A Natural Vanadate–Arsenate Isomorphous Series with Jeffbenite-Type Structure: New Fumarolic Minerals Udinaite, NaMg4(VO4)3, and Arsenudinaite, NaMg4(AsO4)3
Abstract
:1. Introduction
2. Occurrence and Mineral Associations
3. Methods
4. Results
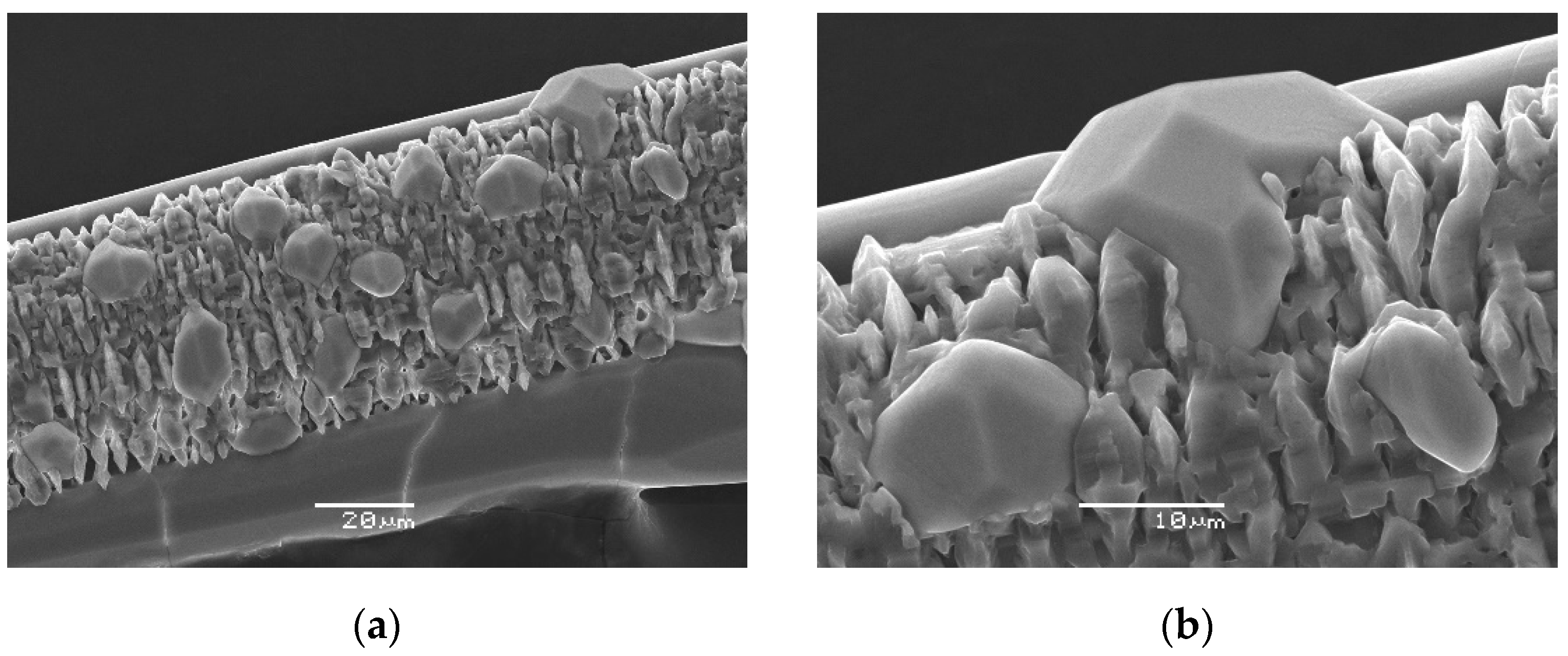
4.1. General Appearance and Physical Properties
4.2. Optical Data
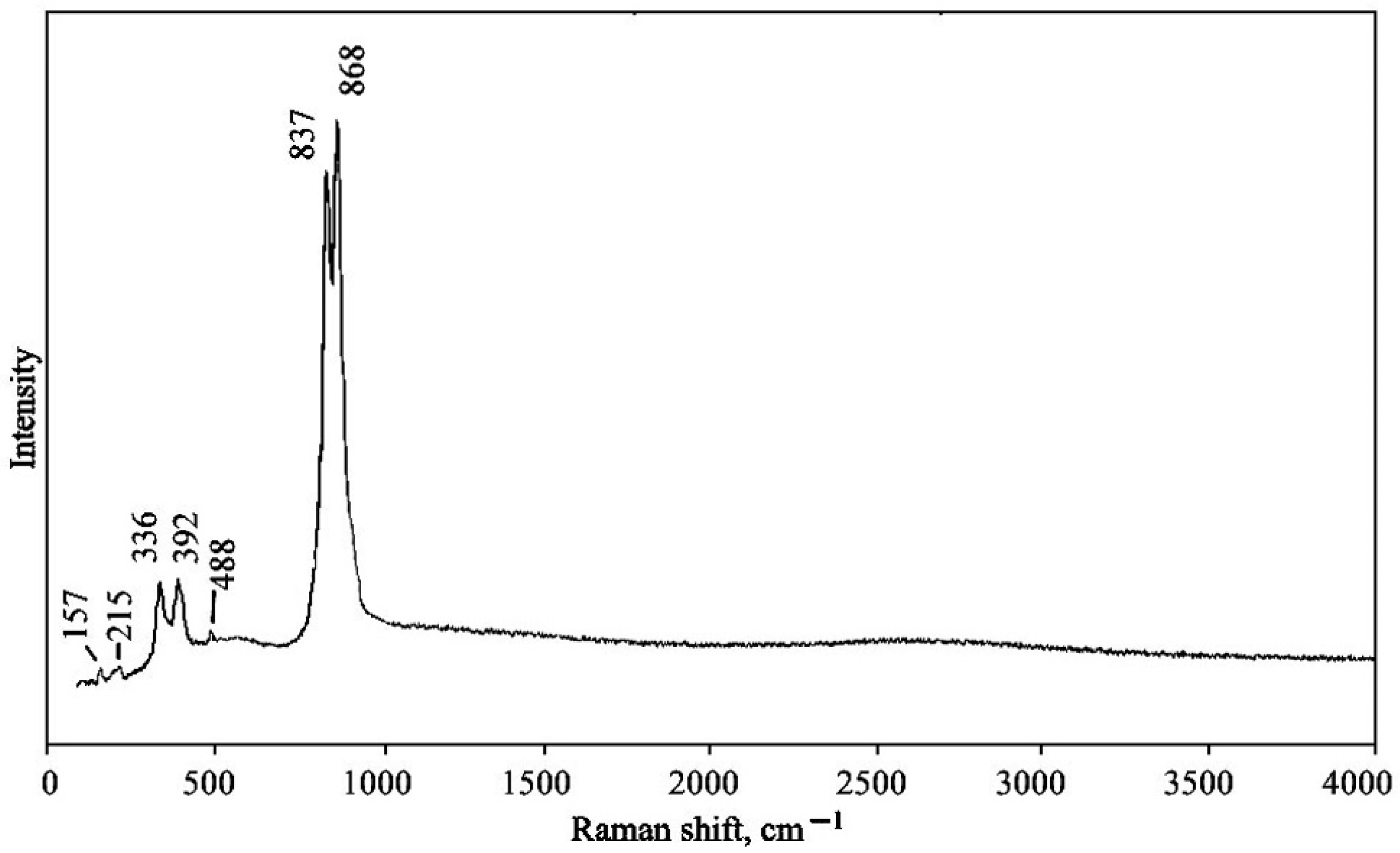
4.3. Raman Spectroscopy
4.4. Chemical Data
4.5. X-ray Crystallography and Crystal Structure
5. Discussion
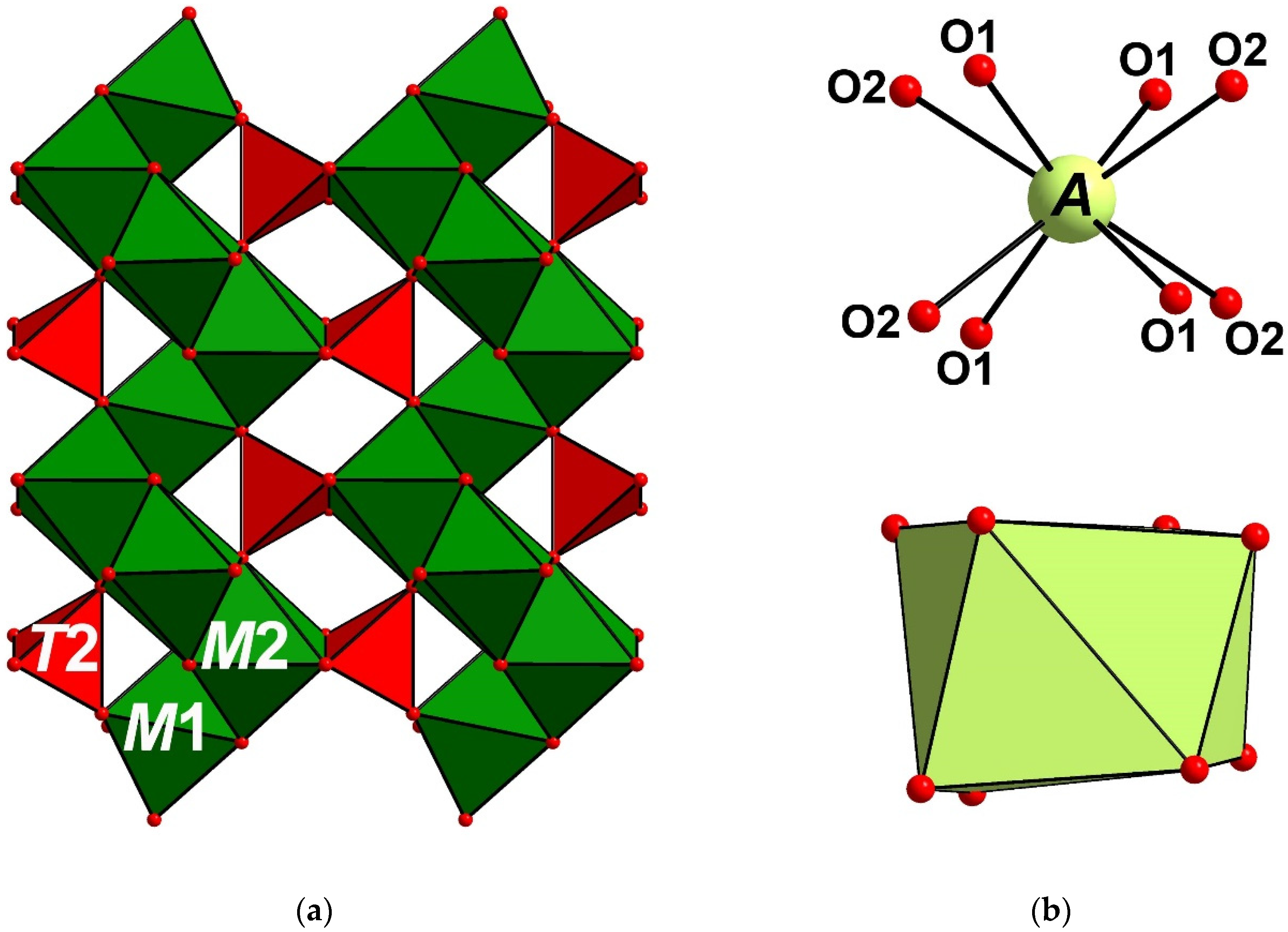
5.1. Structure Description
5.2. Comparative Crystal Chemistry
5.3. Notes on Genetic Features
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fedotov, S.A.; Markhinin, Y.K. (Eds.) The Great Tolbachik Fissure Eruption; Cambridge University Press: New York, NY, USA, 1983. [Google Scholar]
- Pekov, I.V.; Koshlyakova, N.N.; Zubkova, N.V.; Lykova, I.S.; Britvin, S.N.; Yapaskurt, V.O.; Agakhanov, A.A.; Shchipalkina, N.V.; Turchkova, A.G.; Sidorov, E.G. Fumarolic Arsenates—A Special Type of Arsenic Mineralization. Eur. J. Mineral. 2018, 30, 305–322. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Britvin, S.N.; Zubkova, N.V.; Varlamov, D.A.; Sidorov, E.G. Unusual Silicate Mineralization in Fumarolic Sublimates of the Tolbachik Volcano, Kamchatka, Russia—Part 1: Neso-, Cyclo-, Ino- And Phyllosilicates. Eur. J. Mineral. 2020, 32, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for Processing the X-Ray Powder Diffraction Data Obtained from the Curved Image Plate Detector of Rigaku RAXIS Rapid II Diffractometer. Zap. Ross. Mineral. Obs. 2017, 146, 104–107. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murashova, E.V.; Velikodnyi, Y.A.; Trunov, V.K. Crystal Structure of NaMg4(VO4)3. J. Struct. Chem. 1988, 29, 648–650. [Google Scholar] [CrossRef]
- Anissa, H.A.; Brahim, A.; Amor, H. NaMg4(AsO4)3. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, E60, i77–i79. [Google Scholar] [CrossRef]
- Krishnamachari, N.; Calvo, C. Magnesium Arsenate, Mg3As2O8. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, B29, 2611–2613. [Google Scholar] [CrossRef]
- Gopal, R.; Rutherford, J.S.; Robertson, B.E. Closest Packing in Dense Oxides: The Structure of a Polymorph of Co3(AsO4)2. J. Solid State Chem. 1980, 32, 29–40. [Google Scholar] [CrossRef]
