Optimizing Acetic Acid Application Strategy Can Effectively Promote the Remediation Performance of Oilseed Sunflower on Cd-Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Crops and Soil
2.2. Experimental Design
2.3. Test Indicators and Methods
- (1)
- Shoot and root dry weights of oilseed sunflower: the shoot and root parts of oilseed sunflower were first rinsed with tap water, then rinsed with deionized water, placed in an oven at 105 °C for 30 min, then continued to dry at 75 °C until the weight remained constant, and the shoot and root dry matter weights were measured, respectively.
- (2)
- Soil pH value: tested with a pH meter (PHSJ-6L) at a soil to water ratio of 1:5.
- (3)
- Soil enzymatic activity: sucrase activity in soils were determined by the colorimetric method using 3,5-dinitrosalicylic acid, peroxidase activity in soils by potassium permanganate titration, and amylase activity in soils by colorimetric method.
- (4)
- Cd in soils: the soil samples were naturally dried, ground, and passed through a 200 mesh sieve; the exchangeable Cd, carbonate Cd, Fe-Mn oxide Cd, and organic Cd in the samples were extracted and determined by the Tessier graded extraction method. The supernatant after extraction was determined by TAS-986 flame atomic absorption spectrophotometer (Sedico, Giza, Egypt).
- (5)
