Abstract
Aluminum recycling generates large amounts of hazardous wastes, known as salt slags, consisting mainly of oxides, metallic aluminum, and salt fluxes. Water leaching is a common technique used for salt removal, being a decisive operation due to water usage and the need to achieve sufficient salt recovery. In this study, water leaching tests under varied operational conditions (water type, slag particle size, solid content, and leaching time) were carried out in salt slag samples obtained from a Brazilian aluminum scrap melting company. Leaching efficiency was assessed by the % chlorine leached. The optimal leaching condition, defined as the one that resulted in the highest chloride removal from the slag together with appropriate operational conditions (larger viable slag size, lower leaching time, etc.), was identified for a slag size below 2.8 mm, 30 wt% of solids in pulp, and a leaching time of 90 min. The results showed that it was possible to recover more than 92% of the salts initially contained in the raw slag, resulting in a leached material with less than 2.5% salts. The recovered salt characteristics showed potential for recycling and could be re-mixed to the salt flux used for aluminum melting. The possibility of achieving higher efficiencies and lower water consumption during leaching was also discussed, as was a preliminary flowsheet for salt slag treatment.
1. Introduction
Aluminum (Al) is the second most used metal worldwide, and recycling currently accounts for about one-third of global metal production; this percentage is predicted to increase to 50% by 2050 [1,2]. Reduced capital costs, environmental advantages, and the requirement of up to 95% less energy than for primary extraction [3] are among the main advantages of the secondary route. Al recycling involves the recovery of Al from wastes originating from the Hall-Héroult process (white and black drosses, from primary and secondary melting, respectively) [4], when alumina is converted to metallic Al, and from the remelting of scrap Al-alloys to produce wrought or foundry alloys. Scraps from manufacturing processes (off-cuts, shavings, etc.) usually have a known composition and may require only simple treatment before recycling (size reduction and classification when necessary). Conversely, scraps from end-of-life products (pots, radiators, packages, etc.) may undergo further beneficiation (gravity, magnetic, and eddy current separation) to remove undesired contaminants [5].
Melting of Al scrap is mostly performed in reverberatory or rotary furnaces, with the latter considered more efficient and economic [6]. The main goal of the melting process is to maximize Al recovery, which can be challenging given the high Al affinity for oxygen. Thus, the process consists of submerging pre-treated scrap into the furnace bath (heated at around 700–800 °C) as quickly as possible to minimize losses due to Al oxidation.
During melting, the bath surface rapidly oxidizes due to contact with air, forming the thin skin of high-Al2O3 slag. As its thickness increases, a considerable amount of metallic Al can become trapped within the slag layer (up to 80% metal content) [4,7]. To minimize such losses, fluxes are mixed with Al scrap before charging into the melting furnace. The fluxes used should meet several requirements, among them: have low vapor pressure and viscosity, have melting point and density lower than that of Al, and be nontoxic and inexpensive. NaCl and KCl, together with cryolite (Na3AlF6), are the standard flux constituents that fulfill these conditions [5]. These salts perform three main functions: (a) they isolate the bath from the atmosphere, reducing metal oxidation; (b) they act as dispersant agents in the slag layer, decreasing the entrainment of metal within the slag and facilitating coalescence; and (c) they remove metallic impurities, such as Mg, Cu, Zn, etc., by forming stable chlorides that float or sink in the bath, depending on their densities [8,9].
The amount of salt flux charged in the furnace depends on the impurity level of the scrap, but can be equivalent to up to 50 wt% of the metal recovered [4,10]. The slag layer formed by salt fluxes, oxides, and contaminants is termed “salt slag” or “salt cake.” It contains 15–30 wt% aluminum oxide, 45–85 wt% salt fluxes, 5–7 wt% metallic aluminum and impurities, and a production reaching up to 500 kg/ton of aluminum [11]. According to Gil [8], Al2O3, MgAl2O4, MgO, and Al(OH)3 are typical constituents of the oxide phase, while SiO2, CaF2, AlN, and others are among the main impurities.
The European Waste Catalogue categorizes salt slags as hazardous waste (code 100308), classified as highly flammable, irritant, harm-full, and leachable [12]. Storage in controlled landfills is particularly challenging due to its high reactivity with water (including air humidity). Reactions with water lead to the formation of toxic and explosive gases, such as CH4, H2, and H2S. NH3, NH4OH, and PH3 are among the other compounds produced by the hydrolysis of nitrites (AlN) and phosphides (AlP) [4,11]. As a result, serious problems of air and groundwater pollution may occur in the vicinities of landfills.
