Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Isomorphism of Extra-Framework Components in Cubic Sodalite-Group Minerals
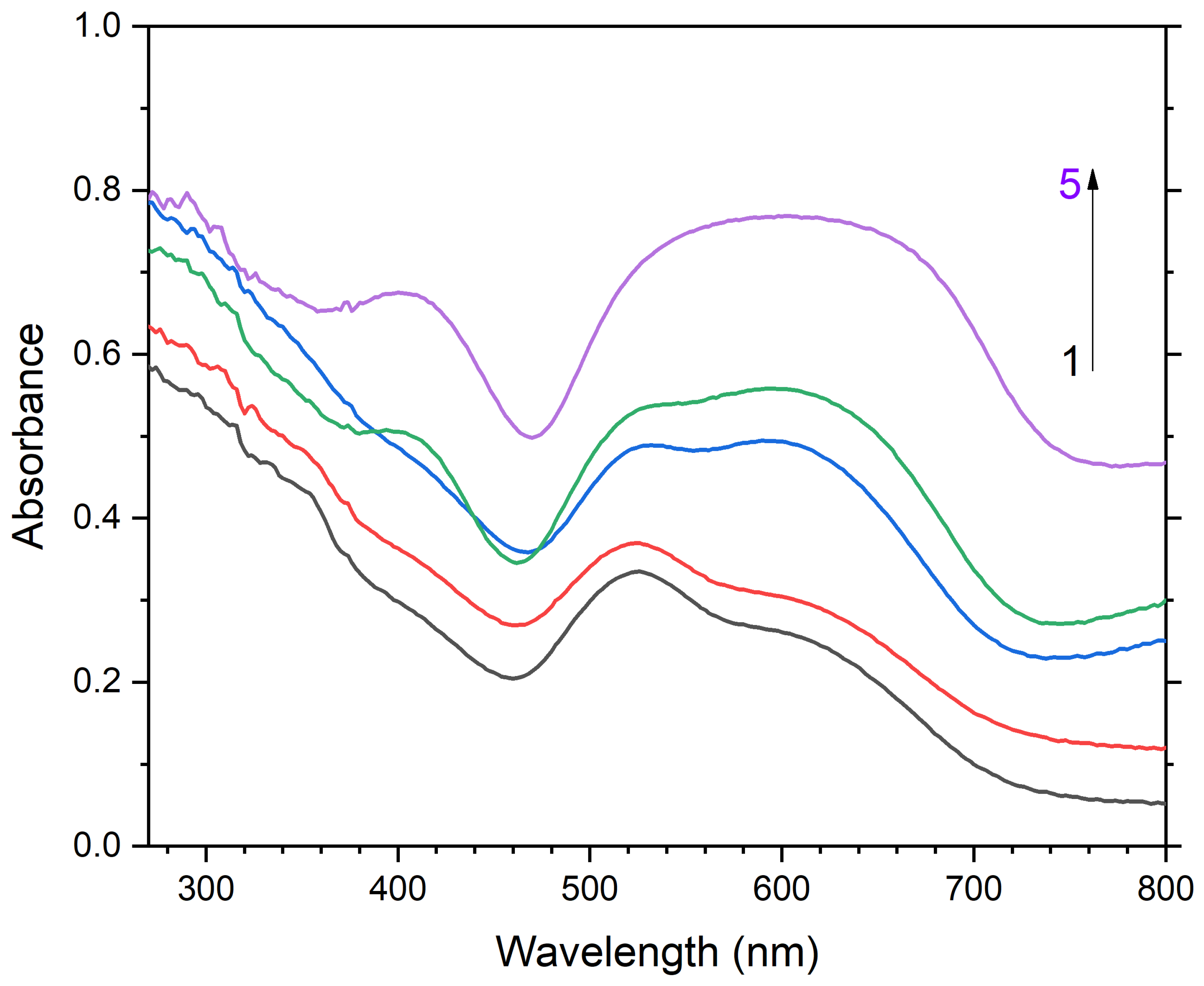
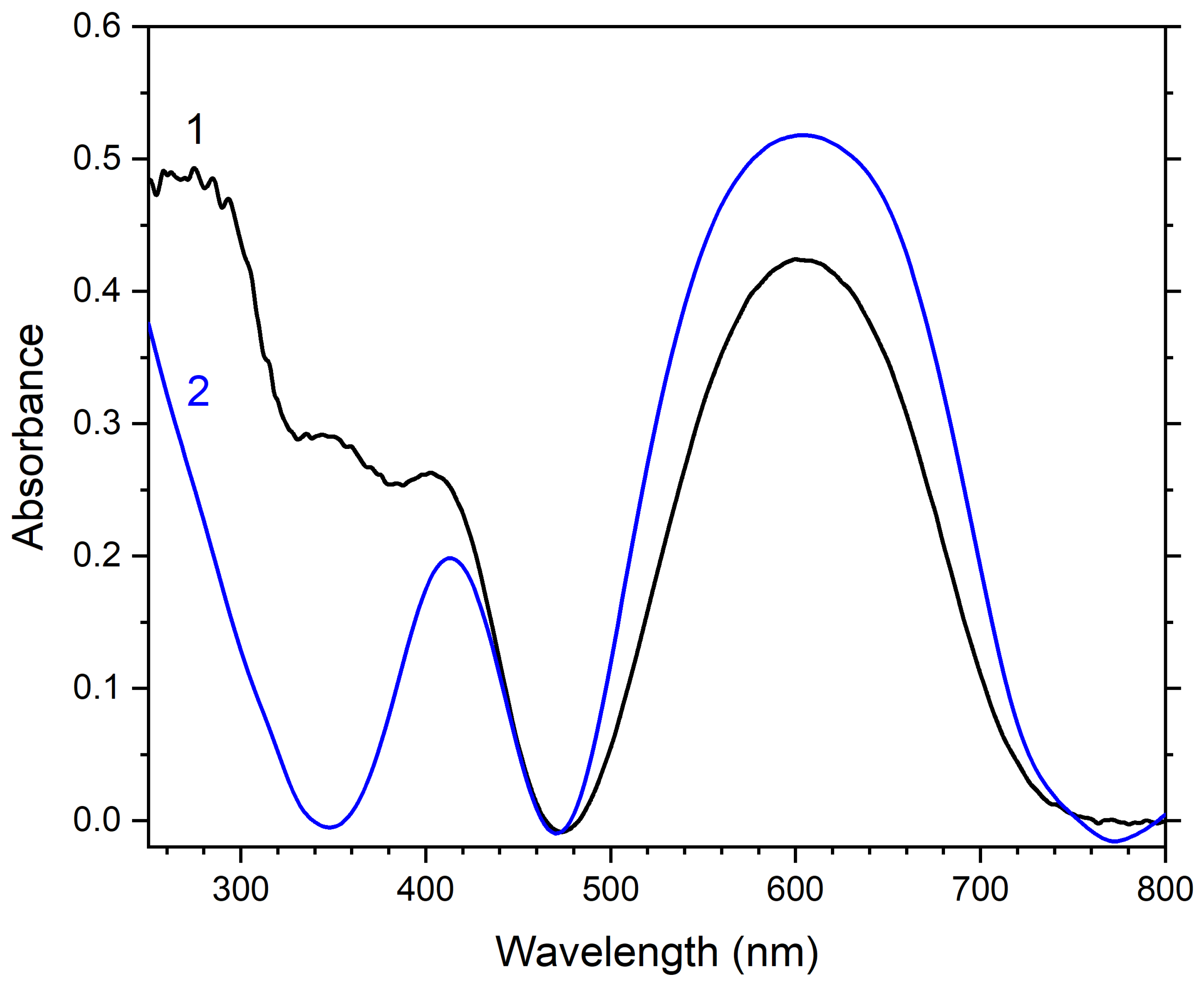
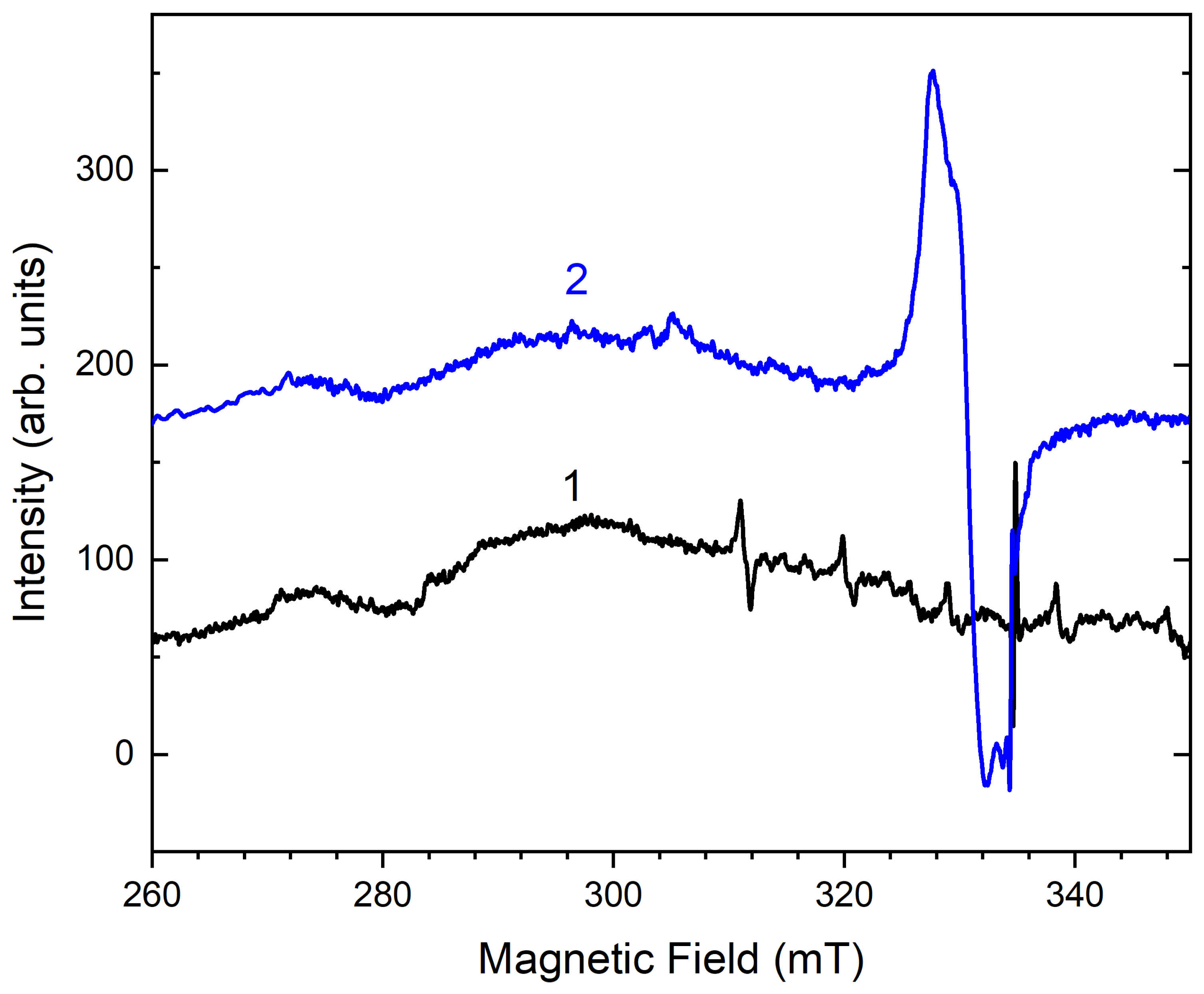
3.2. Crystal Chemistry, Isomorphism, and Thermal Conversions of Haüyne
