Origin of Historical Ba-Rich Slags Related to Pb-Ag Production from Jihlava Ore District (Czech Republic)
Abstract
1. Introduction
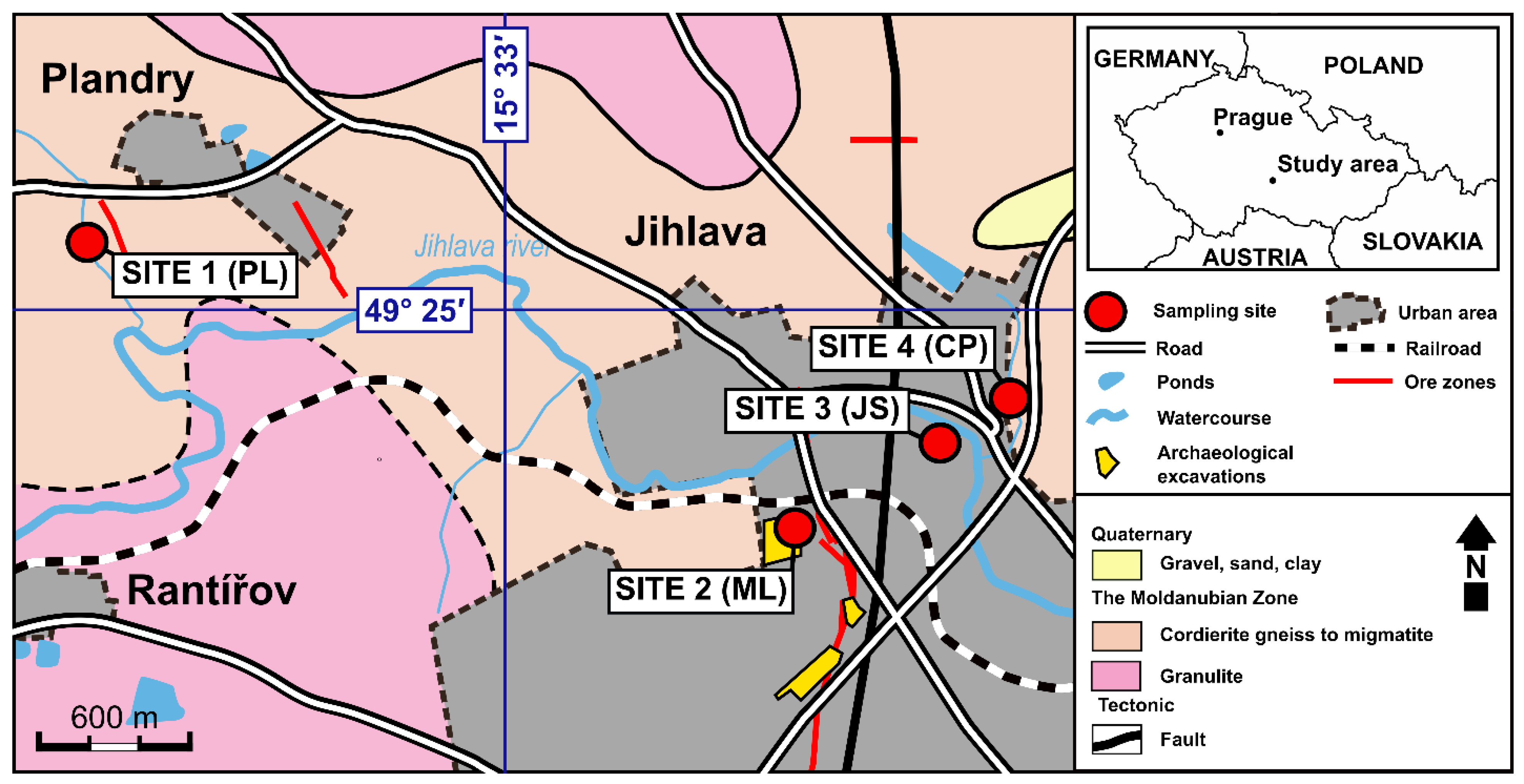
2. History and Ore Mineralization of Studied Sites
2.1. Historical Background
2.2. Ore Mineralization in the JOD
3. Studied Sites and Evidence of Metallurgical Activities
4. Materials and Methods
4.1. Slag Selection and Preparation
4.2. Optical Microscopy and Electron Microprobe
4.3. Bulk Chemical Analyses
4.4. X-ray Powder Diffraction
4.5. Determination of Smelting Temperature
5. Results
5.1. Bulk Chemical Composition
| Sample | PL5 | PL7 | PL8 | PL15 | ML2 | ML7 | ML24 | ML26 | JS4 | JS6 | JS9 | CP1 | CP4 | CP7 | Av | Min | Max | Av | Min | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 4 | n = 19 | n = 18 | ||||
| Type | B | A | A | A | A | A | B | B | B | B | B | B | B | B | A | A | A | B | B | B |
| P2O5 wt.% | 0.67 | 0.94 | 0.95 | 0.55 | 0.53 | 0.59 | 0.44 | 1.43 | 0.41 | 0.43 | 0.32 | 0.39 | 0.39 | 0.36 | 0.60 | 0.37 | 0.99 | 0.51 | 0.30 | 1.43 |
| SiO2 | 41.55 | 45.76 | 42.83 | 43.34 | 44.43 | 44.95 | 38.52 | 42.34 | 42.20 | 44.56 | 59.38 | 56.97 | 49.63 | 55.90 | 44.58 | 38.33 | 53.56 | 48.31 | 38.52 | 59.38 |
| TiO2 | 1.00 | 0.42 | 0.27 | 0.42 | 0.38 | 0.38 | 0.97 | 0.94 | 0.54 | 0.55 | 0.39 | 0.24 | 0.65 | 0.33 | 0.37 | 0.27 | 0.45 | 0.57 | 0.24 | 1.00 |
| Al2O3 | 5.91 | 8.68 | 6.07 | 7.10 | 6.76 | 6.84 | 6.35 | 6.46 | 6.21 | 6.20 | 6.06 | 5.53 | 6.25 | 5.85 | 6.98 | 5.57 | 8.68 | 6.08 | 5.24 | 6.76 |
| BaO | 23.20 | 0.42 | 1.32 | 2.05 | 1.04 | 1.16 | 34.50 | 27.69 | 22.60 | 19.20 | 11.61 | 8.16 | 19.54 | 5.44 | 0.94 | 0.08 | 2.10 | 18.92 | 5.44 | 34.50 |
| CaO | 7.68 | 4.15 | 3.07 | 3.90 | 2.74 | 2.63 | 6.51 | 6.20 | 10.46 | 10.59 | 5.71 | 4.77 | 7.16 | 4.85 | 3.09 | 2.49 | 4.15 | 7.77 | 4.57 | 10.59 |
| FeOtot. | 8.80 | 22.62 | 31.29 | 24.97 | 38.66 | 37.29 | 4.51 | 4.89 | 11.19 | 10.96 | 10.91 | 16.67 | 11.26 | 20.49 | 31.93 | 21.85 | 41.01 | 11.07 | 4.51 | 20.49 |
| MgO | 1.49 | 1.40 | 1.39 | 1.74 | 1.10 | 1.11 | 1.42 | 1.28 | 1.41 | 1.36 | 0.97 | 0.79 | 1.24 | 1.02 | 1.16 | 0.84 | 1.81 | 1.24 | 0.79 | 1.68 |
| MnO | 0.34 | 3.15 | 5.87 | 2.36 | 0.30 | 0.37 | 0.13 | 0.20 | 0.34 | 0.35 | 0.27 | 0.63 | 0.32 | 0.59 | 1.76 | 0.27 | 5.87 | 0.34 | 0.13 | 0.63 |
| PbO | 5.74 | 5.57 | 3.25 | 3.91 | 0.91 | 0.92 | 3.65 | 4.47 | 0.54 | 0.93 | 0.20 | 0.23 | 0.72 | 0.23 | 2.77 | 0.81 | 5.77 | 1.24 | 0.06 | 5.74 |
| ZnO | 1.53 | 3.72 | 2.25 | 5.68 | 1.10 | 1.11 | 0.75 | 0.19 | 1.29 | 1.24 | 1.17 | 0.72 | 0.95 | 0.41 | 2.59 | 0.64 | 6.65 | 0.83 | 0.18 | 1.68 |
| K2O | 2.26 | 3.32 | 2.09 | 2.79 | 2.36 | 2.38 | 1.85 | 2.56 | 2.02 | 2.11 | 1.97 | 2.22 | 2.08 | 2.33 | 2.64 | 2.09 | 3.48 | 2.10 | 1.63 | 2.71 |
| Na2O | 0.52 | 0.80 | 0.45 | 0.95 | 0.54 | 0.53 | 0.46 | 0.66 | 0.69 | 0.52 | 0.44 | 0.38 | 0.59 | 0.45 | 0.60 | 0.43 | 1.03 | 0.53 | 0.38 | 0.71 |
| Stot. | 0.31 | 0.73 | 1.05 | 0.79 | 1.21 | 1.34 | 0.88 | 0.17 | 0.54 | 0.49 | 0.65 | 0.80 | 0.45 | 0.65 | 1.02 | 0.62 | 1.36 | 0.53 | 0.17 | 0.88 |
| Total | 101.00 | 101.68 | 102.15 | 100.54 | 102.06 | 101.59 | 100.94 | 99.49 | 100.44 | 99.49 | 100.06 | 98.50 | 101.22 | 98.89 | 101.04 | 98.30 | 102.15 | 100.04 | 98.50 | 101.34 |
| Ag ppm | 38 | 32 | 112 | 23 | 77 | 78 | 331 | 91 | 77 | 93 | 11 | 51 | 28 | 64 | 69 | 3 | 144 | 79 | 2 | 331 |
| As | 39 | 80 | 86 | 49 | 7 | 8 | 23 | 24 | b.d. | 9 | 46 | 18 | 70 | 14 | 47 | 3 | 269 | 28 | 6 | 71 |
| Au * | b.d. | 20 | 40 | b.d. | 34 | 41 | 5 | 5 | b.d. | 5 | 0 | 4 | b.d. | 9 | 39 | 8 | 86 | 5 | 0 | 9 |
| Cd | 39.5 | 1.5 | 2.1 | 2.2 | 0.4 | 0.8 | 0.3 | b.d. | b.d. | 1.6 | 4.9 | 4.0 | 9.3 | 1.1 | 1.4 | b.d. | 2.2 | 5.7 | 0.3 | 39.5 |
| Co | 60 | 26 | 22 | 31 | 12 | 16 | 4 | 10 | 82 | 26 | 137 | 36 | 82 | 28 | 27 | 10 | 53 | 60 | 4 | 137 |
| Cr | 90 | 120 | 80 | b.d. | 60 | 60 | 80 | 120 | b.d. | 60 | 50 | 60 | 90 | 70 | 87 | 42 | 147 | 79 | 40 | 120 |
| Cu | 740 | 2470 | 1120 | 1530 | 684 | 706 | 138 | 717 | 923 | 910 | 1860 | 763 | 974 | 961 | 1220 | 652 | 2550 | 833 | 138 | 1860 |
| Ni | 11 | 107 | 37 | 28 | 10 | 32 | 5 | 15 | b.d. | 75 | 84 | 9 | 84 | 96 | 25 | 9 | 107 | 38 | 5 | 96 |
