The Origin of Synchysite-(Ce) and Sources of Rare Earth Elements in the Rožná Uranium Deposit, Czech Republic
Abstract
1. Introduction
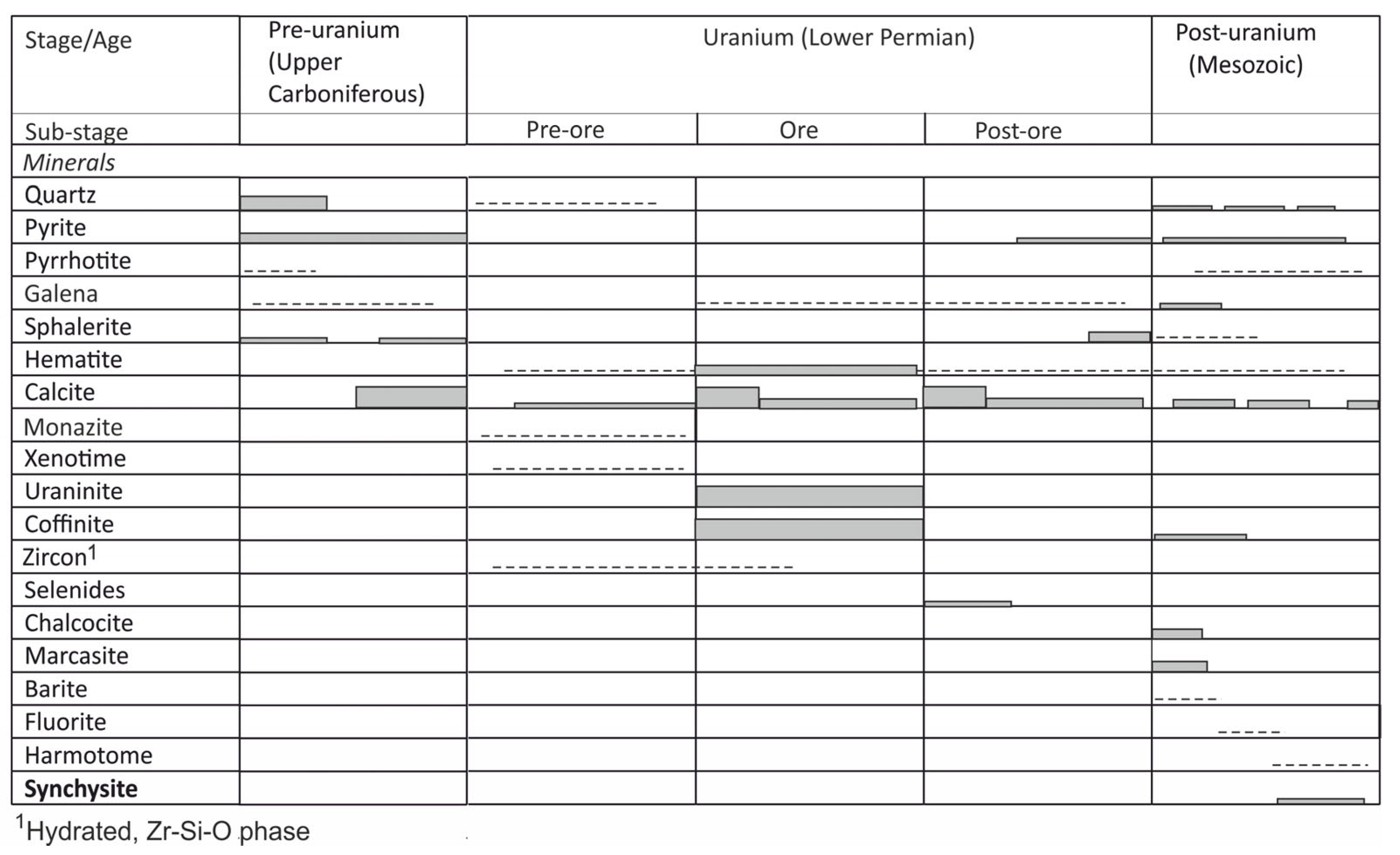
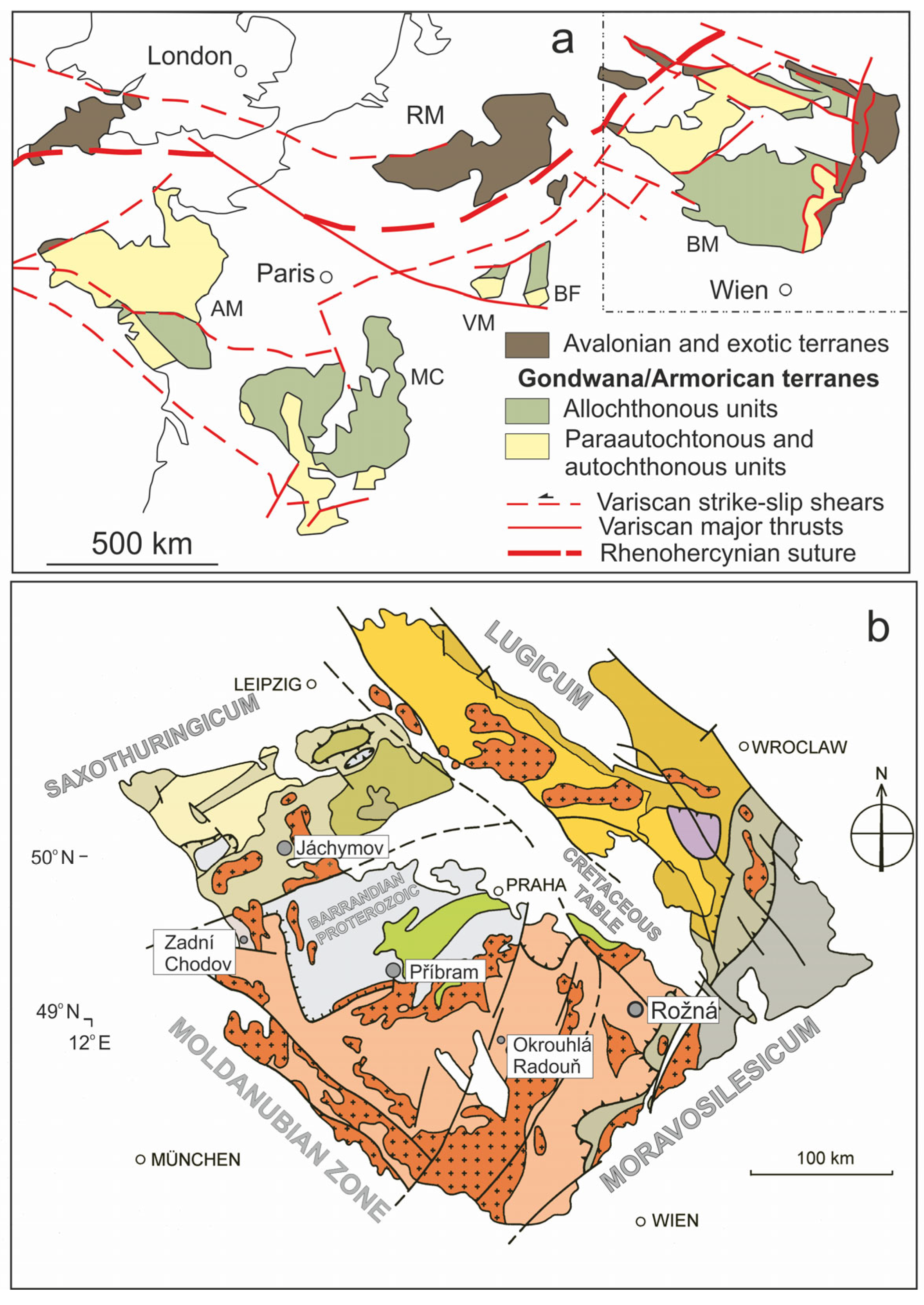
2. Geological Setting and Uranium Mineralization in the Rožná Deposit
3. Materials and Methods
4. Results
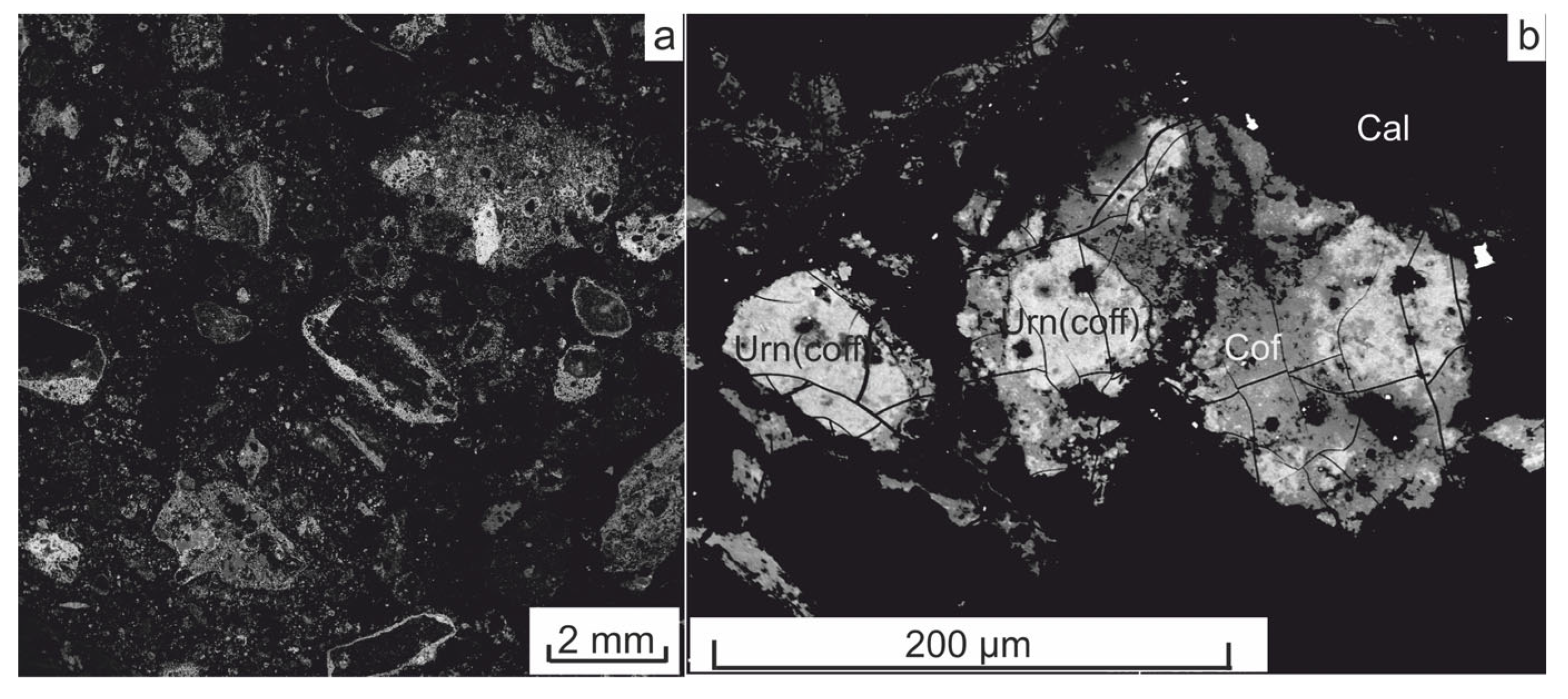
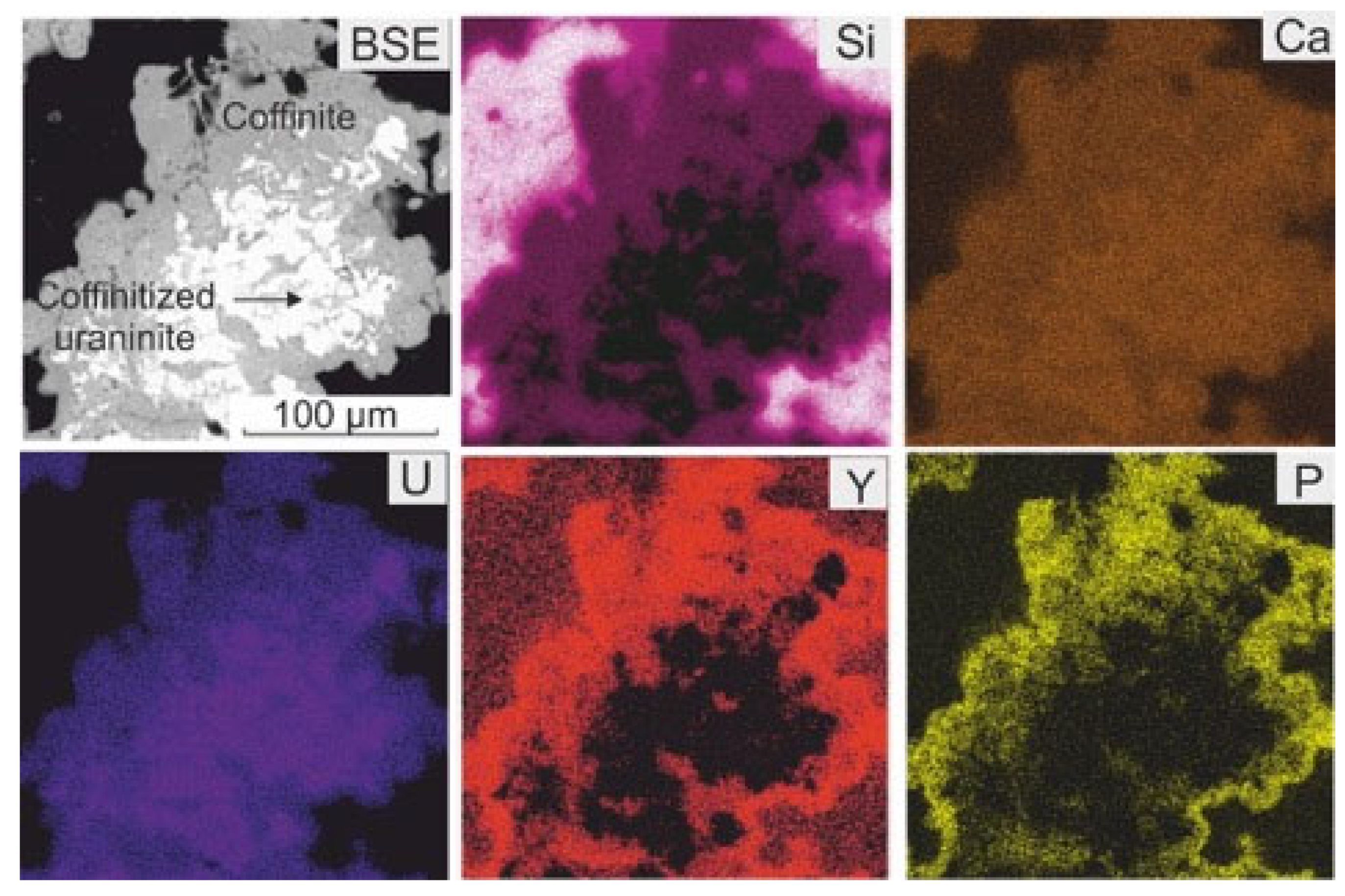
4.1. Petrography and Mineralogy of the Synchysite-Bearing Vein and Host Rocks
4.1.1. The Synchysite-Bearing Vein