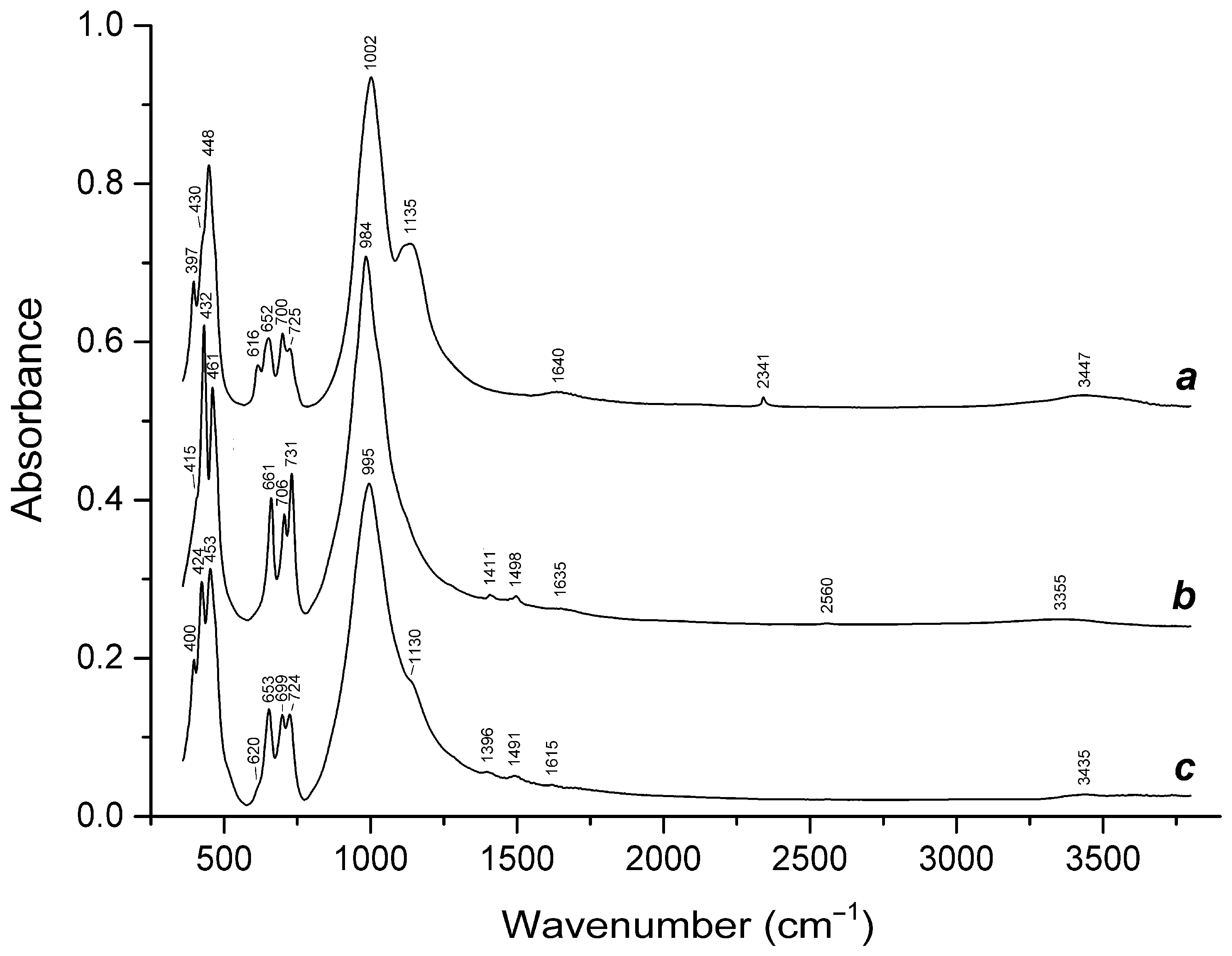
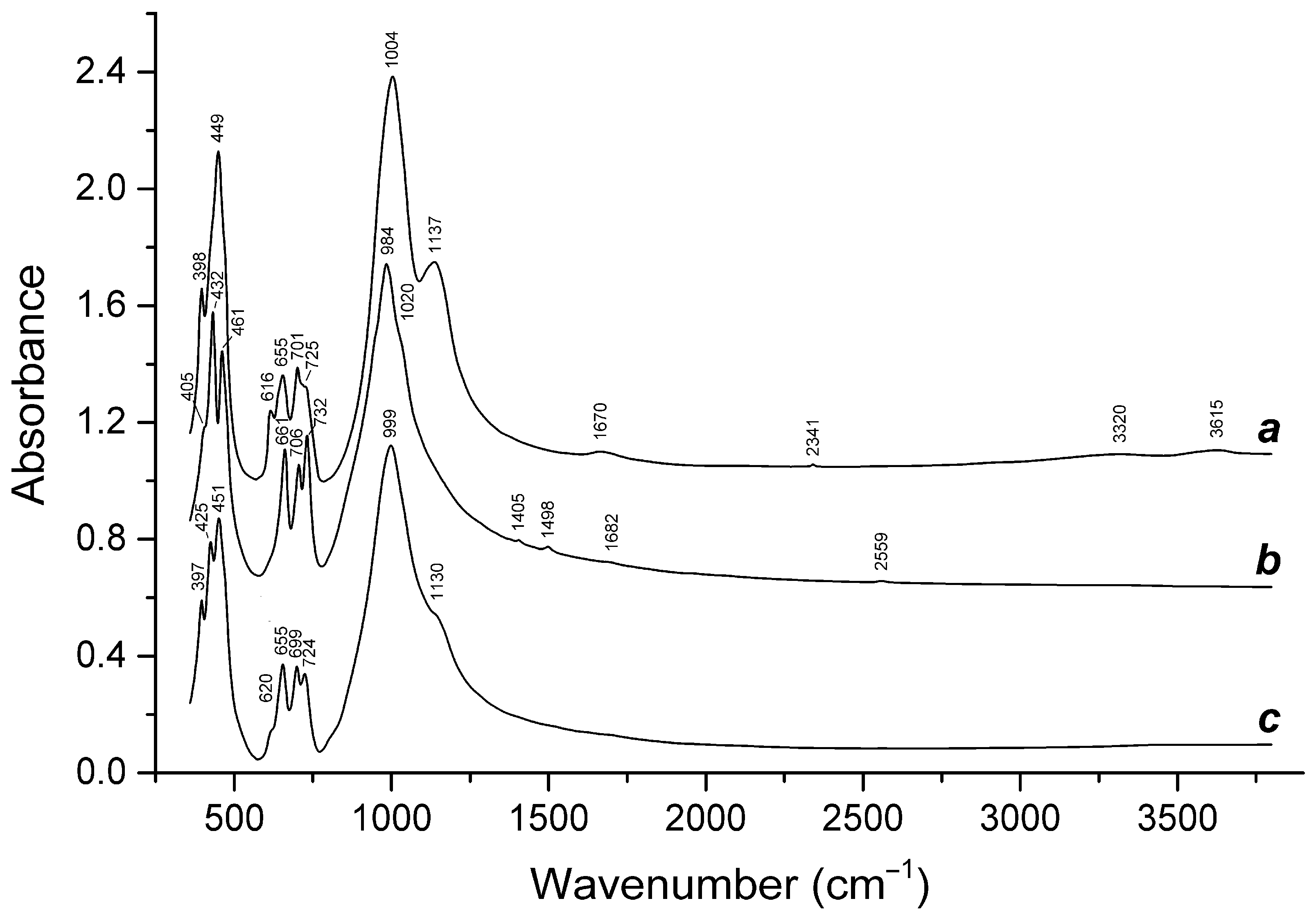
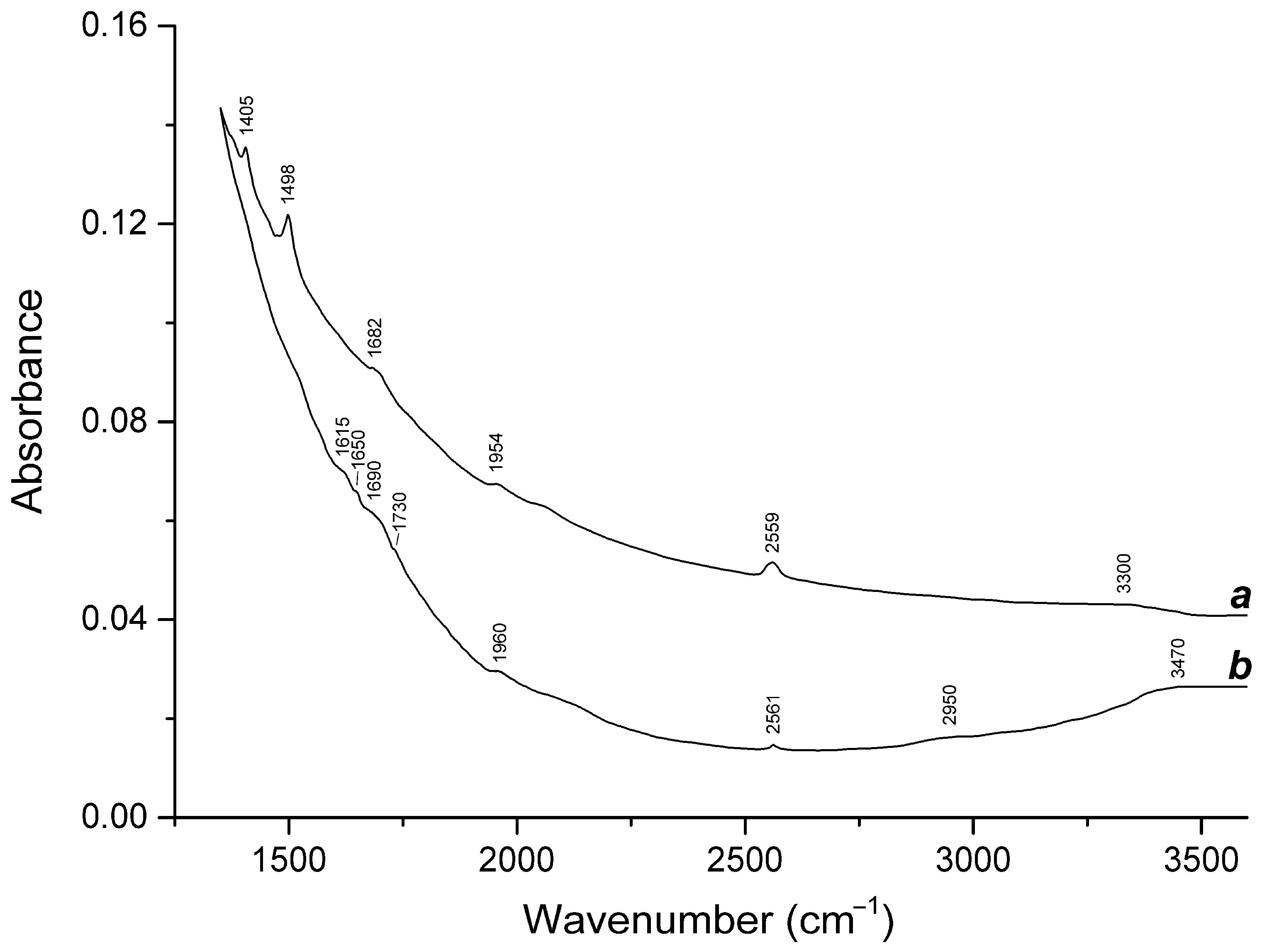
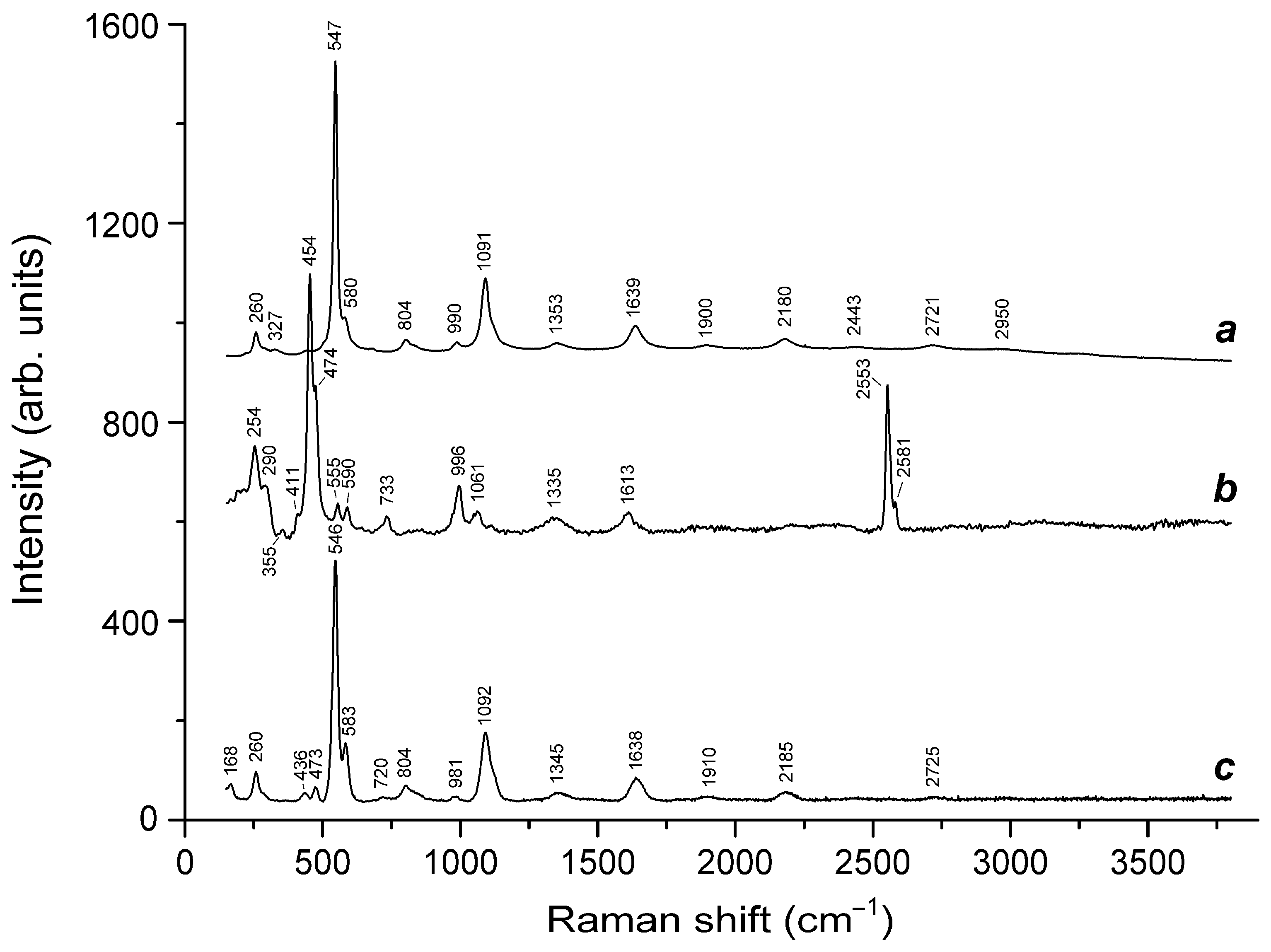
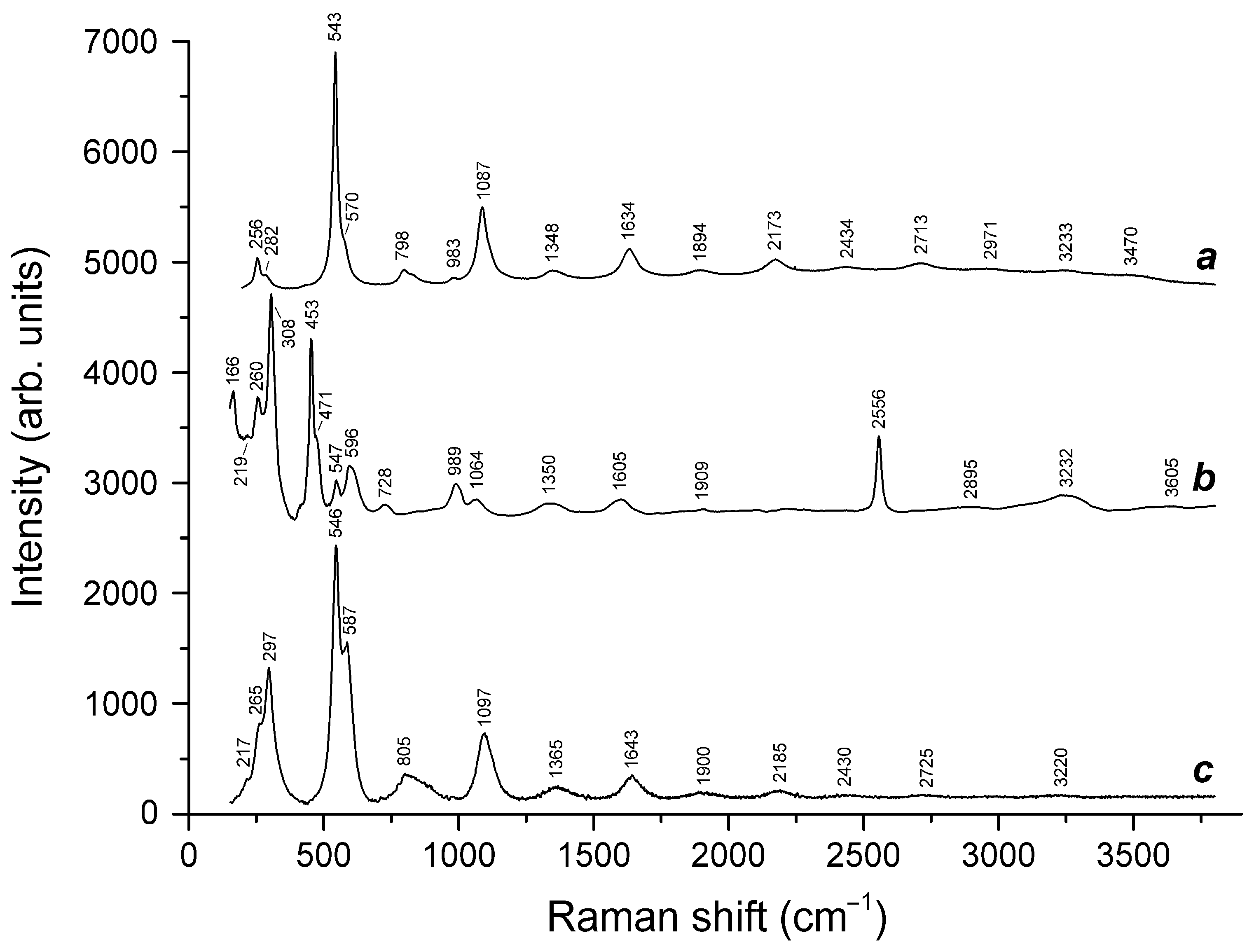
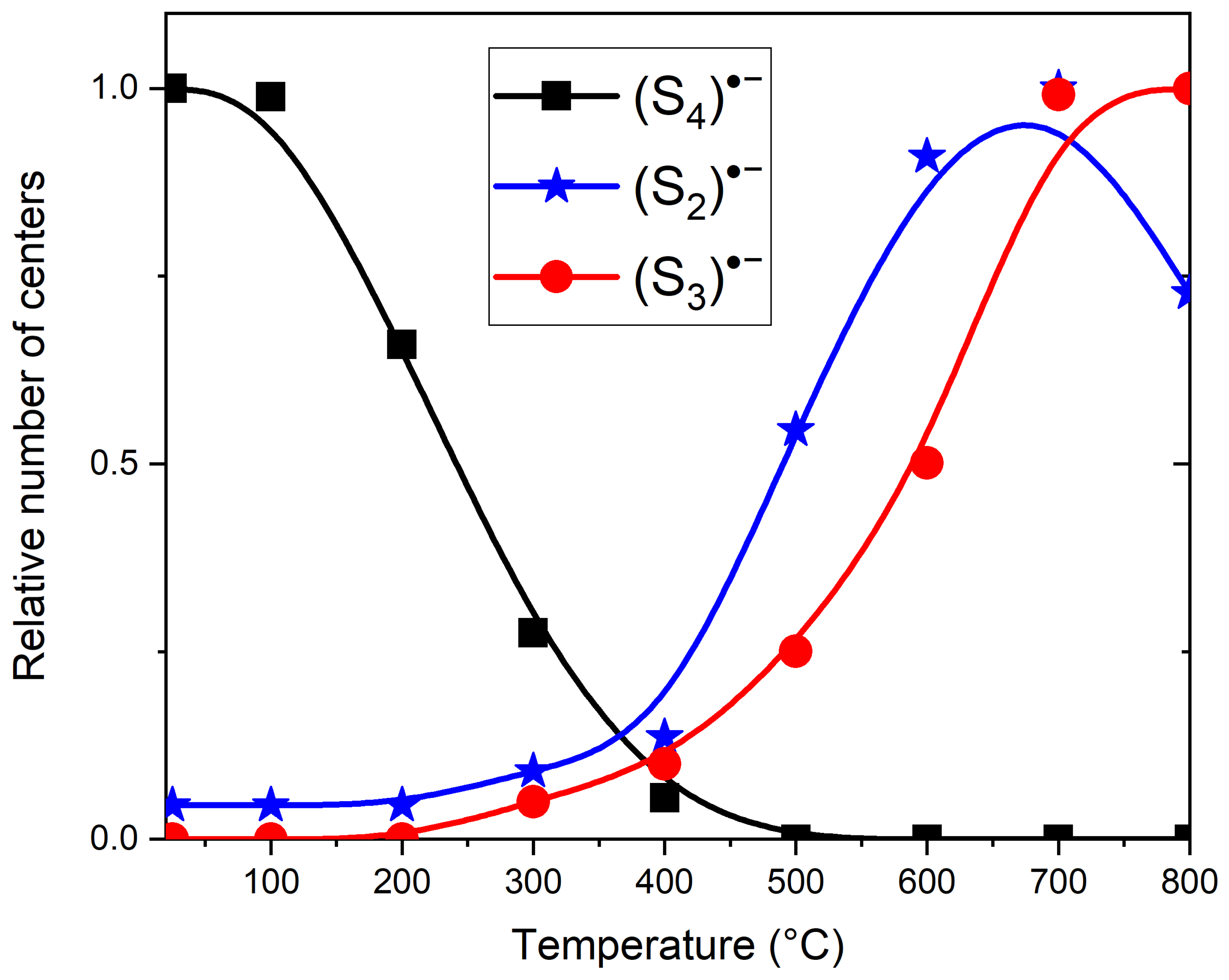
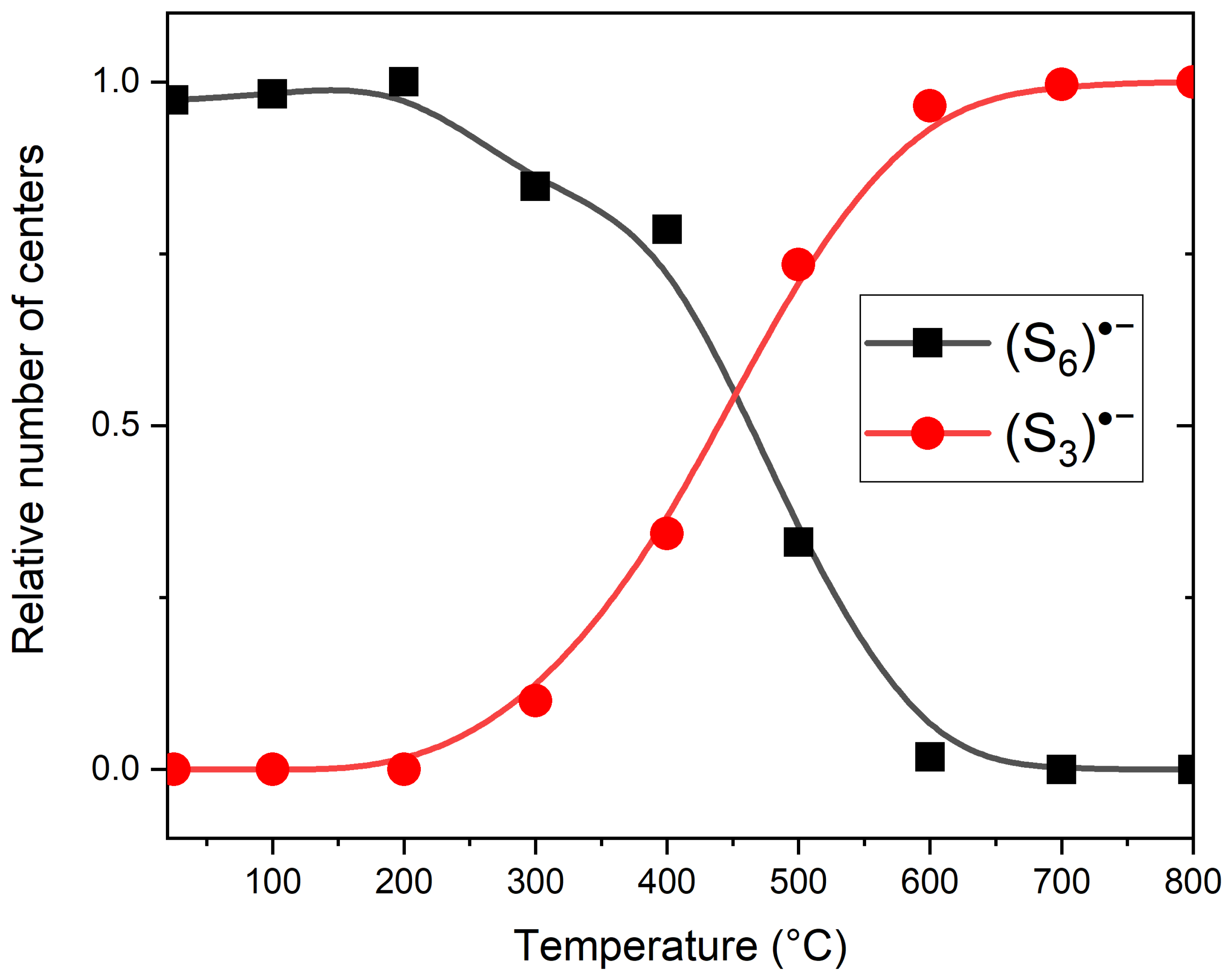
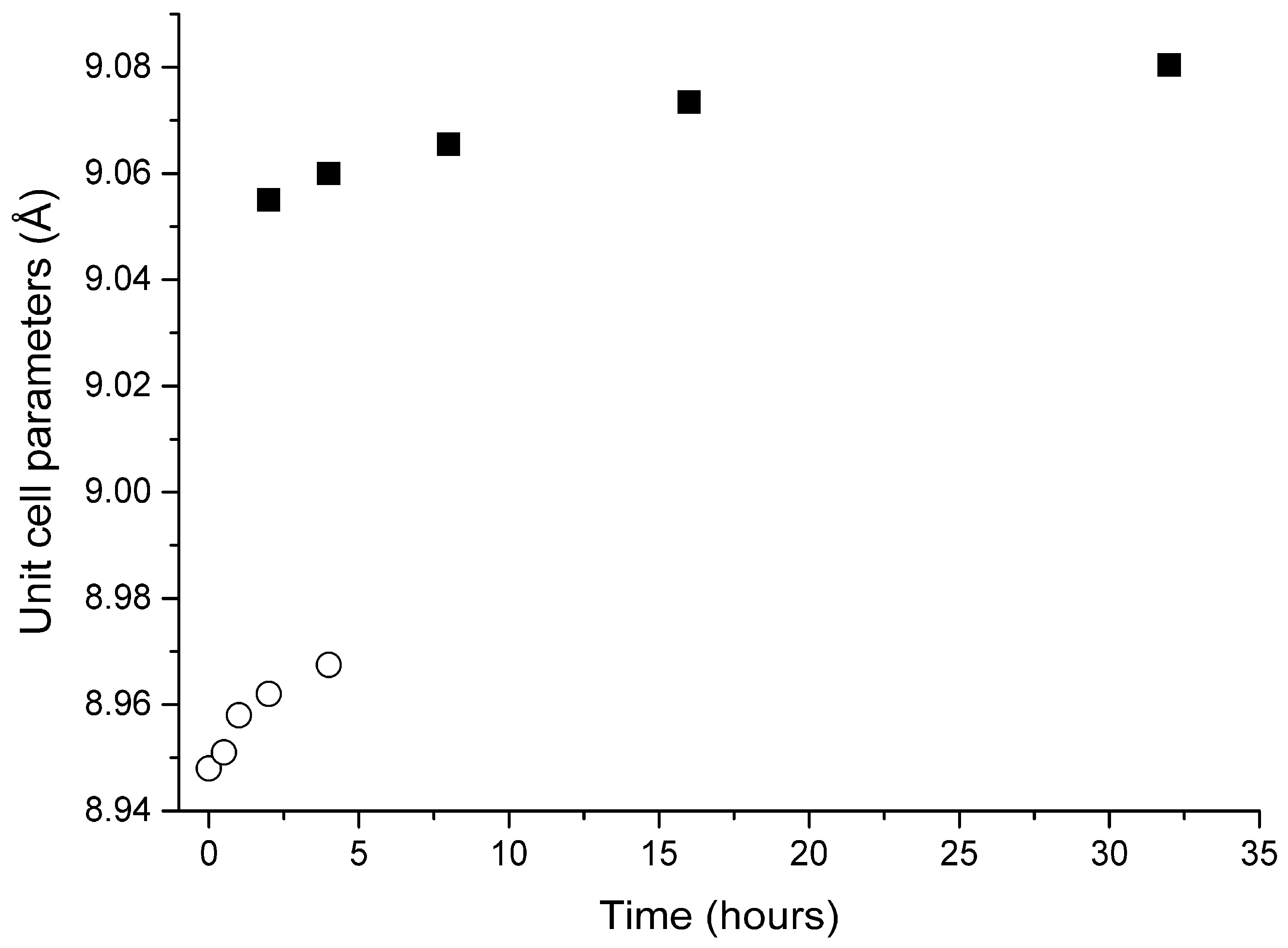