- Cd removal rate: Cd removal rate = (total Cd in soils before planting − total Cd in soils after planting)/total Cd in soils before planting × 100%.
3. Results
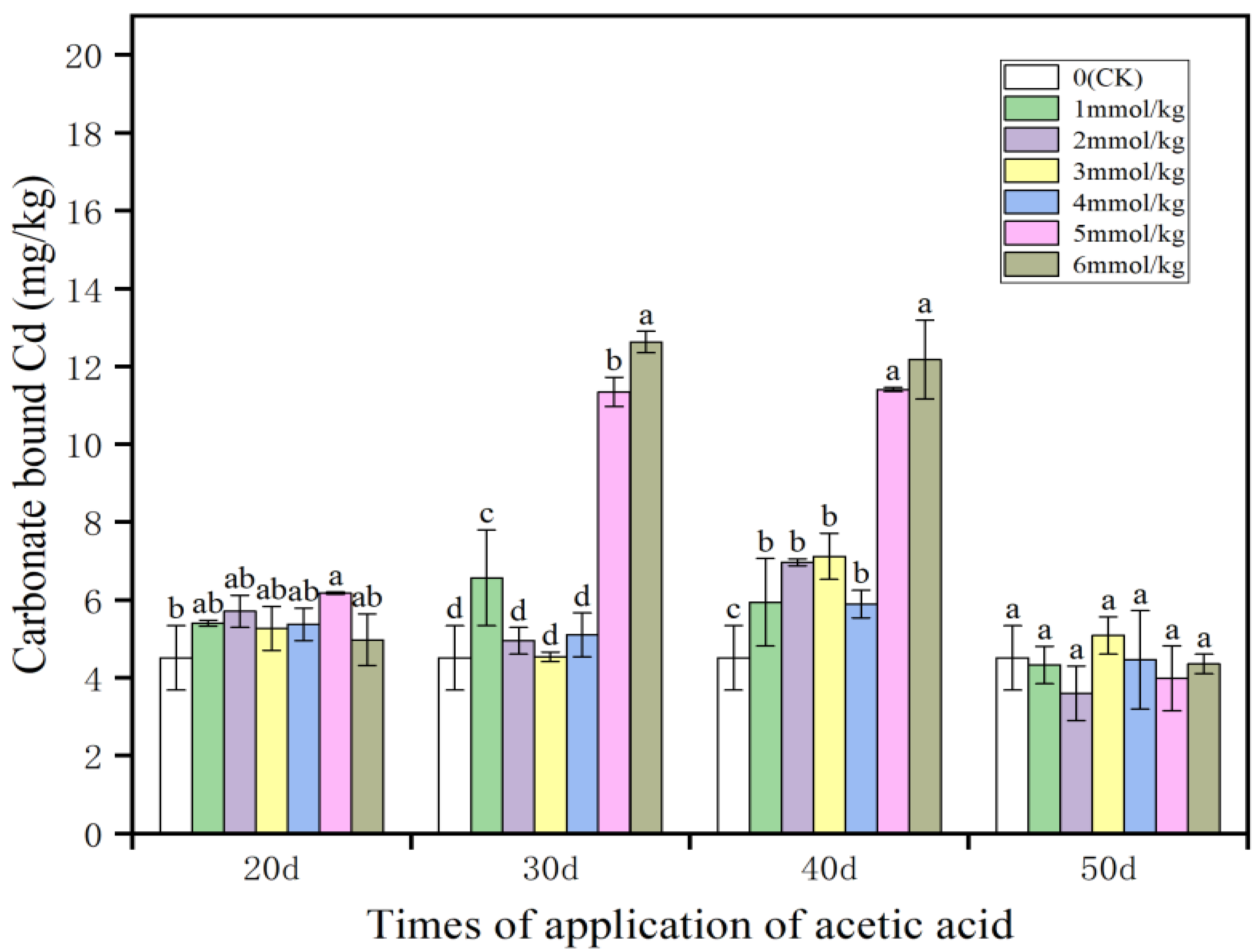
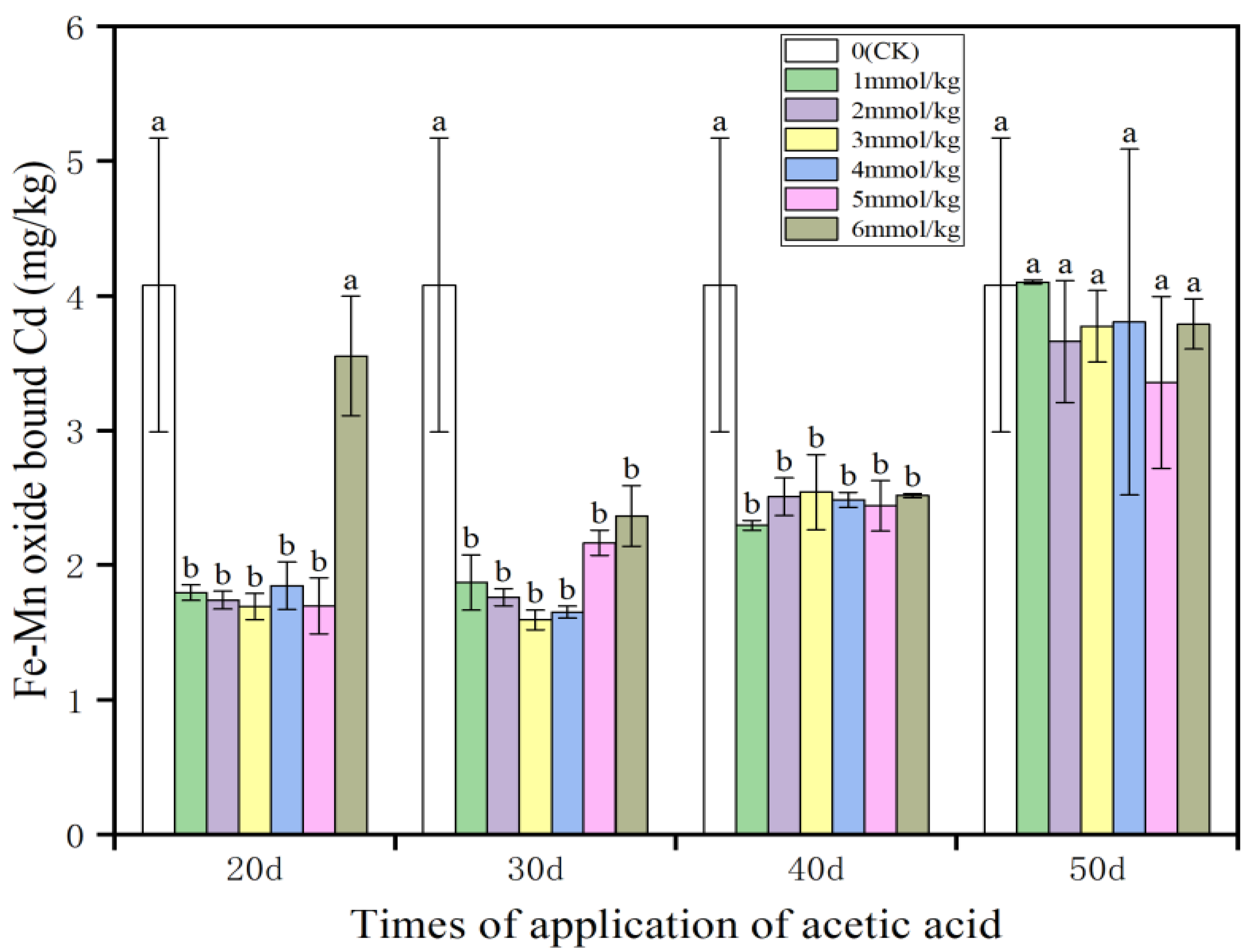
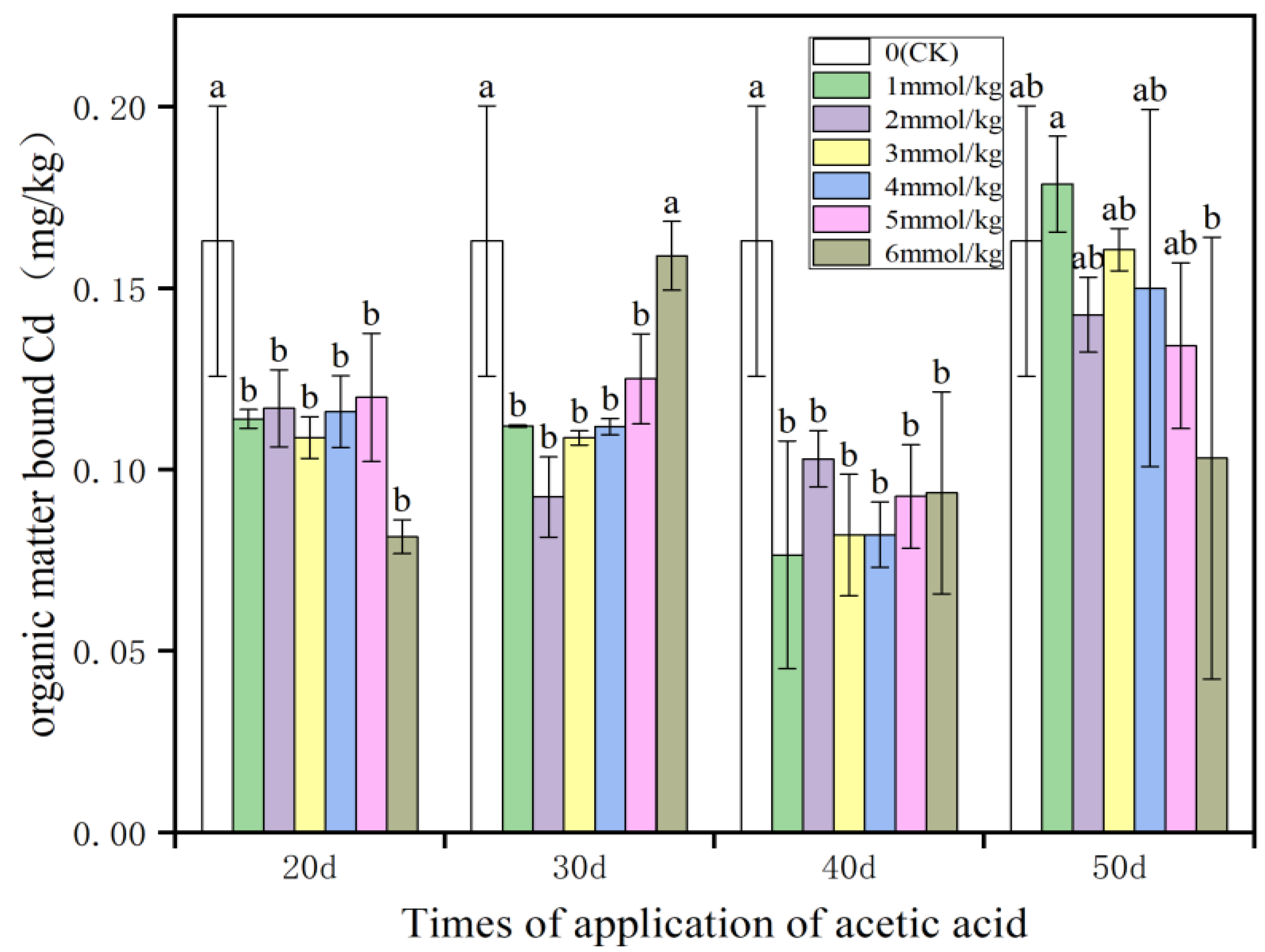
3.1. Cd Forms and Cd Removal Rate in Rhizosphere Soils
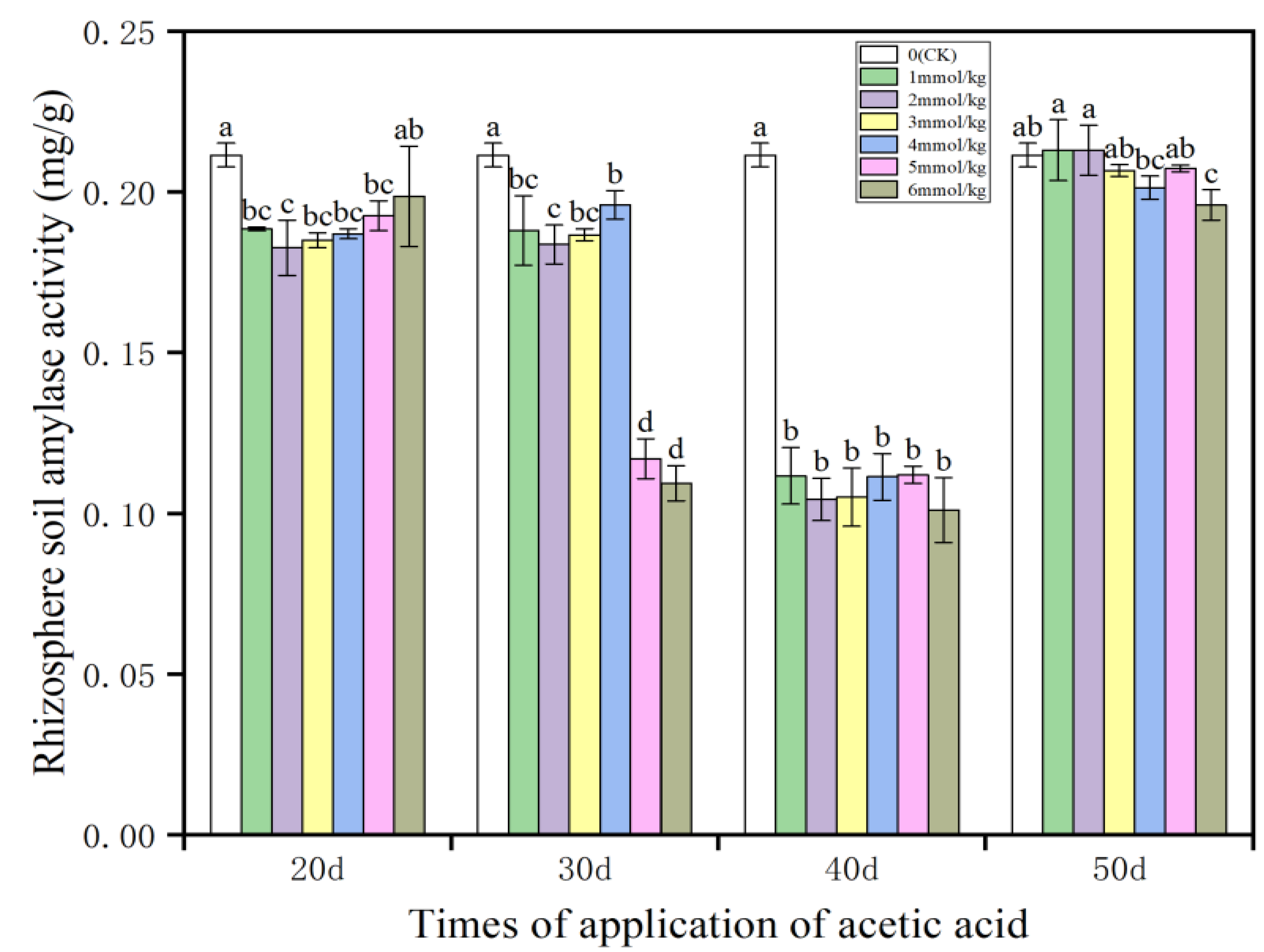
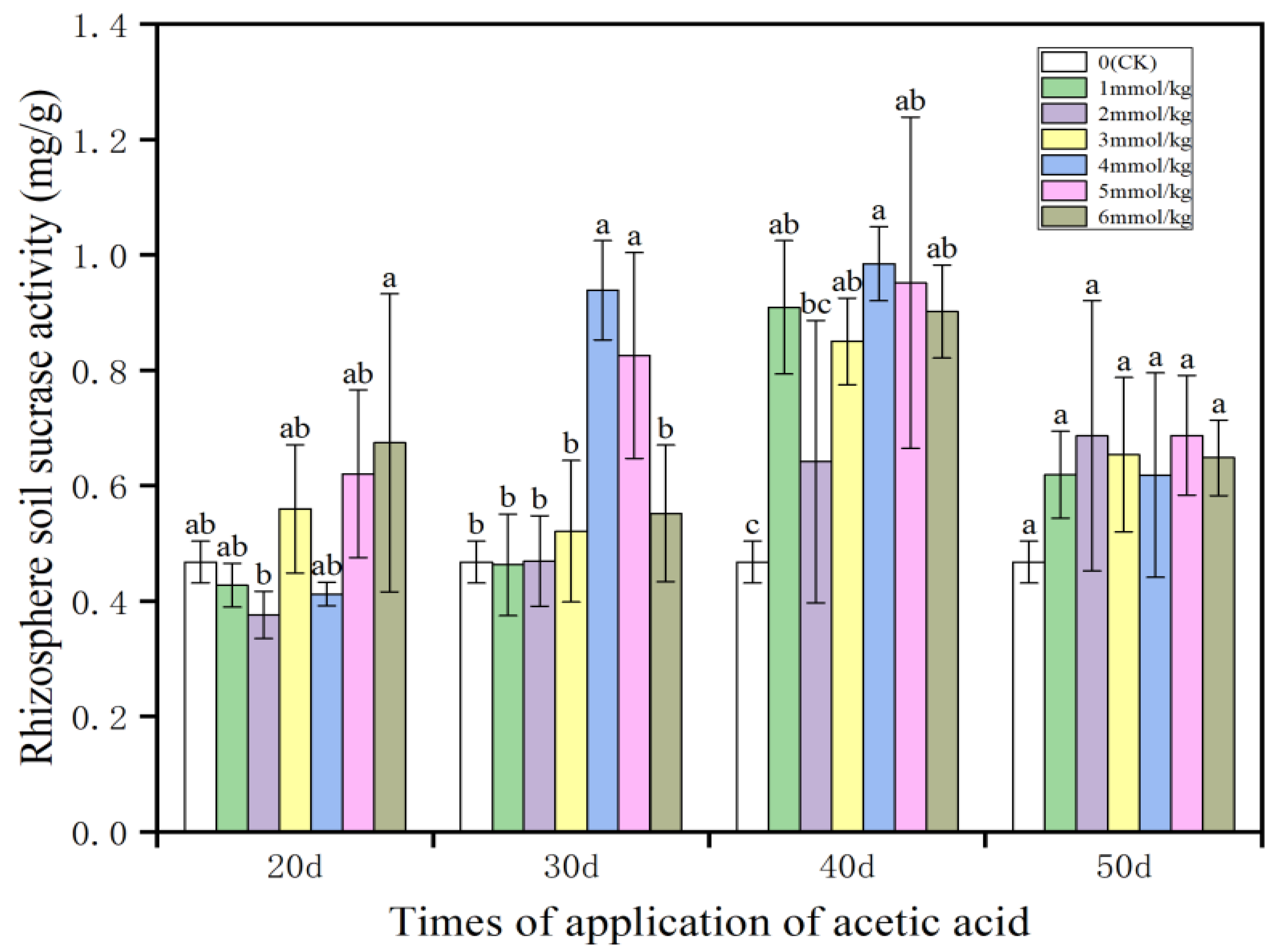
3.2. Soil Enzyme Activity
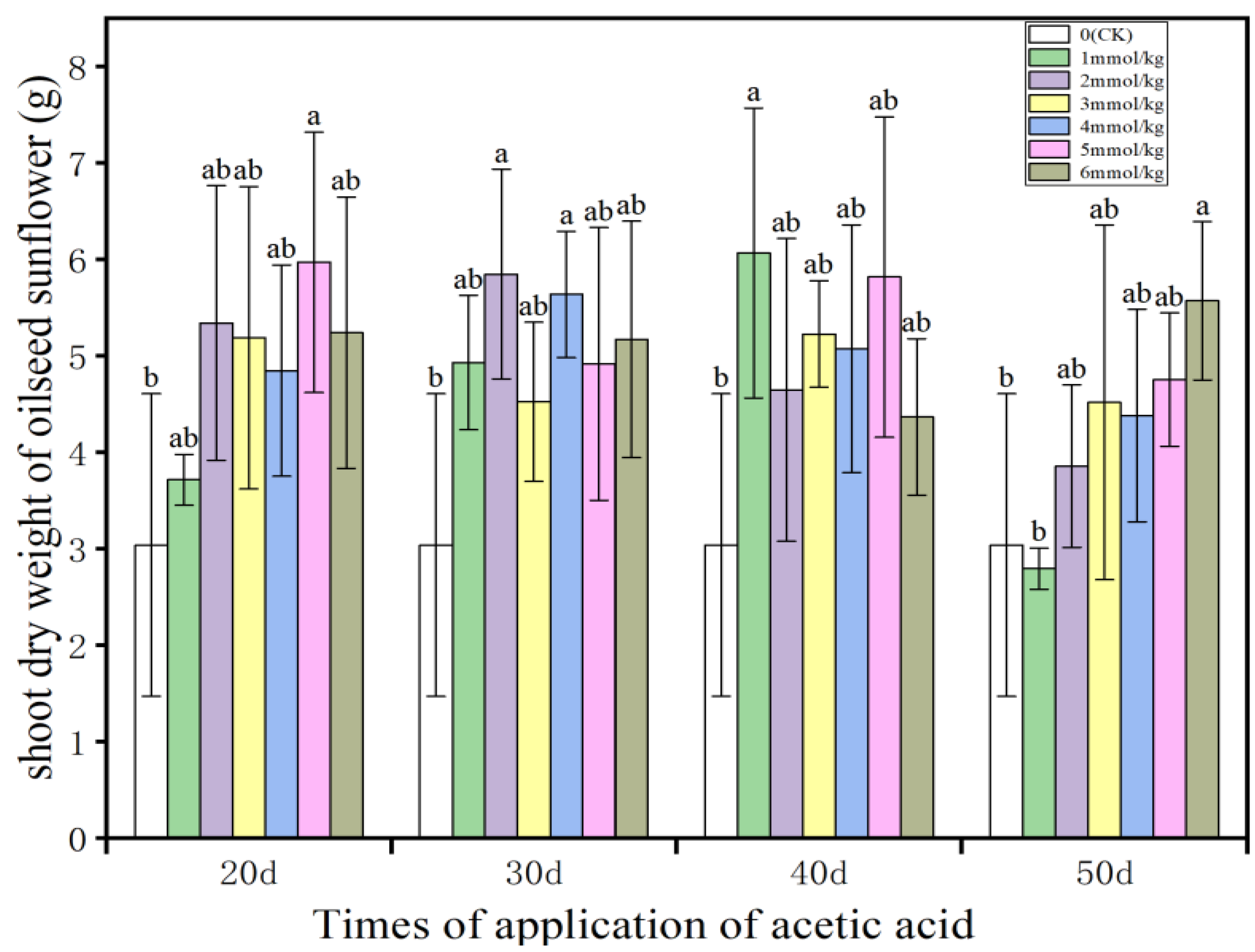
3.3. Shoot and Root Dry Weight of Oilseed Sunflower
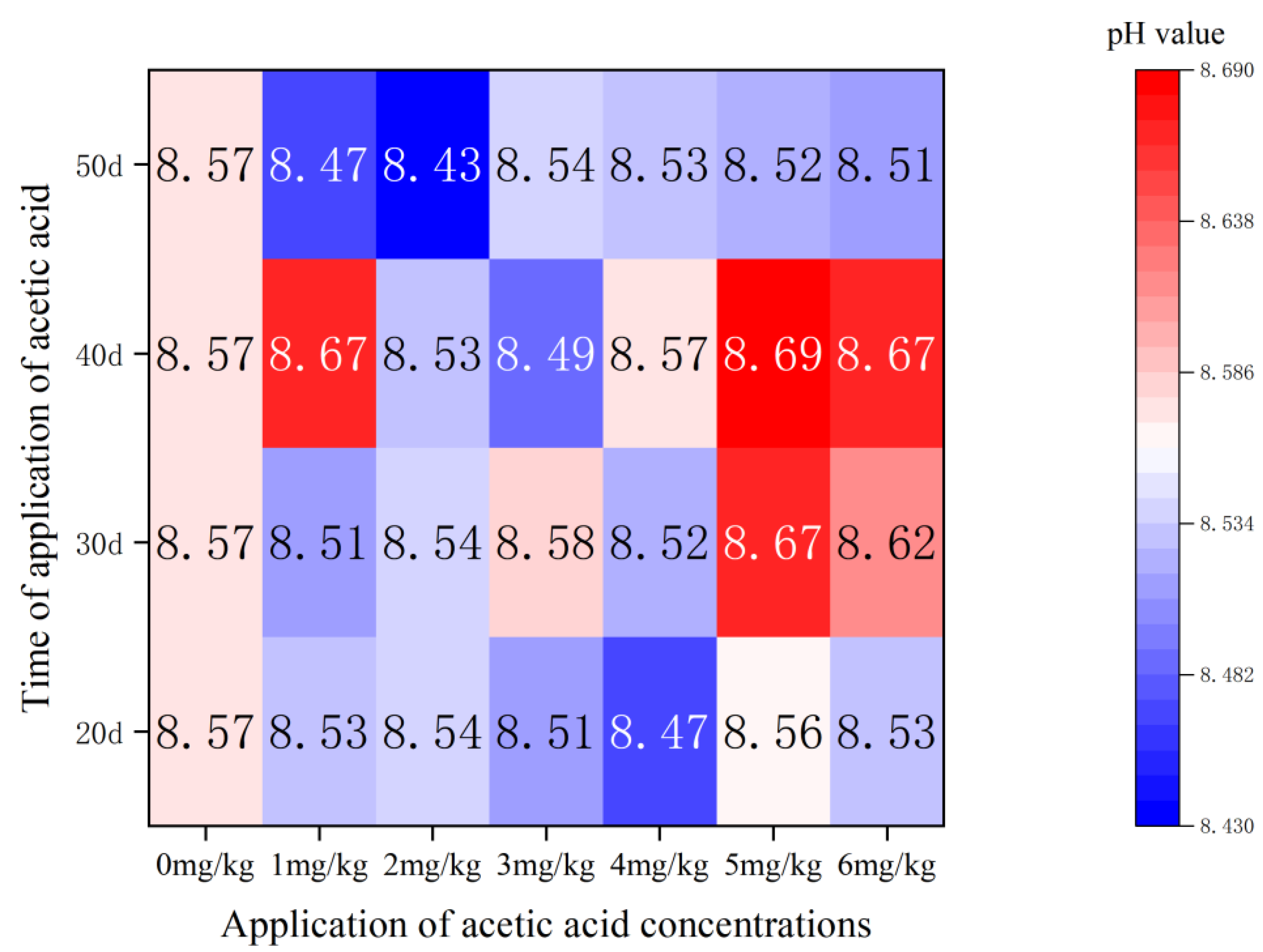
3.4. pH Value in Rhizosphere Soils
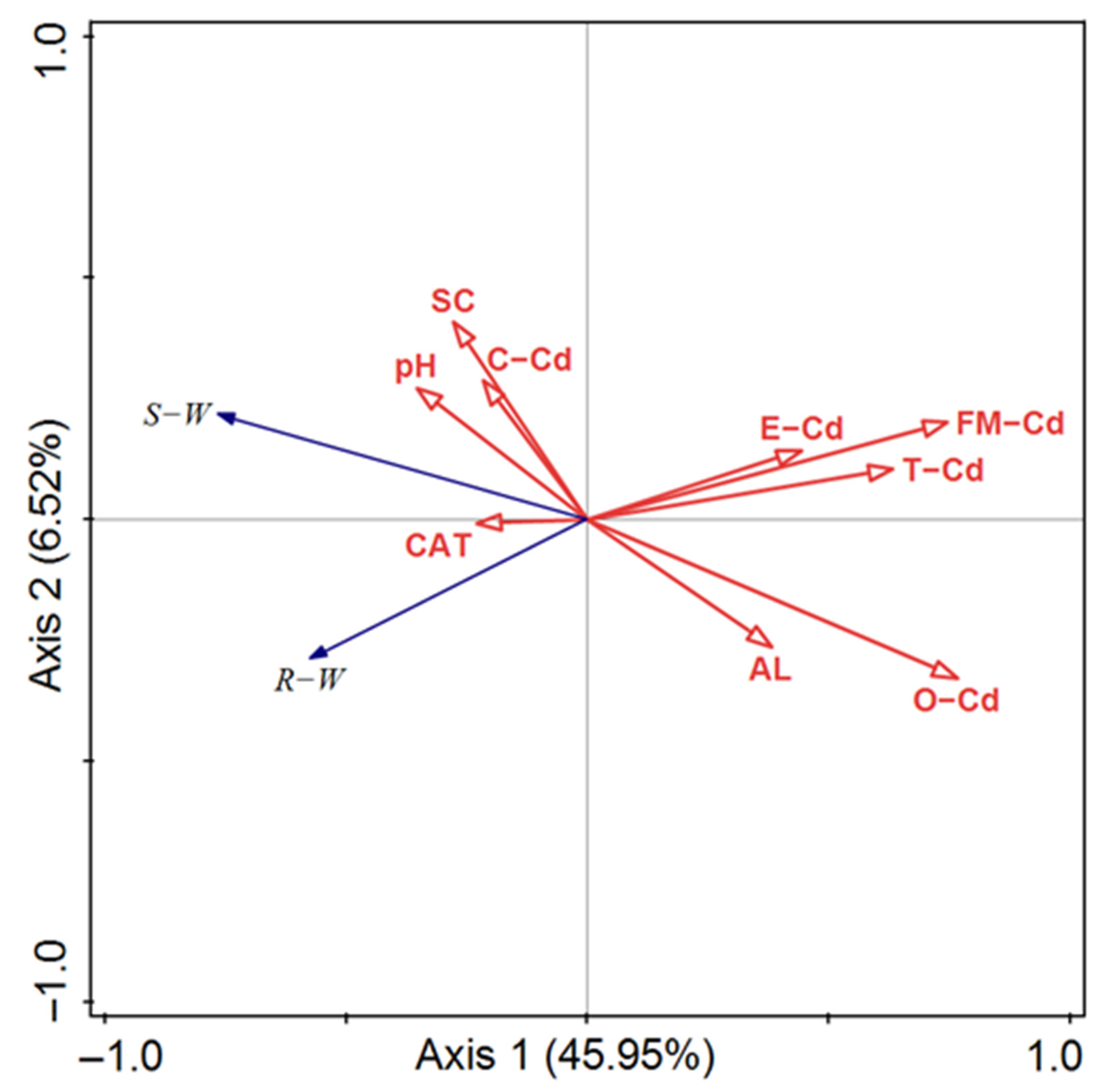
3.5. Correlation between Soil Indicators and Dry Weight of Oilseed Sunflower
3.6. Correlation between Soil Indicators and Availability of Cd
4. Discussion
5. Conclusions
- (1)
- When 4 mmol/kg acetic acid was applied at DASE 20 and 2 mmol/kg acetic acid was applied at DASE 50, the pH value in rhizosphere soils decreased significantly compared to CK. Acetic acid promoted the activity of rhizosphere soil sucrase and catalase, and amylase activity tended to decrease and then increase with the time of application.
- (2)
- The application of acetic acid significantly promoted the increase of shoot and root dry weight of oilseed sunflower. Except for the application of acetic acids at DASE 50, the trend of increasing shoot and root dry weight of oilseed sunflower