Sulfate and Freeze-Thaw Resistance of Porous Geopolymer Based on Waste Clay and Aluminum Salt Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mixture Composition
2.3. Test Methods
3. Results
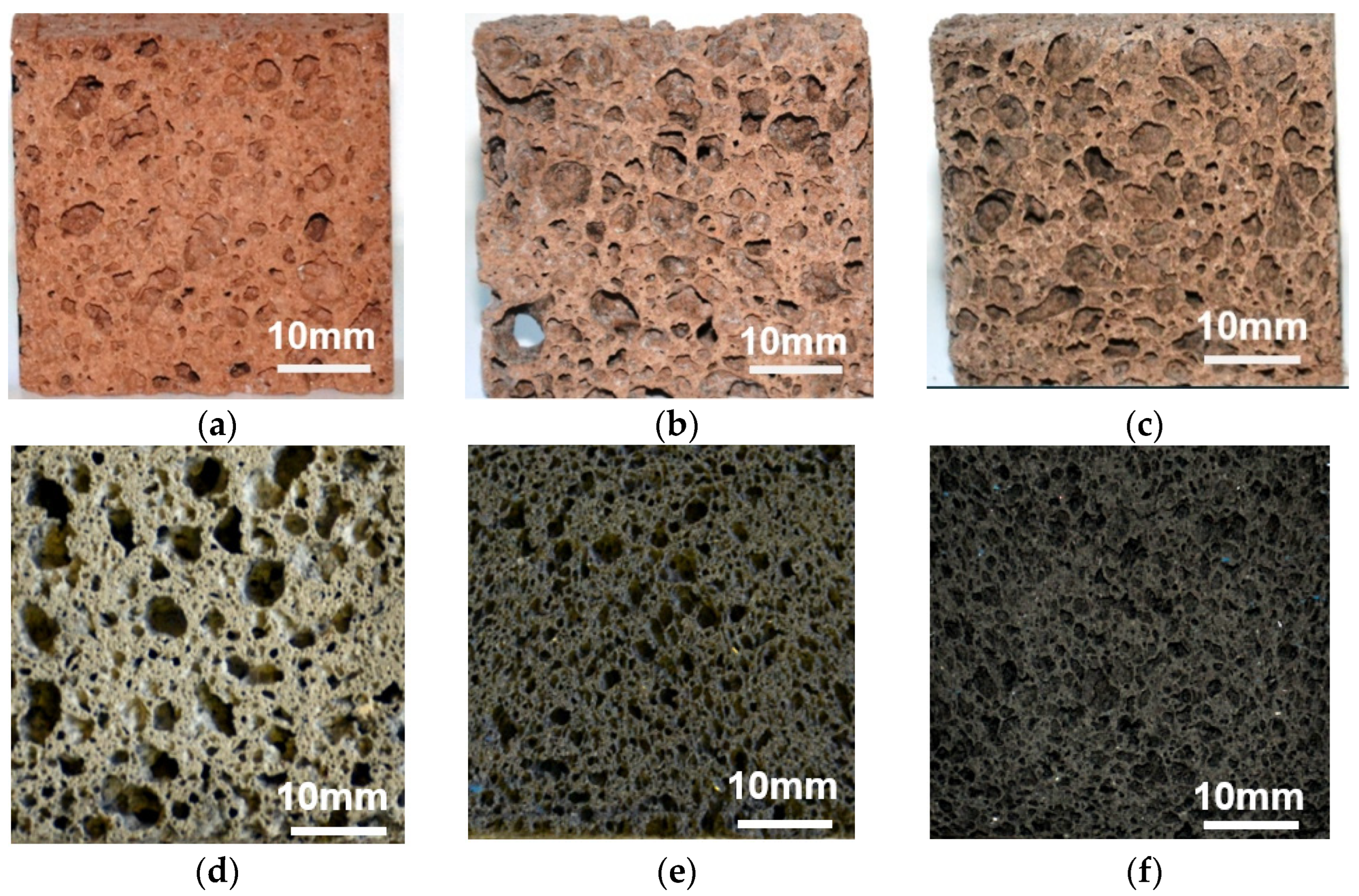
3.1. Macrostructure
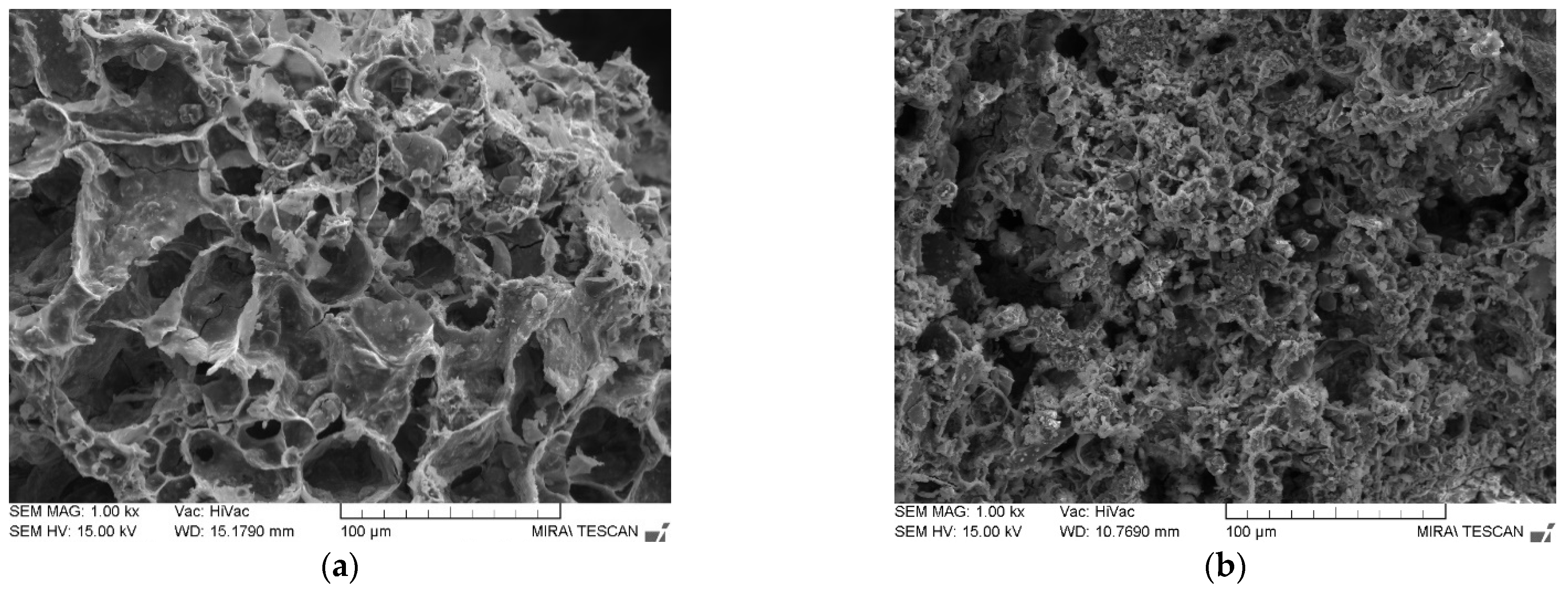
3.2. Microstructure
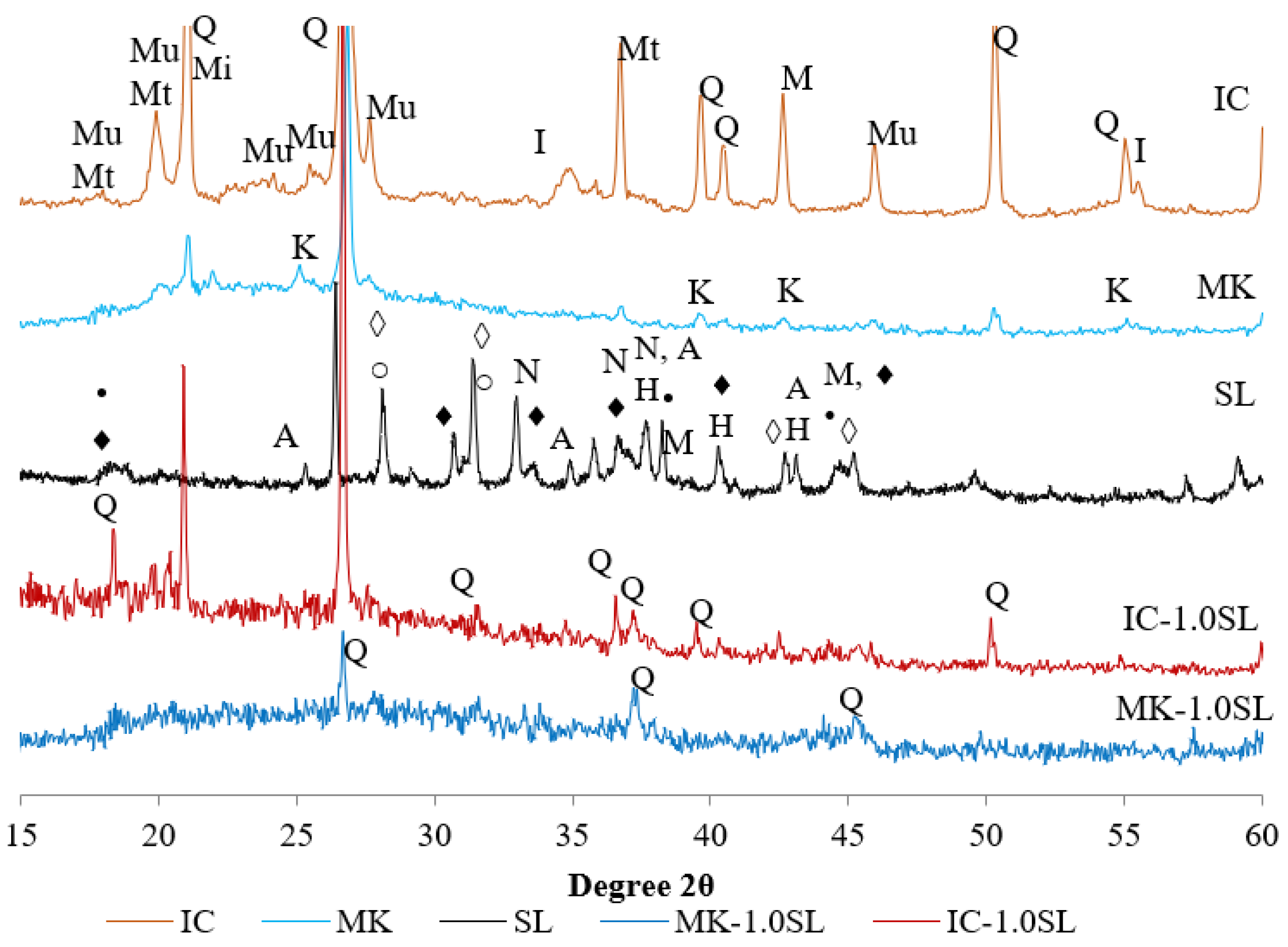
3.3. Mineralogical Composition
3.4. Physical and Mechanical Properties
3.5. Freeze-Thaw Resistance
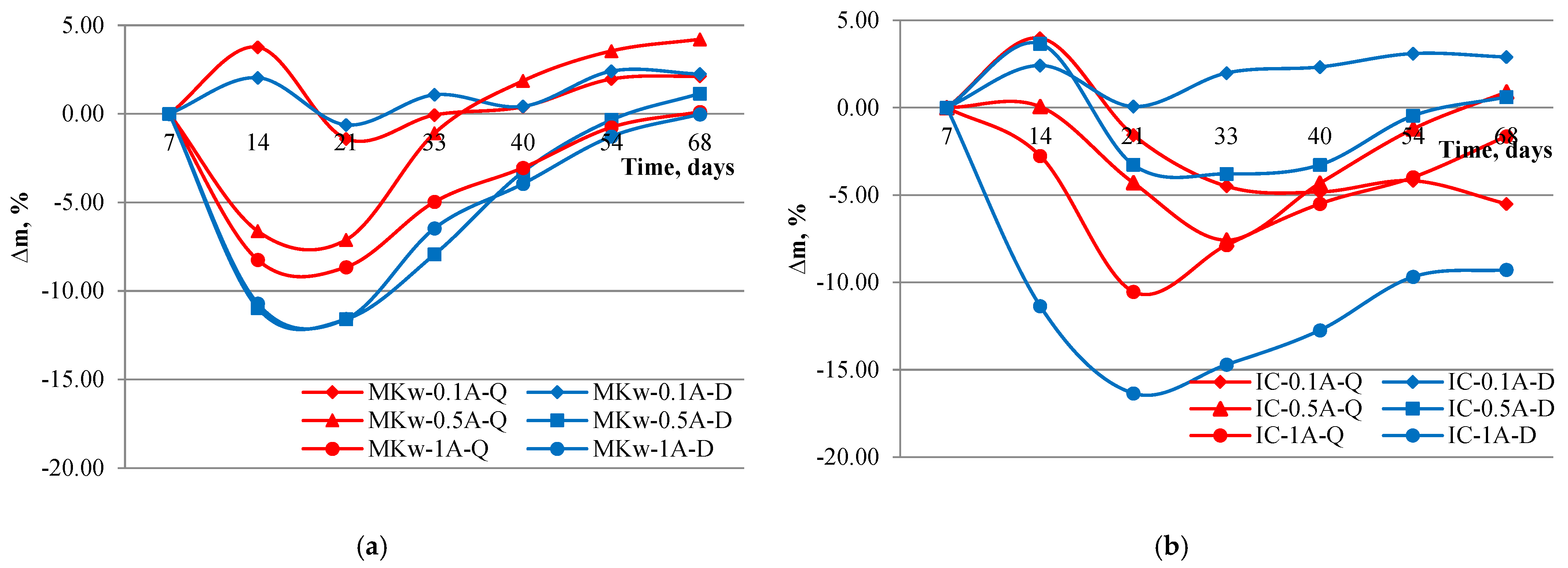
3.6. Sulfate Attack
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Williams, I.; Riessen, A. Van thermal barriers. Appl. Clay Sci. 2009, 46, 6–11. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Zain, H.; Abdullah, M.M.A.B.; Hussin, K.; Ariffin, N.; Bayuaji, R. Review on Various Types of Geopolymer Materials with the Environmental Impact Assessment. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 97. [Google Scholar]
- Sgarlata, C.; Formia, A.; Siligardi, C.; Ferrari, F.; Leonelli, C. Mine Clay Washing Residues as a Source for Alkali-Activated Binders. Materials 2021, 15, 83. [Google Scholar] [CrossRef]