3.3. Thermal Conversions of Slyudyankaite
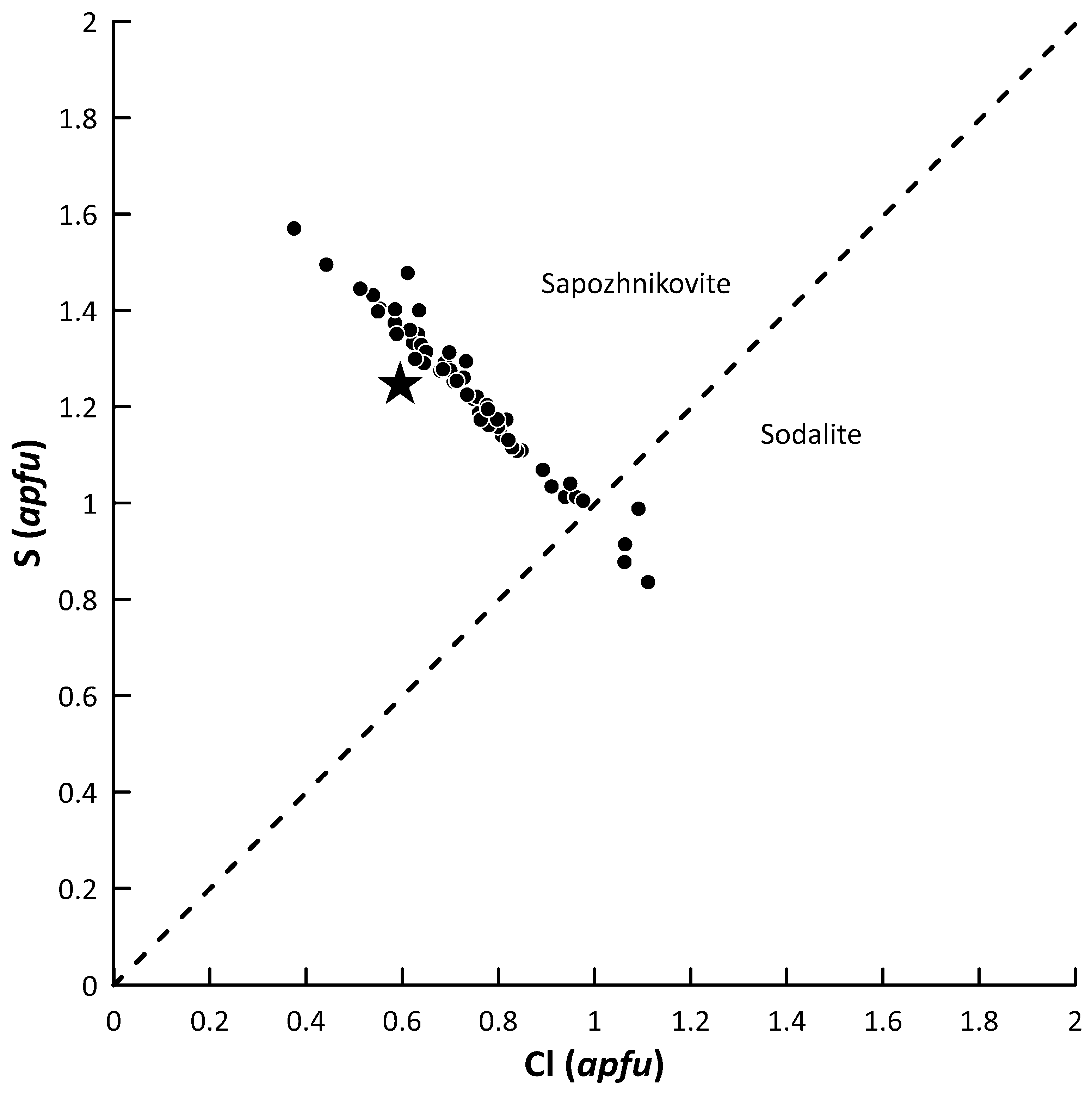
3.4. Sodalite—Sapozhnikovite Solid-Solution Series
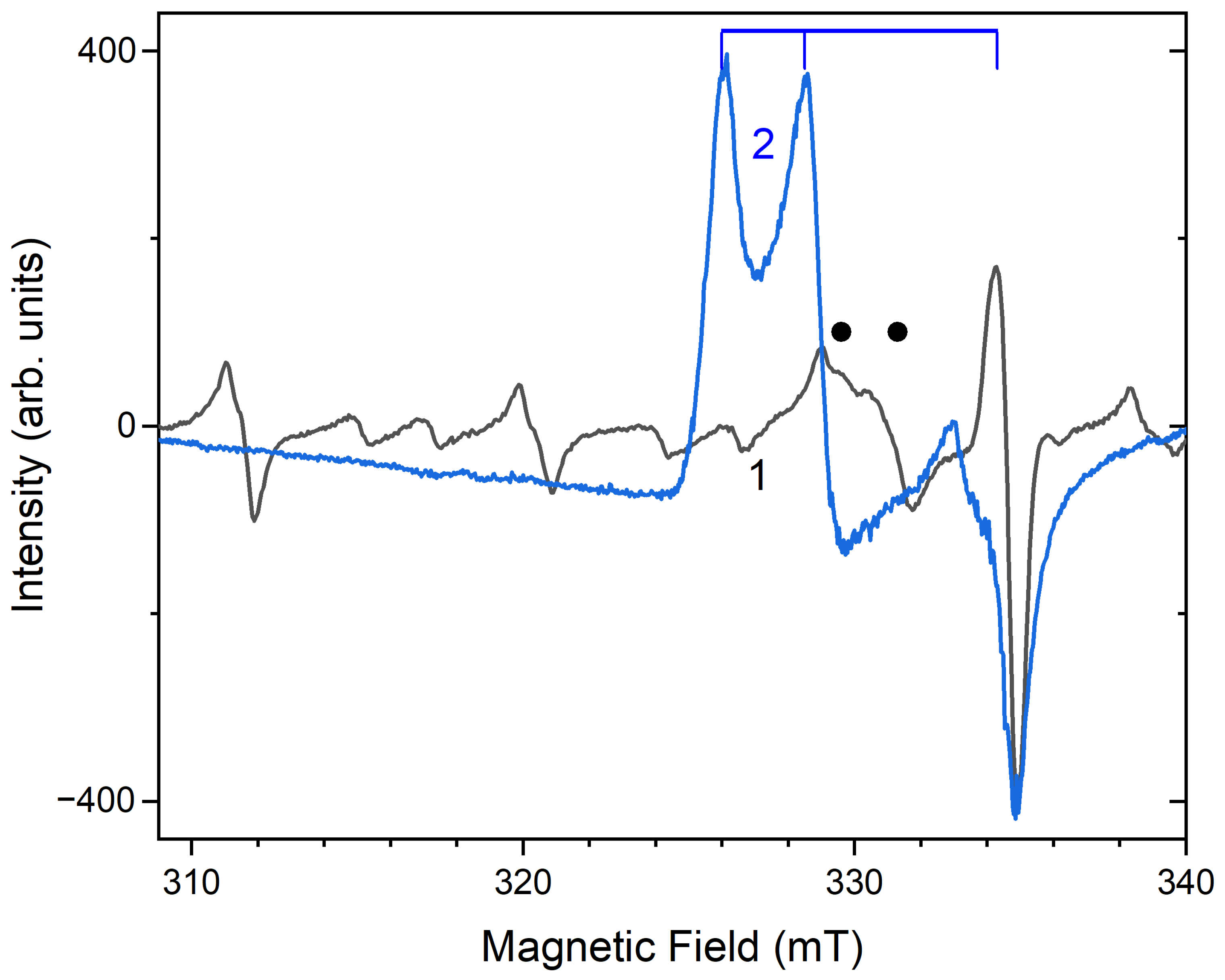
4. Discussion
4.1. General Remarks on the Isomorphism of Extra-Framework Components in Sodalite-Group Minerals
4.2. Sapozhnikovite as a Marker of Reducing Conditions
