A Multi-Methodological Investigation of Natural and Synthetic Red Beryl Gemstones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Description and Gemmological Testing
2.2. LA-ICP-MS Measurements
2.3. Infrared Spectroscopy
2.4. Raman Spectroscopy
2.5. UV–vis–NIR Spectroscopy
2.6. X-ray Diffraction Data Collection and Treatment
3. Results and Discussion
3.1. Gemmological Properties
3.2. Chemical Composition
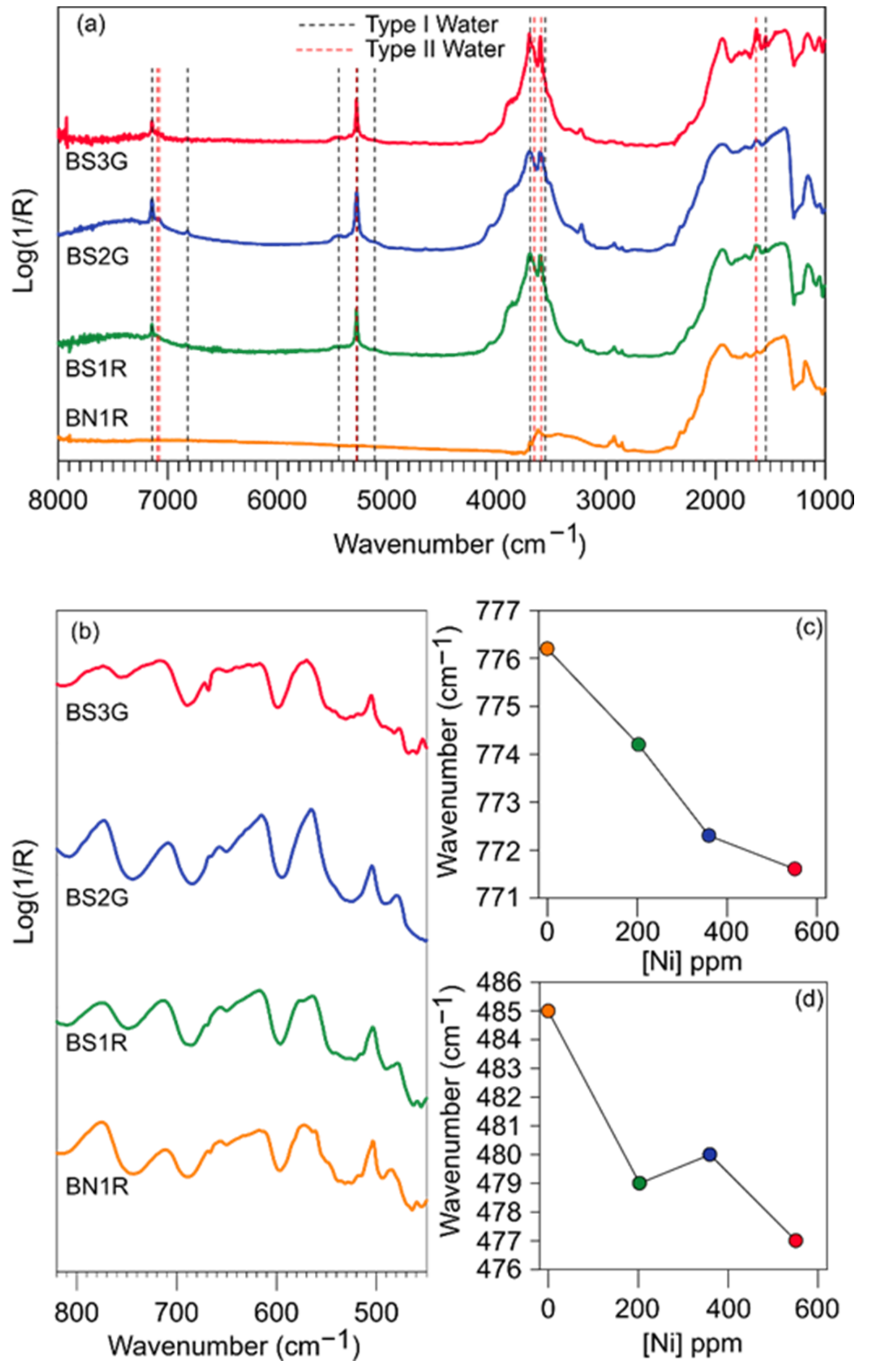
3.3. Infrared Spectra
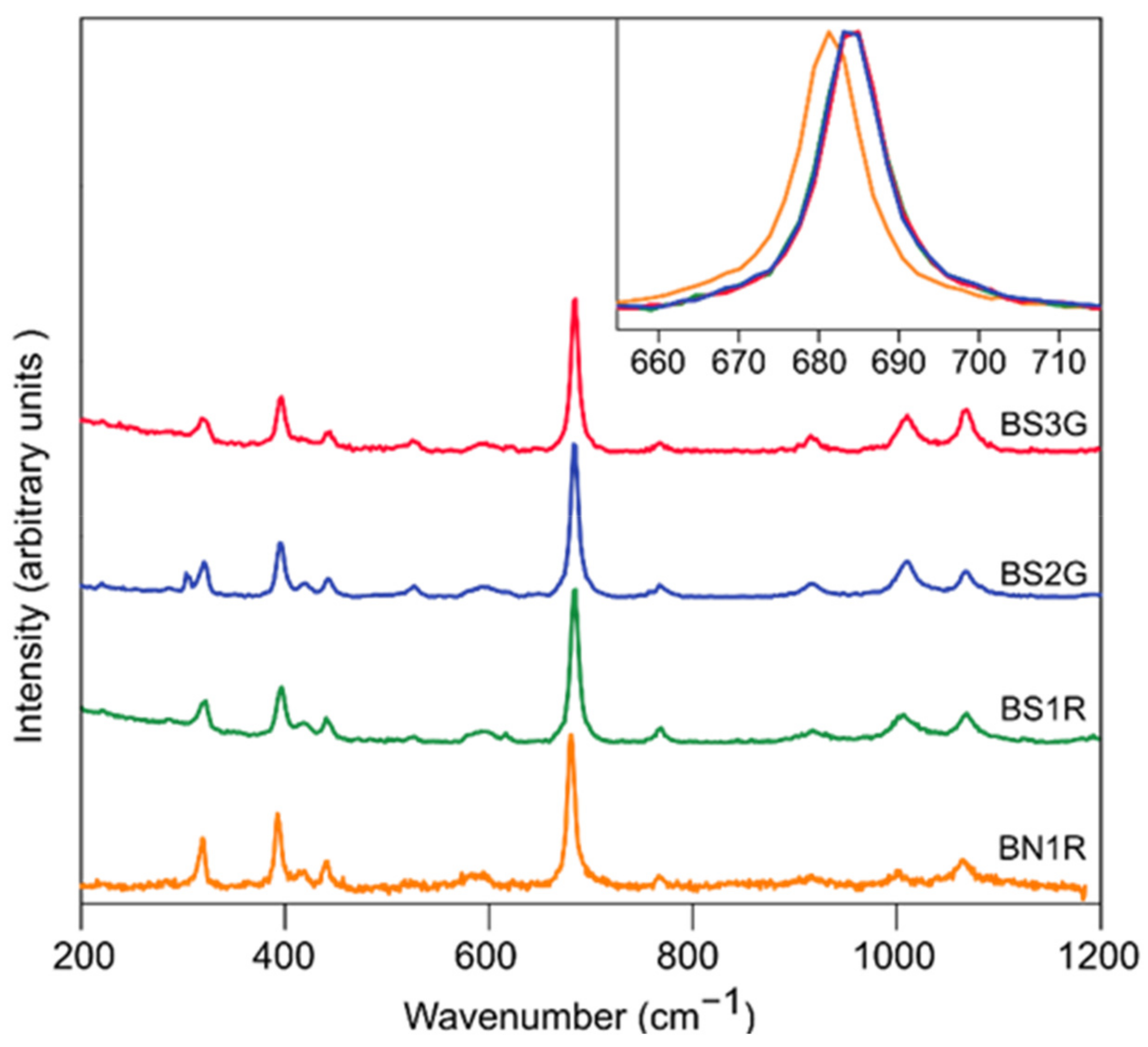
3.4. Raman Spectra
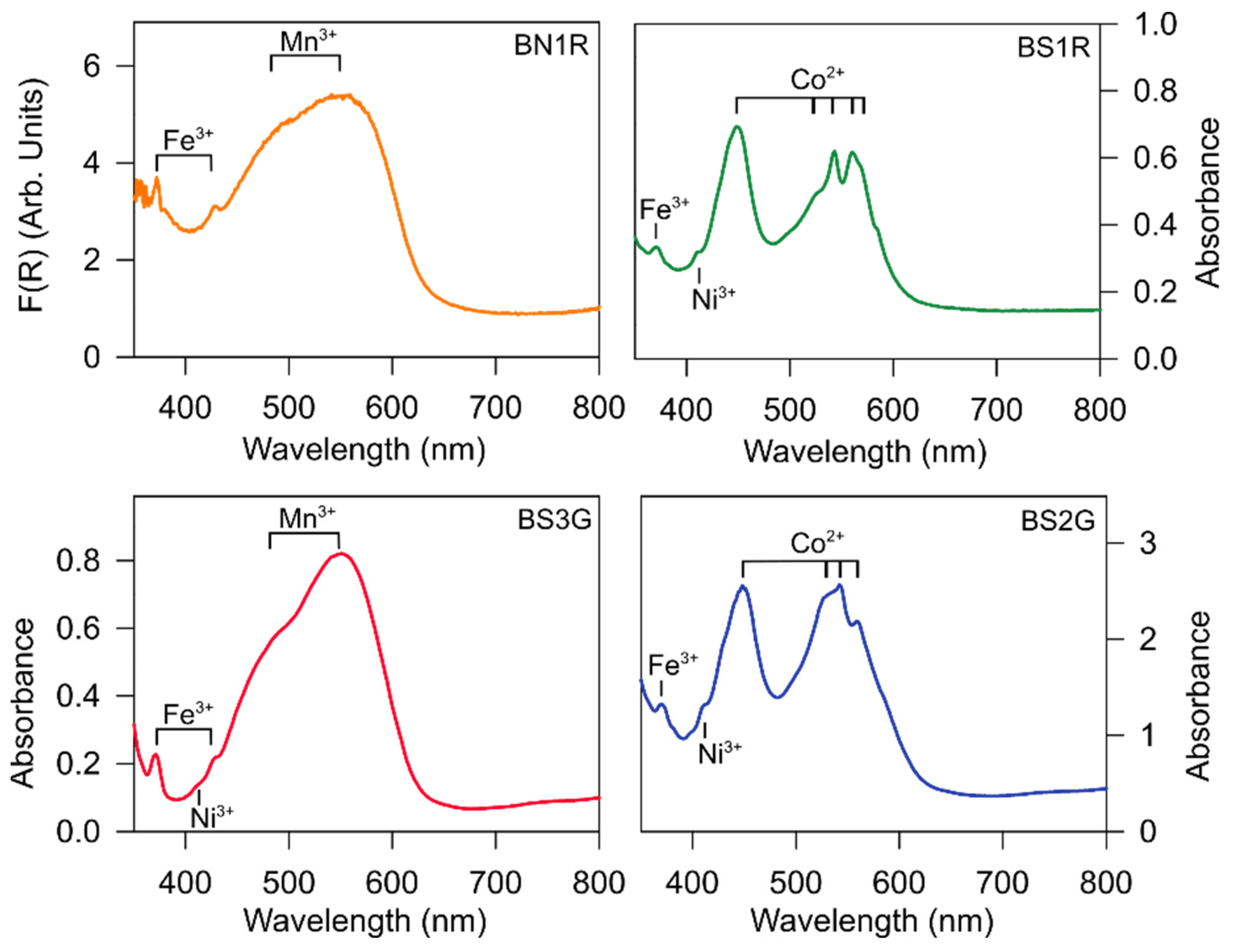
3.5. UV–vis–NIR Spectra
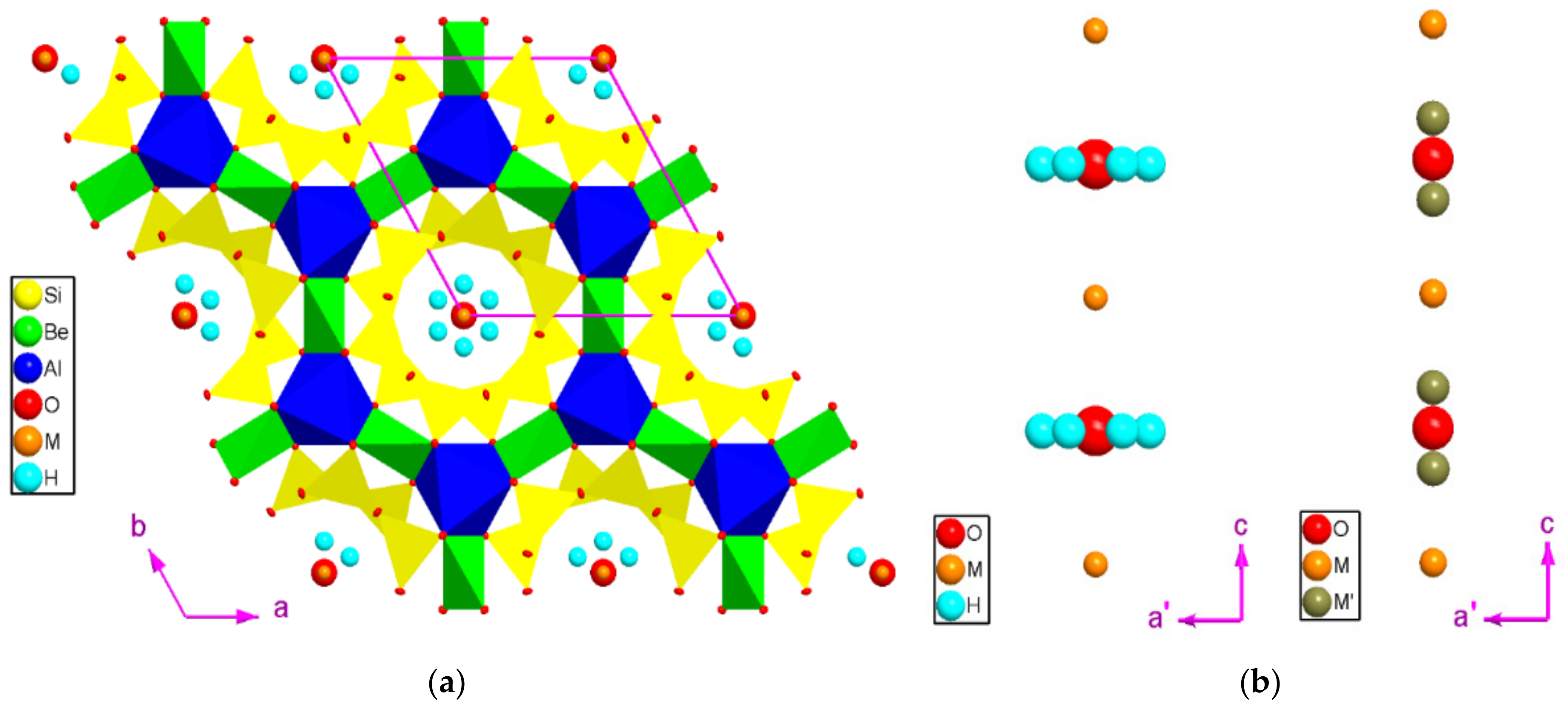
3.6. Crystallographic Data
4. Conclusions
- -