Because of its toxicity and landfilling issues, salt slags require further treatment. As metallic aluminum shows plastic behavior compared to salts and oxides, the standard process for salt slag recycling starts with crushing and screening to remove coarse metallic aluminum, which is fed back to the melting furnace. Aqueous leaching to dissolve the salts (mostly NaCl and KCl) is then applied to the finer fraction, and is typically carried out at 80 °C for 2–3 h [11,13]. The produced brine is then filtered, and water is removed through multi-stage evaporation, resulting in salt crystals (about 70% NaCl and 30% KCl) that can be subsequently recycled as flux [5,11,13,14,15,16]. The remaining low-salt solids can be landfilled, recycled to the primary extraction process for Al recovery [17], or even used as clinker additives for cement production [11]. There is still the possibility of using the produced gases (generated due to reactions with water) for heat generation and energy recovery systems. Hydrogen generation during hydrolysis reactions has been of particular interest, and boosting its production during leaching steps has been the subject of previous works [18]. More detail about the industrial processing of salt slags and management of the output products can be found in Gil and Korili [13].
A few studies have focused on optimizing the salt removal/recovery step, the most expensive operation due to the costs associated with water usage and evaporation. Seeking to integrate maximum solubilization efficiency with minimum use of water, Graziano et al. [14] studied salt leaching under standard and high temperature and pressure conditions (250 °C and 51 atm), concluding that the recycling of salt slags was uneconomical due to the relatively high costs associated.
Davies et al. [19] aimed to evaluate the efficacy of water leaching at 25 °C and 60 °C versus Bayer-type leaching (alkaline solution with 16 vol% NaOH) at 100 °C and 145 °C. The results showed that it was possible to extract 55% of the Na, 45% of the K, and 90% of the Cl from −2 mm salt slag through aqueous leaching for 1 h at 25 °C. Similarly, Bruckard and Woodcock [17] analyzed aqueous treatments at different salt slag particle sizes (−2 mm or finer), leaching time, and temperature, proposing a flowsheet involving milling, classification, and cold leaching of the −150 µm fraction, followed by solar evaporation. It is worth emphasizing that both studies examined only salt slags from dross melting operations (which may or may not include scrap mixed with drosses from primary Al production).
The current scenario encourages recycling practices oriented toward materials until recently considered low added value, as the disruption of the war in Ukraine strongly affected the commodities market [20]. For instance, KCl has experienced one of the sharpest increases, from 221.0 U$/ton (January 2022) to 562.5 U$/ton (March 2022) [21], since Russia and Belarus are among the major exporters. Also, although the forecast of increasing oil prices may help to boost energy transition, it should increase landfill fees, especially hazardous wastes.
Within the context mentioned above, and as part of a project focused on the valorization of salt slags, this study aimed to map and identify the efficient water leaching conditions of salt slags, comparing the characteristics of treated and non-treated slags under optimum conditions, and thus proposing a preliminary treatment route.
2. Materials and Methods
2.1. Sample Preparation
A sample of salt slag of 481 kg was obtained from a Brazilian aluminum scrap melting company consisting of the total mass of slag produced in a batch of rotary furnaces. In this batch, a charge of 14 tons of Al waste composed of a mixture of manufacturing process wastes (ingots, shavings, stampings, etc.), end-of-life products (radiators, pistons, pots, etc.), dross from primary Al production, and salt flux (NaCl and KCl, fed at a mass proportion of 3:1) was melted. The sample was collected in loco after four days of cooling at room temperature, being subsequently crushed in its entirety to −19 mm in a jaw crusher (Plangg J58, Electro Aços Plangg, Porto Alegre, Brazil).
After crushing, the material was homogenized and subsampled using the elongated pile method [22], thus producing a 5 kg subsample. From this, three aliquots of 1.25 kg each, plus one for stocking, were obtained through riffling (Jones Riffle Splitter): (A) −19 mm aliquot; (B) −2.8 mm aliquot, produced by roller crushing (Maqbrit roller crusher); and (C) −0.5 mm aliquot, produced by ceramic ball milling. Milling was conducted over 2 h in a closed circuit with a 0.5 mm aperture screen and having about 35% charge filling. Figure 1 shows a picture of the three aliquots, while the sample preparation scheme is illustrated in Figure 2.
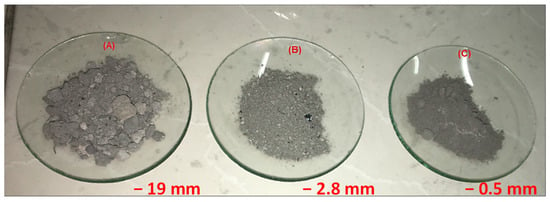
Figure 1.
Salt slag aliquots produced after initial preparation.
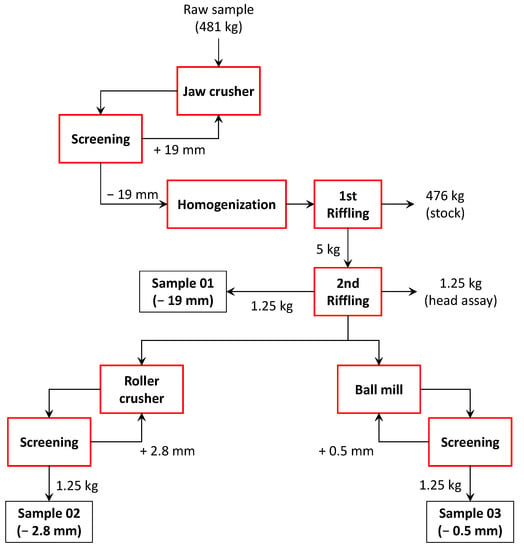
Figure 2.