| Sb | 2 | 122 | 21 | 38 | 21 | 19 | 71 | 103 | 36 | 55 | 9 | 15 | 2 | 13 | 29 | 8 | 122 | 31 | 2 | 103 |
| Sn | 7 | 4 | 17 | b.d. | 2 | 2 | b.d. | 19 | b.d. | b.d. | 6 | 5 | 5 | 2 | 6 | b.d. | 17 | 10 | 1 | 31 |
| Sr | 4470 | 253 | 344 | 459 | 249 | 266 | 6680 | 5550 | 4310 | 3820 | 2280 | 1516 | 3570 | 1090 | 260 | 158 | 529 | 3520 | 1090 | 6680 |
| v.i.mod | 1.09 | 0.83 | 1.04 | 0.96 | 0.95 | 0.92 | 1.20 | 0.99 | 1.04 | 0.93 | 0.51 | 0.55 | 0.78 | 0.58 | 0.93 | 0.64 | 1.15 | 0.84 | 0.51 | 1.20 |
| v.i. | 0.93 | 0.66 | 0.93 | 0.77 | 0.91 | 0.88 | 1.10 | 0.89 | 1.01 | 0.89 | 0.49 | 0.54 | 0.75 | 0.57 | 0.83 | 0.53 | 0.99 | 0.80 | 0.49 | 1.13 |
| SG g/cm3 | 3.47 | 3.15 | 3.38 | 3.44 | 3.31 | 3.28 | 3.24 | 3.04 | 3.27 | 3.13 | 2.99 | 3.02 | 2.86 | 3.04 | 3.27 | 2.77 | 3.53 | 3.07 | 1.83 | 3.47 |
| liquidus °C | 1122 | 1164 | 1188 | 1162 | 1183 | 1182 | 1121 | 1087 | 1114 | 1139 | 1081 | 1093 | 1093 | 1105 | 1178 | 1147 | 1260 | 1110 | 1075 | 1153 |
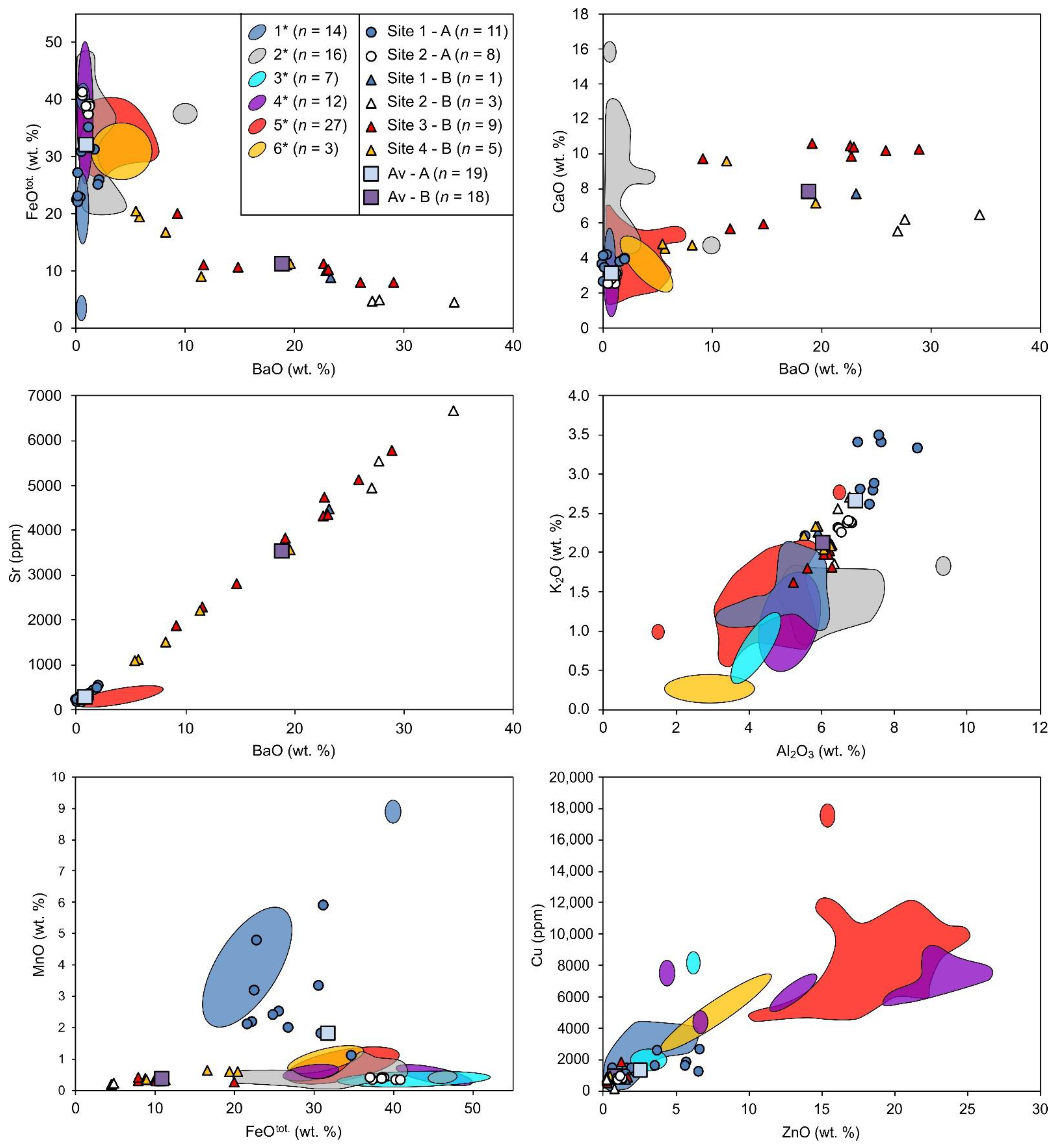
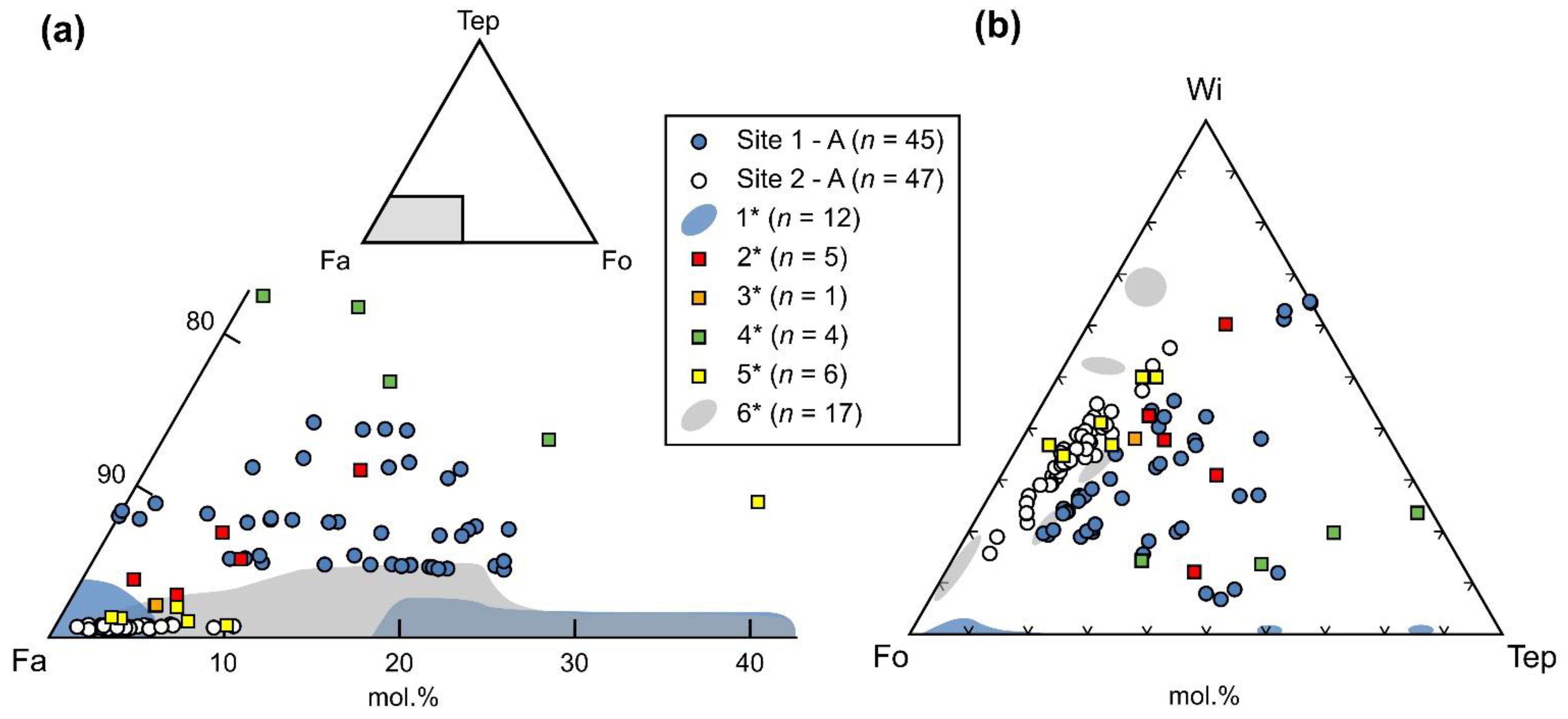
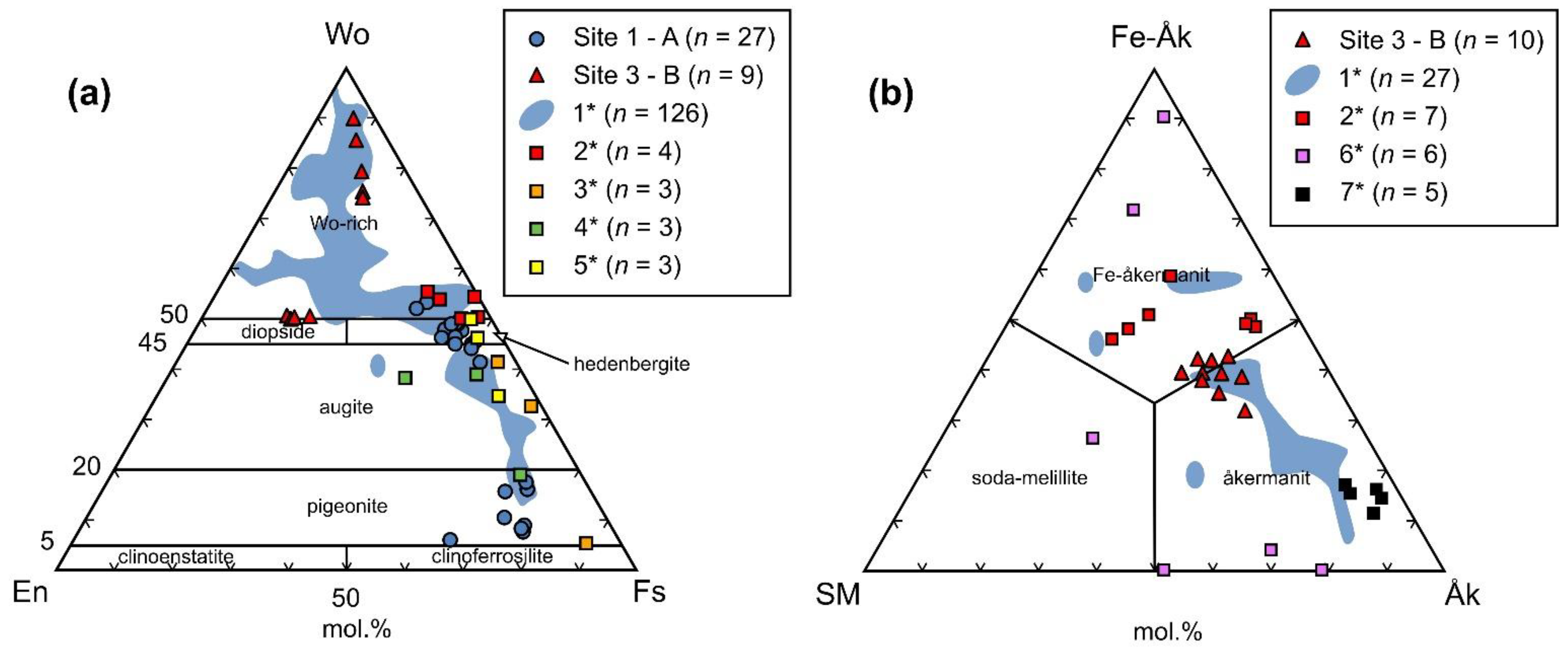
5.2. Slag Mineralogy and Crystal Chemistry
5.3. Smelting Experiments
6. Discussion
6.1. Origin of Barium-Rich Slags
6.2. Ore Processing and Composition of Charge
6.3. The Smelting Temperature and Efficiency of Metal Recovery
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachmann, H.G. The Identification of Slags from Archeological Sites; Routledge: London, UK, 1988. [Google Scholar]
- Tylecote, R.F. A History of Metallurgy, 2nd ed.; Maney Publishing: London, UK, 1992. [Google Scholar]
- Gong, W.; Chen, Q.; Miao, J. Bond behaviors between copper slag concrete and corroded steel bar after exposure to high temperature. J. Build. Eng. 2021, 44, 103312. [Google Scholar] [CrossRef]
- Manasse, A.; Mellini, M.; Viti, C. The copper slags of the Capattoli Valley, Campiglia Marittima, Italy. Eur. J. Mineral. 2001, 13, 949–960. [Google Scholar] [CrossRef]
- Manasse, A.; Mellini, M. Chemical and textural characterisation of medieval slags from the Massa Marittima smelting sites (Tuscany, Italy). J. Cult. Herit. 2002, 3, 187–198. [Google Scholar] [CrossRef]
- Manasse, A.; Mellini, M. Archaeometallurgic slags from Kutná Hora. Neues Jahrb. für Mineral. Monatshefte 2002, 8, 369–384. [Google Scholar] [CrossRef]
- Sáez, R.; Nocete, F.; Nieto, J.M.; Capitán, M.Á.; Rovira, S. The extractive metallurgy of copper from Cabezo Juré, Huelva, Spain: Chemical and mineralogical study of slags dated to the third millenium B.C. Can. Mineral. 2003, 44, 627–638. [Google Scholar] [CrossRef]
- Ettler, V.; Červinka, R.; Johan, Z. Mineralogy of medieval slags from lead and silver smelting (Bohutín, Příbram District, Czech Republic): Toward estimation of historical smelting conditions. Archaeometry 2009, 51, 987–1007. [Google Scholar] [CrossRef]
- Ströbele, F.; Wenzel, T.; Kronz, A.; Hildebrandt, H.; Markl, G. Mineralogical and geochemical characterization of high-medieval lead-silver smelting slags from Wiesloch near Heidelberg (Germany)—An approach to process reconstruction. Archaeol. Anthropol. Sci. 2010, 2, 191–215. [Google Scholar] [CrossRef][Green Version]
- Kądziołka, K.; Pietranik, A.; Kierczak, J.; Potysz, A.; Stolarczyk, T. Towards better reconstruction of smelting temperatures: Methodological review and the case of historical K-rich Cu-slags from the Old Copper Basin, Poland. J. Archaeol. Sci. 2020, 118, 1–19. [Google Scholar] [CrossRef]
- Cabała, J.; Warchulski, R.; Rozmus, D.; Środek, D.; Szełęg, E. Pb-rich slags, minerals, and pollution resulted from a Medieval Ag-Pb smelting and mining operation in the Silesian-Cracowian region (southern Poland). Minerals 2020, 10, 28. [Google Scholar] [CrossRef]
- Derkowska, K.; Świerk, M.; Nowak, K. Reconstruction of Copper smelting technology based on 18-20th-century slag remains from the Old Copper Basin, Poland. Minerals 2021, 11, 926. [Google Scholar] [CrossRef]
- Warchulski, R.; Szczuka, M.; Kupczak, K. Reconstruction of 16th–17th century lead smelting processes on the basis of slag properties: A case study from Sławków, Poland. Minerals 2020, 10, 1039. [Google Scholar] [CrossRef]