4.1.2. Host Rocks
4.2. Textures of Synchysite-Bearing Quartz Filling of the Vein
4.3. Chemical Composition of Quartz–Carbonate–Sulfide Vein and Host Rocks (Whole Rock Analyses)
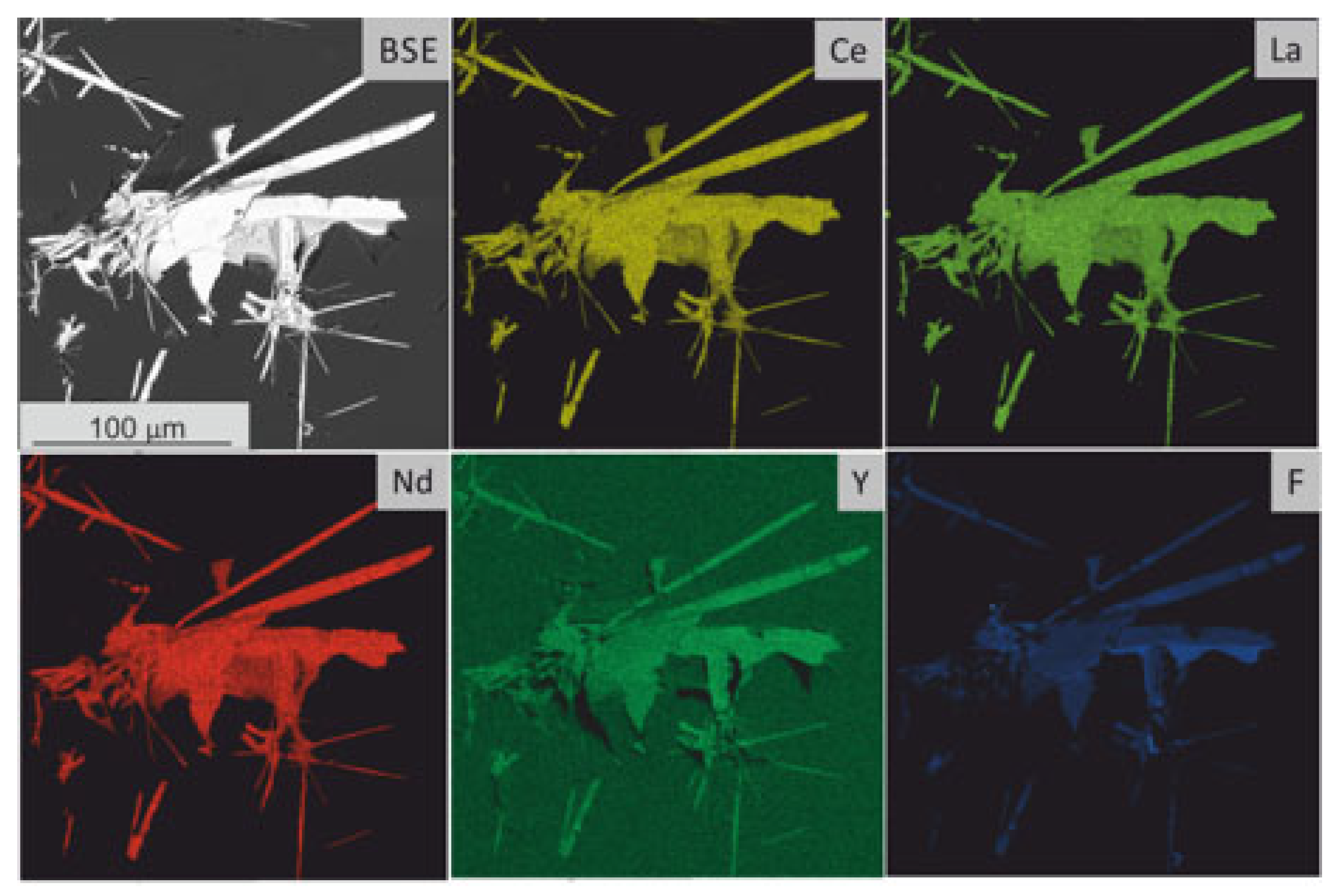
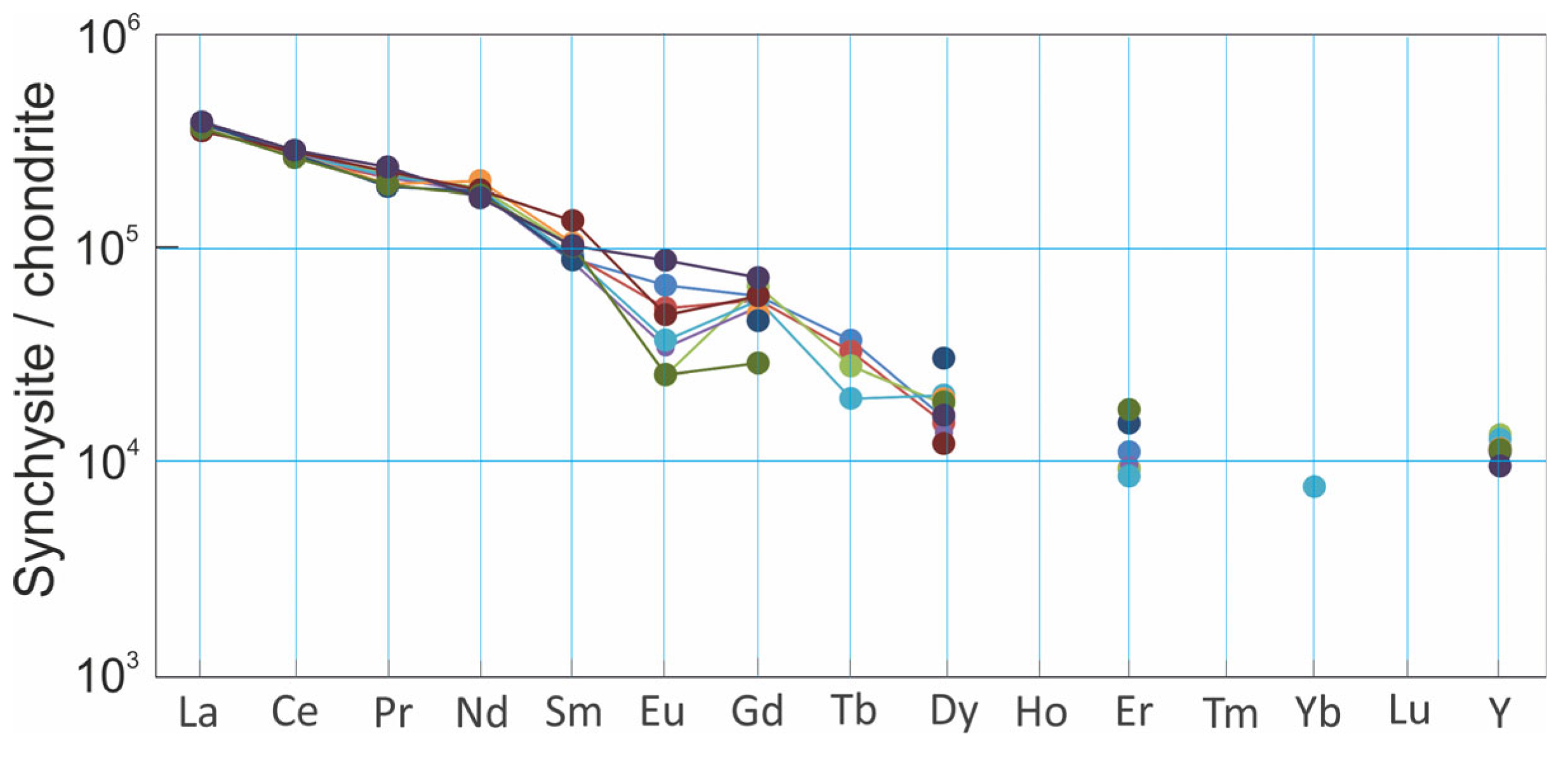
4.4. Synchysite Raman Spectrum and Chemistry
4.5. Fluid Inclusion Studies
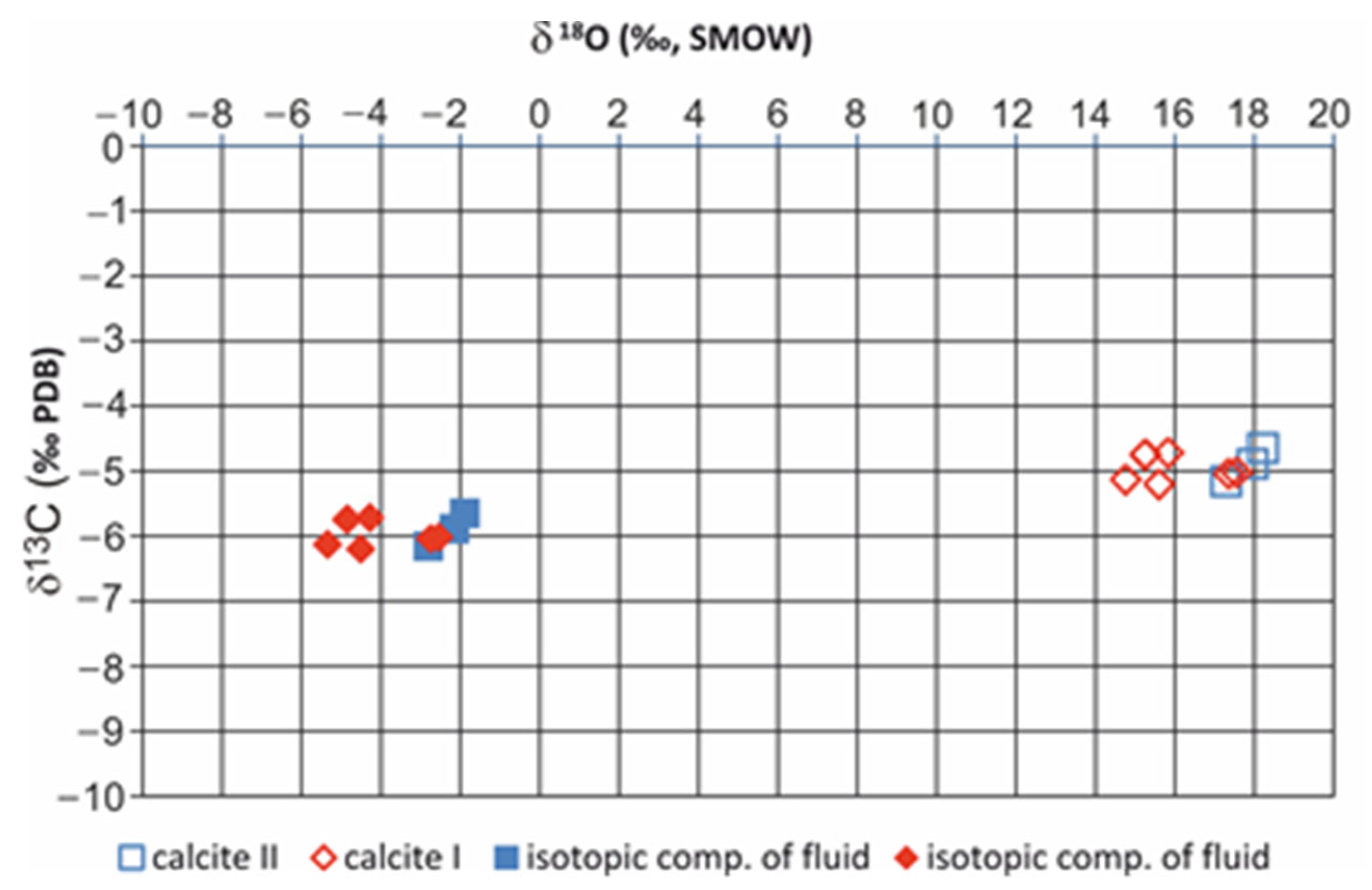
4.6. Stable Isotopes
5. Discussion
5.1. Silica Gel Formation and the Origin of Synchysite
5.2. Sources of REE for Synchysite Formation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COM/2020/474 Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability. Communication from the Comission to the European Parlament, the Council, the European Economic and Social Committee and the Committee of thef Regions. COM(2020) 474 Final. PDF, Brussels, 3 September 2020. Available online: https://eurlex.europa.eu/search.html?scope=EURLEX&text=Critical+Raw+Materials+Resilience+2020&lang=en&type=quick&qid=1649519818132&page=2 (accessed on 21 November 2021).
