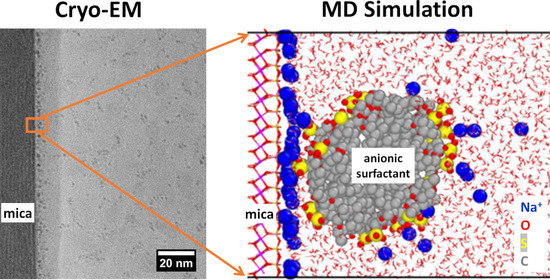
Molecular Dynamics Simulation and Cryo-Electron Microscopy Investigation of AOT Surfactant Structure at the Hydrated Mica Surface
Abstract
:1. Introduction
2. Methods
2.1. MD Simulation
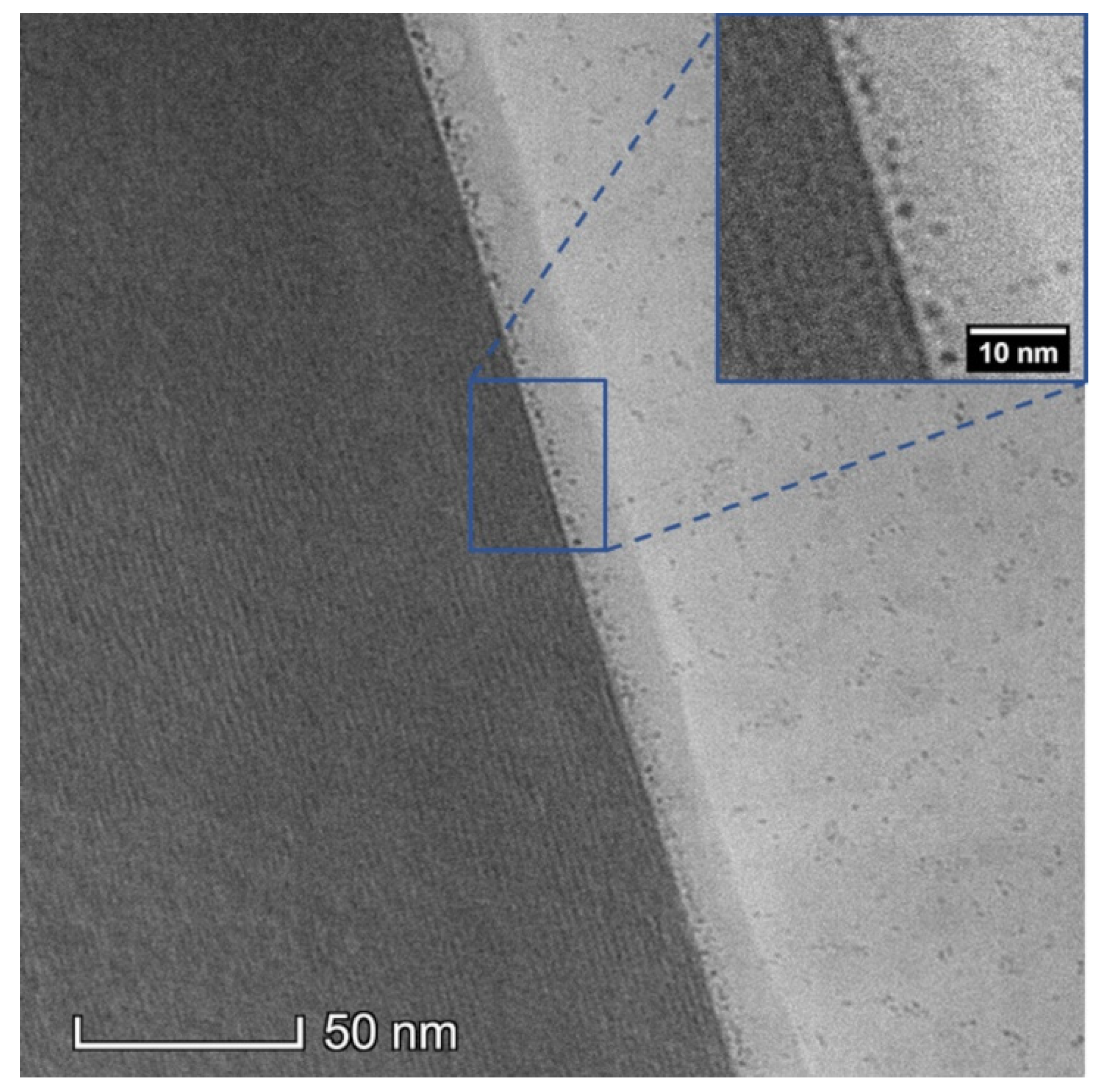
2.2. Sample Preparation and Cryogenic Electron Microscopy
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.F.; Zhang, Y.Y.; Chen, G.P.; Gai, Z.Y. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xie, L.; Cui, X.W.; Chen, J.S.; Zeng, H.B. Intermolecular and surface forces at solid/oil/water/gas interfaces in petroleum production. J. Colloid Interface Sci. 2019, 537, 505–519. [Google Scholar] [CrossRef]
- Liang, Y.; Tsuji, S.; Jia, J.; Tsuji, T.; Matsuoka, T. Modeling CO2–Water–Mineral Wettability and Mineralization for Carbon Geosequestration. Acc. Chem. Res. 2017, 50, 1530–1540. [Google Scholar] [CrossRef]
- Zhang, S.N.; Huang, J.Y.; Chen, Z.; Yang, S.; Lai, Y.K. Liquid mobility on superwettable surfaces for applications in energy and the environment. J. Mater. Chem. A 2019, 7, 38–63. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.H.; Straub, A.P.; Tong, T.Z.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Israelachvili, J.; Ruths, M. Brief History of Intermolecular and Intersurface Forces in Complex Fluid Systems. Langmuir 2013, 29, 9605–9619. [Google Scholar] [CrossRef]
- Kumar, G.; Prabhu, K.N. Review of non-reactive and reactive wetting of liquids on surfaces. Adv. Colloid Interface Sci. 2007, 133, 61–89. [Google Scholar] [CrossRef]
- Khandoozi, S.; Sharifi, A.; Riazi, M. Enhanced Oil Recovery Using Surfactants. In Chemical Methods; Sarapardeh, A.H., Schaffie, M., Ranjbar, M., Dong, M., Li, Z.L., Eds.; Gulf Professional Publishing: Houston, TX, USA, 2021; pp. 95–139. [Google Scholar]
- Tummala, N.R.; Shi, L.; Striolo, A. Molecular dynamics simulations of surfactants at the silica-water interface: Anionic vs. nonionic headgroups. J. Colloid Interface Sci. 2011, 362, 135–143. [Google Scholar] [CrossRef]
- Zhou, Q.; Shen, W.; Zhu, J.X.; Zhu, R.L.; He, H.P.; Zhou, J.H.; Yuan, P. Structure and dynamic properties of water saturated CTMA-montmorillonite: Molecular dynamics simulations. Appl. Clay Sci. 2014, 97–98, 62–71. [Google Scholar] [CrossRef]
- Peng, C.L.; Min, F.F.; Liu, L.Y. Effect of pH on the adsorption of dodecylamine on montmorillonite: Insights from experiments and molecular dynamics simulations. Appl. Surf. Sci. 2017, 425, 996–1005. [Google Scholar] [CrossRef]
- Bai, S.X.; Kubelka, J.; Piri, M. Wettability Reversal on Dolomite Surfaces by Divalent Ions and Surfactants: An Experimental and Molecular Dynamics Simulation Study. Langmuir 2021, 37, 6641–6649. [Google Scholar] [CrossRef]