Subsample preparation scheme.
2.2. Experimental Procedure
Each of the salt slag aliquots previously described was riffled to produce 70–160 g subaliquots for leaching essays. These were performed using distilled and tap water at 25 °C, pH of 6.9–7.0 (measured by pH meter) and three different solid pulp contents: 20 wt%, 30 wt%, and 40 wt% solid in the pulp. Each leaching system was agitated for 2 h at 55 RPM in a laboratory stirrer. A 1 mL solution sample was extracted every 30 min to determine the evolution of chlorine removal. Leaching tests covered 72 essays with combinations of different operational conditions (solid size, water type, solid content, and leaching time). After leaching, the material was paper-filtered, separating the leachate from the solid. The leachate was then heated in an oven at 85 °C for 24 h to guarantee the total removal of water and crystallization of the leachate. The solid fraction was heated in an oven at 50 °C for 8 h to remove humidity. All leaching tests were carried out in duplicate. Figure 3 shows a schematic of the procedures adopted.
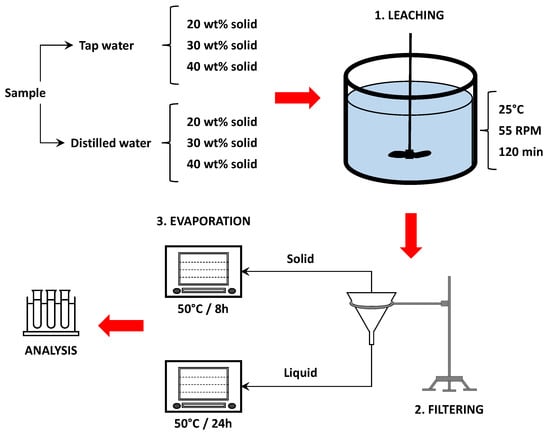
Figure 3.
Procedures and conditions used in leaching essays.
Leaching efficiency was assessed by the % chlorine leached, given by:
where and are the normalized concentrations of chloride (g/L) in leachate and non-treated slag, respectively.
The optimal leaching condition was defined as the one that resulted in the highest chlorine removal from the slag, considering the following conditions:
Leaching at the largest possible slag particle size to minimize comminution costs.
Leaching with the lowest possible water consumption (% Cl leached/mL of water).
Leaching in the shortest possible time.
Minimum leaching of 90% of chlorine, a value similar to that reported by Davies et al. [19] and Bruckard and Woodcock [17] for aqueous and alkaline leaching of Al salt slags.
Once the essay was defined with the best efficiency, the washed and crystallized fractions obtained were qualitatively and quantitatively analyzed.
2.3. Chemical Analysis
A chloride assay was carried out using the silver nitrate titration method (ASTM D 512-89) [23]. This method is recommended for water where chloride content is higher than 5 mg/L, which is the case with leachate solutions from salt slags (concentrations in the range of g/L order). The head chloride assay in the raw slag sample was performed as follows: after homogenization and riffling (see Figure 2), 1.25 kg of material was milled and pulverized in a Fritsch planetary mill (model Pulverisette 6) to a size 100% below 0.075 mm to maximize chloride liberation. The pulverized material was then riffled to obtain two portions of 50 g. Each portion was separately leached in water with a dilution ratio of 1:10 (10 wt% solid) and leaching time of 4 h, the other conditions being the same as described in Section 2.2. The salt solution thus produced, as well as all aliquots generated in the leaching tests, were titrated with AgNO3 (0.025 N) using K2CrO4 as an indicator of chloride content determination.
Elemental analysis of the head sample and the products of leaching at optimum conditions (as defined in Section 2.2) was carried out using energy dispersive X-ray fluorescence spectrometry (ED-XRF) in a spectrometer (EDX-700 Na-U, Shimadzu, Kyoto, Japan) through the loose powder method calibrated with the Al-Cu standard. For this, the head sample and the total mass of the non-leached solids and the crystallized salts from the optimum essay were pulverized, as previously described. Then, a 1 g subsample of each (in triplicate) was placed inside the spectrometer cup. A Rhodium target was used as an X-ray source, operating up to 50 kV and 1000 mA. The detection was conducted under a vacuum atmosphere, and the results were stored in PCEDX Navi software (Shimadzu, Kyoto, Japan).
The non-leached and leached solids, and the crystallized salts from the optimum essay were also submitted for further analysis. Changes in microstructure were evaluated through scanning electron microscopy (SEM) using a Tescan microscope model C3, coupled with an Oxford energy dispersive spectrometer (EDS).
X-ray diffraction data sets were collected from 5 to 80° 2θ at 0.05°/s on a Siemens Bruker AXS D-5000 diffractometer using Cu radiation (λ = 1.5406 Å), operating at 40 kV and 30 mA in the primary beam. A graphite monochromator, a 1° anti-scatter slit, and a 0.2 mm receiving slit were used on the diffracted beam. Finally, sodium (Na+) and potassium (K+) concentrations were assayed in triplicate by flame atomic absorption spectrometry (FAAS) in Perkin-Elmer A Analyst 200 equipment using a Lumina Hollow Cathode Lamp.