- Boca Santa, R.A.A.; Soares, C.; Riella, H.G. Geopolymers with a high percentage of bottom ash for solidification/immobilization of different toxic metals. J. Hazard. Mater. 2016, 318, 145–153. [Google Scholar] [CrossRef]
- Deevasan, K.K.; Ranganath, R.V. Geopolymer concrete using industrial byproducts. Proc. ICE-Constr. Mater. 2011, 164, 43–50. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Tamošaitis, G.; Kantautas, A.; Nizevičienė, D.; Pupeikis, D. Porous alkali-activated materials based on municipal solid waste incineration ash with addition of phosphogypsum powder. Constr. Build. Mater. 2021, 301, 123962. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Lin, H.; Lv, J.; Feng, S.; Ci, J. Early properties and microstructure evolution of alkali-activated brick powder geopolymers at varied curing humidity. J. Build. Eng. 2022, 54, 104674. [Google Scholar] [CrossRef]
- Borg, R.P.; Vaičiukynienė, D.; Gurskis, V.; Nizevičienė, D.; Skominas, R.; Ramukevičius, D.; Sadzevičius, R. Alkali-Activated Material Based on Red Clay and Silica Gel Waste. Waste Biomass Valorization 2020, 11, 2973–2982. [Google Scholar] [CrossRef]