- Tsagkaropoulou, G.; Allen, F.J.; Clarke, S.M.; Camp, P.J. Self-assembly and adsorption of cetyltrimethylammonium bromide and didodecyldimethylammonium bromide surfactants at the mica-water interface. Soft Matter 2019, 15, 8402–8411. [Google Scholar] [CrossRef] [Green Version]
- Kessenich, B.L.; Pokhrel, N.; Nakouzi, E.; Newcomb, C.J.; Flury, M.; Maibaum, L.; De Yoreo, J.J. Connecting wettability, topography, and chemistry in a simple lipid-montmorillonite system. J. Colloid Interface Sci. 2019, 555, 498–508. [Google Scholar] [CrossRef]
- Paria, S.; Khilar, K.C. A review on experimental studies of surfactant adsorption at the hydrophilic solid-water interface. Adv. Colloid Interface Sci. 2004, 110, 75–95. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhao, G.; Brewer, M.; Lv, Q.C.; Sudholter, E.J.R. Comprehensive review on surfactant adsorption on mineral surfaces in chemical enhanced oil recovery. Adv. Colloid Interface Sci. 2021, 294, 23. [Google Scholar] [CrossRef]
- Atkin, R.; Craig, V.S.J.; Wanless, E.J.; Biggs, S. Mechanism of cationic surfactant adsorption at the solid-aqueous interface. Adv. Colloid Interface Sci. 2003, 103, 219–304. [Google Scholar] [CrossRef]
- Wang, X.F.; Lee, S.Y.; Miller, K.; Welbourn, R.; Stocker, I.; Clarke, S.; Casford, M.; Gutfreund, P.; Skoda, M.W.A. Cation Bridging Studied by Specular Neutron Reflection. Langmuir 2013, 29, 5520–5527. [Google Scholar] [CrossRef]
- Sposito, G. The Surface Chemistry of Soils; Oxford University Press: New York, NY, USA, 1984. [Google Scholar]
- Tang, G.Q.; Morrow, N.R. Influence of Brine Composition and Fines Migration on Crude Oil/Brine/Rock Interactions and Oil Recovery. J. Pet. Sci. Eng. 1999, 24, 99–111. [Google Scholar] [CrossRef]
- Allen, F.J.; Griffin, L.R.; Alloway, R.M.; Gutfreund, P.; Lee, S.Y.; Truscott, C.L.; Welbourn, R.J.L.; Wood, M.H.; Clarke, S.M. An Anionic Surfactant on an Anionic Substrate: Monovalent Cation Binding. Langmuir 2017, 33, 7881–7888. [Google Scholar] [CrossRef]
- Griffin, L.R.; Browning, K.L.; Lee, S.Y.; Skoda, M.W.A.; Rogers, S.; Clarke, S.M. Multilayering of Calcium Aerosol-OT at the Mica/Water Interface Studied with Neutron Reflection: Formation of a Condensed Lamellar Phase at the CMC. Langmuir 2016, 32, 13054–13064. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.J.; Truscott, C.L.; Gutfreund, P.; Welbourn, R.J.L.; Clarke, S.M. Potassium, Calcium, and Magnesium Bridging of AOT to Mica at Constant Ionic Strength. Langmuir 2019, 35, 5753–5761. [Google Scholar] [CrossRef]
- Hensel, J.K.; Carpenter, A.P.; Ciszewski, R.K.; Schabes, B.K.; Kittredge, C.T.; Moore, F.G.; Richmond, G.L. Molecular characterization of water and surfactant AOT at nanoemulsion surfaces. Proc. Natl. Acad. Sci. USA 2017, 114, 13351–13356. [Google Scholar] [CrossRef] [Green Version]
