The Impact of Municipal Waste on Seasonal and Spatial Changes in Selected Macro- and Micro-Nutrient Contents on the Background of Soil Biological Activity—A Case Study
Abstract
1. Introduction
2. Materials and Methods
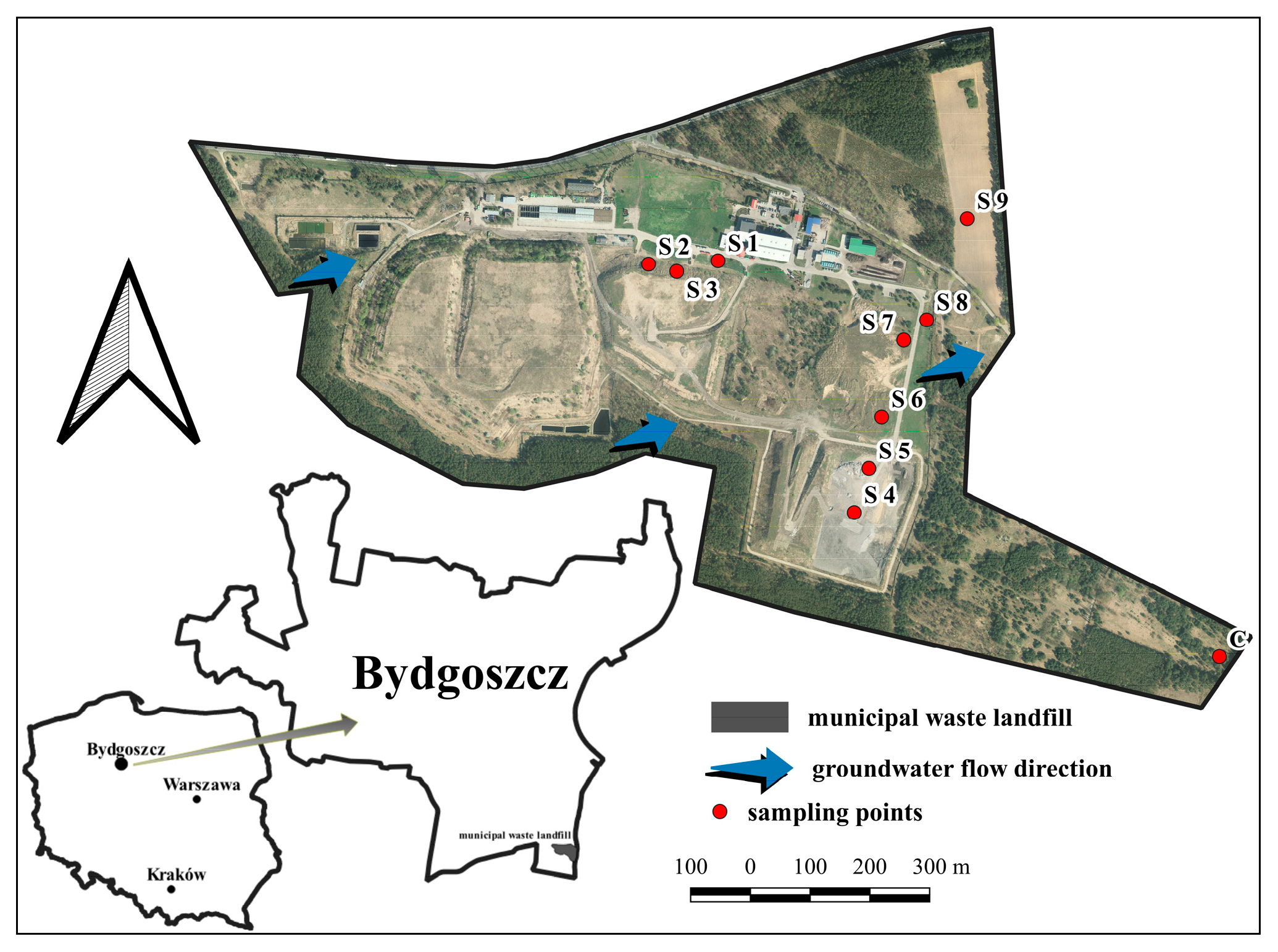
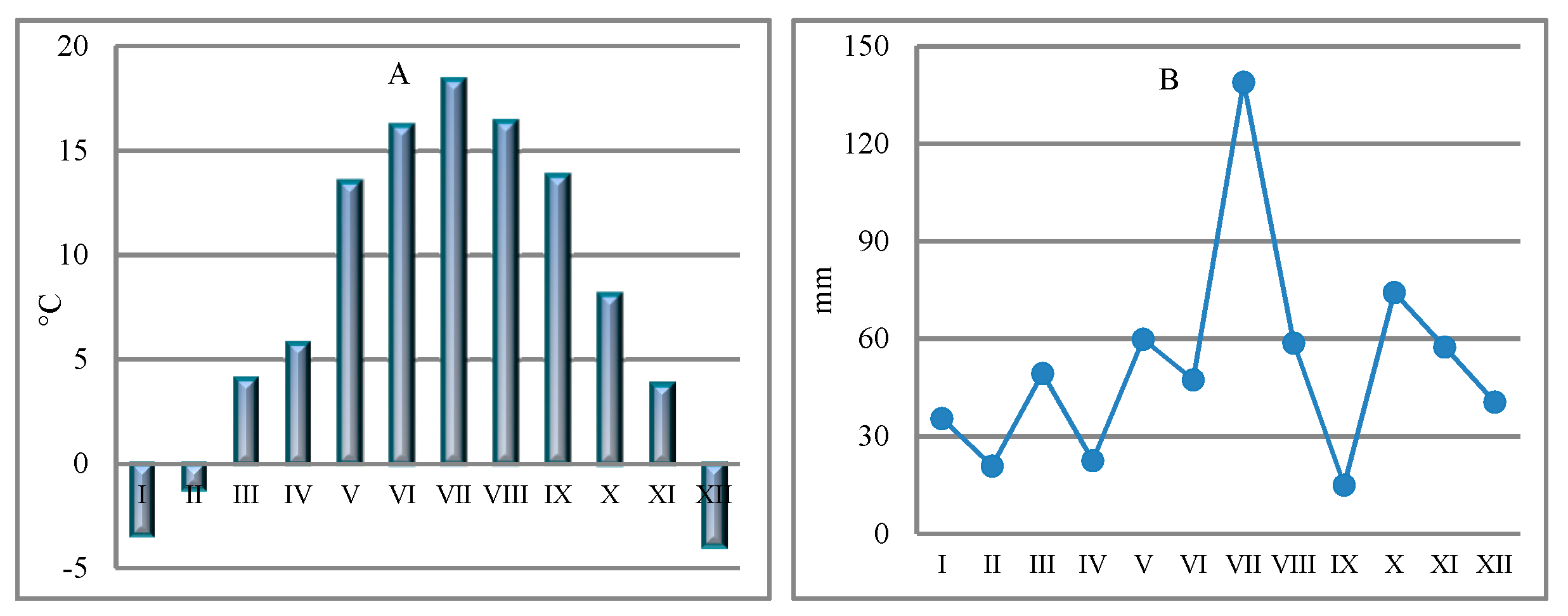
2.1. Study Area
2.2. Soil Sampling
2.3. Soil Analysis
2.3.1. Physicochemical Parameters of the Soil
2.3.2. Microbial and Biochemical Analyses
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koda, E.; Rybak-Niedziółka, K.; Winkler, J.; Černý, M.; Osiński, P.; Podlasek, A.; Kawalec, J.; Vaverková, M.D. Space Redevelopment of Old Landfill Located in the Zone between Urban and Protected Areas: Case Study. Energies 2021, 15, 146. [Google Scholar] [CrossRef]
- GUS. 2021. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2021,1,22.html (accessed on 30 November 2021).
- Silva, A.; Rosano, M.; Stocker, L.; Gorissen, L. From waste to sustainable materials management: Three case studies of the transition journey. Waste Manag. 2017, 61, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Concha, D.M.; Sandoval-Cobo, J.J.; Whiting, K. An experimental study on the impact of two dimensional materials in waste disposal sites: What are the implications for engineered landfills? Sustain. Environ. Res. 2016, 26, 255–261. [Google Scholar] [CrossRef][Green Version]
- Eurostat. Eurostat: The Statistical Office of the European Union Situated in Luxembourg. 2015. Available online: https://ec.europa.eu/eurostat (accessed on 15 May 2015).
- Brennan, R.; Healy, M.; Morrison, L.; Hynes, S.; Norton, D.; Clifford, E. Management of landfill leachate: The legacy of European Union Directives. Waste Manag. 2016, 55, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Breza-Boruta, B. The assessment of airborne bacterial and fungal contamination emitted by a municipal landfill site in Northern Poland. Atmos. Pollut. Res. 2016, 7, 1043–1052. [Google Scholar] [CrossRef]
- Breza-Boruta, B.; Lemanowicz, J.; Bartkowiak, A. Variation in biological and physicochemical parameters of the soil affected by uncontrolled landfill sites. Environ. Earth Sci. 2016, 75, 201. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Gozdowski, D.; Koda, E.; Osiecka, R.; Borzyszkowski, J. Influence of a municipal waste landfill on the spatial distribution of mercury in the environment. PLoS ONE 2015, 10, e0133130. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Breza-Boruta, B.; Lemanowicz, J. Assessment of the content of heavy metals and potential pathogenic microorganisms in soil under illegal dumping sites. Environ. Earth Sci. 2016, 75, 1401. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Breza-Boruta, B. Changes in phosphorus content, phosphatase activity and some physicochemical and microbiological parameters of soil within the range of impact of illegal dumping sites in Bydgoszcz (Poland). Environ. Earth Sci. 2016, 75, 510. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Zloch, J.; Radziemska, M.; Adamcová, D. Environmental impact of landfill on soils—The example of the Czech Republic. Pol. J. Soil Sci. 2017, 50, 93–105. [Google Scholar] [CrossRef][Green Version]
