Abstract
The presence of aluminum in the weathering crust leaching rare earth ore harms the subsequent extraction and separation of rare earths. High-quality rare earth production processes must reduce the aluminum content in their feed liquid. Groups containing lone pairs of electrons can form stable insoluble complexes with metal ions under certain conditions. In this paper, 3-hydroxyphenylphosphoryl propionic acid is used to selectively separate rare earths by complexation in feed liquid. The results show that: using 3-hydroxyphenylphosphoryl propionic acid as the complexing agent, and when the amount of 3-hydroxyphenyl phosphoryl propionic acid is six times the theoretical complete reaction amount, the reaction time is 10 min, the reaction temperature is 50 °C, and the solution is adjusted to pH 1, the extraction rate of RE3+ is 90.48%, and the extraction rate of Al3+ is nearly 9.52%. The separation of rare earth and aluminum is well realized. 3-hydroxyphenyl phosphoryl propionic acid has good water solubility and low cost, and the product after complexation reaction with metal ions is solid and easy to separate. It has potential as an alternative complexing agent in the industry.
1. Introduction
Rare earth is a gift of nature to human beings. People use its unique optical, electrical, magnetic and biological activity to apply it in many high-tech fields such as information technology, energy technology, biotechnology, and green technology products. At the same time, traditional agriculture, metallurgy, machinery, national defense, and many other industries benefit from the use of rare earths. The development of human society has long been inseparable from the existence of rare earth elements [1,2,3]. China’s rare earth reserves are the largest in the world, and among their advantages in addition to their total reserves, they are also rich in species, have diverse minerals, and are easy to mine. Weathered crust leaching-type rare earth ore is unique to China. It was first discovered in southern China and has long been coveted by Western countries. This type of medium and heavy rare earth ore with low radioactivity, formerly known as ion-adsorption rare earth ore, is formed by the physical, chemical, and biological effects of various ores containing rare earth elements which form ionic rare earth resources with clay, rocks, and other adsorptive substances acting as carriers [2,4,5,6,7,8]. The special structure and low grade of the ore phase make it impossible to effectively extract rare earths using traditional physical beneficiation processes. For this reason, much research has been conducted in China with the discovery that strong electrolyte ions in the aqueous phase can exchange with the rare earth ions on the adsorbent carrier to leach them into the aqueous phase [9,10,11]. When using this method to leach rare earths, a large number of impurity ions are also leached, especially aluminum ions, which are the third most abundant element in the earth’s crust. When the rare earth ions are leached from the adsorbent carrier with a strong electrolyte solution, a large number of impurity aluminum ions will also pour into the leaching solution, forming Al3+ with an ionic radius of approximately 57 pm. However, due to the lanthanide contraction, the ionic radius of rare earth elements in the lanthanide series did not change much, and only decreased from 117 to 78 pm. The ionic radius of Y, which is in the same subgroup as the lanthanide rare earths, is 89 pm, and the ionic radius of Sc is 67 pm. They are all close to the radius of aluminum ions, resulting in very similar physical and chemical properties between the elements. In addition, aluminum also has unique amphoteric properties which make the separation between elements extremely difficult [12]. At present, there is no effective method of completely separating it from rare earth elements, which will accompany the entire rare earth separation process and have a huge impact on the entire separation process, which is a problem that cannot be ignored [13,14,15,16].
Some groups containing lone pairs of electrons can form stable complexes with metal ions in aqueous solutions under certain conditions [17,18]. Some of the complexes are extremely insoluble as chelates and can be used to separate rare earth and aluminum elements in the leaching solution. 3-hydroxyphenylphosphoryl propionic acid (CEPPA) contains functional groups such as phosphoryl, hydroxyl, and carboxylic acid. According to the relevant theoretical knowledge of organic coordination chemistry and the relevant literature reports on the separation and purification production process of weathered crust leaching rare earth ore, it can be inferred that CEPPA has different complexing abilities to different metal ions under different process conditions, and can be used as an organic ligand in the separation and purification production process of weathered crust leaching rare earth ores [19,20,21,22]. Compared with some new aluminum removal methods [23], this method has better selectivity and environmental protection. CEPPA has good water solubility and low price, and the product after complexation reaction with metal ions is solid and easy to separate. This may represent a potential method of separating Al and RE in the industry.
