Abstract
The properties of porous and lightweight ceramic foam that can be cured at room temperature using metakaolin-based geopolymers were studied. A geopolymer slurry was prepared using metakaolin and a potassium-based alkaline medium at room temperature, and the obtained viscous paste was expanded via gaseous methods, by means of the decomposition of peroxide at room temperature. Therefore, geopolymer (GP) foam developed in this study through multivariate geopolymer, foaming agents, and surfactants can be cured at room temperature (within 5 days) without a separate heat treatment process. The homogeneous micropores were obtained through the stabilization of the interface between geopolymer slurry and oxygen gas bubbles generated through the base-catalyzed decomposition of hydrogen peroxide. The porosity was confirmed to be 29% and 54% before and after using sodium dodecyl sulfate (SDS). The compressive strengths and densities were 1.57 MPa and 0.75 g/cm3 for GP foam without SDS, and 3.63 MPa and 0.48 g/cm3 for GP foam with SDS. Through the mercury intrusion porosimetry analysis, the pores were further refined from 100 µm to 30 µm when SDS was used, and at the same time, the variation of pore size was minimized, so that a relatively uniform pore size was maintained. In addition, the thermal conductivity is 0.0803 W/m·K and the pore size is 33.2 , which is smaller in pore diameter than the geopolymer containing only hydrogen peroxide. As a result, although the hydrogen peroxide alone sample has excellent thermal conductivity, the use of a surfactant is recommended for fine micropore size control. While reducing the non-uniform distribution of pores and the size of micropores generated through the direct foaming method as an inorganic binder, the possibility of an insulation finish was also confirmed by reducing the weight.
1. Introduction
In recent decades, geopolymers have attracted extensive attention owing to their fascinating properties such as excellent thermal stability, good early mechanical strength, good acid resistance, low permeability, and controllable kinetics of curing at relatively low production costs [1,2]. In general, geopolymer (hereinafter referred as GP) is an amorphous binder composed of aluminosilicate (such as fly ash, kaolin, metakaolin, and slag) that is formed through polycondensation by mixing with an alkaline solution. A geopolymer’s structure is made up of disordered connected Si tetrahedrons with part of their Si cations replaced by Al, and a charge imbalance caused by these cation substitutions is balanced by alkali metals contained in alkali activators. One of the most fascinating properties of geopolymer over traditional ceramic for structural material is that its production does not necessitate high-temperature sintering, resulting in lower carbon dioxide emissions.
Porous geopolymers are another potential source of interesting applications based on their hierarchical role. Geopolymer foams (highly porous materials) are a porous material with low shrinkage and excellent heat resistance after molding at room temperature based on mechanical and chemical stability [3,4]. Most of all, GP foam is more sustainable and eco-friendly than general Portland cement-based foam as it emits less carbon during processing. GP foams are used for photocatalytic degradation applications [5,6,7], membrane supports, catalyst supports and heavy metal adsorption [8,9], humidity control and sound absorption [10,11]; however, most traditional applications for porous ceramics is for the construction application, where it possesses high strength, sound-proof ability, and high thermal insulation [12]. In particular, if foamed cement is used in a building as thermal insulation materials (TIMs), it can improve insulation properties and increase air-conditioning efficiency [13]. It can be said that the control of porosity is the key to determining the desired properties for porous GPs. The porous ceramic GP is controlled by various parameters and determines properties such as pore size and distribution, mechanical strength, and thermal conductivity due to pore interconnection [14,15]. As porosity increases and the density decreases, the strength also decreases significantly. As the porosity and the average pore size (micro) increase, the strength and thermal conductivity decrease [16]. In general, an increase in porosity degrades the mechanical properties of all porous materials, and sufficient strength must be ensured for specific applications [5].
Traditional TIMs such as glass wools [17] are flammable (because it inevitably contains organic chemicals), and its function deteriorates even when it reaches 80 °C. These materials emit toxic gas when caught on fire, jeopardizing a firefighter’s health as well as the neighbors’ health. On the other hand, GP foam has high thermal stability and fire resistance with a porous structure, so it can be an excellent alternative to TIM [18,19,20]. One method to synthesize the foam is a gaseous method [21,22] using various blowing agents. Inorganic polymer foam GP is also a porous material that can be used for thermal insulation, which is made from a mixed solution with a foaming agent such as hydrogen peroxide, NaOCl, silica fume, or metal powder (Al) [23,24,25,26]. The thermal conductivity () of porous geopolymer mainly depends on the type of synthesis additives, where it is typically composed of a dispersant of polymer materials with = 1.0–1.2 W/(m·K) and geopolymer foam possessing air in the pores with = ~0.026 W/(m·K) [27]. Davidovits et al. [28] fabricated porous GP using hydrogen peroxide and sodium perborate as foaming agents, and achieved GP foam with a density of 0.2–0.8 g/cm3 with a maximum thermal resistance of 1200 °C, and a minimum of 0.037 W/(m·K) [29]. The key to improving the thermal conductivity coefficient is to increase the amount of blowing agent to decrease the density [23]. Considering the weight reduction, it can be widely used not only in the construction field but also in the overall industrial field. Therefore, it is necessary to develop a TIM with improved characteristics using GP.