3. Results and Discussion
The results include the characterization of the head sample, analysis of leaching essays, and evaluation of the influence of optimum leaching conditions on product composition.
3.1. Head Sample
Table 1 shows the average concentrations of the major elements obtained through ED-XRF. The high aluminum content (68%) stands out above the values reported for most salt slags in the literature [11]. Factors that may contribute to this high Al content include furnace type, charge composition, and operational issues, which can cause significant retention of Al in the slag. Although not analyzed, it is expected that most of the Al is in the form of oxides [11], with a minor portion as entrained metallic Al. The salt fraction (Cl, Na, and K) composed 23.8% of the slag, the balance consisting of Si, Fe, Ca, and minor elements (mainly Mn, Mg, Ti, and Zn).

Table 1.
Major element concentration (weight %) in raw salt slag.
The Cl, Na, and K concentrations obtained in titration/FAAS analyses are summarized in Table 2. The total salt fraction was similar to that found in the ED-XRF analysis (23.51% versus 23.82% for titration/FAAS and ED-XRF, respectively). Conversely, the mass fractions of individual elements were slightly different, with titration/FAAS showing higher precision (lower standard deviation values) than ED-XRF. It is worth noting that for a Cl− content of 13.63 wt% and considering that all Cl− introduced in the furnace comes from the salt flux only, the theoretical contents of Na+ and K+ should be 6.63 wt% and 3.76 wt%, respectively, for a NaCl:KCl proportion of 3:1 (as informed by the recycler). However, it was known beforehand that drosses from primary aluminum production were mixed with the charge, presumably influencing the salt balance in the slag.

Table 2.
Raw salt slag analyses.
3.2. Leaching Results
The chlorine concentration in the head sample was used as the basis for calculating the leaching efficiency (Equation (1)). Figure 4 exhibits the leaching results for the −19 mm slag after washing in distilled and tap water. In both cases, leaching with 40 wt% solids in pulp was not attainable due to operating problems. The low water column present in this system (2:3 solid to liquid proportion), together with the presence of coarse-sized particles, made it difficult to run the agitator over the essays. On the other hand, the systems with 30 wt% and 20 wt% solids in pulp were susceptible to leaching, with leaching efficiencies slightly higher for washing with distilled water. Leaching efficiencies were also slightly higher for the more diluted system (20 wt% solids). More than 84% of Cl was leached in the first 30 min in the distilled water system, whereas in the tap water system, this threshold was 77%. The last case (tap water) showed little evolution of leaching over time, with overall efficiencies after 2 h of 84.6% and 85.6% (30 wt% and 20 wt% solids, respectively). Conversely, the distilled water system reached 94% (30 wt% solids) and 97.4% (20 wt% solids) of Cl removal after 2 h of washing.
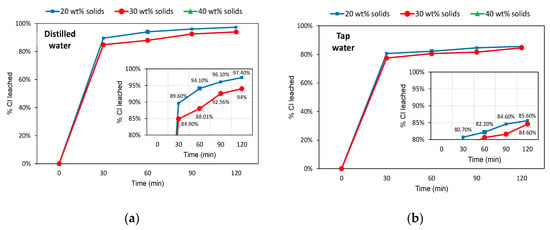
Figure 4.
Leaching efficiencies (% Cl removed) over time for the −19 mm slag samples. (a) Distilled water system; (b) Tap water system.
The leaching results for the −2.8 mm slag samples are shown in Figure 5. In all cases, more than 80% of Cl was leached after just 30 min of washing, and no practical constraint occurred for the test with 40 wt% solids in the pulp. Both leaching systems exhibited a similar evolution of Cl removal over time, as can be seen in the inner graphs highlighted in Figure 5. For more diluted systems (30 wt% and 20 wt% solids), a leaching extent higher than 90% was observed for 90 min of washing. At 2 h of testing, the maximum difference between the systems did not exceed 1% Cl leached, thus not justifying the use of distilled water. The solid pulp content had a remarkable impact on the maximum Cl leached, being below 87% for 40 wt% solids and above 92% for the other cases (30 wt% solids and 20 wt% solids).
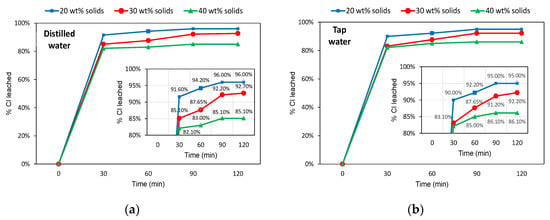
Figure 5.
Leaching efficiencies (% Cl removed) over time for the −2.8 mm slag samples. (a) Distilled water system; (b) Tap water system.