at different concentrations of acetic acid at all other time points firstly increased and then decreased, which showed that a moderate concentration of acetic acid was more suitable for the increase of the dry weight of all parts of oilseed sunflower.
- (3)
- Application of different concentrations of acetic acid at different times reduced the exchangeable Cd, Fe-Mn oxide Cd, and organic Cd contents and increased the carbonate Cd content of the rhizosphere soil. In comparison, the application of acetic acids at DASE 30 and 40 were more beneficial to the reduction of rhizosphere soils’ exchangeable Cd, Fe-Mn oxide Cd and organic Cd, and the application of 5 and 6 mmol/kg acetic acids at DASE 30 and 40 were beneficial to the increase of rhizosphere soils’ carbonate Cd.
- (4)
- CAT, SC, C-Cd, and pH were positively correlated with the shoot and root dry weight of oilseed sunflower; O-Cd, FM-Cd, T-Cd, E-Cd, and AL were negatively correlated with the shoot and root dry matter weight of oilseed sunflower. Among them, O-Cd and FM-Cd had significant effects on the dry weight of oilseed sunflower, while CAT also had obvious effects.
- (5)
- Combining the dry weight of oilseed sunflower and soil indicators, the application of 1 mmol/kg acetic acid at DASE 40 had the best effect on the remediation of Cd-contaminated soil under the conditions of this experiment.