Recently, N. Jiang et al. demonstrated that GP foam is successfully fabricated by using sodium dodecyl sulfate (SDS), which provided the surface activity, air bubble stability, and pore stability [30]. Herein, we have developed fast-curing GP foam derived by the gaseous method, followed by analysis on porosity, compressive strength, and thermal conductivity. Herein, the effect of the contents of a foaming agent (hydrogen peroxide, H2O2) and a surfactant (Sodium dodecyl sulfate (SDS), NaC12H25SO4) was conducted [31].
2. Experimental
2.1. Materials
For use as a thermal insulation building material, it is important to improve constructability through rapid hardening of ceramic foam. Therefore, the high-speed curing formulation developed in the previous study, a formulation composition capable of hardening (i.e., initial curing) within 10 min was used. This formulation is designed to balance the trade-off between curing speed and mechanical strength: Ca(OH)2 and n-SiO2 (i.e., fumed silica) are added to endow it with high strength in a short period of time.
Metakaolin (MK) was used as the aluminosilicate source, which can be achieved by calcinating commercially available kaolin chemical (H2Al2Si2O8∙H2O, DJ 5041-1400, Daejung Chemicals & Metals Co., Ltd., S. Korea) at 800 °C for 4 h [32]. Then, hydroxide and silicate containing K+ alkali ions were mixed in a 1: 0.75 wt% ratio and used as an alkali activator [33,34]. Additionally, Ca(OH)2, carbon fiber, and n-SiO2 were used to accelerate the curing speed and strengthen the GP material. They are added with 11.1, 0.498, and 2.91 wt% of metakaolin, respectively. Such composition with additives is developed in our previous study, where we optimized the design for a fast-curing GP with high mechanical properties, through the design of experiments method. In Supplementary Table S1, we briefly introduced the composition to make porous GP. In Figure 1a, the chemical composition is analysed by X-ray fluorescence (ZSX Primus 4, Rigaku Co., Ltd., Tokyo, Japan), and in Figure 1b, the X-ray diffraction pattern of the geopolymer is presented to compare crystallinity of MK and GP foam. As it can be seen in Figure 1a, the majority of the constituents of MK are found as Al2O3 and SiO2. In Figure 1b, XRD analysis of MK exhibits a typical non-crystalline structure (2θ = 18–35°), with minor but distinctive peaks for kaolinite and quartzite phases observed. MK powder consists of the crystalline phases of Kaolinite and Quartzile; some amorphous phases were observed, and had a low crystalline phase. After geopolymerization, the characteristics of the amorphous phase become clearer, whereas kaolinite and quartzite phases become more insignificant. The increase in the amorphous phase due to the polymerization reaction was increased with the mixing of additives. The crystalline peak seen in metakaolin became weaker and the amorphous peak increased. Therefore, it can be assumed that geopolymerization has been sufficiently performed.
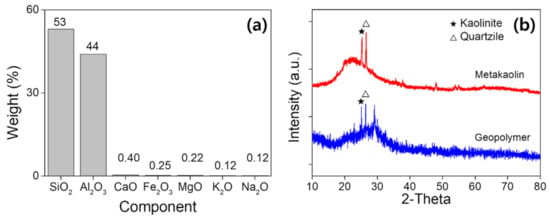
Figure 1.
(a) X-ray fluorescence component analysis of metakaolin powder. (b) XRD results of metakaolin and cured geopolymer.
2.2. Mix Proportions and Fabrication of Specimens
For the alkali activator solution, KOH pellets (KOH, DJ 6597-4405, Daejung Chemicals & Metals Co., Ltd., Siheung-si, Korea) and potassium silicate (K2SiO3, DJ 6617-4405, Daejung Chemicals & Metals Co., Ltd., Korea) were used. Because K+ has high reactivity with aluminosilicate materials [35], potassium-based activators were chosen to achieve a denser structure [36] and increased strength [37,38]. It was made by dissolving potassium silicate pellets in distilled water to 6M KOH solution, then adding potassium silicate solution. To enable fast curing, calcium hydroxide (Ca(OH)2, DJ 2511-4400, Daejung Chemicals & Metals Co., Ltd., Korea) was used. Chopped Cf (C118-3K, HD fiber Co., Ltd., Milyang-si, Korea) with a length of 4 ± 1 mm and f-SiO2 (Konasil, 112945-52-5, OCI Co., Ltd., Seoul, Korea) were used as structural additives.
In order to induce pores in the GP, we have chosen to use the direct foaming method in Figure 2, which employs blowing agents that generates gaseous bubbles to create crates. This method is the most direct, simple, and inexpensive way to create pores. Furthermore, it can be processed at room temperature. Along with the fast-curing formulation of GP, the rapid formability of GP foam at low temperature is a great advantage for construction applications. However, because the gas bubbles in wet foams are likely to undergo spontaneous drainage, constant Ostwald ripening, and coalescence in order to reduce the overall Gibbs free energy of the slurry-air system, direct foaming is a thermodynamically unstable process. Thus, large pores (hundreds of microns) and inhomogeneous pore size are commonly observed in the final GP foam body due to the instability of wet foams [39]. For this reason, stabilizing agents (SAs), such as surfactants [40] or particles that can be added to the slurry, are used to lower the air/slurry system’s surface tension and stabilize the wet foam by reducing Ostwald ripening and drainage [41]. Furthermore, their presence allows for more precise control of cell size and distribution, as well as the ratio of open to closed cells.
