; Nairn, A.E.M. Chapter 6—The End of the Paleozoic and the Early Mesozoic of the Middle East: The Absaroka Cycle. In Sedimentary Basins and Petroleum Geology of the Middle East; Alsharhan, A.S., Nairn, A.E.M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; pp. 161–233. [Google Scholar]
- Loosveld, R.J.H.; Bell, A.; Terken, J.J.M. The Tectonic Evolution of Interior Oman. Geoarabia 1996, 1, 28–51. [Google Scholar] [CrossRef]
- Cooper, D.J.W.; Ali, M.Y.; Searle, M.P. Structure of the northern Oman Mountains from the Semail Ophiolite to the Foreland Basin. Geol. Soc. Lond. Spec. Publ. 2014, 392, 129. [Google Scholar] [CrossRef]
- Sattler, U.T.E.; Immenhauser, A.; HillgÄRtner, H.; Esteban, M. Characterization, lateral variability and lateral extent of discontinuity surfaces on a Carbonate Platform (Barremian to Lower Aptian, Oman). Sedimentology 2005, 52, 339–361. [Google Scholar] [CrossRef]
- Beydoun, Z.R.; Bamahmoud, M.O.; Nani, A.S.O. The Qishn Formation, Yemen: Lithofacies and hydrocarbon habitat. Mar. Pet. Geol. 1993, 10, 364–372. [Google Scholar] [CrossRef]
- Alsharhan, A.S. Geology and reservoir characteristics of Lower Cretaceous Kharaib Formation in Zakum Field, Abu Dhabi, United Arab Emirates. Geol. Soc. Lond. Spec. Publ. 1990, 50, 299. [Google Scholar] [CrossRef]
- Barata, J.; Vahrenkamp, V.; Van Laer, P.J.; Swart, P.; Murray, S. A Regional Analysis of Clumped Isotope Geochemistry to Define the Timing of Creation of Micro-Porosity in a Lower Cretaceous Giant Reservoir. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 9–12 November 2015. [Google Scholar]
- Ehrenberg, S.N.; Zhang, J.; Gomes, J.S. Regional porosity variation in Thamama-B reservoirs of Abu Dhabi. Mar. Pet. Geol. 2020, 114, 104245. [Google Scholar] [CrossRef]
- Bechennec, F.; Le Metour, J.; Rabu, D.; Bourdillon-de-Grissac, C.; de Wever, P.; Beurrier, M.; Villey, M. The Hawasina Nappes: Stratigraphy, palaeogeography and structural evolution of a fragment of the south-Tethyan passive continental margin. Geol. Soc. Lond. Spec. Publ. 1990, 49, 213. [Google Scholar] [CrossRef]
- Searle, M.; Cox, J. Tectonic setting, origin, and obduction of the Oman ophiolite. GSA Bull. 1999, 111, 104–122. [Google Scholar] [CrossRef]
- Hanna, S.S. The Alpine deformation of the central Oman Mountains. Geol. Soc. Lond. Spec. Publ. 1990, 49, 341–359. [Google Scholar] [CrossRef]
- Vandeginste, V.; John, C.M.; van de Flierdt, T.; Cosgrove, J.W. Linking process, dimension, texture, and geochemistry in dolomite geobodies: A case study from Wadi Mistal (northern Oman). AAPG Bull. 2013, 97, 1181–1207. [Google Scholar] [CrossRef]
- Hönig, M.R.; John, C.M. Sedimentological and isotopic heterogeneities within a Jurassic carbonate ramp (UAE) and implications for reservoirs in the Middle East. Mar. Pet. Geol. 2015, 68, 240–257. [Google Scholar] [CrossRef]
- Ali, M.Y.; Aidarbayev, S.; Searle, M.P.; Watts, A.B. Subsidence History and Seismic Stratigraphy of the Western Musandam Peninsula, Oman–United Arab Emirates Mountains. Tectonics 2018, 37, 154–181. [Google Scholar] [CrossRef]
- Schütz, F.; Winterleitner, G.; Huenges, E. Geothermal exploration in a sedimentary basin: New continuous temperature data and physical rock properties from northern Oman. Geotherm. Energy 2018, 6, 5. [Google Scholar] [CrossRef]
- Sena, C.M.; John, C.M.; Jourdan, A.L.; Vandeginste, V.; Manning, C. Dolomitization of Lower Cretaceous Peritidal Carbonates by Modified Seawater: Constraints from Clumped Isotopic Paleothermometry, Elemental Chemistry, and Strontium Isotopes. J. Sediment. Res. 2014, 84, 552–566. [Google Scholar] [CrossRef]
- Ehrenberg, S.N.; Lokier, S.W.; Yaxin, L.; Chen, R. Depositional Cycles in a Lower Cretaceous Limestone Reservoir, Onshore Abu Dhabi, U.A.E. J. Sediment. Res. 2018, 88, 753–776. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ottinger, G.; Zinati, O.A.; Takayanagi, H.; Yamamoto, K.; Iryu, Y. Geochemical, petrographical, and petrophysical evaluations of a heterogeneous, stratiform dolomite from a Barremian oil field, offshore Abu Dhabi (United Arab Emirates). AAPG Bull. 2018, 102, 129–152. [Google Scholar] [CrossRef]
- Ehrenberg, S.N.; Morad, S.; Yaxin, L.; Chen, R. Stylolites and Porosity in A Lower Cretaceous Limestone Reservoir, Onshore Abu Dhabi, U.A.E. J. Sediment. Res. 2016, 86, 1228–1247. [Google Scholar] [CrossRef]
- Henry, D.G.; Watson, J.S.; John, C.M. Assessing and calibrating the ATR-FTIR approach as a carbonate rock characterization tool. Sediment. Geol. 2017, 347, 36–52. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Sibley, D.F. Direct physical evidence of dolomite recrystallization. Sedimentology 2014, 61, 1862–1882. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Thornton, B.P. The effect of temperature on stoichiometry, cation ordering, and reaction rate in high-temperature dolomitization experiments. Chem. Geol. 2017, 468, 32–41. [Google Scholar] [CrossRef]
- Royse, C.F.; Wadell, J.S.; Petersen, L.E. X-ray determination of calcite-dolomite; an evaluation. J. Sediment. Res. 1971, 41, 483–488. [Google Scholar] [CrossRef]
- Lumsden, D.N. Discrepancy between thin-section and X-ray estimates of dolomite in limestone. J. Sediment. Res. 1979, 49, 429–435. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Graf, D.L. Structural and compositional variations in some natural dolomites. J. Geol. 1958, 66, 678–693. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Gregg, J.M.; Bish, D.L.; Machel, H.G.; Fouke, B.W. Dolomite, very-high magnesium calcite, and microbes—Implications for the microbial model of dolomitization. In Characterization and Modeling of Carbonates—Mountjoy Symposium 1; Society for Sedimentary Geology: Tulsa, OK, USA, 2017; Volume 109, pp. 1–14. [Google Scholar] [CrossRef]
- Adlan, Q.; Davies, A.J.; John, C.M. Effects of oxygen plasma ashing treatment on carbonate clumped isotopes. Rapid Commun. Mass Spectrom. 2020, 34, e8802. [Google Scholar] [CrossRef]
- Eiler, J.M.; Schauble, E. 18O13C16O in Earth’s atmosphere. Geochim. Cosmochim. Acta 2004, 68, 4767–4777. [Google Scholar] [CrossRef]
- Huntington, K.W.; Eiler, J.M.; Affek, H.P.; Guo, W.; Bonifacie, M.; Yeung, L.Y.; Thiagarajan, N.; Passey, B.; Tripati, A.; Daeron, M.; et al. Methods and limitations of ‘clumped’ CO2 isotope (Delta(47)) analysis by gas-source isotope ratio mass spectrometry. J. Mass Spectrom. 2009, 44, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Eiler, J.M. Temperatures of aqueous alteration and evidence for methane generation on the parent bodies of the CM chondrites. Geochim. Cosmochim. Acta 2007, 71, 5565–5575. [Google Scholar] [CrossRef]