- Hellsing, M.S.; Rennie, A.R.; Hughes, A.V. Effect of Concentration and Addition of Ions on the Adsorption of Aerosol-OT to Sapphire. Langmuir 2010, 26, 14567–14573. [Google Scholar] [CrossRef]
- Hellsing, M.S.; Rennie, A.R.; Hughes, A.V. Adsorption of Aerosol-OT to Sapphire: Lamellar Structures Studied with Neutrons. Langmuir 2011, 27, 4669–4678. [Google Scholar] [CrossRef]
- Stocker, I.N.; Miller, K.L.; Welbourn, R.J.L.; Clarke, S.M.; Collins, I.R.; Kinane, C.; Gutfreund, P. Adsorption of Aerosol-OT at the calcite/water interface—Comparison of the sodium and calcium salts. J. Colloid Interface Sci. 2014, 418, 140–146. [Google Scholar] [CrossRef]
- Holmberg, K.; Jonsson, B.; Kronberg, B.; Lindman, B. Adsorption of Surfactants at Solid Surfaces. In Surfactants and Polymers in Aqueous Solution; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 357–387. [Google Scholar]
- Striolo, A.; Grady, B.P. Surfactant Assemblies on Selected Nanostructured Surfaces: Evidence, Driving Forces, and Applications. Langmuir 2017, 33, 8099–8113. [Google Scholar] [CrossRef]
- Santos-Carballal, D.; Du, Z.M.; King, H.E.; de Leeuw, N.H. A computational study of the interaction of organic surfactants with goethite alpha-FeO(OH) surfaces. RSC Adv. 2016, 6, 91893–91903. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yu, J.G.; O’Rear, E.A.; Striolo, A. Aqueous Dual-Tailed Surfactants Simulated on the Alumina Surface. J. Phys. Chem. B 2014, 118, 9695–9707. [Google Scholar] [CrossRef]
- Darkins, R.; Sushko, M.L.; Liu, J.; Duffy, D.M. Adhesion of Sodium Dodecyl Sulfate Surfactant Mono layers with TiO2 (Rutile and Anatase) Surfaces. Langmuir 2013, 29, 11609–11614. [Google Scholar] [CrossRef]
- Stewart, M.; Vigers, G. Electron microscopy of frozen-hydrated biological material. Nature 1986, 319, 631–636. [Google Scholar] [CrossRef]
- Dubochet, J.; Adrian, M.; Lepault, J.; McDowall, A.W. Cryo-electron microscopy of vitrified biological specimens. Trends Biochem. Sci. 1985, 10, 143–146. [Google Scholar] [CrossRef]
- Marko, M.; Hsieh, C.; Schalek, R.; Frank, J.; Mannella, C. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat. Methods 2007, 4, 215–217. [Google Scholar] [CrossRef]
- Lin, Z.; Hill, R.M.; Davis, H.T.; Scriven, L.E.; Talmonx, Y. Cryo Transmission Electron Microscopy Study of Vesicles and Micelles in Siloxane Surfactant Aqueous Solutions. Langmuir 1994, 10, 1008–1011. [Google Scholar] [CrossRef]
- Wang, K.; Karlsson, G.; Almgren, M.; Asakawa, T. Aggregation Behavior of Cationic Fluorosurfactants in Water and Salt Solutions. A CryoTEM Survey. J. Phys. Chem. B 1999, 103, 9237–9246. [Google Scholar] [CrossRef]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloids Surf. A Physicochem. Eng. Asp. 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Newcomb, C.J.; Moyer, T.J.; Lee, S.S.; Stupp, S.I. Advances in cryogenic transmission electron microscopy for the characterization of dynamic self-assembling nanostructures. Curr. Opin. Colloid Interface Sci. 2012, 17, 350–359. [Google Scholar] [CrossRef] [Green Version]