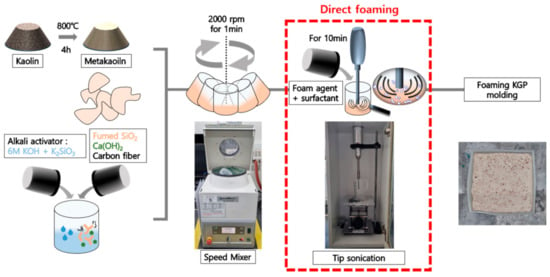
Figure 2.
Geopolymer foam manufacturing process.
In this study, we have used hydrogen peroxide (H2O2, 000H0297, Samchun Chemical Co., Ltd., Seoul, Korea) to derive the GP foam structure. Since the fast-GP curing formula includes various additives (i.e., nanoparticle, fiber), the performance of a blowing agent may not follow the guidance from previous studies; therefore, we have tested various blowing agents including propyl gallate, citric acid, metal particles, and hydrogen peroxide. From our preliminary test, we found the performance of hydrogen peroxide (hereafter denoted as H) in terms of foaming was superior to other candidates for the particular formulation of fast-curing GP used in this study (Supplementary Figure S1). Hydrogen peroxide decomposes naturally at a very slow rate to form water and oxygen gas (2H2O2 → 2H2O + O2(g)) and the oxygen gas enables the generation of crates while GP is being cured. For the surfactant, sodium dodecyl sulfate (SDS, NaC12H25SO4, 000D1069, Samchun Chemical Co., Ltd., Korea) was used, owing to its affordable cost for building materials. In Table 1, the mixture composition of materials for fast-curing GP, blowing agent, and surfactant are summarized.

Table 1.
Geopolymer formulation and foam agent mass ratio.
2.3. Testing Methods
Two terms are used to evaluate pore formation in the system: the porosity based on relative density, and mercury intrusion porosity (MIP). The porosity is defined as porosity (%) = (1 − (density of GP foam)/(density of control GP)) 100. The MIP is performed using mercury porosimetry (Autopore IV 9500 V1.10, Micromeritics instrument Corp., Norcross, GA, USA.), which is based on the intrusion of mercury into a porous structure under stringently controlled pressures.
The thermal conductivity is measured via hot-plate method (HC-074-300, Eko Instruments Co., Ltd., Tokyo, Japan), with the following equation: . Here, is the output value of the upper heat flux converter, is the output of the lower heat flux converter, the specimen thickness, and is the temperature difference between the specimen surfaces. The surface temperatures of the two transducers at the top and bottom are recorded at the end of the test, and the thermal conductivity value is calculated. It is shown in the schematic diagram of thermal conductivity test of Figure 3.
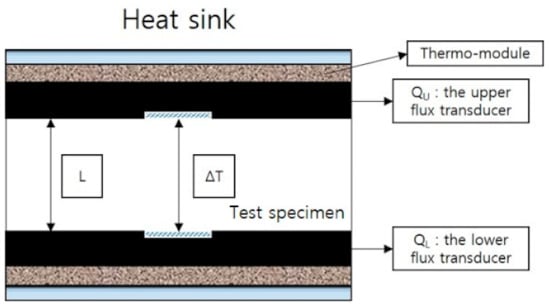
Figure 3.
A schematic of thermal conductivity measurement using hot-plate method.
The compressive test is carried out with a universal testing machine (UTM 5982, Instron Co., Ltd., Norwood, MA, USA). ASTM C39 was referenced for the specimen specification used in the test [42]. The compressive strength is calculated as , where is exerted load on the specimen, and is the radius of cylinder. Furthermore, we have carried out the non-combustible materials test to evaluate the inflammability of the GP foam, with Non-Comb 2005 (FESTEC Co., Ltd., Seoul, Korea), which is designed on the basis of ASTM E 136-19a standards [43].
In order to use it as a heat-resisting, a flame-retardant, or a fire-spread-prevention material following ASTM E2652-18 [44], the weight reduction at 750 °C must be 30% or less, and the internal residual temperature must rise 20 °C or less as the compliance standards for non-combustible materials.
3. Results
Figure 4 shows the visual appearance of the GP foams. For the porosity analysis, it was made in a 30 mm diameter mass cylinder (shrinkage error ± 1 mm) of a confined geometry. As shown in the figure, the size and the apparent volume of pores increase as the H content increases, regardless of whether SDS is used or not. When samples with SDS (Figure 4d–f)) are compared to ones without SDS (Figure 4a–c), the pore size significantly became smaller than when SDS was used.
