Abstract
In this study, SDS is used to enhance the sulfuric acid leaching of chalcopyrite in aqueous and isopropanol media. The presence of SDS increased copper extraction into the solution in both solvents. However, it was the “isopropanol–sulfuric acid–SDS” system that proved to be particularly effective for copper recovery from chalcopyrite. The positive effect of SDS can be attributed to the reduction in the solution’s surface tension and the enhancement of mineral wetting. Additionally, the presence of SDS as a surfactant induces changes in the adsorption patterns of formed sulfur species on the mineral surface. SDS competes with sulfur for occupancy on the surface binding sites. This competitive interaction has the potential to diminish the formation of a substantial sulfur layer on the mineral surface. Under optimal conditions (isopropanol media, 2 M H2SO4, 65 °C, 120 min, 0.6 g/L SDS), copper recovery into the solution was 83%, and this is a considerable achievement for chalcopyrite leaching at ambient pressure in the absence of strong oxidizers.
1. Introduction
The extraction of copper from its primary sources has long been a linchpin of economic development, as it serves as a conduit for conducting electricity, a building block for infrastructure, and a key ingredient in countless consumer products. While the pyrometallurgical method has historically stood as the primary means of processing copper concentrate, it is imperative to acknowledge its intrinsic limitations. The intense heat required for metallurgical processing not only demands significant energy inputs but also contributes to substantial greenhouse gas emissions, thus exacerbating environmental concerns in an era defined by sustainability imperatives [1,2,3]. Moreover, the smelting process associated with pyrometallurgy often leads to the release of noxious sulfur dioxide fumes, contributing to air pollution and necessitating extensive and costly gas treatment systems. Consequently, significant attention has been directed toward the hydrometallurgical processing of copper concentrate for the past half-century, and this remains an ongoing and pertinent topic [4,5].
The target components of copper concentrates are sulfide minerals, predominantly chalcopyrite (CuFeS2), bornite (Cu5FeS4), chalcocite (Cu2S), and covellite (CuS). Among the minerals mentioned, chalcopyrite is generally considered the most difficult to leach using conventional hydrometallurgical methods [6,7,8]. This is due to its complex crystal structure and the presence of iron within the mineral lattice. Chalcopyrite’s resistance to leaching is a significant challenge in the extraction of copper from certain ore deposits. Chalcopyrite is often surrounded by a thin layer of iron sulfide minerals, which can act as a barrier to the leaching solution, limiting its access to the copper-bearing mineral. Additionally, the iron content in chalcopyrite can lead to the formation of passivating layers on the mineral surface, further impeding the leaching process. To address this issue, a range of ways is used, including high-temperature pressure leaching [9,10,11], bioleaching [12,13,14,15], chemical pretreatment [16,17], and a combination of leaching methods [18,19,20]. The use of sulfate, nitrate, chloride, and other leaching agents has been investigated. Yet, the more favorable approach involves subjecting chalcopyrite to sulfuric acid media leaching due to its convenient manageability and the alignment of the process with well-established solvent extraction–electrowinning technology.
Recently, non-polar organic solvents have been employed to enhance the efficiency of chalcopyrite leaching [21,22]. Ethylene glycol has demonstrated its prowess as an exceptional medium for this objective, as it prevented the formation of a passivation layer on the mineral’s surface, thereby guaranteeing an almost complete retrieval of copper into the solution [23,24,25,26,27,28,29,30,31,32]. Solis-Marcial and co-workers conducted experiments using alcoholic acid media—specifically, isopropanol and methanol—for the purpose of acidically leaching copper from chalcopyrite [33,34]. Their findings revealed that alcohol serves a constructive role in the oxidative leaching of chalcopyrite, involving the stabilization of Cu+ ions through alcohol interaction.
To facilitate the extraction of copper from chalcopyrite at lower temperatures, the presence of potent oxidizing agents in the solution is necessary. One of the most commonly used oxidizing agents for this purpose is hydrogen peroxide, which has a redox potential of 1.77 V [35,36,37,38]. It has been found that the combination of non-polar organic solvents with hydrogen peroxide is effective for leaching chalcopyrite [25,27,39]. However, hydrogen peroxide’s use in mineral leaching presents challenges. High cost, intricate chemistry, potential side reactions, safety risks, and environmental concerns are drawbacks. Selectivity issues, scalability challenges, and equipment compatibility further complicate its application in industrial processes.
A promising reagent for leaching minerals is sodium dodecyl sulfate (SDS) (Figure 1).
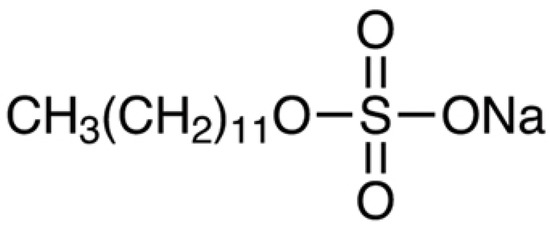
Figure 1.
Chemical formula of sodium dodecyl sulfate.
It was found that the addition of a small amount of SDS (mass fraction of 0.04%) increased the leaching rate of rare earth, and reduced the consumption of the leaching agent [40].
SDS significantly enhanced the leaching of potassium from phosphorus–potassium associated ore [41]. Kolmachikhina and co-workers demonstrated that sodium lingosulfonate and SDS mixtures positively influence the high-temperature oxidative pressure leaching of zinc concentrates and enhance the process [42]. SDS was successfully exploited for the biooxidation of copper mine tailings using Acidithiobacillus ferrooxidans [43].
The SDS leaching of minerals operates through a mechanism called “collective adsorption”, where molecules of the surfactant form a monolayer on the mineral surface. This layer reduces the surface tension of the leaching solution, allowing for better contact between the mineral and the leaching reagents [44]. Additionally, the hydrophobic tail of SDS interacts with hydrophobic mineral surfaces, aiding in the detachment of minerals from the ore. This process enhances the mass transfer of leaching reagents to the mineral surfaces, increasing the leaching efficiency.
