Experimental Setup for Evaluating Rock Volume Alteration and Its Application for Studying Shale Rock Swelling in Various Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pyrolysis
2.2.2. X-ray Diffractometry
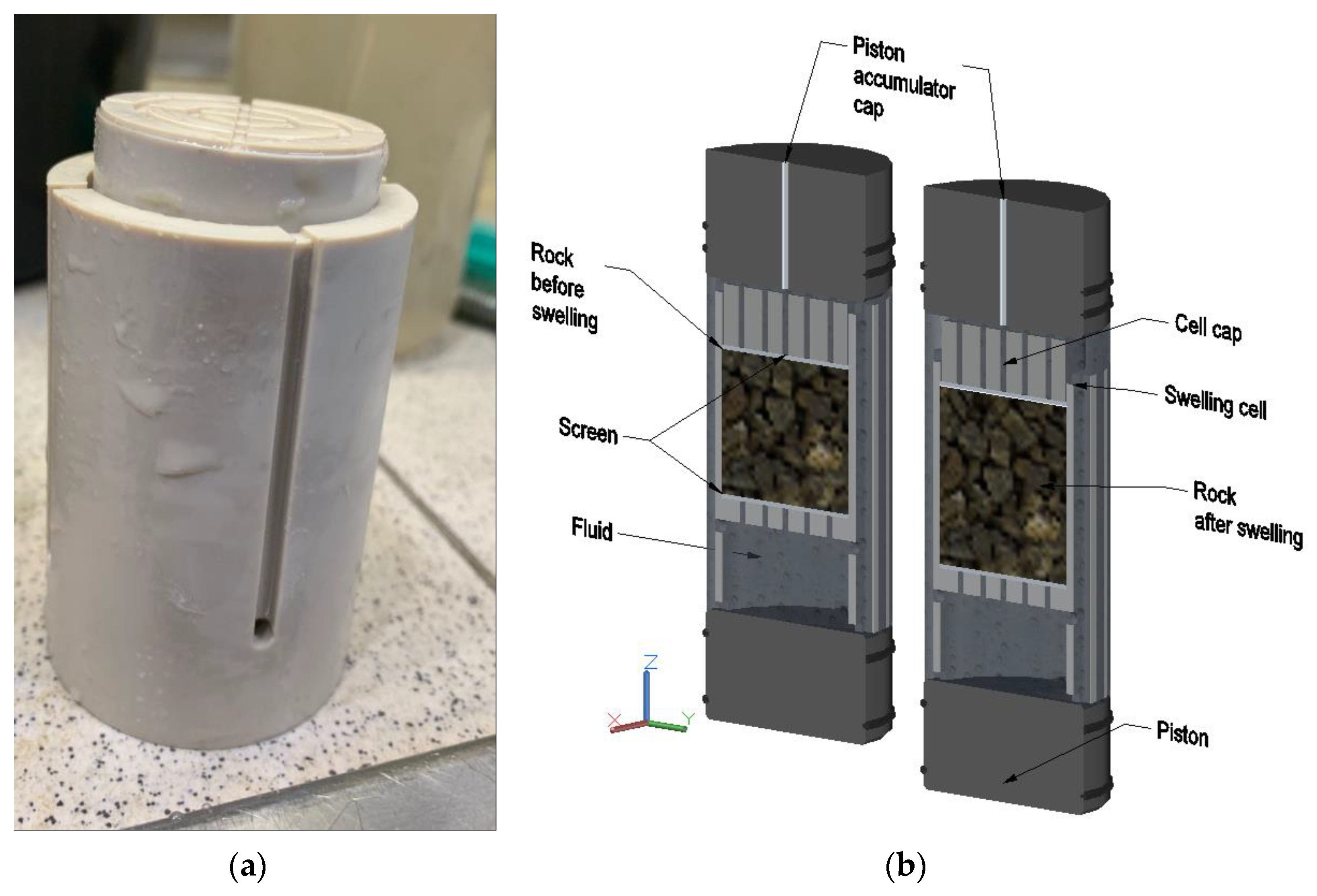
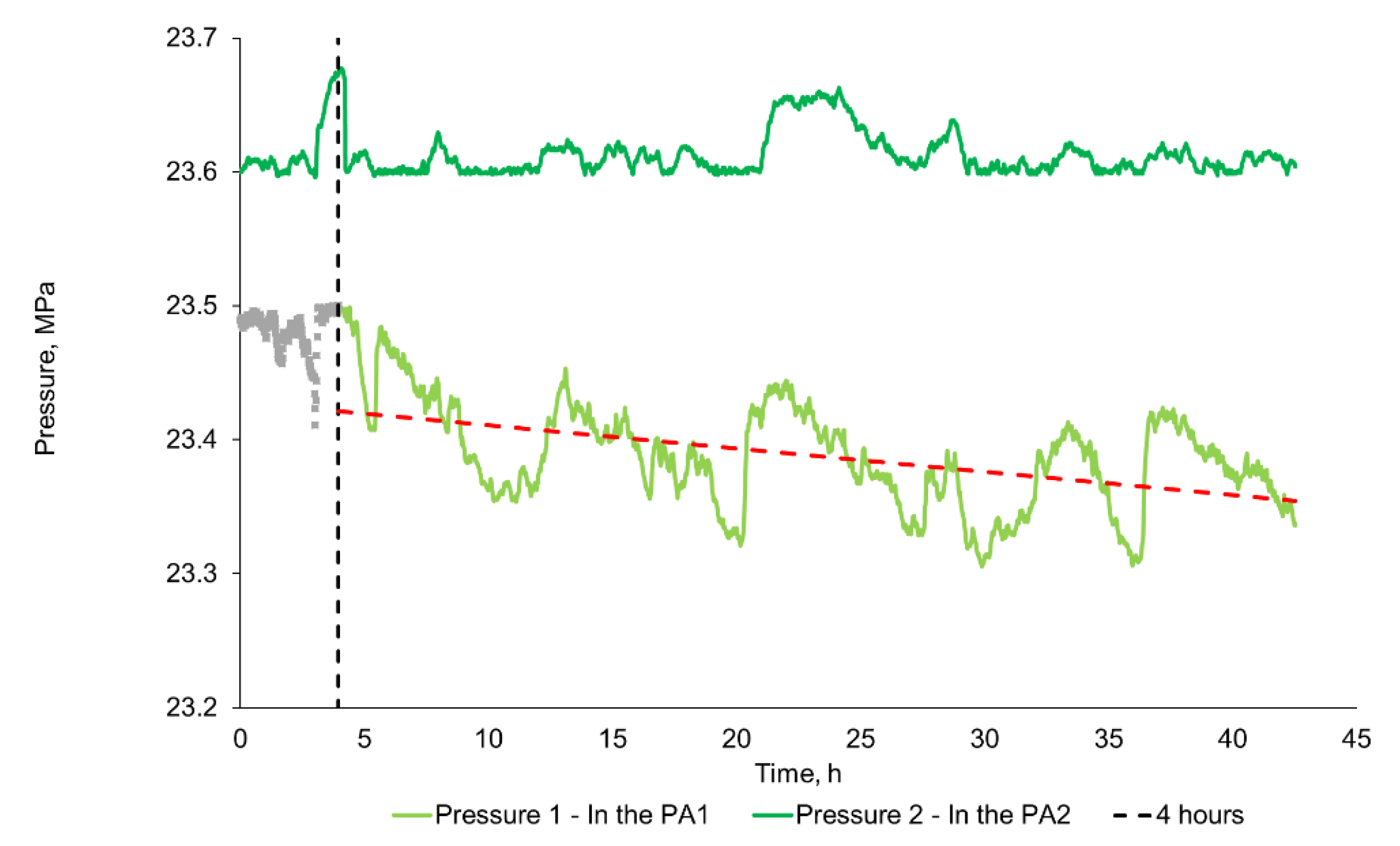
2.2.3. Rock Swelling Study
3. Results and Discussion
3.1. Rock Properties
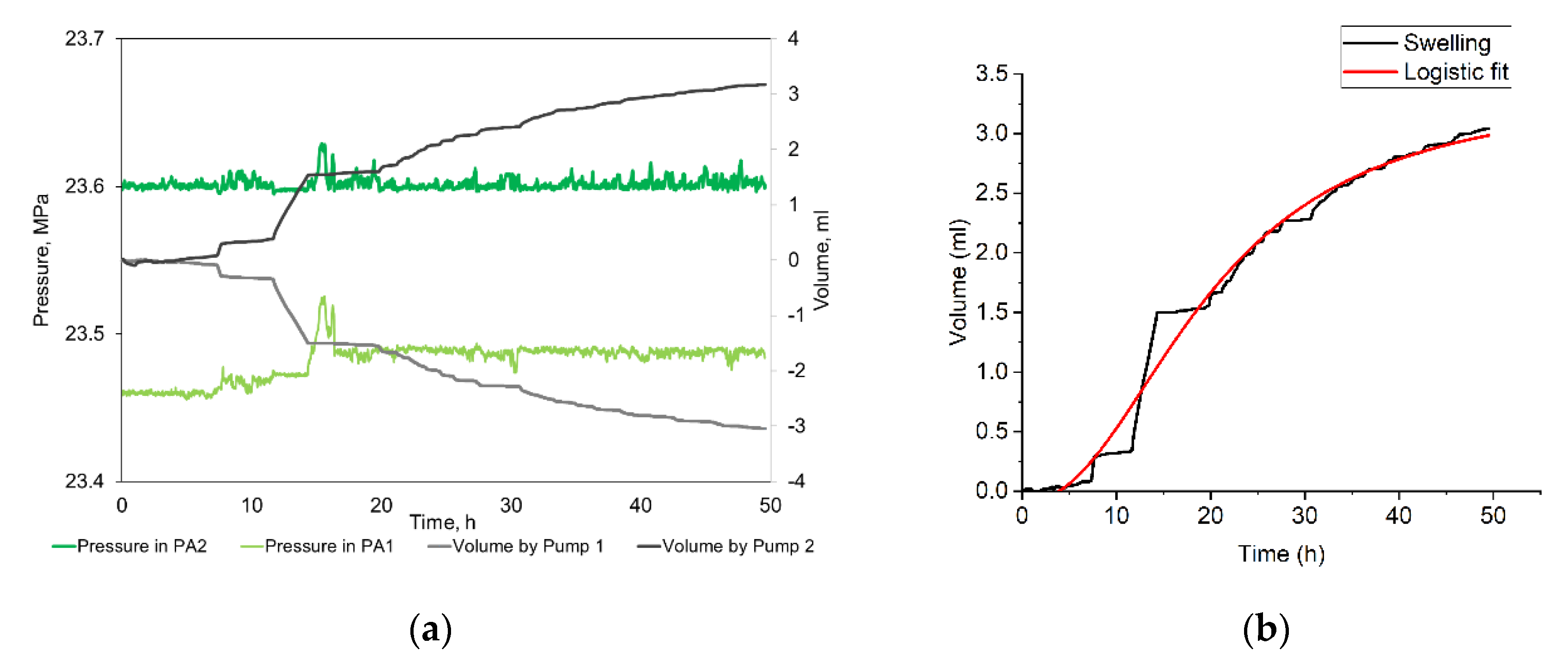
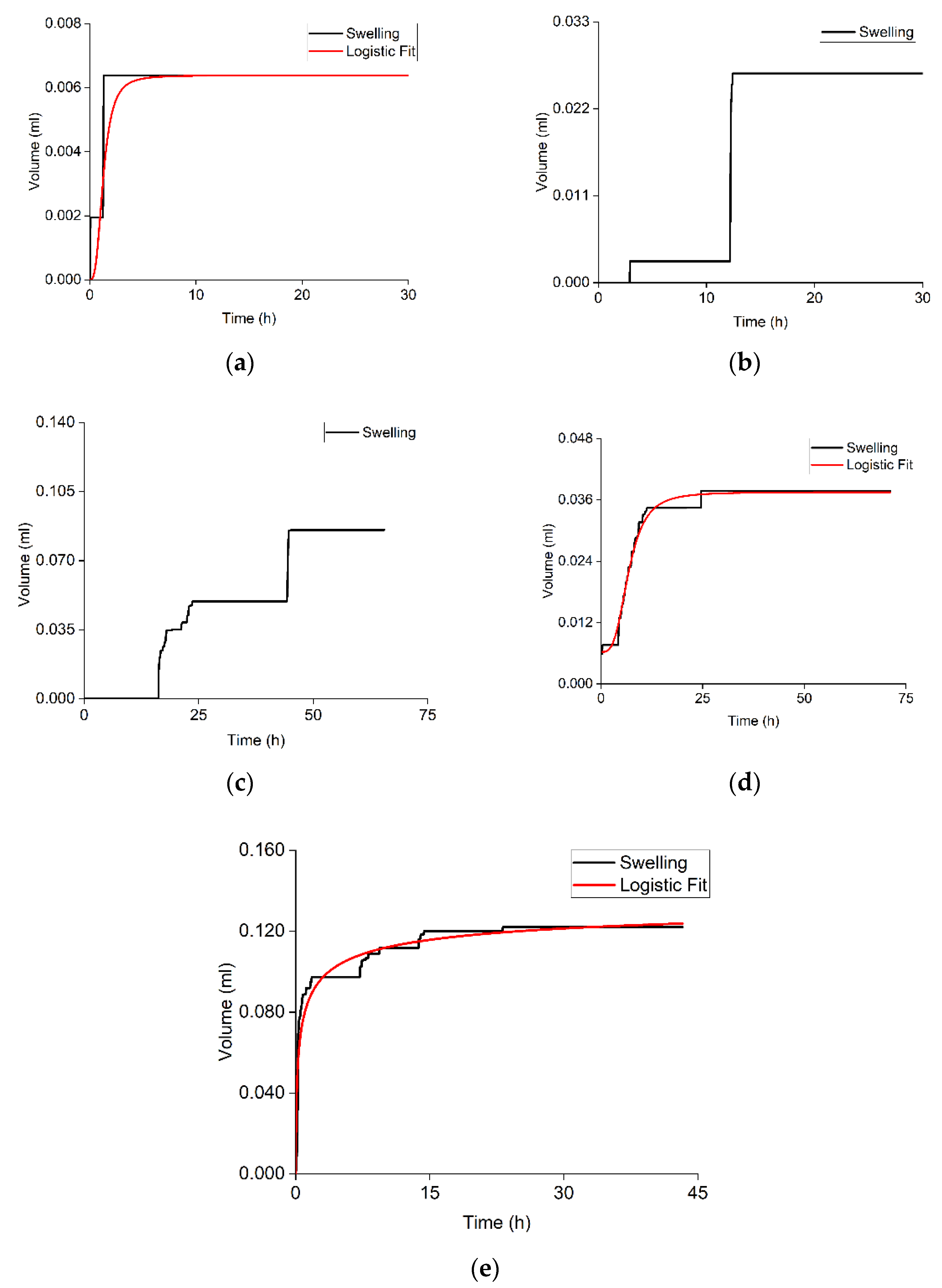
3.2. Bentonite Swelling
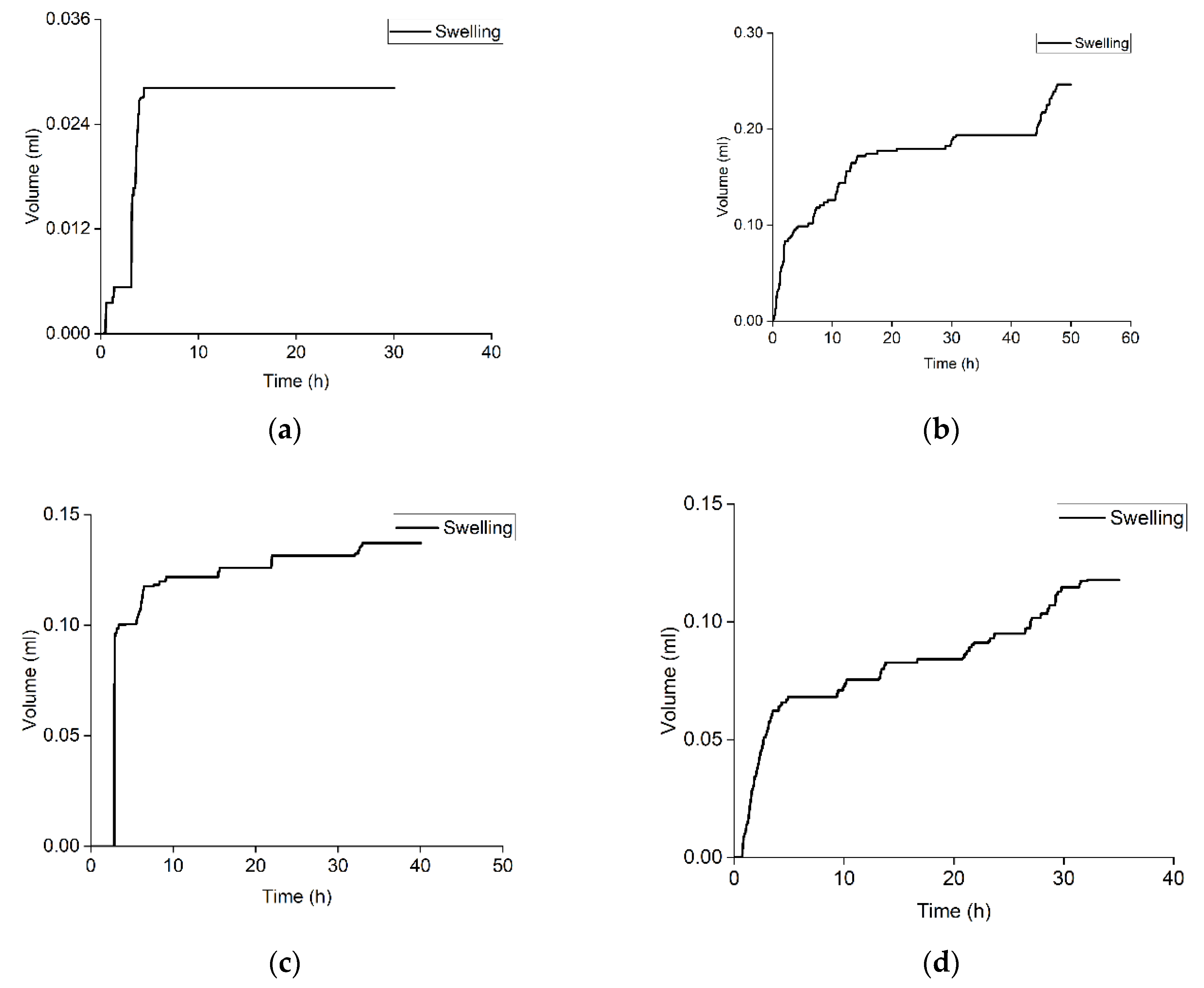
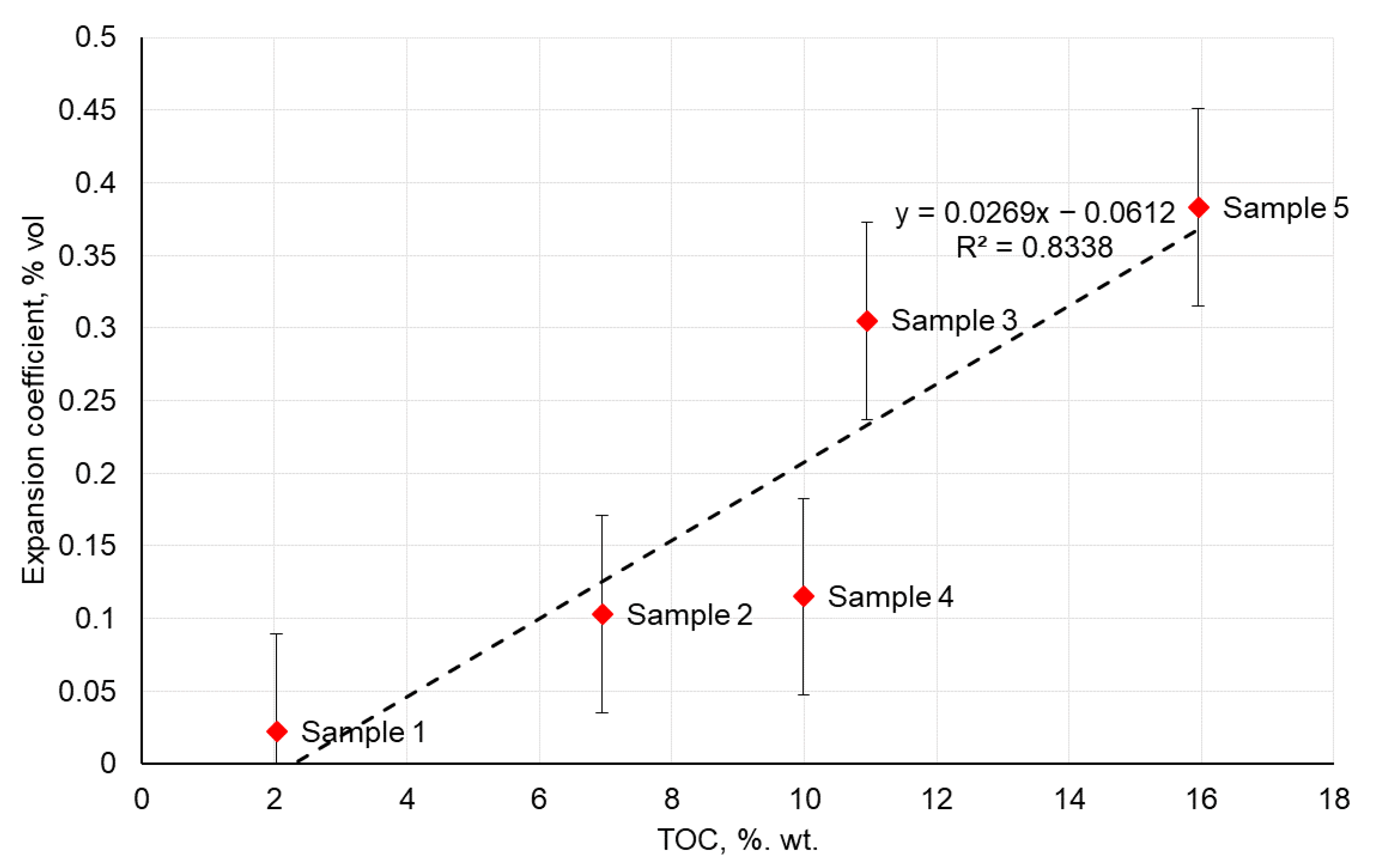
3.3. Swelling of Crushed Rock Samples in Alkaline Water Solution
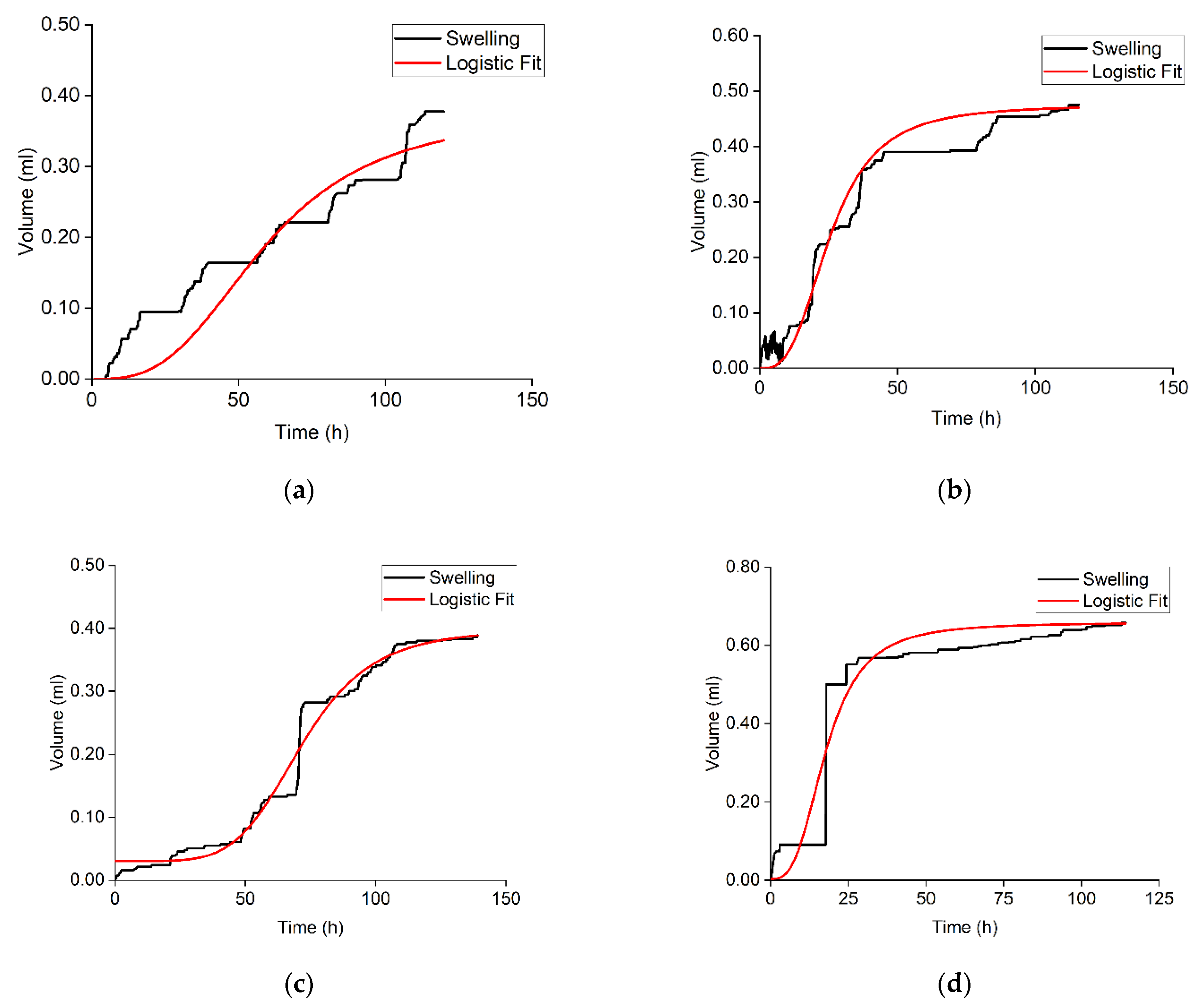
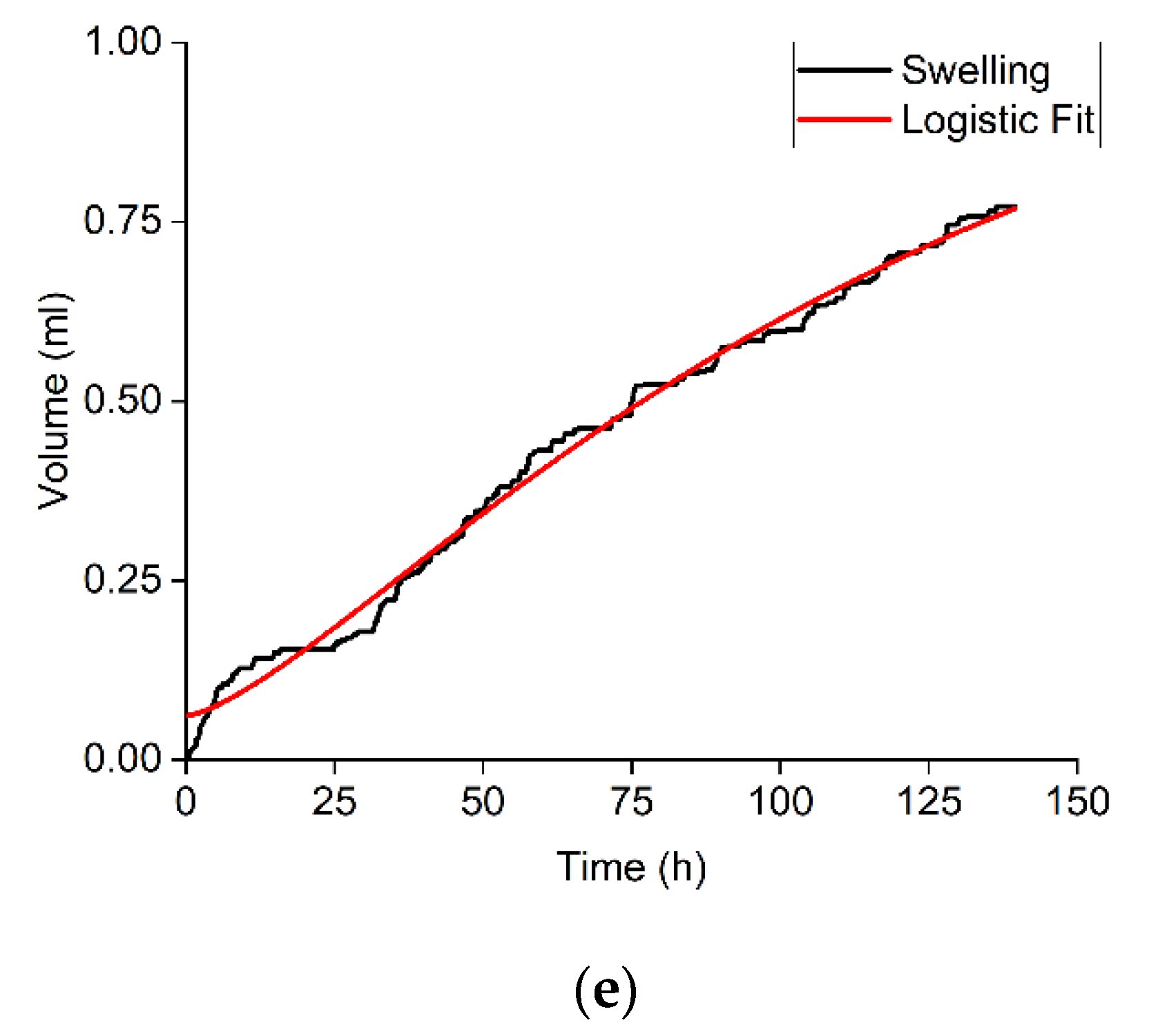
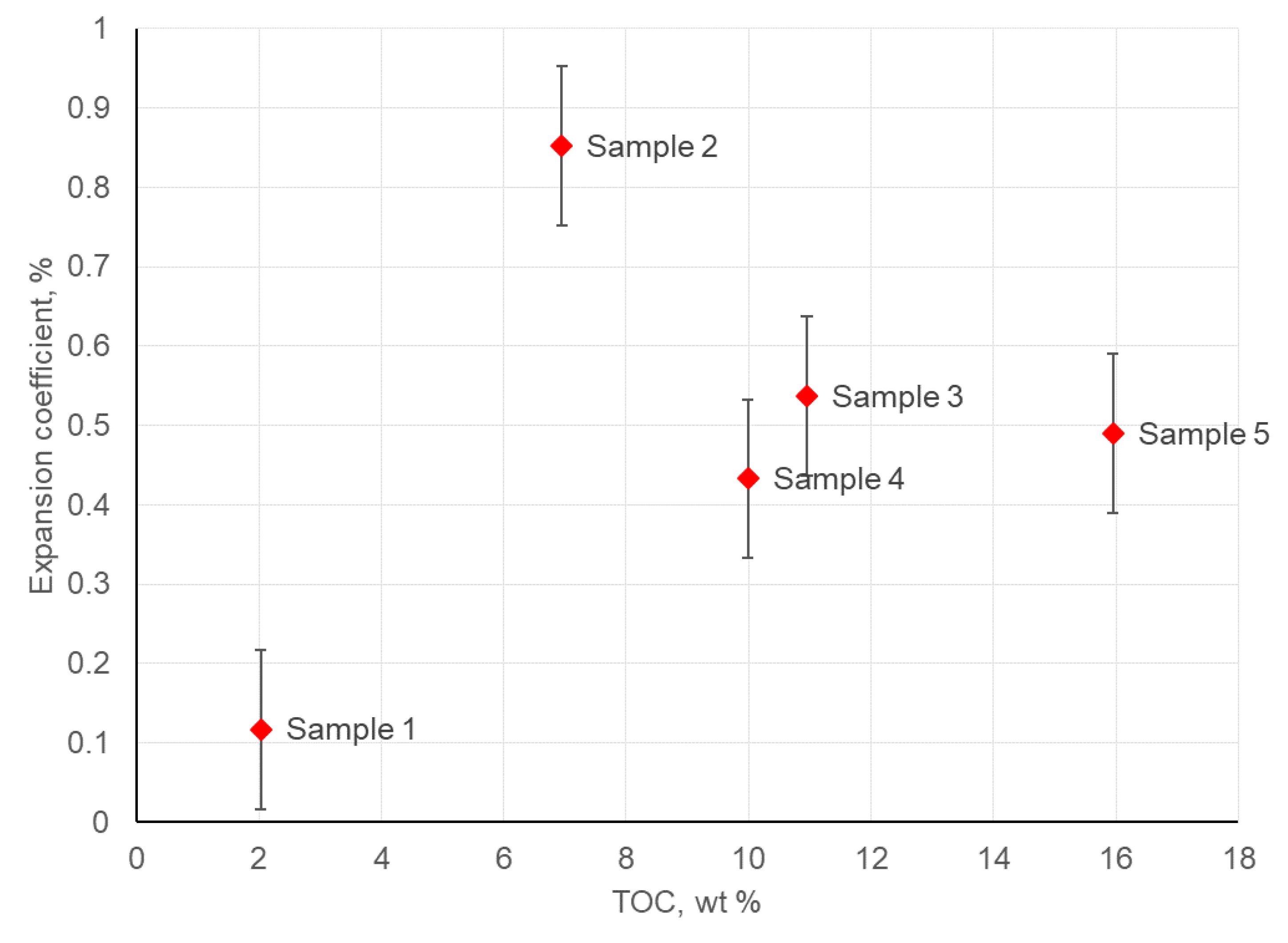
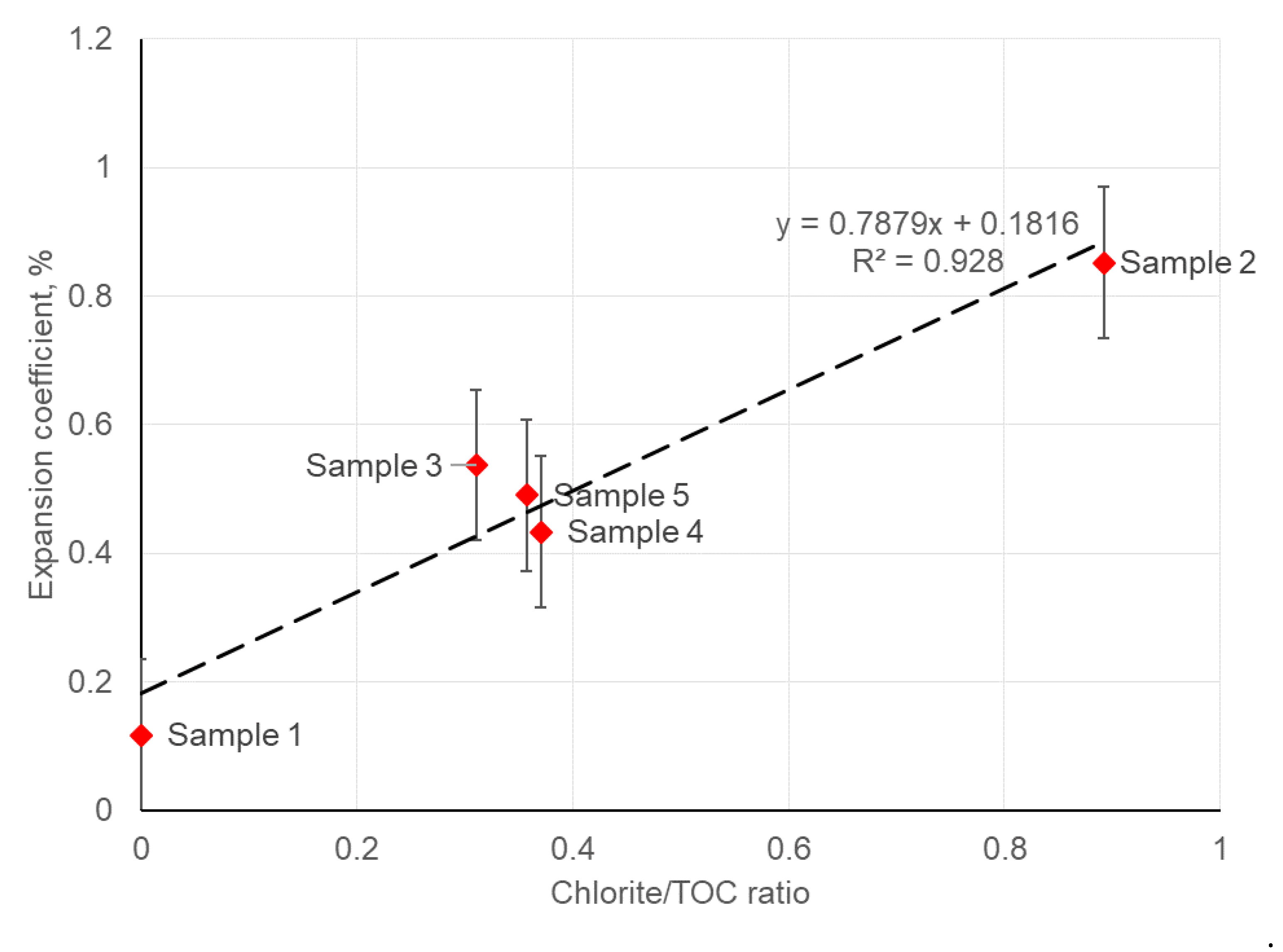
3.4. Swelling of Crushed Rock Samples in Hydrocarbon-Based Fracturing Fluid
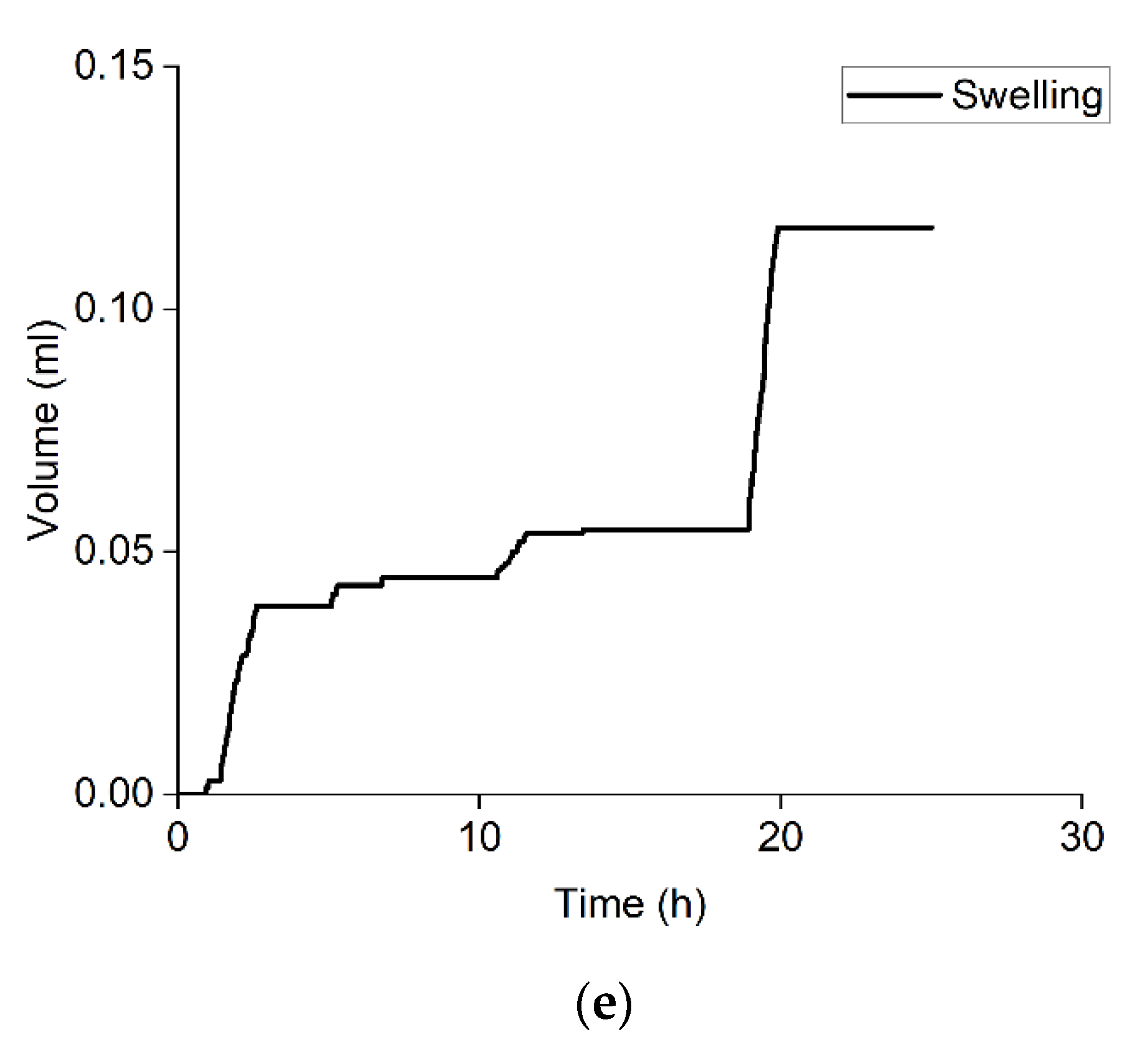
3.5. Rock Swelling in CO2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omran, M.; Berg, C.F. Applying New Rock Typing Methods, and Modelling for Conventional & Unconventional Reservoirs. In Proceedings of the International Petroleum Technology Conference, Society of Petroleum Engineers, Dhahran, Saudi Arabia, 13–15 January 2020. [Google Scholar] [CrossRef]
- Arthur, M.A.; Cole, D.R. Unconventional Hydrocarbon Resources: Prospects and Problems. Elements 2014, 10, 257–264. [Google Scholar] [CrossRef]
- Ilgen, A.G.; Heath, J.E.; Akkutlu, I.Y.; Bryndzia, L.T.; Cole, D.R.; Kharaka, Y.K.; Kneafsey, T.J.; Milliken, K.L.; Pyrak-Nolte, L.J.; Suarez-Rivera, R. Shales at All Scales: Exploring Coupled Processes in Mudrocks. Earth-Sci. Rev. 2017, 166, 132–152. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Surface Area and Pore-Size Distribution in Clays and Shales. In Proceedings of the SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar] [CrossRef]