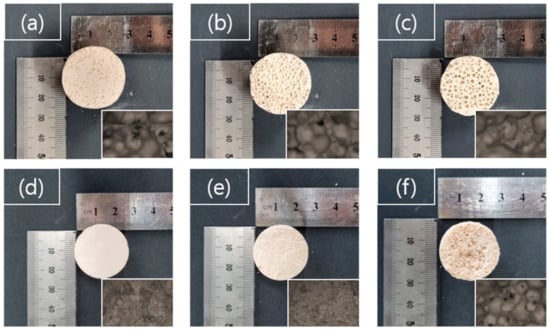
Figure 4.
Appearance of GP foams derived by (a) H-5%, (b) H-10%, (c) H-15% and (d) HS-5%, (e) HS-10%, (f) HS-15% optical microscope images.
The decomposition of H2O2 in liquid water emits O2 gas and its bubble generates pores while the GP cures. Therefore, the total pore volume increases with an increasing amount of generated O2 gas; in other words, increased H contents are in the range of 5% to 15%. The pores, which have a very irregular size distribution and often contain large sizes, are generally not trivial for controlling the uniformity; however, when a surfactant is added, it presumably supports the interface of a gas–solid (geopolymer) and endows a formation of a fine-pore structure. It can be precluded that the growth of pores via spontaneous drainage, constant Ostwald ripening, and coalescence had been impeded due to the influence of the surfactant. To support this idea, the change in the pore distribution of formed GPs was evaluated by the density and the mercury intrusion measurements in the following section.
Figure 5a illustrates densities for fabricated GP foams H and HS. Whereas the density of the fast-curing control GP is 1.05 g/cm3, the density is lowered by ~20%–30% for H and by ~45%–55% for HS, respectively. Such an observation is obvious, judging from the apparent pore formation as shown in Figure 3. For both H and HS, the density tends to decrease by approximately 15%–20% with varying foaming agent content from 5% to 15%. However, if 15% or more is added, we found the demolding is troublesome, and it is expected such formulation is impossible to be utilized due to the handling of the materials. When used with a surfactant, low-density GP foams can be achieved even with the same mass of additives. Thus, it is recommended to use them together to have an efficient porous structure. For H-10 and HS-10, Mercury intrusion porosimetry (MIPS) were found to be 33% and 44%, respectively. It should be noted that the HS possess much lower density than H, whereas the porosity is greater for H; one can conjecture that there are many finer pores than visible ones. Figure 5b quantifies the pore distribution presented in H and HS. The log differential intrusion is a quantity representing the amount of mercury impregnation per unit volume by converting the size of the pores into volume, and measures the degree of pores due to mercury under a constant pressure. Scanned data with continuously increasing pressure can determine the pore structure of the material over a pore size range from 360 μm to 3 nanometers. The H sample has a pore size distribution of 100 , and the HS sample has pores of 30 , which is consistent with our previous argument that there are finer pores presented when SDS is used. Such conclusion is also supported by the field emission scanning electron microscopy (JSM 7610F, JEOL, Japan) presented in Supplementary Figure S2.
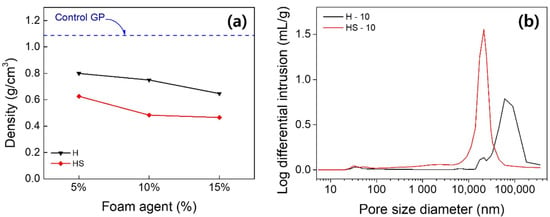
Figure 5.
(a) Density of GP foam with varying activator content, and (b) local differential intrusion of mercury (i.e., net density of mercury) for pore sizes of H-10 and HS-10 (H + SDS).
In Figure 6, the compressive strengths of GP foams are evaluated. The compressive strength of control GP was as high as 24.1 MPa, but the compressive strength after foaming decreased sharply. When H is used alone, it shows a low value of 1.8 MPa at the 5% foaming agent, and the strength becomes weaker as the addition amount increases, ultimately reaching negligible strength at the 15% base point. On the other hand, for the HS cases, the compressive strength was 3 to 4 MPa for 5 to 10% of the foaming agents, respectively. When the content was 15% or more, the strength became equally negligible as H case. The ratio was effective in the 5–10% interval, and no effect of the surfactant was shown in the 15% sample with excessive hydrogen peroxide added. Given that the GP foams with over 15% have poor formability as discussed earlier, the 15% content can be concluded to be the upper bound of viable contents for GP foams. Speaking of the influence of surfactant, the intensity of the sample with the SDS was two-folds greater for 5 to 10% of the foaming agents. The 10% addition showed the greatest strength for additive contents of 5 to 15%. The H-10 GP foam had compressive strengths and densities of 1.57 MPa and 0.75 g/cm3, whereas HS-10 GP foam had 3.63 MPa and 0.48 g/cm3 for GP foam with SDS. It can be understood that the strength is relatively high as the porous GP with the stable pores and closed porous structure is made in balance with the SDS addition amount at the 10% addition condition.