- The gemmological properties of the stones under investigation allow us to distinguish the synthetic samples from the natural counterparts, as proved by the observation under the gemmological microscope. The most distinctive features of the hydrothermal synthetic stones are the strong inhomogeneous growth structures, with chevron-like patterns.
- -
- Chemical data of the natural and synthetic stones of this study show significant (and diagnostic) difference for the minor/trace elements content (i.e., from 102 to 104 wt ppm, Table 2), in particular for the transition metals as Fe3+, Mn3+, Co2+, Ti4+, and Ni3+. Ni3+ and Co2+ are always substantially absent in the natural red beryl and are considered the most diagnostic elements for identifying synthetic red beryls. Significant difference pertains also to the alkalis content: the synthetic stones show a relatively higher fraction of Li+ (i.e., 3–4·103 wt ppm, Table 2) and Na+ (i.e., 0.6–1.8·103 wt ppm, Table 2) if compared to the natural red beryl (i.e., Li+ 0.8·103 wt ppm, Na+ 0.3·103 wt ppm) (Table 2), whereas the fraction of K+ in the synthetic samples is drastically lower (i.e., 0.04–0.12·103 wt ppm) than the natural one (i.e., 1.4·103 wt ppm) (Table 2). As proved by UV–vis–NIR spectroscopy, cobalt is one of the common dopants used to generate red coloration in synthetic beryls, and its occurrence is considered a clear indication of the artificial origin of a gem. In addition, the red hue of Co-doped samples is deeper than the typical red of natural beryls, while higher levels of Mn3+ better mimic the pinkish color of the natural samples.
- -
- Vibrational spectroscopy data, collected on the natural and synthetic red beryls of this study, provide a series of complementary information. Raman spectra proved how local structural distortions, generated by heteroatomic substitution in octahedral coordination, can be efficiently detected even when generated by transition metals with modest concentration (i.e., Ni3+ 2–5·102 wt ppm, Table 2). IR spectra provide evidence about the presence (and, therefore, the content), the orientation and the interaction of the structurally incorporated H2O molecules, lying into the [001] channel. Natural red beryls from Utah have a very low H2O content if compared to other varieties of natural beryls and to the synthetic ones of this study, and its IR spectrum clearly shows that.