- Davies, A.J.; John, C.M. Reducing contamination parameters for clumped isotope analysis: The effect of lowering Porapak (TM) Q trap temperature to below −50 °C. Rapid Commun. Mass Spectrom. 2017, 31, 1313–1323. [Google Scholar] [CrossRef]
- John, C.M.; Bowen, D. Community software for challenging isotope analysis: First applications of “Easotope” to clumped isotopes. Rapid Commun. Mass Spectrom. 2016, 30, 2285–2300. [Google Scholar] [CrossRef]
- Bernasconi, S.M.; Hu, B.; Wacker, U.; Fiebig, J.; Breitenbach, S.F.M.; Rutz, T. Background effects on Faraday collectors in gas-source mass spectrometry and implications for clumped isotope measurements. Rapid Commun. Mass Spectrom. 2013, 27, 603–612. [Google Scholar] [CrossRef]
- Bernasconi, S.M.; Daëron, M.; Bergmann, K.D.; Bonifacie, M.; Meckler, A.N.; Affek, H.P.; Anderson, N.; Bajnai, D.; Barkan, E.; Beverly, E.; et al. InterCarb: A Community Effort to Improve Interlaboratory Standardization of the Carbonate Clumped Isotope Thermometer Using Carbonate Standards. Geochem. Geophys. Geosystems 2021, 22, e2020GC009588. [Google Scholar] [CrossRef]
- Muller, I.A.; Violay, M.E.S.; Storck, J.C.; Fernandez, A.; van Dijk, J.; Madonna, C.; Bernasconi, S.M. Clumped isotope fractionation during phosphoric acid digestion of carbonates at 70 °C. Chem. Geol. 2017, 449, 1–14. [Google Scholar] [CrossRef]
- Anderson, N.T.; Kelson, J.R.; Kele, S.; Daeron, M.; Bonifacie, M.; Horita, J.; Mackey, T.J.; John, C.M.; Kluge, T.; Petschnig, P.; et al. A Unified Clumped Isotope Thermometer Calibration (0.5–1100 °C) Using Carbonate-Based Standardization. Geophys. Res. Lett. 2021, 48, e2020GL092069. [Google Scholar] [CrossRef]
- Kim, S.T.; Mucci, A.; Taylor, B.E. Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75 °C: Revisited. Chem. Geol. 2007, 246, 135–146. [Google Scholar] [CrossRef]
- Rosenbaum, J.; Sheppard, S.M.F. An isotopic study of siderites, dolomites and ankerites at high temperatures. Geochim. Cosmochim. Acta 1986, 50, 1147–1150. [Google Scholar] [CrossRef]
- O’Brien, C.L.; Robinson, S.A.; Pancost, R.D.; Sinninghe Damsté, J.S.; Schouten, S.; Lunt, D.J.; Alsenz, H.; Bornemann, A.; Bottini, C.; Brassell, S.C.; et al. Cretaceous sea-surface temperature evolution: Constraints from TEX86 and planktonic foraminiferal oxygen isotopes. Earth-Sci. Rev. 2017, 172, 224–247. [Google Scholar] [CrossRef]
- Hemingway, J.D. Isotopylog: Open-Source Tools for Clumped Isotope Kinetic Data Analysis. 2020. Available online: http://pypi.python.org/pypi/isotopylog (accessed on 25 August 2021).
- Kim, S.-T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Patel, A.; John, C.; Veillard, C. Identification of Meteoric Diagenetic Transformation of Fine-Grained Carbonates Using Clumped Isotopes. In Proceedings of the Goldschmidt 2020, Honolulu, HI, USA, 21–26 June 2020; p. 2042. [Google Scholar]
- Flügel, E. Diagenesis, Porosity, and Dolomitization. In Microfacies of Carbonate Rocks: Analysis, Interpretation and Application; Flügel, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 267–338. [Google Scholar]
- Sirat, M.; Al-Aasm, I.S.; Morad, S.; Aldahan, A.; Al-Jallad, O.; Ceriani, A.; Morad, D.; Mansurbeg, H.; Al-Suwaidi, A. Saddle dolomite and calcite cements as records of fluid flow during basin evolution: Paleogene carbonates, United Arab Emirates. Mar. Pet. Geol. 2016, 74, 71–91. [Google Scholar] [CrossRef]
- Hashim, M.S.; Kaczmarek, S.E. Experimental stabilization of carbonate sediments to calcite: Insights into the depositional and diagenetic controls on calcite microcrystal texture. Earth Planet. Sci. Lett. 2020, 538, 116235. [Google Scholar] [CrossRef]
- Lambert, L.; Durlet, C.; Loreau, J.-P.; Marnier, G. Burial dissolution of micrite in Middle East carbonate reservoirs (Jurassic–Cretaceous): Keys for recognition and timing. Mar. Pet. Geol. 2006, 23, 79–92. [Google Scholar] [CrossRef]
- Deville de Periere, M.; Durlet, C.; Vennin, E.; Lambert, L.; Bourillot, R.; Caline, B.; Poli, E. Morphometry of micrite particles in cretaceous microporous limestones of the Middle East: Influence on reservoir properties. Mar. Pet. Geol. 2011, 28, 1727–1750. [Google Scholar] [CrossRef]
- Alsuwaidi, M.; Mohamed, A.A.I.; Mansurbeg, H.; Morad, S.; Alsuwaidi, A.; Al-Shalabi, E.W.; Gomes, J.; Al-Ramadan, K.; Mohammed, I.Q.; Farouk, S. Depositional and diagenetic controls on reservoir quality of microporous basinal lime mudstones (Aptian), United Arab Emirates. Sediment. Geol. 2021, 420, 105925. [Google Scholar] [CrossRef]
- Kırmacı, M.Z. Origin of dolomite in the Late Jurassic platform carbonates, Bolkar Mountains, Central Taurides, Turkey: Petrographic and geochemical evidence. Geochemistry 2013, 73, 383–398. [Google Scholar] [CrossRef]
- Nielsen, P.; Swennen, R.; Keppens, E. Multiple-step recrystallization within massive ancient dolomite units: An example from the Dinantian of Belgium. Sedimentology 1994, 41, 567–584. [Google Scholar] [CrossRef]
- Reinhold, C. Multiple episodes of dolomitization and dolomite recrystallization during shallow burial in Upper Jurassic shelf carbonates: Eastern Swabian Alb, southern Germany. Sediment. Geol. 1998, 121, 71–95. [Google Scholar] [CrossRef]
- Scholle, P.A.; Ulmer-Scholle, D.S. Carbonate Diagenesis: Meso- and Telogenetic Burial Diagenesis. In A Color Guide to the Petrography of Carbonate Rocks: Grains, Textures, Porosity, Diagenesis; American Association of Petroleum Geologists: Tulsa, OK, USA, 2003; Volume 77. [Google Scholar] [CrossRef]
- Al-Mojel, A.; Dera, G.; Razin, P.; Le Nindre, Y.-M. Carbon and oxygen isotope stratigraphy of Jurassic platform carbonates from Saudi Arabia: Implications for diagenesis, correlations and global paleoenvironmental changes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 511, 388–402. [Google Scholar] [CrossRef]
- Nelson, C.S.; Smith, A.M. Stable oxygen and carbon isotope compositional fields for skeletal and diagenetic components in New Zealand Cenozoic nontropical carbonate sediments and limestones: A synthesis and review. N. Z. J. Geol. Geophys. 1996, 39, 93–107. [Google Scholar] [CrossRef]
- Moore, C.H. Chapter 9—Burial Diagenetic Environment. In Developments in Sedimentology; Moore, C.H., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 46, pp. 237–284. [Google Scholar]
- Epstein, S.; Buchsbaum, R.; Lowenstam, H.A.; Urey, H.C. Revised Carbonate-Water Isotopic Temperature Scale. GSA Bull. 1953, 64, 1315–1326. [Google Scholar] [CrossRef]
- Friedman, I.; O’Neil, J.R. Compilation of Stable Isotope Fractionation Factors of Geochemical Interest; US Government Printing Office: Washington, DC, USA, 1977; Volume 440.
