Systematic Study of Legacy Phosphorus (P) Desorption Mechanisms in High-P Agricultural Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site Descriptions
2.2. Sample Collection and Characterization
2.2.1. Delmarva Soil Samples
2.2.2. Transect Soils
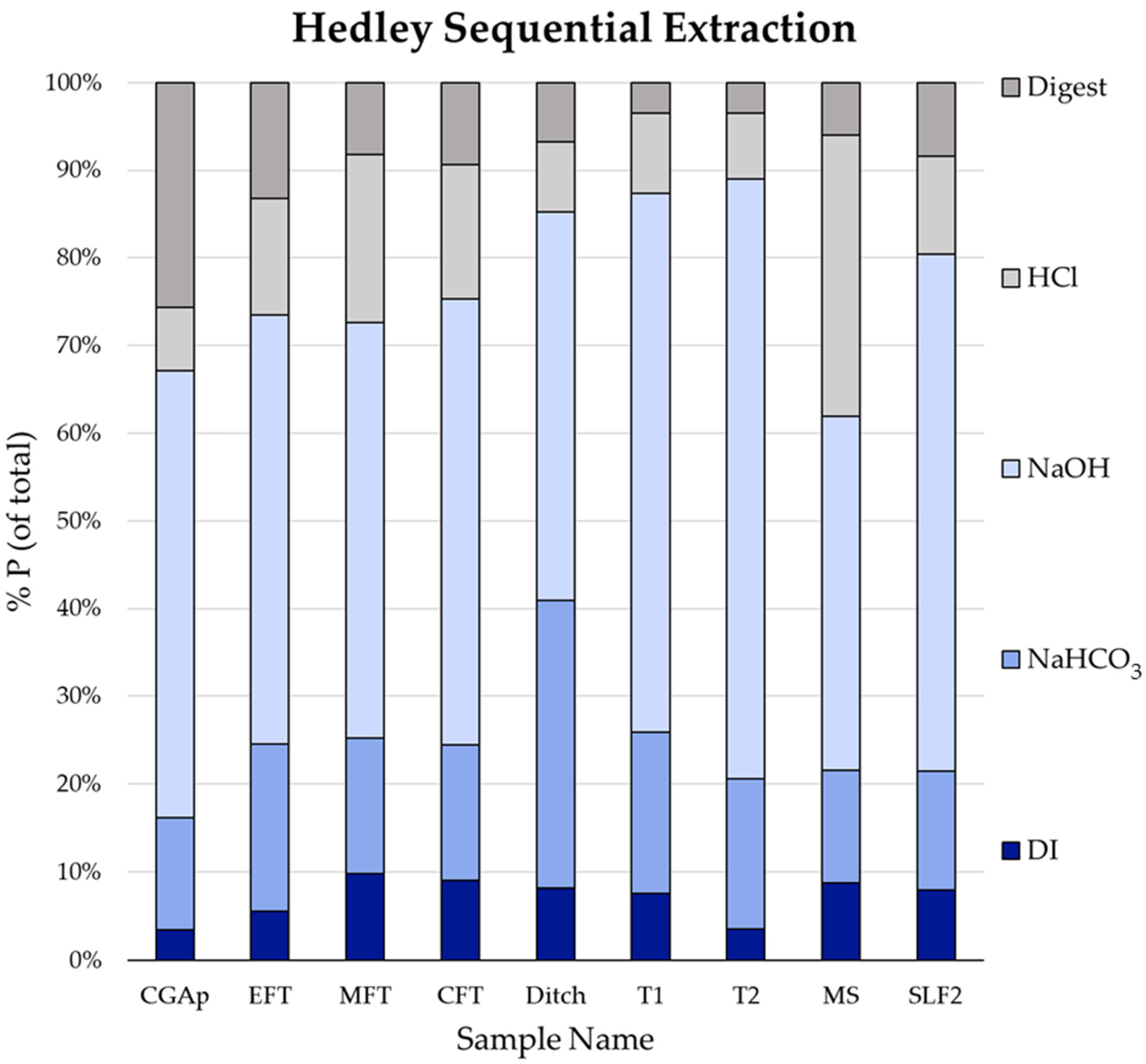
2.3. Hedley Sequential Extraction
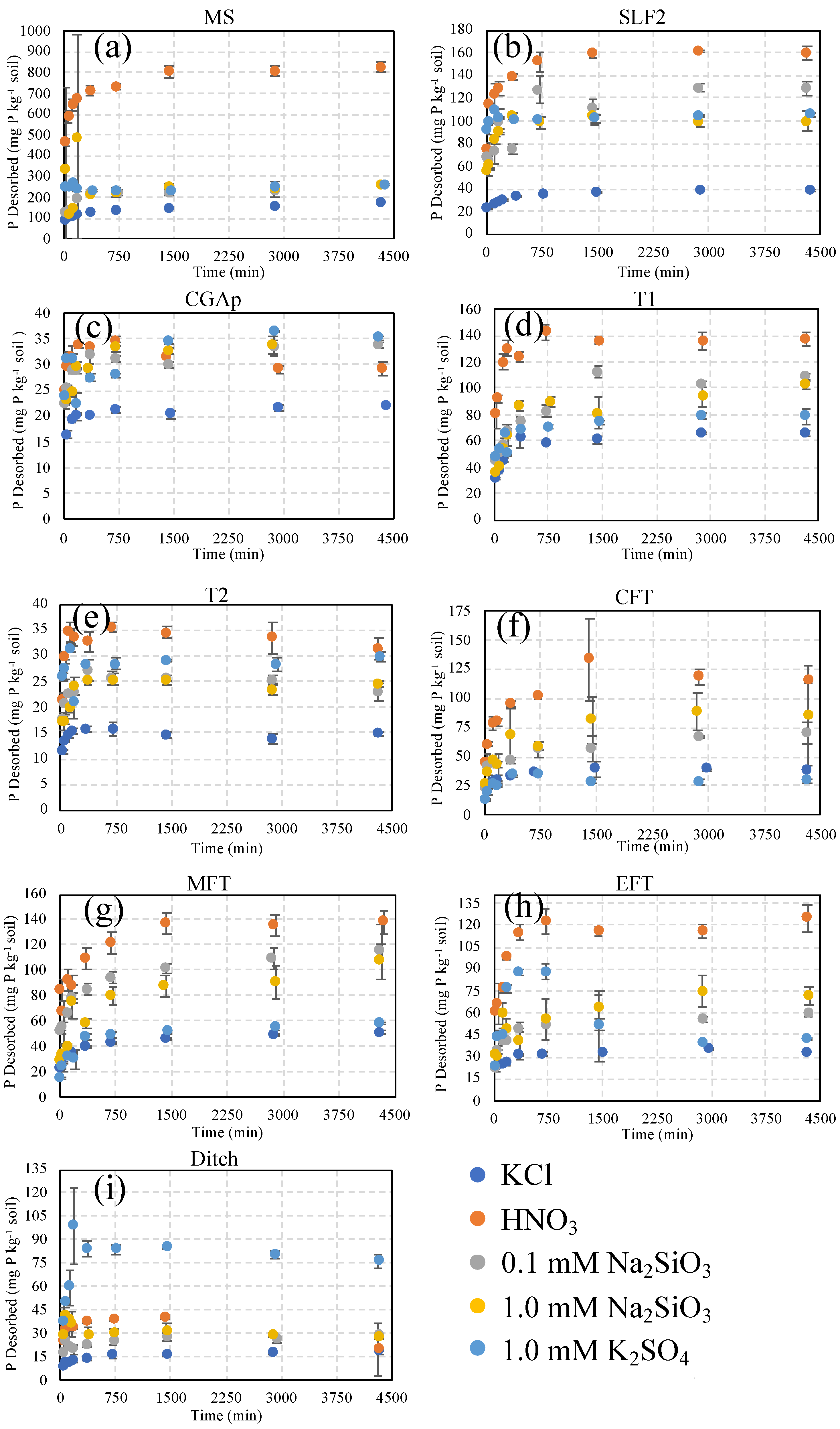
2.4. Desorption Studies
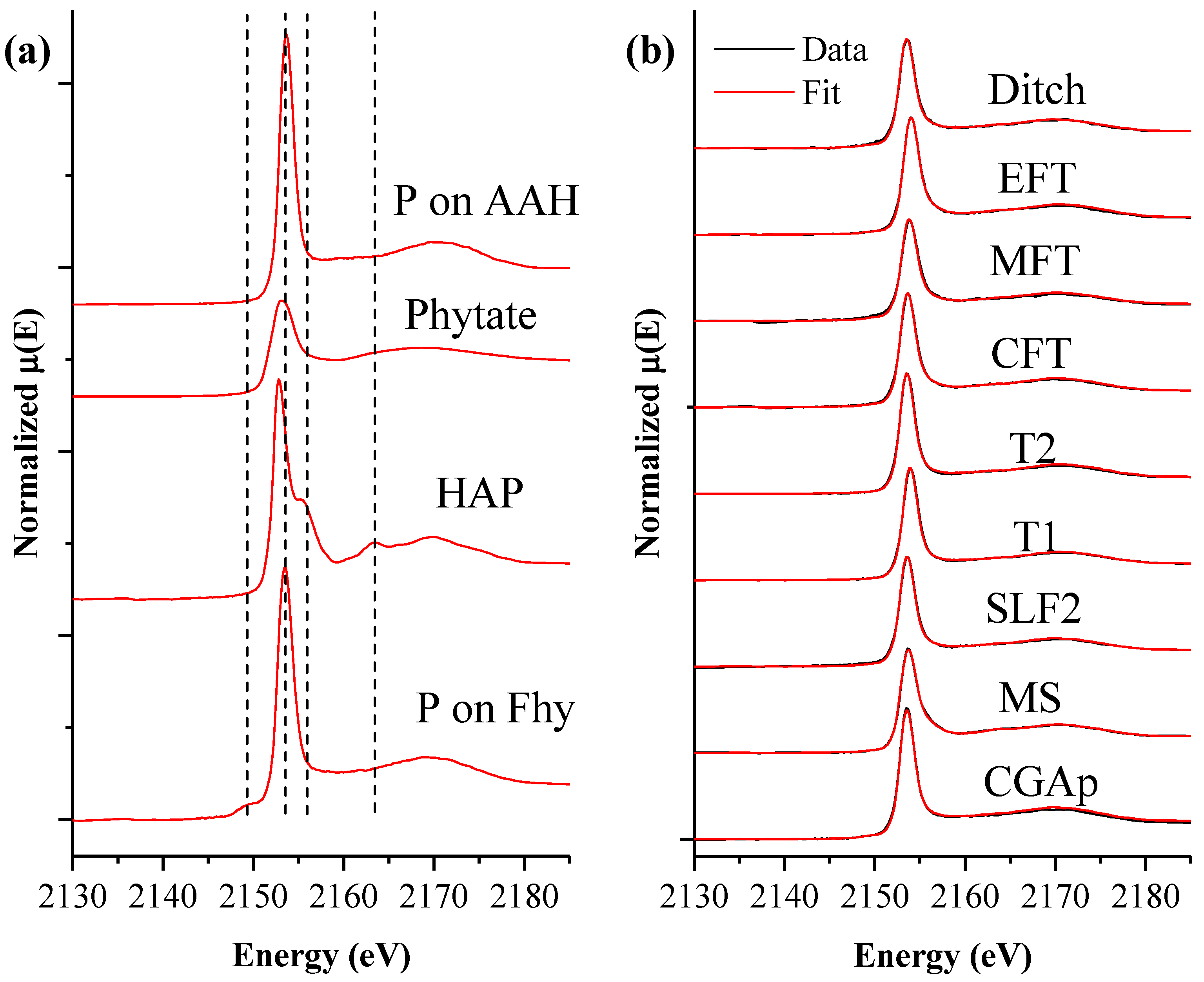
2.5. Bulk P K-Edge XANES and Linear Combination Fitting
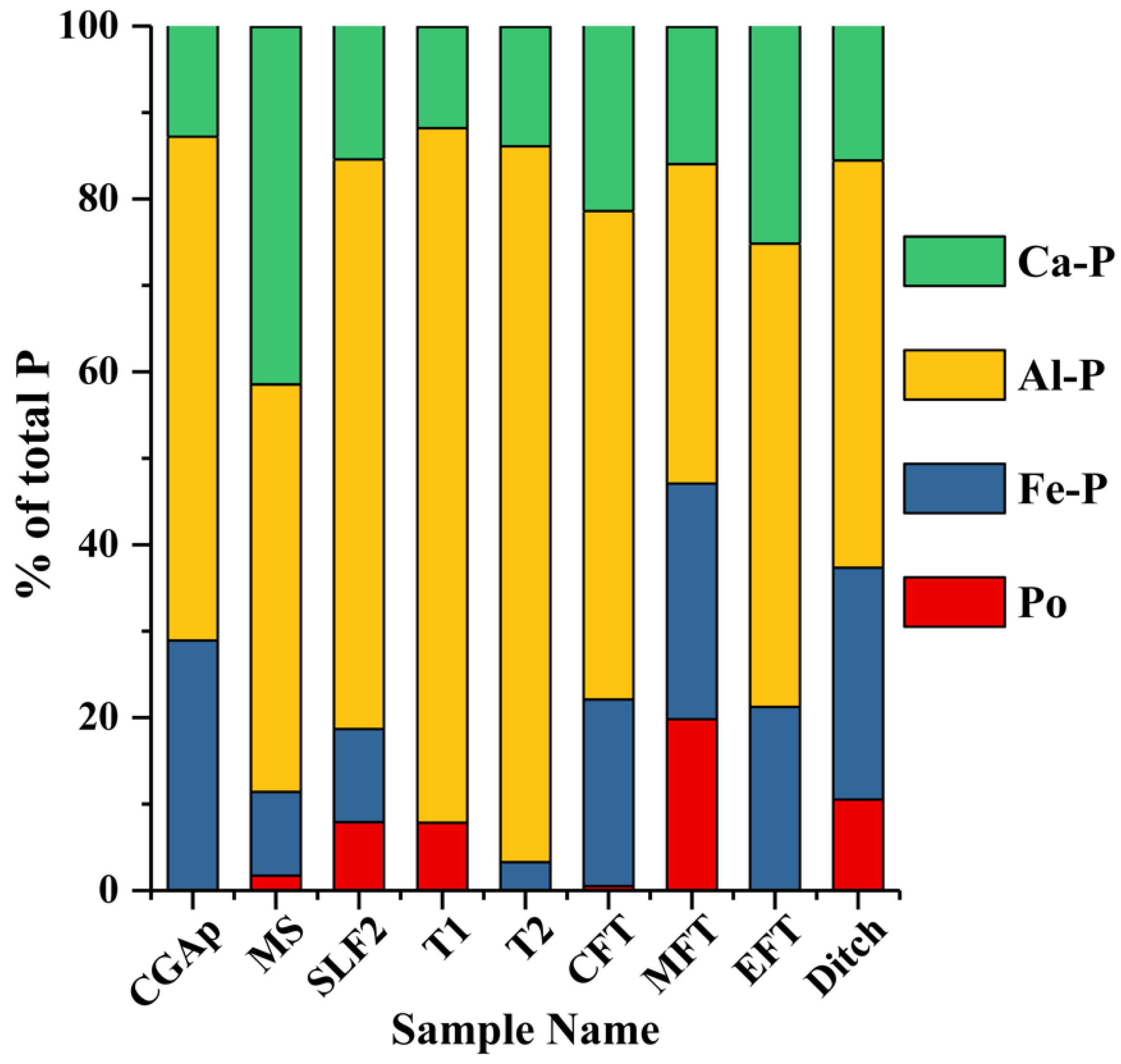
3. Results and Discussion
3.1. MS: Silt Loam
3.2. SLF2: Silt Loam
3.3. CGAp: Silt Loam
3.4. T1 and T2: Loamy Sand Soils
3.5. Transect (CFT, MFT, EFT, and Ditch): Loam and Loamy Sand
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shober, A.L.; Maguire, R.O. Reference Module in Earth Systems and Environmental Sciences. In Manure Management; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Qin, Z.; Shober, A.L. The challenges of managing legacy phosphorus losses from manure-impacted agricultural soils. Curr. Pollut. Rep. 2018, 4, 265–276. [Google Scholar] [CrossRef]
- Sharpley, A.; Jarvie, H.P.; Buda, A.; May, L.; Spears, B.; Kleinman, P. Phosphorus legacy: Overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, J.T.; Edwards, A.C.; Schoumans, O.F.; Simard, R.R. Integrating soil phosphorus testing into environmentally based agricultural management practices. J. Environ. Qual. 2000, 29, 60–71. [Google Scholar] [CrossRef]