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.M.; Xu, Z.; Jin, C.Y.; Bai, H.T.; Wang, L.; Zhao, Z.; Sun, H.W. Source Apportionment and Risk Assessment of Cd and Hg Pollution in Farmland. J. Agric. Environ. Sci. 2016, 35, 1314–1320. Available online: https://kns.cnki.net/kcms/detail/12.1347.s.20160715.0834.040.html (accessed on 15 July 2016).
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.H.; Zhang, Z.Q. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Malik, A. Seasonal variation of different microorganisms with nickel and cadmium in the industrial wastewater and agricultural soil. Environ. Monit. Assess. 2010, 167, 151–163. [Google Scholar] [CrossRef]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S.H. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef]
- Koptsik, G.N. Problems and prospects concerning the phytoremediation of heavy metal polluted soils: A review. Eurasian Soil Sci. 2014, 47, 923–939. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Report on the National General Survey of Soil Contamination. 2015. Available online: https://www.mee.gov.cn/hjzl/hjzlqt/trhj/201605/t20160526_347133.shtml (accessed on 5 June 2015).
- Abdu, N.; Abdullahi, A.A.; Abdulkadir, A. Heavy metals and soil microbes. Environ. Chem. Lett. 2017, 15, 65–84. [Google Scholar] [CrossRef]