- Vaverková, M.D.; Adamcová, D.; Radziemska, M.; Voběrková, S.; Mazur, Z.; Zloch, J. Assessment and evaluation of heavy metals removal from landfill leachate by Pleurotus ostreatus. Waste Biomass Valori. 2017, 9, 503–511. [Google Scholar] [CrossRef]
- Voběrková, S.; Vaverková, M.D.; Burešová, A.; Adamcová, D.; Vršanská, M.; Kynický, J.; Brtnický, M.; Adam, V. Effect of inoculation with white-rot fungi and fungal consortium on the composting efficiency of municipal solid waste. Waste Manag. 2017, 61, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Nikraz, H.; Hung, Y.T. Influence of Waste Age on Landfill Leachate Quality. Int. J. Environ. Sci. Dev. 2010, 1, 347–350. [Google Scholar] [CrossRef]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and Long-Term Composition of MSW Landfill Leachate: A Review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Frączek, K.J.; Ropek, D.; Lenart-Boroń, A.M. Assessment of microbiological and chemical properties in a municipal landfill area. J. Environ. Sci. Health Part A 2014, 49, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557–558, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, H.A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-32714-1. [Google Scholar] [CrossRef]
- Al Sabahi, E.; Rahim, S.A.; Zuhairi, W.W.; Al Nozaily, F.; Alshaebi, F. The Characteristics of Leachate and Groundwater Pollution at Municipal Solid Waste Landfill of Ibb City, Yemen. Am. J. Environ. Sci. 2009, 5, 256–266. [Google Scholar] [CrossRef]
- Christensen, T.H.; Kjeldsen, P.; Bjerg, P.L.; Jensen, D.L.; Christensen, J.B.; Baun, A.; Albrechtsen, H.-J.; Heron, G. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 2001, 16, 659–718. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Adjei, R.; Anokye, J.; Banunle, A. Municipal waste dumpsite: Impact on soil properties and heavy metal concentrations, Sunyani, Ghana. Sci. Afr. 2020, 8, e00390. [Google Scholar] [CrossRef]
- Rasool, B.; Rahman, M.U.; Zubair, M.; Khan, M.A.; Ramzani, P.M.A.; Dradrach, A.; Turan, V.; Iqbal, M.; Khan, S.A.; Tauqeer, H.M.; et al. Synergetic Efficacy of Amending Pb-Polluted Soil with P-Loaded Jujube (Ziziphus mauritiana) Twigs Biochar and Foliar Chitosan Application for Reducing Pb Distribution in Moringa Leaf Extract and Improving Its Anti-cancer Potential. Water Air Soil Pollut. 2022, 233, 344. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; Basharat, Z.; Ramzani, P.M.A.; Farhad, M.; Lewińska, K.; Turan, V.; Karczewska, A.; Khan, S.A.; Faran, G.-E.; Iqbal, M. Aspergillus niger-mediated release of phosphates from fish bone char reduces Pb phytoavailability in Pb-acid batteries polluted soil, and accumulation in fenugreek. Environ. Pollut. 2022, 313, 120064. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Tabatabai, M.A.; Abdel-Moneim, A.-M.A.; Loynachan, T.E. Inhibition of Nodulation and Nitrogen Nutrition of Leguminous Crops by Selected Heavy Metals. Air Soil Water Res. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Shang, Z.; Wu, Z.; Li, D.; Zhu, P.; Gao, H.; Zhang, L.; Gong, P. The activity and kinetic parameters of oxidoreductases in phaeozem in response to long-term fertiliser management. J. Soil Sci. Plant Nutr. 2012, 12, 597–607. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Samuela, A.D.; Domutab, C.; Ciobanub, G.; Sandorb, M.; Ciobanub, C.; Brejeab, R. Enzymological study of the evolution of the technogenic soil submitted to biological recultivation in the Bauxite Mine from Padurea Craiului (Romania). J. Environ. Prot. Ecol. 2011, 12, 535–542. [Google Scholar]
- Bartkowiak, A.; Lemanowicz, J.; Hulisz, P. Ecological risk assessment of heavy metals in salt-affected soils in the Natura 2000 area (Ciechocinek, north-central Poland). Environ. Sci. Pollut. Res. 2017, 24, 27175–27187. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Haddad, S.A.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P. The role of an urban park’s tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil. Sci. Total Environ. 2020, 741, 140446. [Google Scholar] [CrossRef]
- Bilen, S.; Turan, V. Enzymatic Analyses in Soils. In Practical Handbook on Agricultural Microbiology. Springer Protocols Handbooks; Amaresan, N., Patel, P., Amin, D., Eds.; Humana: New York, NY, USA, 2021; pp. 377–385. [Google Scholar] [CrossRef]
- Hadley, P.W.; Sedman, R.M. A health-based approach for sampling shallow soils at hazardous waste sites using the AALsoil contact criterion. Environ. Health Perspect. 1990, 84, 203–207. [Google Scholar] [CrossRef][Green Version]
- PN-ISO 10390; Chemical and Agricultural Analysis: Determining Soil pH. Polish Standards Committee: Warszawa, Poland, 1997.
- PN-R-04023; Chemical and Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- PN-R-04022; Chemical and Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- PN-R-04020; Chemical and Agricultural Analysis. Determination of the Content Available Magnesium. Polish Standards Committee: Warszawa, Poland, 1994.
- Crock, J.; Severson, R. Four reference soil and rock samples for measuring element availability in the Western Energy Regions. Geochem. Surv. Circ. 1980, 841, 1–16. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Obrador, A.; Alvarez, J.M.; Lopez-Valdivia, L.M.; Gonzalez, D.; Novillo, J.; Rico, M.I. Relationships of soil properties with Mn and Zn distribution in acidic soils and their uptake by a barley crop. Geoderma 2007, 137, 432–443. [Google Scholar] [CrossRef]
- Orwin, K.; Wardle, D. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Statistica. Data Analysis Software System, Version 12; TIBCO Software Inc.: Palo Alto, CA, USA, 2019; Available online: https://www.tibco.com/products/data-science (accessed on 28 September 2021).