- Immenhauser, A. On the delimitation of the carbonate burial realm. Depos. Rec. 2022, 8, 524–574. [Google Scholar] [CrossRef]
- Moore, C.H.; Wade, W.J. Chapter 10—Burial Diagenetic Environment. In Developments in Sedimentology; Moore, C.H., Wade, W.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 67, pp. 239–284. [Google Scholar]
- Choquette, P.W.; James, N.; McIlreath, I.; Morrow, D. Limestones—The burial diagenetic environment. Geosci. Can. Repr. Ser. 1990, 4, 75–111. [Google Scholar]
- Moore, C.H.; Druckman, Y. Burial diagenesis and porosity evolution, upper Jurassic Smackover, Arkansas and Louisiana. AAPG Bull. 1981, 65, 597–628. [Google Scholar]
- Anderson, T.F.; Arthur, M.A. Stable isotopes of oxygen and carbon and their application to sedimentologic and paleoenvironmental problems. In Stable Isotopes in Sedimentary Geology; SEPM: Claremore, OK, USA, 1983. [Google Scholar]
- O’Neil, J.R.; Clayton, R.N.; Mayeda, T.K. Oxygen isotope fractionation in divalent metal carbonates. J. Chem. Phys. 1969, 51, 5547–5558. [Google Scholar] [CrossRef]
- Onuma, N.; Clayton, R.N.; Mayeda, T.K. Oxygen Isotope Fractionation between Minerals and an Estimate of the Temperature of Formation. Science 1970, 167, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.R. Electrostatic characterization of oxygen sites in minerals. Geochim. Cosmochim. Acta 1989, 53, 1101–1110. [Google Scholar] [CrossRef]
- Horita, J. Oxygen and carbon isotope fractionation in the system dolomite–water–CO2 to elevated temperatures. Geochim. Cosmochim. Acta 2014, 129, 111–124. [Google Scholar] [CrossRef]
- Lu, C.; Zou, H.; Wang, G.; Cong, F.; Quan, Y.; Swart, P.K. Clumped isotopes of paired dolomite and calcite constraining alteration histories of ancient carbonate successions. Chem. Geol. 2023, 617, 121264. [Google Scholar] [CrossRef]
- Swart, P.K.; Melim, L.A. The Origin of Dolomites in Tertiary Sediments from the Margin of Great Bahama Bank. J. Sediment. Res. 2000, 70, 738–748. [Google Scholar] [CrossRef]
- Roberts, L.R.; Holmes, J.A.; Leng, M.J.; Sloane, H.J.; Horne, D.J. Effects of cleaning methods upon preservation of stable isotopes and trace elements in shells of Cyprideis torosa (Crustacea, Ostracoda): Implications for palaeoenvironmental reconstruction. Quat. Sci. Rev. 2018, 189, 197–209. [Google Scholar] [CrossRef]