In the present work, the effect of SDS on the sulfuric acid leaching of chalcopyrite in aqueous and isopropanol media was studied for the first time. A comparative investigation of the effectiveness of water and isopropanol as the solvents was performed. The effects of the SDS and sulfuric acid concentration, leaching duration, as well as temperature on copper recovery were investigated.
2. Materials and Methods
2.1. Raw Material
A sample of copper concentrate was received from the «Kazakhmys Smelting» copper plant (Kazakhstan). The milled and sieved (~90% of particles ≤ 74 μm) concentrate was subjected to leaching.
2.2. Reagents
The isopropanol (>99.5%), sulphuric acid (96%), sodium dodecyl sulfate (>99%), and sodium hydroxide (≥97%) were purchased from Sigma-Aldrich (Merck Group, St.Louis, Missouri, United States) and used without further purification. Bidistilled water was used for the aqueous leaching and in all washing and cleaning procedures.
2.3. Leaching Procedure
The batch leaching experiments were performed in a 200-mL round-bottom glass reactor, equipped with a thermometer. The leaching solutions were prepared by adding a predetermined amount of reagents into the solvent (isopropanol or water). In the reactor, a predetermined amount of sulfuric acid and SDS (if required) was added into the 50 mL of solvent, and the total volume was then filled to 100 mL with solvent. Then, the reactor was placed on the magnetic stirrer IKA RT 5 (IKA-Werke GmbH & Co. KG, Staufen, Germany) and the required temperature was set. Once the desired temperature was reached, 5 g of the concentrate was placed into the solution; thus, the pulp density was 5% in all leaching tests. The copper concentrations were determined by taking liquid samples of the solution (micropipette was used) every 30 min.
To evaluate the efficiency of leaching, the recovery of copper (α) was determined using the following formula:
where and are the masses of metal in the solution and initial sample, respectively.
Once the leaching procedure was completed, the dried solid residue was subjected to XRD analysis.
2.4. Surface Tension Measurements
The surface tensions of the pre-leaching solution and post-leaching solution were measured using a surface tension meter (A101, USA King Industry).
2.5. Contact Angel Measurement
The chalcopyrite mineral samples were selected for analysis. The glass slides were cleaned thoroughly. Initially, droplets of isopropanol were placed on the mineral samples. Subsequently, for the experimental group, droplets of SDS solution (of concentration of 0.2, 0.4, 0.6 g/L) were placed on the same mineral samples previously exposed to isopropanol. The temperature of all solutions was 45 °C. The contact angles formed by the droplets on the mineral surface were measured immediately using a goniometer (KSV SAM 101, KSV Instruments). There were 3 measurements taken for each mineral sample, and visual observations of the wetting behavior were noted.
2.6. Analytical Techniques
The XRD patterns of the initial concentrate and leaching residue were recorded using a Bruker D8 Advance diffractometer (Billerica, MA, USA) with CuKα (40 kV, 40 mA) radiation.
Scanning electron microscopy (SEM) imaging was performed by using a Quanta 200i 3D (FEI Company, Hillsboro, OR, USA) electron microscope.
The elemental composition of the concentrate was determined using atomic absorption spectrometry (AAS) on an AA-6200 spectrometer (Shimadzu, Japan); preliminary decomposition of the sample with concentrated nitric acid at 90–95 °C and a pressure of 10 atm was performed prior the copper content determination in a resulting solution.
3. Results and Discussion
3.1. Characterization of Initial Copper Concentrate
The chemical composition (wt. %) of the concentrate was Cu 26.2, Fe 23.3, and Si 11.5.
Chalcopyrite (CuFeS2) and quartz (SiO2) were identified as the crystalline phases in the starting concentrate according to XRD analysis.
The SEM image of the initial concentrate is presented in Figure 2 and indicates that the concentrate is characterized by a particle size in the range of 5–60 µm with different shapes, having a smooth surface, and without conglomerates formed. The particles of the concentrate are not covered with any film.

Figure 2.
SEM image of the initial concentrate.
3.2. Leaching Experiments
The influence of the following factors on the extraction of copper into solution was studied: sulfuric acid concentration; temperature; leaching duration; and SDS concentration. Water and isopropanol were used as solvents.
Figure 3 illustrates the interdependencies within the copper extraction process into an aqueous solution of sulfuric acid, considering variations in acid concentration and leaching duration at a temperature of 45 °C. Generally, augmenting both the acid concentration and leaching duration resulted in an amplified recovery of copper into the solution. Nevertheless, the upper limits of this enhancement were modest, reaching 7% under conditions of 1.0 M H2SO4 and 13% under 2.0 M H2SO4 concentration. These findings align with the research presented by Olubambi and Potgieter [37]. Substituting water with isopropanol as the solvent demonstrated a marginal elevation in the copper extraction efficiency (Figure 4). Notably, at a 1.0 M acid concentration, the maximal copper extraction into the solution reached 21%, maintaining a constant trend despite the escalation of acid concentration to 2.0 M. The extracted copper manifested in the form of a soluble complex, as elucidated in prior studies [33,34]. However, this level of copper recovery remains suboptimal from an economic standpoint. Elevating the temperature to 75 °C induced a notable upswing in copper extraction, reaching 19% and 28% in the aqueous and isopropanol media, respectively.
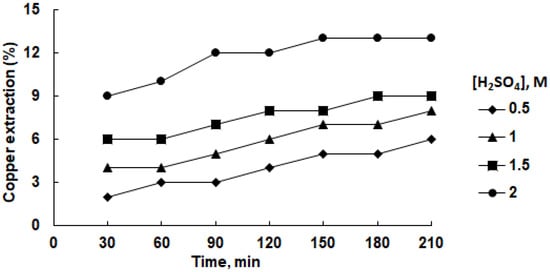
Figure 3.
Effect of sulfuric acid concentration on copper recovery in aqueous solution at 45 °C.