- Neuzil, C.E. Permeability of Clays and Shales. Annu. Rev. Earth Planet. Sci. 2019, 47, 247–273. [Google Scholar] [CrossRef]
- Odusina, E.; Sondergeld, C.; Rai, C. An NMR Study on Shale Wettability. In Proceedings of the Canadian Unconventional Resources Conference, Society of Petroleum Engineers, Calgary, AB, Canada, 15–17 November 2011. [Google Scholar] [CrossRef]
- Xu, M.; Dehghanpour, H. Advances in Understanding Wettability of Tight and Shale Gas Formations. Energy Fuels 2014, 18, 4362–4375. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of Gas Shale Pore Systems by Porosimetry, Pycnometry, Surfacearea, Andfield Emission Scanning Electron Microscopy/Transmission Electron Microscopy Image Analyses: Examples from the Barnett, Woodford, Haynesville, Marcellus, And Doig Units. Am. Assoc. Pet. Geol. Bull. 2012, 96, 1099–1119. [Google Scholar] [CrossRef]
- Fu, C.; Liu, N. Waterless Fluids in Hydraulic Fracturing–A Review. J. Nat. Gas Sci. Eng. 2019, 67, 214–224. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, G.; Bai, Y.; Wang, X.; Li, D. Probing the Mechanism of External Fluids Invasion of Nanopores in Fractured Tight Sandstone Reservoir. J. Dispers. Sci. Technol. 2022, 0, 1–13. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J.; Emadibaladehi, H.; Tu, J. Experimental Study of the Stimulating Mechanism of Shut-in after Hydraulic Fracturing in Unconventional Oil Reservoirs. Fuel 2021, 300, 120982. [Google Scholar] [CrossRef]
- Khan, H.J.; Spielman-Sun, E.; Jew, A.D.; Bargar, J.; Kovscek, A.; Druhan, J.L. A Critical Review of the Physicochemical Impacts of Water Chemistry on Shale in Hydraulic Fracturing Systems. Environ. Sci. Technol. 2021, 55, 1377–1394. [Google Scholar] [CrossRef]