- Reeder, R.J. Constraints on Cation Order in Calcium-rich Sedimentary Dolomite. Aquat. Geochem. 2000, 6, 213–226. [Google Scholar] [CrossRef]
- Jones, B.; Luth, R.W.; MacNeil, A.J. Powder X-Ray Diffraction Analysis of Homogeneous and Heterogeneous Sedimentary Dolostones. J. Sediment. Res. 2001, 71, 790–799. [Google Scholar] [CrossRef]
- Ryan, B.H.; Kaczmarek, S.E.; Rivers, J.M.; Manche, C.J. Extensive recrystallization of Cenozoic dolomite during shallow burial: A case study from the Palaeocene–Eocene Umm er Radhuma formation and a global meta-analysis. Sedimentology 2022, 69, 2053–2079. [Google Scholar] [CrossRef]
- Reeder, R.J.; Wenk, H.-R. Structure refinements of some thermally disordered dolomites. Am. Miner. 1983, 68, 769–776. [Google Scholar]
- Lumsden, D.N.; Chimahusky, J.S. Relationship between Dolomite Nonstoichiometry and Carbonate Facies Parameters. In Concepts and Models of Dolomitization; SEPM: Claremore, OK, USA, 1980; Volume 28. [Google Scholar] [CrossRef]
- Manche, C.J.; Kaczmarek, S.E. A global study of dolomite stoichiometry and cation ordering through the Phanerozoic. J. Sediment. Res. 2021, 91, 520–546. [Google Scholar] [CrossRef]
- Eiler, J.M. Paleoclimate reconstruction using carbonate clumped isotope thermometry. Quat. Sci. Rev. 2011, 30, 3575–3588. [Google Scholar] [CrossRef]
- Ferry, J.M.; Passey, B.H.; Vasconcelos, C.; Eiler, J.M. Formation of dolomite at 40-80 degrees C in the Latemar carbonate buildup, Dolomites, Italy, from clumped isotope thermometry. Geology 2011, 39, 571–574. [Google Scholar] [CrossRef]
- McKenzie, D. Some remarks on the development of sedimentary basins. Earth Planet. Sci. Lett. 1978, 40, 25–32. [Google Scholar] [CrossRef]
- Waples, D.W. A New Model for Heat Flow in Extensional Basins: Radiogenic Heat, Asthenospheric Heat, and the McKenzie Model. Nat. Resour. Res. 2001, 10, 227–238. [Google Scholar] [CrossRef]
- Morgan, P. Constraints on Rift Thermal Processes from Heat Flow and Uplift. In Developments in Geotectonics; Morgan, P., Baker, B.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 19, pp. 277–298. [Google Scholar]