- De Yoreo, J.J.; Sommerdijk, N.A.J.M. Investigating materials formation with liquid-phase and cryogenic TEM. Nat. Rev. Mater. 2016, 1, 16035. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.-L.; Joubert, L.-M.; Chin, R.; Koh, A.L.; Yu, Y.; et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, W.; Li, Y.; Pei, A.; Boyle, D.T.; Cui, Y. Correlating Structure and Function of Battery Interphases at Atomic Resolution Using Cryoelectron Microscopy. Joule 2018, 2, 2167–2177. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, D.K.; Perea, D.E.; Ryan, J.V.; Evans, J.E.; Vienna, J.D. A method for site-specific and cryogenic specimen fabrication of liquid/solid interfaces for atom probe tomography. Ultramicroscopy 2018, 194, 89–99. [Google Scholar] [CrossRef]
- Zachman, M.J.; Tu, Z.; Archer, L.A.; Kourkoutis, L.F. Nanoscale Elemental Mapping of Intact Solid–Liquid Interfaces and Reactive Materials in Energy Devices Enabled by Cryo-FIB/SEM. ACS Energy Lett. 2020, 5, 1224–1232. [Google Scholar] [CrossRef]
- Zachman, M.J.; Tu, Z.; Choudhury, S.; Archer, L.A.; Kourkoutis, L.F. Cryo-STEM mapping of solid-liquid interfaces and dendrites in lithium-metal batteries. Nature 2018, 560, 345–349. [Google Scholar] [CrossRef]
- Zachman, M.J.; Asenath-Smith, E.; Estroff, L.A.; Kourkoutis, L.F. Site-Specific Preparation of Intact Solid-Liquid Interfaces by Label-Free In Situ Localization and Cryo-Focused Ion Beam Lift-Out. Microsc. Microanal. 2016, 22, 1338–1349. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Boyle, D.T.; Li, Y.; Li, Y.; Pei, A.; Chen, H.; Cui, Y. Nanostructural and Electrochemical Evolution of the Solid-Electrolyte Interphase on CuO Nanowires Revealed by Cryogenic-Electron Microscopy and Impedance Spectroscopy. ACS Nano 2019, 13, 737–744. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.; Zhang, M.; Zhang, Y.; Yang, F.; Lee, J.Z.; Lee, M.-H.; Alvarado, J.; Schroeder, M.; Yang, Y.; et al. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Z.; Wynn, T.A.; Schroeder, M.A.; Alvarado, J.; Wang, X.; Xu, K.; Meng, Y.S. Cryogenic Focused Ion Beam Characterization of Lithium Metal Anodes. ACS Energy Lett. 2019, 4, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, W.; Li, Y.; Chiu, W.; Cui, Y. Opportunities for Cryogenic Electron Microscopy in Materials Science and Nanoscience. ACS Nano 2020, 14, 9263–9276. [Google Scholar] [CrossRef]
- Nishikawa, K.; Shinoda, K. Characterization of Electrodeposited Li Metal by Cryo-Scanning Transmission Electron Microscopy/Electron Energy Loss Spectroscopy. J. Phys. Chem. Lett. 2021, 12, 3922–3927. [Google Scholar] [CrossRef]