- Yang, Z.; Woolley, A. Carbonatites in China: Policies and narratives of reinventing an industry. Res. Policy 2006, 38, 384–394. [Google Scholar]
- Yang, X.Y.; Sun, W.D.; Zhang, Y.X.; Zheng, Y.F. Geochemical constraints on the genesis of the Bayan Obo Fe-Nb-REE deposit in Inner Mongolia, China. Geochim. Cosmochim. Acta 2009, 73, 1417–1435. [Google Scholar] [CrossRef]
- Fan, H.-R.; Yang, K.-F.; Hu, F.-F.; Liu, S.; Wang, K.-Y. The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Kynicky, J.; Smith, M.P.; Xu, C. Diversity of rare earth deposits: The key example of China. Elements 2012, 8, 361–367. [Google Scholar] [CrossRef]
- Elliott, H.A.L.; Wall, F.; Chakhmouradian, A.R.; Siegfried, P.R.; Dahlgren, S.; Weatherley, S.; Finch, A.A.; Marks, M.A.W.; Dowman, E.; Deady, E. Fenites associated with carbonatite complexes: A review. Ore Geol. Rev. 2018, 93, 38–59. [Google Scholar] [CrossRef]
- Hatch, G.P. Dynamics in the global market for rare earths. Elements 2012, 8, 341–346. [Google Scholar] [CrossRef]
- Simandl, G.J. Geology and market-dependent significance of rare earth element resources. Miner. Depos. 2014, 49, 889–904. [Google Scholar] [CrossRef]
- Groves, D.I.; Bierlein, F.P.; Meinert, L.D.; Hitzman, W. Iron Oxide Copper-Gold (IOCG) Deposits through Earth history: Implications for origin, lithospheric setting, and distinction from other epigenetic iron oxide deposits. Econ. Geol. 2010, 105, 641–654. [Google Scholar] [CrossRef]
- Reynolds, L.J. Geology of the Olympic Dam Cu-U-Ag-REE deposit. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective; Porter, T.D., Ed.; Australian Mineral Foundation: Adelaide, Australia, 2000; pp. 93–104. [Google Scholar]
- Oreskes, N.; Einaudi, M.T. Origin of Origin of rare earth element-enriched hematite breccias at the Olympic Dam Cu-U-Au-Ag deposit, Roxby Downs, South Australia. Econ. Geol. 1990, 85, 1–28. [Google Scholar] [CrossRef]
- Fayek, M.; Kyser, T.K. Characterization of multiple fluid-flow events and rare-earth-element mobility associated with formation of unconformity-type uranium deposits in the Athabasca basin, Saskatchewan. Can. Miner. 1997, 35, 627–658. [Google Scholar]
- Mercadier, J.; Cuney, M.; Cathelineau, M.; Lacorde, M. Origin of uranium deposits revealed by their rare earth element signature. Terra Nova 2011, 23, 264–269. [Google Scholar] [CrossRef]
- Roscoe, S.M. Paleoplacer uranium gold. In Geology of Canadian Minerals International Workshop, Geology of Rare Metals, Deposit Types; Geology of Canada Series no. 8; Eckstrand, O.R., Sinclair, W.D., Thorp, R.I., Eds.; Geological Survey of Canada: Otawa, ON, Canada, 1996; pp. 10–23. [Google Scholar]
- Cox, J.J.; Ciuculescu, T.; Hwozdyk, T.; Altman, L. Technical Report on the Eco Ridge Mine Rare Earths and Uranium Project, Elliot Lake, Ontario, Canada; Roscoe Postle Associates Inc.: Toronto, ON, Canada, 2011; Available online: http://www.pelemountain.com/pdf/0092011pea.pdf (accessed on 20 November 2020).