- Sims, J.T. Phosphorus soil testing: Innovations for water quality protection. Commun. Soil Sci. Plant Anal. 1998, 29, 1471–1489. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Sharpley, A.N.; Spears, B.; Buda, A.R.; May, L.; Kleinman, P.J.A. Water quality remediation faces unprecedented challenges from “legacy phosphorus”. Environ. Sci. Technol. 2013, 47, 8997–8998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpley, A.N.; Kleinman, P.J.A.; Flaten, D.N.; Buda, A.R. Critical source area management of agricultural phosphorus: Experiences, challenges and opportunities. Water Sci. Technol. 2011, 64, 945–952. [Google Scholar] [CrossRef]
- Kleinman, P.J.A.; Sharpley, A.; Buda, A.; McDowell, R.; Allen, A. Soil controls of phosphorus in runoff: Management barriers and opportunities. Can. J. Soil Sci. 2011, 91, 329–338. [Google Scholar] [CrossRef]
- McDowell, R.W.; Sharpley, A.N.; Chalmers, A.T. Land use and flow regime effects on phosphorus chemical dynamics in the fluvial sediment of the Winooski River, Vermont. Ecol. Eng. 2002, 18, 477–487. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Mohanty, S.; Kumar, A.; Raja, R.; Panda, B.B.; Lal, B.; Gautam, P.; et al. Effects of 42-year long-term fertilizer management on soil phosphorus availability, fractionation, adsorption-desorption isotherm and plant uptake in flooded tropical rice. Crop J. 2015, 3, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Dou, Z.X.; Ramberg, C.F.; Toth, J.D.; Wang, Y.; Sharpley, A.N.; Boyd, S.E.; Chen, C.R.; Williams, D.; Xu, Z.H. Phosphorus speciation and sorption-desorption characteristics in heavily manured soils. Soil Sci. Soc. Am. J. 2009, 73, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Whalen, J.K.; Hendershot, W.H. Phosphate sorption and release in a sandy-loam soil as influenced by fertilizer sources. Soil Sci. Soc. Am. J. 2007, 71, 118–124. [Google Scholar] [CrossRef]