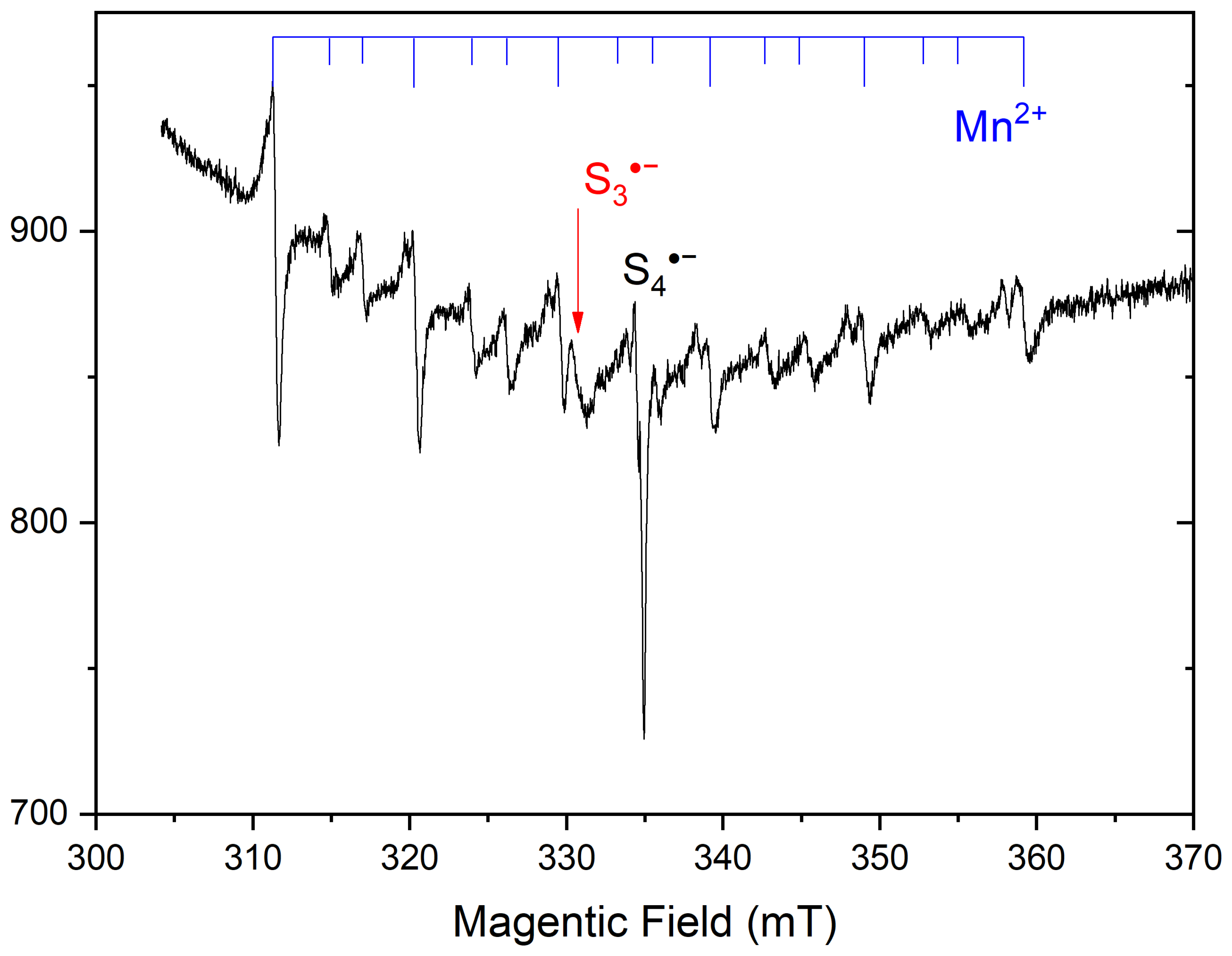
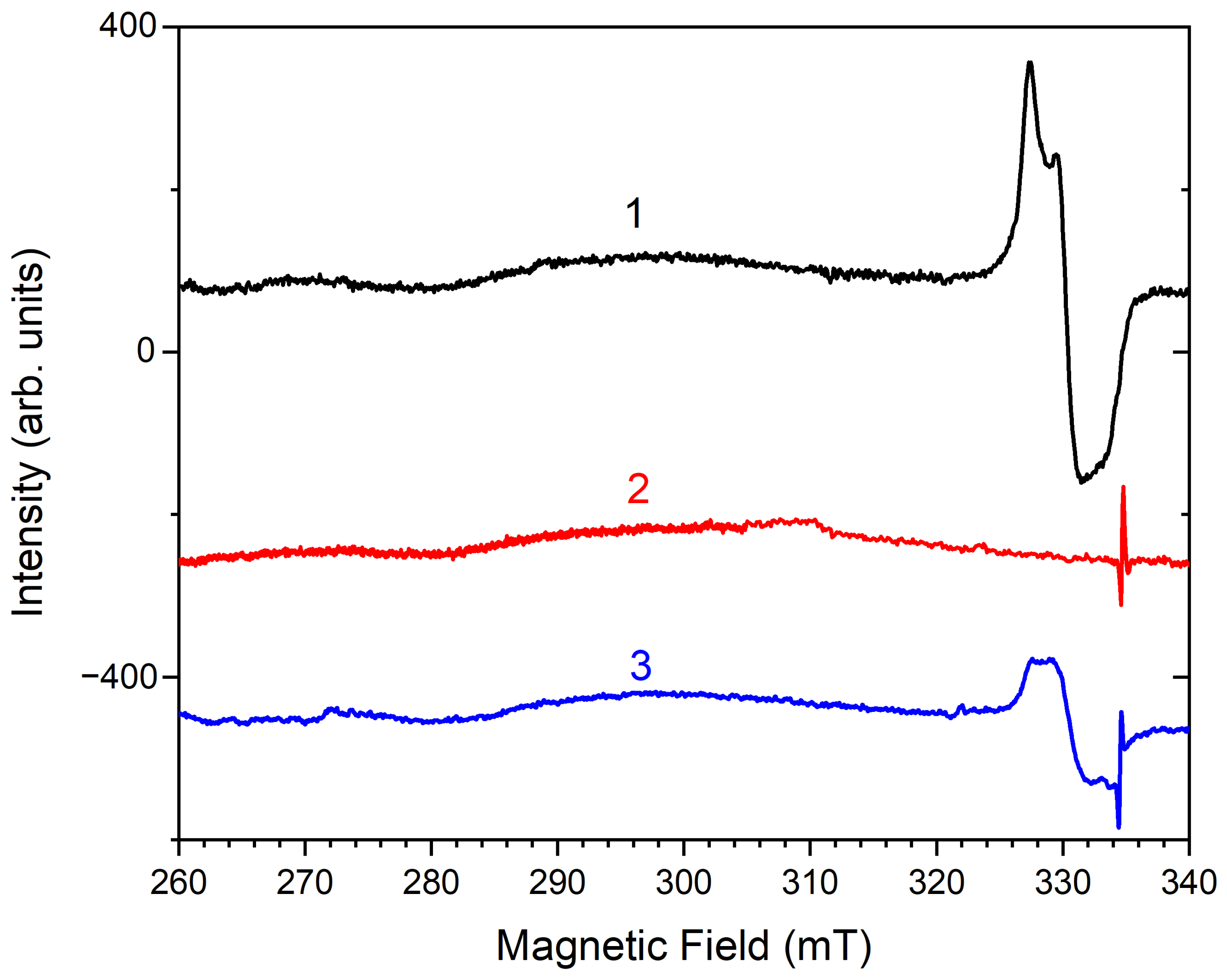
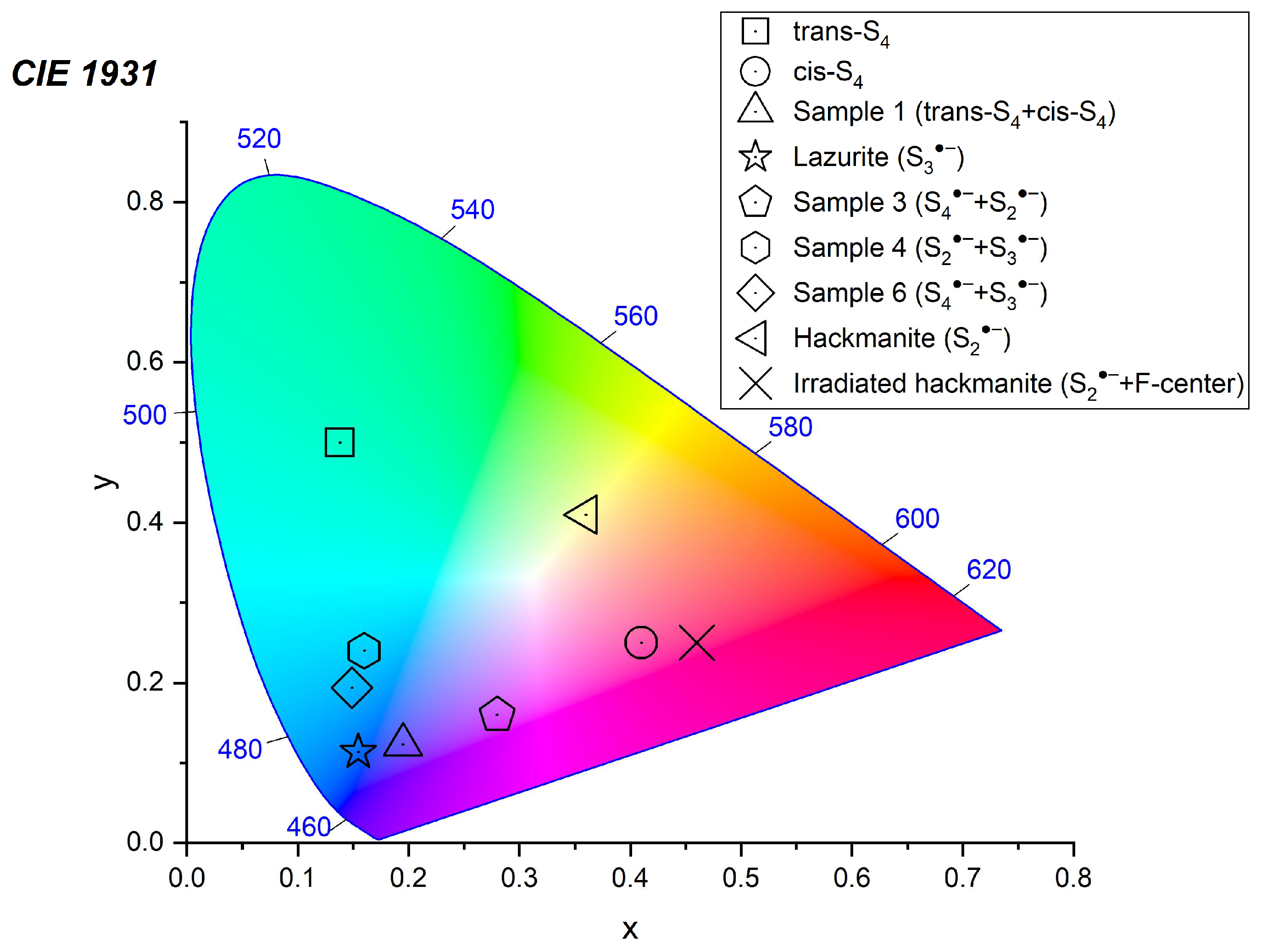
4.3. Color Centers in Sodalite-group Minerals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures, (n.d.). Available online: http://www.iza-structure.org/databases/ (accessed on 16 June 2022).