- Wheildon, J.; Morgan, P.; Williamson, K.H.; Evans, T.R.; Swanberg, C.A. Heat flow in the Kenya rift zone. Tectonophysics 1994, 236, 131–149. [Google Scholar] [CrossRef]
- Hantschel, T.; Kauerauf, A.I. Heat Flow Analysis. In Fundamentals of Basin and Petroleum Systems Modeling; Springer: Berlin/Heidelberg, Germany, 2009; pp. 103–150. [Google Scholar]
- Saddiqi, O.; Michard, A.; Goffe, B.; Poupeau, G.; Oberhänsli, R. Fission-track thermochronology of the Oman Mountains continental windows, and current problems of tectonic interpretation. Bull. Soc. Géol. Fr. 2006, 177, 127–134. [Google Scholar] [CrossRef]
- Grobe, A.; Urai, J.L.; Littke, R.; Lünsdorf, N.K. Hydrocarbon generation and migration under a large overthrust: The carbonate platform under the Semail Ophiolite, Jebel Akhdar, Oman. Int. J. Coal Geol. 2016, 168, 3–19. [Google Scholar] [CrossRef]
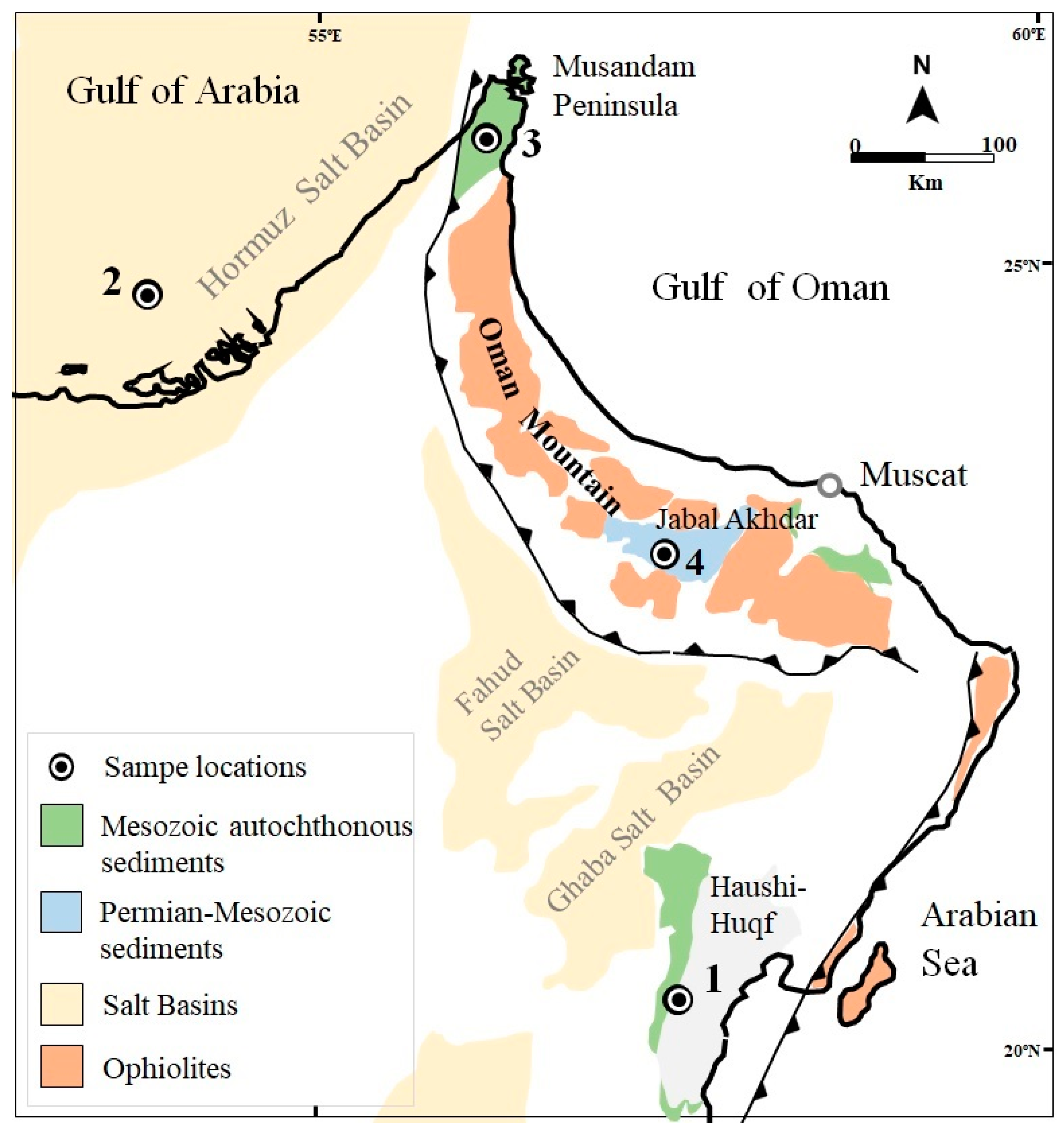

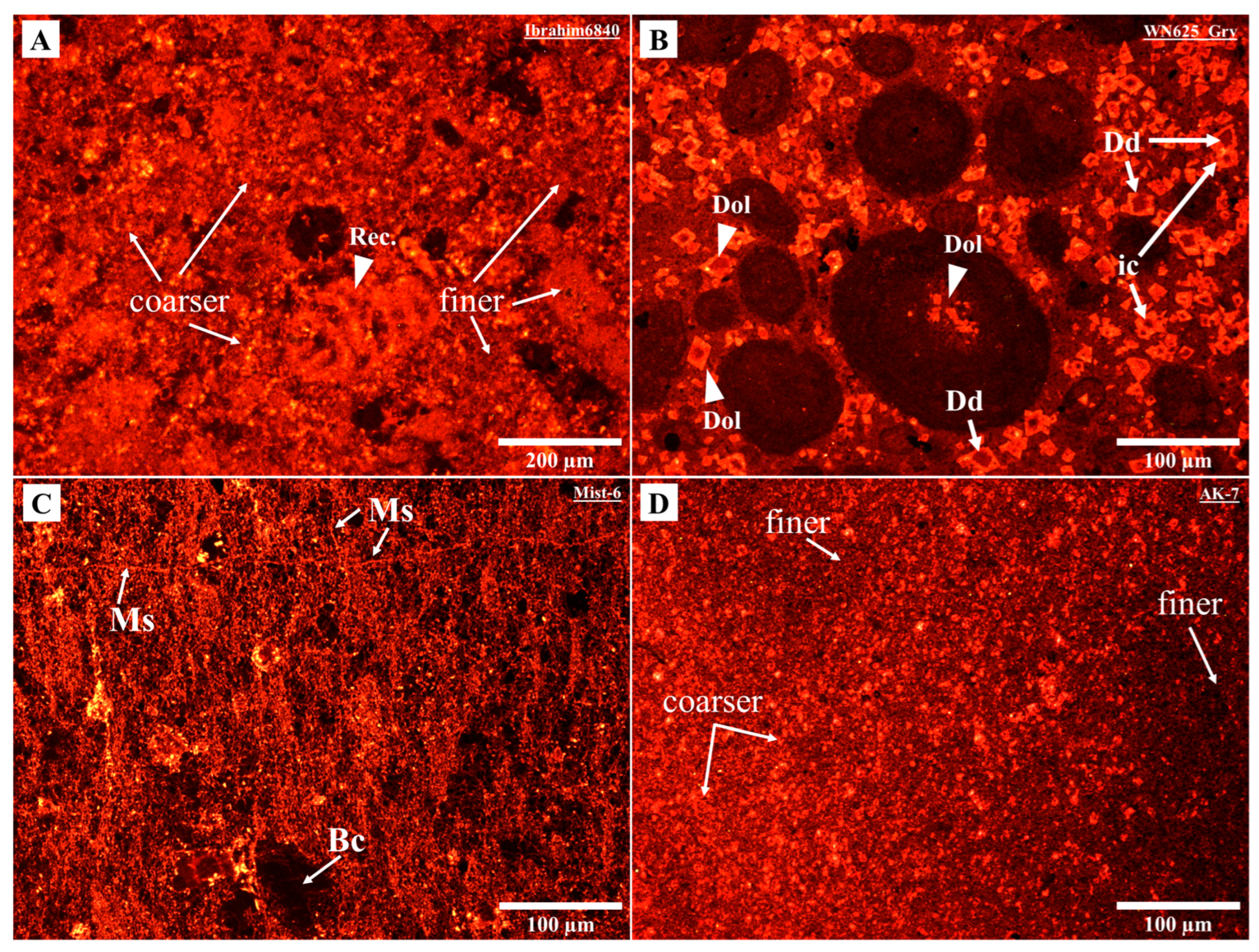
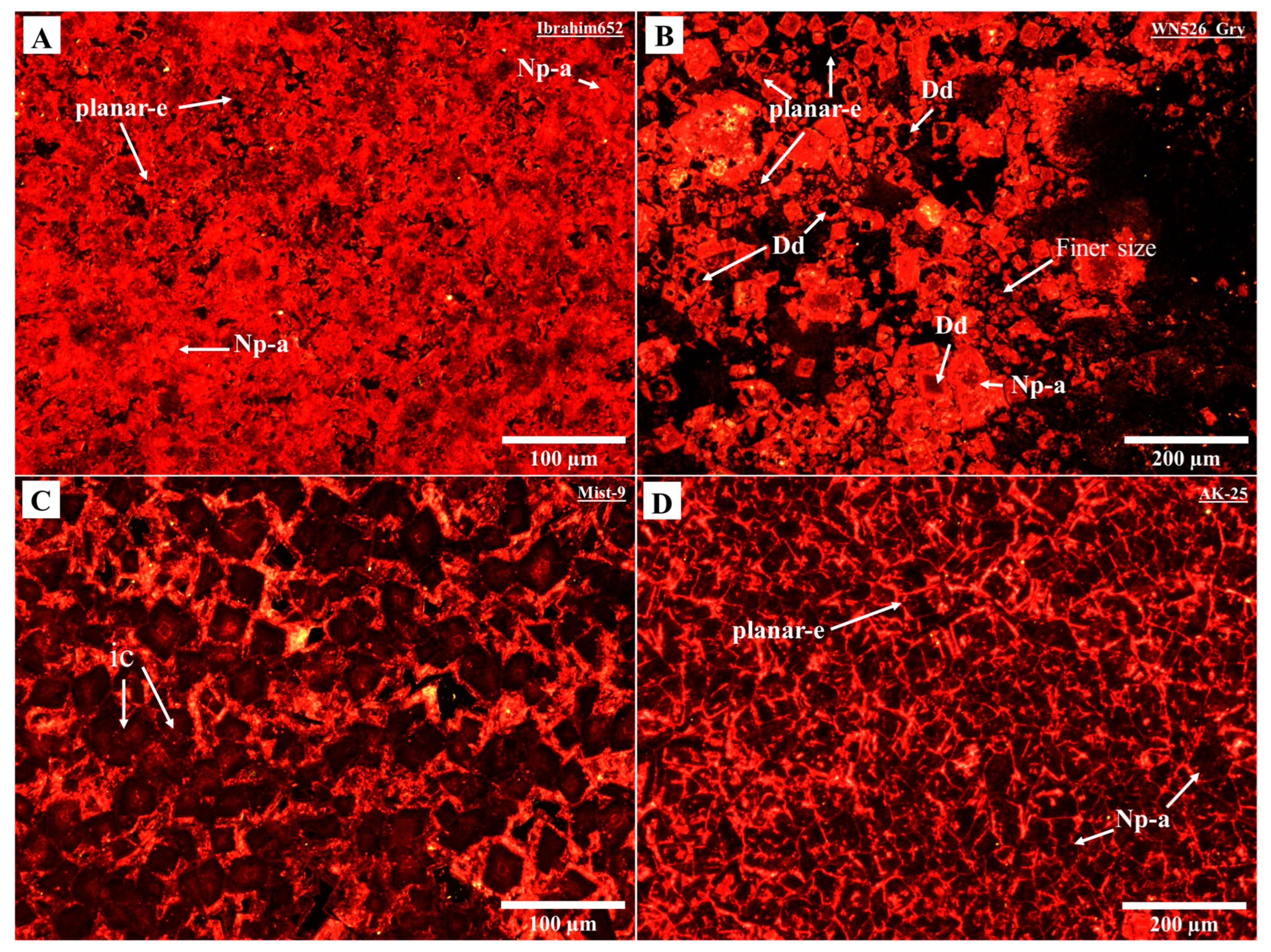


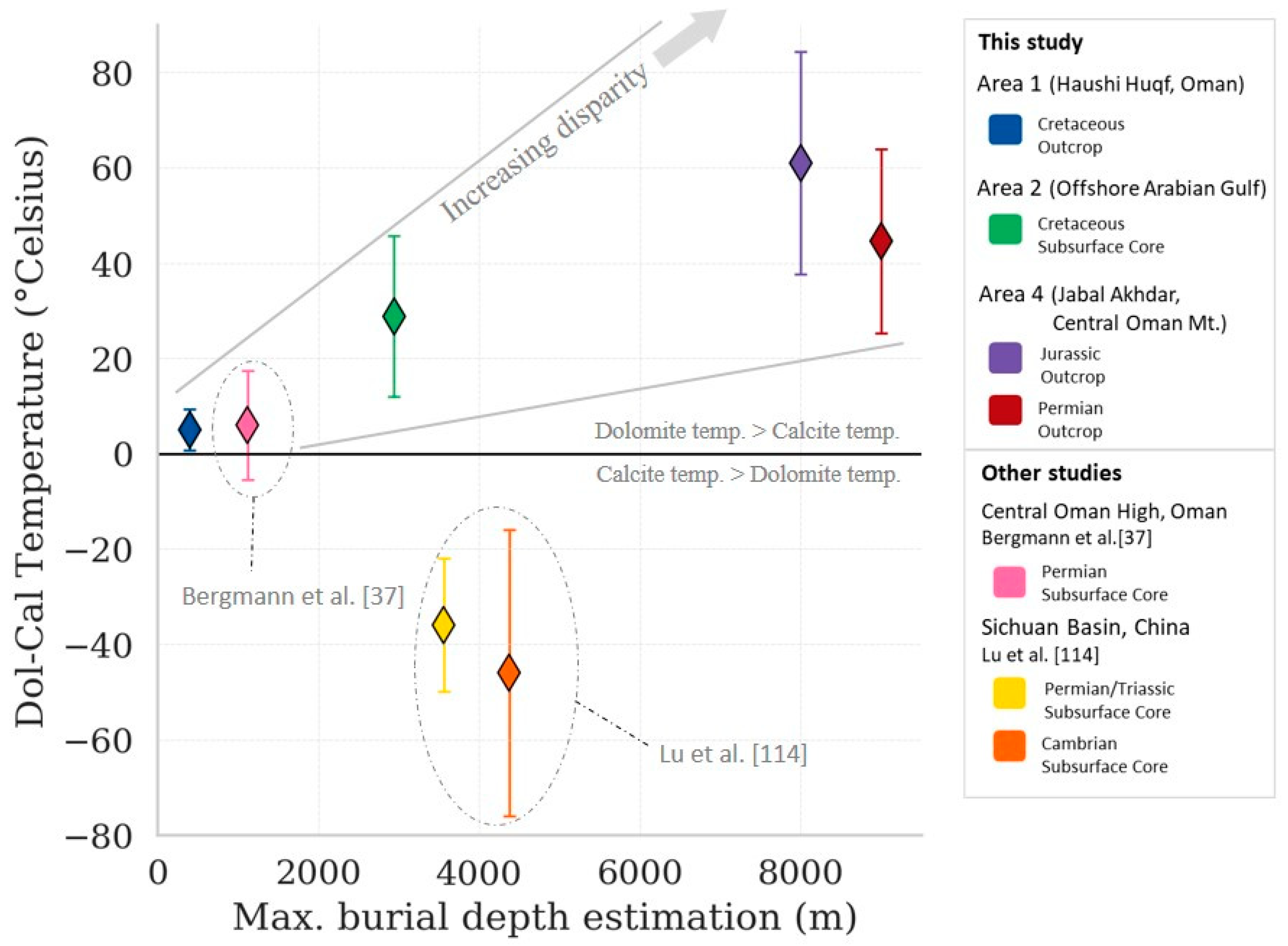
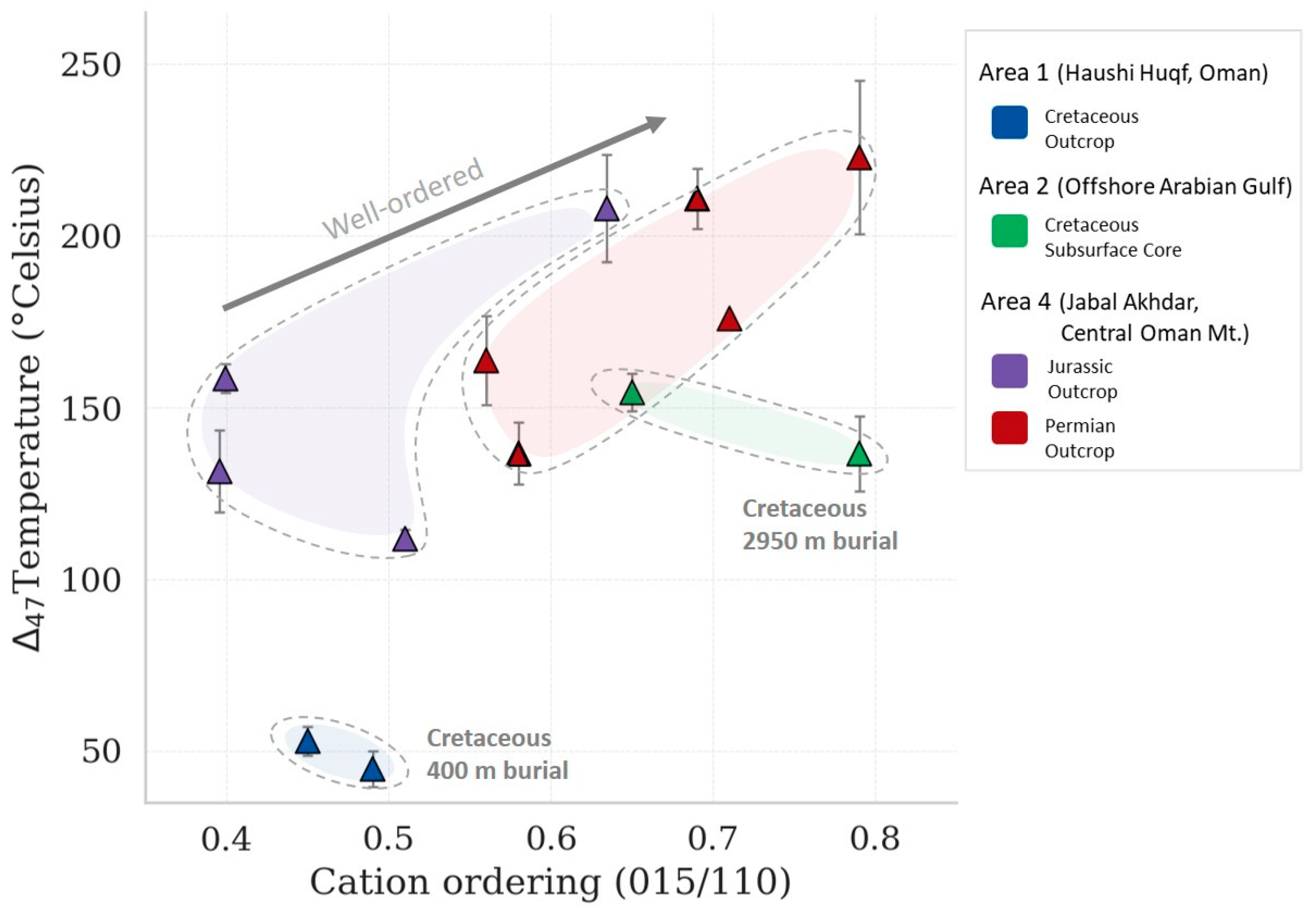
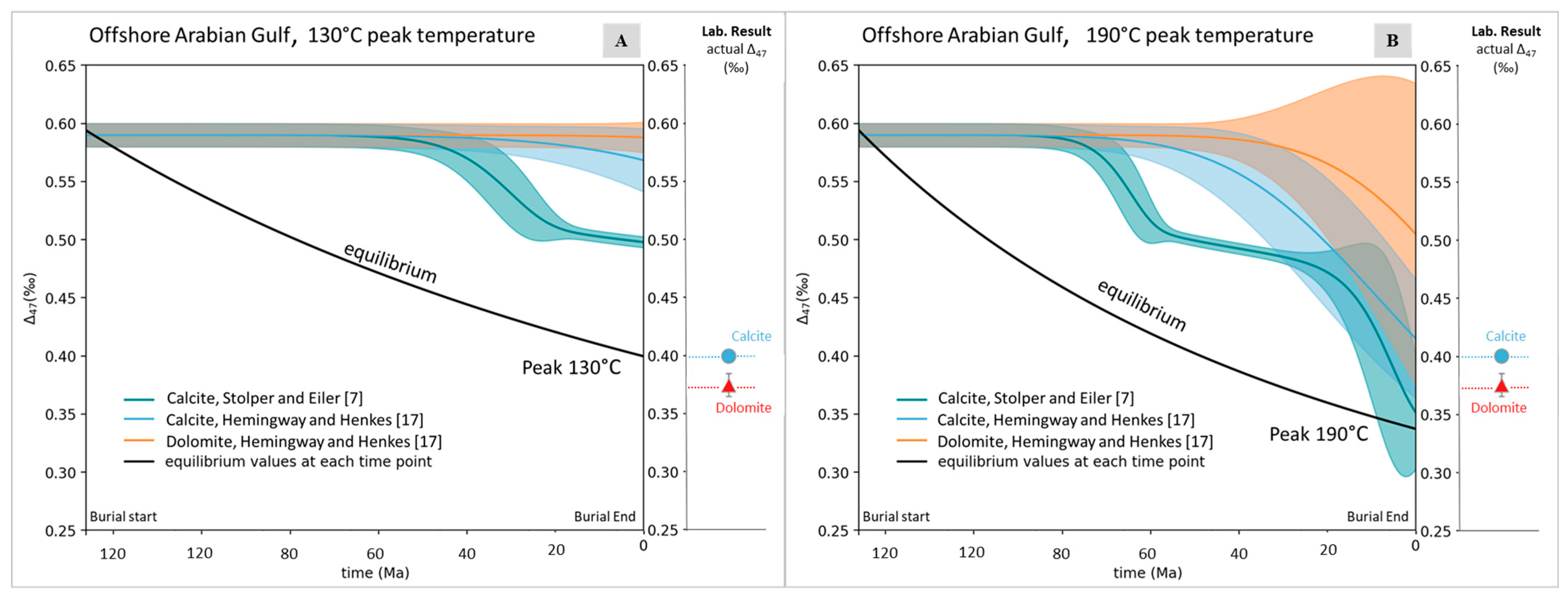

| Area | Formation or Group | Age of Interval | Maximum Burial Depth (a) (m) | Total Sample | Temp. Estimation (b) (°C) | Specimen |
|---|---|---|---|---|---|---|
| 1. Haushi-Huqf, Oman | Qishn Fm. | Cretaceous | 400 | 3 | 47 | Outcrop |
| 2. Offshore Arabian Gulf | Kharaib Fm. | Cretaceous | 2943–2950 | 8 | 130 | Core |
| 3. Wadi Naqab, Musandam Peninsula | Musandam II | Jurassic | 5100 | 7 | 168 | Outcrop |
| 4. Jabal Akhdar, Central Oman Mt. | Sahtan Gr. | Jurassic | 8000 | 6 | 169 | Outcrop |
| 4. Jabal Akhdar, Central Oman Mt. | Saiq Fm. | Permian | 9000 | 16 | 187 | Outcrop |
| Sample Information | Mineralogy-FTIR (a) | Depth (b) (m) | n | δ13C (‰, VPDB) | δ13C SE (‰) | δ18O (‰, VPDB) | δ18O SE (‰) | δ18O fl. (c) (‰, SMOW) | δ18O fl. SE (‰) | Δ47 (‰, I-CDES) | Δ47 SE (‰) | Δ47 Temp. (d) (°C) | Δ47 Temp. SE (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area 1 Haushi-Huqf outcrop, Oman | |||||||||||||
| Cretaceous | |||||||||||||
| SGF37 (e) | Calcite | 400 | 3 | 1.34 | 0.04 | −4.87 | 0.02 | 2.08 | 0.36 | 0.535 | 0.008 | 47 | 2 |
| E37 | Dolomite | 400 | 3 | 1.70 | 0.01 | −5.78 | 0.04 | 2.00 | 0.69 | 0.522 | 0.009 | 53 | 2 |