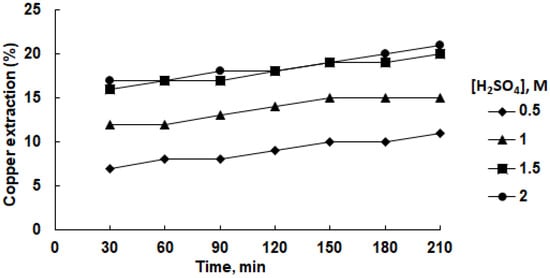
Figure 4.
Effect of sulfuric acid concentration on copper recovery in isopropanol solution at 45 °C.
The incorporation of 0.6 g/L SDS into an aqueous sulfuric acid solution yielded a notable upsurge in copper retrieval (see Figure 5).
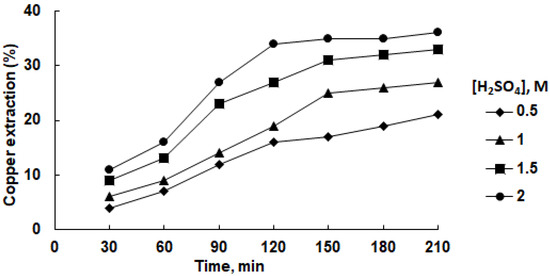
Figure 5.
Effect of sulfuric acid concentration on copper recovery in aqueous solution at 45 °C in the presence of 0.6 g/L SDS.
As the sulfuric acid concentration increases, there is a general trend of higher copper recovery percentages. Additionally, the incorporation of 0.6 g/L SDS enhances the copper recovery across all sulfuric acid concentrations. As the leaching time progresses, the copper recovery tends to increase, with the highest values achieved at longer leaching durations. These trends indicate that both sulfuric acid concentration and SDS presence play pivotal roles in promoting effective copper recovery from the concentrate. At a H2SO4 concentration of 2.0 M and a leaching duration of 210 min, the highest attainable copper extraction into the solution reached 36%. In comparison, without the presence of SDS but under identical conditions, the copper recovery was a mere 12% (refer to Figure 3).
The impact of SDS on copper recovery from chalcopyrite ore through the leaching process can be explored from a physicochemical perspective. SDS, possessing amphiphilic characteristics, exhibits a pronounced affinity for mineral surfaces due to its hydrophilic head and hydrophobic tail configuration [45,46]. Its introduction into the leaching solution facilitates preferential adsorption onto chalcopyrite surfaces, an action that restructures surface properties to facilitate leaching interactions [47].
The effect of the presence of SDS on the sulfuric acid leaching of copper from chalcopyrite was also investigated using isopropanol as a solvent (Figure 6).
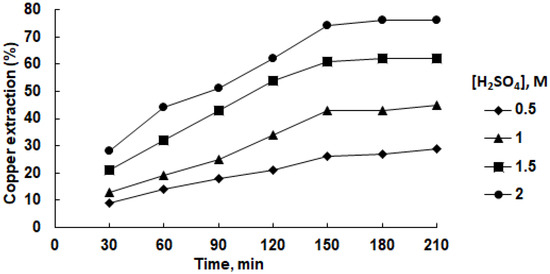
Figure 6.
Effect of sulfuric acid concentration on copper recovery in isopropanol solution at 45 °C in the presence of 0.6 g/L SDS.
The copper recovery percentages exhibited remarkable disparities between the two solvents. Isopropanol consistently yielded higher recoveries across varied sulfuric acid concentrations and leaching durations compared to water. Notably, the water-based recoveries were lower, particularly at lower acid concentrations and shorter leaching intervals. For instance, at a sulfuric acid concentration of 1.0 M and a leaching time of 210 min, the recovery with isopropanol was 45%, while with water, it was only 27%.
The choice of solvent appeared pivotal. Isopropanol, being an organic solvent, demonstrated superior solubilizing capabilities and penetration efficiency, contributing to more efficient copper extraction. Conversely, water’s polar nature appeared to limit its interaction with mineral surfaces, leading to reduced copper recoveries. The maximal copper recovery achieved with isopropanol was 76% (2.0 M sulfuric acid, 210 min), whereas water resulted in a maximal recovery of only 36%.
Isopropanol’s organic properties were also implicated in its enhanced mineral breakdown efficiency. This led to greater exposure of the copper-bearing phases within the chalcopyrite mineral, facilitating better leaching agent access. In contrast, water’s ability to disrupt the mineral structures seemed less pronounced, resulting in incomplete copper extraction.
The leaching kinetics were notably affected. Isopropanol-based leaching showed faster increases in copper recovery percentages over time, indicating accelerated copper dissolution. Water-based leaching, however, exhibited comparatively slower rate increments, suggesting a less efficient process.
The chemical interactions were distinct between the solvents. Isopropanol’s organic nature introduced the possibility of unique chemical complexes with copper ions or different interactions with mineral surfaces. Conversely, water’s interactions relied primarily on physical processes and hydrogen bonding, potentially less effective in driving copper dissolution.
Several rounds of testing were conducted in order to enhance the sulfuric acid leaching process of chalcopyrite within an isopropanol medium. The parameters under investigation included the concentration of SDS (ranging from 0.2 to 0.8 g/L) and the solution’s temperature (ranging from 45 to 75 °C). Throughout all the experiments, the sulfuric acid concentration was maintained at 2.0 M, and the leaching process lasted for 180 min.
The first series of experiments was carried out to identify the effect of the SDS concentration (Figure 7).
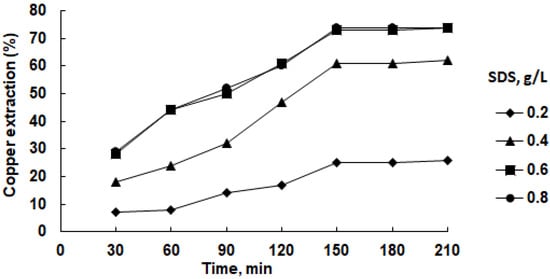
Figure 7.