- Barbier, J. IUCr Tetragonal Ni4.35As3O11.7(OH)0.3. Acta Crystallogr. Sect. C 1999, C55, IUC9900080. [Google Scholar] [CrossRef]
- Levy, D.; Barbier, J. VIII(Mg,Fe)0.85VI(Mg,Fe)4IV(Fe,Ge)3O12: A New Tetragonal Phase and Its Comparison with Garnet. Am. Mineral. 2000, 85, 1053–1060. [Google Scholar] [CrossRef]
- Clemens, O.; Haberkorn, R.; Beck, H.P. New Phases in the System LiMnVO4Mn3(VO4)2. J. Solid State Chem. 2011, 184, 2640–2647. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Fleischer, M.; Wilcox, R.E.; Matzko, J.J. Microscopic Determination of Nonopaque Minerals; US Geological Survey Bulletin: Washington, DC, USA, 1984. [Google Scholar]
- Modaressi, A.; Gerardin, R.; Malaman, B.; Gleitzer, C. Structure et Propriétés d’un Germanate de Fer de Valence Mixte Fe4Ge2O9. Etude Succincte de GexFe3−xO4 (x ≤ 0.5). J. Solid State Chem. 1984, 53, 22–34. [Google Scholar] [CrossRef]
- Tyutyunnik, A.P.; Zubkov, V.G.; Surat, L.L.; Slobodin, B.V. Synthesis and Crystal Structure of LiMg4(VO4)3. Russ. J. Inorg. Chem. 2004, 49, 553–559. [Google Scholar]
- Clemens, O.; Haberkorn, R.; Springborg, M.; Beck, H.P. Neutron and X-ray Diffraction Studies on the High Temperature Phase of Mn3(VO4)2, the New Isostructural Compound NaMn4(VO4)3 and Their Mixed Crystals NaxMn4.5−x/2(VO4) 3 (0≤ x≤ 1). J. Solid State Chem. 2012, 194, 409–415. [Google Scholar] [CrossRef]
- Ben Yahia, H.; Gaudin, E.; Rodewald, U.C.; Pottgen, R. Synthesis and Structure of the Ternary Vanadate NaMn4(VO4)3. Zeitschrift Naturforsch. Sect. B J. Chem. Sci. 2011, 66, 437–440. [Google Scholar] [CrossRef]
- Harris, J.; Hutchison, M.T.; Hursthouse, M.; Light, M.; Harte, B. A New Tetragonal Silicate Mineral Occurring as Inclusions in Lower-Mantle Diamonds. Nature 1997, 387, 486–488. [Google Scholar] [CrossRef]
- Finger, L.W.; Conrad, P.G. The Crystal Structure of “Tetragonal Almandine-Pyrope Phase” (TAPP): A Reexamination. Am. Mineral. 2000, 85, 1804–1807. [Google Scholar] [CrossRef]
- Nestola, F.; Burnham, A.D.; Peruzzo, L.; Tauro, L.; Alvaro, M.; Walter, M.J.; Gunter, M.; Anzolini, C.; Kohn, S.C. Tetragonal Almandine-Pyrope Phase, TAPP: Finally a Name for It, the New Mineral Jeffbenite. Mineral. Mag. 2016, 80, 1219–1232. [Google Scholar] [CrossRef] [Green Version]