| I5 | Dolomite | 400 | 3 | −0.04 | 0.03 | −0.31 | 0.13 | 6.22 | 0.97 | 0.541 | 0.012 | 45 | 2 |
| Area 2 offshore, subsurface core, Arabian Gulf | |||||||||||||
| Cretaceous | |||||||||||||
| Z94708-m (f) | Calcite | 2886 | 3 | 3.14 | 0.09 | −7.04 | 0.18 | 4.17 | 0.86 | 0.478 | 0.012 | 74 | 7 |
| Z94708-sk (f) | Calcite | 2886 | 3 | 3.09 | 0.10 | −6.83 | 0.05 | 3.11 | 0.56 | 0.494 | 0.007 | 66 | 4 |
| Ibrahim484M | Calcite | 2950 | 4 | 3.40 | 0.20 | −6.36 | 0.05 | 4.09 | 0.57 | 0.488 | 0.008 | 69 | 4 |
| Ibrahim484R | Calcite | 2950 | 3 | 3.43 | 0.03 | −7.11 | 0.07 | 6.24 | 0.52 | 0.452 | 0.005 | 89 | 3 |
| Ibrahim6840 | Calcite | 2950 | 4 | 2.92 | 0.04 | −4.86 | 0.06 | 4.39 | 0.40 | 0.504 | 0.006 | 61 | 3 |
| Ibrahim708S | Calcite | 2950 | 3 | 3.23 | 0.03 | −8.37 | 0.08 | 9.43 | 0.70 | 0.400 | 0.007 | 125 | 6 |
| Ibrahim652 | Dolomite | 2943 | 5 | 4.61 | 0.16 | −6.5 | 0.09 | 12.63 | 1.13 | 0.387 | 0.013 | 136 | 11 |
| Ibrahim653 | Dolomite | 2943 | 3 | 4.85 | 0.01 | −6.33 | 0.05 | 14.62 | 0.60 | 0.368 | 0.005 | 154 | 5 |
| Area 3 Wadi Naqab outcrop, Musandam Peninsula | |||||||||||||
| Jurassic | |||||||||||||
| WN526 | Calcite | 5100 | 3 | −3.97 | 0.02 | −2.90 | 0.06 | 8.37 | 0.44 | 0.479 | 0.005 | 74 | 3 |
| WN453_Gry | Calcite | 5100 | 4 | −0.27 | 0.02 | −3.67 | 0.02 | 10.71 | 1.61 | 0.441 | 0.019 | 96 | 13 |
| WN627 | Calcite | 5100 | 3 | 2.01 | 0.05 | −3.03 | 0.06 | 16.68 | 1.27 | 0.382 | 0.013 | 141 | 12 |
| WN625_Gry | Calcite | 5100 | 4 | 2.32 | 0.16 | −2.74 | 0.13 | 20.36 | 1.25 | 0.348 | 0.013 | 176 | 15 |
| WN625_Red | Dolomite | 5100 | 5 | 2.30 | 0.13 | −3.99 | 0.22 | 16.55 | 1.20 | 0.373 | 0.012 | 150 | 11 |
| WN526_Gry | Dolomite | 5100 | 4 | 0.09 | 0.04 | −3.17 | 0.11 | 19.81 | 0.94 | 0.349 | 0.008 | 175 | 10 |
| WN453_Wsk | Calcite | 5100 | 3 | 1.75 | 0.03 | −4.24 | 0.07 | 19.33 | 1.26 | 0.343 | 0.012 | 182 | 15 |
| Area 4 Jabal Akhdar outcrop, Central Oman Mountain | |||||||||||||
| Jurassic | |||||||||||||
| Mist-2 | Calcite | 8000 | 3 | 0.05 | 0.00 | −8.14 | 0.03 | 9.82 | 1.10 | 0.399 | 0.012 | 126 | 10 |
| Mist-6 | Calcite | 8000 | 3 | 1.27 | 0.01 | −4.50 | 0.02 | 15.74 | 1.30 | 0.376 | 0.014 | 147 | 13 |