- Lyu, Q.; Ranjith, P.G.; Long, X.; Kang, Y.; Huang, M. A Review of Shale Swelling by Water Adsorption. J. Nat. Gas Sci. Eng. 2015, 27, 1421–1431. [Google Scholar] [CrossRef]
- Cheng, B.; Li, J.; Li, J.; Su, H.; Tang, L.; Yu, F.; Jiang, H. Pore-Scale Formation Damage Caused by Fracturing Fluids in Low-Permeability Sandy Conglomerate Reservoirs. J. Pet. Sci. Eng. 2022, 208, 109301. [Google Scholar] [CrossRef]
- Anderson, R.L.; Ratcliffe, I.; Greenwell, H.C.; Williams, P.A.; Cliffe, S.; Coveney, P.V. Clay Swelling-A Challenge in the Oilfield. Earth-Sci. Rev. 2010, 98, 201–216. [Google Scholar] [CrossRef]
- Karpiński, B.; Szkodo, M. Clay Minerals–Mineralogy and Phenomenon of Clay Swelling in Oil & Gas Industry. Adv. Mater. Sci. 2015, 15, 37–55. [Google Scholar] [CrossRef]
- Minh, D.N.; Gharbi, H.; Rejeb, A.; Vale, F. Experimental Study of the Influence of the Degree of Saturation on Physical and Mechanical Properties in Tournemire Shale (France). Appl. Clay Sci. 2004, 26, 197–207. [Google Scholar] [CrossRef]
- Mohan, K.K.; Fogler, H.S. Colloidally Induced Smectitic Fines Migration: Existence of Microquakes. AIChE J. 1997, 43, 565–576. [Google Scholar] [CrossRef]
- Civan, F. Reservoir Formation Damage: Fundamentals, Modelling, Assessment and Mitigation; Gulf Publishing Company: Houston, TX, USA, 2000. [Google Scholar]
- Razzaghi-Koolaee, F.; Zargar, G.; Soltani Soulgani, B.; Mehrabianfar, P. Application of a Non-Ionic Bio-Surfactant Instead of Chemical Additives for Prevention of the Permeability Impairment of a Swelling Sandstone Oil Reservoir. J. Pet. Explor. Prod. Technol. 2021, 12, 1523–1539. [Google Scholar] [CrossRef]