- Ash, C.; Borůvka, L.; Tejnecký, V.; Nikodem, A.; Šebek, O.; Drábek, O. Potentially toxic element distribution in soils from the Ag-smelting slag of Kutná Hora (Czech Republic): Descriptive and prediction analyses. J. Geochem. Explor. 2013, 144, 328–336. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z. 12 years of leaching of contaminants from Pb smelter slags: Geochemical/mineralogical controls and slag recycling potential. Appl. Geochem. 2014, 40, 97–103. [Google Scholar] [CrossRef]
- Yin, N.H.; Sivry, Y.; Avril, C.; Borensztajn, S.; Labanowski, J.; Malavergne, V.; Lens, P.N.L.; Rossano, S.; Hullebusch, E.D. Bioweathering of lead blast furnace metallurgical slags by Pseudomonas aeruginosa. Int. Biodeterior. Biodegrad. 2014, 86, 372–381. [Google Scholar] [CrossRef]
- Tyszka, R.; Kierzak, J.; Pietranik, A.; Ettler, V.; Mihaljevič, M. Extensive weathering of zinc smelting slag in a heap in Upper Silesia (Poland): Potentional environmental risks posed by mechanical disturbance of slag deposits. Appl. Geochem. 2014, 40, 70–81. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Pietranik, A.; Kądziołka, K. Mineralogical, geochemical, and leaching study of historical Cu-slags issued from processing of the Zechstein formation (Old Copper Basin, southwestern Poland). Appl. Geochem. 2018, 98, 22–35. [Google Scholar] [CrossRef]
- Derner, K.; Hrubý, P.; Malina, O.; Večeřa, J. Hornické revíry vrcholného středověku a raného novověku ve srovnávacím pohledu—Mining regions in the high Middle Ages and the early modern age viewed in terms of comparison. Archaeol. Hist. 2019, 44, 925–947. [Google Scholar] [CrossRef]
- Derner, K.; Hrubý, P. Farming and food production in medieval mining communities. Archaeol. Hist. 2018, 43, 207–239. (In Czech) [Google Scholar]
- Hrubý, P.; Hejhal, P.; Kočár, P.; Libor, P.; Malý, K. Central Bohemian-Moravian Highlands on the Threshold of the High Middle Ages Archaeology, Geochemistry and the Analyses of Alluvial Sediments; Opera Universitatis Masarykianae Brunensis, Facultas Philosophica; Masarykova Univerzita: Brno, Czech Republic, 2014; p. 266. (In Czech) [Google Scholar]
- Pluskal, O.; Vosáhlo, J. Jihlavský rudní obvod. Vlastivěd. Sb. Vysoč. 1988, 13, 157–191. (In Czech) [Google Scholar]
- Hrubý, P. Středověká hornická aglomerace na Starých Horách u Jihlavy. Stříbrná Jihlava 2004, 5–21. (In Czech) [Google Scholar]
- Zajíček, P. Evaluation of Ag reserves in the Jihlava ore district. Čas. Mineral. Geol. 1983, 28, 197–207. (In Czech) [Google Scholar]
- Bernard, J.H. Empirical Types of Ore Mineralizations in the Bohemian Massif; Geological Survey: Prague, Czech Republic, 1991. [Google Scholar]
- Koutek, J. O rudních žilách a starém dolování u Jihlavy. Sbor. Ústř. Úst. Geol. Odd. Geol. 1952, 19, 77–116. (In Czech) [Google Scholar]
- Němec, D. Geologische und paragenetische Verhältnisse der Erzgänge des Jihlava-Jezdovicer Reviers. Tschermaks Mineral. Petrogr. Mitt. 1964, 9, 42–85. (In German) [Google Scholar]
- Malý, K. Chemistry of carbonates from Jihlava Ore District. Acta Rer. Natur. Přír. Čas. Vysočiny 2009, 7, 57–62. (In Czech) [Google Scholar]
- Malý, K.; Dolníček, Z. Pb-Zn-Ag vein mineralization of central part of the Českomoravská vrchovina Upland (Czech Republic): S, C and O stable isotope study. Bull. Geosci. 2005, 80, 307–319. [Google Scholar]
- Hrubý, P. Metalurgická Produkční Sféra a Neagrární Sídelní Struktura v Závěru Přemyslovské Éry na Centrální Českomoravské Vrchovině. Habilitation Thesis, Masaryk University, Brno, Czech Republic, May 2017. (In Czech). [Google Scholar]
- Hoover, H.C.; Hoover, I.H. Georgius Agricola de Re Metallica; Dover Publications: New York, NY, USA, 1950. [Google Scholar]
- Hrubý, P. From Crude Silver to Coins, or from Smelteries to Mints. Archaeol. Hist. 2015, 39, 609–637. (In Czech) [Google Scholar]
- Malý, K.; Vilímek, L.; Vokáč, M.; Zimola, D. Mining Settlement Evidence in the Alluvial Plain of the Bělokamenský Creek. Archeol. Výzk. Vysoč. 2007, 1, 125–144. (In Czech) [Google Scholar]