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- PTG. Particle size distribution and textural classes of soil and mineral materials—Classification of Polish Society of Soil Sciences 2008. Soil Sci. Annu. 2009, 60, 5–16. [Google Scholar]
- Odom, F.; Gikunoo, E.; Arthur, E.K.; Agyemang, F.O.; Mensah-Darkwa, K. Stabilization of heavy metals in soil and leachate at Dompoase landfill site in Ghana. Environ. Chall. 2021, 5, 100308. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A. Soil Enzyme Activity Response under the Amendment of Different Types of Biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2019, 703, 135468. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420093681. [Google Scholar] [CrossRef]
- Alloway, B.J. Trace metals and metalloids in soil and their bioavailability. In Heavy Metals in Soil; Blackie and Son Ltd.: London, UK, 1990; p. 339. [Google Scholar] [CrossRef]
- Łabętowicz, J.; Rutkowska, B. Factors affecting the concentration of microelements in soil solution. Adv. Agric. Sci. 2001, 6, 75–85. [Google Scholar]
- Fijałkowski, K.; Kacprzak, M.; Grobelak, A.; Placek, A. The influence of selected soil parameters on the mobility of heavy metals in soils. Eng. Protec. Environ. 2012, 15, 81–92. [Google Scholar]
- Liu, H.-H.; Sang, S.-X. Study on the law of heavy metal leaching in municipal solid waste landfill. Environ. Monit. Assess. 2009, 165, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Tałałaj, I.A. Release of heavy metals on selected municipal landfill duping the calendar year. Annu. Environ. Protec. 2014, 16, 404–420. [Google Scholar]
- Datta, A.; Gujre, N.; Gupta, D.; Agnihotri, R.; Mitra, S. Application of enzymes as a diagnostic tool for soils as affected by municipal solid wastes. J. Environ. Manag. 2021, 286, 112169. [Google Scholar] [CrossRef]
- Tripathy, S.; Bhattacharyya, P.; Mohapatra, R.; Som, A.; Chowdhury, D. Influence of different fractions of heavy metals on microbial ecophysiological indicators and enzyme activities in century old municipal solid waste amended soil. Ecol. Eng. 2014, 70, 25–34. [Google Scholar] [CrossRef]
- Błońska, E. Seasonal changeability of enzymatic activity in soils of selected forest sites. Acta Sci. Pol. Silv. Colendar Rat. Ind. Lignar. 2010, 9, 5–15. [Google Scholar]