- Tariq, Z.; Murtaza, M.; Kamal, M.S.; Muhammad, S.; Hussain, S. Clay Swelling Mitigation During Fracturing Operations Using Novel Magnetic Surfactants. In Proceedings of the International Petroleum Technology Conference, Riyadh, Saudi Arabia, 21–23 February 2022. [Google Scholar] [CrossRef]
- Bedrikovetsky, P.; Siqueira, F.D.; Furtado, C.A.; Souza, A.L.S. Modified particle detachment model for colloidal transport in porous media. Transp. Porous Media 2011, 86, 353–383. [Google Scholar] [CrossRef]
- Tangparitkul, S.; Saul, A.; Leelasukseree, C.; Yusuf, M.; Kalantariasl, A. Fines migration and permeability decline during reservoir depletion coupled with clay swelling due to low-salinity water injection: An analytical study. J. Pet. Sci. Eng. 2020, 194, 107448. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Guo, J.; Su, Q.; Zhuo, X. Effects of Inhibitor KCl on Shale Expansibility and Mechanical Properties. Petroleum 2019, 5, 407–412. [Google Scholar] [CrossRef]
- Fu, L.; Liao, K.; Ge, J.; He, Y.; Huang, W.; Du, E. Preparation and Inhibition Mechanism of Bis-Quaternary Ammonium Salt as Shale Inhibitor Used in Shale Hydrocarbon Production. J. Mol. Liq. 2020, 309, 113244. [Google Scholar] [CrossRef]
- Mojid, M.R.; Negash, B.M.; Abdulelah, H.; Jufar, S.R.; Adewumi, B.K. A State–of–Art Review on Waterless Gas Shale Fracturing Technologies. J. Pet. Sci. Eng. 2021, 196, 108048. [Google Scholar] [CrossRef]
- Fu, M.; Huang, Q.; Gu, Y.; Xu, L.; Chen, L. Development of Novel Silicon-Based Thickeners for a Supercritical CO2 Fracturing Fluid and Study on Its Rheological and Frictional Drag Behavior. Energy Fuels 2020, 34, 15752–15762. [Google Scholar] [CrossRef]
- Wang, Y.; Bedrikovetsky, P.; Yin, H.; Othman, F.; Zeinijahromi, A.; Le-Hussain, F. Analytical model for fines migration due to mineral dissolution during CO2 injection. J. Nat. Gas Sci. Eng. 2022, 100, 104472. [Google Scholar] [CrossRef]