2. Experiment
2.1. Experimental Materials
CEPPA was purchased from MACLIN, AR, pKa = 2.21 ± 0.50 (predicted). A leaching rare earth solution was used as the experimental raw material, as shown in Table 1.

Table 1.
Typical chemical analysis of solution.
2.2. Analytical Method
Rare earth element concentration was detected by the EDTA volumetric method, and the aluminum ion concentration was detected by the chromium azure S colorimetric method using a UV-6300 (MAPADA, Shanghai, China) type UV–Visible spectrophotometer [19]. The infrared spectra were obtained using a Nicolet 5700 (Thermo Fisher Scientific, Waltham, MA, USA) type intelligent FTIR spectrometer.
2.3. Experimental Method
A quantitative amount of leaching rare earth solution was measured in a beaker with a measuring cylinder, an appropriate amount of CEPPA was added under constant temperature and stirring, and after, it was completely dissolved. Slowly adjust the pH of the feed liquid with the buffer solution of ammonia water and ammonium chloride to form a precipitate. Under the condition that the temperature and pH of the reaction system are unchanged, the reaction solution is filtered and separated after a certain time of reaction. According to the principle of CEPPA for the different coordination selectivity of metal ions, rare earth and aluminum were separated, and the reaction mechanism was analyzed by Gaussian software. The process flow of the experiment is shown in Figure 1.
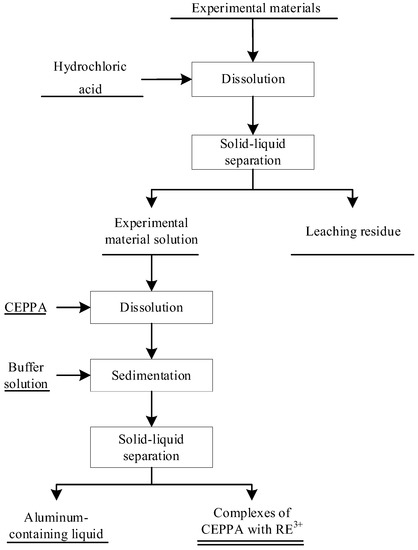
Figure 1.
Flow chart of CEPPA aluminum removal experiment.
CEPPA is a white crystal which can easily absorb water at room temperature and needs to be stored after drying. The molecular formula is C9H11O4P. Its physical and chemical properties are stable. The structure of the substance is shown in Figure 2.
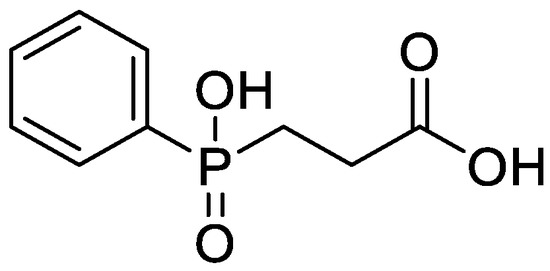
Figure 2.
CEPPA structural formula.
In this paper, CEPPA was selected as the organic complexing agent. Various reaction conditions and related mechanisms that affect the coordination reaction between organic ligands and metal ions are also studied in detail. Therefore, the related process experimental research is carried out.
3. Results and Discussion
3.1. Study on the Process of CEPPA Separation of Rare Earth and Aluminum
3.1.1. Effect of pH on Separation of Rare Earths and Aluminum
According to the relevant books and literature reports of coordination chemistry, it is known that the coordination reaction between organic ligands and metal ions is affected by many factors. The organic ligand CEPPA is acidic, and the pH of the solution becomes the primary factor affecting the coordination reaction. For this reason, the effect of the pH of the rare earth feed liquid on the separation of rare earth and aluminum by CEPPA is firstly studied. The experiment was carried out as follows: five equal portions of 100 mL of rare earth solution were placed in five 250 mL beakers labeled 1–5 with a measuring cylinder, and the temperature of the solution was controlled at 40 °C. Under the condition of magnetic stirring, add MCEPPA:MRE3+ = 6:1 equivalent of CEPPA, until it is completely dissolved. The pH of the rare earth solution in beakers labeled 1–5 was adjusted to 1, 2, 3, 4, and 5 with pre-prepared buffer solutions of ammonia water and ammonium chloride, and filtered after 10 min of reaction. The experimental results of our investigation into the effect of pH on the separation of rare earths and aluminum are shown in Figure 3.