- Xiong, Z.Y.; Lian, J.J.; Pi, W.; Zhang, Z.L.; He, Z.F.; Feng, J.R. Research progress of phytoremediation technology for cadmium-contaminated soil. J. Green Sci. Technol. 2020, 24, 92–94. [Google Scholar] [CrossRef]
- Rossi, G.; Beni, C. Effects of medium-term amendment with diversely processed sewage sludge on soil humification-mineralization processes and on Cu, Pb, Ni, and Zn bioavailability. Plants 2018, 7, 16. [Google Scholar] [CrossRef]
- Feller, U.; Anders, I.; Wei, S.H. Distribution and redistribution of 109Cd and 65Zn in the heavy metal hyperaccumulator Solanum nigrum L.: Influence of cadmium and zinc concentrations in the root medium. Plants 2019, 8, 340. [Google Scholar] [CrossRef]
- Yang, G.D.; Zhang, M.Z.; Feng, T.; Li, M.; Zhang, H.L.; Deng, Y.X.; Yan, J. Research status and prospect of remediation technology for heavy metal polluted soil. Mod. Chem. Ind. 2020, 40, 50–54+58. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Ye, S.J.; Zeng, G.M.; Wu, H.P.; Zhang, C.; Dai, J.; Liang, J.; Yu, J.F.; Ren, X.Y.; Yi, H.; Cheng, M.; et al. Biological technologies for the remediation of co-contaminated soil. Crit. Rev. Biotechnol. 2017, 37, 1062–1076. [Google Scholar] [CrossRef]
- Xiao, P.F.; Wu, D.D. A bibliometric Analysis of Global Phytoremediation Research. J. Ecol. 2021, 41, 8685–8695. Available online: https://kns.cnki.net/kcms/detail/11.2031.Q.20210705.1455.022.html (accessed on 5 July 2021).