- Tesson, S.; Firoozabadi, A. Deformation and Swelling of Kerogen Matrix in Light Hydrocarbons and Carbon Dioxide. J. Phys. Chem. C 2019, 123, 29173–29183. [Google Scholar] [CrossRef]
- Lopatin, N.V.; Zubairaev, S.L.; Kos, I.M.; Emets, T.P.; Romanov, E.A.; Malchikhina, O.V. Unconventional Oil Accumulations in the Upper Jurassic Bazhenov Black Shale Formation, West Siberian Basin: A Self-Sourced Reservoir System. J. Pet. Geol. 2003, 26, 225–244. [Google Scholar] [CrossRef]
- Postnikova, O.V.; Postnikov, A.V.; Zueva, O.A.; Kozionov, A.E.; Milovanova, E.V.; Savinova, L.A. Types of Void Space in the Bazhenov Reservoir Rocks. Geosciences 2021, 11, 269. [Google Scholar] [CrossRef]
- Palyanitsina, A.; Sukhikh, A. Peculiarities of Assessing the Reservoir Propties of Clayish Reservoirs Depending on the Water of Reservoir Pressure Maintenance System Properties. J. Appl. Eng. Sci. 2020, 18, 10–14. [Google Scholar] [CrossRef][Green Version]
- Kuznetsova, A.N.; Rogachev, M.K.; Sukhih, A.S. Surfactant Solutions for Low-Permeable Polimictic Reservoir Flooding. IOP Conf. Ser. Earth Environ. Sci. 2018, 194, 042011. [Google Scholar] [CrossRef]
- Reynolds, M.A. A Technical Playbook for Chemicals and Additives Used in the Hydraulic Fracturing of Shales. Energy Fuels 2020, 34, 15106–15125. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Huang, R.Z.; Chen, Y.F. Constitutive Equations of Shale and Clay Swelling. Theoretical Model and Laboratory Test under Confining Pressure. In Proceedings of the International meeting on Petroleum Engineering, Beijing, China, 24–27 March 1992. [Google Scholar] [CrossRef]