- Jungjohann, K.L.; Gannon, R.N.; Goriparti, S.; Randolph, S.J.; Merrill, L.C.; Johnson, D.C.; Zavadil, K.R.; Harris, S.J.; Harrison, K.L. Cryogenic Laser Ablation Reveals Short-Circuit Mechanism in Lithium Metal Batteries. ACS Energy Lett. 2021, 6, 2138–2144. [Google Scholar] [CrossRef]
- Harrison, K.L.; Goriparti, S.; Merrill, L.C.; Long, D.M.; Warren, B.; Roberts, S.A.; Perdue, B.R.; Casias, Z.; Cuillier, P.; Boyce, B.L.; et al. Effects of Applied Interfacial Pressure on Li-Metal Cycling Performance and Morphology in 4 M LiFSI in DME. ACS Appl. Mater. Interfaces 2021, 13, 31668–31679. [Google Scholar] [CrossRef]
- Harrison, K.L.; Merrill, L.C.; Long, D.M.; Randolph, S.J.; Goriparti, S.; Christian, J.; Warren, B.; Roberts, S.A.; Harris, S.J.; Perry, D.L.; et al. Cryogenic electron microscopy reveals that applied pressure promotes short circuits in Li batteries. iScience 2021, 24, 103394. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Scriven, L.E.; Davis, H.T. Stabilization of aqueous clay suspensions with AOT vesicular solutions. Colloids Surf. A Physicochem. Eng. Asp. 1996, 106, 149–159. [Google Scholar] [CrossRef]
- Sahai, N.; Kaddour, H.; Dalai, P.; Wang, Z.; Bass, G.; Gao, M. Mineral Surface Chemistry and Nanoparticle-aggregation Control Membrane Self-Assembly. Sci. Rep. 2017, 7, 43418. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, M.L.; Nagy, K.L.; Fenter, P.; Cheng, L.; Sturchio, N.C.; Jacobsen, S.D. Cation sorption on the muscovite (001) surface in chloride solutions using high-resolution X-ray reflectivity. Geochim. Cosmochim. Acta 2006, 70, 3549–3565. [Google Scholar] [CrossRef]
- Bryndzia, L.T.; Braunsdorf, N.R. From Source Rock to Reservoir: The Evolution of Self-Sourced Unconventional Resource Plays. Elements 2014, 10, 271–276. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.-J.; Kalinichev, A.G. Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Teleman, O.; Jonsson, B.; Engstrom, S. A Molecular Dynamics Simulation of a Water Model with Intramolecular Degrees of Freedom. Mol. Phys. 1987, 60, 193–203. [Google Scholar] [CrossRef]
- Aqvist, J. Ion-Water Interaction Potentials Derived from Free Energy Perturbation Simulations. J. Phys. Chem. 1990, 94, 8021–8024. [Google Scholar] [CrossRef]
- Smith, D.R.; Dang, L.X. Computer Simulations of NaCl Association in Polarizable Water. J. Chem. Phys. 1994, 100, 3757–3766. [Google Scholar] [CrossRef]