- Kwak, T.A.P.; Abeysinge, P.B. Rare earth and uranium minerals present present as daugthther crystals in fluid inclusion, Mary Kathleen U-REE skarn, Queensland, Australia. Miner. Mag. 1987, 51, 665–670. [Google Scholar] [CrossRef]
- René, M. Anomalous rare earth element, yttrium and zirconium mobility associated with uranium mineralization. Terra Nova 2008, 20, 52–58. [Google Scholar] [CrossRef]
- Bukovská, Z.; Švagera, O.; Chabr, T.; Leichmann, J.; Sosna, K. Data Mining from the Deep Horizons of the Rožná Mine; Final Report; Czech Geological Survey: Prague, Czech Republic, 2020; p. 691, unpublished work. [Google Scholar]
- Deiller, P.; Štípská, P.; Ulrich, M.; Schulmann, K.; Collett, S.; Peřestý, V.; Hacker, B.; Kylander-Clark, A.; Whitechurch, H.; Lexa, O.; et al. Eclogite subduction wedge intruded by arc-type magma: The earliest record of Variscan arc in the Bohemian Massif. Gondwana Res. 2021, 99, 220–246. [Google Scholar] [CrossRef]
- OECD. Uranium 2020: Resources, Production and Demand. In A Joint Report by the Nuclear Energy Agency and the International Atomic Energy Agency; NEA No. 7551; OECD: Boulogne-Billancourt, France, 2020; p. 47. [Google Scholar]
- Arapov, J.A.; Bojcov, V.J.; Czesnokov, N.I.; Djakonov, A.V.; Halbrštát, J.; Yakovenko, M.; Kolek, M.; Komínek, J.; Kozyrev, G.A.; Lazanský, M.; et al. Uranium Deposits of the Czechoslovakia; Czechoslovak Uranium Industry: Prague, Czech Republic, 1984; p. 420. (In Czech) [Google Scholar]
- Anderson, E.B.; Ivanov, P.A.; Komínek, J. Ore metasomatism at the uranium veins of the Rožná deposit. Geol. Hydrometal Uranium 1988, 12, 70–88. (In Czech) [Google Scholar]
- Hein, U.; Lehmann, B.; Křibek, B.; René, M. Evolution of ore-forming fluids along the Rožná- shear zone, Bohemian Massif, Czech Republic: Implication for local uranium deposition and comparison with U-mineralization at Schlema, Erzgebirge, Germany. In Proceedings of the International Symposium Uranium Deposits: From their Genesis to their Environmental Aspects, Prague, Czech Republic, 10–11 September 2002; Kříbek, B., Zeman, J., Eds.; Czech Geological Survey: Prague, Czech Republic, 2002; pp. 61–64. [Google Scholar]
- Kříbek, B.; Žák, K.; Dobeš, P.; Leichmann, J.; Pudilová, M.; René, M.; Scharm, B.; Scharmová, M.; Hájek, A.; Holeczy, D.; et al. The Rožná uranium deposit (Bohemian Massif, Czech Republic): Shear zone-hosted, late Variscan and post-Variscan hydrothermal mineralization. Miner. Depos. 2009, 44, 99–128. [Google Scholar] [CrossRef]
- Kříbek, B.; Hájek, T. (Eds.) The Rožná Uranium Deposit: A Model of Late-Variscan and Post-Variscan Mineralization; Czech Geological Survey: Prague, Czech Republic, 2005; p. 89. ISBN 80-7075-629-2. (In Czech) [Google Scholar]
- Dempírová, L.; Vitková, H. Methods of Certified Chemical Analyses; Czech Geological Survey: Prague, Czech Republic, 2002; p. 230. (In Czech) [Google Scholar]
- Merlet, C. An accurate Computer correction program for quantitative electron probe microanalysis. Microchim. Acta 1994, 114, 363–376. [Google Scholar] [CrossRef]
- Bruker, A.X.S. Topas 5; Bruker AXS GmbH: Karlsruhe, Germany, 2015; p. 247. [Google Scholar]
- Bruker, A.X.S. DIFFRAC.EVA, Version 4.1; Bruker AXS GmbH: Karlsruhe, Germany, 2017; p. 230. [Google Scholar]
- Bodnar, R.J.; Vitik, M.O. Interpretation of microthermometric data for H2O-NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications. Short Course of the Working Group “Inclusions in Minerals”; Blacksburg, V.A., De Vivo, B., Frezzotti, M.L., Eds.; Virginia Polytechnic Institute: Blacksburg, VA, USA, 1994; pp. 117–130. [Google Scholar]
- Davis, D.W.; Lowenstein, T.M.; Spencer, R.J. Melting behavior of fluid inclusions in laboratory-grown halite crystals in the systems NaCl-H2O, NaCl-KCl-H2O, NaCl-MgCl2-H2O, and NaCl-CaCl2-H2O. Geochim. Cosmochim. Acta 1990, 54, 591–601. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of fluid inclusions in diagenetic minerals. In SEPM Short Course No. 31; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1994; p. 199. [Google Scholar]
- McCrea, J.M. On the isotopic chemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Kim, S.T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Boynton, W.V. Cosmochemistry of the rare earth elements: Meteorite studies. Dev. Geochem. 1984, 2, 63–114. [Google Scholar]
- Irber, W. The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu*, Sr/Eu, Y/Ho, and Zr/Hf of evolving peraluminous granite suites. Geochim. Cosmochim. Acta 1999, 63, 489–508. [Google Scholar] [CrossRef]
- Scheetz, B.E.; White, W.B. Vibrational spectra of the alkaline earth double carbonates. Am. Miner. 1977, 62, 36–50. [Google Scholar]
- Wehrmeister, U.; Soldati, A.L.; Jacob, D.E.; Häger, T.; Hofmeister, W. Raman spectroscopy of synthetic, geological and biological vaterite: A Raman spectroscopic study. J. Raman Spectrosc. 2010, 41, 193–201. [Google Scholar] [CrossRef]
- Frost, R.L.; Dickfos, M.J. Raman spectroscopy of halogen-containing carbonates. J. Raman Spectrosc. 2007, 38, 1516–1522. [Google Scholar] [CrossRef]
- Alles, J.; Ploch, A.M.; Schirmer, T.; Nolte, N.; Liessmann, W.; Lehmann, B. Rare-earth-element enrichment in post-Variscan polymetallic vein systems of the Harz Mountains, Germany. Miner. Depos. 2019, 54, 307–328. [Google Scholar] [CrossRef]
- Herrington, R.J.; Wilkinson, J.J. Colloidal gold and silica in mesothermal vein systems. Geology 1993, 21, 539–542. [Google Scholar] [CrossRef]
- Sibson, I.R.H.; Scott, J. Stress/fault controls on the containment and release of overpressured fluids: Examples from gold-quartz vein systems in Juneau, Alaska; Victoria, Australia and Otago, New Zealand. Ore Geol. Rev. 1998, 13, 293–306. [Google Scholar] [CrossRef]
- Oehler, J.H. Hydrothermal crystallization of silica gel. Geol. Soc. Am. Bull. 1976, 87, 1143–1152. [Google Scholar] [CrossRef]
- Williams, L.A.; Crerar, D.A. Silica diagenesis. II. General mechanisms. J. Sediment. Petrol. 1985, 55, 312–321. [Google Scholar]
- Williams, L.A.; Pasts, G.A.; Crerar, D.A. Silica diagenesis, I. Solubility controls. J. Sediment. Petrol. 1985, 55, 301–311. [Google Scholar]
- Rimstidt, J.D.; Barnes, H.L. The kinetics of silica-water reactions. Geochim. Cosmochim. Acta 1980, 44, 1683–1699. [Google Scholar] [CrossRef]
- Onasch, C.M.; Farver, J.R.; Dunne, W.M. The role of dilatation and cementation in the formation of cataclasite in low temperature deformation of well cemented quartz-rich rocks. J. Struct. Geol. 2010, 32, 1912–1922. [Google Scholar] [CrossRef]