- Wang, W.; Page-Dumroese, D.; Lv, R.; Xiao, C.; Li, G.; Liu, Y. Soil Enzyme Activities in Pinus tabuliformis (Carriére) Plantations in Northern China. Forests 2016, 7, 112. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Li, H.; Zhou, Z.; Wang, S.; Wang, Z.; Song, C.; Zhou, Y. Distribution of phosphorus-solubilizing bacteria in relation to fractionation and sorption behaviors of phosphorus in sediment of the Three Gorges Reservoir. Environ. Sci. Pollut. Res. 2017, 24, 17679–17687. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, G.; Ma, P.; Lin, Y.; Yang, X.; Cao, C. Isolation and Characterization of a Phosphorus-Solubilizing Bacterium from Rhizosphere Soils and Its Colonization of Chinese Cabbage (Brassica campestris ssp. chinensis). Front. Microbiol. 2017, 8, 1270. [Google Scholar] [CrossRef]
- Yuan, Z.; Yi, H.; Wang, T.; Zhang, Y.; Zhu, X.; Yao, J. Application of phosphate solubilizing bacteria in immobilization of Pb and Cd in soil. Environ. Sci. Pollut. Res. 2017, 24, 21877–21884. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and Characterization of Phosphorus Solubilizing Bacteria with Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- de Miera, L.E.S.; Pinto, R.; Calvo, L.; Ansola, G. A new index of resilience applicable to external pulse-disturbances that considers the recovery of communities in the short term. Ecol. Indic. 2021, 130, 108051. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. S1), 11512–11519. [Google Scholar] [CrossRef]
- Martyn, W.; Niemczuk, B. Content of iron and aluminium in the profiles of variously utilized rusty soils. Environ. Protec. Nat. Res. 2011, 48, 287–296. [Google Scholar]
- Jaworska, H. Evaluation of the impact of the Copperwork „Głogów on the total content of manganese and its mobile forms in the vicinity of arable soils. Ecol. Chem. Eng. A 2012, 19, 403–410. [Google Scholar] [CrossRef]
- Łukasiewicz, S. The physical structure of the land, organic substances content, and the chemical composition of soil comprising the subsoil of 21 urban greenery locations in the territory of Poznań. Part IV. Content of microelements: Cl, Fe, Mn, Zn, Cu, B and Na Pb, Cd. The “EC” salinity index. Physiogr. Res. 2012, 63, 49–75. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Schwab, A.P. The chemistry of iron in soils and its availability to plants. J. Plant Nutr. 1982, 5, 821–840. [Google Scholar] [CrossRef]
- Xian, Y.; Wang, M.; Chen, W. Quantitative assessment on soil enzyme activities of heavy metal contaminated soils with various soil properties. Chemosphere 2015, 139, 604–608. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Wang, X.; Zhang, X.; Fang, L. Evaluation methods of heavy metal pollution in soils based on enzyme activities: A review. Soil Ecol. Lett. 2021, 3, 169–177. [Google Scholar] [CrossRef]
- Slunjski, S.; Coga, L.; Ćustić, M.H.; Petek, M.; Spoljar, A. Phosphorus, manganese and iron ratios in grapevine (vitis vinifera L.) leaves on acid and calcareous soils. Acta Hortic. 2012, 938, 299–306. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. For. Res. Pap. 2017, 78, 39–44. [Google Scholar] [CrossRef]