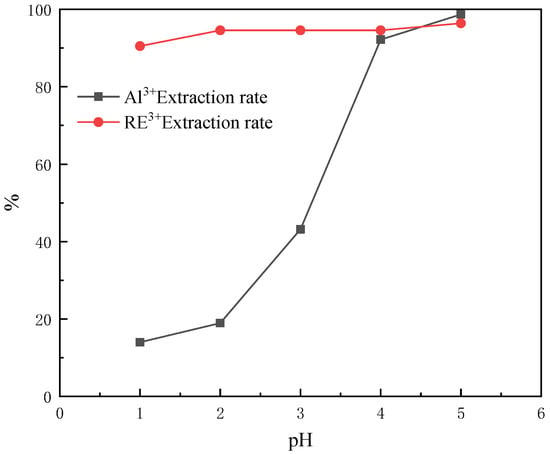
Figure 3.
Effect of pH on separation of rare earths and aluminum.
According to Figure 3, when the pH of the feed liquid is equal to 1, the extraction rate of rare earth ions is much higher than that of aluminum ions. It can be seen that the selectivity of CEPPA to rare earth ions is stronger under the condition of a low pH of rare earth feed liquid. When the pH of the feed liquid is in the range of 2–4, with the increase in the pH of the feed liquid, the extraction rate of rare earth ions slightly increases, but the extraction rate of aluminum ions sharply increases within this range. It is speculated that the hydrogen ions of the hydroxyl groups on the CEPPA branch start to fall off during the process of the pH increase, which makes CEPPA more selective for aluminum ions than rare earth ions in the coordination reaction and rapidly precipitates. For the simultaneous increase in the extraction rate of rare earth ions and aluminum ions when the pH of the feed liquid is equal to 4–5, it should be the precipitation of hydroxides under higher pH conditions. Through the comprehensive consideration and analysis of the experiments, pH = 1 was selected as the optimal reaction condition. Under this condition, the extraction rate of rare earth ions was 90.48%, and the extraction rate of aluminum ions was 14.02%.
3.1.2. Effect of CEPPA Amount on Separation of Rare Earth and Aluminum
In the coordination reaction, the effect of the amount of the reaction substrate on the reaction process cannot be ignored. For this purpose, the effect of the amount of CEPPA on the separation of rare earth and aluminum was explored. The experiment was carried out as follows: 5 equal portions of 100 mL of the rare earth solution were placed in 5250 mL beakers labeled 1–5 with a measuring cylinder, the temperature of the solution was controlled at 40 °C. Under the condition of magnetic stirring, add MCEPPA:MRE3+ = 3:1, 4:1, 5:1, 6:1, 7:1 equivalent of CEPPA in beakers 1–5, respectively, until it is completely dissolved. Slowly adjust the pH of the feed liquid to 1 with a buffer solution of ammonia water and ammonium chloride, and filter after 10 min of reaction. The experimental results of our investigation into the effect of the CEPPA amount on the separation of rare earths and aluminum are shown in Figure 4.
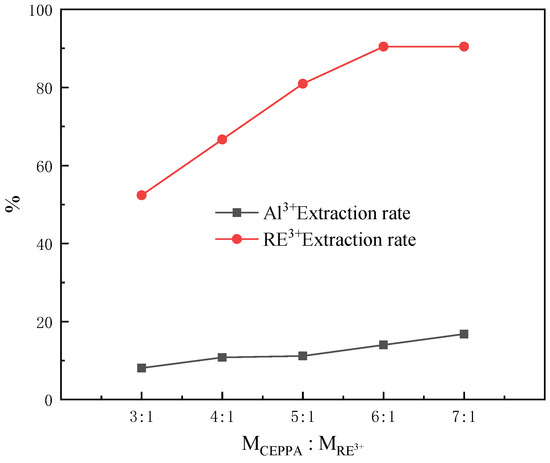
Figure 4.