- Singh, V.; Dhillon, N.; Brar, B. Influence of long-term use of fertillizers and farmyard manure on the adsorption-desorption behavior and bioavailability of phosphorus in soils. Nutr. Cycl. Agroecosyst. 2006, 75, 67–78. [Google Scholar] [CrossRef]
- Breeuwsma, A.; Reijerink, J.G.A.; Schoumans, O.F. Impact of manure on accumulation and leaching of phosphate in areas of intensive livestock farming. In Animal Waste and the Landwater Interface; Steele, K., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 239–249. [Google Scholar]
- Sample, E.C.; Soper, R.J.; Racz, G.J. Reactions of phosphate fertilizers in soils. In The Role of Phosphorus in Agriculture; Khasawneh, F.E., Sample, E.C., Kamprath, E.J., Eds.; ASA and SSSA: Madison, WI, USA, 1980; pp. 263–310. [Google Scholar]
- Wang, Q.; Li, Y. Phosphorus adsorption and desorption behavior on sediments of different origins. J. Soils Sediments 2010, 10, 1159–1173. [Google Scholar] [CrossRef]
- Gamble, A. Speciation and Reactivity of Phosphorus and Arsenic in Mid-Atlantic Soils. Ph.D. Thesis, University of Delaware, Newark, DE, USA, 2017. [Google Scholar]
- Violante, A. Elucidating mechanisms of competitive sorption at the mineral/water interface. Adv. Agron. 2013, 118, 111–176. [Google Scholar]