- Roy, S.; Bhattacharyya, P.; Ghosh, A.K. Influence of toxic metals on activity of acid and alkaline phosphatase enzymes in metal-contaminated landfill soils. Soil Res. 2004, 42, 339–344. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action; Soil Biology, 26; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 26, pp. 215–243. [Google Scholar] [CrossRef]
- Vasconcelos, R.D.L.; de Almeida, H.J.; Prado, R.D.M.; dos Santos, L.F.J.; Júnior, J.M.P. Filter cake in industrial quality and in the physiological and acid phosphatase activities in cane-plant. Ind. Crop. Prod. 2017, 105, 133–141. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Kumar, A.; Rai, L.C. Soil organic carbon and phosphorus availability regulate abundance of culturable phosphate-solubilizing bacteria in paddy fields. Pedosphere 2017, 30, 405–413. [Google Scholar] [CrossRef]

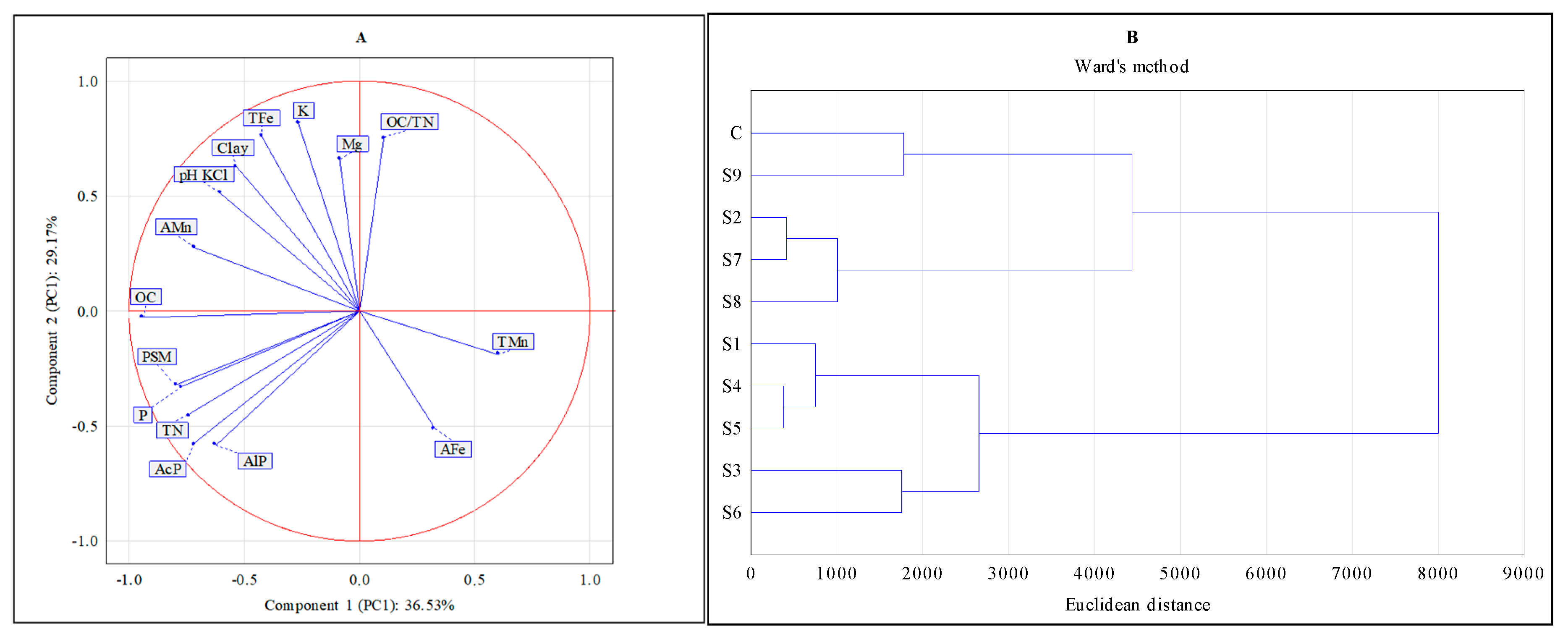
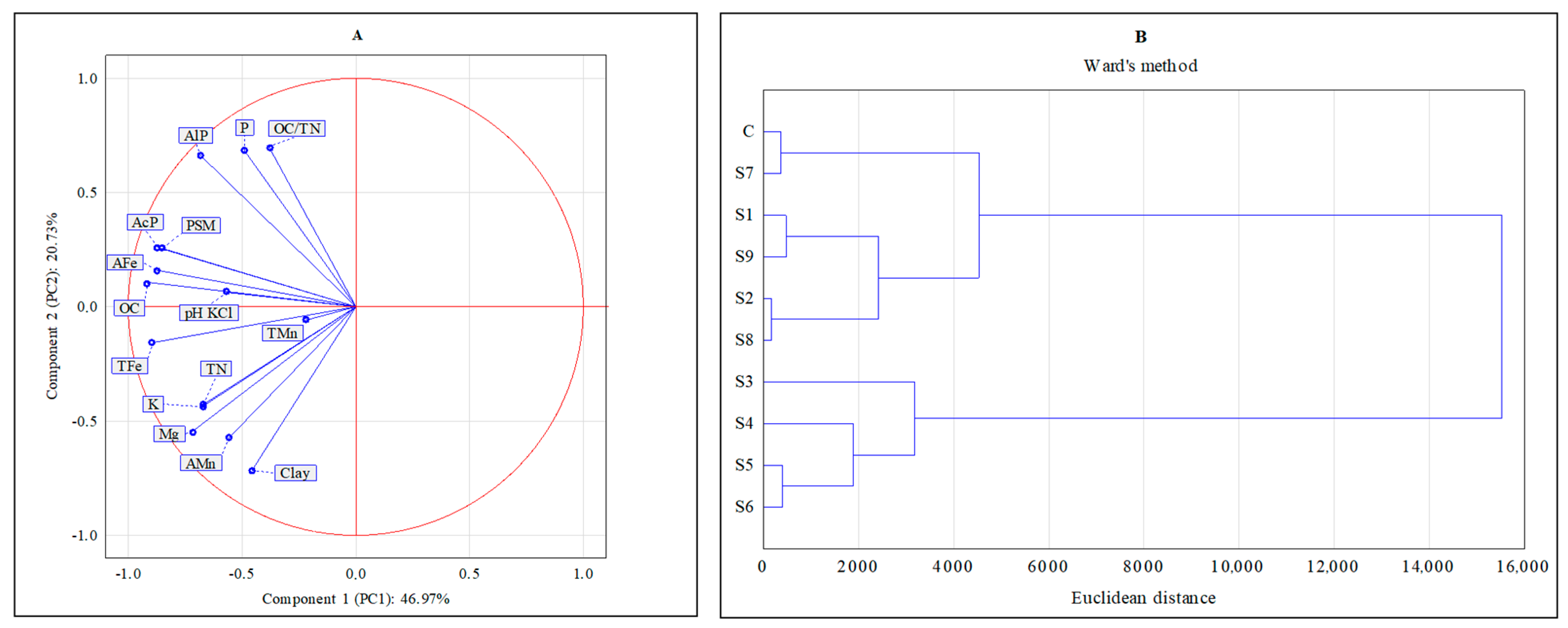
| Sites * | Clay % | pH KCl | OC [g kg−1] | TN [g kg−1] | |||
|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | ||
| C | 0.64 | 4.18 | 4.37 | 7.23 (±0.14) j | 6.81 (±0.01) g | 0.40 (±0.01) e | 0.37 (±0.03) f |
| S1 | 3.55 | 7.09 | 7.21 | 14.60 (±0.02) d | 7.39 (±0.02) f | 0.98 (±0.02) bc | 0.47 (±0.08) e |
| S2 | 4.70 | 7.39 | 7.45 | 20.4 (±0.28) b | 10.02 (±0.01) c | 1.79 (±0.02) a | 0.82 (±0.02) b |
| S3 | 5.18 | 7.58 | 7.64 | 23.32 (±0.05) a | 27.04 (±0.06) a | 1.02 (±0.03) b | 1.62 (±0.10) a |
| S4 | 0.58 | 7.61 | 7.69 | 17.14 (±0.01) c | 20.71 (±0.01) b | 0.37 (±0.01) f | 0.39 (±0.01) f |
| S5 | 4.97 | 7.57 | 7.68 | 12.23 (±0.05) f | 10.06 (±0.10) c | 0.31 (±0.01) f | 0.36 (±0.02) f |
| S6 | 5.10 | 7.55 | 7.71 | 9.54 (±0.08) h | 8.93 (±0.01) e | 0.14 (±0.03) g | 0.27 (±0.02) g |
| S7 | 1.69 | 7.6 | 7.59 | 13.89 (±0.06) e | 3.53 (±0.09) h | 0.89 (±0.02) c | 0.20 (±0.002) h |
| S8 | 3.54 | 7.65 | 7.51 | 10.21 (±0.02) g | 10.06 (±0.01) c | 0.59 (±0.03) d | 0.61 (±0.02) d |
| S9 | 1.59 | 5.40 | 5.41 | 8.90 (±0.01) i | 9.78 (±0.02) d | 0.56 (±0.02) d | 0.71 (±0.01) c |
| Sites * | P [mg kg−1] | K [mg kg−1] | Mg [mg kg−1] | |||
|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | |
| C | 8.52 (±0.26) d | 11.92 (±0.09) ef | 24.42 (±0.68) f | 43.28 (±0.91) f | 16.09 (±0.40) e | 32.00 (±1.06) e |
| S1 | 9.59 (±0.31) d | 10.09 (±0.01) f | 54.68 (±0.72) d | 73.13 (±0.47) de | 12.25 (±0.11) fg | 24.55 (±0.33) gh |
| S2 | 30.13 (±0.37) a | 16.70 (±0.26) d | 55.11 (±0.52) d | 75.73 (±0.42) de | 14.22 (±0.19) ef | 28.70 (±0.22) ef |