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Strength and Durability of Granular Soil Stabilized with FA-GGBS Geopolymer. J. Mater. Civ. Eng. 2021, 33, 06021003. [Google Scholar] [CrossRef]
- Min, Y.; Wu, J.; Li, B.; Zhang, M.; Zhang, J. Experimental study of freeze–thaw resistance of a one-part geopolymer paste. Case Stud. Constr. Mater. 2022, 17, e01269. [Google Scholar] [CrossRef]
- Tan, J.; Dan, H.; Ma, Z. Metakaolin based geopolymer mortar as concrete repairs: Bond strength and degradation when subjected to aggressive environments. Ceram. Int. 2022, 48, 23559–23570. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Hu, X. Reaction mechanism of sulfate attack on alkali-activated slag/fly ash cements. Constr. Build. Mater. 2022, 318, 126052. [Google Scholar] [CrossRef]
- Yang, T.; Gao, X.; Zhang, J.; Zhuang, X.; Wang, H.; Zhang, Z. Sulphate resistance of one-part geopolymer synthesized by calcium carbide residue-sodium carbonate-activation of slag. Compos. Part B Eng. 2022, 242, 110024. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.; Polyakov, P. A Review of Secondary Aluminum Production and Its Byproducts. JOM 2021, 73, 2603–2614. [Google Scholar] [CrossRef]