Figure 6 presents the leaching results for the −0.5 mm slag samples. In general, the leaching extent was slightly higher than that observed for the −2.8 mm samples, which was expected beforehand due to the higher particles’ liberation. This difference was more remarkable for the 40 wt% solids sample, whose Cl− leached reached approximately 90% in 2 h of washing. On the other hand, differences in maximum Cl− leaching were less perceptible for more dilute pulps. The highest difference observed was +3% (compared to the −2.8 mm sample, 30 wt% solids in tap water after 2 h of washing). Relative variations of cumulative chlorine leached over time were somewhat similar to the −2.8 mm samples, and not notably different between distilled and tap water systems.
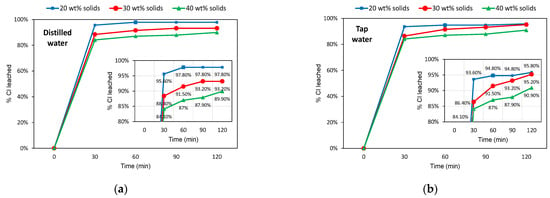
Figure 6.
Leaching efficiencies (% Cl removed) over time for the −0.5 mm slag samples. (a) Distilled water system; (b) Tap water system.
The obtained results allow us to assume that there is no significant difference between using distilled or tap water for slag leaching. Less surprisingly, there is evidence that slags of finer sizes are more extensively leached, which is the standard behavior in leaching processes [24]. However, as previously discussed, the roller crushing process (i.e., size reduction to −2.8 mm) has proven sufficient for chlorine compound liberation, allowing the achievement of leaching levels similar to those of ball-milled samples (−0.5 mm slag). This implies that no further comminution is required after crushing to −2.8 mm, thus eliminating the well-known costs related to grinding [25]. This evidence is also comparable to those by Davies et al. [19] and Bruckard and Woodcock [17], which find water leaching to be efficient for −2 mm aluminum salt slags.
As pointed out by Graziano et al. [14], provided that the efficient removal of salts is achieved, water consumption during leaching should be minimized to reduce evaporation costs. In this respect, Figure 7 presents the ratio between chlorine leached and the volume of consumed water. As can be seen, beyond the direct evidence that more concentrated pulps resulted in higher ratios (meaning less water per % Cl removed), the data show that leaching conducted with larger particle sizes required less water for a given pulp concentration. This reinforces the advantage of leaching salts in not-too-fine particle sizes (−0.5 mm in this case). The one-near exception occurs for 30 wt% solids in pulp, in which the % Cl leached/mL of water values remain approximately constant (0.41–0.44 range) for −19 mm and −2.8 mm sizes. That is, within this range, the use of −2.8 mm slag for washing does not entail significant water consumption compared to larger particle sizes.
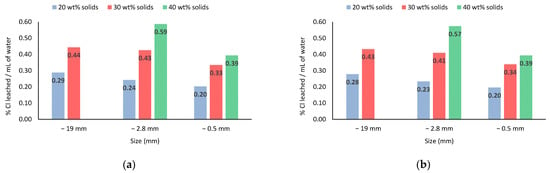
Figure 7.
Cl− leached per volume of water used. (a) Distilled water system; (b) Tap water system.
Based on the previous analysis and considering the prerequisites defined in Section 2.2 for the selection of the optimum conditions, the optimum leaching set tested was leaching with tap water of −2.8 mm size slag, 30 wt% solids in pulp, and 90 min of washing, whose leaching efficiency was 91.2%. Thus, after filtration, drying (solid residue), and evaporation (salt liquid), the leaching products of this essay were analyzed in detail.
3.3. Characteristics of Leaching Products
Figure 8 displays the SEM images and elemental analysis (EDS) of the slag before and after treatment under previously defined optimum leaching conditions. In its raw state, the slag micrograph illustrates a condensed phase rich in Al, O, Cl, Mg, Na, Ca, and K, which also contains small amounts of Si, Fe, and Cu. The occurrence of this continuous phase may be due to the fact that Al can act as a binder for oxide particles, causing the formation of agglutinates after solidification [10]. A similar composition can be observed for the solid after leaching, except for the non-detection of Cl, Na, and K. The micrograph of the crystals clearly shows a cubic distribution characteristic of salts in which only Cl, Na, and K were detected, suggesting that leaching was selective for the flux salts (NaCl and KCl).
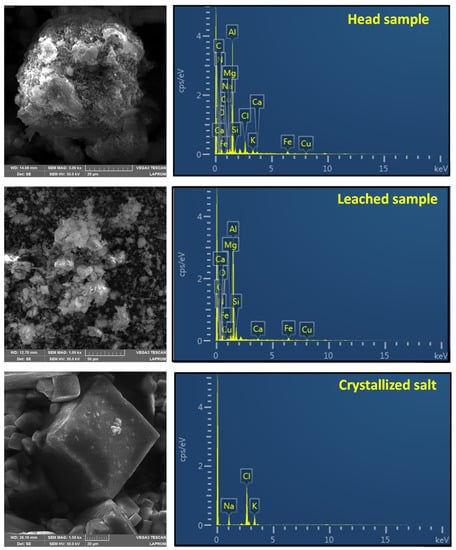
Figure 8.