| S3 | 19.01 (±0.38) b | 22.18 (±0.40) c | 387.08 (±5.46) b | 531.75 (±8.24) a | 46.97 (±0.89) a | 162.3 (±0.57) a |
| S4 | 28.34 (±0.61) a | 54.62 (±1.13) a | 56.33 (±0.68) d | 85.75 (±0.53) d | 11.79 (±0.16) g | 23.55 (±0.49) h |
| S5 | 10.10 (±0.43) d | 13.93 (±0.52) de | 74.10 (±1.01) c | 139 (±1.16) c | 15.11 (±0.45) e | 49.80 (±0.48) d |
| S6 | 4.91 (±0.40) e | 15.74 (±0.37) d | 406.8 (±1.90) a | 515 (±1.86) b | 40.24 (±0.41) b | 79.25 (±0.76) b |
| S7 | 5.905 (±0.11) e | 12.41 (±0.30) ef | 41.32 (±0.61) e | 68.01 (±0.49) e | 14.99 (±0.27) e | 27.65 (±0.74) fg |
| S8 | 15.92 (±0.48) c | 41.83 (±0.86) b | 42.81(±0.52) e | 68.68 (±0.55) e | 27.89 (±0.29) c | 55.20 (±0.67) c |
| S9 | 6.335 (±0.16) e | 10.62 (±0.19) f | 26.12 (±0.49) f | 52.65 (±0.47) f | 25.49 (±0.50) d | 50.51 (±0.91) d |
| Sites * | MnT [mg kg−1] | FeT [mg kg−1] | ||
|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | |
| C | 299 (±7.21) a | 258 (±10.14) b | 5671 (±51.37) f | 3423 (±9.84) g |
| S1 | 228 (±10.11) b | 134 (±17.36) d | 7053 (±6.69) c | 5224 (±21.08) e |
| S2 | 219 (±12.76) b | 219 (±8.05) bc | 6188 (±11.78) d | 6361 (±29.31) d |
| S3 | 220 (±11.67) b | 261 (±22.86) b | 8745 (±23.72) a | 10738 (±28.72) a |
| S4 | 224 (±1.24) b | 250 (±16.91) bc | 7581 (±13.15) b | 8431 (±19.57) c |
| S5 | 205 (±4.16) bc | 341 (±15.21) a | 7768 (±16.20) b | 9138 (±6.88) b |
| S6 | 227 (±0.279) b | 208 (±7.49) c | 8842 (±18.19) a | 9193 (±10.15) b |
| S7 | 161 (±4.23) c | 120 (±7.30) d | 5889 (±68.54) e | 3652 (±29.33) g |
| S8 | 301 (±5.09) a | 310 (±8.92) a | 5690 (±63.78) f | 6394 (±36.75) d |
| S9 | 290 (±8.09) a | 344 (±3.96) a | 4003 (±33.34) g | 4806 (±5.91) f |
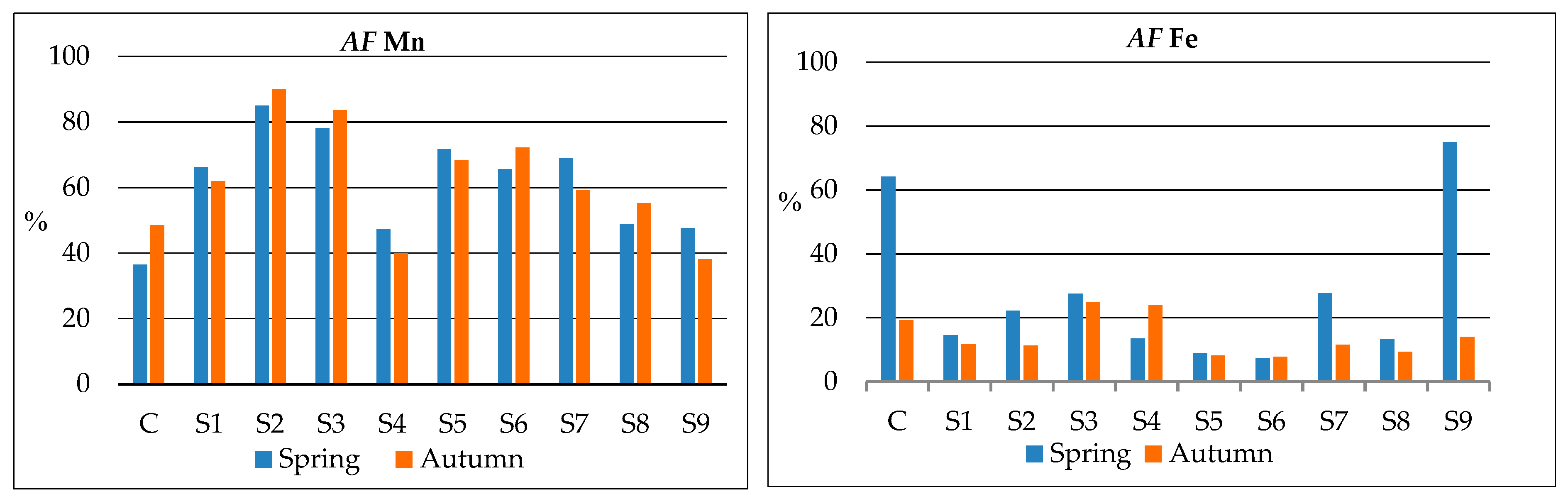
| Sites * | AMn [mg kg−1] | AFe [mg kg−1] | ||
|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | |
| C | 109 (±9.28) de | 125 (±8.28) de | 3641 (±20.98) a | 656 (±19.2) def |
| S1 | 151 (±6.20) ab | 83 (±4.00) f | 1023 (±19.95) f | 613 (±12.95) ef |
| S2 | 186 (±8.09) a | 197 (±4.40) ab | 1377 (±22.82) e | 721 (±12.12) cd |
| S3 | 172 (±3.58) ab | 218 (±5.64) a | 2406 (±14.77) c | 2684 (±21.41) a |
| S4 | 106 (±2.65) e | 100 (±6.28) ef | 1024 (±15.85) f | 2015 (±29.73) b |
| S5 | 147 (±5.30) bcd | 233 (±9.89) a | 696 (±11.49) g | 749(±10.70) c |
| S6 | 149 (±9.38) abc | 150 (±7.49) cd | 657 (±13.73) gh | 714 (±10.41) cd |
| S7 | 111 (±9.21) cde | 71 (±9.42) f | 1629 (±23.84) d | 422 (±9.86) g |
| S8 | 147 (±2.28) bcd | 171 (±5.44) bc | 758 (±16.13) h | 593 (±10.12) f |
| S9 | 138 (±9.60) bcde | 131 (±4.28) de | 3003 (±20.34) b | 674 (±9.28) de |
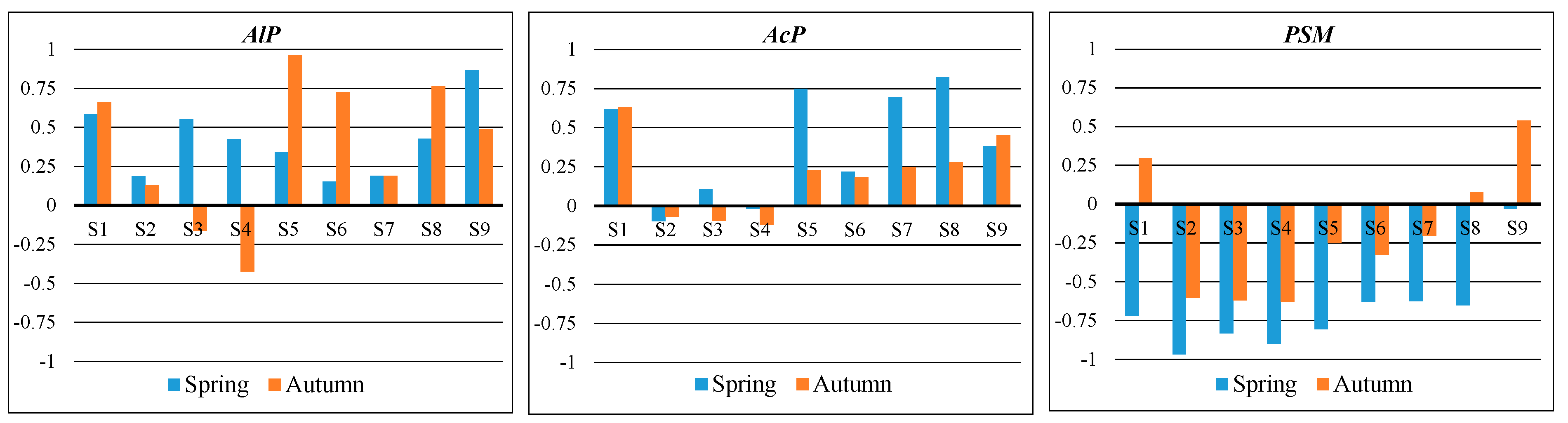