- Lee, Y.B.; Hoon, C.; Hwang, J.Y.; Lee, I.B.; Kim, P.J. Enhancement of phosphate desorption by silicate in soils with salt accumulation. Soil Sci. Plant Nutr. 2004, 50, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Owino-Gerroh, C.; Gascho, G.J. Effect of silicon on low pH soil phosphorus sorption and on uptake and growth of maize. Commun. Soil Sci. Plant Anal. 2005, 35, 2369–2378. [Google Scholar] [CrossRef]
- Koski-Vähälä, J.; Hartikainen, H.; Tallberg, P. Phosphorus mobilization from various sediment pools in response to increased pH and silicate concentration. J. Environ. Qual. 2001, 30, 546–552. [Google Scholar] [CrossRef]
- Jianfeng, M.A.; Takahashi, E. Effect of silicate on phosphate availability for rice in a P-deficient soil. Plant Soil 1991, 133, 151–155. [Google Scholar] [CrossRef]
- Pigna, M.; Violante, A. Adsorption of sulfate and phosphate on andisols. Commun. Soil Sci. Plant Anal. 2003, 34, 2099–2113. [Google Scholar] [CrossRef]
- Lamers, L.P.; Tomassen, H.B.; Roelofs, J.G. Sulfate-induced eutrophication and phytotoxicity in freshwater wetlands. Environ. Sci. Technol. 1998, 32, 199–205. [Google Scholar] [CrossRef]
- DE.gov. Available online: https://de.gov/topics/facts/geo.shtml (accessed on 30 March 2022).
- MODIS. Satellite Image of the Chesapeake Bay. 2000, NASA. Available online: https://visibleearth.nasa.gov/images/54295/chesapeake-bay-from-modis (accessed on 3 October 2021).
- Eckert, D.; Sims, J.T. Recommended soil pH and lime requirement tests. In Recommended Soil Testing Procedures for the Northeastern United States; Sims, J.T., Wolf, A., Eds.; University of Delaware: Newark, DE, USA, 1995; pp. 11–16. [Google Scholar]