- Kamran, M.A.; Bibi, S.; Xu, R.K.; Benizri, E.; Hussain, S.; Mehmood, K.; Chaudhary, H.J. Phyto-extraction of Chromium and Influence of Plant Growth Promoting Bacteria to Enhance Plant Growth. J. Geochem. Explor. 2017, 182, 269–274. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, J.X.; Guo, J.J.; Guo, J.M.; Zheng, G.D.; Lu, Y.F. Effect of Fertilizers on Cadmium Uptake and Accumulation by Sunflowers. Environ. Sci. 2018, 39, 5189–5197. [Google Scholar] [CrossRef]
- Ren, L.T. The Effect of Plant Endophyte on Tolerance and Enrichment of Heavy Metals of Oil Sunflower in Jingchang Mining Area. Master’s Thesis, Lan Zhou University, Lanzhou, China, 2018. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201802&filename=1018979793.nh&uniplatform=NZKPT&v=yjUmwwznTCA118XpDhZohOs1m2hGCOi9ZUEPTwk6A5ZcpbVz0J6qyXeBXi-C8f1- (accessed on 16 August 2018).
- Ma, H.; Li, X.D.; Hou, S.Y.; Peng, D.H.; Wang, Y.; Xu, F.; Xu, H. The activation and extraction systems using organic acids and Lentinus edodes to remediate cadmium contaminated soil. Environ. Pollut. 2019, 255, 113252. [Google Scholar] [CrossRef]
- Chauhan, P.; Mathur, J. Potential of Helianthus annuus for phytoremediation of multiple pollutants in the environment: A review. J. Biol. Sci. Med. 2018, 4, 5–16. Available online: http://refhub.elsevier.com/S2352-1864(19)30695-9/sb9 (accessed on 6 September 2022).
- Chen, H.; Cutright, T. EDTA and HEDTA effects on Cd, Cr, and Ni uptake by Helianthus annuus. Chemosphere 2001, 45, 21–28. [Google Scholar] [CrossRef]
- Sun, Y.M.; Ning, G.H.; Liu, S.Q.; Wang, Q.Q.; Yang, S.S.; Yang, Z.X. Accumulation Characteristics of Cadmium in 2 Tolerant Plant Species, Oil Sunflower and Cotton. J. Soil Water Conserv. 2015, 29, 281–286. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.P.; Li, H.L.; Liao, B.H.; Zeng, Q.R. The potential of two agricultural cropping patterns for remediating heavy metals from soils. J. Ecol. 2016, 36, 688–695. Available online: https://kns.cnki.net/kcms/detail/11.2031.q.20150612.1511.012.html (accessed on 12 June 2015).
- Wang, Y.Y.; Xu, J.B.; Sheng, L.X. Comparative Research on Cd Removal from Water by Different Kinds of Seedlings. Environ. Sci. 2007, 5, 987–992. [Google Scholar] [CrossRef]
- Guo, P.; Liu, C.; Zhang, H.B.; Song, X.J.; Bao, G.Z. Studies on Enrichment and Tolerance Ability to Pb, Cu of Sunflower Seedlings. J. Soil Water Conserv. 2007, 6, 92–95, 113. [Google Scholar] [CrossRef]
- Chang, Y.C. Screening of Highly Enriched Plants and Comparison of the Phytoremediation Potential by Different Crop Rotation Models in Mild and Moderate Cadmium Polluted Farmland. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2019. [Google Scholar] [CrossRef]
- Hassan, S.E.; Hijri, M.; St-Arnaud, M. Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. New Biotechnol. 2013, 30, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Prasher, S.O.; Simpson, B.K. Extraction of metals from a contaminated sandy soil using citric acid. Environ. Prog. 2000, 19, 275–282. [Google Scholar] [CrossRef]
- Ansari, A.A.; Gill, S.S.; Gill, R.; Lanza, G.R.; Newman, L. Role of Phytochelatins in Phytoremediation of Heavy Metals Contaminated Soils. In Environmental Bioremediation Technologies; Singh, S.N., Tripathi, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Available online: http://refhub.elsevier.com/S0147-6513(21)00013-0/sbref3 (accessed on 6 September 2022).
- Qi, Y.C.; Zhou, C.; He, X.R.; Wang, P.; Wei, H. Effects of exogenous organic acid on Cd forms in soil and Cd accumulation in salix variegate franch. Res. Environ. Sci. 2021, 34, 2220–2227. [Google Scholar] [CrossRef]
- Komarek, M.; Tlustos, P.; Szakova, J.; Chrastny, V.; Ettler, V. The use of maize and poplar in chelant-enhanced phytoextraction of lead from contaminated agricultural soils. Chemosphere 2007, 67, 640–651. [Google Scholar] [CrossRef]
- Schwab, A.P.; Zhu, D.S.; Banks, M.K. Influence of organic acids on the transport of heavy metals in soil. Chemosphere 2008, 72, 986–994. [Google Scholar] [CrossRef]
- Hu, N.; Chen, K.H.; Li, Q.S.; Xu, Z.M.; Guo, S.H.; Yu, D.P.; Luo, T. Effect of salinity-inducted organic acid variation on Cd accumulation and salinity tolerance of edible amaranth. J. Agric. Environ. Sci. 2016, 35, 858–864. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2016&filename=NHBH201605006&uniplatform=NZKPT&v=jWOYW2T5v-EAgc9n7ceSI2V5hKK9Qcq9AqL25UOK_ErGtD48azB1JjtZxn87euJn (accessed on 23 December 2015).
- Bao, T.; Sun, T.H.; Sun, L.N. Low molecular weight organic acids in root exudates and cadmium accumulation in cadmium hyperaccumulator Solanum nigrum L. and non-hyperaccumulator Solanum lycopersicum L. Afr. J. Biotechnol. 2011, 10, 17180–17185. [Google Scholar] [CrossRef]
- Fischer, K.; Bipp, H.P. Removal of heavy metals from soil components and soils by natural chelating agents. Part II. Soil extraction by sugar acids. Water Air Soil Pollut. 2002, 138, 271–288. [Google Scholar] [CrossRef]
- Qiao, D.M.; Lu, H.F.; Zhang, X.X. Change in phytoextraction of Cd by rapeseed (Brassica napus L.) with application rate of organic acids and the impact of Cd migration from bulk soil to the rhizosphere. Environ. Pollut. 2021, 267, 115452. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Pan, W.; Liu, T.T.; Tie, M.; Deng, B.; Sun, P.; Xing, Z.Q.; Zang, S.L. Study on effect of organic acid on cadmium-contaminated soil remediation. Environ. Sci. Manag. 2006, 8, 76–78. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2006&filename=BFHJ200608025&uniplatform=NZKPT&v=Os7dfB0DEEI4wE0NaAX-KjXLEt1YMGNdcBKQ9qEw5LYVZ9lBwNzHj6CffMrzBXv- (accessed on 8 May 2006).