Effect of the CEPPA amount on the separation of rare earths and aluminum.
According to Figure 4, it can be seen that with the increase in the amount of CEPPA, the extraction rate of rare earth ions in the rare earth feed liquid will sharply increase, and the increase in the extraction rate of rare earth ions is no longer obvious after MCEPPA:MRE3+ = 6:1. The extraction rate of aluminum ions in the rare earth feed liquid is different from that of rare earth ions. With the increase in the ratio of MCEPPA:MRE3+, the extraction rate of aluminum ions in the feed liquid has always shown an upward trend, but the increase is not evident. Through the comprehensive consideration and analysis of the experiment: the amount of CEPPA with MCEPPA:MRE3+ = 6:1 is selected as the best reaction amount. Under this condition, the extraction rate of rare earth ions in the rare earth feed liquid is 90.48%, and the extraction rate of aluminum ions is 14.02%.
3.1.3. Effect of Reaction Temperature on Separation of Rare Earth and Aluminum
In the coordination reaction, the temperature of the reaction system can affect the progress of the reaction through the cleavage and combination of chemical bonds, which holds great influence over the progress of the reaction. Therefore, the influence of the reaction temperature on the coordination reaction of CEPPA needs to be considered. The experiment was carried out as follows: five equal portions of 100 mL of the solution were measured in five 250 mL beakers labeled 1–5 with a measuring cylinder. Under the condition of magnetic stirring, add MCEPPA:MRE3+ = 6:1 equivalent of CEPPA until it is completely dissolved. The temperature of the rare earth feed liquid in the beakers labeled 1–5 was controlled to be 30 °C, 40 °C, 50 °C, 60 °C, and 70 °C, respectively. Slowly adjust the pH of the feed liquid to 1 with a buffer solution of ammonia water and ammonium chloride, and filter after 10 min of reaction. The experimental results of our investigation into the effect of the reaction temperature on the separation of rare earths and aluminum are shown in Figure 5.
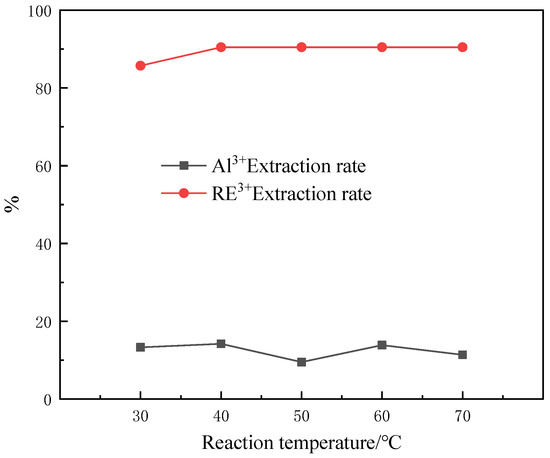
Figure 5.
Effect of reaction temperature on the separation of rare earths and aluminum.
According to Figure 5, it can be seen that the effect of the reaction temperature on the separation of rare earth and aluminum by CEPPA is not apparent, and with the increase in reaction temperature, the extraction rate of rare earth ions in the rare earth feed liquid slightly increased while the extraction rate of aluminum ions in the feed liquid randomly fluctuates. Through the comprehensive consideration and analysis of the experiment, the reaction temperature of 50 °C was selected as the optimal reaction temperature for this experiment. Under this condition, the extraction rate of rare earth ions in the rare earth feed liquid is 90.48%, and the extraction rate of aluminum ions is 9.52%.
3.1.4. Effect of Reaction Time on Separation of Rare Earth and Aluminum
The effect of the reaction time on chemical reactions is self-evident. Proper control cannot only save resources and reduce production costs, but also affect the final reaction product. For this reason, the effect of reaction time on the CEPPA coordination reaction process was explored through experiments. The experiment was carried out as follows: five equal portions of 100 mL of rare earth solutions were measured in five 250 mL beakers labeled 1–5 with a measuring cylinder and the temperature of the solution was controlled at 50 °C. Under the condition of magnetic stirring, add MCEPPA:MRE3+ = 6:1 equivalent of CEPPA until it is completely dissolved. Slowly adjust the pH of the feed liquid to 1 with a buffer solution of ammonia water and ammonium chloride. The reaction times in the beakers with control labels 1–5 were 10 min, 20 min, 30 min, 40 min, and 50 min, respectively, and filtered after the reaction was over. The experimental results of our investigation into the effect of the reaction time on the separation of rare earths and aluminum are shown in Figure 6.