- Smith, D.E.; Dang, L.X. Computer-Simulations of Cesium Water Clusters—Do Ion Water Clusters Form Gas-Phase Clathrates. J. Chem. Phys. 1994, 101, 7873–7881. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Madura, J.D.; Swenson, C.J. Optimized intermolecular potential functions for liquid hydrocarbons. J. Am. Chem. Soc. 1984, 106, 6638–6646. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Hart, D.B.; Ochs, M.E. Alcohol and Thiol Adsorption on (Oxy)hydroxide and Carbon Surfaces: Molecular Dynamics Simulation and Desorption Experiments. J. Phys. Chem. C 2012, 116, 26756–26764. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Geatches, D.I.; Pike, D.Q.; Greenwell, H.C.; Johnston, C.T.; Wilcox, J.; Cygan, R.T. Methylene Blue Adsorption on the Basal Surfaces of Kaolinite: Structure and Thermodynamics from Quantum and Classical Molecular Simulation. Clays Clay Miner. 2015, 63, 185–198. [Google Scholar] [CrossRef]
- Szczerba, M.; Kalinichev, A.G. Intercalation of Ethylene Glycol in Smectites: Several Molecular Simulation Models Verified by X-ray Diffraction Data. Clays Clay Miner. 2016, 64, 488–502. [Google Scholar] [CrossRef] [Green Version]
- Fazelabdolabadi, B.; Alizadeh-Mojarad, A. A molecular dynamics investigation into the adsorption behavior inside {001} kaolinite and {1014} calcite nano-scale channels: The case with confined hydrocarbon liquid, acid gases, and water. Appl. Nanosci. 2017, 7, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.G.; Leng, Y.S. Squeezing and stick-slip friction behaviors of lubricants in boundary lubrication. Proc. Natl. Acad. Sci. USA 2018, 115, 6560–6565. [Google Scholar] [CrossRef] [Green Version]
- Lopes, J.N.C.; Padua, A.A.H.; Shimizu, K. Molecular force field for ionic liquids IV: Trialkylimidazolium and alkoxycarbonyl-imidazolium cations; alkylsulfonate and alkylsulfate anions. J. Phys. Chem. B 2008, 112, 5039–5046. [Google Scholar] [CrossRef]
- Mao, X.W.; Brown, P.; Červinka, C.; Hazell, G.; Li, H.; Ren, Y.; Chen, D.; Atkin, R.; Eastoe, J.; Grillo, I.; et al. Self-assembled nanostructures in ionic liquids facilitate charge storage at electrified interfaces. Nat. Mater. 2019, 18, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Teich-McGoldrick, S.L.; Greathouse, J.A.; Cygan, R.T. Molecular Dynamics Simulations of Uranyl Adsorption and Structure on the Basal Surface of Muscovite. Mol. Simul. 2014, 40, 610–617. [Google Scholar] [CrossRef]
- Liang, J.J.; Hawthorne, F.C.; Swainson, I.P. Triclinic Muscovite: X-ray Diffraction, Neutron Diffraction and Photo-Acoustic FTIR Spectroscopy. Can. Mineral. 1998, 36, 1017–1027. [Google Scholar]
- Loewenstein, W. The Distribution of Aluminum in the Tetrahedra of Silicates and Aluminates. Am. Miner. 1954, 39, 92–96. [Google Scholar]
- Plimpton, S.J. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comp. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Plimpton, S.J.; Pollock, R.; Stevens, M. Particle-Mesh Ewald and rRESPA for Parallel Molecular Dynamics Simulations. In Proceedings of the Eighth SIAM Conference on Parallel Processing for Scientific Computing, Minneapolis, MN, USA, 14–17 March 1997. [Google Scholar]