- Kampf, A.R.; Nash, B.P.; Plášil, J.; Smith, J.B.; Feinglos, M.N. Niasite and Johanngeorgenstadtite, Ni2+4.5(AsO4)3 Dimorphs from Johanngeorgenstadt, Germany. Eur. J. Mineral. 2020, 32, 373–385. [Google Scholar] [CrossRef]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. Nomenclature of the Garnet Supergroup. Am. Mineral. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Hatert, F. A New Nomenclature Scheme for the Alluaudite Supergroup. Eur. J. Mineral. 2019, 31, 807–822. [Google Scholar] [CrossRef]
- Koshlyakova, N.N.; Pekov, I.V.; Zubkova, N.V.; Agakhanov, A.A.; Turchkova, A.G.; Kartashov, P.M.; Sidorov, E.G.; Pushcharovsky, D.Y. A New Solid Solution with Garnet Structure: Berzeliite–Schäferite Isomorphic Series from the Fumarole Exhalation of the Tolbachik Volcano, Kamchatka. Geol. Ore Depos. 2021, 63, 857–868. [Google Scholar] [CrossRef]
- Pekov, I.V.; Koshlyakova, N.N.; Agakhanov, A.A.; Zubkova, N.V.; Belakovskiy, D.I.; Vigasina, M.F.; Turchkova, A.G.; Sidorov, E.G.; Pushcharovsky, D.Y. New Arsenate Minerals from the Arsenatnaya Fumarole, Tolbachik Volcano, Kamchatka, Russia. XV. Calciojohillerite, NaCaMgMg2(AsO4)3, a Member of the Alluaudite Group. Mineral. Mag. 2021, 85, 215–223. [Google Scholar] [CrossRef]
- Pekov, I.V.; Koshlyakova, N.N.; Belakovskiy, D.I.; Vigasina, M.F.; Zubkova, N.V.; Agakhanov, A.A.; Britvin, S.N.; Sidorov, E.G.; Pushcharovsky, D.Y. New Arsenate Minerals from the Arsenatnaya Fumarole, Tolbachik Volcano, Kamchatka, Russia. XVII. Paraberzeliite, NaCaCaMg2(AsO4)3, an Alluaudite-Group Member Dimorphous with Berzeliite. Mineral. Mag. 2022, 86, 103–111. [Google Scholar] [CrossRef]
- Symonds, R.B.; Reed, M.H. Calculation of Multicomponent Chemical Equilibria in Gas-Solid-Liquid Systems; Calculation Methods, Thermochemical Data, and Applications to Studies of High-Temperature Volcanic Gases with Examples from Mount St. Helens. Am. J. Sci. 1993, 293, 758–864. [Google Scholar] [CrossRef]





| Mineral | Udinaite | Arsenudinaite |
|---|---|---|
| Ideal, end-member formula | NaMg4(VO4)3 | NaMg4(AsO4)3 |
| Crystal system Space group | Tetragonal I-42d | |
| a, Å c, Å V, Å3 Z | 6.8011 (2) 19.1839 (12) 887.35 (7) 4 | 6.8022 (1) 19.1843 (6) 887.66 (4) 4 |
| Dcalc., g cm−3 | 3.613 | 3.816 |
| Strongest reflections of the powder X-ray diffraction pattern:d, Å—I | 4.654–19 4.294–22 3.340–28 3.003–48 2.774–100 2.747–17 2.663–16 1.699–26 | 4.657–26 4.300–24 3.341–29 3.007–46 2.775–100 2.750–17 2.663–17 1.698–27 |