- Schulte, E.E. Recommended soil organic matter tests. In Recommended Soil Testing Procedures for the Northeastern United States; Sims, J.T., Wolf, A., Eds.; University of Delaware: Newark, DE, USA, 1995; pp. 47–56. [Google Scholar]
- Wolf, A.; Beegle, D. Recommended soil tests for macro and micronutrients. In Recommended Soil Testing Procedures for the Northeastern United States, 3rd ed.; Wolf, A., Ed.; University of Delaware: Newark, DE, USA, 2011; pp. 39–48. [Google Scholar]
- Ross, D. Recommended soil tests for determining soil cation exchange capacity. In Recommended Soil Testing Procedures for the Northeastern United States; Sims, J.T., Wolf, A., Eds.; University of Delaware: Newark, DE, USA, 1995; pp. 62–69. [Google Scholar]
- USEPA. Test Methods for Evaluating Solid Waste, 3rd ed.; National Technical Information Service: Springfield, VA, USA, 1986. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Sims, J.; Maguire, R.O.; Leytem, A.B.; Gartley, K.L.; Pautler, M.C. Evaluation of Mehlich 3 as an Agri-Environmental Soil Phosphorus Test for the Mid-Atlantic United States of America. Soil Sci. Soc. Am. J. 2002, 66, 2016–2032. Available online: https://www.researchgate.net/publication/255643736_Evaluation_of_Mehlich_3_as_an_Agri-Environmental_Soil_Phosphorus_Test_for_the_Mid-Atlantic_United_States_of_America (accessed on 2 April 2022). [CrossRef]
- Sims, J.T.; Gartley, K.L. Note 18: Phosphorus management and the phosphorus saturation ratio. In Soil Test Notes; University of Delaware: Newark, DE, USA, 2001. [Google Scholar]