- Jafari, N.H.; Stark, T.D.; Roper, R. Classification and Reactivity of Secondary Aluminum Production Waste. J. Hazard. Toxic Radioact. Waste 2014, 18, 04014018. [Google Scholar] [CrossRef]
- Shinzato, M.C.; Hypolito, R. Solid waste from aluminum recycling process: Characterization and reuse of its economically valuable constituents. Waste Manag. 2005, 25, 37–46. [Google Scholar] [CrossRef]
- Calder, G.V.; Stark, T.D. Aluminum Reactions and Problems in Municipal Solid Waste Landfills. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2010, 14, 258–265. [Google Scholar] [CrossRef]
- Rugele, K.; Bumanis, G.; Mezule, L.; Juhna, T.; Bajare, D. Application of industrial wastes in renewable energy production. Agron. Res. 2015, 13, 526–532. [Google Scholar]
- Gruskevica, K.; Bumanis, G.; Tihomirova, K.; Bajare, D.; Juhna, T. Alkaline activated material as the adsorbent for uptake of high concentration of zinc from wastewater. In Key Engineering Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2017; Volume 721 KEM, ISBN 9783035710724. [Google Scholar]
- Novais, R.M.; Buruberri, L.H.H.; Seabra, M.P.P.; Labrincha, J.A.A. Novel porous fly-ash containing geopolymer monoliths for lead adsorption from wastewaters. J. Hazard. Mater. 2016, 318, 631–640. [Google Scholar] [CrossRef]