| Optical data ω ε | Uniaxial (–) 1.785 (8) 1.830 (6) | Uniaxial (–) 1.777 (10) 1.820 (6) |
| No. | 1 * | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 * | 11 | 12 | 13 * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | Udn (ht) | Udn | Udn | Udn | Udn | AsUdn | AsUdn | AsUdn | AsUdn | AsUdn (ht) | AsUdn | AsUdn | AsUdn |
| wt. % | |||||||||||||
| Na2O | 3.51 (3.37–3.63) | 3.63 | 3.00 | 3.57 | 4.08 | 3.67 | 3.63 | 2.86 | 3.59 | 3.43 (3.18–3.89) | 3.18 | 3.04 | 6.11 |
| K2O | 0.01 | 0.01 | 0.01 | ||||||||||
| CaO | 1.8 (1.69–1.94) | 1.76 | 1.62 | 1.54 | 2.27 | 1.45 | 1.53 | 1.62 | 1.04 | 1.41 (0.93–2.40) | 2.40 | 0.98 | |
| SrO | 0.35 | 0.24 | 0.05 | ||||||||||
| PbO | 0.14 (0.00–0.36) | ||||||||||||
| MgO | 33.54 (32.51–34.16) | 32.51 | 33.48 | 32.84 | 33.12 | 32.52 | 31.82 | 31.40 | 31.17 | 31.48 (30.61–32.56) | 31.40 | 30.61 | 30.04 |
| MnO | 0.29 (0.25–0.37) | 0.25 | 0.26 | 0.20 | 0.42 | 0.21 | 0.16 | 0.10 | 0.26 | 0.17 (0.10–0.26) | 0.17 | 0.14 | |
| CuO | 0.03 (0.00–0.08) | 0.08 | 0.05 | 0.10 | 0.15 | 0.09 | 0.05 | 0.03 | 0.03 (0.00–0.06) | 0.01 | |||
| ZnO | 0.01 | 0.02 | |||||||||||
| Fe2O3 | 0.21 (0.18–0.27) | 0.20 | 0.10 | 0.13 | 0.31 | 0.10 | 0.21 | 0.04 | 0.23 | 0.09 (0.00–0.23) | 0.04 | ||
| SiO2 | 0.38 (0.22–0.65) | 0.22 | 0.14 | 0.24 | 0.95 | 0.26 | 0.07 | 0.09 | 0.06 | 0.1 (0.06–0.21) | 0.07 | 0.21 | |
| P2O5 | 4.11 (3.59–4.82) | 4.08 | 2.75 | 3.42 | 5.38 | 3.70 | 1.83 | 0.47 | 2.20 | 1.33 (0.22–2.33) | 2.03 | 0.22 | 1.10 |
| V2O5 | 30.51 (29.25–31.14) | 30.13 | 28.12 | 25.80 | 24.11 | 22.37 | 22.26 | 18.83 | 15.01 | 14.82 (11.47–18.83) | 13.01 | 12.56 | 1.55 |
| As2O5 | 24.75 (24.07–25.60) | 25.60 | 31.41 | 31.74 | 28.22 | 35.00 | 36.95 | 44.06 | 45.26 | 46.34 (43.67–50.61) | 46.50 | 50.61 | 60.53 |
| SO3 | 0.22 (0.21–0.24) | 0.22 | 0.03 | 0.18 | 0.16 | 0.14 | 0.16 | 0.18 | 0.07 | 0.14 (0.07–0.28) | 0.09 | 0.13 | |
| Total | 99.49 | 99.03 | 100.97 | 100.0 | 99.20 | 99.56 | 98.62 | 99.71 | 98.92 | 99.34 | 98.85 | 98.56 | 99.33 |
| Formula calculated on the basis of 12 O atoms per formula unit | |||||||||||||
| Na | 0.55 | 0.57 | 0.47 | 0.57 | 0.65 | 0.59 | 0.59 | 0.47 | 0.59 | 0.57 | 0.53 | 0.51 | 1.05 |
| Ca | 0.16 | 0.15 | 0.14 | 0.13 | 0.20 | 0.13 | 0.14 | 0.15 | 0.09 | 0.13 | 0.22 | 0.09 | |