Effect of SDS concentration on copper recovery in isopropanol solution at 45 °C in the presence of 2.0 M H2SO4.
An optimal recovery was observed with an SDS concentration of 0.6 g/L, reaching a plateau of approximately 74% after 150 min and remaining constant thereafter. Lower concentrations exhibited incremental increases in recovery over time, while higher concentrations demonstrated quicker attainment of peak recovery percentages.
In subsequent experiments, the temperature of the solution varied, while the concentrations of sulfuric acid and SDS remained constant (2.0 M and 0.6 g/L, respectively). The results of the experiments are presented in Figure 8.
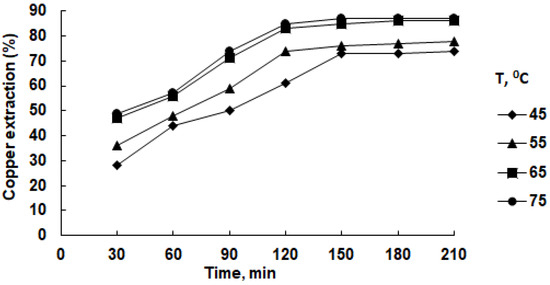
Figure 8.
Effect of temperature on copper recovery in isopropanol solution in the presence of 2.0 M H2SO4 and 0.6 g/L SDS.
The influence of temperature on copper extraction is evident; a rise in temperature up to 65 °C notably boosted the copper solubility. In 120 min, approximately 83% of the copper dissolved, in contrast to around 60% at 45 °C within the same timeframe. Subsequent increments in temperature and leaching duration showed minimal impact on copper extraction into the solution.
3.3. Kinetic Study
A shrinking core model is widely used to describe the leaching process [48,49,50]. The process of the sulfuric acid leaching of chalcopyrite in an isopropanol medium is usually limited by the chemical reaction between the target mineral and the leaching agent [21,22]. For this kind of processes, the relationship between the metal fraction extracted into the solution (XMe) and the rate constant of the chemical reaction (k) at a certain leaching time (τ) is described by the following models [48]:
The apparent rate constant for the chemical reaction was determined by utilizing the data from Figure 8 in conjunction with Equation (2). Only the portions of the curves showing an upward linear trend were selected, specifically the segments of the curves from 0 min to 120 min. Since increasing the temperature from 65 to 75 °C had virtually no effect on copper extraction, the extraction values at 75 °C were not included in the calculations.
The resulting graphical representation, shown in Figure 9, depicts 1 − (1 − XMe)1/3 plotted against the leaching duration.
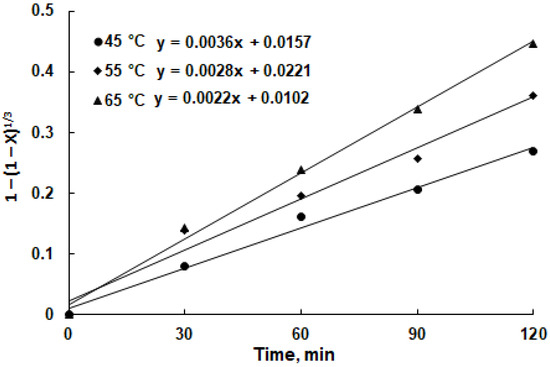
Figure 9.
A plot of 1 − (1 − XMe)1/3 vs. leaching duration for copper recovery from chalcopyrite (2.0 M H2SO4 in isopropanol; pulp density of 5%).
The chemical reaction rate constants at different temperatures were as follows: 0.0022 min−1 at 45 °C, 0.0028 min−1 at 55 °C, and 0.0036 min−1 at 65 °C. These rate constants were employed to construct the Arrhenius plot for copper recovery from copper smelter slag, as illustrated in Figure 10. The linear fit of the data affirms that the copper recovery in the solution is predominantly governed by the chemical reaction occurring on the mineral surface.
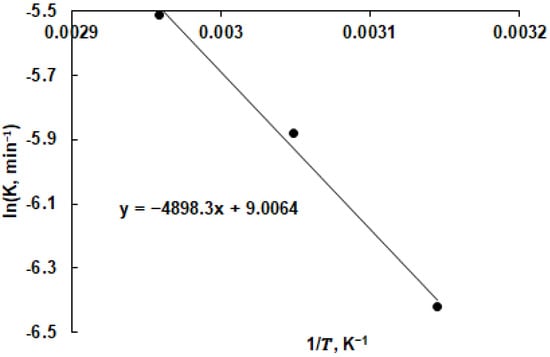
Figure 10.
Arrhenius plot for copper leaching from chalcopyrite (2.0 M H2SO4 in isopropanol; pulp density of 5%).
The activation energy (Ea) and pre-exponential factor (A) governing the overall chemical reaction responsible for copper extraction into the solution were determined using Arrhenius’s law (Equation (3)) [49]:
lnk = −Ea/(RT) + lnA
Here, k represents the rate constant of the chemical reaction, R stands for the universal gas constant, and T denotes the absolute temperature.
The calculated values for Ea and A were 40.72 kJ/mol and 8103 min−1, respectively. Both of these values are consistent with the range of values reported for chalcopyrite leaching [50].
3.4. Influence of SDS on the Solution Surface Tension
In Figure 11, the values of the surface tension of aqueous solutions of 2.0 M H2SO4 before and after leaching (120 min, 65 °C) are presented, both in the absence and presence of 0.6 g/L SDS.