- Nakamura, Y.; Muto, J.; Nagahama, H.; Shimizu, I.; Miura, T.; Arakawa, I. Amorphization of quartz by friction: Implication to silica-gel lubrication of fault surfaces. Geophys. Res. Lett. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Borhara, K.; Onasch, C.M. Evidence for silica gel and its role in faulting in the Tuscarora sandstone. 2020. J. Struct. Geol. 2020, 139, 104140. [Google Scholar] [CrossRef]
- Hayashi, N.; Tsutsumi, A. Deformation textures and mechanical behavior of a hydrated amorphous silica formed along an experimentally produced fault in chert. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Schubel, K.A.; Simonson, B.M. Petrography and diagenesis of chert formation from lake Magadi, Kenya. J. Sediment. Petrol. 1990, 60, 761–776. [Google Scholar]
- Pauchard, L.; Parisse, F.; Allain, C. Influence of salt content on crack patterns formed through colloidal suspension desiccation. Phys. Rev. E 1999, 59, 3737. [Google Scholar] [CrossRef]
- Scherer, G.W. Mechanics of syneresis I. Theory. J. Non-Cryst. Solids 1989, 108, 18–27. [Google Scholar] [CrossRef]
- Elliston, J. Hydration of silica and its role in the formation of quartz veins—Part 1. Substantia 2018, 2, 43–71. [Google Scholar] [CrossRef]
- Plummer, P.S.; Gostin, A.V. Shrinkage cracks: Desiccation or synaeresis? J. Sediment. Res. 1981, 51, 1147–1156. [Google Scholar] [CrossRef]
- Krug, H.-J.; Brandtstadter, H.; Jacob, K.H. Morphological instabilities in pattern formation by precipitation and crystallization processes. Geol. Rundsch. 1996, 85, 19–28. [Google Scholar] [CrossRef]
- Ostwald, W. A-Linien von R. E. Liesegang (paper review). Z. Phys. Chem. 1897, 23, 365. [Google Scholar]
- Lagzi, I. Simulation of Liesegang patterns: Effect of reversible complex formation of precipitate. J. Phys. Chem. B 2003, 107, 13750–13753. [Google Scholar] [CrossRef]
- George, J.; Varghese, G. Intermediate colloidal formation and the varying width of periodic precipitation bands in reaction–diffusion systems. J. Colloid Interface Sci. 2005, 282, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Liesegang, M.; Milke, R. Australian sedimentary opal-A and its associated minerals: Implications for natural silica sphere formation. Am. Miner. 2014, 99, 1488–1499. [Google Scholar] [CrossRef]
- Bethke, C.M. Geochemical Reaction Modeling. In Concepts and Applications; Oxford University Press: New York, NY, USA; Oxford, UK, 1996; p. 397. [Google Scholar]
- Migdisov, A.; Williams-Jones, A.E.; Brugger, J.; Caporuscio, F.A. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef]
- Loges, A.; Migdisov, A.A.; Wagner, T.; Williams-Jones, A.E.; Markl, G. An experimental study of the aqueous solubility and speciation of Y(III) fluoride at temperatures up to 250 °C. Geochim. Cosmochim. Acta 2013, 123, 403–415. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine bearing aqueous liquids. Miner. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Perry, P.E.; Gysi, A.P. Rare earth elements in mineral deposits: Speciation in hydrothermal fluids and partitioning in calcite. Geofluids 2018, 5382480. [Google Scholar] [CrossRef]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Cosmochim. Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]
- Fei, Y.; Hua, J.; Liu, C.; Li, F.; Zhu, Z.; Xiao, T.; Chen, M.; Gao, T.; Wei, Z.; Hao, L. Aqueous Fe(II)-induced phase transformation of ferrihydrite coupled adsorption/immobilization of rare earth elements. Minerals 2018, 8, 357. [Google Scholar] [CrossRef]
- Yang, M.; Liang, X.; Li, Y.; He, H.; Zhu, R.; Arai, Y. Ferrihydrite transformation impacted by adsorption and structural incorporation of rare earth elements. ACS Earth Space Chem. 2021, 5, 2768–2777. [Google Scholar] [CrossRef]
- Piasecki, W.; Sverjensky, D.A. Speciation of adsorbed yttrium and rare earth elements on oxide surfaces. Geochim. Cosmochim. Acta 2008, 72, 3964–3979. [Google Scholar] [CrossRef]
- Ortoleva, P.; Merino, E.; Moore, C.; Chadam, J. Geochemical self-organisation 1: Reaction-transport feedbacks and modeling approach. Am. J. Sci. 1987, 287, 979–1007. [Google Scholar] [CrossRef]
- Barker, S.L.L.; Cox, S.F.; Eggins, S.M.; Gagan, M.K. Microchemical evidence for episodic growth of individual growth of antitaxial veins during fracture-controlled fluid flow. Earth Planet. Sci. Lett. 2006, 250, 331–344. [Google Scholar] [CrossRef]
- Verdugo-Ihl, M.R.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.J.; Courtney-Davies, L.; Gilbert, S. Textures and U-W-Sn-Mo signatures in hematite from the Olympic Dam Cu- U-Au-Ag deposit, South Australia: Defining the archetype for IOCG deposits. Ore Geol. Rev. 2017, 91, 173–195. [Google Scholar] [CrossRef]
- Capitani, G. Synchysite-(Ce) from Cinquevalli (Trento, Italy): Stacking Disorder and the Polytypism of (Ca,REE)-Fluorcarbonates. Minerals 2020, 10, 77. [Google Scholar] [CrossRef]
- Huang, W.-L. The nucleation and growth of polycrystalline quartz: Pressure effect from 0.05 to 3 GPa. Eur. J. Miner. 2003, 15, 843–853. [Google Scholar] [CrossRef]
- Lagoerio, L.; Barbosa, P. Nucleation and growth of new grains in recrystallized quartz vein: An example from banded ore formation in Iron Quadrangle, Brazil. J. Struct. Geol. 2010, 32, 595–604. [Google Scholar] [CrossRef][Green Version]
- Buckley, P.; Hargreaves, N.; Cooper, S. Nucleation of quartz under ambient conditions. Commun. Chem. 2018, 1, 49. [Google Scholar] [CrossRef]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Leichmann, J.; Matula, M.; Broska, I.; Holeczy, D. Low-degree partial melting of metapelites—Another possible implement for selective concentration of uranium: Example from the Rožná uranium deposit, Bohemian Massif. In Proceedings of the International Workshop Uranium Deposits: From Their Genesis to Their Environmental Aspects, Prague, Czech Republic, 10–11 September 2002; Kříbek, B., Zeman, J., Eds.; Czech Geological Survey: Prague, Czech Republic, 2002; pp. 75–78. [Google Scholar]
- Ni, Y.; Huges, J.M.; Marianno, M.N. Crystal chemistry of the monazite and xenotime structures. Am. Miner. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Kříbek, B.; Veselovský, F.; Knésl, I.; Pour, O.; Pořádek, P.; Hak, J.; Škoda, R.; Leichmann, J. Coffinite-Zr-Ti-(REE-Y) mineralization in deeper parts of the Rožná uranium deposit, Czech Republic. In The Critical Role of Minerals in the Carbon-Free Future, Proceedings of the 16th SGA Biennial Meeting, Rotorua, New Zealand, 28–31 March 2022; Cristie, A., Ed.; Society of Geology Applied to Mineral Deposits: Rotorura, New Zealand, 2022; Volume 1, pp. 188–191. [Google Scholar]