- -
- Chemical and crystallographic data consistently show that, for all the samples (natural or synthetic) here investigated, the potential substituents at the tetrahedral sites, occupied by Be (6f) and Si (12l), are only modest, at least for the natural sample. A significant fraction of Li is observed in the synthetic samples (i.e., 3–4·103 wt ppm, Table 2), and we cannot exclude a replacement of Be, though with no crystallographic evidence. In contrast, Al at the octahedral site (4c) is partially replaced by elements with higher atomic number: Fe, Co, and Mn (along with Ti for the natural and Ni for the synthetic stones), and the effect of such a replacement is manifested at the unit-cell level (with c/a ranging between the domain of the “octahedral beryls”) and at the atomic level (in terms of electron density at the 4c site and Al-O bond distances, Table 4 and Table 5). The similar atomic number of Mn, Fe, and Co (and Ni) does not allow any reliable discrimination among these transition metals in X-ray structure refinements, but the aggregate effect of these substituents is observable (e.g., a virtually identical Al-O2 bond distance is generated either by ~2.1·104 wt ppm of Fe or by ~1.5·104 Fe + 0.4·104 Co wt ppm).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webster, R. Gems: Their Sources, Descriptions and Identification, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2006; p. 1072. [Google Scholar]
- Adamo, I.; Gatta, G.D.; Rotiroti, N.; Diella, V.; Pavese, A. Gemmological investigation of a synthetic blue beryl: A multi-methodological study. Min. Mag. 2008, 72, 799–808. [Google Scholar] [CrossRef]
- Bragg, W.L.; West, I. The structure of beryl. Proc. R. Soc. Lond. 1926, 3A, 691–714. [Google Scholar]
- Wood, D.L.; Nassau, K. Infrared spectra of foreign molecules in beryl. J. Chem. Phys. 1967, 42, 2220–2228. [Google Scholar] [CrossRef]
- Wood, D.L.; Nassau, K. The characterization of beryl and emerald by visible and infrared absorption spectroscopy. Am. Miner. 1968, 53, 777–800. [Google Scholar]
- Morosin, B. Structure and thermal expansion of beryl. Acta Crystallogr. 1972, B28, 1899–1903. [Google Scholar] [CrossRef]
- De Almeida Sampaio Filho, H.; Sighinolfi, G.; Galli, E. Contribution to crystal chemistry of beryl. Contr. Miner. Petr. 1973, 38, 279–290. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Breck, D.W.; Meagher, E.P. Structural refinement of hydrous and anhydrous beryl, Al2(Be3Si6)O18 and emerald, Al1.9Cr0.1(Be3Si6)O18. Lithos 1968, 1, 275–285. [Google Scholar] [CrossRef]
- Goldman, D.S.; Rossman, G.R.; Parkin, K.M. Channel constituents in beryl. Phys. Chem. Miner. 1978, 3, 225–235. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Černý, P. The alkali-metal position in Cs-Li beryl. Can. Miner. 1977, 15, 414–421. [Google Scholar]
- Aurisicchio, C.; Fioravanti, G.; Grubessi, O.; Zanazzi, P.F. Reapprasail of the crystal chemistry of beryl. Am. Miner. 1988, 73, 826–837. [Google Scholar]
- Sheriff, B.L.; Grundy, D.H.; Hartman, J.S.; Hawthorne, F.C.; Černý, P. The incorporation of alkalis in beryl: Multi-nuclear MAS NMR and crystal structure study. Can. Miner. 1991, 29, 271–285. [Google Scholar]
- Artioli, G.; Rinaldi, R.; Ståhl, Z.; Zanazzi, P.F. Structure refinement of beryl by single-crystal neutron and X-ray diffraction. Am. Mineral. 1993, 78, 762–768. [Google Scholar]
- Artioli, G.; Rinaldi, R.; Wilson, C.C.; Zanazzi, P.F. Single-crystal pulsed neutron diffraction of a highly hydrous beryl. Acta Crystallogr. 1995, B51, 733–737. [Google Scholar] [CrossRef]
- Kolesov, B. Vibrational states of H2O in beryl: Physical aspects. Phys. Chem. Miner. 2008, 35, 271–278. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. The orientation and vibrational states of H2O in synthetic alkali-free beryl. Phys. Chem. Miner. 2000, 27, 557–564. [Google Scholar] [CrossRef]
