Abstract
Currently, the use of called “green solvents” is a challenge that must be assumed by the industry, since they contribute to a friendly process and where its use has been extended, causing research needs, and recognition of application and analysis of potential new uses. The main objective of this study was to analyze the global scientific production related to the use of glycine or glutamate, amino acids that are used in mineral/waste leaching processes for the recovery of metals of interest, in an environmentally friendly manner. A literature search was performed using the Web of science database. Australia is identified as the country that occupies the first place with 17 (41.4%) published documents. For its part, the Hydrometallurgy journal with 13 (34.2%) documents and the Minerals Engineering journal with 10 (26.6%) documents are the journals with the highest number of documents published. In turn, Curtin University 14 (36.8%) is identified as the institution that presents the greatest leadership with respect to the number of publications. This study offers a first approach to the global efforts aimed at this new area of research, which in the last 5 years (2018–2022) has developed most of its publications and where an effort to increase scientific productivity is necessary.
1. Introduction
Currently, sustainable industrial processes are gaining great relevance worldwide [1,2,3]. In Chile, the development of green or sustainable processes in the mining industry is being discussed. The use of clean technologies, the certification of production and export of minerals in a sustainable manner, efficient use of resources such as water and energy, consideration of issues of security and quality of life of society, are aspects that must be implemented in the short-term and medium-term. A responsible extraction of resources is expected [4,5,6].
On the other hand, the United Nations in 2015 established global objectives as part of the 2030 agenda, where 17 objectives and 169 goals were indicated to be achieved by the year 2030 [7,8]. Mining can play a relevant role in promoting decent work and economic growth, developing affordable and non-polluting energy, and encouraging the development of industry, innovation and infrastructure, among other things [9]. For that reason, green mining processes should be developed to reduce adverse environmental impacts. An alternative to implementation is the use of so-called green solvents (environmentally friendly solvents [10]). Green solvents are related to the principles of green chemistry where Gu and Jerôme [11] established 12 criteria to be met by these solvents: synthesis, availability, price, flammability, recyclability, biodegradability, grade, toxicity, performance, stability, storage and renewability. Moreover, Anastas and Warner [12] proposed another definition of a green solvent. A green solvent is a new and apparently benign solvent from the point of view of the environment and the health of living beings, compared to the conventionally used solvent that must be replaced. In addition, Jessop [13] established that it is necessary to determine the environmental impact of a solvent through life cycle analysis (LCA), considering from its manufacture to its recycling or disposal. Häckl and Kunz [14] classified new green solvents as: ionic liquids (IL), ephemeral solvents such as deep eutectic solvent (DES), switchable solvents and hydrotropes. Moreover, Cvjetko et al. [10] added supercritical and sub-critical fluids.
Within the so-called green solvents are the amino acids that are structural units of peptides that form proteins. Two amino acids can combine, with the loss of a water molecule, to form a peptide containing an amide bond (CO-NH) between the two amino acids. There are 20 common amino acids of great importance. Since they contain acidic (COOH) and basic (NH2) groups in the same molecule, they exist as zwitterions. The pH of an aqueous solution of an amino acid determines the predominant form in which it is present. Many amino acids can be obtained in industrial quantities by hydrolysis of proteins or as by-products of fermentation. They can also be obtained in laboratories by synthetic procedures.
Glycine is the smallest of the amino acids. It consists of a single carbon molecule attached to an amino acid and a carboxyl group whose formula is NH2-CH2-COOH (global formula, C2H5NO2). Glycine can exist in aqueous solutions in three different forms: NH3CH2COOH+ (cationic glycinium ion), NH3+CH2COO− (neutral zwitterion) and NH2CH2COO− (anionic glycinate) [15,16]. Glycine has been used in leaching processes for the recovery of different metals from different solid matrices such as minerals, mineral residues, electrical and electronic waste [17,18,19].
Monosodium glutamate is one of the most abundant non-essential amino acids in nature derived from glutamic acid. Its molecular formula is C5H8NNaO4. It is a non-volatile reagent. Currently, the efficacy of glutamate as a leachate has been tested due its low price, availability, biodegradability, reusability. In alkaline conditions, it is a powerful ligand with metals (Cu, Au, Zn, Mg, Ca) [19,20,21].
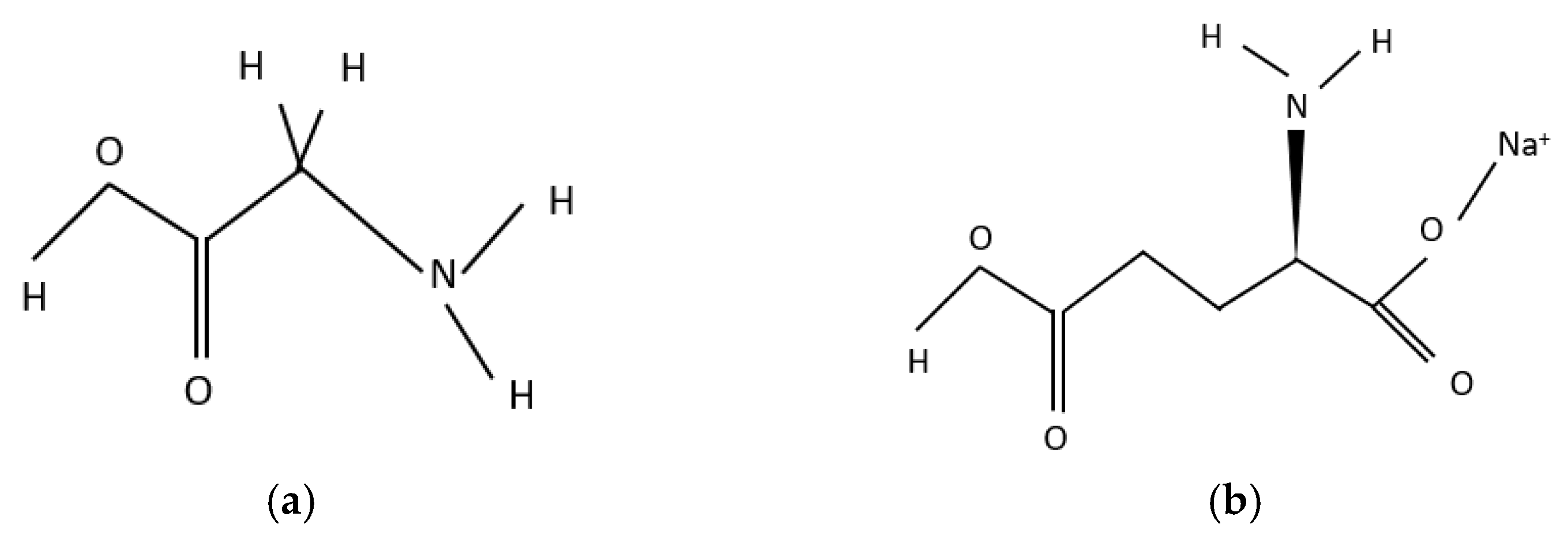
In Figure 1, the molecular structures are shown.