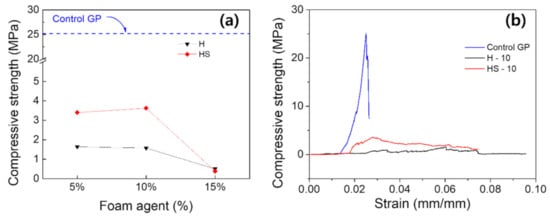
Figure 6.
(a) The compressive strength as a function of the content of foaming agents with or without the presence of surfactant (H and HS), and (b) as shown below, stress–strain curve for Control GP, H-10, HS-10.
In a view of the microstructure, in other words the pore distributions, it is expected the strength logarithmically decreases as the density decreases, because the percolating porous network will severely deteriorate the capability to sustain exerted stress. In previous discussions, it was found that the pore size was reduced when surfactant was used. Even with the lower density (i.e., higher number density of pores) for the GP foam derived with surfactant, the compressive strength was measured as significantly higher as they construct far less percolation network by pores. Our analysis suggests that the 10% foaming agent provides favorable pore distribution to maintain high compressive strength for both H and HS cases, thus 10% was set as a standard for comparison in further analyses.
To evaluate the applicability of GP foam as an insulating construction material, these H and HS are evaluated for thermal conductivity and a non-combustible test, as shown in Figure 7a. The control GP, a reference structure, exhibited thermal conductivity of 0.2651 W/m·K. For H-10; the thermal conductivity was as low as 0.0654 W/m·K due to the greater pore size and presumably percolating pores. For HS-10, it slightly increased to 0.0803 W/m·K. It can be inferred that higher thermal conductivity of HS-10 owes to the homogeneously distributed micropores, forcing the specimen to transfer heat via matter (i.e., geopolymer), whereas H-10 with percolating pores allows heat dissipation through the air trapped in the porous network. The difference in thermal conductivity between control GP and GP foams can be explained in the same manner. Figure 7b shows the nonflammability test according to ISO 1182 [45] of the developed GP foam. For fire-spread prevention structures, the requirements at 750 °C are: (i) weight loss of 30% or less and (ii) temperature rise of internal core of 20 °C or less. The (i) weight losses were found to be 2.26 and 0.42% and (ii) the temperature rises were 0.1 and 0.0 °C, for H and HS, respectively. GP foam, an inorganic material, is expected to have a low mass reduction rate due to its porous structure in both H and HS. This would have reduced the area in contact with actual heat due to the pore structure, which led to a low core temperature improvement. As with these factors, the rising temperature was also insignificant.
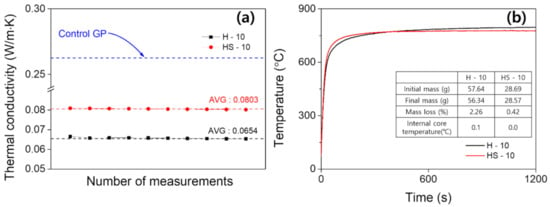
Figure 7.
(a) Thermal conductivity of H, HS samples, and (b) time-dependent temperature change of the materials in non-combustible test according to ISO 1182.
4. Discussion
Herein, we compare the thermos-physical properties of developed GP foam to those from previous studies in the literature. In Table 2, the fundamental properties of GP foams as an insulating construction material are compared. It should be noted that some of the literature properties in Table 2 are reproduced based on articles, including the porosity, which is defined as porosity (%) = (1 − (density of GP foam)/(density of control GP)) 100. The diameter of the pore is set to be the most frequent diameter that has been measured.

Table 2.
Comparative summary of thermo-physical and mechanical properties of GP foams.
It is clear that when the porosity increases, it is common for the density to decrease. In this work, the porosity through the apparent density of HS-10 is 54%. However, the difference in the porosity (44%) measured by the mercury intrusion porosimetry is expected to be related to the pore shape. The HS-10 samples are found to have reduced the pore size and pores which cannot be accessed by mercury intrusion. Therefore, although the apparent porosity of the HS-10 has increased, the porosity measure by MIP appears to have decreased due to the closed pores, and the density has also decreased, so the actual pore rate is expected to be more than 54%. The higher the porosity with closed pore is, the lower the thermal conductivity is expected.
Comparing with other geopolymer properties in Table 2, the density of the developed GP foam (HS) is 0.48 g/cm3, and the density is equivalent or relatively high compared to the case where metakaolin or fly-ash is made without surfactant. Analogously, the compressive strength is at a similar level compared to the same density level (0.5 g/cm3). For the cases where surfactant is used, the density is higher than when it is not used, and the thermal conductivity is also observed to be greater. The thermal conductivity of GP foam in this study, 0.08030 W/m·K, is quite low compared to other studies with surfactant, which makes it as a good candidate for insulation material (the range of thermal conductivity used as a general insulator is 1.7–2.5 W/m·K, and if the thermal conductivity of GP foam is sufficient, it can be used [51]). Although our sample has a high density, it exhibits relatively low thermal conductivity compared to a similar density with surfactant [46]. This is consistent with our previous analysis that despite having the same pore volume, the pores are closed, smaller, and more homogeneous, resulting in low thermal conductivity. Therefore, our GP foam has low thermal conductivity compared to the same density and complies with compressive strength, so its usability as an insulating material is promising.