- Fischer, R.X.; Baur, W.H. Symmetry relationships of sodalite (SOD)-type crystal structures. Z. Krist. 2009, 224, 185–197. [Google Scholar] [CrossRef]
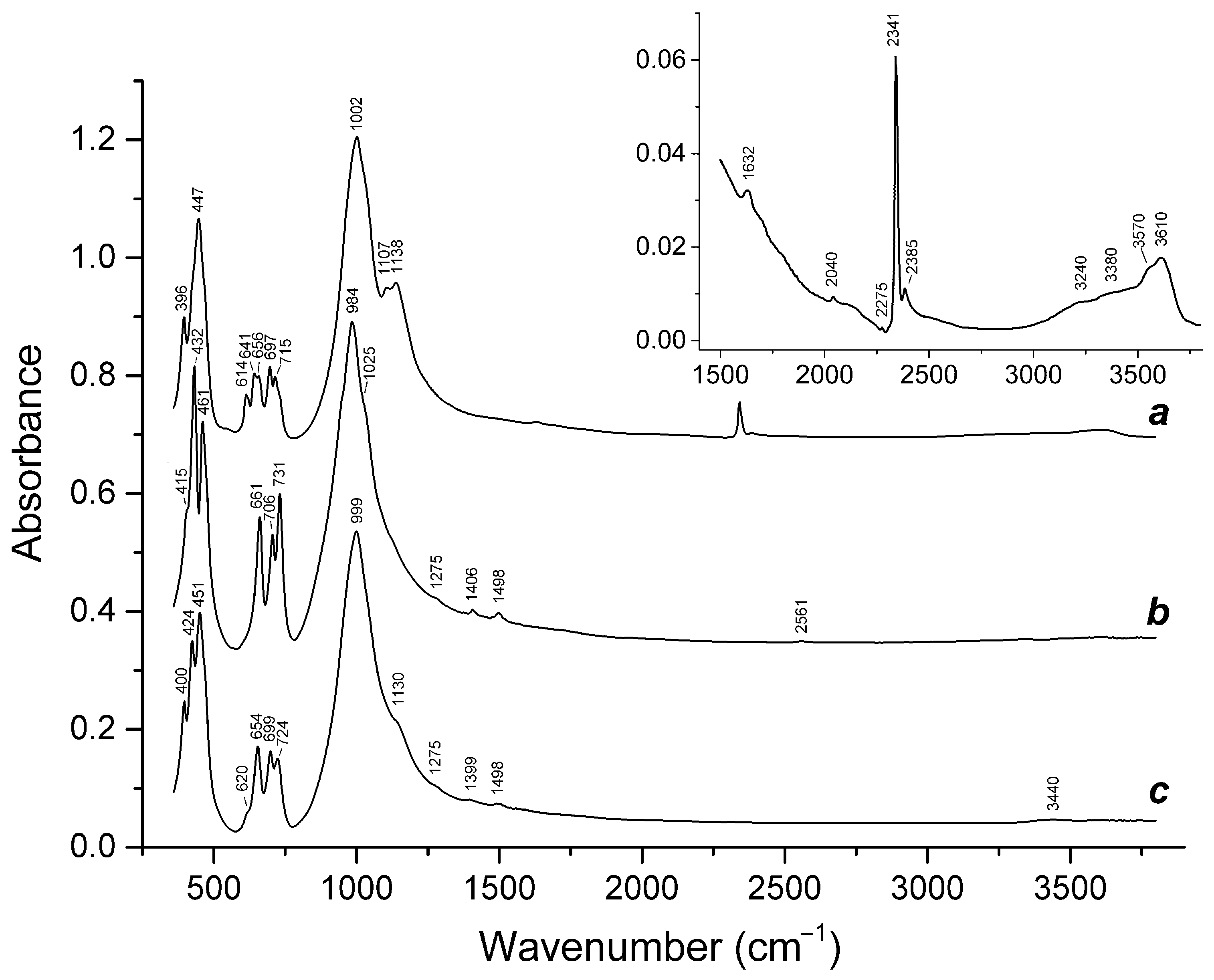
- Chukanov, N.V.; Vigasina, M.F.; Zubkova, N.V.; Pekov, I.V.; Schäfer, C.; Kasatkin, A.V.; Yapaskurt, V.O.; Pushcharovsky, D.Y. Extra-framework content in sodalite-group minerals: Complexity and new aspects of its study using infrared and Raman spectroscopy. Minerals 2020, 10, 363. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Sapozhnikov, A.N.; Shendrik, R.Y.; Vigasina, M.F.; Steudel, R. Spectroscopic and crystal-chemical features of sodalite-group minerals from gem lazurite deposits. Minerals 2020, 10, 1042. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Chukanov, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Tauson, V.L.; Lipko, S.V.; Belakovskiy, D.I.; Levitskiy, V.I.; Suvorova, L.F.; Ivanova, L.A. Lazurite: Confirmation of the status as a mineral species with the formula Na7Ca(Al6Si6O24)(SO4) S3•–·H2O and new data. Zap. Ross. Mineral. Obs. (Proc. Russ. Mineral Soc.) 2021, 150, 92–102. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Britvin, S.N.; Zubkova, N.V.; Varlamov, D.A.; Sidorov, E.G. Unusual silicate mineralization in fumarolic sublimates of the Tolbachik volcano, Kamchatka, Russia—Part 2: Tectosilicates. Eur. J. Mineral. 2020, 32, 121–136. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K. Structural chemistry, IR spectroscopy, properties, and genesis of natural and synthetic microporous cancrinite- and sodalite-related materials: A review. Micropor. Mesopor. Mater. 2021, 323, 111098. [Google Scholar] [CrossRef]
- Gobeltz-Hautecoeur, N.; Demortier, A.; Lede, B.; Lelieur, J.P.; Duhayon, C. Occupancy of the sodalite cages in the blue ultramarine pigments. Inorg. Chem. 2002, 41, 2848–2854. [Google Scholar] [CrossRef]
- Heil, C.; Cataldo, S.; Bachelet, G.B.; Boeri, L. Superconductivity in sodalite-like yttrium hydride clathrates. Phys. Rev. B Condens. Matter. 2019, 99, 220502(R). [Google Scholar] [CrossRef]
- Ogura, M.; Morozumi, K.; Elangovan, S.P.; Tanada, H.; Ando, H.; Okubo, T. Potassium-Doped sodalite: A tectoaluminosilicate for the catalytic material towards continuous combustion of carbonaceous matters. Appl. Catal. B 2008, 77, 294–299. [Google Scholar] [CrossRef]
- Shanbhag, G.V.; Choi, M.; Kim, J.; Ryoo, R. Mesoporous sodalite: A novel, stable solid catalyst for base-catalyzed organic transformations. J. Catal. 2009, 264, 88–92. [Google Scholar] [CrossRef]
- Sachse, A.; Galarneau, A.; Renzo, F.D.; Fajula, F.; Coq, B. Synthesis of zeolite monoliths for flow continuous processes. The case of sodalite as a basic catalyst. Chem. Mater. 2010, 22, 4123–4125. [Google Scholar] [CrossRef]
- Hiyoshi, N. Nanocrystalline sodalite: Preparation and application to epoxidation of 2-cyclohexen-1-one with hydrogen peroxide. Appl. Catal. 2012, 419–420, 164–169. [Google Scholar] [CrossRef]
- Manique, M.C.; Lacerda, L.V.; Alves, A.K.; Bergmann, C.P. Biodiesel production using coal fly ash-derived sodalite as a heterogeneous catalyst. Fuel 2017, 190, 268–273. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.-J.; Chang, X.; Zhao, J.; Tian, H.; Yang, C.; Li, M.; Fu, Q.; Mu, R.; Gong, J. Activation and spillover of hydrogen on sub-1 nm palladium nanoclusters confined within sodalite zeolite for the semi-hydrogenation of alkynes. Angew. Chem. Int. Ed. 2019, 58, 7668–7672. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Production of ultra pure water by desalination of seawater using a hydroxyl sodalite membrane. J. Membr. Sci. 2010, 356, 52–57. [Google Scholar] [CrossRef]
- Nabavi, M.S.; Mohammadi, T.; Kazemimoghadam, M. Hydrothermal synthesis of hydroxyl sodalite zeolite membrane: Separation of H2/CH4. Ceram. Int. 2014, 40, 5889–5896. [Google Scholar] [CrossRef]
- Kalantari, N.; Vaezi, M.J.; Yadollahi, M.; Babaluo, A.A.; Bayati, B.; Kazemzadeh, A. Synthesis of nanostructure hydroxysodalite composite membranes via hydrothermal method: Support surface modification and synthesis method effects. Asia-Pac. J. Chem. Eng. 2015, 10, 45–55. [Google Scholar] [CrossRef]
- Wei, X.-L.; Pan, W.-Y.; Li, X.; Pan, M.; Huo, C.-F.; Yang, R.; Chao, Z.-S. MCM-22 zeolite-induced synthesis of thin sodalite zeolite membranes. Chem. Mater. 2020, 32, 333–340. [Google Scholar] [CrossRef]
- Yang, G.; Guo, H.; Kang, Z.; Feng, S.; Zhao, L.; Mintova, S. Sandwich-type H2/CO2 membranes comprising of graphene oxide and sodalite crystals with adjustable morphology and size. Micropor. Mesopor. Mater. 2020, 300, 110120. [Google Scholar] [CrossRef]
- Eterigho-Ikelegbe, O.; Bada, S.O.; Daramola, M.O. Preparation and evaluation of nanocomposite sodalite/α-Al2O3 tubular membranes for H2/CO2 separation. Membranes 2020, 10, 312. [Google Scholar] [CrossRef]
- Eden, C.L.; Daramola, M.O. Evaluation of silica sodalite infused polysulfone mixed matrix membranes during H2/CO2 separation. Mater. Today Proc. 2021, 38, 522–527. [Google Scholar] [CrossRef]
- Ntshangase, N.C.; Sadare, O.O.; Daramola, M.O. Effect of silica sodalite functionalization and PVA coating on performance of sodalite infused PSF membrane during treatment of acid mine drainage. Membranes 2021, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.A.R.; Barea, E.; Salas, J.M.; Masciocchi, N.; Galli, S.; Sironi, A.; Ania, C.O.; Parra, J.B. H2, N2, CO, and CO2 sorption properties of a series of robust sodalite-type microporous coordination polymers. Inorg. Chem. 2006, 45, 2397–2399. [Google Scholar] [CrossRef] [PubMed]
- Asgari, A.; Jawahery, S.; Bloch, E.D.; Hudson, M.R.; Flacau, R.; Vlaisavljevich, B.; Long, J.R.; Brown, C.M.; Queen, W.L. An experimental and computational study of CO2 adsorption in the sodalite-type M-BTT (M = Cr, Mn, Fe, Cu) metal–organic frameworks featuring open metal sites. Chem. Sci. 2018, 9, 4579–4588. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Hu, S.; Peng, S.; Xu, C.; Lu, A. Dehydrated Na6[AlSiO4]6 sodalite as a promising SO2 sorbent material: A first principles thermodynamics prediction. J. Amer. Ceram. Soc. 2019, 102, 3663–3672. [Google Scholar] [CrossRef]
- Asgari, M.; Semino, R.; Schouwink, P.A.; Kochetygov, I.; Tarver, J. Understanding how ligand functionalization influences CO2 and N2 adsorption in a sodalite metal-organic framework. Chem. Mater. 2020, 32, 1526–1536. [Google Scholar] [CrossRef]
- Dickson, J.O.; Harsh, J.B.; Lukens, W.W.; Pierce, E.M. Perrhenate incorporation into binary mixed sodalites: The role of anion size and implications for technetium-99 sequestration. Chem. Geol. 2015, 395, 138–143. [Google Scholar] [CrossRef]
- Gilbert, M.R. Pressureless sintering of sodalite waste-forms for the immobilization of pyroprocessing wastes. In MRS Online Proceedings Library (OPL): Symposium EE—Scientific Basis for Nuclear Waste Management XXXVIII; Cambridge University Press: cambrige, UK, 2015; Volume 1744, pp. 61–66. [Google Scholar] [CrossRef]
- Luksic, S.A.; Riley, B.J.; Parker, K.E.; Hrma, P. Sodalite as a vehicle to increase Re retention in waste glass stimulant during vitrification. J. Nucl. Mater. 2016, 479, 331–337. [Google Scholar] [CrossRef]
- Vance, E.R.; Gregg, D.J.; Grant, C.; Stopic, A.; Maddrell, E.R. Silver iodide sodalite for 129I immobilization. J. Nucl. Mater. 2016, 480, 177–181. [Google Scholar] [CrossRef]