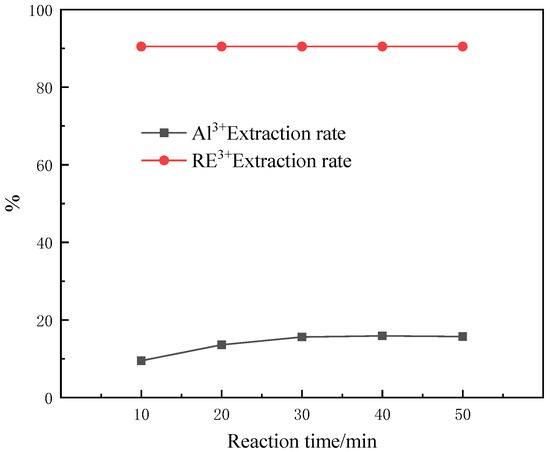
Figure 6.
Effect of reaction time on the separation of rare earths and aluminum.
According to Figure 6, it can be seen that the effect of the reaction time on the CEPPA coordination reaction is not obvious, and that the extraction rate of aluminum ions in the feed liquid has a slight upward trend. Through the comprehensive consideration and analysis of the experiment, the reaction time of 10 min was selected as the optimal reaction time for this experiment. Under this condition, the extraction rate of rare earth ions in the rare earth feed liquid is 90.48% and the extraction rate of aluminum ions is 9.52%.
3.1.5. Effect of Al3+ Concentration on the Separation of Rare Earth and Aluminum
In the separation and purification process of weathered crust leaching type rare earth ore, the aluminum content in the feed liquid holds great influence on the whole production process. Therefore, the concentration of aluminum ions in the feed liquid is essential to the relevant experimental research on the coordination reaction of CEPPA. The experiment was carried out as follows: first, prepare rare earth feed liquids containing different aluminum ion concentrations according to the experimental requirements. Because the selectivity of CEPPA ligands to rare earth ions is greater than that of aluminum ions, control the concentration of rare earth ions in the rare earth feed liquid to remain unchanged, and just change the concentration of the aluminum element in the feed liquid. The concentration of aluminum ions in the feed liquid was 1 g/L, 2 g/L, 5 g/L, and 10 g/L; take 100 mL of each measuring cylinder and place them in four 250 mL beakers labeled 1–4—the temperature of the solution being controlled at 50 °C. Under the condition of magnetic stirring, add MCEPPA:MRE3+ = 6:1 equivalent of CEPPA, until it is completely dissolved. Slowly adjust the pH of the feed liquid to 1 with a buffer solution of ammonia water and ammonium chloride, and filter after 10 min of reaction. The experimental results of our investigation into the effect of Al3+ concentration on the separation of rare earths and aluminum are shown in Figure 7.
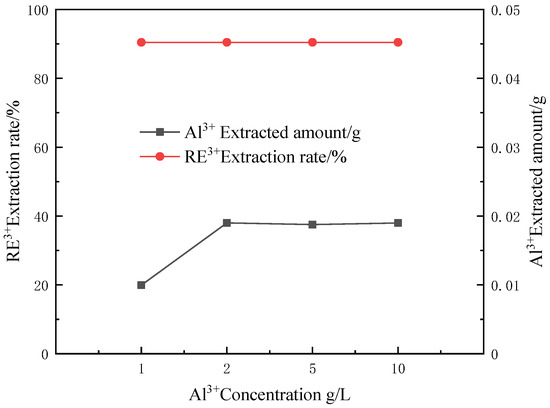
Figure 7.
Effect of Al3+ concentration on the separation of rare earths and aluminum.