It should be noted that this is not the first study revealing GP foam as an excellent property for insulating material. For instance, Duan et al. [55] reported GP foam derived from a polystyrene foaming agent resulted in a lower density, adequate strength, and even lower thermal conductivity than the GP foam in this study. Yet, our preliminary testing suggested that polystyrene showed a low porosity and strength for the particular formulation of the fast-curing geopolymer. This suggests that there is a more suitable foaming agent depending on the formulation of the geopolymer.
Beyond the aforementioned properties, the developed GP foam has a greater advantage in that it can be cured in a short time at room temperature. As can be seen from Table 2, curing (the manifestation of the maximum strength) of GP foam takes more than 2 weeks, or can often be cured at elevated temperatures to shorten the curing time, in general. In our case, it can be confirmed that the maximum strength is established in about 5 days at room temperature. In fast curing of geopolymer, it can be used as a repair sealant, a plastic finish, and an emergency repair material for construction, and through pore and foam formation, it can be used as sound insulation, insulation, a moisture hygroscopic agent, and a heavy metal ion adsorbent. In addition, the size of the pores is not only homogeneous but also as small as 30 , which is smaller than the previous studies, even though it hardened faster. For this reason, although the density is relatively high, it is sufficiently useful as an insulator in terms of thermal conductivity.
5. Conclusions
In this study, metakaolin-based geopolymers were used to investigate the properties of porous and lightweight ceramic foam that can be cured at room temperature. Hydrogen peroxide was used as a foaming agent, along with sodium lauryl sulfate (SDS) as a surfactant. The homogeneous few tens of micro-size pores were created as SDS stabilizing oxygen gas bubbles produced by base-catalyzed hydrogen peroxide decomposition. For the optimal design, H-10 and HS-10, the porosity by mercury porosimetry were found to be 33% and 44%, respectively. The porosity estimated by relative density to the control GP was 29% and 54%, respectively, and pore structures of H-10 are interconnected to become open pores; on the other hand, HS-10 consists of closed pores, whereas mercury may not be able to intrude. The H-10 GP foam had compressive strengths and densities of 1.57 MPa and 0.75 g/cm3, whereas HS-10 GP foam had 3.63 MPa and 0.48 g/cm3 for GP foam with SDS. With the addition of SDS, the density becomes lower but the compressive strength is higher than when hydrogen peroxide only was used. The estimated porosity from relative density is 29% and 54%, so that the discrepancy in porosity predicts the presence of a closed pore. With the beneficial fast- and ambient-curing behavior, the developed GP foam exhibited TC = 0.0803 W/m·K, along with other good performance for TIMs. The pore analysis indicates that SDS endowed a formation of homogeneous and small micropores, of which microstructures resulted in the above properties. The foaming properties of geopolymers through stabilization of foaming agents and surfactants suggest that this material, which has a tremendous advantage of curing rapidly at room temperature, is expected to be a good candidate for insulation material. However, since the strength is low, the addition of a filler capable of reinforcing strength and lowering thermal conductivity at the same time should be considered in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min12070821/s1, Figure S1: (a) Density of GP with varying foaming agnet content, and (b) compressive strength as a function of the content of foaming agents with 3 types of foaming agents. Figure S2: (a–c) and (d–f) show surfaces at different magnifications of the HS sample. The pore distribution and size due to the effect of adding a surfactant (SDS) can be visually estimated; Figure S3: Mercury intrusion porosimetry test cumulative volume curve graph of Control GP, H-10, HS-10; Table S1: Formulation molar ratio of fast curing geopolymer discussed in previous study.
Author Contributions
Conceptualization, H.M.L.; methodology, H.K.; software, H.K.; validation, H.M.L. and H.K.; formal analysis, K.W.K.; investigation, K.W.K.; resources, K.W.K.; data curation, K.W.K.; writing—original draft preparation, K.W.K. and H.K.; writing—review and editing, H.M.L. and H.K.; visualization, K.W.K.; supervision, S.-Y.Y.; project administration, H.M.L.; funding acquisition, H.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Technology Business Innovation Program of Ministry of Land, Infrastructure and Transport of Korean government, number 21CTAP-C152123-03.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Silico-Aluminophosphate and Alkali-Aluminosilicate Geopolymers: A Comparative Review. Front. Mater. 2019, 6, 106. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.; Labrincha, J. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Shimizu, T.; Matsuura, K.; Furue, H.; Matsuzak, K. Thermal conductivity of high porosity alumina refractory bricks made by a slurry gelation and foaming method. J. Eur. Ceram. Soc. 2013, 33, 3429–3435. [Google Scholar] [CrossRef]