Figure 1.
Molecular structure: (a) glycine, (b) monosodium glutamate. Source: Pubchem https://pubchem.ncbi.nlm.nih.gov/compound/750; https://pubchem.ncbi.nlm.nih.gov/compound/23672308 (accessed on 24 October 2022).
Considering that it is necessary to develop environmentally friendly mining processes, the use of amino acids in the leaching process can provide a sustainable alternative to traditional processes. It is for this reason that the objective of this study is to make a global review, with current data, of the scientific articles published in recent years that include these amino acids (glycine and glutamate) as leaching media, to learn about the advances related to this topic and identify future challenges for their use implementation on an industrial scale. These amino acids were chosen because they have been used in several mineral/waste dissolution processes. Moreover, this review considers different metals extraction such as Cu, Au, Zn, Pd, Ag, U, Cd, Li, Co, Ni, Pb and Fe using these solvents.
2. Methodology
For the search and identification of the documents, the Web of Science (WOS) database, commonly used for bibliometric analyses [22], was used. This process was performed on 11 August 2022 using the following search algorithm: [[Glycine] OR [Glutamate] AND [leaching]] linked to article content, abstract and keywords. The search included documents from the last 5 years (January 2018–August 2022).
Initially, 150 results were found and the following types of documents were recorded: articles, reviews and short communications. Publications called, letters, prologues and those that were not related to the proposed topics were excluded. The eliminated documents were related to bioleaching, agriculture, geology, health and environment topics. Thus, the final sample included 38 documents, which were divided into 37 original articles and 1 review article.
With the extracted documents, the following data were organized in a database: name of the signatory authors, title of the publication, type of publication, year of publication, type of access to the publication, abstract, institutions of affiliation of the signatory authors, journal of publication, country of edition, number of citations received, together with the technical study performed by each article as a type of solid used, metal to be recovered, experimental conditions such as particle size, stirring speed, temperature, use of oxidants, among other variables.
This information initially allowed a descriptive analysis to be carried out and to identify the countries, journals and institutions that are publishing the most on the subject.
Secondly, the total number of authors (n = 105) were considered and the collaboration indices of Lawani [23] and Subramanyam [24] were estimated.
The Lawani collaboration index (IL) corresponds to the weighted average of authors per article, distributed in the defined publication period. It is calculated from the following Equation (1):
where N is the total number of articles, j represents the number of authors per article, fj is the number of articles with multiple authorship.
The degree of collaboration (GC) or Subramanyan index (IS) corresponds to the proportion of articles with multiple authorship (2 or more authors). It is calculated from the following Equation (2):
where Nm is number of articles with multiple authorship, Ns is number of articles with single authorship (one author). The maximum value that this index can reach is 1 and it corresponds to all published articles having at least two authors.
The most cited publications were then identified and the mean citations were estimated. Finally, with the support of the VOSviewer software [25], a network was developed with the main thematic axes associated with the keywords of the publications.
3. Results
3.1. Bibliometric Analyses
The documents were published by authors who signed with institutions of affiliation from 14 countries; the year with the highest number of articles was 2021 with a total of 13; there was a small growth from 2018 to date in the number of publications, with an increase of approximately 3 articles per year on average, and 8 of the publications were open access. Australia ranked first in the number of documents published with respect to green solvents, with 17 publications and 41.4% of world production. Australia was followed by Egypt with 11 publications (27%). The list of the first 6 countries can be seen in Table 1 (considering that an article can belong to two or more different countries).

Table 1.
Countries versus number of publications (data from 2018 to 2022).
Table 2 shows the top 3 journals in which articles related to green solvent were published. Thirteen papers (31.7%) were published in Hydrometallurgy, while 11 (27%) were published in Minerals Engineering and 4 (9.8%) in Separation and Purification Technology.

Table 2.
Main journals where articles have been published considering glycine in leaching (data from 2018 to 2022).
Of this ranking, all journals are in quartile Q1 of WOS. It is important to note that 30 articles correspond to the Publisher Elsevier. In addition, according to WOS, most of the journals that have published on the subject are classified in the areas of Metallurgy &Metallurgical Engineering (17), Engineering Chemically (14), Mineralogy (12), Mining Mineral Processing (12). On the other hand, the top 6 institutions with the highest number of documents correspond to Curtin University 14 (34%), Egyptian Knowledge Bank Ekb 11 (27%) and Assiut University 10 (24%), Amirkabir University of Technology 5 (12.2%) and Aalto University 4 (9.8%). They reach being in this list with 44 publications in total (Table 3).

Table 3.
Top 6 institutions with the highest number of publications (data from 2018 to 2022).