- Šamalová, E. Defunct medieval metallurgical site near Jihlava. Stříbrná Jihlava 2007, 228–237. (In Czech) [Google Scholar]
- Havlíček, J. The Discovery of Ore Millstone Fragments Near the Metallurgy Plant in Plandry. Archeol. Výzk. Vysoč. 2015, 4, 170–172. (In Czech) [Google Scholar]
- Hrubý, P.; Hejhal, P.; Malý, K. Těžba a úprava rudy na Jihlavských Starých Horách ve 13. století (Montánní archeologický výzkum a aplikace přírodovědných analýz). Stříbrná Jihlava 2007, 238–269. (In Czech) [Google Scholar]
- Zimola, D. Analýza keramických artefaktů ze Starých Hor u Jihlavy. Acta Rer. Natur. Přír. Čas. Vysočiny 2014, 16, 121–143. (In Czech) [Google Scholar]
- Crkal, J.; Derner, K.; Hrubý, P.; Milo, P. Architecture of mining settlements in the late Přemyslid era. Archaeol. Hist. 2019, 44, 887–923. (In Czech) [Google Scholar] [CrossRef][Green Version]
- Gualda, G.A.R.; Ghiorso, M.S. MELTS_Excel: A Microsoft Excel-based MELTS interface for research and teaching of magma properties and evolution. Geochem. Geophys. Geosyst. 2015, 16, 315–324. [Google Scholar] [CrossRef]
- Eggers, T.; Ruppert, H.; Kronz, A. Change of copper smelting techniques during medieval times in the Harz-Mountains (Germany). In Applied Mineralogy; Rammlmair, D., Mederer, J., Oberthür, T., Heimann, R.B., Pentinghaus, H., Eds.; Balkema: Rotterdam, The Netherlands, 2000; Volume 5, pp. 971–974. [Google Scholar]
- Asmus, B. Medieval Copper Smelting in the Harz Mountains, Germany; Deutsches Bergbau-Museum: Bochum, Germany, 2012. [Google Scholar]
- Chaudhuri, J.N.B.; Newesely, H. Mineralogical characterization of old Harz Mountain slags. Can. Metall. Q. 1993, 32, 1–12. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Vítková, M.; Skála, R.; Kotrlý, M.; Habler, G.; Klementová, M. Reliability of chemical microanalyses for solid waste materials. J. Hazard. Mater. 2012, 221–222, 298–302. [Google Scholar] [CrossRef]
- Ettler, V.; Legendre, O.; Bodénan, F.; Touray, J.C. Primary phase and natural weathering of old lead-zinc pyrometallurgical slag from Příbram, Czech Republic. Can. Mineral. 2001, 39, 873–888. [Google Scholar] [CrossRef]
- Morimoto, N. Nomenclature of pyroxenes. Am. Mineral. 1988, 73, 1123–1133. [Google Scholar]
- Ettler, V.; Johan, Z.; Zavřel, J.; Wallisová, M.S.; Mihaljevič, M.; Šebek, O. Slag remains from the Na Slupi site (Prague, Czech Republic): Evidence for early medieval non-ferrous metal smelting. J. Archaeol. Sci. 2015, 53, 72–83. [Google Scholar] [CrossRef]
- Kolitsch, U.; Brandstätter, F.; Schreiber, F.; Fink, R.; Auer, C. Die Mineralogie der weltweit einzigartigen Schlacken von Waitschach, Kärnten. Ann. Naturhist. Mus. Wien Serie A 2013, 115, 19–87. (In German) [Google Scholar]
- Smith, R. A typology of lead-bale slags based on their physico-chemical properties. Hist. Metall. 2006, 40, 115–128. [Google Scholar]
- Hamilton, K.; McDonnell, J.G.; Schmidt, A. Assessment of early lead working sites in the Yorkshire Dales by geophysical prospection. British Mining 1999, 63, 156–164. [Google Scholar]
- Kiernan, D.T. The Derbyshire Lead Industry in the Sixteenth Century; Derbyshire Record Society: Chesterfield, UK, 1989. [Google Scholar]
- Zori, C.; Tropper, P. Silver lining: Evidence for Inka silver refining in northern Chile. J. Archaeol. Sci. 2013, 40, 3282–3292. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Chen, X.; Li, H.; Xu, Y.; Li, X. The research of burning ancient Chinese lead-barium glass by using mineral raw materials. J. Cult. Herit. 2016, 21, 796–801. [Google Scholar] [CrossRef]
- Cui, J.; Wu, X.; Huang, B. Chemical and lead isotope analysis of some lead-barium glass wares from the Warring States Period, unearthed from Chu tombs in Changed City, Hunan Province, China. J. Archaeol. Sci. 2011, 38, 1671–1679. [Google Scholar] [CrossRef]
- Wedepohl, K.H.; Simon, K. The chemical composition of medieval wood ash glass from Central Europe. Geochemistry 2010, 70, 89–97. [Google Scholar] [CrossRef]
- Pánová, K.; Rohanová, D.; Randáková, S. Modelling of Bohemian and Moravian glass recipes from Gothic to Baroque periods. Herit. Sci. 2020, 8, 117. [Google Scholar] [CrossRef]