- Yeh, I.C.; Berkowitz, M.L. Ewald summation for systems with slab geometry. J. Chem. Phys. 1999, 111, 3155–3162. [Google Scholar] [CrossRef]
- Rubino, S.; Akhtar, S.; Melin, P.; Searle, A.; Spellward, P.; Leifer, K. A site-specific focused-ion-beam lift-out method for cryo Transmission Electron Microscopy. J. Struct. Biol. 2012, 180, 572–576. [Google Scholar] [CrossRef]
- Strunk, K.M.; Wang, K.; Ke, D.; Gray, J.L.; Zhang, P. Thinning of large mammalian cells for cryo-TEM characterization by cryo-FIB milling. J. Microsc. 2012, 247, 220–227. [Google Scholar] [CrossRef]
- Mahamid, J.; Schampers, R.; Persoon, H.; Hyman, A.A.; Baumeister, W.; Plitzko, J.M. A focused ion beam milling and lift-out approach for site-specific preparation of frozen-hydrated lamellas from multicellular organisms. J. Struct. Biol. 2015, 192, 262–269. [Google Scholar] [CrossRef]
- Parmenter, C.D.; Nizamudeen, Z.A. Cryo-FIB-lift-out: Practically impossible to practical reality. J. Microsc. 2021, 281, 157–174. [Google Scholar] [CrossRef]
- Parmenter, C.D.; Fay, M.W.; Hartfield, C.; Eltaher, H.M. Making the practically impossible “Merely difficult”—Cryogenic FIB lift-out for “Damage free” soft matter imaging. Microsc. Res. Tech. 2016, 79, 298–303. [Google Scholar] [CrossRef]
- He, J.; Hsieh, C.; Wu, Y.; Schmelzer, T.; Wang, P.; Lin, Y.; Marko, M.; Sui, H. Cryo-FIB specimen preparation for use in a cartridge-type cryo-TEM. J. Struct. Biol. 2017, 199, 114–119. [Google Scholar] [CrossRef]
- Chang, Y.; Lu, W.; Guénolé, J.; Stephenson, L.T.; Szczpaniak, A.; Kontis, P.; Ackerman, A.K.; Dear, F.F.; Mouton, I.; Zhong, X.; et al. Ti and its alloys as examples of cryogenic focused ion beam milling of environmentally-sensitive materials. Nat. Commun. 2019, 10, 942. [Google Scholar] [CrossRef]
- Bassim, N.D.; De Gregorio, B.T.; Kilcoyne, A.L.D.; Scott, K.; Chou, T.; Wirick, S.; Cody, G.; Stroud, R.M. Minimizing damage during FIB sample preparation of soft materials. J. Microsc. 2012, 245, 288–301. [Google Scholar] [CrossRef]
- Henderson, R. The Potential and Limitations of neutrons, electrons and X-Rays for atomic resolution microscopy of unstained biological molecules. Q. Rev. Biophys. 1995, 28, 171–193. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.L. Thermodynamics of AOT Micelle Formation in Ethylammonium Nitrate. J. Dispers. Sci. Technol. 2009, 30, 100–103. [Google Scholar] [CrossRef]
- Chem, J.; Noble, A.J.; Kang, J.Y.; Darst, S.A. Eliminating effects of particle adsorption to the air/water interface in single-particle cryo-electron microscopy: Bacterial RNA polymerase and CHAPSO. J. Struct. Biol. X 2019, 1, 100005. [Google Scholar]
- Noble, A.J.; Dandey, V.P.; Wei, H.; Brasch, J.; Chase, J.; Acharya, P.; Tan, Y.Z.; Zhang, Z.; Kim, L.Y.; Scapin, G.; et al. Routine single particle CryoEM sample and grid characterization by tomography. elife 2018, 7, e34257. [Google Scholar] [CrossRef]