| Sr | 0.02 | 0.01 | 0.02 | ||||||||||
| Mg | 4.04 | 3.95 | 4.03 | 4.00 | 4.03 | 4.00 | 4.00 | 3.97 | 3.97 | 4.01 | 4.03 | 3.98 | 3.99 |
| Mn | 0.02 | 0.02 | 0.02 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | |
| Cu | - | 0.01 | - | 0.01 | 0.01 | 0.01 | - | - | - | - | |||
| Fe | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | - | 0.01 | 0.01 | - | ||
| Si | 0.03 | 0.02 | 0.01 | 0.02 | 0.08 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | |
| P | 0.28 | 0.28 | 0.19 | 0.24 | 0.37 | 0.26 | 0.13 | 0.03 | 0.16 | 0.10 | 0.15 | 0.02 | 0.08 |
| V | 1.63 | 1.62 | 1.50 | 1.39 | 1.30 | 1.22 | 1.24 | 1.05 | 0.85 | 0.84 | 0.74 | 0.72 | 0.09 |
| As | 1.05 | 1.09 | 1.33 | 1.36 | 1.20 | 1.51 | 1.63 | 1.95 | 2.02 | 2.07 | 2.09 | 2.31 | 2.82 |
| S | 0.01 | 0.01 | - | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | - | 0.01 | 0.01 | 0.01 | |
| ΣA | I | 0.74 | 0.61 | 0.71 | 0.85 | 0.74 | 0.73 | 0.62 | 0.68 | 0.70 | 0.75 | 0.60 | 1.05 |
| ΣM | 4.07 | 3.99 | 4.06 | 4.03 | 4.09 | 4.03 | 4.02 | 3.98 | 4.00 | 4.03 | 4.04 | 3.99 | 3.99 |
| ΣT | 3.00 | 3.02 | 3.03 | 3.02 | 2.96 | 3.02 | 3.02 | 3.05 | 3.04 | 3.03 | 3.00 | 3.08 | 2.99 |
| Udinaite | Arsenudinaite | hkl | ||||||
|---|---|---|---|---|---|---|---|---|
| Imeas | dmeas | Icalc * | dcalc ** | Imeas | dmeas | Icalc * | dcalc ** | |
| 1 | 6.406 | 1 | 6.410 | 1 | 6.441 | 101 | ||
| 7 | 4.792 | 5 | 4.796 | 9 | 4.792 | 5 | 4.796 | 004 |
| 19 | 4.654 | 16 | 4.659 | 26 | 4.657 | 16 | 4.659 | 103 |
| 22 | 4.294 | 15 | 4.299 | 24 | 4.300 | 17 | 4.300 | 112 |
| 28 | 3.340 | 23 | 3.342 | 29 | 3.341 | 23 | 3.342 | 105 |
| 7 | 3.204 | 6 | 3.205 | 9 | 3.208 | 6 | 3.206 | 202 |
| 48 | 3.003 | 40 | 3.004 | 46 | 3.007 | 40 | 3.005 | 211 |
| 100 | 2.774 | 100 | 2.774 | 100 | 2.775 | 100 | 2.774 | 204 |
| 17 | 2.747 | 14 | 2.747 | 17 | 2.750 | 14 | 2.747 | 213 |
| 16 | 2.663 | 14 | 2.663 | 17 | 2.663 | 14 | 2.663 | 116 |
| 10 | 2.400 | 3 9 | 2.405 2.398 | 8 | 2.399 | 4 9 | 2.405 2.398 | 220 008 |
| 2 | 2.383 | 4 | 2.387 | 2 | 2.384 | 215 | ||
| 11 | 2.328 | 8 | 2.329 | 12 | 2.330 | 7 | 2.330 | 206 |
| 3 | 2.037 | 3 | 2.036 | 4 | 2.037 | 3 | 2.036 | 217 |
| 3 | 1.962 | 3 | 1.962 | 3 | 1.963 | 3 | 1.963 | 314 |
| 10 | 1.878 | 7 | 1.877 | 5 | 1.874 | 7 | 1.878 | 321 |