As for the collaboration between the signatory authors, a Lawani index of 97% and a Subramanyam index of 97% were obtained. The first index indicates the weighted average of authors who sign an article [23], observing that 4.5 on average authors signed the 38 documents analyzed.
For its part, the second index indicates the proportion of publications with multiple authorships, indicating the values close to 1 that most of the works were published by two or more authors [24]. Thirty-four (89.47%) documents had 3 or more authors, the maximum number of authors corresponds to 10 and 1 publication with 1 signatory author was also identified, which corresponds to a scientific article from the WOS area of Metallurgy & Metallurgical Engineering. The total number of citations from all publications was 1626 and there was an average of 42.79 citations. There are 2 articles with more than 80 citations. The most cited article is called “Chalcopyrite leaching in novel lixiviants”, with 137 citations, in the journal Hydrometallurgy, corresponding to a review paper. Four of the 6 most cited articles were published in Hydrometallurgy, an Elsevier editorial journal. The most cited articles can be viewed in Table 4.

Table 4.
Articles most cited (data from 2018 to 2022).
The evolution of citations in the last 4 years is presented in Table 5, which highlights the growth presented in 2021; they are 67.8 on average per year.

Table 5.
Citations of the last 4 years (data from 2018 to 2022).
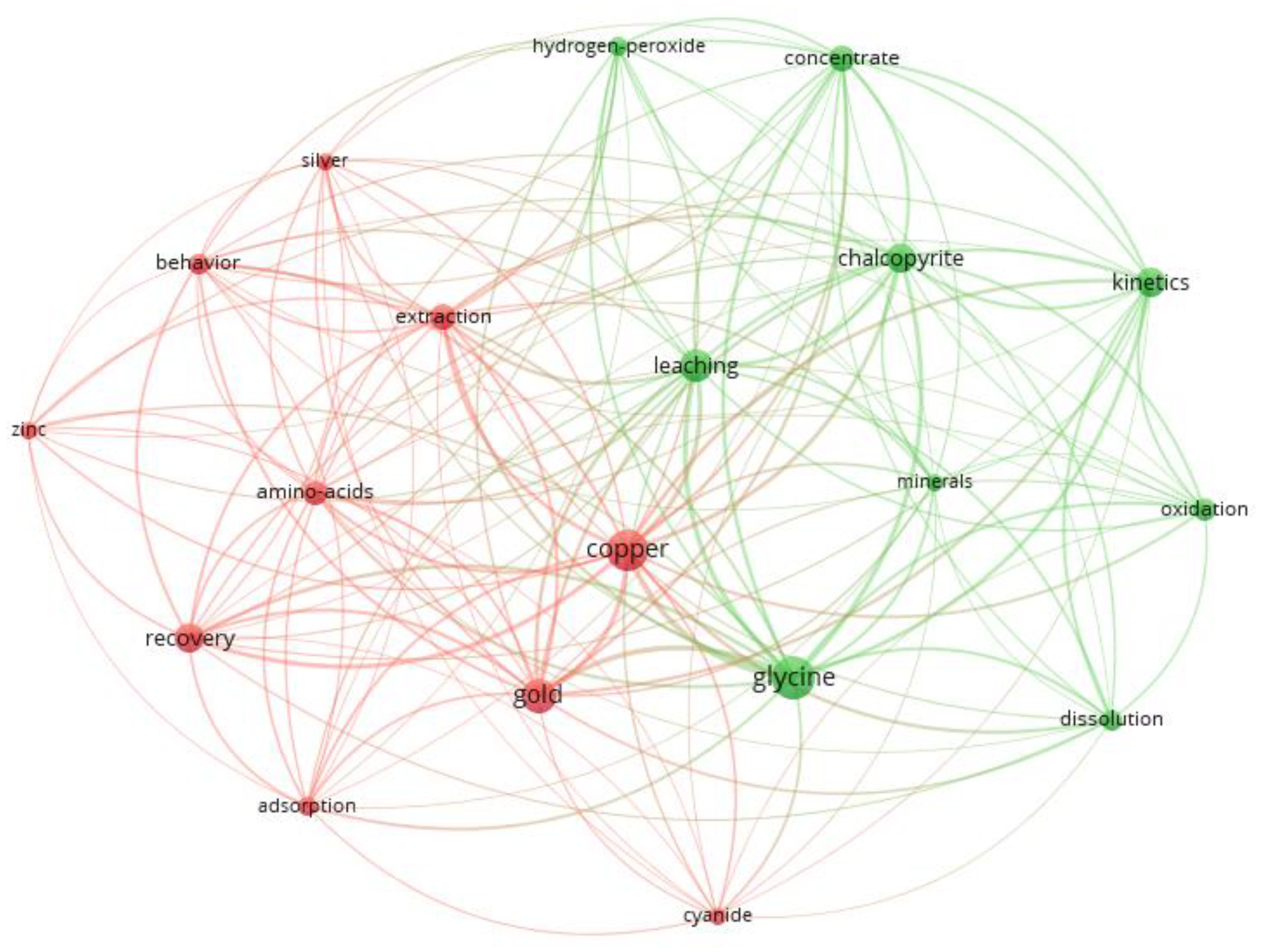
Figure 2 shows the main keywords associated with research with glycine. You can see 2 clusters represented by 2 different colors that group the various relationships of the concepts and in which the recitation networks can be visualized. The size of the nodes, added to the central and peripheral of these, allows one to appreciate the links of one concept with another.
Figure 2.
Keywords associated with research in glycine: cluster 1 (red) and cluster 2 (green).