- Spasennykh, M.; Maglevannaia, P.; Kozlova, E.; Bulatov, T.; Leushina, E.; Morozov, N. Geochemical Trends Reflecting Hydrocarbon Generation, Migration and Accumulation in Unconventional Reservoirs Based on Pyrolysis Data (On the Example of the Bazhenov Formation). Geosciences 2021, 11, 307. [Google Scholar] [CrossRef]
- Bazhenova, T.K. Osnovy Regionalnoy Organicheskoy Geokhimii; GEOS: Moscow, Russia, 2020. [Google Scholar]
- Ivanov, K.S.; Maslennikov, V.V.; Artemyev, D.A.; Tseluiko, A.S. Highly Metalliferous Potential of Framboidal and Nodular Pyrite Varieties from the Oil-Bearing Jurassic Bazhenov Formation, Western Siberia. Minerals 2020, 10, 449. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Saaid, I.M.; Akhir, N.A.M.; Rashedi, M. Influence of Various Cation Valence, Salinity, PH and Temperature on Bentonite Swelling Behaviour. AIP Conf. Proc. 2016, 1774, 040005. [Google Scholar] [CrossRef]
- Wersin, P.; Curti, E.; Appelo, C.A.J. Modelling Bentonite-Water Interactions at High Solid/Liquid Ratios: Swelling and Diffuse Double Layer Effects. Appl. Clay Sci. 2004, 26, 249–257. [Google Scholar] [CrossRef]
- Shirazi, S.M.; Kazama, H.; Kuwano, J.; Rashid, M.M. The Influence of Temperature on Swelling Characteristics of Compacted Bentonite for Waste Disposal. Environ. Asia 2010, 3, 60–64. [Google Scholar]
- Zhang, C.L.; Wieczorek, K.; Xie, M.L. Swelling Experiments on Mudstones. J. Rock Mech. Geotech. Eng. 2010, 2, 44–51. [Google Scholar] [CrossRef]
- Yilmaz, I. Gypsum/Anhydrite: Some Engineering Problems. Bull. Eng. Geol. Environ. 2001, 60, 227–230. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, H.; Tesson, S.; Firoozabadi, A. Absolute Adsorption of Light Hydrocarbons and Carbon Dioxide in Shale Rock and Isolated Kerogen. Fuel 2019, 235, 855–867. [Google Scholar] [CrossRef]
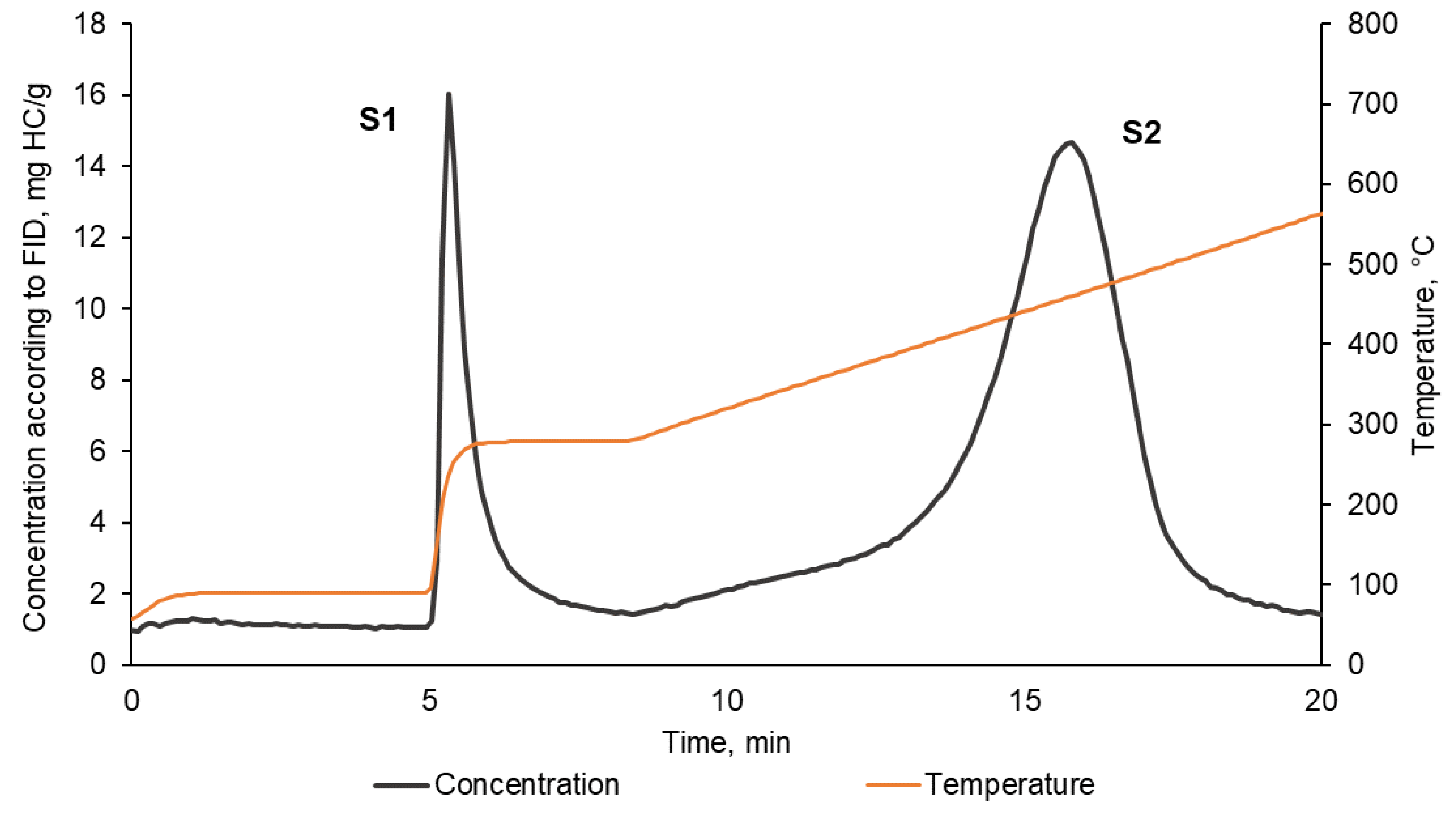