| 5 | 1.810 | 4 | 1.809 | 4 | 1.811 | 4 | 1.809 | 323 |
| 4 | 1.784 | 3 2 | 1.785 1.782 | 4 | 1.784 | 3 2 | 1.785 1.782 | 316 1.1.10 |
| 9 | 1.749 | 2 8 | 1.747 1.746 | 8 | 1.749 | 2 8 | 1.747 1.746 | 307 219 |
| 26 | 1.699 | 15 23 13 | 1.700 1.698 1.693 | 27 | 1.698 | 15 23 13 | 1.701 1.698 1.693 | 400 228 325 |
| 1 | 1.675 | 2 | 1.674 | 2 | 1.676 | 2 | 1.674 | 402 |
| 6 | 1.599 | 3 1 2 | 1.601 1.599 1.597 | 6 | 1.599 | 3 1 2 | 1.601 1.599 1.597 | 318 0.0.12 413 |
| 9 | 1.555 | 6 6 | 1.554 1.553 | 8 | 1.555 | 6 6 | 1.554 1.553 | 327 309 |
| 7 | 1.524 | 3 | 1.521 | 7 | 1.525 | 3 | 1.521 | 420 |
| 9 | 1.517 | 7 | 1.515 | 8 | 1.517 | 7 | 1.516 | 415 |
| 4 | 1.503 | 2 2 | 1.502 1.501 | 4 | 1.503 | 2 3 | 1.502 1.501 | 422 406 |
| 10 | 1.450 | 10 9 | 1.450 1.447 | 10 | 1.449 | 10 10 | 1.450 1.447 | 424 2.0.12 |
| 5 | 1.433 | 3 3 | 1.433 1.432 | 5 | 1.434 | 4 3 | 1.433 1.432 | 336 3.1.10 |
| 2 | 1.389 | 4 | 1.387 | 2 | 1.388 | 4 | 1.387 | 408 |
| 3 | 1.375 | 5 | 1.373 | 3 | 1.375 | 5 | 1.374 | 426 |
| 3 | 1.358 | 3 | 1.357 | 2 | 1.359 | 4 | 1.357 | 501 |
| 3 | 1.334 | 4 | 1.334 | 2 | 1.334 | 4 | 1.334 | 510 |
| Sample | (1) Holotype Udinaite | (2) Holotype Arsenudinaite | (3) As-Richest Arsenudinaite |
|---|---|---|---|
| Crystal chemical formula | (Na0.70Ca0.15)∑0.85 | (Na0.70Ca0.15)∑0.85 | Na |
| Mg4 | Mg4 | Mg4 | |
| (V1.35As1.33P0.32)∑3.00O12 | (As1.64V0.96P0.40)∑3.00O12 | (As2.76V0.12P0.12)∑3.00O12 | |
| Formula weight | 489.67 | 495.38 | 528.84 |
| Crystal system, space group, Z | Tetragonal, I-42d, 4 | ||
| a, Å | 6.8011 (2) | 6.8022 (1) | 6.79122(12) |
| c, Å | 19.1839 (12) | 19.1843 (6) | 19.1590(6) |
| V, Å3 | 887.35 (7) | 887.66 (4) | 883.63(4) |
| F(000) | 938 | 947 | 1003 |
| µ (mm−1) | 6.923 | 7.684 | 10.94 |
| Absorption correction | gaussian | ||
| Crystal dimensions (mm) | 0.06 × 0.08 × 0.10 | 0.08 × 0.13 × 0.16 | 0.15 × 0.21 × 0.32 |
| Diffractometer | Xcalibur S CCD | ||
| Temperature (K) | 293 | ||
| Radiation | MoKα, λ = 0.71073 Å | ||
| θ range (°) | 3.18–32.13 | 3.18–28.28 | 4.25–29.92 |
| Range of h, k, l | −9 → 9, −9 → 9, −25 → 25 | −9 → 9, −9 → 9, −25 → 25 | −9 → 9, −9 → 9, −25 → 25 |
| Numbers of measured, independent and observed [I > 2σ(I)] reflections | 7155, 552, 544 | 6977, 557, 554 | 6193, 546, 543 |
| Rint | 0.0815 | 0.0359 | 0.0469 |
| Structure solution | direct methods | ||