According to Figure 7, it can be seen that the change in the concentration of aluminum ions in the rare earth feed liquid has no effect on the extraction rate of rare earth ions while the extraction amount of aluminum ions in the feed liquid remains basically unchanged after 2 g/L. The main reason is that the change of aluminum ion concentration has little effect on the selective coordination reaction of CEPPA, and the total amount of aluminum ions that can be complexed by such an equivalent of CEPPA remains unchanged under the experimental conditions. Therefore, the separation of rare earths and aluminum via a complexation process can be carried out using CEPPA for the rare earth solution with a high aluminum content.
3.2. Analysis of the Experimental Procedure
In previous experiments, it was found during the process of the separation of rare earths and aluminum using CEPPA that CEPPA mainly binds Y3+ in the feed liquid. It is determined that the white solid obtained by filtration is the complex product of CEPPA and Y3+. The complex product was placed in a muffle furnace and calcined at 900 °C for a certain period of time, and the gray-white powder after calcination was analyzed and detected by XRD. The XRD characterization is shown in Figure 8.
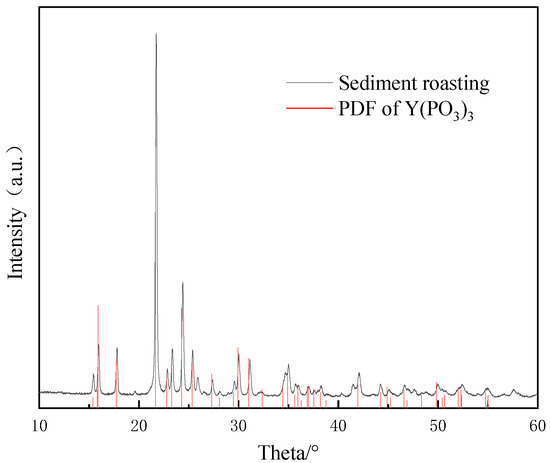
Figure 8.
XRD characterization of a complex product after roasting.
The red line PDF card in Figure 8 is the standard PDF card for Y(PO3)3, It can be seen that the white solid obtained in the previous experiment is Y(PO3)3 after calcination, which verifies the conjecture that it is a complex product of Y3+ and CEPPA.
To further investigate the mechanism of the complexation reaction of rare earths with CEPPA, the CEPPA and the white solid obtained after filtration during the experiment were characterized by infrared spectroscopy after drying. The obtained infrared spectrum is shown in Figure 9.
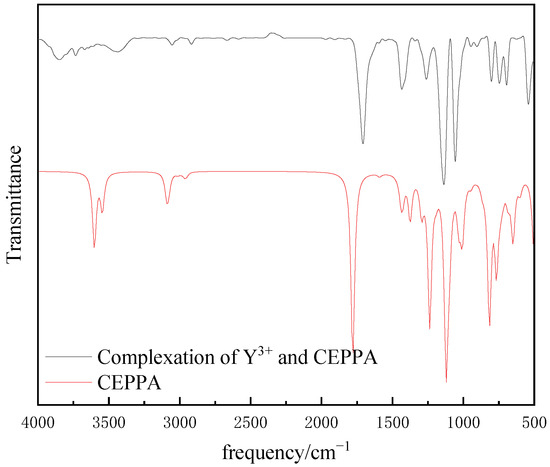
Figure 9.
FTIR spectra of CEPPA and CEPPA complexation products with Y3+.
From Figure 9, it can be seen that the vibration absorption peak of the hydroxyl group is mainly missing after the reaction of CEPPA and Y3+. Therefore, it is speculated that the reaction between CEPPA and Y3+ is shown in Formula (1):
The Gaussian16 software was used to simulate and calculate the infrared spectrum of Y2(CEPPA)3. The calculation model shown in Figure 10 (left) was established with Chem3D and Gauss View in the experiment. Under the condition that the DFT/B3LYP/6-31G* solvent is water, the molecular structure of Y2(CEPPA)3 was optimized and then characterized by infrared simulation (using def2tzvp macronuclear pseudopotential basis set for Y atom); after the optimization, the structure is shown in Figure 10 (right), and the calculated infrared spectrum is shown in Figure 11.