- Lilova, K.; Pierce, E.M.; Wu, L.; Jubb, A.M.; Subramani, T.; Navrotsky, A. Energetics of salt-bearing sodalites, Na8Al6Si6O24X2 (X = SO4, ReO4, Cl, I): A treatment option for pertechnetate-enriched nuclear waste streams. ACS Earth Space Chem. 2020, 4, 2153–2161. [Google Scholar] [CrossRef]
- Grajciar, L. PbS clusters embedded in sodalite zeolite cavities of different compositions: Unraveling the structural evolution and optical properties using ab initio calculations. J. Phys. Chem. 2016, C120, 27050–27065. [Google Scholar] [CrossRef]
- Van den Berg, A.W.C.; Bromley, S.T.; Jansen, J.C. Thermodynamic limits on hydrogen storage in sodalite framework materials: A molecular mechanics investigation. Micropor. Mesopor. Mater. 2005, 78, 63–71. [Google Scholar] [CrossRef]
- Zheng, Z.; Guliants, V.V.; Misture, S. Sodalites as ultramicroporous frameworks for hydrogen separation at elevated temperatures: Thermal stability, template removal, and hydrogen accessibility. J. Porous Mater. 2009, 16, 343–347. [Google Scholar] [CrossRef]
- Rüscher, C.H.; Stemme, F.; Schomborg, L.; Buhl, J.-C. Low temperature hydrogen release from borontetrahydride-sodalite and its reloading: Observations in in situ and ex situ TIR experiments. In Ceramics for Environmental and Energy Applications; Boccaccini, A., Marra, J., Dogan, F., Lin, H.-T., Watanabe, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 65–70. [Google Scholar]
- Gong, Y.-N.; Meng, M.; Zhong, D.-C.; Huang, Y.-L.; Jiang, L.; Lu, T.-B. Counter-Cation modulation of hydrogen and methane storage in a sodalite-type porous metal–organic framework. Chem. Commun. 2012, 48, 12002–12004. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Bolotina, N.B.; Chukanov, N.V.; Kaneva, E.V.; Shendrik, R.Y.; Vigasina, M.F.; Ivanova, L.A. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC)—Newsletter 65. Eur. J. Mineral. 2022, 34, 143–148. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Zubkova, N.V.; Pekov, I.V.; Shendrik, R.Y.; Varlamov, D.A.; Vigasina, M.F.; Belakovskiy, D.I.; Britvin, S.N.; Yapaskurt, V.O.; Pushcharovsky, D.Y. Sapozhnikovite, Na8(Al6Si6O24)(HS)2, a new sodalite-group mineral from the Lovozero alkaline massif, Kola Peninsula. Mineral. Mag. 2022, 86, 49–59. [Google Scholar] [CrossRef]
- Rejmak, P. Computational refinement of the puzzling red tetrasulfur chromophore in ultramarine pigments. Phys. Chem. Chem. Phys. 2020, 22, 22684–22698. [Google Scholar] [CrossRef]
- Eckert, B.; Steudel, F. Molecular spectra of sulfur molecules and solid sulfur allotropes. Top. Curr. Chem. 2003, 231, 31–97. [Google Scholar] [CrossRef]
- Hettmann, K.; Wenzel, T.; Marks, M.; Markl, G. The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals. Amer. Mineral. 2012, 97, 1653–1661. [Google Scholar] [CrossRef]
- Ling, Z.C.; Wang, A.; Jolliff, B.L. Mineralogy and geochemistry of four lunar soils by laser-Raman study. Icarus 2011, 211, 101–113. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Tauson, V.L.; Lipko, S.V.; Shendrik, R.Y.; Levitskii, V.I.; Suvorova, L.F.; Chukanov, N.V.; Vigasina, M.F. On the crystal chemistry of sulfur-rich lazurite, ideally Na7Ca(Al6Si6O24)(SO4)(S3)−·nH2O. Amer. Mineral. 2021, 106, 226–234. [Google Scholar] [CrossRef]
- Steudel, R. Inorganic polysulfides Sn2− and radical anions Sn●−. In Elemental Sulfur und Sulfur-Rich Compounds II. Topics in Current Chemistry; Steudel, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 231. [Google Scholar]
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319 and 4338. [Google Scholar] [CrossRef]
- Wong, M.W.; Steudel, R. Structure and spectra of tetrasulfur S4—An ab initio MO study. Chem. Phys. Lett. 2003, 379, 162–169. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Mangone, A.; Aquafredda, P. Blue coloured haüyne from Mt. Vulture (Italy) volcanic rocks: SEM-EDS and Raman investigation of natural and heated crystals. J. Raman Spectrosc. 2022, 53, 956–968. [Google Scholar] [CrossRef]
- Bény, C.; Guilhaumou, N.; Touray, J.-C. Native-Sulphur-Bearing fluid inclusions in the CO2-H2S-H2O-S system—Microthermometry and Raman microprobe (MOLE) analysis—Thermochemical interpretations. Chem. Geol. 1982, 37, 113–127. [Google Scholar] [CrossRef]
- Dubessy, J.; Boiron, M.-C.; Moissette, A.; Monnion, C.; Sretenskaya, N. Determination of water, hydrates and pH in fluid inclusions by micro-Raman spectrometry. Eur. J. Mineral. 1992, 4, 885–894. [Google Scholar] [CrossRef]
- Kaneva, E.; Shendrik, R.; Mesto, E.; Bogdanov, A.; Vladykin, N. Spectroscopy and crystal chemical properties of NaCa2[Si4O10]F natural agrellite with tubular structure. Chem. Phys. Lett. 2020, 738, 136868. [Google Scholar] [CrossRef]
- Weser, G.; Hensel, F.; Warren, W.W. The optical absorption spectrum of fluid sulfur up to supercritical conditions. Ber. Bunsenges. Phys. Chem. 1978, 82, 588–594. [Google Scholar] [CrossRef]
- Paniz, H.; Lester, A. Vibronic absorption spectra of S3 and S4, in solid argon. J. Phys. Chem. 1992, 96, 6579–6585. [Google Scholar]
- Annersten, H.; Haseeb, A. Blue sodalite. Canad. Mineral. 1979, 17, 39–46. [Google Scholar]
- Radomskaya, T.A.; Kaneva, E.V.; Shendrik, R.Y.; Suvorova, L.F.; Vladykin, N.V. Sulfur-Bearing sodalite, hackmanite, in alkaline pegmatites of the Inagli massif (Aldan Shield): Crystal chemistry, photochromism, and luminescence. Geol. Ore Depos. 2021, 63, 696–704. [Google Scholar] [CrossRef]
- Tauson, V.L.; Akimov, V.V.; Sapozhnikov, A.N.; Kuznetzov, K.E. Investigation of the stability conditions and structural-chemical transformations of Baikal lazurite. Geochem. Int. 1998, 36, 717–733. [Google Scholar]
- Rastsvetaeva, R.K.; Sapozhnikov, A.N.; Tauson, V.L.; Kashaev, A.A. Crystal structure of sulfide sodalite, a product of sulfate sulfur reduction in lazurite. Dokl. Akad. Nauk. 1997, 356, 773–776. (In Russian) [Google Scholar]
- Chukanov, N.V.; Vigasina, M.F.; Shendrik, R.Y.; Varlamov, D.A.; Pekov, I.V.; Zubkova, N.V. Nature and Isomorphism of Extra-Framework Components in cancrinite- and sodalite-related minerals: New data. Minerals 2022, 12, 729. [Google Scholar] [CrossRef]
- Steudel, R.; Jensen, D.; Göbel, P.; Hugo, P. Optical absorption spectra of the homocyclic sulfur molecules Sn (n = 6, 7, 8, 9, 10, 12, 15, 20) in solution. Ber. Bunsenges. Phys. Chem. 1988, 92, 118–122. [Google Scholar] [CrossRef]
- Raulin, K.; Gobeltz, N.; Vezin, H.; Touat, N.; Ledé, B.; Moissette, A. Identification of the EPR signal of S2− in green ultramarine pigments. Phys. Chem. Chem. Phys. 2011, 13, 9253–9259. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Dubrovinsky, L.S. The S3− ion is stable in geological fluids at elevated temperatures and pressures. Science 2011, 331, 1052–1054. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Dubessy, J. Stability and abundance of the trisulfur radical ion S3− in hydrothermal fluids. Earth Planet. Sci. Lett. 2015, 411, 298–309. [Google Scholar] [CrossRef]
- Arieli, D.; Vaughan, D.E.W.; Goldfarb, D. New synthesis and insight into the structure of blue ultramarine pigments. J. Amer. Chem. Soc. 2004, 126, 5776–5788. [Google Scholar] [CrossRef]
- Ostroumov, M.; Fritsch, E.; Faulques, E.; Chauvet, O. Etude spectrometrique de la lazurite du Pamir, Tajikistan. Canad. Mineral. 2002, 40, 885–893. [Google Scholar] [CrossRef][Green Version]
- Ballirano, O.; Maras, A. Crystal chemical and structural characterization of an unusual CO3-bearing sodalite-group mineral. Eur. J. Mineral. 2005, 17, 805–812. [Google Scholar] [CrossRef]