- Yang, X.L.; Luo, Y.; Sun, L.; Hua, T.M. Effects of three organic acids on the growth and cadmium absorption of solanum nigrum L. in cadmium polluted yellow soil. Liaoning Chem. Ind. 2021, 50, 1451–1454, 1473. [Google Scholar] [CrossRef]
- Ma, H.H.; Qiao, D.M.; Qi, X.B.; Hu, C. Bioavailability of Cadmium to Oil Sunflower after Addition of Organic Acids. J. Irrig. Drain. 2017, 36, 9–14. [Google Scholar] [CrossRef]
- Lu, H.F.; Qiao, D.M.; Qi, X.B.; Hu, C.; Zhao, Z.J.; Bai, F.F.; Zhao, Y.L.; Han, Y. Effects of exogenous organic acids on soil pH, enzyme activity and cadmium migration and transformation. J. Agric. Environ. Sci. 2020, 39, 542–553. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=NHBH202003012&uniplatform=NZKPT&v=kSXBwTORyzmelnTvrpY7HlxfyJWpNeP9hB8OsaYSZdE1r_9N20HLGsJaSr7vAfmn (accessed on 26 September 2019).
- Li, W. Synthesis of Acetoin, 2,3-Butanedioland Glycolate from Acetate as Carbon Source. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2019. [Google Scholar] [CrossRef]
- Crawford, J.W.; Deacon, L.; Grinev, D.; Harris, J.A.; Ritz, K.; Singh, B.K.; Young, I. Microbial diversity affects self-organization of the soil-microbe system with consequences for function. J. R. Soc. Interface 2012, 9, 1302–1310. [Google Scholar] [CrossRef]
- Zhu, X.L.; Lv, B.X.; Shang, X.Q.; Wang, J.Q.; Li, M.; Yu, X.Y. The immobilization effects on Pb, Cd and Cu by the inoculation of organic phosphorus-degrading bacteria (OPDB) with rapeseed dregs in acidic soil. Geoderma 2019, 350, 1–10. [Google Scholar] [CrossRef]
- Javed, M.T.; Stoltz, E.; Lindberg, S.; Greger, M. Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements. Environ. Sci. Pollut. Res. 2013, 20, 1876–1880. [Google Scholar] [CrossRef]
- Wang, J.M. Research on the Remediation of Pb-Zn Contaminated Soil by Ultrasoni Enhanced Organic Acids Washing Method. Master’s Thesis, Tsinghua University, Beijing, China, 2016. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201801&filename=1017817733.nh&uniplatform=NZKPT&v=e8mwzuoovRGecxu7ZWeamjquB7_Xxd38vOmRfGOwRZRSfadV1GFWvxYUgoriOobV (accessed on 16 March 2018).
- Huang, G.Y.; You, J.W.; Zhou, X.P.; Ren, C.; Islam, M.S.; Hu, H.Q. Effects of low molecular weight organic acids on Cu accumulation by castor bean and soil enzyme activities. Ecotoxicol. Environ. Saf. 2020, 203, 110983. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.X. Effects of leaching with low molecular weight organic acids on soil pH and exchangeable aluminum. Soils 2005, 5, 669–673. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2005&filename=TURA200506017&uniplatform=NZKPT&v=POcvkPUIcSI9KhSFb8z8hDuSBuhqh3n_8ZNtPh-8V7S25q_0_lhMOqnk_JtUeEDb (accessed on 6 September 2022).
- Chen, H.C.; Wu, K.J.; Li, R.; Wang, T.; Zhou, C.; Ma, W.C.; Wei, H. Effects of exogenous organic acids on the characteristics of Cd accumulation of salix variegata under Cd stress. J. Agric. Environ. Sci. 2019, 39, 4510–4518. Available online: https://kns.cnki.net/kcms/detail/11.2031.Q.20190401.0913.060.html (accessed on 1 April 2019).
- Mao, W.; Li, W.X.; Gao, H.; Chen, X.; Jiang, Y.; Hang, T.W.; Gong, X.X.; Chen, M.; Zhang, Y.P. pH Variation and driving factors of farmlands in Yangzhou for 30 years. J. Plant Nutr. Fertil. 2017, 23, 883–893. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2017&filename=ZWYF201704005&uniplatform=NZKPT&v=N1LiZOvsOJMTvVZ_DDU0vIcqLsop7_WiIdG01LQHMYEKf6QaCsavjDw_ENAi06aq (accessed on 22 November 2016).
- Romkens, P.; Bouwman, L.; Japenga, J.; Draaisma, C. Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ. Pollut. 2002, 116, 109–121. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.D.; Wei, M.Y.; Zeng, G.Q.; Hou, S.Y.; Li, D.; Xu, H. Elucidation of the mechanisms into effects of organic acids on soil fertility, cadmium speciation and ecotoxicity in contaminated soil. Chemosphere 2020, 239, 124706. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Mitra, A.; Chakrabarti, K.; Chattopadhyay, D.J.; Chakraborty, A.; Kim, K. Effect of heavy metals on microbial biomass and activities in century old landfill soil. Environ. Monit. Assess. 2008, 136, 299–306. [Google Scholar] [CrossRef]
- Shen, G.Q.; Lu, Y.T.; Hong, J.B. Combined effect of heavy metals and polycyclic aromatic hydrocarbons on urease activity in soil. Ecotoxicol. Environ. Saf. 2006, 63, 474–480. [Google Scholar] [CrossRef]
- Chowardhara, B.; Saha, B.; Borgohain, P.; Awasthi, J.P.; Panda, S.K. Differential amelioration of cadmium toxicity by sodium nitroprusside and citric acid in Brassica juncea (L.) Czern and Coss. Biocatal. Agric. Biotechnol. 2021, 35, 102091. [Google Scholar] [CrossRef]
- Ma, S.J.; Nan, Z.R.; Zang, F.; Yang, X.Y.; Chen, S. Oil Crops Remediate Heavy Metal-contaminated Farmland Soil: A Review. Chin. Agric. Sci. Bull. 2019, 35, 80–84. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2019&filename=ZNTB201936015&uniplatform=NZKPT&v=4bC2kVCCW5JC7fzISaNlqceSonFfXbycDUIX9uhYwOjHxzpk3d0aq1YxBi9gxmcb (accessed on 23 July 2018).
- Lynd, L.R.; Laser, M.S.; Bransby, D.; Dale, B.E.; Davison, B.; Hamilton, R.; Himmel, M.; Keller, M.; McMillan, J.D.; Sheehan, J.; et al. How biotech can transform biofuels. Nat. Biotechnol. 2008, 26, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.M.; Hao, J.S.; Zhou, J.; Gao, Q.Y.; Zhu, H.H.; Xu, L.; Cui, H.B.; Li, H.X. Effects of four amendments on Cu and Cd forms and soil enzyme activity in Cu-Cd polluted soil. Ecol. Environ. Sci. 2011, 20, 1507–1512. [Google Scholar] [CrossRef]
- Chen, L.L. Study on the Effect of Exogenous Organic Acids on Phytoremediation of Cadmium Contaminated Soil. Master’s Thesis, Shihezi University, Xinjiang, China, 2021. [Google Scholar] [CrossRef]
- Yu, H.Y.; Song, X.L.; Wang, S.S.; Cao, L.J.; Guo, L.; Wang, X.L.; Peng, G.Y. Effects of low molecular weight organic acids on soil enzymes activities and bacterial community structure. Chin. Agric. Sci. 2015, 48, 4936–4947. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2016&filename=ZNYK201524009&uniplatform=NZKPT&v=CRTgYka7Dy2l2UXb-lxx9pj_onyP_mvxEivZjPSyNUdku0W-7t6s5GCL7sqNN2Qk (accessed on 27 May 2015).