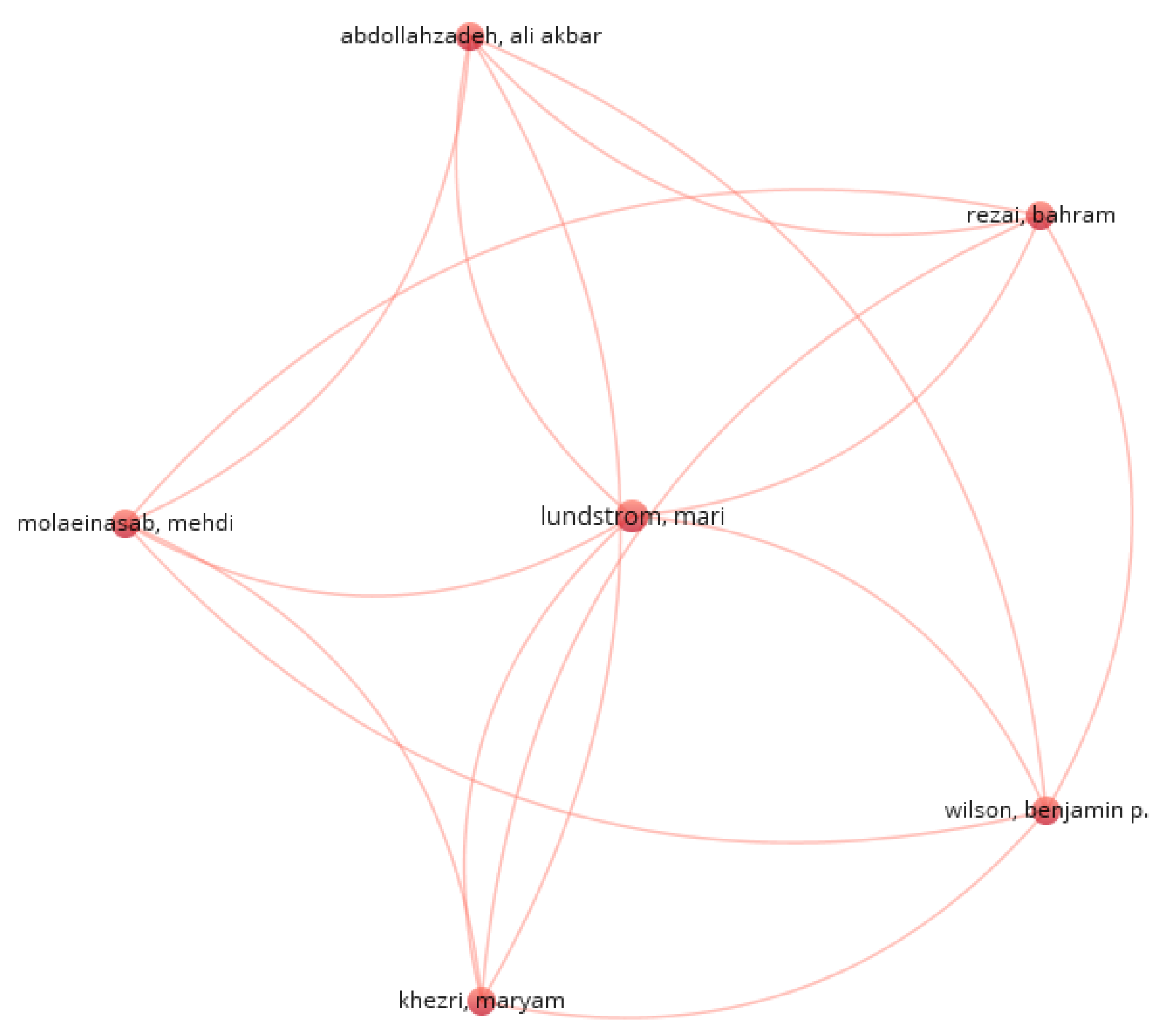
Considering a relationship between authors of at least 2 works together, a relationship of 9 items and 2 clusters that are not related to each other is obtained. It can be seen in Figure 3.

Figure 3.
Relationship between authors.
Only two manuscripts were found using glutamate as the leaching medium [19,20].
3.2. Content Analyses
In the Table 6, a resume of operational conditions of several laboratory test using glycine to leach metals is shown.

Table 6.
Operational leaching conditions of laboratory test using glycine (data from 2018 to 2022).
According to Table 6, different metals have been chosen to be studied in these systems using amino acids.
Several studies using glycine have been related to gold dissolution as an alternative to traditional cyanide or as a mixed cyanide/glycine system.
Eksteen et al. [15] presented a review of articles that use glycine for the dissolution of gold and copper in different solid matrices (concentrates, ores and wastes) compared to the traditional system using cyanide. Through the study, the authors determined that glycine produces slow dissolution kinetics which may be suitable for industrial use in heaps, vats and in situ leaching. In agitation leaching, the use of cyanide and glycine as a synergistic system is adequate, which produces high extractions of the metal to be recovered.
Wang et al. [58] studied the thermodynamics of glycine and thiosulfate systems for gold and copper recovery as an alternative to cyanide. Through Pourbaix and speciation diagrams, the authors determined the predominant complex species under certain conditions. Glycine has a wide availability zone at alkaline and neutral pH which helps stabilize cupric in thiosulfate solutions. In addition, glycine can also serve as a lixiviant for the dissolution of gold in a thiosulfate medium thanks to the formation of cupric–glycine complexes that could act as catalysts.
Altinkaya et al. [46] and Wu et al. [53] conducted studies using gold concentrate. In general, extractions of the order of 90% Au and 93% Au, respectively, were obtained.
Altinkaya et al. [46] determined that under the conditions studied (see Table 6), the glycine concentration did not have a significant effect on gold dissolution, contrary to temperature and pH. Through a statistical model, they determined that the optimal conditions for the dissolution of gold were 1.25 M of glycine, pH = 12 and T = 60 °C, for a gold extraction of 87%, which was corroborated experimentally obtaining 90% Au.
Wu et al. [53] studied the leaching of a gold concentrate in a mixed system of ammonium thiocyanate and glycine. The authors determined that the mixed system achieved an efficiency of 93% Au at 25 °C, thanks to the addition of ferric in the solution, forming a ferric–glycine complex.