| Refinement on | F2 | ||
| R1 and wR2 for I > 2σ(I) | 0.0287, 0.0614 | 0.0119, 0.0348 | 0.0151, 0.0430 |
| R1 and wR2 for all data | 0.0295, 0.0617 | 0.0118, 0.0348 | 0.0152, 0.0430 |
| Number of parameters refined | 49 | 49 | 47 |
| Δρmax, Δρmin (e Å−3) | 0.545, −0.713 | 0.217, −0.510 | 0.267, −0.443 |
| GooF | 1.160 | 1.281 | 1.209 |
| Weighting scheme | w = 1/[σ2(Fo2) + (0.0250P)2 + 2.2343P] | w = 1/[σ2(Fo2) + (0.0153P)2 + 0.8144P] | w = 1/[σ2(Fo2) + (0.0175P)2 + 2.2732P] |
| p = ([max of (0 or Fo2)] + 2Fc2)/3 | |||
| Site | № | x | y | z | Ueq | s.o.f. | Q |
|---|---|---|---|---|---|---|---|
| T(1) | (1) | 0 | 0 | 0 | 0.0078(2) | As0.60V0.28P′0.12 | 4 |
| (2) | 0.00643(12) | As0.72V0.12P′0.16 | |||||
| (3) | 0.0046(2) | As0.92V0.04P′0.04 | |||||
| T(2) | (1) | 0.34580(12) | 0.25 | 0.125 | 0.00720(19) | V0.57As0.35P′0.08 | 8 |
| (2) | 0.34557(6) | 0.00550(10) | As0.46V0.42P′0.12 | ||||
| (3) | 0.34481(10) | 0.00697(17) | As0.92V0.04P′0.04 | ||||
| M(1) | (1) | 0 | 0.5 | 0.02167(10) | 0.0117(4) | Mg1.00 | 8 |
| (2) | 0.02167(5) | 0.0102(2) | |||||
| (3) | 0.02064(10) | 0.0066(4) | |||||
| M(2) | (1) | 0.7598(3) | 0.25 | 0.125 | 0.0114(4) | Mg1.00 | 8 |
| (2) | 0.75977(17) | 0.0090(2) | |||||
| (3) | 0.7591(3) | 0.0072(4) | |||||
| A | (1) | 0 | 0 | 0.5 | 0.0172(8) | Na0.70Ca0.15□0.15 | 4 |
| (2) | 0.0163(4) | Na0.70Ca0.15□0.15 | |||||
| (3) | 0.0126(8) | Na1.00 | |||||
| O(1) | (1) | 0.5056(5) | 0.2032(4) | 0.05940(14) | 0.0118(6) | O1.00 | 16 |
| (2) | 0.5060(3) | 0.2033(2) | 0.05939(8) | 0.0114(3) | |||
| (3) | 0.5058(5) | 0.2055(5) | 0.05960(15) | 0.0085(7) | |||
| O(2) | (1) | 0.2250(5) | 0.4544(4) | 0.09941(15) | 0.0142(7) | O1.00 | 16 |
| (2) | 0.2247(3) | 0.4544(2) | 0.09947(8) | 0.0123(3) | |||
| (3) | 0.2234(5) | 0.4529(4) | 0.10007(15) | 0.0090(6) | |||
| O(3) | (1) | 0.9439(4) | 0.2034(4) | 0.04413(15) | 0.0119(7) | O1.00 | 16 |
| (2) | 0.9440(2) | 0.2047(2) | 0.04406(8) | 0.0114(3) | |||
| (3) | 0.9418(5) | 0.2054(5) | 0.04375(16) | 0.0082(6) |
| (1) Holotype Udinaite | (2) Holotype Arsenudinaite | (3) As-Richest Arsenudinaite | |
|---|---|---|---|
| T(1)–O(3) | 1.666(3) × 4 | 1.6727(16) × 4 | 1.6749(31) × 4 |
| <T(1)–O> | 1.666 | 1.673 | 1.675 |
| T(2)–O(2) | 1.688(3) × 2 | 1.6877(16) × 2 | 1.6753(30) × 2 |
| −O(1) | 1.693(3) × 2 | 1.6961(16) × 2 | 1.6901(31) × 2 |