- Meille, S.; Lombardi, M.; Chevalier, J.; Montanaro, L. Mechanical properties of porous ceramics in compression: On the transition between elastic, brittle, and cellular behavior. J. Eur. Ceram. Soc. 2012, 32, 3959–3967. [Google Scholar] [CrossRef]
- Bagherian, E.; Ariffin, M.K.; Sulaiman, S. Development of a Ceramic Foam Filter for Filtering Molten Aluminum Alloy in Casting Processes. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 2009. [Google Scholar]
- Geus, J.W.; Van Giezen, J. Catalysts supported by porous ceramic layers on ceramic or metallic substrates. MRS Online Proc. Libr. 1996, 454, 147. [Google Scholar] [CrossRef]
- Barhate, R.S.; Ramakrishna, S. Nanofibrous filtering media: Filtration problems and solutions from tiny materials. J. Membr. Sci. 2007, 296, 1–8. [Google Scholar] [CrossRef]
- Chou, K.-S.; Lee, T.-K.; Liu, F.-J. Sensing mechanism of a porous ceramic as humidity sensor. Sens. Actuators B Chem. 1999, 56, 106–111. [Google Scholar] [CrossRef]
- Wongkvanklom, A.; Posi, P.; Kasemsiri, P. Strength, thermal conductivity and sound absorption of cellular lightweight high calcium fly ash geopolymer concrete. Eng. Appl. Sci. Res. 2021, 48, 487–496. [Google Scholar]
- Zhu, L.; Li, S.; Li, Y.; Xu, N. Novel applications of waste ceramics on the fabrication of foamed materials for exterior building walls insulation. Constr. Build. Mater. 2018, 180, 291–297. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zou, S.; Zhang, G. Experimental research on a feasible rice husk/geopolymer foam building insulation material. Energy Build. 2020, 226, 110358. [Google Scholar] [CrossRef]
- Seeber, B.S.M.; Gonzenbach, U.T.; Gauckler, L.J. Mechanical properties of highly porous alumina foams. J. Mater. Res. 2013, 28, 2281–2287. [Google Scholar] [CrossRef]
- Sarkar, N.; Park, J.G.; Mazumder, S.; Pokhrel, A.; Aneziris, C.G.; Kim, I.J. Effect of amphiphile chain length on wet foam stability of porous ceramics. Ceram. Int. 2015, 41, 4021–4027. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Li, Y.; Sang, S.; Li, S. Effects of pore structure on thermal conductivity and strength of alumina porous ceramics using carbon black as pore-forming agent. Ceram. Int. 2016, 42, 8221–8228. [Google Scholar] [CrossRef]
- Brown, R.C.; Harrison, P.T. Alkaline earth silicate wools–A new generation of high temperature insulation. Regul. Toxicol. Pharmacol. 2012, 64, 296–304. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Łach, M.; Mierzwiński, D.; Korniejenko, K.; Mikuła, J. Geopolymer foam as a passive fire protection. MATEC Web Conf. 2018, 247, 00031. [Google Scholar] [CrossRef][Green Version]
- Oo, H.M.; Mohamed-Kamari, H.; Wan-Yusoff, W.M.D. Optical Properties of Bismuth Tellurite Based Glass. Int. J. Mol. Sci. 2012, 13, 4623–4631. [Google Scholar] [CrossRef]
- Kovářík, T.; Hájek, J.; Pola, M.; Rieger, D.; Svoboda, M.; Beneš, J.; Šutta, P.; Deshmukh, K.; Jandová, V. Cellular ceramic foam derived from potassium-based geopolymer composite: Thermal, mechanical and structural properties. Mater. Des. 2021, 19, 109355. [Google Scholar] [CrossRef]
- Pokhrel, A.; Seo, D.N.; Lee, S.T.; Kim, I.J. Processing of porous ceramics by direct foaming: A review. J. Korean Ceram. Soc. 2013, 50, 93–102. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Ducman, V.; Korat, L. Characterization of geopolymer fly-ash based foams obtained with the addition of Al powder or H2O2 as foaming agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Ascensão, G.; Seabra, M.P.; Labrincha, J. Porous biomass fly ash-based geopolymers with tailored thermal conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Prud’homme, E.; Michaud, P.; Joussein, E.; Peyratout, C.; Smith, A.; Arrii-Clacens, S.; Clacens, J.; Rossignol, S. Silica fume as porogent agent in geo-materials at low temperature. J. Eur. Ceram. Soc. 2010, 30, 1641–1648. [Google Scholar] [CrossRef]
- Natali Murri, A.; Medri, V.; Papa, E.; Laghi, L.; Mingazzini, C.; Landi, E. Porous geopolymer insulating core from a metakaolin/biomass ash composite. Environments 2017, 4, 86. [Google Scholar] [CrossRef]
- Davidovits, J.G. Geopolymer Chemistry and Applications; Institute Geopolymer: Saint-Quentin, France, 2008. [Google Scholar]
- Łach, M.; Korniejenko, K.; Mikuła, J. Thermal insulation and thermally resistant materials made of geopolymer foams. Procedia Eng. 2016, 151, 410–416. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, X.; Sheng, Y.; Zong, R.; Li, C.; Lu, S. Role of salts in performance of foam stabilized with sodium dodecyl sulfate. Chem. Eng. Sci. 2020, 216, 115474. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Jung, M.; Han, S.; Kim, S.; Jeon, H.-S. Controlling the pore size and connectivity of alumina-particle-stabilized foams using sodium dodecyl sulfate: Role of surfactant concentration. Langmuir 2020, 36, 10331–10340. [Google Scholar] [CrossRef]