- Gerasimovskiy, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyalov, A.I.; Saprykina, T.V.; Balashov, Y.A. Geochemistry of the Lovozero Alkaline Massif; Nauka: Moskow, Russia, 1966. (In Russian) [Google Scholar]
- Zhang, B.; Wu, J.; Gu, J.; Li, S.; Yan, T.; Gao, X.-P. The fundamental understanding of lithium polysulfides in ether-based electrolyte for lithium-sulfur batteries. ACS Energy Lett. 2021, 6, 537–546. [Google Scholar] [CrossRef]
- Finch, A.A.; Friis, H.; Maghrabi, M. Defects in sodalite-group minerals determined from X-ray-induced luminescence. Phys. Chem. Miner. 2016, 43, 481–491. [Google Scholar] [CrossRef]
- Agamah, C.; Vuori, S.; Colinet, P.; Norrbo, I.; Miranda de Carvalho, J.; Key, L.; Nakamura, O.; Lindblom, J.; van Goethem, L.; Emmermann, A.; et al. Hackmanite—The natural glow-in-the-dark material. Chem. Mater. 2020, 32, 8895–8905. [Google Scholar] [CrossRef]
- Huie, R.E.; Clifton, C.L.; Altstein, N. A pulse radiolysis and flash photolysis study of the radicals SO2−, SO3−, SO4− and SO5−. Int. J. Radiat. Appl. Instr. Part C Radiat. Phys. Chem. 1989, 33, 361–370. [Google Scholar] [CrossRef]
- McElroy, W.J.; Waygood, S.J. Kinetics of the reactions of the SO4− radical with SO4−, S2O82−, H2O and Fe2+. J. Chem. Soc. Faraday Trans. 1990, 86, 2557. [Google Scholar] [CrossRef]
- Olysych, L.V.; Vigasina, M.F.; Melchakova, L.V.; Pekov, I.V.; Chukanov, N.V. Study of thermal decomposition of the cancrinite-kyanoxalite solid-solution series minerals. In Proceedings of the Abstracts of XXVII International Conference “Geochemistry of Magmatic Rocks”, Moscow, Russia, 9–16 September 2010; pp. 135–136. [Google Scholar]
- Chukanov, N.V.; Zubkova, N.V.; Buhl, J.-C.; Pekov, I.V.; Ksenofontov, D.A.; Depmeier, W.; Pushcharovskii, D.Y. Crystal structure of nitrate cancrinite synthesized under low-temperature hydrothermal conditions. Dokl. Earth Sci. 2011, 438, 669–672. [Google Scholar] [CrossRef]
| Sample No. | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Color | Bluish lilac | Deep blue | ||||||
| Na2O | 18.04 | 17.92 | ||||||
| K2O | 0.21 | 0.74 | ||||||
| CaO | 7.83 | 7.60 | ||||||
| Al2O3 | 28.63 | 27.79 | ||||||
| Fe2O3 | 0 | 0.19 | ||||||
| SiO2 | 33.65 | 33.46 | ||||||
| CO2 a | 0.67 | 0.25 | ||||||
| SO3 b | 14.58 | 15.33 | ||||||
| F | 0.42 | 0 | ||||||
| Cl | 0.32 | 0.31 | ||||||
| –O=Cl,F | −0.25 | −0.07 | ||||||
| Total | 104.10 | 103.52 | ||||||
| Analysis No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Contents, wt. % | ||||||||
| Na2O | 23.60 | 24.20 | 24.60 | 24.28 | 24.49 | 24.34 | 24.01 | 24.21 |
| Al2O3 | 30.84 | 31.50 | 31.37 | 31.38 | 31.69 | 31.29 | 31.16 | 31.33 |
| Fe2O3 | 0.36 | - | 0.27 | - | - | 0.13 | - | - |
| SiO2 | 36.03 | 36.88 | 36.95 | 36.61 | 36.82 | 36.50 | 36.48 | 36.26 |
| HS * | 2.78 | 3.10 | 3.52 | 3.74 | 4.17 | 4.48 | 4.86 | 5.27 |
| Cl | 3.97 | 3.41 | 3.32 | 3.07 | 2.68 | 2.31 | 1.85 | 1.35 |
| –O=(Cl,HS) | –1.57 | –1.52 | –1.60 | –1.59 | –1.61 | –1.60 | –1.60 | –1.58 |
| Total | 96.01 | 97.57 | 98.43 | 97.49 | 98.24 | 97.45 | 96.76 | 96.84 |
| Formula Calculated on the Basis of Al + Fe + Si = 12 Atoms Per Formula Unit | ||||||||
| Na | 7.56 | 7.61 | 7.72 | 7.68 | 7.68 | 7.71 | 7.56 | 7.70 |
| Al | 6.00 | 6.02 | 5.99 | 6.03 | 6.04 | 6.02 | 6.02 | 6.05 |
| Fe | 0.04 | - | 0.03 | - | - | 0.02 | - | - |
| Si | 5.96 | 5.98 | 5.98 | 5.97 | 5.96 | 5.96 | 5.98 | 5.95 |
| S | 0.84 | 0.91 | 1.03 | 1.11 | 1.22 | 1.33 | 1.45 | 1.57 |
| Cl | 1.11 | 1.06 | 0.91 | 0.85 | 0.73 | 0.64 | 0.51 | 0.38 |
| S + Cl | 1.95 | 1.97 | 1.94 | 1.96 | 1.95 | 1.97 | 1.96 | 1.95 |
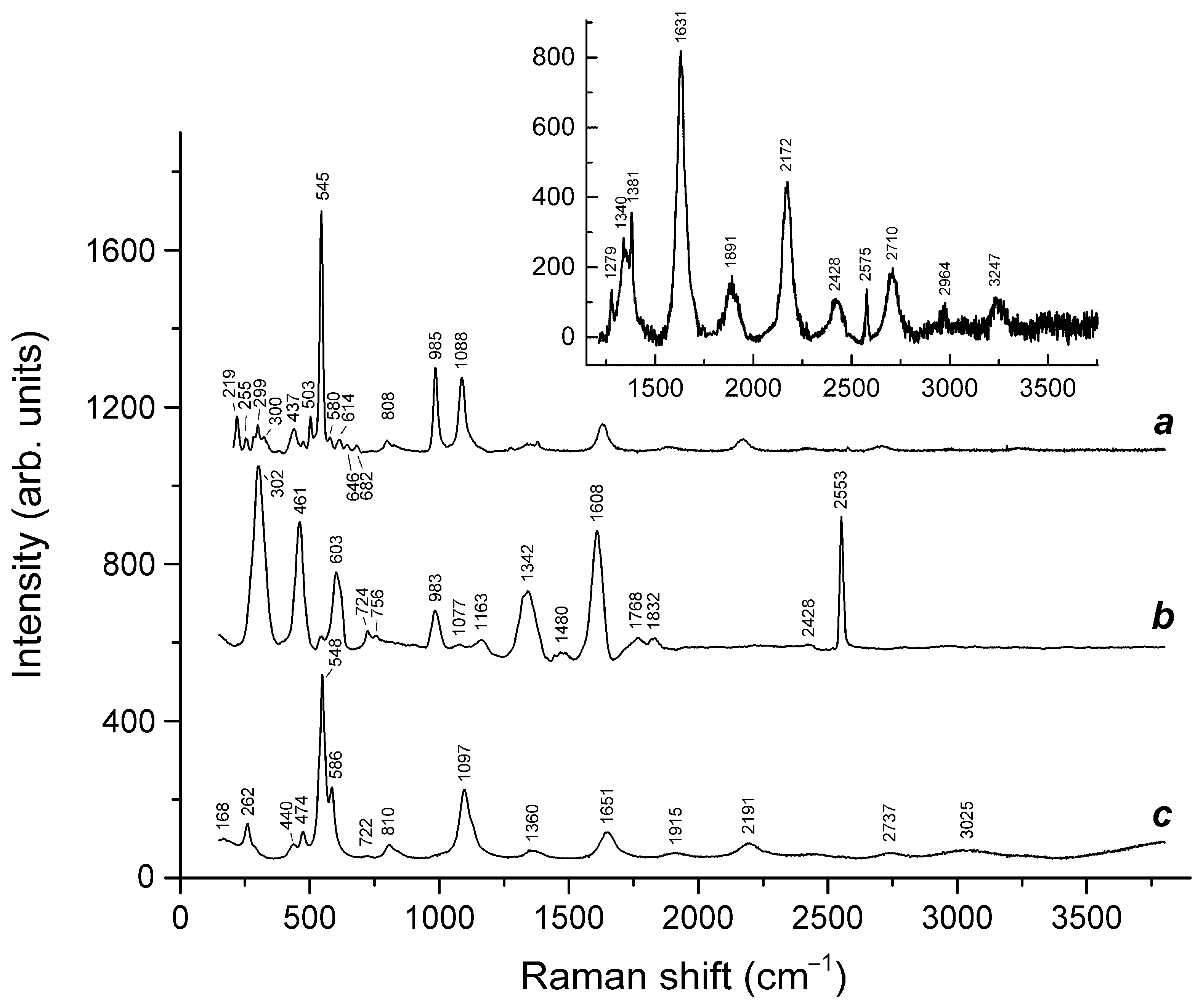
| Raman Shift (cm−1) | Assignment | |||||
|---|---|---|---|---|---|---|
| Sample No. | ||||||
| 1 | 7 | 8 | 9 | 10 | 11 | |
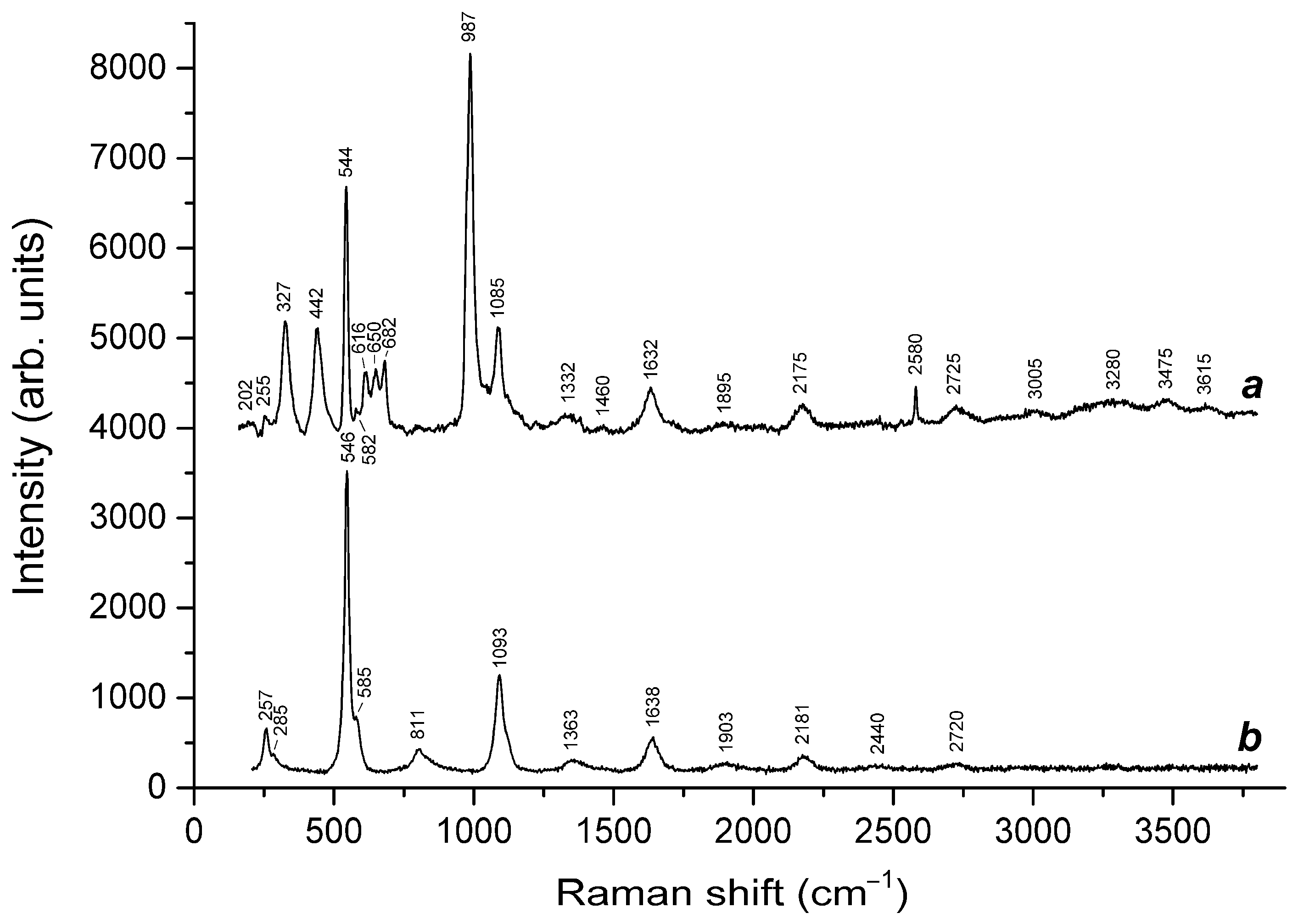
| 202w | - | - | - | 210 | - | Combination of low-frequency lattice modes and/or trans-S4 bending mode |
| 255 | - | 257 | - | - | S3●− bending mode (ν2) | |
| - | - | 260 | - | - | 266 | Bending vibrations of the [ClNa4]3+ and [(HS)Na4]3+ clusters |
| - | - | 294 | 285w | 283w | - | Combination of low–frequency lattice modes involving Na+ cations and/or S6 bending mode |
| 327 | - | - | - | - | - | cis-S4 mixed (bending + stretching) ν4 mode |
| - | - | 417w | - | - | 410w | Bending vibrations of the aluminosilicate framework |
| 442 | 439 | - | 441s | - | SO42− [the E(ν2) mode] and/or δ[O–Si(Al)–O] bending vibrations | |
| - | - | 459s | - | - | 463s | Stretching vibrations of the [ClNa4] and [(HS)Na4] clusters |
| 544s | - | - | 546s | 548w | - | S3●− symmetric stretching (ν1) mode |
| 582w | - | - | 585 | - | - | S3●− antisymmetric stretching (ν3), possibly, overlapping with the stretching band of S2●− |
| - | - | - | - | 580w, 605w | - | S2●− stretching mode |
| - | - | 611w | - | - | 611w | Overtone of vibrations involving Na+ cations? |
| 616 | 621 | - | - | - | - | SO42− bending vibrations [F2(ν4) mode] |
| 650 | - | - | - | - | - | gauche-S4 symmetric stretching A1(ν1) mode |
| 682 | - | - | - | 673w | - | trans-S4 symmetric stretching ν3 mode |
| - | 724w | 732w | - | - | 732w | Mixed vibrations of the aluminosilicate framework |
| 987s | 983s | 978, 989 | - | 986s | 970, 986 | SO42− symmetric stretching vibrations [A1(ν1) mode] |
| 1053w | 1049 | 1062 | - | - | 1060 | Stretching vibrations of the framework and/or CO32− symmetric stretching vibrations |
| - | - | - | - | 1074 | - | HF libration |
| 1085 | - | - | 1093s | - | - | S3●− overtone (2 × ν1) |
| - | 1138 | - | - | - | - | SO42− asymmetric stretching vibrations [F2(ν3) mode], possibly, overlapping with S2●− overtone (2 × ν1) |
| - | - | - | - | 1271 | - | CO2 Fermi resonance |
| 1332 | - | - | - | - | - | Overtone of the cis-S4 antisymmetric stretching mode (2 × ν3) |
| - | - | - | - | 1350 | - | H+ translation |
| - | - | - | 1363 | - | - | S3●− combination mode (2ν1 + ν2) |
| - | - | - | - | 1381 | - | CO2 Fermi resonance |
| 1460w | - | - | - | - | - | CO3 asymmetric stretching mode |
| 1632 | - | - | 1638 | - | - | S3●− overtone (3 × ν1) |
| 1895 | - | - | - | - | - | S3●− combination mode (3 × ν2 + ν1) |
| 2175 | - | - | 2181 | - | - | S3●− overtone (4 × ν1) |
| - | - | - | 2440w | - | - | S3●− combination mode (4 × ν2 + ν1) |
| - | - | 2553 | - | - | - | HS− stretching mode |
| 2580 | - | - | - | - | - | H2S symmetric stretching mode |
| 2725 | - | - | 2720 | - | - | S3●− overtone (5 × ν1) |
| 3005, 3280 | - | - | - | - | 3130 | H3O+ stretching mode |
| 3475, 3615 | - | - | - | 3540 | - | H2O stretching vibrations |
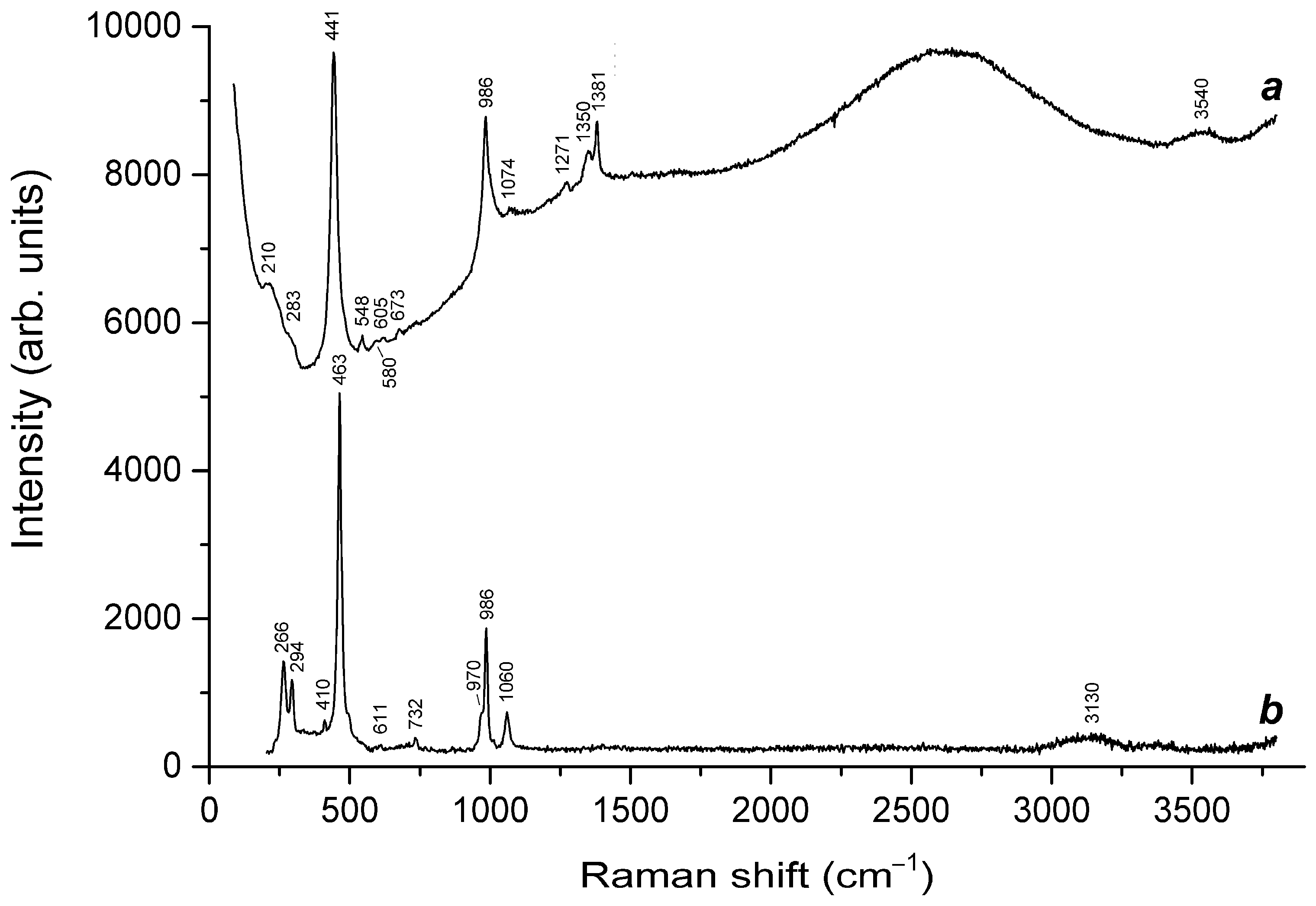
| Raman Shift (cm−1) | Assignment | ||
|---|---|---|---|
| Initial Sample | Preheated Sample | Sample Heated at 800 °C in Air | |
| - | 194w | 168w | Combination of low-frequency lattice modes |
| - | 213w | 219 | trans-S4 bending mode |
| - | 254 | - | Bending vibrations of the [(HS)−Na4]3+ cluster |
| 260 | - | 260 | S3●− bending mode (ν2) |
| 287w | 290 | - | Low–frequency lattice modes involving Na+ cations and/or S4●− bending vibrations |
| - | - | - | cis-S4 mixed ν4 mode (combined symmetric bending + stretching vibrations) |
| 327w | 355w | - | cis-S4●− mixed ν3 mode |
| - | - | 380 | cis-S4 mixed ν3 mode |
| 448w | - | - | SO42− [E(ν2) mode] and/or δ[O–Si(Al)–O] bending vibrations |