- Pánová, K.; Jílková, K.; Rohanová, D.; Lahodný, F.; Galusková, D.; Míka, M. Melting Process and Viscosity of Bohemian Historical Glasses Studied on Model Glasses. Minerals 2021, 11, 829. [Google Scholar] [CrossRef]
- Scheinert, M.; Kupsch, H.; Bletz, B. Geochemical investigations of slags from the historical smelting in Freiberg, Erzgebirge (Germany). Chem. Erde 2009, 69, 81–90. [Google Scholar] [CrossRef]
- Nováček, K. The mineral resources of medieval Bohemia as an archaeological problem: The state and perspectives of research into metal production and working. Archeol. Rozhl. 2001, 53, 279–309. (In Czech) [Google Scholar]
- Sano, N. Thermodynamics of BaO bearing fluxes. In Proceedings of the 4th International Conference on Molten Slags and Fluxes, Sendai, Japan, 8–11 June 1992. [Google Scholar]
- Vaněk, V.; Velebil, D. Early Metallurgy of silver. Stříbrná Jihlava 2007, 188–205. (In Czech) [Google Scholar]
- Kierczak, J.; Pietranik, A. Mineralogy and composition of historical Cu slags from the Rudawy Janowickie Mountains, southwestern Poland. Can. Mineral. 2011, 49, 1281–1296. [Google Scholar] [CrossRef]
- Kotková, J.; Schaltegger, U.; Leichmann, J. Two types of ultrapotassic plutonic rocks in the Bohemian Massif—Coeval intrusions at different crustal levels. Lithos 2010, 115, 163–176. [Google Scholar] [CrossRef]
- René, M. Evolution of two mica granites in the area between Mrákotín and Řásná. Geol. Res. Morav. Silesia 2000, 8, 82–84. (In Czech) [Google Scholar]
- Buriánek, D.; Soukup, M.; Ivanov, M. Migration of alkali metals in the weathering profiles of migmatites from the Svratka Crystalline Unit and Moldanubicum. Geol. Res. Morav. Silesia 2020, 27, 88–97. (In Czech) [Google Scholar]
- Joosten, I. Technology of Early Historical Iron Production in The Netherlands; Institute for Geo- and Bioarchaeology, Vrije Universiteit Amsterdam: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Breiter, K.; Scharbert, S. Two-mica and biotite granites in the Weitra-Nové Hrady area, Austria—Czech Republic. J. Czech Geol. Soc. 2006, 51, 217–230. [Google Scholar] [CrossRef]
- Muan, A.; Osborn, E.F. Phase Equilibria among Oxides in Steelmaking; Addison-Wesley Publishing Company, Inc.: Boston, MA, USA, 1965. [Google Scholar]
- Hall, F.P.; Insley, H. Phase Diagrams for Ceramists; The American Ceramic Society Inc.: Columbus, OH, USA, 1947. [Google Scholar]


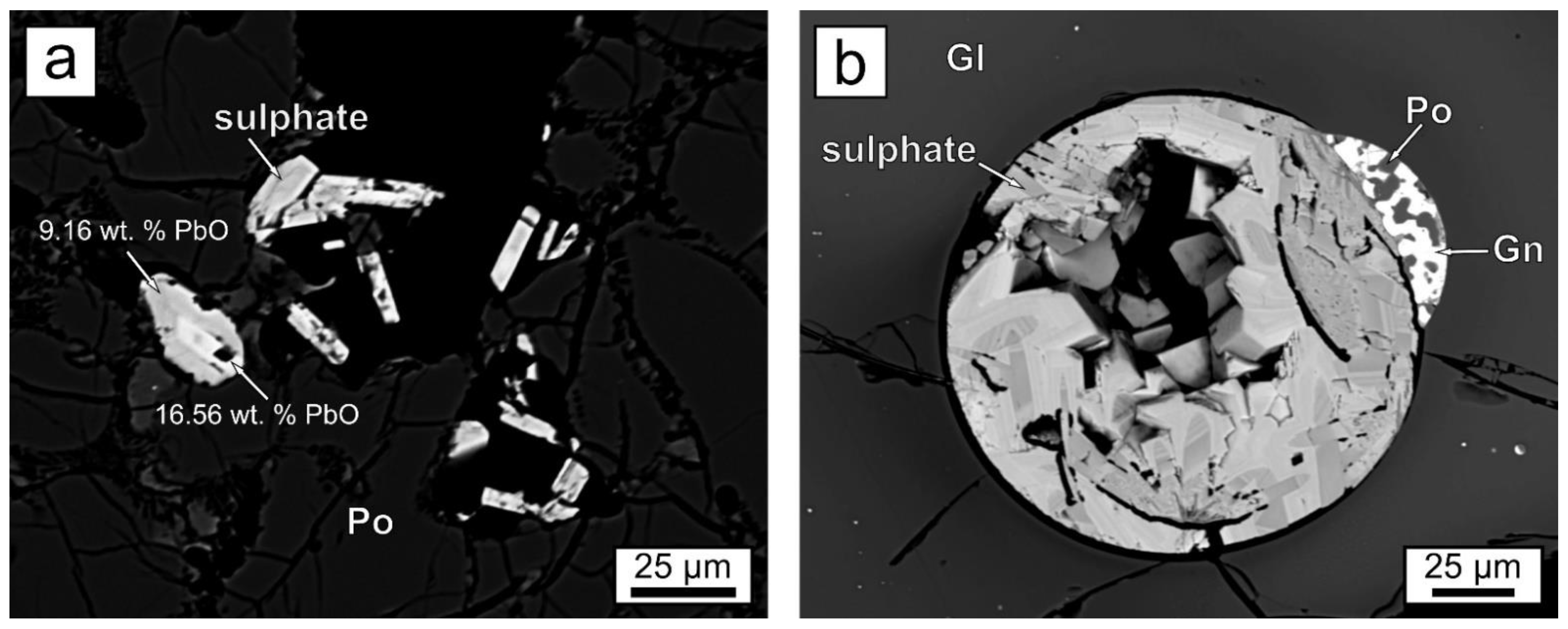
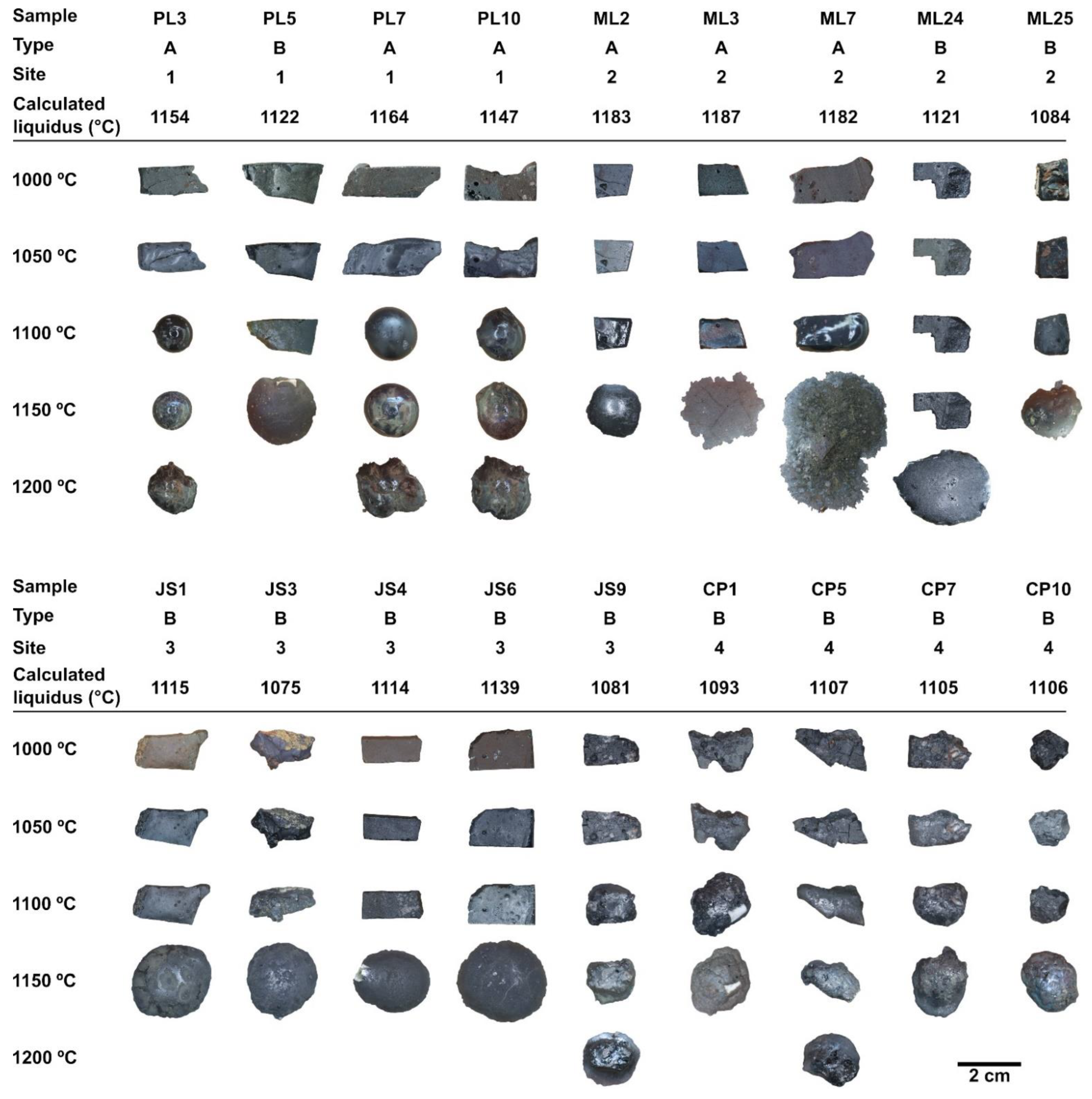
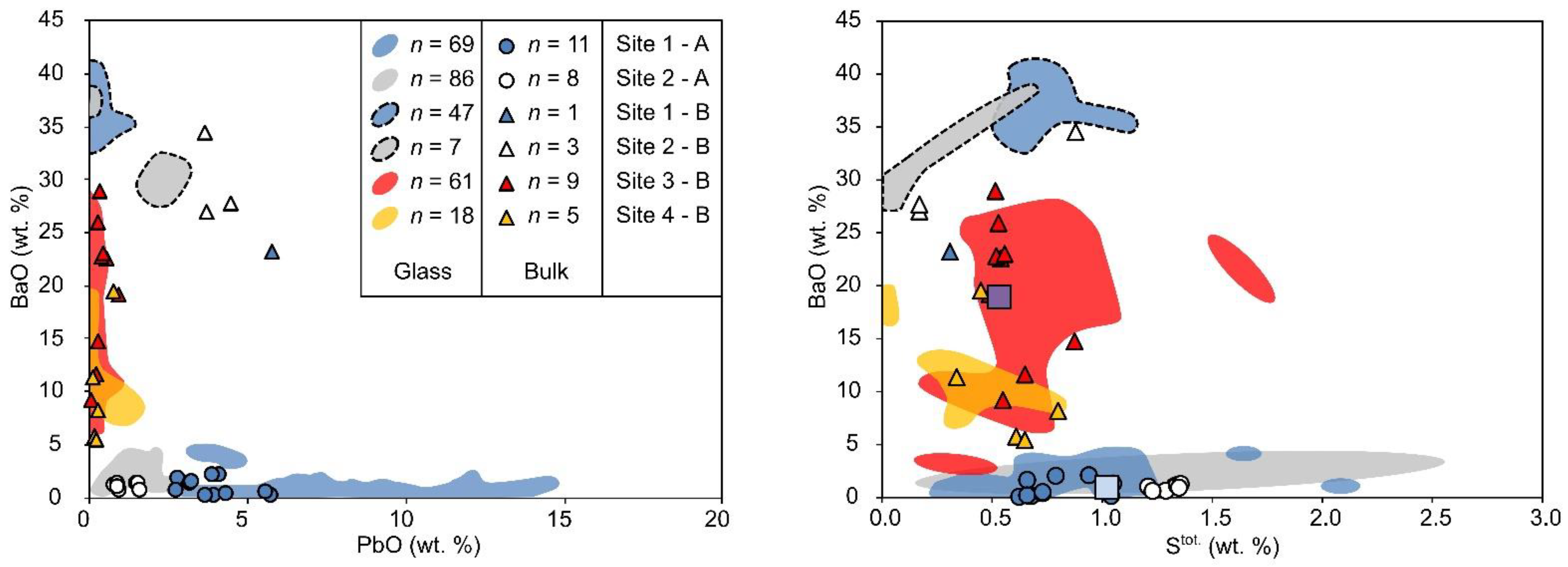
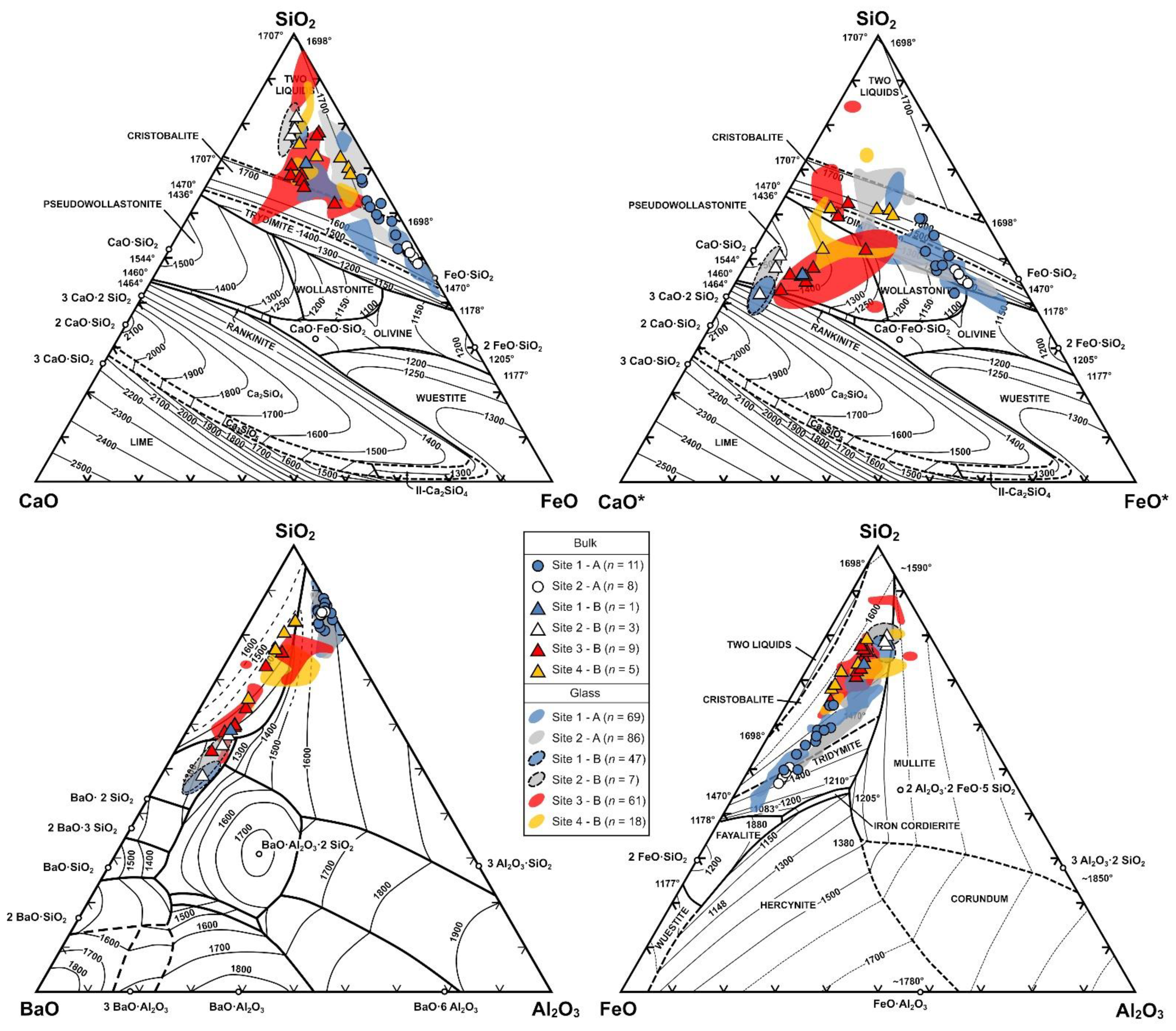
| Assemblage | Phases | Slag Type | Site |
|---|---|---|---|
| I | Gl + Ol | A | 1, 2 |
| II | Gl + Fld ± Wo ± Px ± Mel | B | 1, 2, 3, 4 |
| III | Gl + Ol + Fld ± Px | A | 1 |
| Phase | Ol | Ol | Ol | Ol | Ol | Ol | Ol | Px | Px | Px | Px | Wo | Wo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An. No. | 18/1. | 17/1. | 56/1. | 1/1. | 99/1. | 82/1. | 114/1. | 67/1. | 46 | 74/1. | 4 | 28/1. | 32/1. |
| Sample | ML1 | ML1 | PL1 | PL1 | PL2 | PL7 | PL8 | PL1 | PL15 | PL7 | JS5 | JS5 | JS5 |
| Site | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 |
| Type | A | A | A | A | A | A | A | A | A | A | B | B | B |
| P2O5 wt.% | 0.50 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.33 | b.d. | b.d. | b.d. | b.d. | b.d. |
| SiO2 | 29.57 | 30.80 | 30.94 | 31.64 | 30.71 | 30.19 | 31.11 | 45.65 | 44.92 | 46.80 | 51.36 | 51.31 | 51.92 |
| TiO2 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 1.23 | 0.27 | b.d. | 0.52 | b.d. | 0.21 |
| Al2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 3.17 | 3.13 | 0.43 | 1.03 | b.d. | 0.62 |
| BaO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.39 |
| CaO | 0.40 | 0.41 | 0.52 | 0.45 | 0.46 | 1.30 | 0.39 | 19.28 | 6.38 | 16.78 | 24.01 | 46.97 | 45.12 |
| FeO | 63.31 | 62.85 | 51.41 | 49.78 | 49.16 | 47.24 | 51.43 | 22.43 | 33.67 | 23.29 | 9.49 | 0.59 | 0.77 |
| MgO | 2.54 | 2.45 | 6.83 | 9.15 | 7.75 | 6.70 | 5.40 | 2.66 | 2.63 | 1.24 | 11.74 | b.d. | b.d. |
| MnO | 0.52 | 0.56 | 3.14 | 3.45 | 4.75 | 7.29 | 9.52 | 1.25 | 3.75 | 6.19 | 0.34 | b.d. | 0.23 |