- Černý, P.; Anderson, A.J.; Tomascak, P.B.; Chapman, R. Geochemical and morphological features of beryl from the Bikita granitic pegmatite, Zimbabwe. Can. Miner. 2003, 41, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Andersoon, L.O. The position of H+, Li+ and Na+ impurities in beryl. Phys. Chem. Miner. 2006, 33, 403–416. [Google Scholar] [CrossRef]
- Gatta, G.D.; Nestola, F.; Bromiley, G.D.; Mattauch, S. The real topological configuration of the extra-framework content in alkali-poor beryl: A multi-methodological study. Am. Miner. 2006, 91, 29–34. [Google Scholar] [CrossRef]
- Bačík, P.; Fridrichová, J. The site occupancy assessment in beryl based on bond-length constraints. Minerals 2019, 9, 641. [Google Scholar] [CrossRef] [Green Version]
- Shigley, J.E.; Foord, E.E. Gem-quality red beryl from the Wah Wah Mountains, Utah. G&G 1984, 20, 208–221. [Google Scholar]
- Keith, J.D.; Christiansen, E.H.; Tingey, D.G. Geological and chemical conditions of formation of red beryl, Wah Wah Mountains, Utah. Utah Geol. Assoc. Publ. 1994, 23, 155–169. [Google Scholar]
- Shigley, J.E.; Thompson, T.J.; Keith, J.D. Red beryl from Utah: A review and update. G&G 2003, 39, 302–313. [Google Scholar]
- Shigley, J.E.; McClure, S.F.; Cole, J.E.; Koivula, J.I.; Lu, T.; Elen, S.; Demiantes, L.N. Hydrothermal synthetic red beryl from the Institute of Crystallography, Moscow. G&G 2001, 37, 42–55. [Google Scholar]
- Henn, U.; Milisenda, C.C. Synthetic red beryl from Russia. J. Gemm. 1999, 26, 481–486. [Google Scholar] [CrossRef]
- Nassau, K.; Wood, D.L. An examination of red beryl from Utah. Am. Miner. 1968, 53, 801–806. [Google Scholar]
- Flamini, A.; Gastaldi, L.; Grubessi, O.; Viticoli, S. Sulle caratteristiche particolari del berillo rosso dell’Utah. Gemmologia 1983, 9, 12–20. (In Italian) [Google Scholar]
- Solntsev, V.P.; Mashkovtsev, R.I. Valent state and coordination of cobalt ions in beryl and chrysoberyl crystals. Phys. Chem. Miner. 2004, 31, 1–11. [Google Scholar] [CrossRef]
- Solntsev, V.P.; Tsvetkov, E.G.; Alimpiev, A.I.; Mashkovtsev, R.I. Coordination and valent state of nickel ions in beryl and chrysoberyl crystals. Phys. Chem. Miner. 2006, 33, 300–313. [Google Scholar] [CrossRef]
- Taran, M.N.; Rossman, G.R. Optical spectra of Co2+ in three synthetic silicate minerals. Am. Mineral. 2001, 86, 889–895. [Google Scholar] [CrossRef]
- Czaja, M.; Radoslaw, L.; Chrobak, A.; Sitko, R.; Mazurak, Z. The absorption- and luminescence spectra of Mn3+ in beryl and vesuvianite. Phys. Chem. Miner. 2018, 45, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Rigaku Oxford Diffraction, CrysAlisPro Software System. Rigaku Corporation. 2019. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 19 March 2022).
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Larson, A.C. Inclusion of secondary extinction in least-squares calculations. Acta Crystallogr. 1967, 23, 664–665. [Google Scholar] [CrossRef]
- Adams, D.M.; Gardner, I.R. Single-crystal vibrational spectra of beryl and dioptase. J. Chem. Soc. Dalton Trans. 1974, 14, 1502–1505. [Google Scholar] [CrossRef]
- Kim, C.C.; Bell, M.; McKeown, D. Vibrational analysis of beryl (Be3Al2Si6O18) and its constituent ring (Si6O18). Phys. B Condens. Matter 1995, 205, 193–208. [Google Scholar] [CrossRef]
- Prencipe, M.; Noel, Y.; Civalleri, B.; Roetti, C.; Dovesi, R. Quantum-mechanical calculation of the vibrational spectrum of beryl (Al4Be6Si12O36) at the Γ point. Phys. Chem. Miner. 2006, 33, 519–532. [Google Scholar] [CrossRef]