Scanning electron microscopy images (SEM) and elemental analysis (EDS) of raw slag (head sample), leached slag, and crystallized salt. The grain size is 20 µm, 50 µm, and 20 µm, respectively.
Figure 9, Figure 10 and Figure 11 show the XRD patterns of slag before and after treatment. A semi-quantitative approximation revealed the predominance of NaCl and KCl in the raw slag. Aluminum was identified in the form of Al2O3, corroborating the overlap between Al and O detected in the SEM-EDS analysis. Fe was also detected in three different phases: K2Fe2O4, KFeO2, and Fe2O3. After leaching, the number of salt peaks significantly decreased, consequently intensifying the characteristic peaks of other phases, especially Al2O3 and Fe2O3. The leached sample presented significant overlapping of peaks, high background noise, and low intensity peak characteristics of a high concentration of amorphous phases. This result is compatible with the increase in element dispersion detected by SEM-EDS (see Figure 8). In the crystallized salt, only NaCl and KCl peaks were detectable, confirming the leaching selectivity for NaCl and KCl dissolution.
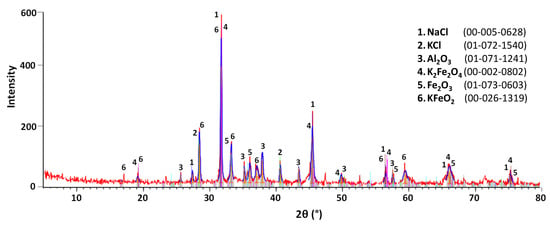
Figure 9.
Mineralogical phases (XRD patterns) of raw salt slag.
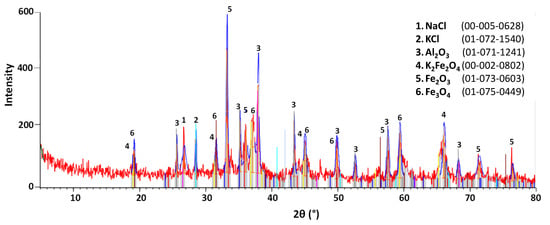
Figure 10.
Mineralogical phases (XRD patterns) of salt slag after leaching.
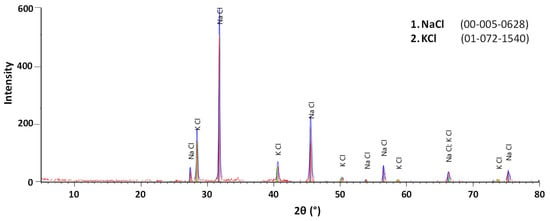
Figure 11.
Mineralogical phases (XRD patterns) of crystallized salts after evaporation.
The XRD results are in general agreement with the analyses performed by Davies et al. [19], in which the salts were presented in the form of NaCl and KCl, and the slag was basically composed of hydrolyzable salts and various metal oxides. The XRD analyses also suggest that the formation of gases during washing was not significant, as hydrolyzable aluminum compounds (Al4C3, AlN, AlP, Al, Al2S3) were not detected, in accordance with MacKenzie [26] but, in this case, differed from the data obtained by Davies et al. [19]. Bruckard & Woodcock [17] state that such differences may be related to the variation of composition of the charge loaded into the furnace during the melting process, as well as to the storage conditions of the slag.
The elemental composition of the leaching products obtained by ED-XRF is shown in Table 3 and Table 4. The results are in general agreement with those of the SEM-EDS and XRD analyses, confirming the generation of a residue with low salt content and enriched with oxides. Potassium (K) was almost fully removed after leaching (non-detected in the non-leached fraction), and the already high Al concentration in the raw slag increased to more than 85% in the treated slag. A proportional increase in the content of Fe, Si, and Ti also occurred as a result of removing the salt-forming elements. The recovered salt consisted of 95.6% NaCl and KCl, which also had about 1% Al. The balance consisted of Fe, Ca, Si, Ti, Mn, Zn, Mg, and Cu in concentrations below 0.45%.

Table 3.
Major element concentration (weight %) in the slag after leaching.

Table 4.
Major element concentration (weight %) in the recovered salt.
Table 5 presents the K+, Na+, and Cl− concentrations from the titration/FAAS analysis. The elements in the recovered salt sum approximately 90%, about 6% lower than that found in the ED-XRF analysis. This resulted from differences in the K and Cl contents detected in each method (Na values were very close). It is worth noting that ED-XRF analysis showed lower precision than the titration (Cl) and FAAS (K) techniques, as indicated by the larger SD values. Considering the characterization through titration/FAAS only (Table 5), the slag could be nearly considered “free of salts,” as defined by Gil [8], since the final salt content in the residue was little more than 2 wt%. Once free of salts, the solid residue can be subjected to further treatment for recovery of aluminum [7] and other processes, such as hydrogen generation [18] and insertion in the clinker of Portland cements [11], among others. Regarding the recovered salt, the results showed that salt composition was not ideal, since about 10 wt% consisted of elements other than Na, K, and Cl. These elements may have occurred below the XRD detection limit, which was about 5% under the conditions used, the reason why peaks other than NaCl and KCl were not identified in the recovered salt. Imperfections during leachate filtration may be a possible source of error, resulting in the entrainment of undissolved material. However, it should be noted that real-scale filtration processes are barely perfect and presumably difficult to attain a pure salt. Future work should therefore include filtration regulation and evaporation control to refine the results.