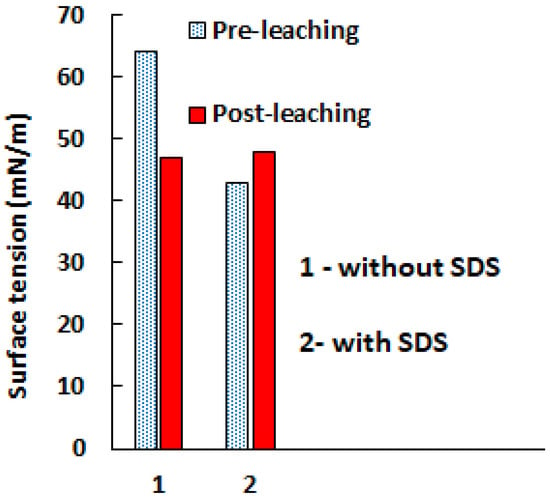
Figure 11.
Effect of 0.6 g/L SDS on the pre-leaching and post-leaching sulfuric acid (2.0 M) aqueous solution.
The addition of SDS to the sulfuric acid solution significantly reduces its surface tension (64 mN/m compared to 43 mN/m), which is attributed to the well-known effect of reducing the surface tension of the solution in the presence of surfactants. In the absence of SDS, the pre-leaching solution had a lower surface tension than the post-leaching solution; a decrease in the surface tension of the solution in the presence of metal ions was also noted by Liu [41] and Zhao [51]. However, in the presence of SDS, a reverse effect was observed: the surface tension of the post-leaching solution increased (37 mN/m compared to 33 mN/m). This fact can be explained by the adsorption of SDS on the mineral surface, which reduces the concentration of surfactants in the solution [52].
Through its ability to lower the surface tension of the leaching solution, SDS facilitates an augmented wetting process, encouraging closer interaction between the solution and the mineral particles [53]. The attendant reduction in surface tension enhances mass transfer and the overall diffusion of reactants to and from the mineral surface. Moreover, SDS manifests a dispersant effect through its hydrophobic tail, deterring particle aggregation and fostering a more dispersed arrangement that exposes a larger surface area for leaching reactions.
Electrostatic alterations arise due to SDS’s charged head groups, influencing the surface charge of the chalcopyrite particles [54]. This modulation in electrostatic repulsion mitigates particle aggregation, bolstering their dispersion within the leaching solution. SDS also engenders a protective layer on the mineral surface, preventing the accumulation of passivating species that could impede effective leaching.
A similar series of experiments was also conducted for an isopropanol solution (Figure 12).
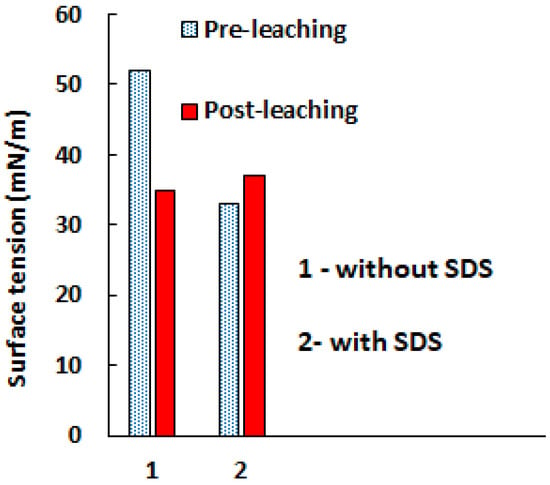
Figure 12.
Effect of 0.6 g/L SDS on the pre-leaching and post-leaching sulfuric acid (2 M) isopropanol solution.
Similar trends in changes in the surface tension of the solutions before and after leaching in the absence and presence of SDS are observed for both the aqueous and isopropanol solutions; however, the absolute values of surface tension in all cases are higher for the aqueous solution compared to isopropanol, which was expected since isopropanol has a lower surface tension than water due to the absence of strong hydrogen bonding between the isopropanol molecules compared to the extensive hydrogen bonding present in water. This circumstance explains the better leaching of copper into an isopropanol solution than into an aqueous one.
3.5. Contact Angle Results
The data in Figure 13 show the contact angle measurements for chalcopyrite at different time intervals (0, 5, 10, 15, 20, 25, and 30 min) when exposed to isopropanol alone (0 min data point) and isopropanol with varying SDS concentrations (0.2, 0.4, and 0.6 g/L) thereafter. Notably, the contact angle decreases progressively over time for all SDS concentrations, indicating improved wetting of the chalcopyrite surface. Higher SDS concentrations correspond to lower contact angles, highlighting the surfactant’s effectiveness in enhancing chalcopyrite wettability. Initially, at 0 min with pure isopropanol, the contact angle is around 85°, but it decreases with longer exposure times. This data underscore that the addition of SDS to isopropanol significantly reduces the chalcopyrite contact angles, suggesting enhanced wettability, particularly when compared to pure isopropanol.
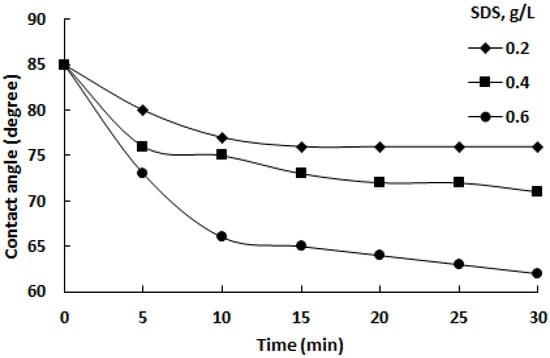
Figure 13.
Effect of SDS concentration in isopropanol on chalcopyrite contact angles over time.
3.6. Analysis of Leaching Residue
In Figure 14, the XRD pattern of the initial sample (1) and the residues after its sulfuric acid leaching (2.0 M H2SO4, 65 °C, 120 min) in both the aqueous (2) and isopropanol (3,4) media are shown, both in the absence (3) and presence (4) of SDS.