- Aines, R.D.; Rossman, G.R. The high-temperature behaviour of water and carbon dioxide in cordierite and beryl. Am. Miner. 1984, 69, 319–327. [Google Scholar]
- Charoy, B.; de Donato, P.; Barres, O.; Pinto-Coelho, C. Channel occupancy in an alkali-poor beryl from Serra Branca (Goias, Brazil): Spectroscopic characterization. Am. Miner. 1996, 81, 395–403. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G. Minerals from Macedonia XXIII. Spectroscopic and structural characterization of scholr and beryl cyclosilicates. Spectrochim. Acta Part A 2009, 73, 460–467. [Google Scholar] [CrossRef]
- Mashkovtsev, R.I.; Thomas, V.G.; Fursenko, D.A.; Zhukova, E.S.; Uskov, V.V.; Gorshunov, B.P. FTIR spectroscopy of D2O and HDO molecules in the c-axis channels of synthetic beryl. Am. Miner. 2016, 101, 175–180. [Google Scholar] [CrossRef]
- Taran, M.N.; Dyar, M.D.; Khomenko, V.M. Spectroscopic study of synthetic hydrothermal Fe3+-bearing beryl. Phys. Chem. Miner. 2017, 31, 489–496. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, R. Color characteristics of blue to yellow beryl from multiple origins. G&G 2020, 56, 54–65. [Google Scholar]
- Hofmeister, A.; Hoering, T.; Virgo, D. Vibrational spectroscopy of beryllium aluminosilicates: Heat capacity calculations from band assignments. Phys. Chem. Miner. 1987, 14, 205–224. [Google Scholar] [CrossRef]
- Łodziński, M.; Sitarz, M.; Stec, K.; Kozanecki, M.; Fojud, Z.; Jurga, S. ICP, IR, Raman, NMR investigations of beryls from pegmatites of the Sudety Mts. J. Mol. Struct. 2005, 744, 1005–1015. [Google Scholar] [CrossRef]
- Aurisicchio, C.; Grubessi, O.; Zecchini, P. Infrared spectroscopy and crystal chemistry of the beryl group. Can. Miner. 1994, 32, 55–68. [Google Scholar]
- Bačík, P.; Fridrichová, J.; Uher, P.; Rybár, S.; Bizovská, V.; Luptákovás, J.; Vrábliková, D.; Pukančik, L.; Vaculovič, T. Octahedral substitution in beryl from weakly fractionated intragranitic pegmatite Predné Solisko, Tatry Mountains (Slovakia): The indicator of genetic conditions. J. Geosci. 2019, 64, 59–72. [Google Scholar] [CrossRef]
- Hagemann, H.; Lucken, A.; Bill, H.; Gysler-Sanz, J.; Stalder, H.A. Polarized Raman spectra of beryl and bazzite. Phys. Chem. Miner. 1990, 17, 395–401. [Google Scholar] [CrossRef]
- Christiansen, E.; Keith, J.; Thompson, T. Origin of gem red beryl in Utah’s Wah Wah Mountains. Min. Engin. 1997, 49, 37–41. [Google Scholar]
- Fridrichová, J.; Bačík, P.; Ertl, A.; Wildner, M.; Dekan, J.; Miglierini, M. Jahn-Teller distortion of Mn3+-occupied octahedra in red beryl from Utah indicated by optical spectroscopy. J. Mol. Struct. 2018, 1152, 79–86. [Google Scholar] [CrossRef]
- Taran, M.N.; Vyshnevskyi, O.A. Be, Fe2+-substitution in natural beryl: An optical absorption spectroscopy study. Phys. Chem. Miner. 2019, 46, 795–806. [Google Scholar] [CrossRef]
- Knop, O.; Reid, K.I.G.; Sutarno; Nakagawa, Y. Chalkogenides of the transition elements. VI. X-Ray, neutron, and magnetic investigation of the spinels Co3O4, NiCo2O4, Co3S4, and NiCo2S4. Can. J. Chem. 1968, 46, 3463–3476. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, R.; Gatta, G.D.; Knight, K.S.; Geiger, C.; Artioli, G. Crystal chemistry, cation ordering and thermoelastic behaviour of CoMgSiO4 olivine at high temperature as studied by in-situ neutron powder diffraction. Phys. Chem. Miner. 2005, 32, 655–664. [Google Scholar] [CrossRef]
- Gatta, G.D.; Hradil, K.; Meven, M. Where is the hydrogen? Elements 2021, 17, 163–168. [Google Scholar] [CrossRef]

| Property | Natural | Synthetic | ||
|---|---|---|---|---|
| BNR1 | BS1R | BS2G | BS3G | |
| Color | Purplish red | Red | Orange red | Purplish red |
| Appearance | Rough with a prismatic habit | Rough with a tabular habit | Oval shape, mixed cut | Rectangular shape, mixed cut |
| Refractive indices | ||||