| - | 454s | - | Stretching vibrations of the [(HS)−Na4]3+ cluster |
| - | 474 | 473 | S6 stretching mode and/or mixed ν4 mode of trans–S4 |
| 547s | - | 546s | S3●− symmetric stretching (ν1) mode |
| 555 | - | S3 neutral molecule and/or gauche-S4 | |
| 580 | - | 583 | S3●− antisymmetric stretching (ν3), possibly, overlapping with the stretching band of S2●− |
| - | 590 | - | Stretching vibrations of the [(S2−)Na4]2+ cluster and/or S2●− stretching mode |
| - | 733 | - | O–C–O bending vibrations of oxalate anions |
| - | - | 720 | Mixed vibrations of the aluminosilicate framework |
| 804 | - | 804 | S3●− combination mode (ν1 + ν2) |
| - | 845w | - | C–C stretching vibrations of oxalate anions |
| 990 | 996 | 981w | SO42− symmetric stretching vibrations [A1(ν1) mode] and/or framework stretching vibrations |
| - | 1061 | - | CO32− symmetric stretching vibrations |
| 1091s | - | 1092s | S3●− overtone (2 × ν1) |
| - | 1335 | - | Symmetric C–O stretching vibrations of oxalate anions |
| 1353 | - | 1345 | S3●− combination mode (2ν1 + ν2) |
| - | 1613 | - | Antisymmetric C–O stretching vibrations of oxalate anions |
| 1639 | - | 1638 | S3●− overtone (3 × ν1) |
| 1900w | - | 1910w | S3●− combination mode (3 × ν2 + ν1) |
| 2180 | - | 2185 | S3●− overtone (4 × ν1) |
| 2443w | - | - | S3●− combination mode (4 × ν2 + ν1) |
| - | 2553s | - | HS− stretching mode |
| - | 2581w | - | H2S symmetric stretching mode |
| 2721 | - | 2725 | S3●− overtone (5 × ν1) |
| 2950w | - | - | S3●− combination mode (5 × ν1 + ν2) |
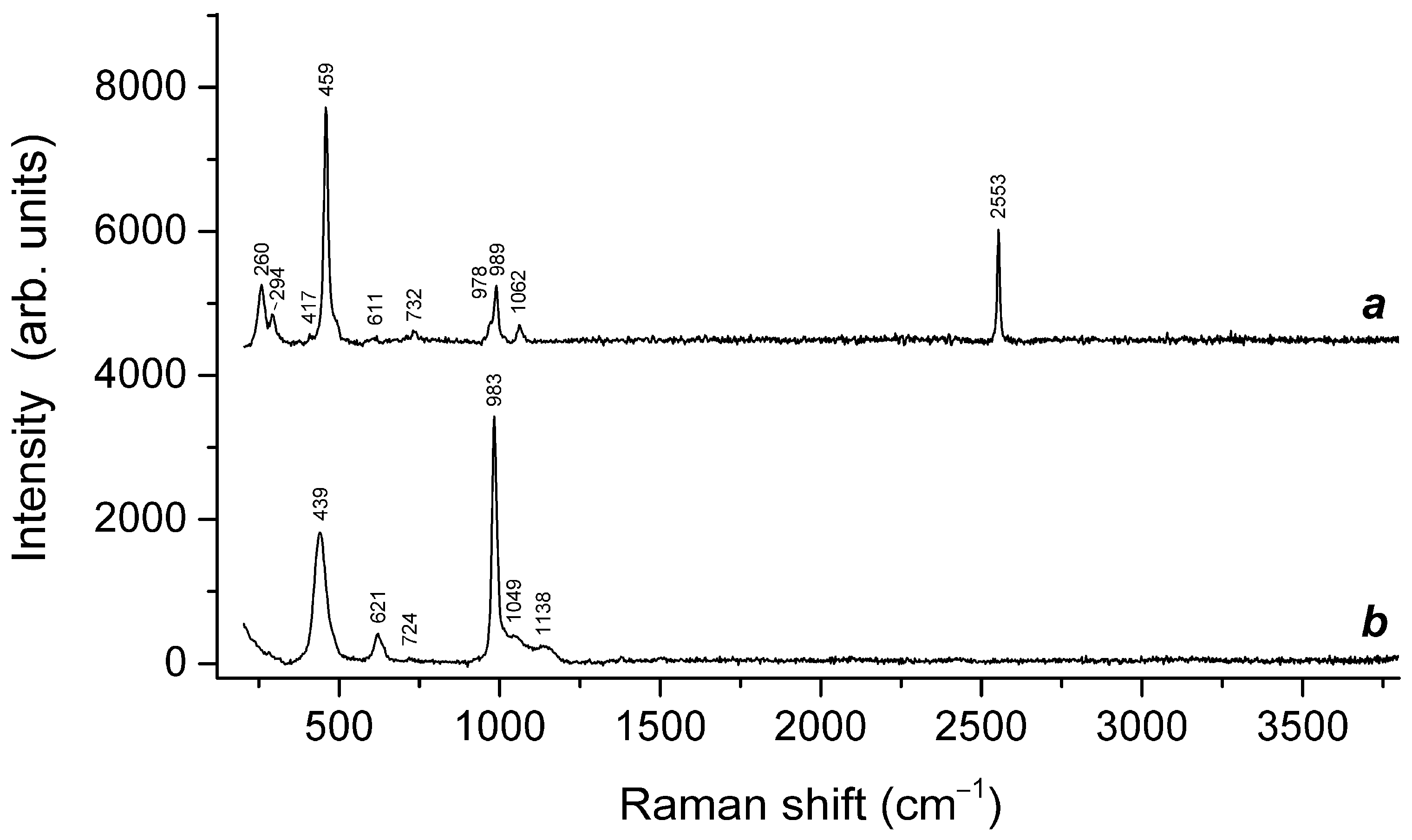
| Raman Shift (cm−1) | Assignment | ||
|---|---|---|---|
| Initial Sample | Preheated Sample | Sample Heated at 800 °C in Air | |
| - | 166 | - | Combination of low-frequency lattice modes |
| - | 219w | 217w | trans-S4 bending mode |
| - | 260 | - | Bending vibrations of the [(HS)−Na4]3+ cluster |
| 256 | - | 265 | S3●− bending mode (ν2) |
| 282w | - | - | Low–frequency lattice modes involving Na+ cations and/or S4●− bending vibrations |
| - | 308s | 297s | S4●− bending vibrations and/or cis-S4 mixed ν4 mode (combined symmetric bending + stretching vibrations) |
| - | 453s | - | Stretching vibrations of the [(HS)−Na4]3+ cluster |
| - | 471 | - | S6 stretching mode and/or mixed ν4 mode of trans–S4 |
| 543s | 547 | 546s | S3●− symmetric stretching (ν1) mode |
| 570 | - | 587 | S3●− antisymmetric stretching (ν3), possibly, overlapping with the stretching band of S2●− |
| - | 596 | - | Stretching vibrations of the [(S2−)Na4]2+ cluster and/or S2●− stretching mode |
| - | 728 | - | O–C–O bending vibrations of oxalate anions |
| 798 | - | 805 | S3●− combination mode (ν1 + ν2) |
| - | 850w | - | C–C stretching vibrations of oxalate anions |
| 983w | 989 | - | SO42− symmetric stretching vibrations [A1(ν1) mode] and/or framework stretching vibrations |
| - | 1064 | - | CO32− symmetric stretching vibrations |
| 1087s | - | 1097s | S3●− overtone (2 × ν1) |
| - | 1350 | - | Symmetric C–O stretching vibrations of oxalate anions |
| 1348 | - | 1365 | S3●− combination mode (2ν1 + ν2) |
| - | 1605 | - | Antisymmetric C–O stretching vibrations of oxalate anions |
| 1634 | - | 1643 | S3●− overtone (3 × ν1) |
| 1894w | - | 1900w | S3●− combination mode (3 × ν2 + ν1) |
| - | 1909w | - | Overtone of SO42− symmetric stretching vibrations? |
| 2173 | - | 2185 | S3●− overtone (4 × ν1) |
| 2434w | - | 2430w | S3●− combination mode (4 × ν2 + ν1) |
| - | 2556s | - | HS− stretching mode |
| 2713 | - | 2725 | S3●− overtone (5 × ν1) |
| 2971w | - | - | S3●− combination mode (5 × ν1 + ν2) |
| 3233w, 3470w | 3232, 3605w | 3220w | O–H stretching modes |
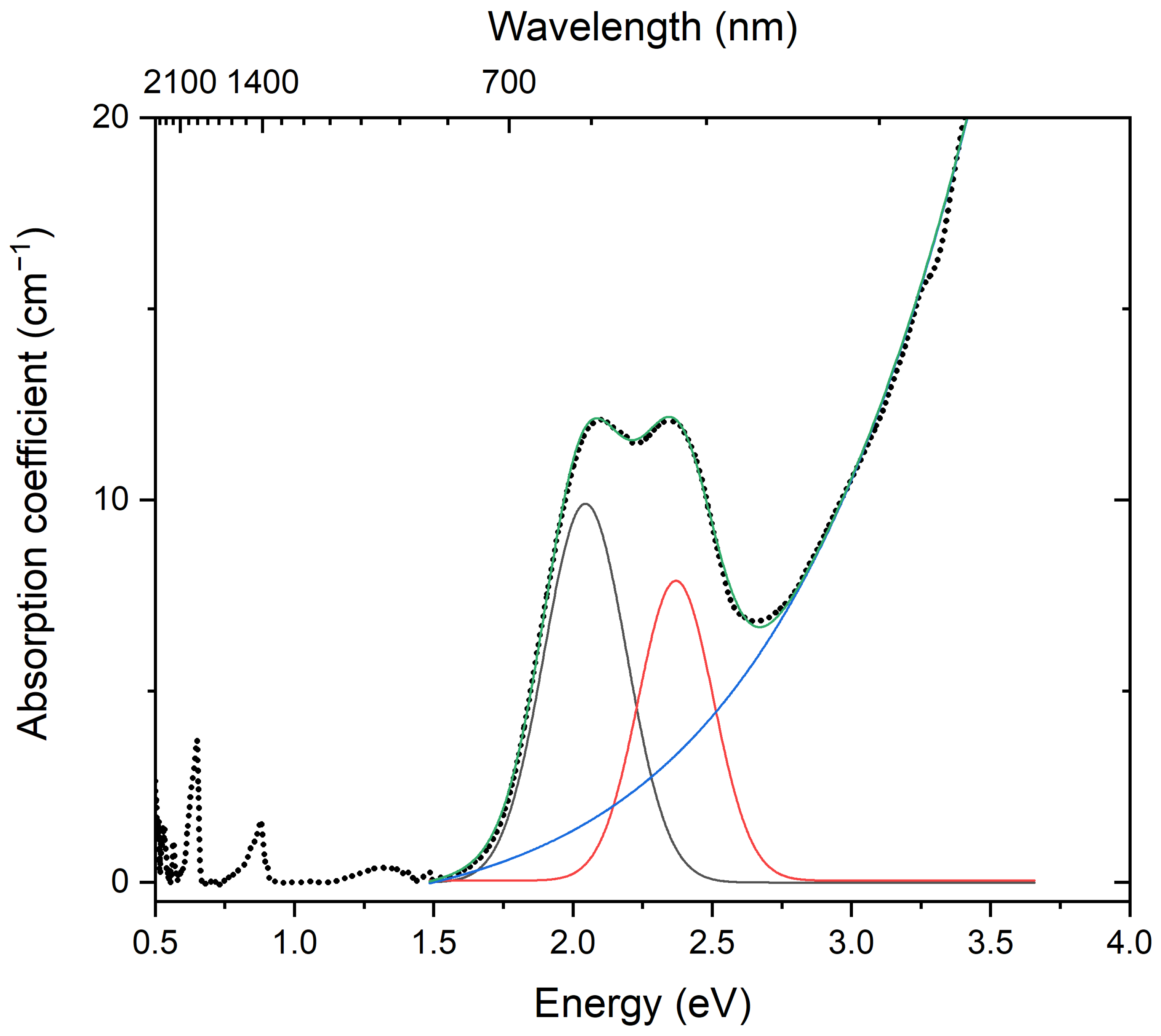
| Raman Shift (cm−1) | Assignment | ||
|---|---|---|---|
| Initial Sample | Preheated Sample | Sample Heated at 800 °C in Air | |
| 219 | - | - | trans-S4 bending |
| 260 | - | 262 | S3●− bending A2 (ν2) and S6 (with D3d symmetry) bending |
| 283 | - | - | Framework bending vibrations (resonance with a S6 bending mode?) |
| 298 | 302s | - | S4●− bending vibrations |
| 330 | - | - | cis-S4 mixed ν4 mode (symmetric bending + stretching) |
| 380w | - | - | cis-S4 mixed ν3 mode |
| 437 | - | 440w | SO4 [bending E (ν2) mode] and/or S6 (mixed mode) |
| - | 461s | - | [(HS)Na4]3+ stretching vibrations |
| 477 | - | 474 | S6 stretching mode and/or mixed ν4 mode of trans-S4 |
| 503 | - | - | Bending vibrations of the framework |
| 545s | - | 548s | S3●− symmetric stretching (ν1) (possibly, overlapping with the stretching band of gauche-S4) |
| 580 | - | 586 | S3●− antisymmetric stretching mode (ν3) |
| - | 603 | - | S2●− stretching mode |
| 614 | - | - | SO42– [bending F2 (ν4) mode] |
| 645 | - | - | cis-S4 stretching |
| 682 | - | - | trans-S4 symmetric stretching ν3 mode |
| - | 724, 756w | 722w | O–C–O bending vibrations of oxalate anions |
| 807 | - | 810 | S3●− combination mode (ν1 + ν2) |
| 985s | 983 | - | SO42− [symmetric stretching A1 (ν1) mode] (possibly, overlapping with the weak band of framework stretching vibrations) |
| - | 1077w | - | CO32− symmetric stretching mode |
| 1088s | - | 1097s | S3●− overtone (2 × ν1) [possibly, overlapping with the SO4●− stretching band (ν3 − F2)] |
| - | 1163 | - | S2●− overtone (2 × ν1) |
| 1279, 1381 | - | - | Symmetric stretching vibrations of CO2 molecules (Fermi doublet, resonance with the overtone of bending vibrations). |
| 1340 | 1342 | 1360 | Symmetric C–O stretching vibrations of CO2 molecules—involved in strong dipole-dipole interactions and/or symmetric C–O stretching vibrations of acid oxalate anions |
| - | 1480w | - | CO32− asymmetric stretching mode |
| - | 1609s | - | Antisymmetric C–O stretching vibrations of acid oxalate anions |
| 1631 | - | - | S3●− overtone (3 × ν1) |
| - | - | 1651 | Symmetric C–O stretching vibrations of oxalate anions |
| - | 1768, 1832 | - | C=O stretching vibrations of acid oxalate groups |
| 1891 | - | 1915 | S3●− combination mode (3 × ν1 + ν2) |
| 2172 | - | 2191 | S3●− overtone (4 × ν1) |
| 2428w | - | - | S3●− combination mode (4 × ν2 + ν1) |
| - | 2553s | - | HS− stretching mode |
| 2575w | - | - | H2S symmetric stretching mode |
| 2710 | - | 2737 | S3●− overtone (5 × ν1) |
| 2964w | - | - | S3●− combination mode (5 × ν1 + ν2) |
| 3025 | - | - | O–H stretching vibrations |
| 3247w | - | - | S3●− overtone (6 × ν1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukanov, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Pekov, I.V.; Sapozhnikov, A.N.; Shcherbakov, V.D.; Varlamov, D.A. Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals. Minerals 2022, 12, 887. https://doi.org/10.3390/min12070887
Chukanov NV, Shendrik RY, Vigasina MF, Pekov IV, Sapozhnikov AN, Shcherbakov VD, Varlamov DA. Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals. Minerals. 2022; 12(7):887. https://doi.org/10.3390/min12070887
Chicago/Turabian StyleChukanov, Nikita V., Roman Yu. Shendrik, Marina F. Vigasina, Igor V. Pekov, Anatoly N. Sapozhnikov, Vasily D. Shcherbakov, and Dmitry A. Varlamov. 2022. "Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals" Minerals 12, no. 7: 887. https://doi.org/10.3390/min12070887
APA StyleChukanov, N. V., Shendrik, R. Y., Vigasina, M. F., Pekov, I. V., Sapozhnikov, A. N., Shcherbakov, V. D., & Varlamov, D. A. (2022). Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals. Minerals, 12(7), 887. https://doi.org/10.3390/min12070887