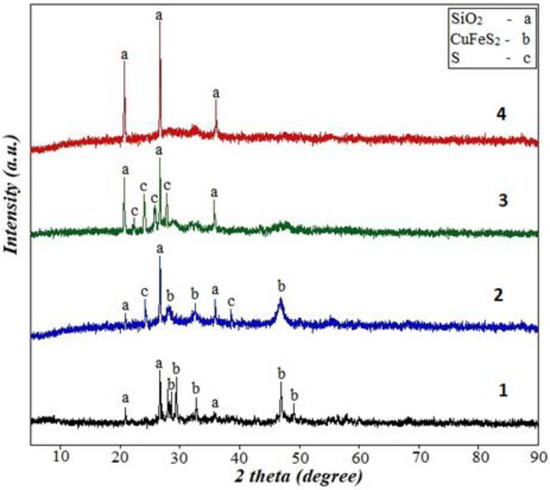
Figure 14.
XRD pattern of the initial sample (1) and leaching residues (2–4) after leaching (2.0 M H2SO4, 65 °C, 120 min). 2—aqueous media, absence of SDS; 3—isopropanol media, absence of SDS; 4—isopropanol media, 0.6 g/L SDS.
After leaching into an aqueous media in the absence of SDS, both components present in the initial sample (chalcopyrite and quartz), as well as sulfur, were detected in the residue (2). The formation of sulfur is a result of the interaction between the chalcopyrite and sulfuric acid in the presence of dissolved oxygen [55]:
CuFeS2 + 2.5O2 + H2SO4 = CuSO4 + FeSO4 + H2O + S°
The sulfur anion in chalcopyrite is oxidized to elemental sulfur (i.e., it acts as a reducing agent), while oxygen serves as the oxidizing agent.
Replacing water with isopropanol during leaching resulted in the disappearance of the chalcopyrite peaks, while the quartz and sulfur peaks were preserved (3). Since the leaching residue was thoroughly washed with water before recording the XRD pattern, the presence of sulfur in the residue may indicate a bond between sulfur and quartz.
In the case of leaching into the isopropanol in the presence of SDS, only quartz was observed in the residue (4). Therefore, the presence of the mentioned surfactant prevented the precipitation and binding of sulfur to the surface of the residue, and sulfur was separated from it during washing. Apparently, SDS as a surfactant alters the adsorption behavior of the sulfur species on the mineral surface, and the surfactant competes with sulfur for the surface binding sites. This competition can potentially reduce the formation of a thick sulfur layer on the mineral surface.
In Figure 15, SEM images of the residues after leaching into the isopropanol media in the absence (a) and presence (b) of SDS are shown; images (a) and (b) correspond to samples (3) and (4) in Figure 14.
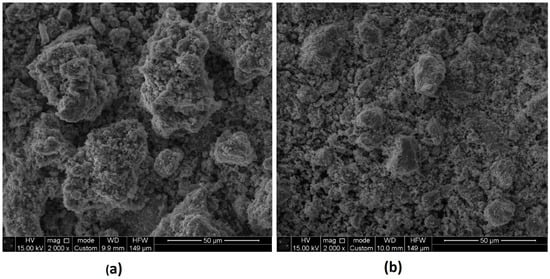
Figure 15.
SEM images of residues after leaching into isopropanol media (2.0 M H2SO4, 65 °C, 120 min) in the absence of SDS (a) and in the presence of 0.6 g/L SDS (b).
In the absence of SDS, the residue particles (Figure 15a) have a higher tendency to aggregate and form larger clusters. This can occur because without a surfactant, particles may have a higher surface tension and a tendency to stick together.
The presence of SDS resulted in smaller and more dispersed residue particles (Figure 15b). SDS reduced the surface tension of the solution and improved the wetting of particles, potentially leading to more efficient dispersion.
4. Conclusions
In conclusion, the study demonstrates that the use of SDS (sodium dodecyl sulfate) in the leaching process of chalcopyrite into isopropanol and aqueous media has proven highly effective for enhancing copper recovery. Specifically, the “isopropanol–sulfuric acid–SDS” system showed exceptional promise in extracting copper from chalcopyrite.
The positive impact of SDS on copper extraction can be attributed to its ability to reduce the surface tension of the leaching solution, which improves mineral wetting and facilitates better contact between the solvent and the chalcopyrite surface. Additionally, SDS as a surfactant alters the adsorption patterns of the sulfur species on the mineral surface, competing with sulfur for binding sites and potentially reducing the formation of a thick sulfur layer.
Under optimized conditions (isopropanol media, 2.0 M H2SO4, 65 °C, 120 min, 0.6 g/L SDS), a remarkable 83% copper recovery into the solution was achieved. This achievement is significant as it was accomplished at ambient pressure without the need for strong oxidizers, offering an environmentally and economically favorable approach to chalcopyrite leaching.
Author Contributions
Conceptualization, B.K.; methodology, T.K.; investigation, A.B. and K.K.; resources, R.N. and B.K.; writing—original draft preparation, R.N. and T.K.; writing—review and editing, B.K.; visualization, A.B. and K.K.; project administration, R.N.; funding acquisition, R.N. and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant no. BR18574006).