| PbO | b.d. | b.d. | b.d. | b.d. | b.d. | 0.21 | b.d. | b.d. | b.d. | 0.37 | b.d. | b.d. | b.d. |
| ZnO | 1.76 | 1.55 | 6.33 | 5.72 | 5.47 | 4.86 | 1.88 | 2.98 | 4.00 | 2.90 | 0.59 | b.d. | b.d. |
| K2O | 0.07 | 0.14 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.09 | b.d. | b.d. | b.d. | b.d. | b.d. |
| Sum | 98.67 | 98.76 | 99.17 | 100.20 | 98.30 | 97.79 | 99.73 | 99.07 | 98.75 | 98.00 | 99.08 | 98.87 | 99.26 |
| P5+ apfu | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Si4+ | 0.996 | 1.028 | 1.007 | 1.003 | 1.002 | 0.998 | 1.010 | 1.877 | 1.914 | 1.989 | 1.960 | 3.010 | 3.023 |
| Ti4+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.038 | 0.009 | 0.000 | 0.015 | 0.000 | 0.009 |
| Al3+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.154 | 0.157 | 0.022 | 0.046 | 0.000 | 0.043 |
| Ba2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 |
| Ca2+ | 0.014 | 0.015 | 0.018 | 0.015 | 0.016 | 0.046 | 0.014 | 0.850 | 0.291 | 0.764 | 0.982 | 2.952 | 2.815 |
| Fe2+ | 1.784 | 1.754 | 1.399 | 1.320 | 1.341 | 1.306 | 1.397 | 0.771 | 1.200 | 0.828 | 0.303 | 0.029 | 0.037 |
| Mg2+ | 0.128 | 0.122 | 0.331 | 0.432 | 0.377 | 0.330 | 0.261 | 0.163 | 0.167 | 0.079 | 0.668 | 0.000 | 0.000 |
| Mn2+ | 0.015 | 0.016 | 0.087 | 0.093 | 0.131 | 0.204 | 0.262 | 0.044 | 0.136 | 0.223 | 0.011 | 0.000 | 0.011 |
| Pb2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 |
| Zn2+ | 0.044 | 0.038 | 0.152 | 0.134 | 0.132 | 0.119 | 0.045 | 0.090 | 0.126 | 0.091 | 0.017 | 0.000 | 0.000 |
| K+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Catsum | 2.981 | 2.972 | 2.993 | 2.997 | 2.998 | 3.002 | 2.990 | 3.999 | 3.999 | 4.000 | 4.002 | 5.990 | 5.947 |
| Phase | Mel | Mel | Mel | Fld | Fld | Fld | Fld | Fld | Fld | Fld | Fld | Fld | Fld |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An. No. | 35/1. | 30/1. | 29/1. | 65/1. | n = 19 | 122/1. | 139/1. | 164/1. | n = 28 | ||||
| Sample | JS5 | JS5 | JS5 | PL1 | Av | Min | Max | JS9 | CP5 | ML26 | Av | Min | Max |
| Site | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 3 | 4 | 2 | 1–4 | 1–4 | 1–4 |
| Type | B | B | B | A | A | A | A | B | B | B | B | B | B |
| P2O5 wt.% | b.d. | b.d. | 0.35 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.16 | 0.18 | 0.16 | 0.19 |
| SiO2 | 41.39 | 41.00 | 40.01 | 52.67 | 56.35 | 51.05 | 61.63 | 47.82 | 41.99 | 45.02 | 43.59 | 32.87 | 52.43 |
| Al2O3 | 4.49 | 3.89 | 4.64 | 20.72 | 21.31 | 18.97 | 26.82 | 16.25 | 24.77 | 17.69 | 21.15 | 16.25 | 24.77 |
| Fe2O3 | - | - | - | 0.77 | 1.20 | 0.51 | 1.84 | 4.77 | 0.37 | 1.72 | 1.35 | 0.27 | 4.77 |
| BaO | 2.29 | 1.64 | 3.35 | 14.13 | 6.33 | 0.55 | 14.13 | 22.38 | 27.11 | 26.45 | 26.08 | 14.88 | 38.82 |
| CaO | 34.96 | 35.07 | 34.47 | 0.36 | 3.12 | 0.12 | 12.08 | 1.14 | 1.16 | 0.95 | 0.58 | 0.07 | 2.53 |
| FeO | 6.27 | 7.54 | 6.62 | - | - | - | - | - | - | - | - | - | - |
| MgO | 4.77 | 4.23 | 4.79 | b.d. | b.d. | b.d. | b.d. | 0.46 | b.d. | 0.86 | 0.47 | 0.13 | 1.00 |
| MnO | 0.53 | b.d. | 0.46 | b.d. | 0.20 | 0.16 | 0.30 | b.d. | b.d. | b.d. | 0.17 | 0.16 | 0.19 |
| PbO | b.d. | b.d. | b.d. | 0.53 | 1.29 | 0.35 | 3.72 | b.d. | b.d. | 0.40 | 0.63 | 0.21 | 1.71 |
| SrO | b.d. | b.d. | b.d. | 0.16 | 0.18 | 0.15 | 0.25 | 0.19 | 0.49 | b.d. | 0.23 | 0.14 | 0.49 |
| ZnO | 2.74 | 4.65 | 2.75 | 0.31 | 0.36 | 0.16 | 0.75 | 1.04 | b.d. | 0.15 | 0.43 | 0.15 | 1.04 |
| K2O | 0.65 | 0.39 | 0.54 | 9.84 | 8.52 | 1.55 | 12.05 | 5.96 | 3.82 | 5.26 | 4.78 | 0.71 | 7.25 |
| Na2O | 1.39 | 1.63 | 0.94 | 0.47 | 1.62 | 0.42 | 5.77 | 0.46 | 0.55 | 0.35 | 0.52 | 0.21 | 1.49 |
| Sum | 99.48 | 100.04 | 98.92 | 99.88 | 99.28 | 97.62 | 101.12 | 99.99 | 100.22 | 98.84 | 98.81 | 97.56 | 100.41 |
| P5+ apfu | 0.000 | 0.000 | 0.009 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.009 |
| Si4+ | 1.989 | 1.980 | 1.954 | 2.702 | 2.734 | 2.424 | 2.936 | 2.628 | 2.344 | 2.502 | 2.463 | 2.093 | 2.670 |
| Al3+ | 0.254 | 0.221 | 0.267 | 1.253 | 1.217 | 1.079 | 1.497 | 1.053 | 1.630 | 1.379 | 1.417 | 1.053 | 1.796 |
| Fe3+ | - | - | - | 0.030 | 0.044 | 0.017 | 0.067 | 0.197 | 0.015 | 0.044 | 0.057 | 0.011 | 0.197 |