- Bumanis, G.; Novais, R.M.; Carvalheiras, J.; Bajare, D.; Labrincha, J.A. Metals removal from aqueous solutions by tailored porous waste-based granulated alkali-activated materials. Appl. Clay Sci. 2019, 179, 105147. [Google Scholar] [CrossRef]
- Strozi, M.; Raymundo, M.; Colombo, P. Effect of process parameters on the physical properties of porous geopolymers obtained by gelcasting. Ceram. Int. 2014, 40, 13585–13590. [Google Scholar] [CrossRef]
- Bajare, D.; Korjakins, A.; Kazjonovs, J.; Rozenstrauha, I. Pore structure of lightweight clay aggregate incorporate with non-metallic products coming from aluminium scrap recycling industry. J. Eur. Ceram. Soc. 2012, 32, 141–148. [Google Scholar] [CrossRef]
- Bajare, D.; Korjakins, A.; Kazjonovs, J. Application of Aluminium Dross and Glass Waste for Production of Expanded Clay Aggregate. Civ. Eng. 2011, 11, 27–31. [Google Scholar]
- LVS 156-1:2009; Concrete—National Annex of Latvian Standard to European Standard EN 206-1—Part 1: Requirements for Classification and Attestation of Conformity. LVS/STK/04: Rīga, Latvia, 2009.