Table 5.
Concentration (mass %) of K+, Na+, and Cl− in the obtained products. The maximum relative standard deviation was ± 2.19% and occurred for Na+ analysis in the recovered salt.
4. Further Discussion
Figure 12 shows a preliminary processing flowsheet and mass balance for salt slag treatment, considering the optimum leaching conditions. In this case, 24.2% of the slag mass was dissolved during leaching, resulting in about 365 kg of treated slag for 481 kg of raw slag produced in the furnace batch. Based on this overall mass balance, material balances were also performed for each major element to calculate the balance accuracy error (residual mass divided by the total mass of the element) and thus check the accuracy of elemental analysis. Considering the ED-XRF analysis data, balance errors ranged from + 1.29% for Si to about −8% for K, where the positive (+) and negative (−) signals mean a surplus and a shortage of elemental mass, respectively. On the other hand, balances carried out for titration/FAAS analysis data (Cl, Na, and K, only) exhibited a maximum error of + 0.15% for K, suggesting an accuracy higher than that of ED-XRF analysis. Thus, the results from titration and FAAS analysis were adopted to estimate the performance indexes (purity and recovery) of the salt recycling process.
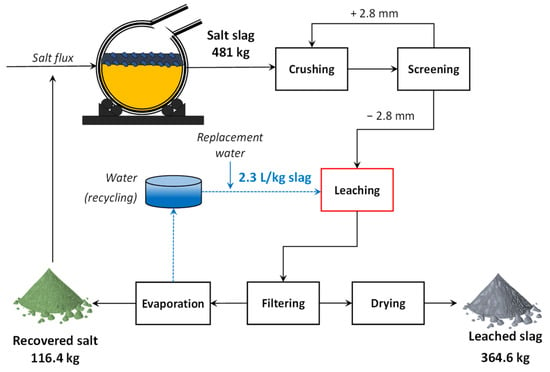
Figure 12.
Preliminary flowsheet and mass balance for salt slag processing.
The purity and overall recovery of salts were 90% and 92%, respectively, with a NaCl:KCl proportion of 4.3:1, allowing its effective recycling with a few additions of KCl to adjust the salt flux proportion adopted by the recycler (NaCl:KCl of 3:1). It is worth noting that despite their low individual concentrations, some impurities detected in the recovered salt may slightly decrease its capacity to form stable chlorides with contaminants (such as Mg and Zn) during its reuse as flux, since the salt could already be partially saturated with one or more of these elements. Analyzing it, however, is beyond the scope of this study. The water demand for leaching with 30 wt% solids in pulp would be 2.3 L/kg of fed slag. This value could be reduced to 1.5 L/kg of slag if 40 wt% solids in pulp were used, but at the cost of a lower leaching efficiency, as discussed in Section 3.2.
The crushing operation indicated in the flowsheet in Figure 12 can mean either the two-stage crushing used in the current study or another arrangement. The salt slag sampled was easily fragmented, so perhaps a single roller crushing operation in close circuit with screening could be enough to achieve the used particle size (−2.8 mm). Gravity sedimentation in tanks could also be used for the solid-liquid separation of leachate and solid residue after leaching, as mentioned by Shinzato [16], although its efficiency would arguably be below filtration systems. Most importantly, minimizing the costs of drying and evaporation is a pivotal point for improving the economic viability of salt slag treatment. When possible, the use of heat recovery systems can be an efficient strategy for decreasing costs, as the sensible heat of flue gases produced in furnaces could be redirected to those operations, thus increasing the thermal efficiency of the process. Solar dry/evaporation ponds could also be an option, as suggested by Bruckard and Woodcook [17].
5. Conclusions
In this study, we focus on optimizing the water leaching of chlorides from Al salt slag and testing practicable operational conditions. Leaching with tap water at 25 °C made it possible to dissolve more than 92% of the chlorides contained in the raw slag under the optimum leaching conditions: particle size below 2.8 mm, 30 wt% of solids in pulp, and leaching time of 90 min.
The leached slag consisted mainly of Al (predominantly as Al2O3), Si, Fe, Ca, and minor elements (Mg, Mn, Ti, and Zn), with less than 2.5% salts after leaching (compared to about 24% before it), and the recovered salt obtained after evaporation consisted of about 90% NaCl and KCl, with the potential to be re-mixed to the salt flux fed to the furnace. The possibility of minimizing water demand by performing leaching with 40 wt% solids in pulp was also presented, although this resulted in a lowering of leaching efficiency.
Finally, the study provides a framework for future studies to assess the performance of other operations that can possibly be used for salt slag treatment. Future work should focus on strategies to optimize water usage, such as the use of heat recovery systems associated with drying and evaporation steps, as well as further treatment of the leached fraction for its reuse and recycling.