| Sites * | AlP [mM pNP kg1 soil h−1] | AcP [mM pNP kg1 soil h−1] | PSM [jtk g−1] | |||
|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | |
| C | 0.847 (±0.01) c | 0.537 (±0.01) ef | 1.32 (±0.04) ef | 1.58 (±0.05) c | 1.77 (±0.61) c | 7.55 (±1.08) b |
| S1 | 1.07 (±0.02) b | 0.428 (±0.01) f | 1.63 (±0.03) e | 1.95 (±0.06) c | 12.59 (±1.64) b | 3.45 (±0.59) b |
| S2 | 1.43 (±0.07) a | 0.957 (±0.03) c | 2.93 (±0.04) a | 3.26 (±0.02) ab | 118.97(±28.98) a | 38.27 (±16.14) a |
| S3 | 1.09 (±0.03) b | 1.29 (±0.01) b | 2.39 (±0.06) b | 3.32 (±0.01) ab | 21.33 (±7.39) b | 39.73 (±14.6) a |
| S4 | 1.19 (±0.02) b | 1.87 (±0.06) a | 2.69 (±0.03) a | 3.50 (±0.05) a | 35.87 (±7.06) b | 40.51(±5.79) a |
| S5 | 0.428 (±0.01) e | 0.529 (±0.02) ef | 1.51 (±0.04) de | 2.36 (±0.03) bc | 18.40 (±3.19) b | 20.17 (±0.04) ab |
| S6 | 0.221 (±0.01) f | 0.625 (±0.03) de | 0.473 (±0.03) g | 2.60 (±0.04) ab | 9.57 (±3.55) b | 22.49 (±1.86) ab |
| S7 | 0.269 (±0.02) f | 0.907 (±0.04) c | 1.56 (±0.06) de | 2.49 (±0.55) bc | 9.46 (±1.26) b | 19.01 (±4.19) ab |
| S8 | 0.507 (±0.01) de | 0.611 (±0.02) de | 1.19 (±0.02) f | 2.35 (±0.01) c | 10.15 (±2.26) b | 14.00 (±4.37) b |
| S9 | 0.786 (±0.01) c | 0.725 (±0.01) d | 1.91 (±0.08) c | 2.09 (±0.02) c | 3.65 (±0.63) c | 5.28 (±4.60) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemanowicz, J.; Bartkowiak, A.; Breza-Boruta, B.; Sowiński, P.; Haddad, S.A.; Jaskulska, I. The Impact of Municipal Waste on Seasonal and Spatial Changes in Selected Macro- and Micro-Nutrient Contents on the Background of Soil Biological Activity—A Case Study. Minerals 2023, 13, 47. https://doi.org/10.3390/min13010047
Lemanowicz J, Bartkowiak A, Breza-Boruta B, Sowiński P, Haddad SA, Jaskulska I. The Impact of Municipal Waste on Seasonal and Spatial Changes in Selected Macro- and Micro-Nutrient Contents on the Background of Soil Biological Activity—A Case Study. Minerals. 2023; 13(1):47. https://doi.org/10.3390/min13010047
Chicago/Turabian StyleLemanowicz, Joanna, Agata Bartkowiak, Barbara Breza-Boruta, Paweł Sowiński, Samir A. Haddad, and Iwona Jaskulska. 2023. "The Impact of Municipal Waste on Seasonal and Spatial Changes in Selected Macro- and Micro-Nutrient Contents on the Background of Soil Biological Activity—A Case Study" Minerals 13, no. 1: 47. https://doi.org/10.3390/min13010047
APA StyleLemanowicz, J., Bartkowiak, A., Breza-Boruta, B., Sowiński, P., Haddad, S. A., & Jaskulska, I. (2023). The Impact of Municipal Waste on Seasonal and Spatial Changes in Selected Macro- and Micro-Nutrient Contents on the Background of Soil Biological Activity—A Case Study. Minerals, 13(1), 47. https://doi.org/10.3390/min13010047