- Welsh, C.; Tenuta, M.; Flaten, D.N.; Thiessen-Martens, J.R.; Entz, M.H. High yielding organic crop management decreases plant-available but not recalcitrant phosphorus. Agron. J. 2009, 101, 1027–1035. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Athena, Artemis, Hephaestus: Data analysis for X-ray absorption spectroscopy using Ifeffit. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Hu, Y.; Tang, Y.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces. Environ. Sci. Nano 2017, 4, 2193–2204. [Google Scholar] [CrossRef]
- Gu, C.; Dam, T.; Hart, S.C.; Turner, B.L.; Chadwick, O.A.; Berhe, A.A.; Hu, Y.; Zhu, M. Quantifying uncertainties in sequential chemical extraction of soil phosphorus using XANES spectroscopy. Environ. Sci. Technol. 2020, 54, 2257–2267. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils; Pearson Education: London, UK, 2016. [Google Scholar]
- Toor, G.S.; Peak, J.D.; Sims, J.T. Phosphorus speciation in broiler litter and turkey manure produced from modified diets. J. Environ. Qual. 2005, 34, 687–697. [Google Scholar] [CrossRef] [Green Version]
- EPA. A Closer Look: Land Loss Along the Atlantic Coast. Available online: https://www.epa.gov/climate-indicators/atlantic-coast (accessed on 9 March 2022).

| Mehlich III Extractible | EPA3051 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | P | Fe | Al | Ca | Mn | S | K | P Sat. | % P | P | Fe | Al | Ca | Mn | S | K |
| mg kg−1 | Ratio | Removed | mg kg−1 | |||||||||||||
| CGAp | 199 | 272 | 982 | 774 | 79 | 9 | 257 | 41.5 | 21.2 | 937 | 16430 | 14342 | 1099 | 390 | 165 | 918 |
| MS | 1122 | 407 | 737 | 2061 | 25 | 17 | 517 | 253.8 | 56.0 | 2004 | 4501 | 12544 | 3381 | 112 | 325 | 1751 |
| SLF2 | 634 | 292 | 1112 | 1279 | 12 | 15 | 212 | 109.5 | 57.2 | 1109 | 4037 | 15913 | 1692 | 51 | 214 | 1522 |