Barani et al. [34], Barani et al. [43], Orabi et al. [45] and Oraby et al. [52] investigated the leaching of gold ores using glycine, at basic pH, obtaining gold extractions of 92%, 89%, 85% and 85%, respectively. A mixed system of cyanide with glycine [34,43] was used adding H2O2 as oxidant and modifying the pH through the use of NaOH or H2SO4.
Orabi et al. [52] studied an in situ leaching system as an alternative to mineral processing that otherwise would not be economically feasible. The authors verified that about 85% Au can be recovered by this route, with low dissolution of impurities and high selectivity.
Azadi et al. [28] developed a microfluidic device for in situ gold leaching using alkaline glycine as leachant, with promising results. The authors indicated that this microchannel system has great potential for future industrial uses in in situ leaching.
Using mining waste such as tailings Li et al. [33], Picazo-Rodríguez et al. [44], Wu et al. [39] and Munive et al. [50] investigated the dissolution of gold.
Li et al. [33] studied the leaching of gold without cyanide, using a system composed of glycine and ammonia in the presence of permanganate for a copper–gold tailings. A maximum extraction of gold and copper of 77% and 65%, respectively, was achieved.
Picazo-Rodríguez et al. [44] conducted studies about the recovery of gold and silver from a residue using cyanide and cyanide–glycine mixtures, finding low recovery of these metals, mainly due to the effect of gangue produced such as jarosite and sulfur. They determined that a pretreatment of the residue improved the extraction of gold from 40% to 88% and silver from 65% to 94%.
Wu et al. [39] studied pressure leaching of a chalcopyrite leach residue for gold recovery. A synergistic effect between glycine and cyanide was observed, increasing gold extraction to 85%.
Munive et al. [50] conducted studies using the leaching of a mining tailings for the recovery of gold and silver for the development of a sustainable mining process. Thiosulfate, glycine and thiosulfate–glycine mixture were used. The mixture of leachants obtained promising results, providing an environmentally friendly alternative to process a mining waste.
Gold and other metals (Ag, Pd, Cu, Zn, Pb) have also been recovered using glycine from e-waste leaching. Li et al. [32], Li et al. [40] and Oraby et al. [48] studied the dissolution of metals from printed circuit boards (PCBs). Extractions over 80% were obtained from different metals at temperatures < 55 °C. Perea and Restrepo [19] studied the leaching of gold from electrical contacts pins and sheets of pure gold using glycine or glutamate in a medium with permanganate or hydrogen peroxide.
With respect to copper extraction using glycine, Barton and Hiskey [16] developed a review of novel lixiviants to chalcopyrite. In that review, several studies using glycine were reported.
Attia and Awny [35] studied the leaching of copper and uranium from an ore using glycine with the help of H2O2 as an oxidant. Extractions of 98% Cu and 85% U were achieved.
Nicol [57] carried out an electrochemical study on the use of ammonium chloride or glycine as leachates in heap leach for chalcopyrite minerals. According to the results obtained, the use of these reagents shows promising results, but it is necessary to evaluate their economic feasibility on an industrial scale.
Hidalgo et al. [26] studied thermal stability of different leaching systems, including glycine, to dissolve copper from chalcopyrite samples (cube of 4 mm by side) at 110 and 170 °C.
O’Connor et al. [27] carried out electrochemical tests using a chalcopyrite electrode in glycine solutions. Passivation zones were not found under the tested conditions.
O’Connor et al. [55] studied the electrochemical properties of metallic copper dissolution in a glycine leaching system. The authors found that there is a stage of maximum corrosion at a pH between 10 and 10.5, 0.3 M glycine with a maximum temperature of 60 °C. The formation of copper glycinate complex help to dissolution due that the specie acts as an oxidant agent.
Tanda et al. [30] conducted studies of leaching of chalcopyrite samples in a leaching medium with glycine and oxygen. According to surface analyses, sulfur is not formed on the surface and an iron hydroxide species was identified.
Tanda et al. [56] carried out tests of leaching of natural chalcocite in glycine solutions with oxygen. They determined the presence of covellite in samples of leached ore. Because of this, the authors postulated that chalcocite leaching occurs in two stages. The maximum copper extraction reached was 78% in particle size under 20 µm at 25 °C.
Khezri et al. [36] studied the effect of mechanical activation and electrochemistry of chalcopyrite in a glycine system of leaching. The authors determined that a glycine leaching of chalcopyrite concentrate pretreated with mechanical activation could be an interesting method of process.
Khezri et al. [47] conducted studied of chalcopyrite concentrate leaching in presence of glycine. Several parameters were studied. Increase in the temperature from 30 to 60 °C, increase in the copper extraction; but the increase above 60 °C produces a decrease in the dissolution of copper due to the formation of glycinate and decrease of oxygen available. A maximum of 90% Cu was achieved.
Shin et al. [54] studied the leaching system glycine–peroxide to dissolve copper from chalcopyrite concentrate. A 42% of copper extraction was achieved.
Copper from printed circuit boards (PCBs) was recovered by leaching using glycine with efficiency of about 94–96% Cu at temperatures < 55 °C [18,29,37].
In the review of 38 articles, 4 of them mention metal recovery processes from batteries. The main metal to recover indicated in the articles is cobalt, which presents recovery percentages from 76% [51], using alkaline glycine solutions in slightly oxidizing environments, up to 97% [17] obtained from lithium-ion batteries (LIB), using glycine in 300 g/L and 10% H2O2. The results for cobalt are interesting, since in traditional smelting processes the recovery rates remain low, together with a high loss in the slag. Lithium is another of the minerals with a high recovery rate where glycine was applied as the leachant for the hydrothermal leaching of lithium-ion battery (LIB) cathode materials, LiCoO2 and LiNiO2, at 90–180 °C for 5–90 min. LiCoO2 was completely leached at 180 °C for 30 min with 100% high efficiency [41].