- Bancroft, G.M.; Metson, J.B.; Kresovic, R.A.; Nesbitt, H.W. Leaching studies of natural and synthetic titanites using secondary ion mass spectrometry. Geochim. Cosmochim. Acta 1987, 51, 911–918. [Google Scholar] [CrossRef]
- Fryer, B.J.; Taylor, R.P. Rare-earth element distributions in uraninites: Implications for ore genesis. Chem. Geol. 1987, 63, 101–108. [Google Scholar] [CrossRef]
- Balboni, E.; Simonetti, A.; Spano, T.; Cook, N.D.; Burns, P.C. Rare-earth element fractionation in uranium ore and its U(VI) alteration minerals. Appl. Geochem. 2017, 87, 84–92. [Google Scholar] [CrossRef]
- Janeczek, R.; Ewing, C. Dissolution and alteration of uraninite under reducing conditions. J. Nucl. Mater. 1992, 190, 157–173. [Google Scholar] [CrossRef]
- Frimmel, H.E.; Schedel, S.; Brätz, H. Uraninite chemistry as forensic tool for provenance analysis. Appl. Geochem. 2014, 48, 104–121. [Google Scholar] [CrossRef]
- Burns, P.C.; Finch, R. Uranium: Mineralogy, Geochemistry and the Environment; Mineralogical Society of America: Washington, DC, USA, 1999. [Google Scholar]
- Stille, P.; Gauthier-Lafaye, F.; Jensen, K.A.; Salah, S.; Bracke, G.; Ewing, R.C.; Louvat, D.; Million, D. REE mobility in groundwater proximate to the natural fission reactor at Bangombé (Gabon). Chem. Geol. 2003, 198, 289–304. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Miner. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]
- René, M. Rare-earth, yttrium and zirconium mobility associated with the uranium mineralisation at Okrouhlá Radouň, Bohemian Massif, Czech Republic. Eur. J. Miner. 2015, 27, 57–70. [Google Scholar] [CrossRef]
- Dolníček, Z.; René, M.; Hermannová, S.; Prochaska, W. Origin of the Okrouhlá Radouň episyenite-hosted uranium deposit, Bohemian Massif, Czech Republic: Fluid inclusion and stable isotope constraints. Miner. Depos. 2014, 49, 409–425. [Google Scholar] [CrossRef]
- Göb, S.; Gühring, J.-E.; Bau, M.; Markl, G. Remobilization of U and REE and the formation of secondary minerals in oxidized U deposits. Am. Miner. 2013, 98, 530–548. [Google Scholar] [CrossRef]
- Zhao, D.; Ewing, R.C. Alteration products of uraninite from the Colorado Plateau. Radiochim. Acta 2000, 88, 739–750. [Google Scholar] [CrossRef]
- Papoutsa, A.; Pe-Piper, G. Variation of REE-hydrothermal circulation in complex shear zones: The Cobequid Highlands, Nova Scotia. Can. Miner. 2014, 52, 943–968. [Google Scholar] [CrossRef]
- Förster, H.J. Synchysite-(Y)-synchysite-(Ce) solid solutions from Markersbach, Erzgebirge, Germany: REE and Th mobility during high-T alteration of highly fractionated aluminous A-type granites. Miner. Petrol. 2001, 72, 259–280. [Google Scholar] [CrossRef]
- Dill, H.G.; Hansen, B.T.; Weber, B. REE contents, REE minerals and Sm/Nd isotopes of granite-and unconformity-related fluorite mineralization at the western edge of the Bohemian Massif: With special reference to the Nabburg-Wölsendorf District, SE Germany. Ore Geol. Rev. 2011, 40, 132–148. [Google Scholar] [CrossRef]







| Sample | Quartz–Carbonate–Sulfide Vein (V) | Host Rocks (H) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V1qtz | V2qtz | V3qtz | V4qtz/carb | V5carb | H1 | H2 | H3 | H4 | H5 | |
| SiO2 | 81.87 | 89.26 | 89.47 | 85.25 | 69.82 | 58.20 | 59.91 | 44.06 | 51.21 | 62.04 |
| TiO2 | 0.04 | 0.04 | 0.06 | 0.21 | 0.06 | 0.83 | 0.06 | 0.48 | 0.49 | 1.18 |
| Al2O3 | 1.40 | 1.60 | 1.66 | 3.64 | 2.24 | 20.72 | 18.32 | 12.44 | 12.81 | 13.52 |
| Fe2O3 | 0.49 | 0.09 | 0.09 | 1.00 | 0.10 | 3.64 | 0.64 | 2.38 | 1.52 | 0.88 |
| FeO | 0.21 | 0.28 | 0.29 | 0.52 | 0.55 | 1.40 | 0.40 | 3.89 | 1.39 | 2.94 |
| MgO | 0.06 | 0.04 | 0.03 | 0.23 | 0.05 | 0.27 | 0.27 | 1.67 | 0.47 | 0.90 |
| MnO | 0.13 | 0.10 | 0.10 | 0.07 | 0.20 | 0.04 | 0.04 | 0.18 | 0.17 | 0.13 |
| CaO | 7.43 | 3.60 | 3.50 | 2.81 | 14.88 | 1.11 | 4.74 | 14.09 | 11.86 | 4.86 |
| SrO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | 0.02 | 0.04 | 0.02 | 0.01 |
| BaO | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.03 | 0.10 | 0.07 | 0.07 | 0.04 |
| Li2O | 0.01 | 0.005 | 0.005 | 0.01 | 0.01 | 0.002 | 0.001 | 0.004 | 0.003 | 0.002 |
| Na2O | 0.03 | 0.09 | 0.07 | 0.08 | 0.03 | 10.67 | 9.27 | 4.75 | 6.49 | 0.11 |
| K2O | 0.18 | 0.14 | 0.10 | 0.87 | 0.09 | 1.12 | 1.02 | 0.33 | 0.13 | 3.39 |
| P2O5 | 0.02 | 0.04 | 0.02 | 0.07 | 0.02 | 0.11 | 0.01 | 0.22 | 0.18 | 0.20 |
| F | 0.05 | 0.06 | 0.05 | 0.05 | 0.02 | 0.09 | 0.02 | 0.05 | 0.03 | 0.10 |
| CO2 | 6.60 | 3.09 | 2.91 | 2.29 | 11.26 | 0.40 | 3.48 | 11.07 | 9.33 | 5.21 |
| C(graphite) | 0.14 | 0.11 | 0.12 | 0.07 | 0.02 | 0.03 | 0.03 | 1.29 | 1.14 | 0.13 |
| S(tot.) | 0.50 | 0.19 | 0.23 | 1.13 | 0.62 | 0.01 | 0.03 | 0.05 | 0.04 | 1.14 |
| H2O(+) | b.d.l. | 0.59 | 0.58 | 0.83 | b.d.l. | 0.91 | 0.81 | 2.45 | 1.41 | 2.65 |
| H2O(−) | 0.20 | 0.21 | 0.23 | 0.47 | 0.25 | 0.30 | 0.19 | 0.56 | 0.50 | 0.59 |
| Total | 99.361 | 99.546 | 99.525 | 99.639 | 100.23 | 99.89 | 99.36 | 100.07 | 99.26 | 100.02 |
| As | 51 | 246 | 126 | 1021 | 36 | 17 | 21 | 18 | 24.8 | 20.42 |
| Ba | 109 | 64 | 82 | 233 | 220 | 78 | 89 | 103 | 159 | 199.38 |
| Bi | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.08 | 0.09 | 0.08 | 0.09 | 0.12 |
| Co | 3 | b.d.l. | b.d.l. | 12 | 6 | 14.6 | 14 | 15 | 15.4 | 14.82 |
| Cr | b.d.l. | b.d.l. | b.d.l. | 16 | 11 | 47 | 57 | 47 | 61 | 56.54 |
| Cu | 4 | 3 | 3 | 19 | 9 | 72.1 | 68.2 | 71.2 | 100.5 | 114.25 |
| Mo | 29 | 13 | 10 | 124 | 153 | 3.62 | 3.6 | 3.32 | 3.39 | 3.63 |
| Nb | 3 | b.d.l. | b.d.l. | b.d.l. | 8 | 6.03 | 6.33 | 6.45 | 7.02 | 6.73 |
| Ni | 9 | 8 | 8 | 26 | 9 | 32.2 | 39 | 38 | 39 | 36 |
| Pb | 1352 | 1520 | 2158 | 473 | 2096 | 134 | 77 | 126 | 104 | 102 |
| Rb | 16 | 11 | 11 | 65 | 13 | 60 | 58 | 59 | 56 | 60 |
| Sr | 28 | 23 | 20 | 41 | 38 | 231 | 227 | 230 | 230 | 224 |
| Th | 16 | 16 | 24 | 6 | 24 | 5 | 5 | 5 | 6 | 5 |
| U | 307 | 152 | 253 | 677 | 329 | 131 | 133 | 137 | 147 | 126 |
| V | 41 | 20 | 15 | 257 | 65 | 118 | 123 | 122 | 144 | 133 |
| Zn | 1493 | 1056 | 654 | 8014 | 2270 | 689 | 383 | 706 | 313 | 469 |
| Zr | 25 | 6 | 6 | 54 | 20 | 157 | 104 | 147 | 164 | 141 |
| Sample | V1 | V2 | V3 | V4 | V5 | H1 | H2 | H3 | H4 | H5 |
|---|---|---|---|---|---|---|---|---|---|---|
| La | 648 | 973 | 1032 | 196 | 142 | 24.3 | 26.3 | 35.6 | 33.7 | 29.9 |
| Ce | 1092 | 1619 | 1701 | 339 | 260 | 55.1 | 57.0 | 78.4 | 73.7 | 66.1 |