- Fang, Z.G.; Xie, J.T.; Yang, Q.; Lu, Y.Z.; Huang, H.; Zhu, Y.X.; Yin, S.M.; Wu, X.T.; Du, S.T. Role and Mechanism of Low Molecular-Weight-Organic Acids in Enhanced Phytoremediation of Heavy Metal Contaminated Soil. Environ. Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Ruley, A.T.; Sharma, N.C.; Sahi, S.V.; Singh, S.R.; Sajwan, K.S. Effects of lead and chelators on growth, photosynthetic activity and Pb uptake in Sesbania drummondii grown in soil. Environ. Pollut. 2006, 144, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Wang, Y.; Yeh, K.C. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, S.J.; Lee, C.H.; Yun, S.G.; Ko, B.G.; Yang, J.E. Effects of organic acids on availability of phosphate and growth of corn in phosphate and salts accumulated soil. Korean J. Soil Sci. Fertil. 2016, 49, 265–270. [Google Scholar] [CrossRef]
- Gao, Y.Z.; He, J.Z.; Ling, W.T.; Hu, H.Q.; Liu, F. Effect of organic acids of copper and cadmium desorption from contaminated soils. Environ. Int. 2003, 29, 613–618. [Google Scholar] [CrossRef]
- Jean, L.; Bordas, F.; Moussard, C.; Vernay, P.; Hitmi, A.; Bollinger, J.C. Effect of citric acid and EDTA on chromium and nickel uptake and translocation by Datura innoxia. Environ. Pollut. 2008, 153, 555–563. [Google Scholar] [CrossRef]
- Li, Y.Q.; Wang, Y.J.; Khan, M.A.; Luo, W.X.; Xiang, Z.C.; Xu, W.J.; Zhong, B.; Ma, J.W.; Ye, Z.Q.; Zhu, Y.W.; et al. Effect of plant extracts and citric acid on phytoremediation of metal contaminated soil. Ecotoxicol. Environ. Saf. 2021, 211, 111902. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.T.; Wang, Y.P.; Jiang, Y.Z.; Wang, Z.Q. Effects of exogenous phenolic acids on root physiologic characteristics and morphologic development of poplar hydroponic cuttings. For. Sci. 2010, 46, 73–80. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2010&filename=LYKE201011011&uniplatform=NZKPT&v=Tp3C0MR0E-322qtDNPgLwNw6hwKRCAvezr7LAwRfSedbEn-LGTOuA3uaQZPjrNLc (accessed on 9 October 2010).
- Najaf, S.; Jalali, M. Effects of organic acids on cadmium and copper sorption and desorption by two calcareous soils. Environ. Monit. Assess. 2015, 187, 585. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.; Hanaf, M.M.; Zia-Ur-Rehman, M.; Saifullah; Ahmad, H.R.; Hakeem, K.R.; Aziz, T. Comparison of low-molecular-weight organic acids and ethylenediaminetetraacetic acid to enhance phytoextraction of heavy metals by maize. Commun. Soil Sci. Plant Anal. 2014, 45, 42–52. [Google Scholar] [CrossRef]
- Han, Y.; Qiao, D.M.; Qi, X.B.; Li, Z.Y.; Hu, C.; Lu, H.F.; Zhao, Y.L.; Bai, F.F.; Pang, Y. Effects of oxalic acid on oil sunflower biomass, enzyme activity, and the Cd speciation of Cd-polluted soils. J. Agric. Environ. Sci. 2020, 39, 1964–1973. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=NHBH202009012&uniplatform=NZKPT&v=kSXBwTORyzkwZ0VPtwbWakMioOksiB-m_wFBORkHJ3Oya_aKmaRnDKSYbTwINhrY (accessed on 2 March 2020).
- Zhang, S.L.; Chen, H.C.; He, D.N.; He, X.R.; Yan, Y.; Wu, K.J.; Wei, H. Effects of Exogenous Organic Acids on Cd Tolerance Mechanism of Salix variegata Franch Under Cd Stress. Front. Plant Sci. 2020, 11, 594352–594365. [Google Scholar] [CrossRef]
- Beven, K.; Germann, P. Macropores and water flow in soils revisited. Water Resour. Res. 2013, 49, 3071–3092. [Google Scholar] [CrossRef] [Green Version]




| Mechanical Composition (%) | Nutrient Element | OM (%) | ||||
|---|---|---|---|---|---|---|
| 0.002 mm | 0.05–0.002 mm | 0.05 mm | TN (g·kg−1) | TP (g·kg−1) | K (mg·kg−1) | |
| 11.53 | 75.37 | 13.10 | 1.14 | 0.63 | 86 | 2.7 |
| Test Indicator | Application of Acetic Acid Concentrations | Time of Application of Acetic Acid | |||
|---|---|---|---|---|---|
| DASE 20 | DASE 30 | DASE 40 | DASE 50 | ||
| Total Cd | 0 (CK) | 14.80 ± 0.28a | 14.80 ± 0.28a | 14.80 ± 0.28ab | 14.80 ± 0.28a |
| 1 mmol/kg | 13.12 ± 0.07a | 13.41 ± 0.60b | 11.95 ± 1.49c | 14.60 ± 0.70a | |
| 2 mmol/kg | 13.74 ± 0.09a | 13.05 ± 0.33b | 15.66 ± 0.28a | 13.21 ± 1.02a | |
| 3 mmol/kg | 12.46 ± 0.92a | 13.27 ± 0.37b | 14.86 ± 0.22ab | 14.19 ± 0.23a | |
| 4 mmol/kg | 12.95 ± 1.24a | 13.59 ± 0.52b | 14.96 ± 0.12ab | 13.88 ± 1.92a | |
| 5 mmol/kg | 12.36 ± 1.89a | 13.25 ± 0.31b | 12.77 ± 2.37bc | 13.30 ± 1.14a | |
| 6 mmol/kg | 12.43 ± 1.20a | 14.67 ± 0.91a | 14.79 ± 0.59ab | 13.86 ± 0.67a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qiao, D.; Han, Y.; Zhang, D. Optimizing Acetic Acid Application Strategy Can Effectively Promote the Remediation Performance of Oilseed Sunflower on Cd-Contaminated Soils. Minerals 2022, 12, 1139. https://doi.org/10.3390/min12091139
Wang Y, Qiao D, Han Y, Zhang D. Optimizing Acetic Acid Application Strategy Can Effectively Promote the Remediation Performance of Oilseed Sunflower on Cd-Contaminated Soils. Minerals. 2022; 12(9):1139. https://doi.org/10.3390/min12091139
Chicago/Turabian StyleWang, Yadan, Dongmei Qiao, Yang Han, and Dengmin Zhang. 2022. "Optimizing Acetic Acid Application Strategy Can Effectively Promote the Remediation Performance of Oilseed Sunflower on Cd-Contaminated Soils" Minerals 12, no. 9: 1139. https://doi.org/10.3390/min12091139
APA StyleWang, Y., Qiao, D., Han, Y., & Zhang, D. (2022). Optimizing Acetic Acid Application Strategy Can Effectively Promote the Remediation Performance of Oilseed Sunflower on Cd-Contaminated Soils. Minerals, 12(9), 1139. https://doi.org/10.3390/min12091139