Oghabi et al. [49] reported a selective leaching of Cd from spent Ni-Cd battery using alkaline glycine solution reaching over 80% Cd extraction.
Only two studies were reported about zinc leaching using glycine. Khodaei et al. [31] proposes a process for the selective leaching of zinc from a low-grade carbonate source through the use of glycine. Response Surface Methodology (RSM) was applied in conjunction with Central Composite Design (CCD) to optimize glycine concentration, pH, and temperature levels. The conditions to achieve more than 90% zinc leaching were glycine concentration = 1.5 M, pH = 9.5 and temperature = 70 °C; the leaching percentages of the main impurities were negligible.
Huang et al. [38] studied the zinc–cobalt separation using glycine as a leaching agent from zinc–cobalt slag. Recovery of zinc and cadmium of 94% and 88%, respectively, were obtained under optimal conditions of pH 10, 45 °C, concentration of glycine of 100 g/L, L/S ratio of 40:1 and a reaction time of 180 min.
Prasetyo et al. [20] studied the leaching of zinc and copper using monosodium glutamate as a lixiviante. High efficiency of dissolution was reached with 99% Zn and 86% Cu. Possibility of monosodium glutamate recycling from pregnant leaching solutions was demonstrated.
3.3. Perspectives of Applications of Use of Glycine/Glutamate
According to the results analyzed, the use of glycine and/or glutamate turns out to be a promising process to continue investigating. This is based on the satisfactory results obtained in the revised manuscripts for the dissolution of the different metals studied.
It is not yet possible to determine if its use at an industrial level is possible with the information collected, but future use can continue to be projected due to the advantages that this type of leachant provides.
In the recovery of several metals, it showed promising results with high extractions, especially of gold, copper, lithium and cobalt, from different solid matrices. The advantages of using these amino acids refer to their biodegradability, which can support sustainable mining through environmentally friendly processes. In addition, these amino acids are non-toxic, non-volatile, stable and environmentally safe reagents. According to the literature review, these systems with amino acids can be used for the dissolution of acid-consuming minerals.
Despite the recent increase in research on the leaching of minerals/concentrates/residues into new leachants such as these two amino acids chosen for this review, much remains to be learned about it. There is a particular need for detailed kinetic studies on different solid samples. It is necessary to consider the effects of multiple fractions of different sizes, agitation speeds, concentrations of amino acids and oxidants, determination of the dissolution mechanism in this type of system, control of the redox potential, consideration of thermodynamic and speciation aspects.
In addition, consider testing on a larger scale, weighing in on a possible future scale-up at an industrial level.
Undoubtedly, more research should be carried out on these systems, including economic aspects.
4. Conclusions
The main conclusions of this article are summarized in the following points:
- (1)
- Australia is the country with the highest number of published documents on green solvents;
- (2)
- The largest number of documents published on this subject is mainly in journals associated with Editorial Elsevier and from Australia, among which stand out the 6 in scientific journals Hydrometallurgy and Minerals Engineering with the highest number of published documents located in quartile 1 (Q1);
- (3)
- The citations of the most cited documents correspond mainly to scientific articles. The article with the highest number of citations is called “Chalcopyrite leaching in novel lixiviants” and was published in Hydrometallurgy;
- (4)
- The institution with the highest number of published documents is Curtin University;
- (5)
- The relationships analyzed through the keywords show how it is still a challenge to account for interdisciplinary in a concentrated field of knowledge;
- (6)
- It is important to hypothesize that the release of a significant number of publications in open access will be possible once the use of green solvents is recognized, recognizing potentialities and achieving competitive prices;
- (7)
- Promissory results of metals dissolutions were achieved by several authors, showing that the amino acids systems can be an alternative to traditional leaching processes;
- (8)
- Kinetic studies on different solid samples are necessary to consider including determination of the dissolution mechanism in this type of system, control of the redox potential, consideration of thermodynamic and speciation aspects;
- (9)
- Larger scale tests should be carried out to provide more information on the dissolution system using amino acids considering the economic aspects and operating conditions of mining plants. This information will be useful in a possible future scale-up at an industrial level;
- (10)
- It will be interesting in future studies to perform this analysis in other databases such as SCOPUS and in databases associated with project and intellectual property registries.
Author Contributions
Conceptualization, content and formal analysis, and writing, P.H.; methodology, literature search and writing, I.J.; content analysis, data curation and writing, P.C.; draft preparation, M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FOVI210007, ANID-Chile.
Data Availability Statement
Not applicable.