| <T(2)–O> | 1.691 | 1.692 | 1.683 |
| M(1)–O(1) | 2.081(3) × 2 | 2.0814(17) × 2 | 2.0765(32) × 2 |
| −O(3) | 2.098(3) × 2 | 2.0894(17) × 2 | 2.0867(31) × 2 |
| −O(2) | 2.159(3) × 2 | 2.1588(17) × 2 | 2.1725(32) × 2 |
| <M(1)–O> | 2.113 | 2.110 | 2.112 |
| M(2)–O(3) | 2.018(3) × 2 | 2.0189(18) × 2 | 2.0136(34) × 2 |
| −O(2) | 2.072(3) × 2 | 2.0724(16) × 2 | 2.0770(30) × 2 |
| −O(1) | 2.162(4) × 2 | 2.1596(19) × 2 | 2.1495(38) × 2 |
| <M(2)–O> | 2.084 | 2.084 | 2.080 |
| A–O(1) | 2.318(3) × 4 | 2.3182(17) × 4 | 2.3035(31) × 4 |
| −O(2) | 2.689(3) × 4 | 2.6917(17) × 4 | 2.7032(32) × 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekov, I.V.; Koshlyakova, N.N.; Zubkova, N.V.; Belakovskiy, D.I.; Vigasina, M.F.; Agakhanov, A.A.; Ksenofontov, D.A.; Turchkova, A.G.; Britvin, S.N.; Sidorov, E.G.; et al. A Natural Vanadate–Arsenate Isomorphous Series with Jeffbenite-Type Structure: New Fumarolic Minerals Udinaite, NaMg4(VO4)3, and Arsenudinaite, NaMg4(AsO4)3. Minerals 2022, 12, 850. https://doi.org/10.3390/min12070850
Pekov IV, Koshlyakova NN, Zubkova NV, Belakovskiy DI, Vigasina MF, Agakhanov AA, Ksenofontov DA, Turchkova AG, Britvin SN, Sidorov EG, et al. A Natural Vanadate–Arsenate Isomorphous Series with Jeffbenite-Type Structure: New Fumarolic Minerals Udinaite, NaMg4(VO4)3, and Arsenudinaite, NaMg4(AsO4)3. Minerals. 2022; 12(7):850. https://doi.org/10.3390/min12070850
Chicago/Turabian StylePekov, Igor V., Natalia N. Koshlyakova, Natalia V. Zubkova, Dmitry I. Belakovskiy, Marina F. Vigasina, Atali A. Agakhanov, Dmitry A. Ksenofontov, Anna G. Turchkova, Sergey N. Britvin, Evgeny G. Sidorov, and et al. 2022. "A Natural Vanadate–Arsenate Isomorphous Series with Jeffbenite-Type Structure: New Fumarolic Minerals Udinaite, NaMg4(VO4)3, and Arsenudinaite, NaMg4(AsO4)3" Minerals 12, no. 7: 850. https://doi.org/10.3390/min12070850
APA StylePekov, I. V., Koshlyakova, N. N., Zubkova, N. V., Belakovskiy, D. I., Vigasina, M. F., Agakhanov, A. A., Ksenofontov, D. A., Turchkova, A. G., Britvin, S. N., Sidorov, E. G., & Pushcharovsky, D. Y. (2022). A Natural Vanadate–Arsenate Isomorphous Series with Jeffbenite-Type Structure: New Fumarolic Minerals Udinaite, NaMg4(VO4)3, and Arsenudinaite, NaMg4(AsO4)3. Minerals, 12(7), 850. https://doi.org/10.3390/min12070850