| Sample | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| S1, mgHC/g | 0.68 | 0.83 | 2.30 | 2.32 | 3.74 |
| S2, mgHC/g | 3.19 | 31.37 | 47.11 | 37.70 | 59.94 |
| S3, mgCO2/g | 0.36 | 0.23 | 0.25 | 0.52 | 0.45 |
| S4, mGCO/g | 41.99 | 108.37 | 184.98 | 191.21 | 314.01 |
| S5, mgCO/g | 3.92 | 1.06 | 4.21 | 30.19 | 2.14 |
| TOC, % wt. | 2.03 | 6.95 | 10.95 | 9.99 | 15.95 |
| HI, mgHC/gTOC | 157 | 452 | 430 | 377 | 376 |
| Tmax, °C | 441 | 439 | 437 | 434 | 435 |
| Minerals | Sample | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Albite | 6.70 | 9.60 | 6.50 | 7.60 | 5.30 |
| Anhydrite | 2.90 | 0.00 | 0.00 | 0.00 | 0.00 |
| Calcite | 1.80 | 0.00 | 0.00 | 4.50 | 0.00 |
| Pyrite | 0.30 | 5.30 | 2.50 | 1.60 | 8.20 |
| Quartz | 85.50 | 53.10 | 56.70 | 28.50 | 24.80 |
| Clays | |||||
| Chlorite | 0.00 | 6.20 | 3.40 | 3.70 | 5.70 |
| Illite | 2.80 | 19.40 | 22.30 | 31.60 | 29.00 |
| Kaolinite | 0.00 | 6.40 | 8.60 | 9.70 | 18.20 |
| Mixed-layer | 0.00 | 0.00 | 0.00 | 12.60 | 8.30 |
| Matrix density, g/mL | 2.11 | 2.36 | 2.54 | 2.31 | 2.72 |
| ωclays, % wt. | 2.74 | 29.60 | 30.40 | 51.57 | 51.58 |
| Sample | Volumetric Expansion Coefficient, % vol. | Adsorbed Water/Rock Mass Ratio, mg/g | R2 for the Approximation with a Logistic Function | Parameters of the Logistic Function | |||
|---|---|---|---|---|---|---|---|
| A1 | A2 | x0 | p | ||||
| 1 | 1.69 | 13.51 | 0.82 | 0 | 0.37 | 59.2 | 3.00 |
| 2 | 2.09 | 9.53 | 0.93 | 0.00 | 0.47 | 25.5 | 3.00 |
| 3 | 1.55 | 17.15 | 0.98 | 0.03 | 0.40 | 71.7 | 5.30 |
| 4 | 2.34 | 96.02 | 0.91 | 0.00 | 0.66 | 17.9 | 3.00 |
| 5 | 3.25 | 18.06 | 0.99 | 0.06 | 1.38 | 126.7 | 1.40 |
| Sample | Volumetric Expansion Coefficient, % vol. | Adsorbed Hydrocarbon/Rock Mass Ratio, mg/g |
|---|---|---|
| 1 | 0.117 | 4.15 |
| 2 | 0.852 | 4.02 |
| 3 | 0.537 | 3.47 |