Acknowledgments
Ingrid Jamett, Paulina Carrasco, Monique Olmos and Pía Hernández thanks Universidad de Antofagasta. Pía Hernández thanks ANID-Chile, for financing this research through the FOVI210007 project, and also, the ANID/ACT210027 project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, H.; Zhu, X. Research on green innovation performance of manufacturing industry and its improvement path in China. Sustainability 2022, 14, 8000. [Google Scholar] [CrossRef]
- Allan, B.; Lewis, J.I.; Oatley, T. Green Industrial Policy and the Global Transformation of Climate Politics. Glob. Environ. Politics 2021, 21, 1–19. [Google Scholar] [CrossRef]
- Shahzad, M.; Qu, Y.; Rehman, S.U.; Zafar, A.U. Adoption of green innovation technology to accelerate sustainable development among manufacturing industry. J. Innov. Knowl. 2022, 7, 100231. [Google Scholar] [CrossRef]
- Corporación-Alta-Ley. Minería Verde. Oportunidades y Desafíos; Corporación-Alta-Ley: Santiago, Chile, 2021. [Google Scholar]
- Andes-Pacific-Technology-Access (APTA). Minería del Futuro para una Operación Inteligente y Sustentable; Andes-Pacific-Technology-Access (APTA): Santiago, Chile, 2021. [Google Scholar]
- Corporación-Alta-Ley. Hoja de Ruta 2.0 de la Minería Chilena. Actualización y Consensos para una Mirada Renovada; Corporación-Alta-Ley: Santiago, Chile, 2019. [Google Scholar]
- Hák, T.; Janoušková, S.; Moldan, B. Sustainable Development Goals: A need for relevant indicators. Ecol. Indic. 2016, 60, 565–573. [Google Scholar] [CrossRef]
- United Nation. Sustainable Development Knowledge Platform. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nation: New York, NY, USA, 2015. [Google Scholar]
- Monteiro, N.B.R.; da Silva, E.A.; Neto, J.M.M. Sustainable development goals in mining. J. Clean. Prod. 2019, 228, 509–520. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Redovniković, I.R.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Gu, Y.; Jerome, F. Bio-based solvents: An emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev. 2013, 42, 9550–9570. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; 135p. [Google Scholar]
- Jessop, P.G. Fundamental properties and practical applications of ionic liquids: Concluding remarks. Faraday Discuss. 2018, 206, 587–601. [Google Scholar] [CrossRef]
- Häckl, K.; Kunz, W. Some aspects of green solvents. Comptes Rendus Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Eksteen, J.; Oraby, E.; Tanda, B.; Tauetsile, P.; Bezuidenhout, G.; Newton, T.; Trask, F.; Bryan, I. Towards industrial implementation of glycine-based leach and adsorption technologies for gold-copper ores. Can. Metall. Q. 2018, 57, 390–398. [Google Scholar] [CrossRef]
- Barton, I.F.; Hiskey, J.B. Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 2022, 207, 105775. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Qi, Y.; Han, Y.; Wang, R.; Fu, J.; Meng, F.; Yi, X.; Huang, J.; Shu, J. Cobalt and lithium leaching from waste lithium ion batteries by glycine. J. Power Sources 2021, 482, 228942. [Google Scholar] [CrossRef]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; Sun, Z.; Sun, S.; Shu, J. Copper extraction from waste printed circuit boards by glycine. Sep. Purif. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Perea, C.; Restrepo, O. Use of amino acids for gold dissolution. Hydrometallurgy 2018, 177, 79–85. [Google Scholar] [CrossRef]
- Prasetyo, E.; Anderson, C.; Nurjaman, F.; al Muttaqii, M.; Handoko, A.S.; Bahfie, F.; Mufakhir, F.R. Monosodium glutamate as selective lixiviant for alkaline leaching of zinc and copper from electric arc furnace dust. Metals 2020, 10, 644. [Google Scholar] [CrossRef]
- Perea, C.; Baena, O.R.; Ihle, C.; Estay, H. Copper leaching from wastes electrical and electronic equipment (WEEE) using alkaline monosodium glutamate: Thermodynamics and dissolution tests. Clean. Eng. Technol. 2021, 5, 100312. [Google Scholar] [CrossRef]
- Moed, H.F. New developments in the use of citation analysis in research evaluation. Arch. Immunol. Ther. Exp. 2009, 57, 13–18. [Google Scholar] [CrossRef]
- Lawani, S.M. Bibliometrics: Its theoretical foundations, methods and applications. Libri 1981, 31, 294–315. [Google Scholar] [CrossRef]
- Subramanyam, K. Bibliometric studies of research collaboration: A review. J. Inf. Sci. 1983, 6, 33–38. [Google Scholar] [CrossRef]
- Van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Hidalgo, T.; McDonald, R.; Beinlich, A.; Kuhar, L.; Putnis, A. Comparative analysis of copper dissolution and mineral transformations in coarse chalcopyrite for different oxidant/lixiviant systems at elevated temperature (110 °C and 170 °C). Hydrometallurgy 2022, 207, 105700. [Google Scholar] [CrossRef]
- O’Connor, G.; Lepkova, K.; Eksteen, J.; Oraby, E. Electrochemical behaviour and surface analysis of chalcopyrite in alkaline glycine solutions. Hydrometallurgy 2018, 182, 32–43. [Google Scholar] [CrossRef]
- Azadi, M.; Karrech, A.; Elchalakani, M.; Attar, M. Microfluidic study of sustainable gold leaching using glycine solution. Hydrometallurgy 2019, 185, 186–193. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of copper and the co-leaching behaviour of other metals from waste printed circuit boards using alkaline glycine solutions. Resour. Conserv. Recycl. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Tanda, B.; Eksteen, J.; Oraby, E.; O’Connor, G. The kinetics of chalcopyrite leaching in alkaline glycine/glycinate solutions. Miner. Eng. 2019, 135, 118–128. [Google Scholar] [CrossRef]
- Khodaei, H.; Haghshenas, D.F.; Firoozi, S. Selective leaching of zinc from carbonate source using glycine as an ecofriendly lixiviant. Miner. Eng. 2022, 185, 107680. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of precious metals from waste printed circuit boards using cyanide-free alkaline glycine solution in the presence of an oxidant. Miner. Eng. 2022, 181, 107501. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J.; Mali, T. Extraction of Gold and Copper from Flotation Tailings Using Glycine-Ammonia Solutions in the Presence of Permanganate. Minerals 2022, 12, 612. [Google Scholar] [CrossRef]