| Mist-5 | Dolomite | 8000 | 3 | 0.52 | 0.03 | −5.93 | 0.09 | 10.37 | 0.43 | 0.418 | 0.004 | 112 | 3 |
| Mist-9 | Dolomite | 8000 | 3 | 1.73 | 0.04 | −3.38 | 0.01 | 15.27 | 1.31 | 0.393 | 0.014 | 131 | 12 |
| Mist-31 | Dolomite | 8000 | 3 | 2.03 | 0.01 | −5.34 | 0.05 | 16.05 | 0.45 | 0.364 | 0.004 | 158 | 4 |
| Mist-8 | Dolomite | 8000 | 3 | 1.69 | 0.02 | −7.35 | 0.11 | 18.28 | 1.28 | 0.323 | 0.011 | 208 | 16 |
| Permian | |||||||||||||
| MPA11A | Calcite | 9000 | 5 | 2.41 | 0.02 | −6.13 | 0.07 | 13.48 | 1.04 | 0.382 | 0.011 | 141 | 10 |
| MPA11B | Calcite | 9000 | 4 | 3.36 | 0.31 | −7.49 | 0.51 | 6.69 | 3.27 | 0.442 | 0.033 | 95 | 22 |
| WSNE2_C | Calcite | 9000 | 4 | 2.00 | 0.06 | −6.40 | 0.16 | 13.02 | 1.41 | 0.384 | 0.014 | 139 | 12 |
| AK-13 | Calcite | 9000 | 3 | 6.04 | 0.09 | −3.35 | 0.10 | 19.94 | 0.05 | 0.346 | 0.001 | 178 | 1 |
| AK-7 | Calcite | 9000 | 3 | 5.46 | 0.01 | −2.82 | 0.02 | 19.65 | 0.76 | 0.354 | 0.013 | 169 | 9 |
| MPA35 | Dolomite | 9000 | 3 | 6.38 | 0.03 | −0.58 | 0.05 | 18.73 | 0.97 | 0.387 | 0.010 | 136 | 9 |
| AK-23 | Dolomite | 9000 | 3 | 6.08 | 0.01 | −1.06 | 0.01 | 24.99 | 0.67 | 0.321 | 0.006 | 211 | 9 |
| AK-24 | Dolomite | 9000 | 3 | 5.92 | 0.05 | −1.05 | 0.05 | 22.10 | 0.04 | 0.348 | 0 | 176 | 1 |
| AK-25 | Dolomite | 9000 | 3 | 6.02 | 0.01 | −0.02 | 0.04 | 26.96 | 1.65 | 0.313 | 0.014 | 223 | 22 |
| AK-27 | Dolomite | 9000 | 3 | 3.21 | 0.03 | 0.38 | 0.09 | 22.45 | 1.29 | 0.359 | 0.012 | 164 | 13 |
| Sample Information | Depth (a) (m) | G.Geo. Temp. (b) (°C) | Δ47 Temp. (c) (°C) | Δ47 Temp. (d) (°C) |Dol. − Cal.| | S.E. Temp. |Dol. − Cal.| | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | S.E. | Median | Min | Max | |||||
| This Study | ||||||||||
| Haushi-Huqf outcrop, Oman (Area 1) | ||||||||||
| Cretaceous | 5 | 4 | ||||||||
| Calcite (e) | 400 | 47.5 | 24 | 34 | 4 | 35 | 21 | 47 | ||
| Dolomite (f) | 400 | 47.5 | 5 | 47 | 2 | 45 | 44 | 53 | ||
| Offshore, subsurface core, Arabian Gulf (Area 2) | ||||||||||
| Cretaceous | 29 | 17 | ||||||||
| Calcite | 2950 | 130.0 | 4 | 86 | 14 | 79 | 61 | 126 | ||
| Dolomite | 2943 | 130.0 | 2 | 145 | 9 | 145 | 136 | 154 | ||
| Wadi Naqab outcrop, Musandam Peninsula (Area 3) | ||||||||||
| Jurassic | −1 | 26 | ||||||||