| T1 | 607 | 156 | 1117 | 646 | 16 | 15 | 100 | 110.1 | 59.3 | 1023 | 1117 | 4758 | 944 | 39 | 184 | 436 |
| T2 | 357 | 273 | 1321 | 820 | 8 | 18 | 100 | 55.5 | 36.7 | 974 | 2061 | 5655 | 1264 | 32 | 195 | 359 |
| CFT | 256 | 195 | 578 | 808 | 9 | 14 | 140 | 83.5 | 53.1 | 482 | 1920 | 7538 | 985 | 29 | 148 | 876 |
| MFT | 331 | 302 | 482 | 699 | 14 | 13 | 123 | 114 | 60.0 | 552 | 1835 | 5134 | 885 | 35 | 140 | 684 |
| EFT | 303 | 410 | 670 | 1063 | 10 | 18 | 152 | 76.9 | 41.7 | 727 | 4841 | 8807 | 1299 | 51 | 192 | 699 |
| Ditch | 181 | 576 | 360 | 578 | 10 | 33 | 63 | 63.4 | 56.6 | 320 | 1922 | 3528 | 589 | 19 | 238 | 336 |
| Soil | Sand | Silt | Clay | Texture | pH | OM | CEC |
|---|---|---|---|---|---|---|---|
| % | % | cmolc kg soil−1 | |||||
| CGAp | 13 | 65 | 22 | Silt loam | 6.1 | 2.6 | 7.1 |
| MS | 29 | 53 | 18 | Silt loam | 6.7 | 3.1 | 15.8 |
| SLF2 | 17 | 63 | 20 | Silt loam | 6.1 | 3.0 | 10.4 |
| T1 | 86 | 10 | 4 | Loamy sand | 5.4 | 3.4 | 7.2 |
| T2 | 81 | 13 | 6 | Loamy sand | 5.7 | 3.6 | 7.7 |
| CFT | 43 | 33 | 24 | Loam | 5.2 | 1.7 | 7.3 |
| MFT | 52 | 28 | 20 | Loam | 5.1 | 1.6 | 6.2 |
| EFT | 51 | 25 | 24 | Loam | 5.4 | 1.7 | 10.4 |
| D1 | 86 | 8 | 6 | Loamy sand | 6.2 | 1.4 | 4.2 |
| P Speciation (%) | CGAP | ± | MS | ± | SLF2 | ± | T1 | ± | T2 | ± | CFT | ± | MFT | ± | EFT | ± | Ditch | ± |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Po | 0.0 | 1.6 | 1.7 | 0.9 | 7.9 | 1.5 | 7.8 | 1.4 | 0.0 | 1.6 | 0.5 | 0.6 | 19.8 | 2.0 | 0.0 | 1.2 | 10.5 | 1.5 |