| Ba2+ | 0.043 | 0.031 | 0.064 | 0.284 | 0.110 | 0.000 | 0.284 | 0.482 | 0.593 | 0.593 | 0.585 | 0.297 | 0.969 |
| Ca2+ | 1.800 | 1.814 | 1.804 | 0.020 | 0.158 | 0.006 | 0.622 | 0.067 | 0.069 | 0.004 | 0.034 | 0.004 | 0.138 |
| Fe2+ | 0.252 | 0.304 | 0.270 | - | - | - | - | - | - | - | - | - | - |
| Mg2+ | 0.342 | 0.304 | 0.349 | 0.000 | 0.000 | 0.000 | 0.000 | 0.038 | 0.000 | 0.043 | 0.024 | 0.000 | 0.085 |
| Mn2+ | 0.022 | 0.000 | 0.019 | 0.000 | 0.003 | 0.000 | 0.012 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.009 |
| Pb2+ | 0.000 | 0.000 | 0.000 | 0.007 | 0.016 | 0.000 | 0.049 | 0.000 | 0.000 | 0.003 | 0.002 | 0.000 | 0.026 |
| Sr2+ | b.d. | b.d. | b.d. | 0.005 | 0.002 | 0.000 | 0.007 | 0.006 | 0.016 | 0.006 | 0.007 | 0.000 | 0.016 |
| Zn2+ | 0.097 | 0.166 | 0.099 | 0.012 | 0.009 | 0.000 | 0.026 | 0.042 | 0.000 | 0.000 | 0.009 | 0.000 | 0.042 |
| K+ | 0.040 | 0.024 | 0.034 | 0.644 | 0.533 | 0.090 | 0.732 | 0.418 | 0.272 | 0.383 | 0.343 | 0.058 | 0.499 |
| Na+ | 0.130 | 0.153 | 0.089 | 0.047 | 0.149 | 0.041 | 0.509 | 0.049 | 0.060 | 0.044 | 0.051 | 0.000 | 0.152 |
| Catsum | 4.969 | 4.998 | 4.959 | 5.002 | 4.976 | 4.890 | 5.019 | 4.980 | 4.999 | 5.000 | 4.996 | 4.885 | 5.025 |
| n = 70 | n = 86 | n = 7 | n = 18 | n = 61 | n = 6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Av | SD | Rep. | Av | SD | Av | SD | Av | SD | Av | SD | Av | Bulk | |
| Sample | PL | PL | PL5 | ML | ML | ML | ML | CP | CP | JS | JS | JS1 | JS1 |
| Site | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 4 | 4 | 3 | 3 | 3 | 3 |
| Type | A | A | B | A | A | B | B | B | B | B | B | B | B |
| SO3 wt.% | 1.22 | 0.79 | 0.77 | 1.26 | 0.59 | 0.55 | 0.38 | 0.65 | 0.38 | 0.88 | 0.32 | 0.55 | 0.53 |
| P2O5 | 0.95 | 0.42 | 0.07 | 1.11 | 0.42 | 1.39 | 0.34 | 0.57 | 0.43 | 0.34 | 0.11 | 0.24 | 0.37 |
| SiO2 | 44.58 | 5.91 | 40.79 | 52.07 | 6.86 | 41.64 | 3.25 | 50.67 | 4.81 | 46.24 | 6.31 | 43.42 | 44.05 |
| TiO2 | 0.64 | 0.37 | b.d. | 0.71 | 0.39 | 1.16 | 0.21 | 0.43 | 0.15 | 0.61 | 0.18 | b.d. | 0.48 |
| Al2O3 | 8.15 | 2.19 | 7.15 | 10.52 | 1.93 | 6.19 | 0.87 | 7.96 | 2.81 | 6.12 | 1.73 | 5.78 | 5.61 |
| BaO | 1.66 | 1.33 | 35.01 | 2.23 | 0.85 | 32.42 | 4.18 | 11.52 | 2.75 | 20.26 | 6.98 | 26.26 | 25.90 |
| CaO | 6.40 | 3.29 | 5.90 | 4.96 | 1.81 | 6.42 | 0.93 | 7.91 | 2.67 | 9.41 | 2.77 | 10.42 | 10.17 |
| FeO | 22.92 | 12.72 | 5.54 | 21.53 | 8.03 | 4.25 | 0.29 | 13.69 | 6.06 | 10.54 | 4.61 | 7.50 | 7.93 |
| MgO | 0.78 | 0.50 | 1.24 | 0.43 | 0.40 | 1.12 | 0.17 | 1.31 | 0.37 | 1.00 | 0.34 | 1.10 | 1.40 |
| MnO | 1.92 | 0.96 | b.d. | 0.26 | 0.05 | 0.31 | 0.00 | 0.67 | 0.43 | 0.23 | 0.16 | 0.34 | 0.28 |
| PbO | 5.59 | 3.59 | 0.57 | 1.18 | 0.45 | 2.16 | 0.54 | 0.45 | 0.43 | 0.17 | 0.34 | 0.14 | 0.25 |
| SrO | b.d. | - | b.d. | 0.05 | 0.07 | 0.44 | 0.11 | b.d. | - | 0.05 | 0.12 | b.d. | 0.61 |
| ZnO | 3.44 | 1.72 | 0.78 | 1.19 | 0.37 | 0.44 | 0.35 | 1.23 | 0.72 | 1.58 | 1.54 | 0.94 | 0.93 |
| K2O | 2.21 | 0.92 | 1.61 | 3.10 | 0.84 | 2.42 | 0.73 | 2.43 | 0.62 | 2.02 | 0.86 | 1.75 | 1.80 |
| Na2O | 0.81 | 0.29 | 0.43 | 0.77 | 0.18 | 0.60 | 0.12 | 0.69 | 0.28 | 0.52 | 0.20 | 0.54 | 0.42 |
| Sum | 99.41 | 1.27 | 99.87 | 100.22 | 1.09 | 98.82 | 0.86 | 98.67 | 1.20 | 99.15 | 0.73 | 99.53 | 100.12 |
| v.i. | 0.74 | 0.40 | 1.04 | 0.55 | 0.20 | 1.00 | 0.19 | 0.66 | 0.18 | 0.87 | 0.20 | 0.97 | 0.99 |
| v.i.mod | 0.91 | 0.39 | 1.07 | 0.59 | 0.20 | 1.05 | 0.17 | 0.69 | 0.18 | 0.90 | 0.21 | 1.00 | 0.96 |
| An. No. | 36/1. | 37/1. | 40/1. | 41/1. | 22/1. | 28/1. | 6/1. | 33/1. | 42/1. | 44/1. | 45/1. | 49/1. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | JS9 | JS9 | JS9 | JS9 | CP5 | CP5 | CP5 | CP5 | CP10 | CP10 | CP10 | CP10 |
| Site | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| SO3 wt.% | 34.05 | 34.12 | 33.39 | 33.17 | 35.26 | 35.05 | 33.45 | 35.20 | 34.15 | 33.95 | 34.77 | 34.17 |
| BaO | 55.43 | 55.29 | 51.97 | 52.07 | 60.12 | 59.90 | 39.93 | 61.55 | 63.37 | 63.44 | 64.35 | 55.41 |
| CaO | 0.05 | b.d. | 0.10 | 0.10 | 0.91 | 0.99 | 0.21 | 0.86 | 0.29 | 0.29 | 0.23 | 0.88 |