| Calcite | 5100 | 168.4 | 4 | 122 | 23 | 118 | 74 | 176 | ||
| Dolomite | 5100 | 168.4 | 2 | 162 | 13 | 162 | 149 | 175 | ||
| Jabal Akhdar outcrop, Central Oman Mountain (Area 4) | ||||||||||
| Jurassic | 61 | 23 | ||||||||
| Calcite | 8000 | 169.3 | 2 | 137 | 11 | 137 | 126 | 147 | ||
| Dolomite | 8000 | 169.3 | 4 | 152 | 21 | 145 | 112 | 208 | ||
| Permian | 45 | 19 | ||||||||
| Calcite | 9000 | 187 | 5 | 145 | 14 | 141 | 95 | 178 | ||
| Dolomite | 9000 | 187 | 5 | 181 | 13 | 177 | 136 | 223 | ||
| Other studies | ||||||||||
| Central Oman High, subsurface core, Oman (Bergmann et al. [37]) | ||||||||||
| Permian | 6 | 11 | ||||||||
| Calcite | 1115 | 55.3 | 18 | 36 | 3 | 35 | 30 | 43 | ||
| Dolomite | 1199 | 56.3 | 3 | 36 | 11 | 33 | 28 | 49 | ||
| Leached samples from Mx13 well in Sichuan Basin, China (Lu et al. [114]) | ||||||||||
| Permian/Triassic | −36 | 14 | ||||||||
| Calcite | 3556–3899 | ~113 | 2 | 119 | 11 | 119 | 117 | 120 | ||
| Dolomite | 3556–3899 | ~113 | 2 | 79 | 11 | 79 | 74 | 83 | ||
| Cambrian | −46 | 30 | ||||||||
| Calcite | 4370–4601 | ~140 | 3 | 130 | 21 | 134 | 121 | 136 | ||
| Dolomite | 4370–4601 | ~140 | 3 | 81 | 21 | 83 | 71 | 90 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adlan, Q.; Kaczmarek, S.E.; John, C.M. Clumped Isotope Reordering and Kinetic Differences in Co-Hosted Calcite and Dolomite Minerals throughout Burial Diagenesis and Exhumation. Minerals 2023, 13, 1466. https://doi.org/10.3390/min13121466
Adlan Q, Kaczmarek SE, John CM. Clumped Isotope Reordering and Kinetic Differences in Co-Hosted Calcite and Dolomite Minerals throughout Burial Diagenesis and Exhumation. Minerals. 2023; 13(12):1466. https://doi.org/10.3390/min13121466
Chicago/Turabian StyleAdlan, Qi, Stephen E. Kaczmarek, and Cédric M. John. 2023. "Clumped Isotope Reordering and Kinetic Differences in Co-Hosted Calcite and Dolomite Minerals throughout Burial Diagenesis and Exhumation" Minerals 13, no. 12: 1466. https://doi.org/10.3390/min13121466
APA StyleAdlan, Q., Kaczmarek, S. E., & John, C. M. (2023). Clumped Isotope Reordering and Kinetic Differences in Co-Hosted Calcite and Dolomite Minerals throughout Burial Diagenesis and Exhumation. Minerals, 13(12), 1466. https://doi.org/10.3390/min13121466