- Wang, M. Geopolymerization Mechanism of Aluminosilicate Geopolymer and Microstructure and Properties of Fly Ash Cenosphere/Geopolymer Composite; Harbin Institute of Technology: Harbin, China, 2011. [Google Scholar]
- Bondar, D.; Lynsdale, C.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A. Effect of type, form, and dosage of activators on strength of alkali-activated natural pozzolans. Cem. Concr. Compos. 2011, 33, 251–260. [Google Scholar] [CrossRef]
- Rocha, T.d.S.; Dias, D.P.; França, F.C.C.; de Salles Guerra, R.R.; de Oliveira, L.R.D.C. Metakaolin-based geopolymer mortars with different alkaline activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Fasihnikoutalab, M.H.; Pourakbar, S.; Ball, R.J.; Huat, B.K. The Effect of Olivine Content and Curing Time on the Strength of Treated Soil in Presence of Potassium Hydroxide. Int. J. Geosynth. Ground Eng. 2017, 3, 12. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Wang, Q.; Zhang, Z.; Ou, Z. Composition design and performance of alkali-activated cements. Mater. Struct. 2017, 50, 178. [Google Scholar] [CrossRef]
- Hafid, K.E.; Hajjaji, M. Geopolymerization of glassand silicate-containing heated clay. Constr. Build. Mater. 2018, 159, 598–609. [Google Scholar] [CrossRef]
- Gomez-Zamorano, L.Y.; Vega-Cordero, E.; Struble, L. Composite geopolymers of metakaolin and geothermal nanosilica waste. Constr. Build. Mater. 2016, 115, 269–276. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Low, A. Fly ash based geopolymer for the adsorption of anionic surfactant from aqueous solution. J. Clean. Prod. 2019, 229, 232–243. [Google Scholar] [CrossRef]
- Ohji, T.; Fukushima, M. Macro-porous ceramics: Processing and properties. Int. Mater. Rev. 2012, 57, 115–131. [Google Scholar] [CrossRef]
- ASTM C39/C39M; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International, American Society for Testing and Materials International (ASTM): West Conshohocken, PA, USA, 2015.
- ASTM E136-19a; Standard Test Method for Assessing Combustibility of Materials Using a Vertical Tube Furnace at 750 °C. American Society for Testing and Materials International (ASTM): West Conshohocken, PA, USA, 2019.
- ASTM E2652-18; Standard Test Method for Assessing Combustibility of Materials Using a Tube Furnace with a Cone-Shaped Airflow Stabilizer, at 750 °C. American Society for Testing and Materials International (ASTM): West Conshohocken, PA, USA, 2018.
- ISO 1182; Reaction to Fire Tests for Products—Non Combustibility Test. International Organization for Standardization: Geneva, Switzerland, 2010.
- Bai, C.; Colombo, P. High-porosity geopolymer membrane supports by peroxide route with the addition of egg white as surfactant. Ceram. Int. 2017, 43, 2267–2273. [Google Scholar] [CrossRef]
- Bai, C.; Franchin, G.; Elsayed, H.; Conte, A.; Colombo, P. High strength metakaolin-based geopolymer foams with variable macroporous structure. J. Eur. Ceram. Soc. 2016, 36, 4243–4249. [Google Scholar] [CrossRef]
- Korat, L.; Ducman, V. The influence of the stabilizing agent SDS on porosity development in alkali-activated fly-ash based foams. Cem. Concr. Compos. 2017, 80, 168–174. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Buruberri, L.; Senff, L.; Labrincha, J. Influence of blowing agent on the fresh-and hardened-state properties of lightweight geopolymers. Mater. Des. 2016, 108, 551–559. [Google Scholar] [CrossRef]
- Samson, G.; Cyr, M. Porous structure optimisation of flash-calcined metakaolin/fly ash geopolymer foam concrete. Eur. J. Environ. Civ. Eng. 2018, 22, 1482–1498. [Google Scholar] [CrossRef]
- Samson, G.; Cyr, M.; Gao, X.X. Thermomechanical performance of blended metakaolin-GGBS alkali-activated foam concrete. Constr. Build. Mater. 2017, 157, 982–993. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Poulesquen, A. Design of lightweight metakaolin based geopolymer foamed with hydrogen peroxide. Ceram. Int. 2019, 45, 1322–1330. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, F.; Liu, J.; Ren, B.; He, P.; Jia, D.; Yang, J. Green synthesis of high porosity waste gangue microsphere/geopolymer composite foams via hydrogen peroxide modification. J. Clean. Prod. 2019, 227, 483–494. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Zhang, Y.; Li, D. Preparation and characterization of ultra-lightweight foamed geopolymer (UFG) based on fly ash-metakaolin blends. Constr. Build. Mater. 2018, 168, 771–779. [Google Scholar] [CrossRef]
- Duan, P.; Song, L.; Yan, C.; Ren, D.; Li, Z. Novel thermal insulating and lightweight composites from metakaolin geopolymer and polystyrene particles. Ceram. Int. 2017, 43, 5115–5120. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).