Data Availability Statement
The data supporting the results can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C.; Mian, M.; Cheema, A.I. Strategies for arsenic pollution control from copper pyrometallurgy based on the study of arsenic sources, emission pathways and speciation characterization in copper flash smelting systems. Environ. Pollut. 2021, 270, 116203. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Gálvez, E.; Robles, P.; Wilson, R.; Navarra, A. Environmental, economic and technological factors affecting Chilean copper smelters—A critical review. J. Mater. Res. Technol. 2021, 15, 213–225. [Google Scholar] [CrossRef]
- Coursol, P.; Mackey, P.J.; Kapusta, J.P.T.; Valencia, N.C. Energy Consumption in Copper Smelting: A New Asian Horse in the Race. JOM 2015, 67, 1066–1074. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Chmielewski, T. Solvent extraction in hydrometallurgical processing of Polish copper concentrates. Physicochem. Probl. Miner. Process. 2011, 46, 207–218. [Google Scholar]
- Yu, S.; Liao, R.; Yang, B.; Fang, C.; Wang, Z.; Liu, Y.; Qiu, G. Chalcocite (bio) hydrometallurgy—Current state, mechanism, and future directions: A review. Chin. J. Chem. Eng. 2022, 41, 109–120. [Google Scholar] [CrossRef]
- Ji, G.; Liao, Y.; Wu, Y.; Xi, J.; Liu, Q. A review on the research of hydrometallurgical leaching of low-grade complex chalcopyrite. J. Sustain. Met. 2022, 8, 964–977. [Google Scholar] [CrossRef]
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Ghosh, M.K.; Ayanda, O.S.; Bale, R.B.; Sheik, A.R.; Pradhan, S.R. A review on novel techniques for chalcopyrite ore processing. Int. J. Min. Eng. Miner. Process. 2012, 1, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- McDonald, R.; Muir, D. Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 2007, 86, 191–205. [Google Scholar] [CrossRef]
- Padilla, R.; Vega, D.; Ruiz, M. Pressure leaching of sulfidized chalcopyrite in sulfuric acid–oxygen media. Hydrometallurgy 2007, 86, 80–88. [Google Scholar] [CrossRef]
- Wang, S. Copper leaching from chalcopyrite concentrates. JOM 2005, 57, 48–51. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.L.; Leão, V.A. Chalcopyrite bioleaching in chloride media: A mini-review. Hydrometallurgy 2022, 216, 105995. [Google Scholar] [CrossRef]
- Liang, C.-L.; Xia, J.-L.; Zhao, X.-J.; Yang, Y.; Gong, S.-Q.; Nie, Z.-Y.; Ma, C.-Y.; Zheng, L.; Zhao, Y.-D.; Qiu, G.-Z. Effect of activated carbon on chalcopyrite bioleaching with extreme thermophile Acidianus manzaensis. Hydrometallurgy 2010, 105, 179–185. [Google Scholar] [CrossRef]
- Martins, F.L.; Patto, G.B.; A Leão, V. Chalcopyrite bioleaching in the presence of high chloride concentrations. J. Chem. Technol. Biotechnol. 2019, 94, 2333–2344. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Dong, K.; Xie, F.; Lu, D.; Chang, Y.; Jiang, K. Effect of microwave pretreatment on chalcopyrite dissolution in acid solution. J. Mater. Res. Technol. 2022, 16, 471–481. [Google Scholar] [CrossRef]
- Faris, N.; Ram, R.; Chen, M.; Tardio, J.; Pownceby, M.I.; Jones, L.A.; McMaster, S.; Webster, N.A.; Bhargava, S. The effect of thermal pre-treatment on the dissolution of chalcopyrite (CuFeS2) in sulfuric acid media. Hydrometallurgy 2017, 169, 68–78. [Google Scholar] [CrossRef]
- Copur, M.; Kizilca, M.; Kocakerim, M.M. Determination of the Optimum Conditions for Copper Leaching from Chalcopyrite Concentrate Ore Using Taguchi Method. Chem. Eng. Commun. 2015, 202, 927–935. [Google Scholar] [CrossRef]
- Mohammadabad, F.K.; Hejazi, S.; Khaki, J.V.; Babakhani, A. Mechanochemical leaching of chalcopyrite concentrate by sulfuric acid. Int. J. Miner. Met. Mater. 2016, 23, 380–388. [Google Scholar] [CrossRef]
- Jafari, M.; Karimi, G.; Ahmadi, R. Improvement of chalcopyrite atmospheric leaching using controlled slurry potential and additive treatments. Physicochem. Probl. Miner. Process. 2017, 53, 73597534. [Google Scholar]
- Solis-Marcíal, O.; Lapidus, G. Improvement of chalcopyrite dissolution in acid media using polar organic solvents. Hydrometallurgy 2013, 131-132, 120–126. [Google Scholar] [CrossRef]
- Nadirov, R.; Karamyrzayev, G. Selective ozone-assisted acid leaching of copper from copper smelter slag by using isopropanol as a solvent. Minerals 2022, 12, 1047. [Google Scholar] [CrossRef]
- Li, X.; Monnens, W.; Li, Z.; Fransaer, J.; Binnemans, K. Solvometallurgical process for extraction of copper from chalcopyrite and other sulfidic ore minerals. Green Chem. 2020, 22, 417–426. [Google Scholar] [CrossRef]
- Carlesi, C.; Harris, R.C.; Abbott, A.P.; Jenkin, G.R.T. Chemical dissolution of chalcopyrite concentrate in choline chloride ethylene glycol deep eutectic solvent. Minerals 2022, 12, 65. [Google Scholar] [CrossRef]
- Mahajan, V.; Misra, M.; Zhong, K.; Fuerstenau, M. Enhanced leaching of copper from chalcopyrite in hydrogen peroxide–glycol system. Miner. Eng. 2007, 20, 670–674. [Google Scholar] [CrossRef]
- Tehrani, M.E.H.N.; Naderi, H.; Rashchi, F. Electrochemical study and XPS analysis of chalcopyrite dissolution in sulfuric acid in the presence of ethylene glycol. Electrochim. Acta 2021, 369, 137663. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Lapidus, G.T. Study of chalcopyrite leaching from a copper concentrate with hydrogen peroxide in aqueous ethylene glycol media. Hydrometallurgy 2017, 169, 192–200. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Lázaro, I.; Lapidus, G. Improvement effect of organic ligands on chalcopyrite leaching in the aqueous medium of sulfuric acid-hydrogen peroxide-ethylene glycol. Hydrometallurgy 2020, 193, 105293. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Lapidus, G. Electrochemical and leaching studies to better understand the role of ethylene glycol in the oxidative acid dissolution of chalcopyrite. Electrochimica Acta 2022, 418, 140343. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Lapidus, G. A study to understand the role of ethylene glycol in the oxidative acid dissolution of chalcopyrite. Miner. Eng. 2022, 180, 107502. [Google Scholar] [CrossRef]