Author Contributions
Conceptualization, A.B.T. and C.H.S.; methodology, A.B.T. and F.L.Q.R.; validation, A.B.T. and W.M.A.; formal analysis, A.B.T. and W.M.A.; investigation, A.B.T. and F.L.Q.R.; resources, C.H.S. and J.O.M.; data curation, A.B.T.; writing—original draft preparation, W.M.A.; writing—review and editing, C.H.S., J.O.M. and I.A.S.D.B.; visualization, W.M.A.; supervision, C.H.S. and I.A.S.D.B.; project administration, C.H.S.; funding acquisition, J.O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- I.A.I. Aluminium Sector Greenhouse Gas Pathways to 2050. Available online: https://international-aluminium.org/resource/aluminium-sector-greenhouse-gas-pathways-to-2050-2021/ (accessed on 24 April 2022).
- Ashkenazi, D. How aluminum changed the world: A metallurgical revolution through technological and cultural perspectives. Technol. Forecast. Soc. Chang. 2019, 143, 101–113. [Google Scholar] [CrossRef]
- I.A.I. Aluminium Recycling Factsheet. Available online: https://international-aluminium.org/resource/aluminium-recycling-fact-sheet/ (accessed on 24 April 2022).
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M.E. Aluminum Recycling; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Capuzzi, S.; Timelli, G. Preparation and melting of scrap in aluminum recycling: A review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Ünlü, N.; Drouet, M.G. Comparison of salt-free aluminum dross treatment processes. Resour. Conserv. Recycl. 2002, 36, 61–72. [Google Scholar] [CrossRef]
- Gil, A. Management of the salt cake from secondary aluminum fusion processes. Ind. Eng. Chem. Res. 2005, 44, 8852–8857. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.; Polyakov, P. A review of secondary aluminum production and its byproducts. JOM 2021, 73, 2603–2614. [Google Scholar] [CrossRef]
- Hazar, A.B.Y.; Saridede, M.N.; Çiğdem, M. A study on the structural analysis of aluminium drosses and processing of industrial aluminium salty slags. Scand. J. Metall. 2005, 34, 213–219. [Google Scholar] [CrossRef]
- Tsakiridis, P. Aluminium salt slag characterization and utilization–A review. J. Hazard. Mater. 2012, 217, 1–10. [Google Scholar] [CrossRef] [PubMed]
- European Waste Catalogue and Hazardous Waste List. 2002. Available online: https://v4r7y5k5.stackpathcdn.com/wp-content/uploads/2016/08/EWC_HWL.pdf (accessed on 20 March 2022).
- Gil, A.; Korili, S. Management and valorization of aluminum saline slags: Current status and future trends. Chem. Eng. J. 2016, 289, 74–84. [Google Scholar] [CrossRef]
- Graziano, D.; Hryn, J.; Daniels, E. The Economics of salt cake recycling. In Proceedings of the Annual Meeting and Exhibition of the Minerals, Metals and Materials Society (TMS), Anaheim, CA, USA, 4–8 February 1996. [Google Scholar]
- Jody, B.; Daniels, E.; Bonsignore, P.; Karvelas, D. Recycling of Aluminum Salt Cake; Argonne National Lab.: Lemont, IL, USA, 1991. [Google Scholar]
- Shinzato, M.; Hypolito, R. Solid waste from aluminum recycling process: Characterization and reuse of its economically valuable constituents. Waste Manag. 2005, 25, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bruckard, W.; Woodcock, J. Characterisation and treatment of Australian salt cakes by aqueous leaching. Miner. Eng. 2007, 20, 1376–1390. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Hydrolysis of aluminum dross material to achieve zero hazardous waste. J. Hazard. Mater. 2012, 209, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Smith, P.; Bruckard, W.; Woodcock, J. Treatment of salt cakes by aqueous leaching and Bayer-type digestion. Miner. Eng. 2008, 21, 605–612. [Google Scholar] [CrossRef]
- Bank, W. The Impact of the War in Ukraine on Commodity Markets. Available online: https://www.worldbank.org/en/research/commodity-markets (accessed on 4 May 2022).
- Bank, W. World Bank Commodities Price Data. Available online: http://www.worldbank.org/commodities (accessed on 4 May 2022).
- D6323-19; Standard Guide for Laboratory Subsampling of Media Related to Waste Management Activities. ASTM International: West Conshohocken, PA, USA, 2019.
- D512-89; Standard Test Methods for Chloride Ion in Water. ASTM International: West Conshohocken, PA, USA, 1999.
- Faraji, F.; Alizadeh, A.; Rashchi, F.; Mostoufi, N. Kinetics of leaching: A review. Rev. Chem. Eng. 2022, 38, 113–148. [Google Scholar] [CrossRef]
- Saramak, D. Challenges in Raw Material Treatment at the Mechanical Processing Stage. Minerals 2021, 11, 940. [Google Scholar] [CrossRef]
- MacKenzie, D.S. Handbook of Aluminum: Alloy Production and Materials Manufacturing; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).