| Pr | 150 | 226 | 238 | 47.1 | 34.1 | 7.51 | 7.26 | 10.7 | 9.36 | 8.68 |
| Nd | 550 | 822 | 862 | 190 | 141 | 24.6 | 26.2 | 35.3 | 32.7 | 29.7 |
| Sm | 97.6 | 145 | 155 | 36 | 25.2 | 5.28 | 5.43 | 7.12 | 6.83 | 6.10 |
| Eu | 19.6 | 27.4 | 29.8 | 7.94 | 5.77 | 1.73 | 1.65 | 1.74 | 1.82 | 1.70 |
| Gd | 64.9 | 96.1 | 103 | 23.6 | 16.3 | 5.33 | 5.52 | 6.84 | 7.16 | 6.18 |
| Tb | 6.17 | 9.21 | 10.6 | 2.99 | 1.89 | 0.73 | 0.82 | 1.05 | 0.92 | 0.85 |
| Dy | 24.8 | 35.3 | 38.4 | 13.6 | 7.71 | 4.56 | 4.73 | 5.62 | 5.05 | 4.95 |
| Y | 126 | 167 | 175 | 73.6 | 41.2 | 27.9 | 27.9 | 30.5 | 29.6 | 28.9 |
| Ho | 3.58 | 4.89 | 6.15 | 2.39 | 1.27 | 1.12 | 0.95 | 1.35 | 1.16 | 1.08 |
| Er | 6.91 | 9.15 | 10.43 | 5.97 | 2.94 | 2.93 | 2.73 | 2.92 | 2.83 | 2.83 |
| Tm | 0.62 | 0.69 | 0.73 | 0.80 | 0.36 | 0.45 | 0.42 | 0.53 | 0.45 | 0.43 |
| Yb | 2.63 | 2.53 | 2.78 | 4.39 | 1.96 | 2.56 | 2.32 | 2.55 | 2.72 | 2.50 |
| Lu | 0.32 | 0.29 | 0.30 | 0.56 | 0.26 | 0.32 | 0.45 | 0.43 | 0.41 | 0.38 |
| ΣREE | 2792 | 4137 | 4366 | 944 | 680 | 165 | 169 | 221 | 208 | 190 |
| LaN/SmN | 4.18 | 4.23 | 4.20 | 3.46 | 3.54 | 2.90 | 3.04 | 3.15 | 3.10 | 3.09 |
| LaN/YN | 166 | 259 | 250 | 30.1 | 48.7 | 6.41 | 7.63 | 9.42 | 8.35 | 8.08 |
| DyN/YbN | 6.13 | 9.05 | 8.97 | 2.01 | 2.56 | 1.16 | 1.32 | 1.43 | 1.21 | 1.29 |
| Ce/Ce* | 0.84 | 0.83 | 0.83 | 0.85 | 0.90 | 0.98 | 0.99 | 0.97 | 1.00 | 0.99 |
| Eu/Eu* | 0.75 | 0.71 | 0.72 | 0.84 | 0.87 | 1.00 | 0.92 | 0.76 | 0.80 | 0.85 |
| t1 | 1.00 | 0.99 | 0.99 | 0.96 | 0.98 | 1.22 | 1.14 | 1.20 | 1.16 | 1.18 |
| t3 | 0.89 | 0.91 | 0.88 | 0.93 | 0.92 | 0.82 | 0.94 | 0.88 | 0.82 | 0.87 |
| t1/t3 | 0.94 | 0.95 | 0.93 | 0.94 | 0.95 | 1.00 | 1.03 | 1.02 | 0.97 | 1.01 |
| Sample | 1V | 1V | 1V | 2V | 2V. | V2A | V2A 2 | V2A 3 | V2A | V2A |
|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | 8/1 | 9/1 | 10/1 | 11/1 | 12/1 | 1/1 | 2/1 | 3/1 | 4/1 | 5/1 |
| SiO2 | 0.14 | 0.20 | 0.18 | 0.76 | 0.22 | 0.25 | 0.13 | 0.11 | 2.55 | 1.02 |
| Y2O3 | 2.34 | 2.26 | 2.67 | 1.88 | 2.54 | 2.30 | 2.22 | 2.27 | 2.28 | 1.91 |
| La2O3 | 10.54 | 10.19 | 10.09 | 10.70 | 9.93 | 9.90 | 10.58 | 9.79 | 10.09 | 10.81 |
| Ce2O3 | 20.09 | 19.13 | 19.57 | 20.12 | 19.47 | 19.75 | 19.71 | 20.20 | 18.99 | 20.55 |
| Pr2O3 | 2.47 | 2.31 | 2.40 | 2.33 | 2.37 | 2.17 | 2.11 | 2.47 | 2.16 | 2.60 |
| Nd2O3 | 9.88 | 9.92 | 10.18 | 9.71 | 9.99 | 11.00 | 9.82 | 9.95 | 9.40 | 9.16 |
| Sm2O3 | 1.53 | 1.59 | 1.76 | 1.47 | 1.58 | 1.81 | 1.51 | 2.31 | 1.75 | 1.76 |
| Eu2O3 | 0.44 | 0.34 | 0.17 | 0.22 | 0.24 | 0.00 | 0.00 | 0.32 | 0.17 | 0.57 |
| Gd2O3 | 1.37 | 1.31 | 1.52 | 1.22 | 1.31 | 1.13 | 1.05 | 1.37 | 0.67 | 1.67 |
| Tb2O3 | 0.15 | 0.14 | 0.12 | b.d.l. | 0.08 | NA | NA | NA | NA | NA |
| Dy2O3 | 0.45 | 0.43 | 0.53 | 0.39 | 0.58 | 0.56 | 0.86 | 0.34 | 0.54 | 0.47 |
| Er2O3 | 0.20 | 0.17 | 0.17 | 0.18 | 0.16 | b.d.l. | 0.28 | 0.00 | 0.32 | 0.00 |
| Yb2O3 | b.d.l. | b.d.l. | b.d.l. | 0.14 | 0.14 | NA | NA | NA | NA | NA |
| CaO | 19.45 | 20.03 | 19.09 | 19.40 | 20.27 | 20.01 | 20.08 | 19.22 | 18.96 | 19.53 |
| F | 5.76 | 5.59 | 5.67 | 5.46 | 5.34 | 5.68 | 5.63 | 5.72 | 5.41 | 5.54 |
| CO2 | 28.67 | 28.67 | 28.35 | 28.29 | 29.05 | 28.89 | 28.80 | 28.37 | 27.46 | 28.69 |
| H2O | 0.01 | 0.00 | 0.05 | 0.09 | 0.16 | 0.01 | 0.00 | 0.01 | 0.01 | 0.11 |
| O=F | −2.43 | −2.35 | −2.39 | −2.30 | −2.25 | −2.39 | −2.37 | −2.41 | −2.28 | −2.33 |
| total | 101.07 | 99.93 | 100.12 | 100.06 | 101.19 | 101.04 | 100.40 | 100.03 | 98.46 | 102.04 |
| The empirical formulae were calculated based on ΣREE = 3 atoms per formula unit (apfu). | ||||||||||
| Y | 0.204 | 0.204 | 0.234 | 0.169 | 0.226 | 0.204 | 0.199 | 0.199 | 0.212 | 0.167 |
| La | 0.637 | 0.637 | 0.612 | 0.664 | 0.613 | 0.609 | 0.657 | 0.597 | 0.650 | 0.655 |
| Ce | 1.205 | 1.188 | 1.178 | 1.238 | 1.192 | 1.205 | 1.216 | 1.223 | 1.214 | 1.237 |
| Pr | 0.148 | 0.143 | 0.144 | 0.143 | 0.144 | 0.132 | 0.129 | 0.149 | 0.137 | 0.156 |
| Nd | 0.578 | 0.601 | 0.598 | 0.583 | 0.596 | 0.654 | 0.591 | 0.588 | 0.586 | 0.538 |
| Sm | 0.086 | 0.093 | 0.100 | 0.085 | 0.091 | 0.104 | 0.088 | 0.132 | 0.105 | 0.100 |
| Eu | 0.024 | 0.020 | 0.009 | 0.013 | 0.014 | 0.000 | 0.000 | 0.018 | 0.010 | 0.032 |
| Gd | 0.074 | 0.074 | 0.083 | 0.068 | 0.072 | 0.062 | 0.058 | 0.075 | 0.038 | 0.091 |
| Tb | 0.008 | 0.008 | 0.006 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Dy | 0.024 | 0.024 | 0.028 | 0.021 | 0.031 | 0.030 | 0.047 | 0.018 | 0.030 | 0.025 |
| Er | 0.010 | 0.009 | 0.009 | 0.009 | 0.008 | 0.000 | 0.015 | 0.000 | 0.018 | 0.000 |
| Yb | 0.000 | 0.000 | 0.000 | 0.007 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca | 3.415 | 3.640 | 3.363 | 3.494 | 3.632 | 3.573 | 3.626 | 3.407 | 3.547 | 3.439 |
| F | 2.987 | 2.998 | 2.946 | 2.904 | 2.823 | 2.994 | 2.999 | 2.992 | 2.985 | 2.879 |
| REE | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 |
| CO3 | 6.415 | 6.640 | 6.363 | 6.494 | 6.632 | 6.573 | 6.626 | 6.407 | 6.547 | 6.439 |
| OH | 0.013 | 0.002 | 0.054 | 0.096 | 0.177 | 0.006 | 0.001 | 0.008 | 0.015 | 0.121 |
| SiO2 | 0.023 | 0.034 | 0.029 | 0.127 | 0.037 | 0.042 | 0.021 | 0.019 | 0.446 | 0.167 |
| tTE1 | 0.952 | 0.912 | 0.933 | 0.926 | 0.940 | 0.864 | 0.871 | 0.987 | 0.906 | 1.012 |
| EuN/EuN* | 0.919 | 0.720 | 0.309 | 0.507 | 0.512 | 0.543 | 0.472 | 1.015 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kříbek, B.; Knésl, I.; Dobeš, P.; Veselovský, F.; Pořádek, P.; Škoda, R.; Čopjaková, R.; Leichmann, J.; Košek, F. The Origin of Synchysite-(Ce) and Sources of Rare Earth Elements in the Rožná Uranium Deposit, Czech Republic. Minerals 2022, 12, 690. https://doi.org/10.3390/min12060690
Kříbek B, Knésl I, Dobeš P, Veselovský F, Pořádek P, Škoda R, Čopjaková R, Leichmann J, Košek F. The Origin of Synchysite-(Ce) and Sources of Rare Earth Elements in the Rožná Uranium Deposit, Czech Republic. Minerals. 2022; 12(6):690. https://doi.org/10.3390/min12060690
Chicago/Turabian StyleKříbek, Bohdan, Ilja Knésl, Petr Dobeš, František Veselovský, Přemysl Pořádek, Radek Škoda, Renata Čopjaková, Jaromír Leichmann, and Filip Košek. 2022. "The Origin of Synchysite-(Ce) and Sources of Rare Earth Elements in the Rožná Uranium Deposit, Czech Republic" Minerals 12, no. 6: 690. https://doi.org/10.3390/min12060690
APA StyleKříbek, B., Knésl, I., Dobeš, P., Veselovský, F., Pořádek, P., Škoda, R., Čopjaková, R., Leichmann, J., & Košek, F. (2022). The Origin of Synchysite-(Ce) and Sources of Rare Earth Elements in the Rožná Uranium Deposit, Czech Republic. Minerals, 12(6), 690. https://doi.org/10.3390/min12060690