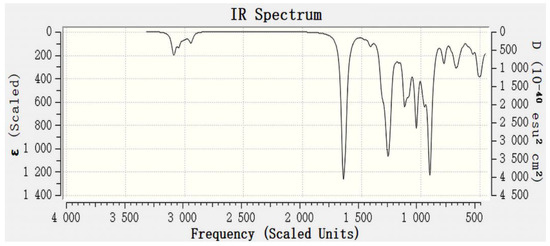
Figure 10.
Calculated FTIR spectrum of Y2(CEPPA)3.
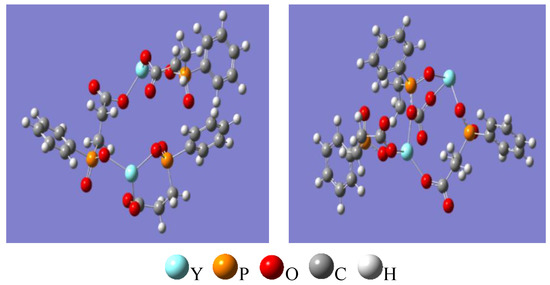
Figure 11.
Building Y2(CEPPA)3 structural model (left). Y2(CEPPA)3 structural optimization model (right).
Comparing Figure 11 with the infrared spectrum of CEPPA and Y3+, it was found that the FTIR spectrum of the white solid measured by the experiment is basically consistent with the FTIR spectrum of Y2(CEPPA)3 obtained by simulation. Therefore, it can be determined that the organic CEPPA is the reaction shown in the above formula 1 to carry out the separation of rare earth and aluminum.
4. Conclusions
(1) CEPPA can be used in the separation of rare earths and aluminum processes. Under the condition that the aluminum ion concentration of the rare earth feed liquid is 2 g/L, the rare earth ion concentration is 5 g/L, the amount of CEPPA is MCEPPA:MRE3+ = 6:1, the pH of the rare earth feed liquid was 1, the reaction temperature was 50 °C, the reaction time was 10 min, the extraction rate of rare earth ions was 90.48%, and the extraction rate of aluminum ions was 9.52%.
(2) Under the condition that the amount of CEPPA remains unchanged, the higher the concentration of aluminum ions in the rare earth feed liquid, the lower the extraction rate of aluminum ions, and the extraction rate of rare earth ions remains unchanged.
(3) The reaction path was determined by Gaussian software calculation, and CEPPA could be used for the separation of high-aluminum rare earths.
Author Contributions
J.L. (Jinhui Li): validation, supervision, funding acquisition, writing—review and editing. Y.W.: investigation, writing—original draft. Y.C.: formal analysis. W.L.: resources. J.L. (Jinbiao Liu): project administration. R.W.: visualization. Z.X.: data curation. All authors have read and agreed to the published version of the manuscript.
Funding
The project was sponsored by the National Natural Science Foundation (51974140, 52064018), Distinguished Professor Program of Jinggang Scholars in institutions of higher learning, Jiangxi Province, and the cultivation project of the State Key Laboratory of Green Development and High-value Utilization of Ionic Rare Earth Resources in Jiangxi Province (20194AFD44003).
Conflicts of Interest
We declare that we have no conflict of interest.