- Barani, K.; Kogani, Y.; Nazarian, F. Leaching of complex gold ore using a cyanide-glycine solution. Miner. Eng. 2022, 180, 107475. [Google Scholar] [CrossRef]
- Attia, R.M.; Awny, E.G. Leaching characterisations and recovery of copper and uranium with glycine solution of sandy dolomite, Allouga area, South Western Sinai, Egypt. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Khezri, M.; Rezai, B.; Abdollahzadeh, A.A.; Wilson, B.P.; Molaeinasab, M.; Lundström, M. Investigation into the effect of mechanical activation on the leaching of chalcopyrite in a glycine medium. Hydrometallurgy 2021, 203, 105492. [Google Scholar] [CrossRef]
- Mokhlis, H.; Daoudi, R.D.; Azzi, M. Selective leaching of copper from waste printed circuit boards (PCBs) using glycine as a complexing agent. Glob. Nest J. 2021, 23, 90–96. [Google Scholar]
- Huang, Y.; Guo, H.; Zhang, C.; Liu, B.; Wang, L.; Peng, W.; Cao, Y.; Song, X.; Zhu, X. A novel method for the separation of zinc and cobalt from hazardous zinc–cobalt slag via an alkaline glycine solution. Sep. Purif. Technol. 2021, 273, 119009. [Google Scholar] [CrossRef]
- Wu, J.; Ahn, J.; Lee, J. Gold deportment and leaching study from a pressure oxidation residue of chalcopyrite concentrate. Hydrometallurgy 2021, 201, 105583. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Cyanide consumption minimisation and concomitant toxic effluent minimisation during precious metals extraction from waste printed circuit boards. Waste Manag. 2021, 125, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Shibazaki, K.; Hirama, S.; Iwatate, Y.; Kishita, A.; Hiraga, Y.; Nakayasu, Y.; Watanabe, M. Glycine-Assisted Hydrothermal Leaching of LiCoO2/LiNiO2 Cathode Materials with High Efficiency and Negligible Acid Corrosion Employing Batch and Continuous Flow System. ACS Sustain. Chem. Eng. 2021, 9, 3246–3257. [Google Scholar] [CrossRef]
- Khezri, M.; Rezai, B.; Abdollahzadeh, A.A.; Wilson, B.P.; Molaeinasab, M.; Lundström, M. Cyclic voltammetry and potentiodynamic polarization studies of chalcopyrite concentrate in glycine medium. Trans. Nonferrous Met. Soc. China 2021, 31, 545–554. [Google Scholar] [CrossRef]
- Barani, K.; Dehghani, M.; Azadi, M.; Karrech, A. Leaching of a polymetal gold ore and reducing cyanide consumption using cyanide-glycine solutions. Miner. Eng. 2021, 163, 106802. [Google Scholar] [CrossRef]
- Picazo, N.G.; Pedroza, F.R.C.; Luévanos, A.M.; Aguilar, M.d.J.S.; Guzmán, I.A. Sº and jarosite behavior during recovery of values from the direct leaching residue of sphalerite using cyanide and glycine. J. Min. Metall. Sect. B Metall. 2021, 57, 349–358. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J.; O’Connor, G. Gold leaching from oxide ores in alkaline glycine solutions in the presence of permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Altinkaya, P.; Wang, Z.; Korolev, I.; Hamuyuni, J.; Haapalainen, M.; Kolehmainen, E.; Yliniemi, K.; Lundström, M. Leaching and recovery of gold from ore in cyanide-free glycine media. Miner. Eng. 2020, 158, 106610. [Google Scholar] [CrossRef]
- Khezri, M.; Rezai, B.; Abdollahzadeh, A.A.; Molaeinasab, M.; Wilson, B.P.; Lundström, M. Glycine leaching of Sarcheshmeh chalcopyrite concentrate at high pulp densities in a stirred tank reactor. Miner. Eng. 2020, 157, 106555. [Google Scholar] [CrossRef]
- Oraby, E.A.; Li, H.; Eksteen, J.J. An alkaline glycine-based leach process of base and precious metals from powdered waste printed circuit boards. Waste Biomass Valorization 2020, 11, 3897–3909. [Google Scholar] [CrossRef]
- Oghabi, H.; Haghshenas, D.F.; Firoozi, S. Selective separation of Cd from spent Ni-Cd battery using glycine as an eco-friendly leachant and its recovery as CdS nanoparticles. Sep. Purif. Technol. 2020, 242, 116832. [Google Scholar] [CrossRef]
- Munive, G.T.; Encinas, M.A.; Campoy, M.M.S.; Álvarez, V.E.; Vazquez, V.M.; Choque, D.C. Leaching Gold and silver with an alternative system: Glycine and thiosulfate from mineral tailings. Jom 2020, 72, 918–924. [Google Scholar] [CrossRef]
- Eksteen, J.; Oraby, E.; Nguyen, V. Leaching and ion exchange based recovery of nickel and cobalt from a low grade, serpentine-rich sulfide ore using an alkaline glycine lixiviant system. Miner. Eng. 2020, 145, 106073. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J.; Karrech, A.; Attar, M. Gold extraction from paleochannel ores using an aerated alkaline glycine lixiviant for consideration in heap and in-situ leaching applications. Miner. Eng. 2019, 138, 112–118. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Y.; Huang, W.; Li, H.; Liao, S. The role of glycine in the ammonium thiocyanate leaching of gold. Hydrometallurgy 2019, 185, 111–116. [Google Scholar] [CrossRef]
- Shin, D.; Ahn, J.; Lee, J. Kinetic study of copper leaching from chalcopyrite concentrate in alkaline glycine solution. Hydrometallurgy 2019, 183, 71–78. [Google Scholar] [CrossRef]
- O’Connor, G.; Lepkova, K.; Eksteen, J.; Oraby, E. Electrochemical behaviour of copper in alkaline glycine solutions. Hydrometallurgy 2018, 181, 221–229. [Google Scholar] [CrossRef]
- Tanda, B.; Eksteen, J.; Oraby, E. Kinetics of chalcocite leaching in oxygenated alkaline glycine solutions. Hydrometallurgy 2018, 178, 264–273. [Google Scholar] [CrossRef]
- Nicol, M.J. A comparative assessment of the application of ammonium chloride and glycine as lixiviants in the heap leaching of chalcopyritic ores. Hydrometallurgy 2018, 175, 285–291. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Pan, Y.; Liu, F.; Xu, Z. Thermodynamic analysis of gold leaching by copper-glycine-thiosulfate solutions using Eh-pH and species distribution diagrams. Miner. Eng. 2022, 179, 107438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).