| Fe-P | 28.9 | 3.8 | 9.7 | 2.1 | 10.8 | 3.5 | 0.0 | 3.2 | 3.3 | 3.7 | 21.6 | 3.3 | 27.3 | 4.6 | 21.2 | 3.0 | 26.8 | 3.6 |
| Al-P | 58.3 | 2.9 | 47.2 | 1.6 | 65.9 | 2.6 | 80.4 | 2.4 | 82.8 | 2.8 | 56.5 | 2.5 | 36.9 | 3.5 | 53.6 | 2.3 | 47.1 | 2.8 |
| Ca-P | 12.8 | 1.6 | 41.4 | 0.8 | 15.5 | 1.3 | 11.7 | 1.2 | 13.8 | 1.4 | 21.4 | 4.2 | 15.9 | 1.8 | 25.2 | 1.2 | 15.5 | 1.4 |
| R factor | 0.0021 | 0.0008 | 0.0020 | 0.0016 | 0.0019 | 0.0017 | 0.0042 | 0.0014 | 0.0022 | |||||||||
| Reduced chi-square | 0.0053 | 0.0013 | 0.0035 | 0.0029 | 0.0040 | 0.0033 | 0.0065 | 0.0029 | 0.0038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szerlag, K.D.; Elavarthi, M.; Siebecker, M.G.; Gu, C.; McCrone, C.; Sparks, D.L. Systematic Study of Legacy Phosphorus (P) Desorption Mechanisms in High-P Agricultural Soils. Minerals 2022, 12, 458. https://doi.org/10.3390/min12040458
Szerlag KD, Elavarthi M, Siebecker MG, Gu C, McCrone C, Sparks DL. Systematic Study of Legacy Phosphorus (P) Desorption Mechanisms in High-P Agricultural Soils. Minerals. 2022; 12(4):458. https://doi.org/10.3390/min12040458
Chicago/Turabian StyleSzerlag, Kathryn Daria, Monica Elavarthi, Matthew G. Siebecker, Chunhao Gu, Conner McCrone, and Donald Lewis Sparks. 2022. "Systematic Study of Legacy Phosphorus (P) Desorption Mechanisms in High-P Agricultural Soils" Minerals 12, no. 4: 458. https://doi.org/10.3390/min12040458
APA StyleSzerlag, K. D., Elavarthi, M., Siebecker, M. G., Gu, C., McCrone, C., & Sparks, D. L. (2022). Systematic Study of Legacy Phosphorus (P) Desorption Mechanisms in High-P Agricultural Soils. Minerals, 12(4), 458. https://doi.org/10.3390/min12040458