- Ercoli, R.; Laskowska, D.; Nguyen, V.V.; Le, V.S.; Louda, P.; Ło´s, P.Ł.; Ciemnicka, J.; Prałat, K.; Renzulli, A.; Paris, E.; et al. Mechanical and Thermal Properties of Geopolymer Foams (GFs) Doped with By-Products of the Secondary Aluminum Industry. Polymers 2022, 14, 703. [Google Scholar] [CrossRef]
- Bumanis, G. Alkali Activated Materials and Its Applications. Ph.D. Thesis, Riga Technical University, Riga, Latvia, 2015. [Google Scholar]
- Hall, C.; Hamilton, A. Porosity–density relations in stone and brick materials. Mater. Struct. Constr. 2015, 48, 1265–1271. [Google Scholar] [CrossRef]
- López, F.J.; Sugita, S.; Tagaya, M.; Kobayashi, T. Metakaolin-Based Geopolymers for Targeted Adsorbents to Heavy Metal Ion Separation. J. Mater. Sci. Chem. Eng. 2014, 2, 16–27. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Zhong, D.; Wang, S.; Gao, Y.; Wang, L.; Feng, K. Experimental study on freeze-thaw resistance of modified magnesium oxychloride cement foam concrete. J. Phys. Conf. Ser. 2021, 1885, 32009. [Google Scholar] [CrossRef]
- Shon, C.S.; Lee, D.; Kim, J.H.; Chung, C.W. Freezing and thawing resistance of cellular concrete containing binary and ternary cementitious mixtures. Constr. Build. Mater. 2018, 168, 73–81. [Google Scholar] [CrossRef]
- Ghanem, G.; Yehia, S.; Mohamed, N.; Helmy, M. Effect of Sulphate Attack on Compressive Strength of Geopolymer Concrete. Int. J. Sci. Eng. Res. 2022, 13, 63–70. [Google Scholar]
- Alyousef, R.; Ebid, A.A.K.; Huseien, G.F.; Mohammadhosseini, H.; Alabduljabbar, H.; Ngian, S.P.; Mohamed, A.M. Effects of Sulfate and Sulfuric Acid on Efficiency of Geopolymers as Concrete Repair Materials. Gels 2022, 8, 53. [Google Scholar] [CrossRef]
| Chemical Component | SL | IC | MK | Q | D |
|---|---|---|---|---|---|
| Al2O3 | 63.19 | 14.60 | 51.7 | 1.42 | - |
| SiO2 | 7.92 | 73.84 | 34.4 | 96.8 | - |
| CaO | 2.57 | 0.91 | 0.09 | - | - |
| SO3 | 0.36 | - | - | - | - |
| TiO2 | 0.53 | 0.63 | 0.55 | - | - |
| MgO | 4.43 | 1.10 | 0.13 | - | - |
| Fe2O3 | 4.54 | 4.08 | 0.53 | 0.34 | - |
| Na2O | 3.84 | 0.06 | 0.63 | - | - |
| K2O | 3.81 | 2.75 | 0.01 | - | - |
| CaCO3*MgCO3 | - | - | - | - | 97.0 |
| Other | 2.60 | 1.05 | 1.96 | 0.49 | - |
| LOI, 1000 °C | 6.21 | 0.98 | 10.1 | 0.95 | 3.0 |
| Composition | IC | MK | SL | D | Q | Sodium Silicate Solution/Solid Ratio | Main Oxide Ratios | |
|---|---|---|---|---|---|---|---|---|
| SiO2/Al2O3 | Na2O/Al2O3 | |||||||