| 4 | 0.433 | 3.44 |
| 5 | 0.490 | 3.40 |
| Sample | Volumetric Expansion Coefficient, % vol. | R2 for the Approximation with a Logistic Function | Parameters of the Logistic Function | |||
|---|---|---|---|---|---|---|
| A1 | A2 | x0 | p | |||
| 1 | 0.022 | 0.99 | 0.00 | 0.006 | 1.27 | 3.00 |
| 2 | 0.103 | - | - | - | - | - |
| 3 | 0.305 | - | - | - | - | - |
| 4 | 0.115 | 0.98 | 0.00 | 0.037 | 6.80 | 3.3 |
| 5 | 0.383 | 0.90 | 0.00 | 0.140 | 0.25 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunusov, T.I.; Smirnov, A.V.; Mukhina, E.D.; Potapenko, D.I.; Bukharov, D.F.; Baluev, A.A.; Cheremisin, A.N. Experimental Setup for Evaluating Rock Volume Alteration and Its Application for Studying Shale Rock Swelling in Various Fluids. Minerals 2022, 12, 714. https://doi.org/10.3390/min12060714
Yunusov TI, Smirnov AV, Mukhina ED, Potapenko DI, Bukharov DF, Baluev AA, Cheremisin AN. Experimental Setup for Evaluating Rock Volume Alteration and Its Application for Studying Shale Rock Swelling in Various Fluids. Minerals. 2022; 12(6):714. https://doi.org/10.3390/min12060714
Chicago/Turabian StyleYunusov, Timur I., Alexey V. Smirnov, Elena D. Mukhina, Dmitriy I. Potapenko, Dinar F. Bukharov, Anatoly A. Baluev, and Alexey N. Cheremisin. 2022. "Experimental Setup for Evaluating Rock Volume Alteration and Its Application for Studying Shale Rock Swelling in Various Fluids" Minerals 12, no. 6: 714. https://doi.org/10.3390/min12060714
APA StyleYunusov, T. I., Smirnov, A. V., Mukhina, E. D., Potapenko, D. I., Bukharov, D. F., Baluev, A. A., & Cheremisin, A. N. (2022). Experimental Setup for Evaluating Rock Volume Alteration and Its Application for Studying Shale Rock Swelling in Various Fluids. Minerals, 12(6), 714. https://doi.org/10.3390/min12060714