- Le, T.T.-Y.; Tsay, R.-Y.; Lin, S.-Y. A study on the dynamic surface tension of surfactant solutions at dilute concentrations. J. Mol. Liq. 2021, 324, 115112. [Google Scholar] [CrossRef]
- Kobayashi, K.; Liang, Y.F.; Murata, S.; Matsuoka, T.; Takahashi, S.; Amano, K.; Nishi, N.; Sakka, T. Stability Evaluation of Cation Bridging on Muscovite Surface for Improved Description of Ion-Specific Wettability Alteration. J. Phys. Chem. C 2017, 121, 9273–9281. [Google Scholar] [CrossRef]
- Sun, W.Y.; Zeng, H.B.; Tang, T. Enhanced Adsorption of Anionic Polymer on Montmorillonite by Divalent Cations and the Effect of Salinity. J. Phys. Chem. A. 2021, 125, 1025–1035. [Google Scholar] [CrossRef]
- Ho, T.A.; Criscenti, L.J.; Greathouse, J.A. Revealing Transition States During the Hydration of Clay Minerals. J. Phys. Chem. Lett. 2019, 10, 3704–3709. [Google Scholar] [CrossRef]
- Barry, P.H.; Lawson, M.; Meurer, W.P.; Cheng, A.; Ballentine, C.J. Noble Gases in Deepwater Oils of the U.S. Gulf of Mexico. Geochem. Geophys. Geosyst. 2018, 19, 4218–4235. [Google Scholar] [CrossRef]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef] [Green Version]

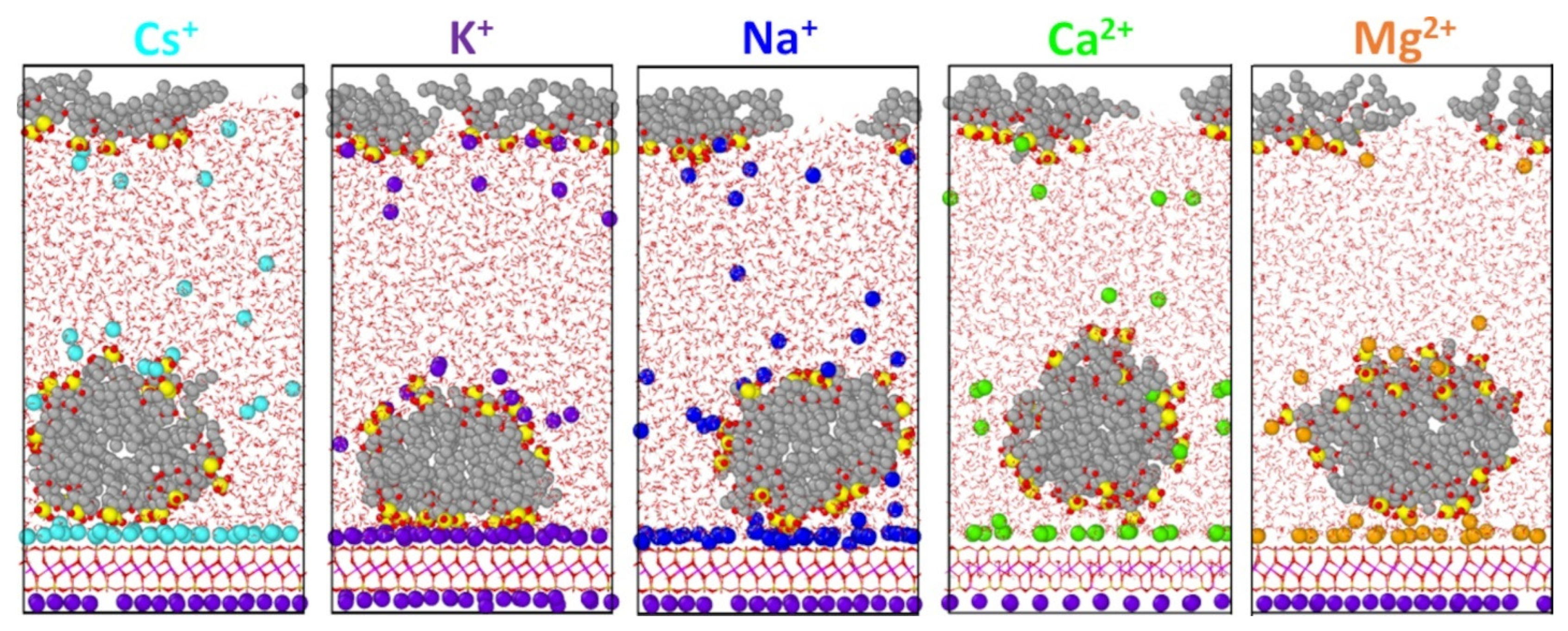
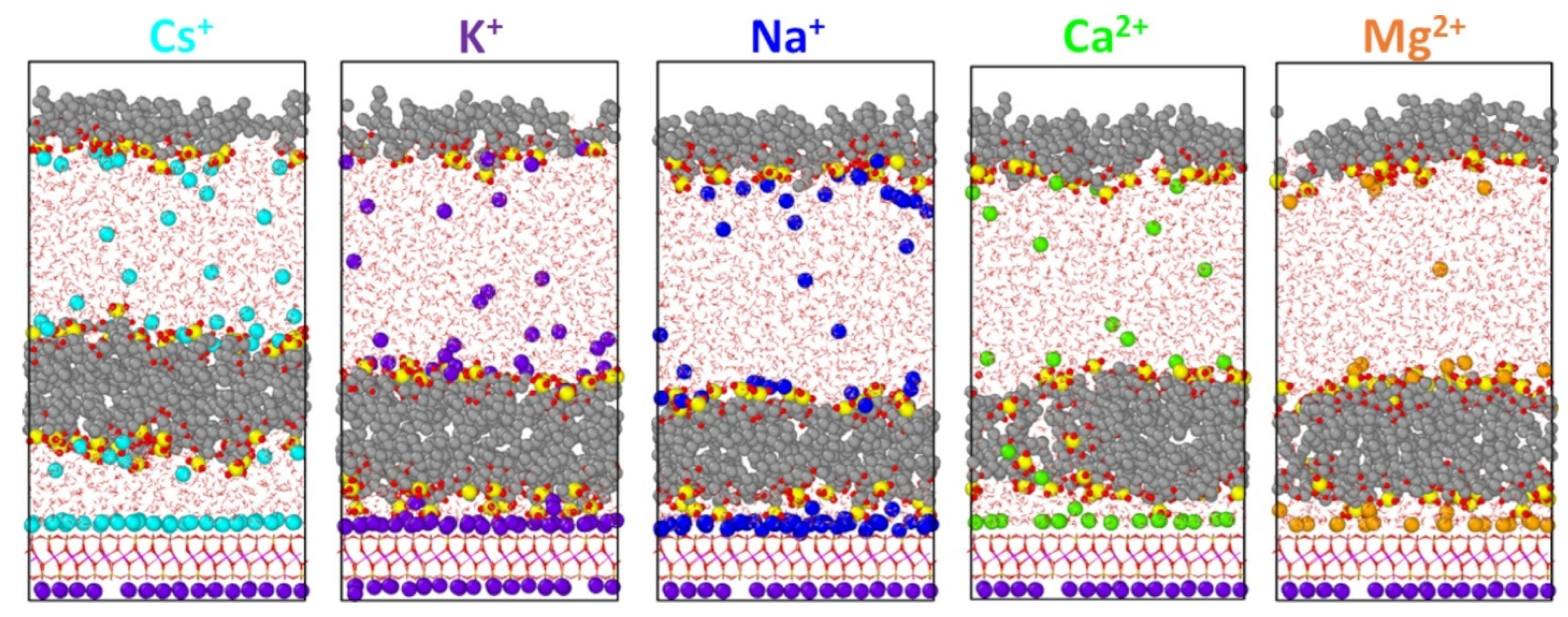
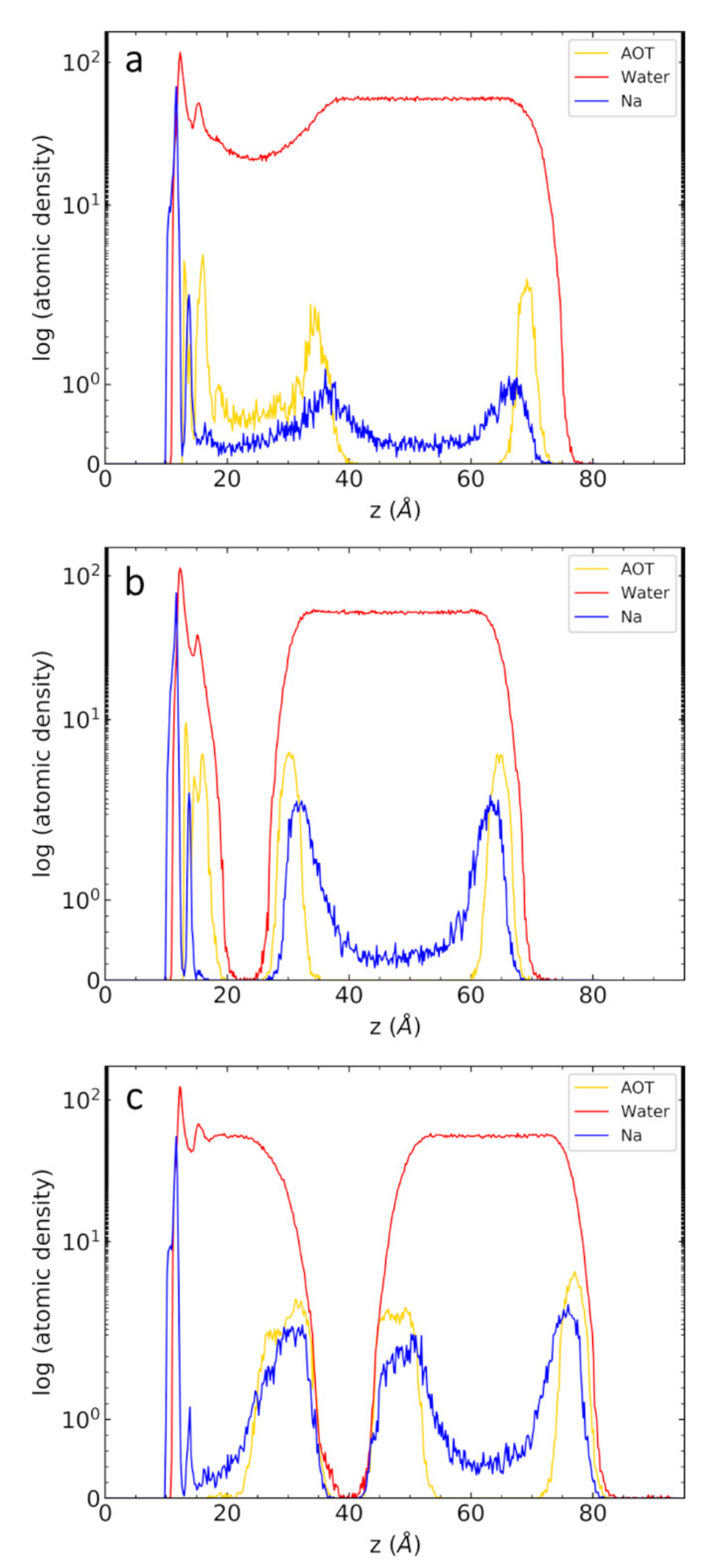
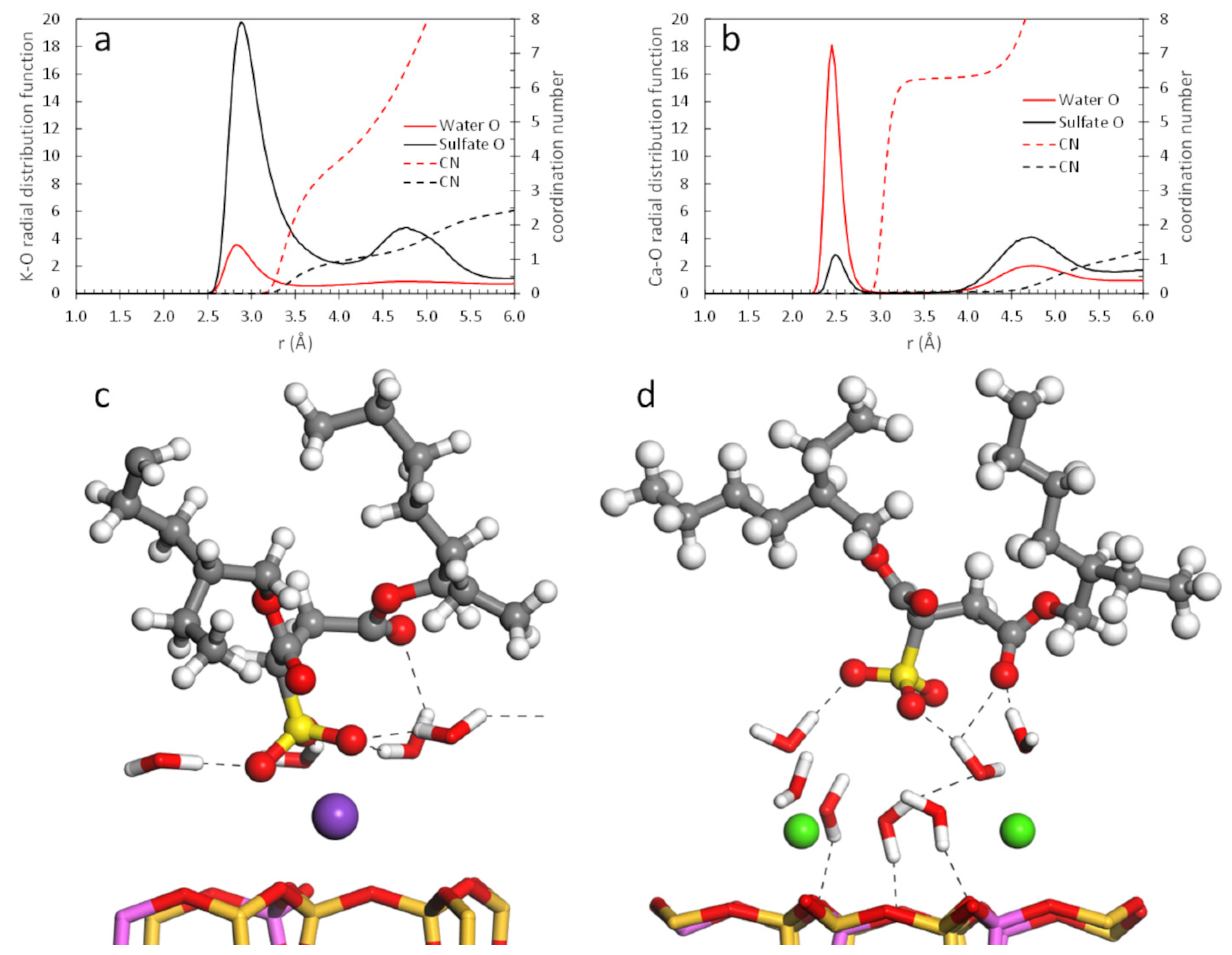

| Counterion | Micelle Thickness a (Å) | Bilayer Thickness b (Å) | Area per Molecule (Å2/AOT) c |
|---|---|---|---|
| Cs+ | 26.6 | 24.5 | 88 |
| K+ | 22.5 | 21.9 | 78 |
| Na+ | 26.1 | 20.4 | 93 |
| Ca2+ | 30.0 | 23.2 | 107 |
| Mg2+ | 26.9 | 22.8 | 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, D.M.; Greathouse, J.A.; Xu, G.; Jungjohann, K.L. Molecular Dynamics Simulation and Cryo-Electron Microscopy Investigation of AOT Surfactant Structure at the Hydrated Mica Surface. Minerals 2022, 12, 479. https://doi.org/10.3390/min12040479
Long DM, Greathouse JA, Xu G, Jungjohann KL. Molecular Dynamics Simulation and Cryo-Electron Microscopy Investigation of AOT Surfactant Structure at the Hydrated Mica Surface. Minerals. 2022; 12(4):479. https://doi.org/10.3390/min12040479
Chicago/Turabian StyleLong, Daniel M., Jeffery A. Greathouse, Guangping Xu, and Katherine L. Jungjohann. 2022. "Molecular Dynamics Simulation and Cryo-Electron Microscopy Investigation of AOT Surfactant Structure at the Hydrated Mica Surface" Minerals 12, no. 4: 479. https://doi.org/10.3390/min12040479
APA StyleLong, D. M., Greathouse, J. A., Xu, G., & Jungjohann, K. L. (2022). Molecular Dynamics Simulation and Cryo-Electron Microscopy Investigation of AOT Surfactant Structure at the Hydrated Mica Surface. Minerals, 12(4), 479. https://doi.org/10.3390/min12040479