| IC-0.1SL-Q | 1.0 | - | 0.1 | - | 1.0 | 0.75 | 4.5 | 0.8 |
| IC-0.5SL-Q | 1.0 | - | 0.5 | - | 1.0 | 0.75 | 2.3 | 0.5 |
| IC-1.0SL-Q | 1.0 | - | 1.0 | - | 1.0 | 0.75 | 1.5 | 0.4 |
| IC-0.1SL-D | 1.0 | - | 0.1 | 1.0 | - | 0.75 | 4.5 | 0.8 |
| IC-0.5SL-D | 1.0 | - | 0.5 | 1.0 | - | 0.75 | 2.3 | 0.5 |
| IC-1.0SL-D | 1.0 | - | 1.0 | 1.0 | - | 0.75 | 1.5 | 0.4 |
| MK-0.1SL-Q | - | 1.0 | 0.1 | - | 1.0 | 0.75 | 2.4 | 0.4 |
| MK-0.5SL-Q | - | 1.0 | 0.5 | - | 1.0 | 0.75 | 1.6 | 0.4 |
| MK-1.0SL-Q | - | 1.0 | 1.0 | - | 1.0 | 0.75 | 1.1 | 0.3 |
| MK-0.1SL-D | - | 1.0 | 0.1 | 1.0 | - | 0.75 | 2.4 | 0.4 |
| MK-0.5SL-D | - | 1.0 | 0.5 | 1.0 | - | 0.75 | 1.6 | 0.4 |
| MK-1.0SL-D | - | 1.0 | 1.0 | 1.0 | - | 0.75 | 1.1 | 0.3 |
| Mixture Composition | Bulk Density, kg/m3 | Water Absorption, Wt,% | Open Porosity, vol.% | Total Porosity, vol.% | Compressive Strength, fc, MPa | Bending Strength, fm, MPa |
|---|---|---|---|---|---|---|
| IC-0.1SL-Q | 655 ± 16 | 41.5 ± 2.5 | 33.4 ± 0.8 | 73.6 ± 1.2 | 1.9 ± 0.1 | 1.0 ± 0.08 |
| IC-0.5SL-Q | 585 ± 12 | 49.0 ± 3.7 | 31.0 ± 2.8 | 76.8 ± 1.5 | 1.5 ± 0.1 | 0.7 ± 0.03 |
| IC-1.0SL-Q | 540 ± 27 | 52.8 ± 2.7 | 29.7 ± 1.6 | 78.6 ± 1.8 | 1.7 ± 0.1 | 0.6 ± 0.05 |
| IC-0.1SL-D | 675 ± 17 | 41.5 ± 1.7 | 30.3 ± 1.3 | 73.0 ± 1.3 | 2.0 ± 0.1 | 0.9 ± 0.02 |
| IC-0.5SL-D | 555 ± 16 | 55.2 ± 3.2 | 33.0 ± 0.7 | 78.0 ± 2.0 | 1.4 ± 0.1 | 0.6 ± 0.03 |
| IC-1.0SL-D | 550 ± 16 | 54.4 ± 4.7 | 29.4 ± 0.8 | 78.4 ± 1.5 | 1.7 ± 0.1 | 0.6 ± 0.02 |
| MK-0.1SL-Q | 675 ± 13 | 31.9 ± 2.3 | 21.7 ± 1.2 | 71.4 ± 0.5 | 3.8 ± 0.2 | 2.1 ± 0.16 |
| MK-0.5SL-Q | 610 ± 13 | 41.1 ± 2.1 | 25.0 ± 1.1 | 74.9 ± 0.5 | 3.1 ± 0.2 | 1.7 ± 0.06 |
| MK-1.0SL-Q | 600 ± 14 | 52.5 ± 2.8 | 30.7 ± 2.1 | 75.9 ± 0.6 | 2.0 ± 0.1 | 1.4 ± 0.14 |
| MK-0.1SL-D | 670 ± 12 | 31.7 ± 1.2 | 21.2 ± 0.6 | 72.0 ± 0.5 | 3.8 ± 0.3 | 2.1 ± 0.11 |
| MK-0.5SL-D | 620 ± 14 | 47.3 ± 1.9 | 29.5 ± 1.0 | 74.5 ± 0.6 | 3.1 ± 0.2 | 1.5 ± 0.13 |
| MK-1.0SL-D | 580 ± 10 | 56.3 ± 2.9 | 33.4 ± 2.4 | 76.0 ± 1.0 | 2.4 ± 0.2 | 1.4 ± 0.07 |
| Composition | ∆m, % | Initial Strength, MPa | Residual Strength, MPa | Strength Change, % | |||
|---|---|---|---|---|---|---|---|
| No. of Cycles | No. of Cycles | No. of Cycles | |||||
| 25 | 50 | 25 | 50 | 25 | 50 | ||
| IC-0.1SL-Q | −8.0 | - | 1.9 ± 0.1 | 1.2 ± 0.2 | - | 36.8 | - |
| IC-0.5SL-Q | −6.9 | - | 1.5 ± 0.1 | 1.3 ± 0.2 | - | 13.3 | - |
| IC-1.0SL-Q | −14.4 | - | 1.7 ± 0.1 | 1.1 ± 0.2 | - | 35.3 | - |