- Ghomi, M.A.; Mozammel, M.; Moghanni, H.; Shahkar, L. Atmospheric leaching of chalcopyrite in the presence of some polar organic reagents: A comparative study and optimization. Hydrometallurgy 2019, 189, 105120. [Google Scholar] [CrossRef]
- Castillo-Magallanes, N.; Cruz, R.; Lázaro, I. Effect of organic agents on the oxidation process of chalcopyrite in a sulfuric acid solution. Electrochimica Acta 2020, 355, 136789. [Google Scholar] [CrossRef]
- Solís-Marcíal, O.; Lapidus, G. Chalcopyrite leaching in alcoholic acid media. Hydrometallurgy 2014, 147–148, 54–58. [Google Scholar] [CrossRef]
- Solís Marcial, O.J.; Nájera Bastida, A.; Bañuelos, J.E.; Valdés Martínez, O.U.; Luevano, L.A.; Serrano Rosales, B. Chalcopyrite leaching kinetics in the presence of methanol. Int. J. Chem. React. Eng. 2019, 17, 20190081. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Stanković, S.; Kamberović, Ž.; Štrbac, N.; Manojlović, V.; Petronijević, N. Kinetics of chalcopyrite leaching by hydrogen peroxide in sulfuric acid. Metals 2019, 9, 1173. [Google Scholar] [CrossRef]
- Agacayak, T.; Aras, A.; Aydogan, S.; Erdemoglu, M. Leaching of chalcopyrite concentrate in hydrogen peroxide solution. Physicochem. Probl. Miner. Process. 2014, 50, 657–666. [Google Scholar]
- Olubambi, P.A.; Potgieter, J.H. Investigations on the mechanisms of sulfuric acid leaching of chalcopyrite in the presence of hydrogen peroxide. Miner. Process. Extr. Metall. Rev. 2009, 30, 327–345. [Google Scholar] [CrossRef]
- Turan, M.D.; Sarı, Z.A.; Nizamoğlu, H. Pressure leaching of chalcopyrite with oxalic acid and hydrogen peroxide. J. Taiwan Inst. Chem. Eng. 2021, 118, 112–120. [Google Scholar] [CrossRef]
- Solís-Marcial, O.; Lapidus, G. Study of the dissolution of chalcopyrite in sulfuric acid solutions containing alcohols and organic acids. Electrochimica Acta 2014, 140, 434–437. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Yang, S.; Zhong, Z.; Zhou, H.; Luo, X. Enhancing the leaching effect of an ion-absorbed rare earth ore by ameliorating the seepage effect with sodium dodecyl sulfate surfactant. Int. J. Min. Sci. Technol. 2021, 31, 995–1002. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Yang, F.; Ma, J.; Yang, W.; Wang, T.; Wang, C. The intensified leaching mechanism of phosphorus-potassium associated ore in HCl-CaF2 system with sodium dodecyl sulfate. Chem. Eng. Process. -Process Intensif. 2020, 149, 107847. [Google Scholar] [CrossRef]
- Kolmachikhina, E.B.; Ryzhkova, E.A.; Dmitrieva, D.V. Sodium Lignosulfonate and Sodium Dodecyl-Sulfate Mixtures Influence on Zinc Concentrate Pressure Leaching and Zinc Electro-Winning. Solid State Phenom. 2020, 299, 1121–1127. [Google Scholar] [CrossRef]
- Dou, L.; Yajie, Y.; Dongwei, L.; Liyan, S.; Yangqing, W.; Wei, T.; Zhonghui, X. Effects of sodium dodecyl sulphate on biooxidation of copper mine tailings by Acidithiobacillus ferrooxidans. Res. J. Biotechnol. 2013, 8, 11. [Google Scholar]
- Ai, C.-M.; Sun, P.-P.; Wu, A.-X.; Chen, X.; Liu, C. Accelerating leaching of copper ore with surfactant and the analysis of reaction kinetics. Int. J. Miner. Met. Mater. 2019, 26, 274–281. [Google Scholar] [CrossRef]
- Bordes, R.; Holmberg, K. Amino acid-based surfactants—Do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rakshit, A.; Acharjee, A.; Saha, B. Novel amphiphiles and their applications for different purposes with special emphasis on polymeric surfactants. ChemistrySelect 2019, 4, 6978–6995. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of finely ground chalcopyrite using emulsified oil stabilized by emulsifiers: Implications for porphyry copper ore flotation. Metals 2020, 10, 912. [Google Scholar] [CrossRef]
- Safari, V.; Arzpeyma, G.; Rashchi, F.; Mostoufi, N. A shrinking particle—Shrinking core model for leaching of a zinc ore containing silica. Int. J. Miner. Process. 2009, 93, 79–83. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: New York, NY, USA, 1999; p. 684. [Google Scholar]
- Hidalgo, T.; Kuhar, L.; Beinlich, A.; Putnis, A. Kinetic study of chalcopyrite dissolution with iron(III) chloride in methanesulfonic acid. Miner. Eng. 2018, 125, 66–74. [Google Scholar] [CrossRef]
- Zhao, G.X.; Zhao, M.Y. The Action Principle of Surfactant; China Light Industry Press: Beijing, China, 2003. [Google Scholar]
- Rosen, M.J. Surfactants and Interfacial Phenomena; John Wiley & Sons, Inc.: London, UK, 2012. [Google Scholar]
- Nie, H.-Q.; Hou, W.-G. Vesicle formation induced by layered double hydroxides in the catanionic surfactant solution composed of sodium dodecyl sulfate and dodecyltrimethylammonium bromide. Colloid Polym. Sci. 2011, 289, 775–782. [Google Scholar] [CrossRef]
- Abeidu, A. The separation of cobaltite from chalcopyrite and pyrite. J. Less Common Met. 1976, 46, 327–331. [Google Scholar] [CrossRef]
- Cháidez, J.; Parga, J.; Valenzuela, J.; Carrillo, R.; Almaguer, I. Leaching chalcopyrite concentrate with oxygen and sulfuric acid using a low-pressure reactor. Metals 2019, 9, 189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).