| CuO | 0.39 | 0.52 | 0.52 | 0.27 | 0.24 | 0.23 | 1.40 | b.d. | b.d. | b.d. | b.d. | b.d. |
| FeO | 1.48 | 1.06 | 1.02 | 1.10 | 0.93 | 0.45 | 3.11 | 0.40 | b.d. | b.d. | 0.18 | 0.51 |
| PbO | 8.82 | 8.82 | 12.23 | 13.13 | b.d. | 2.44 | 20.98 | 1.40 | 1.61 | 1.30 | 1.53 | 8.58 |
| SrO | b.d. | b.d. | b.d. | b.d. | 1.62 | 0.99 | b.d. | 0.94 | b.d. | b.d. | b.d. | 0.67 |
| Na2O | b.d. | 0.28 | b.d. | b.d. | 0.14 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.18 | b.d. |
| Sum | 100.22 | 100.09 | 99.23 | 99.84 | 99.22 | 100.05 | 99.08 | 100.35 | 99.42 | 98.98 | 101.24 | 100.22 |
| Ba2+ apfu | 0.848 | 0.844 | 0.813 | 0.817 | 0.888 | 0.892 | 0.622 | 0.914 | 0.969 | 0.975 | 0.963 | 0.845 |
| Ca2+ | 0.002 | 0.000 | 0.004 | 0.004 | 0.037 | 0.040 | 0.009 | 0.035 | 0.012 | 0.012 | 0.009 | 0.037 |
| Cu2+ | 0.012 | 0.015 | 0.016 | 0.008 | 0.007 | 0.007 | 0.042 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Fe2+ | 0.048 | 0.035 | 0.034 | 0.037 | 0.029 | 0.014 | 0.103 | 0.013 | 0.000 | 0.000 | 0.006 | 0.017 |
| Pb2+ | 0.093 | 0.092 | 0.131 | 0.142 | 0.000 | 0.025 | 0.225 | 0.014 | 0.017 | 0.014 | 0.016 | 0.090 |
| Sr2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.035 | 0.022 | 0.000 | 0.021 | 0.000 | 0.000 | 0.000 | 0.015 |
| Na+ | 0.000 | 0.011 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 | 0.000 |
| Catsum | 1.003 | 0.996 | 0.998 | 1.008 | 1.001 | 1.000 | 1.002 | 0.996 | 0.998 | 1.001 | 1.001 | 1.003 |
| S6+ | 0.999 | 0.998 | 1.001 | 0.997 | 0.998 | 1.000 | 0.999 | 1.001 | 1.001 | 1.000 | 0.998 | 0.999 |
| Ansum | 0.999 | 0.998 | 1.001 | 0.997 | 0.998 | 1.000 | 0.999 | 1.001 | 1.001 | 1.000 | 0.998 | 0.999 |
| Type | A | B | A | B | B | B | |
|---|---|---|---|---|---|---|---|
| Site | 1 | 1 | 2 | 2 | 3 | 4 | |
| SiO2-CaO-FeO | 1600–1700 | 1700 | 1550–1600 | ~1700 | ~1700 | ~1700 | |
| SiO2-CaO*-FeO* | 1100–1600 | 1400 | 1150–1300 | ~1500 | 1250–1500 | 1400–1600 | |
| SiO2-Al2O3-FeO | 1400–1500 | 1500 | 1400–1470 | ~1500 | 1550–1590 | ~1580 | |
| SiO2-Al2O3-BaO | 1600–1700 | 1300 | ~1650 | ~1250 | 1250–1450 | 1400–1500 | |
| bulk liquidus | 1147–1260 | 1122 | 1182–1191 | 1084–1121 | 1075–1152 | 1093–1107 | |
| glass liquidus | 1068–1158 | 1101 | 1129–1173 | 1081–1142 | 1088–1172 | 1091–1137 | |
| bulk liquidus (Av) | 1173 | 1122 | 1186 | 1098 | 1119 | 1101 | |
| glass liquidus (Av) | 1106 | 1101 | 1146 | 1114 | 1117 | 1111 | |
| experimental smelts | 1150–1200 # | 1150 | 1150 | 1150–1200 | 1150–1200 # | 1150–1200 # | |
| 1* | 2* | 3* | 4* | 5* | 6* | ||
|---|---|---|---|---|---|---|---|
| Location | Příbram | Wiesloch | Kutná Hora | Harz | Harz | Harz | |
| SiO2-CaO-FeO | 1300–1700 | 1110–1600 | 1150–1300 | 1175–1600 | 1125–1500 | 1175–1700 | |
| SiO2-CaO*-FeO* | 1300–1700 | 1100–1200 | 1125–1200 | 1150–1200 | 1100–1250 | 1150–1450 | |
| SiO2-Al2O3-FeO | 1400–1600 | 1180–1500 | 1300–1400 | 1180–1470 | 1150–1400 | 1200–1550 | |
| SiO2-Al2O3-BaO | 1600–1700 | 1300–1700 | 1600–1700 | 1650–1700 | 1350–1700 | 1550 | |
| bulk liquidus | 1127–1438 | 1094–1403 | 1040–1307 | 1149–1661 | 1179–1540 | 1164–1300 | |
| bulk liquidus (Av) | 1171 | 1237 | 1194 | 1355 | 1393 | 1211 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusta, J.; Dolníček, Z.; Sracek, O.; Malý, K. Origin of Historical Ba-Rich Slags Related to Pb-Ag Production from Jihlava Ore District (Czech Republic). Minerals 2022, 12, 985. https://doi.org/10.3390/min12080985
Kapusta J, Dolníček Z, Sracek O, Malý K. Origin of Historical Ba-Rich Slags Related to Pb-Ag Production from Jihlava Ore District (Czech Republic). Minerals. 2022; 12(8):985. https://doi.org/10.3390/min12080985
Chicago/Turabian StyleKapusta, Jaroslav, Zdeněk Dolníček, Ondra Sracek, and Karel Malý. 2022. "Origin of Historical Ba-Rich Slags Related to Pb-Ag Production from Jihlava Ore District (Czech Republic)" Minerals 12, no. 8: 985. https://doi.org/10.3390/min12080985
APA StyleKapusta, J., Dolníček, Z., Sracek, O., & Malý, K. (2022). Origin of Historical Ba-Rich Slags Related to Pb-Ag Production from Jihlava Ore District (Czech Republic). Minerals, 12(8), 985. https://doi.org/10.3390/min12080985