| IC-0.1SL-D | −9.3 | - | 2.0 ± 0.1 | 1.1 ± 0.1 | - | 45.0 | - |
| IC-0.5SL-D | −9.7 | - | 1.4 ± 0.1 | 1.0 ± 0.2 | - | 28.6 | - |
| IC-1.0SL-D | −11.6 | - | 1.7 ± 0.1 | 1.1 ± 0.2 | - | 35.3 | - |
| MK-0.1SL-Q | −6.5 | −15.5 | 3.8 ± 0.2 | 1.7 ± 0.2 | 1.3 ± 0.1 | 55.3 | 65.8 |
| MK-0.5SL-Q | −3.5 | −6.2 | 3.1 ± 0.2 | 2.4 ± 0.2 | 2.2 ± 0.4 | 22.6 | 29.0 |
| MK-1.0SL-Q | −3.3 | −8.4 | 2.0 ± 0.1 | 1.8 ± 0.2 | 1.5 ± 0.2 | 10.0 | 25.0 |
| MK-0.1SL-D | −3.6 | −8.8 | 3.8 ± 0.3 | 2.6 ± 0.3 | 1.9 ± 0.2 | 31.6 | 50.0 |
| MK-0.5SL-D | −3.7 | −6.6 | 3.1 ± 0.2 | 2.6 ± 0.2 | 2.4 ± 0.3 | 16.1 | 22.6 |
| MK-1.0SL-D | −3.6 | −9.9 | 2.4 ± 0.2 | 2.0 ± 0.1 | 1.1 ± 0.1 | 16.7 | 54.2 |
| Mixture | Weight Change, % | Initial Compressive Strength, MPa | Residual Compressive Strength, Mpa | Compressive Strength Change, % |
|---|---|---|---|---|
| IC-0.1SL-Q | −5.5 | 1.9 ± 0.1 | 0.8 ± 0.1 | −57.9 |
| IC-0.5SL-Q | 0.9 | 1.5 ± 0.1 | 1.0 ± 0.1 | −33.3 |
| IC-1.0SL-Q | −1.6 | 1.7 ± 0.1 | 1.1 ± 0.1 | −35.3 |
| IC-0.1SL-D | 2.9 | 2.0 ± 0.1 | 1.8 ± 0.4 | −10.0 |
| IC-0.5SL-D | 0.61 | 1.4 ± 0.1 | 1.1 ± 0.2 | −21.4 |
| IC-1.0SL-D | −9.3 | 1.7 ± 0.1 | 1.2 ± 0.1 | −29.4 |
| MK-0.1SL-Q | 2.1 | 3.8 ± 0.2 | 2.1 ± 0.2 | −44.7 |
| MK-0.5SL-Q | 4.2 | 3.1 ± 0.2 | 2.6 ± 0.2 | −16.1 |
| MK-1.0SL-Q | 2.2 | 2.0 ± 0.1 | 1.9 ± 0.3 | −5.0 |
| MK-0.1SL-D | 1.2 | 3.8 ± 0.3 | 2.8 ± 0.3 | −26.3 |
| MK-0.5SL-D | 0.1 | 3.1 ± 0.2 | 2.7 ± 0.3 | −12.9 |
| MK-1.0SL-D | 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumanis, G.; Bajare, D.; Korjakins, A.; Vaičiukynienė, D. Sulfate and Freeze-Thaw Resistance of Porous Geopolymer Based on Waste Clay and Aluminum Salt Slag. Minerals 2022, 12, 1140. https://doi.org/10.3390/min12091140
Bumanis G, Bajare D, Korjakins A, Vaičiukynienė D. Sulfate and Freeze-Thaw Resistance of Porous Geopolymer Based on Waste Clay and Aluminum Salt Slag. Minerals. 2022; 12(9):1140. https://doi.org/10.3390/min12091140
Chicago/Turabian StyleBumanis, Girts, Diana Bajare, Aleksandrs Korjakins, and Danutė Vaičiukynienė. 2022. "Sulfate and Freeze-Thaw Resistance of Porous Geopolymer Based on Waste Clay and Aluminum Salt Slag" Minerals 12, no. 9: 1140. https://doi.org/10.3390/min12091140
APA StyleBumanis, G., Bajare, D., Korjakins, A., & Vaičiukynienė, D. (2022). Sulfate and Freeze-Thaw Resistance of Porous Geopolymer Based on Waste Clay and Aluminum Salt Slag. Minerals, 12(9), 1140. https://doi.org/10.3390/min12091140