References
- Liu, F. Synthesis of Rare Earth Metal Organophosphorus Extractants. Master’s Thesis, Hubei University, Wuhan, China, 2014. [Google Scholar]
- Hu, Y.; Wang, L.; Cao, Z.; Zhang, W. Research progress on rare earth ore metallurgy and separation technology in China. Conserv. Util. Miner. Resour. 2020, 40, 151–161. [Google Scholar]
- Lu, Y. Synthesis of Phosphorus-Containing Extractants and Their Application in the Extraction and Separation of Rare Earths and Thorium. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. [Google Scholar]
- He, Y.; Cheng, L.; Li, Y.; Ran, D.; Wei, Q. Mineralization mechanism and mineralization signatures of ion adsorption rare earth ores. Chin. Rare Earths 2015, 36, 98–103. [Google Scholar]
- Fan, F.; Xiao, H.; Chen, L.; Bao, X.; Cai, Y.; Zhang, J.; Zhu, Y. Geological characteristics of rare earth ore formation in the weathered crust drenching zone of Pitou, Ganan. J. Chin. Rare Earth Soc. 2014, 32, 101–107. [Google Scholar]
- Chi, R.a.; Liu, X. Current status and outlook of the development of weathered crust drenching rare earth ore. J. Chin. Rare Earth Soc. 2019, 37, 129–140. [Google Scholar]
- Zhang, S.; Zhang, L.; Zhang, Y.; Shang, L.; Chen, X. Overview of rare earth mineral resources and their distribution at home and abroad. Inorg. Chem. Ind. 2020, 52, 9–16. [Google Scholar]
- Luo, X.; Zhang, Y.; Zhou, H.; He, K.; Luo, C.; Liu, Z.; Tang, X. Review on the development and utilization of ionic rare earth ore. Minerals 2022, 12, 554. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, H.; Fang, X.; Li, X. Status and progress of extraction and decontamination technology for weathered crust drenched rare earth ores. Chin. Rare Earths 2012, 33, 81–85. [Google Scholar]
- Chi, R.A.; Li, L.; Wang, D. Study on the ion-exchange equilibrium of clay ores with adsorption of rare earths. J. Cent. South Inst. Min. Metall. 1991, 2, 81–85. [Google Scholar]
- Wang, L.; Liao, C.; Yang, Y.; Xu, H.; Xiao, Y.; Yan, C. Effects of organic acids on the leaching process of ion-adsorption type rare earth ore. J. Rare Earths 2017, 35, 1233–1238. [Google Scholar] [CrossRef]
- Song, T.; Xu, J.; Cheng, G. Inorganic Chemistry, 4th ed.; Higher Education Press: Beijing, China, 2004; pp. 733–735. [Google Scholar]
- Chen, B.; Wang, Z.; Huang, L.; Wu, F.; Chen, J.; Xu, W. The microbial metallogeny of weathering crust REE deposits in South China. Chin. Sci. Bull. 1999, 44, 71–72. [Google Scholar]
- Wu, H.; Yin, Y.; Fang, X. Current status and development of mining and separation technology of weathered crust drenching rare earth ores. Nonferrous Met. Sci. Eng. 2010, 1, 73–76. [Google Scholar]
- Zhang, L. Research on the Leaching Process and Separation of Weathered Crust Drenched Rare Earth Ore. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2012. [Google Scholar]
- Qian, M. Effect of Aluminum on the Extraction of Rare Earth Elements by P507 System. Master’s Thesis, Northeastern University, Shenyang, China, 2010. [Google Scholar]
- Chen, Z. Study on the Alumina Removal Process of High Alumina Rare Earth Feed Liquor. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2018. [Google Scholar]
- Tian, L.; Gong, A.; Wu, X.; Xu, Z.; Zhang, T.A.; Liu, Y.; Wei, K.; Yu, Z. Oxygen pressure acid leaching of artificial sphalerite catalyzed by Fe3+/Fe2+ self-precipitation. J. Cent. South Univ. 2020, 27, 1703–1713. [Google Scholar] [CrossRef]
- Xu, Z. Modern Coordination Chemistry; Chemical Industry Press: Beijing, China, 1987; pp. 129–170. [Google Scholar]
- Wang, Y.; Li, J.; Gao, Y.; Yang, Y.; Gao, Y.; Xu, Z. Removal of aluminum from rare-earth leaching solutions via a complexation-precipitation process. Hydrometallurgy 2020, 191, 105220. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Chi, R.; Feng, J.; Ding, Y.; Liu, Q. Preparation of modified montmorillonite and its application to rare earth adsorption. Minerals 2019, 9, 747. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, Y.; Sang, X.; Wang, Y.; Lu, G. Process study on the removal of aluminum from praseodymium chloride solution in P507-hydrochloric acid-kerosene extraction system. Sci. Technol. Innov. Herald 2020, 20, 87–88. [Google Scholar]
- Li, J.; Gao, Y.; Chen, Z.; Wang, R.; Xu, Z. Study on aluminum removal through 5-sulfosalicylic acid targeting complexing and D290 resin adsorption. Miner. Eng. 2020, 147, 106–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).