| nε | 1.566 | 1.572 | 1.570 | 1.572 |
| nω | 1.574 | 1.580 | 1.578 | 1.580 |
| Birefringence | 0.008 | 0.008 | 0.008 | 0.008 |
| Pleochroism | ||||
| Parallel to c-axis | Purplish red | Purplish red | Purplish red | Purplish red |
| Perpendicular to c-axis | Orange red | Orange red | Orange red | Orange red |
| Specific gravity | 2.67 | 2.69 | 2.68 | 2.70 |
| UV fluorescence | Inert | Inert | Inert | Inert |
| Microscopic features | Fractures | Inhomogeneous growth structures, liquid veils, seed plate of synthetic emerald | Inhomogeneous growth structures, liquid veils | Inhomogeneous growth structures, liquid veils |
| Elements | Natural | Synthetic | ||
|---|---|---|---|---|
| BNR1 | BS1R | BS2G | BS3G | |
| Li | 819 | 4021 | 3072 | 3624 |
| Be | 45,422 | 45,422 | 45,569 | 46,566 |
| Na | 274 | 1823 | 648 | 1749 |
| Mg | 3101 | 1780 | 1759 | 2252 |
| Al | 79,923 | 87,323 | 87,496 | 91,788 |
| K | 1378 | 125 | 89 | 38 |
| Sc | 76 | 28 | 30 | 19 |
| Ti | 2595 | 15 | 69 | 132 |
| V | <1 | <1 | <1 | <1 |
| Cr | <1 | 9 | 20 | 15 |
| Mn | 3057 | 1477 | 1097 | 1670 |
| Fe | 21,921 | 14,654 | 8796 | 8822 |
| Co | <1 | 3404 | 2393 | 7 |
| Ni | <1 | 203 | 360 | 551 |
| Cu | 6 | 11 | 13 | 11 |
| Zn | 600 | 6 | 3 | 43 |
| Ga | 87 | 24 | 21 | 25 |
| Rb | 1319 | 15 | 4 | 7 |
| Sr | <1 | <1 | <1 | <1 |
| Y | 1 | <1 | <1 | <1 |
| Zr | 155 | 3 | 2 | 46 |
| Nb | 21 | <1 | 78 | 111 |
| Sn | 130 | <1 | <1 | <1 |
| Cs | 5103 | 209 | 48 | 134 |
| BS1R | BS2G | BS3G | BN1R | |
|---|---|---|---|---|
| T (K) | 293 | 293 | 293 | 293 |
| Crystal shape | Prism | Prism | Prism | Prism |
| Crystal volume (mm) | 0.28 × 0.20 × 0.12 | 0.45 × 0.37 × 0.28 | 0.45 × 0.45 × 0.18 | 0.40 × 0.35 × 0.30 |
| Unit-cell parameters | a = 9.2251(1) Å | a = 9.2231(1) Å | a = 9.2218(1) Å | a = 9.2279(1) Å |
| c = 9.1877(1) Å | c = 9.1927(2) Å | c = 9.1894(2) Å | c = 9.1904(1) Å | |
| V = 677.14(2) Å3 | V = 677.22(2) Å3 | V = 676.78(2) Å3 | V = 677.76(2) Å3 | |
| c/a = 0.9959 | c/a = 0.9967 | c/a = 0.9958 | c/a = 0.9959 | |
| Space Group | P6/mcc | P6/mcc | P6/mcc | P6/mcc |
| Reference formula | Al2Be3Si6O18 | Al2Be3Si6O18 | Al2Be3Si6O18 | Al2Be3Si6O18 |
| Z | 2 | 2 | 2 | 2 |
| Radiation type, λ (Å) | X-ray, MoKα | X-ray, MoKα | X-ray, MoKα | X-ray, MoKα |
| Diffractometer | Rigaku XtaLABSynergy-i | Rigaku XtaLABSynergy-i | Rigaku XtaLABSynergy-i | Rigaku XtaLABSynergy-i |
| Data-collection method | ω-scans | ω-scans | ω-scans | ω-scans |
| θmax (°) | 39.3 | 39.2 | 39.2 | 39.3 |
| −16 ≤ h ≤ +16 | −16 ≤ h ≤ +16 | −16 ≤ h ≤ +16 | −13 ≤ h ≤ +13 | |
| −16 ≤ k ≤ +16 | −16 ≤ k ≤ +16 | −16 ≤ k ≤ +16 | −16 ≤ k ≤ +16 | |
| −12 ≤ l ≤ +13 | −12 ≤ l ≤ +13 | −13 ≤ l ≤ +13 | −16 ≤ l ≤ +16 | |
| Measured reflections | 27,192 | 26,987 | 27,042 | 26,385 |
| Unique reflections | 638 | 596 | 647 | 707 |
| Unique refl. Fo > 4σ(Fo) | 569 | 575 | 622 | 672 |
| Refined parameters | 35 | 35 | 35 | 36 |
| Extinction coeff. | 0.032(2) | 0.048(3) | 0.052(2) | 0.023(2) |
| RInt | 0.0546 | 0.0351 | 0.0493 | 0.0271 |
| R1 (F) with Fo > 4σ(Fo) | 0.0198 | 0.0170 | 0.0166 | 0.0167 |
| R1 (F) for all reflections | 0.0276 | 0.0183 | 0.0178 | 0.0185 |
| wR2(F2) | 0.0423 | 0.0442 | 0.0374 | 0.0422 |
| GooF | 1.278 | 1.414 | 1.349 | 1.343 |
| Residuals (e−/Å3) | −0.4/+0.5 | −0.3/+0.4 | −0.4/+0.4 | −0.3/+0.4 |
| Site (Whyc. pos.) | x | y | z | Site Occupancy | U11 | U22 | U33 | U23 | U13 | U12 | Ueq/Uiso |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BS1R | |||||||||||
| Si (12l) | 0.38690(3) | 0.11526(3) | 0 | Si 1.0 | 0.0051(1) | 0.0045(1) | 0.0049(1) | 0 | 0 | 0.0026(1) | 0.00477(8) |
| Be (6f) | ½ | 0 | 1/4 | Be 1.0 | 0.0076(4) | 0.0056(6) | 0.0074(8) | 0 | 0 | 0.0028(3) | 0.0071(3) |
| Al (4c) | 2/3 | 1/3 | 1/4 | Al 0.935(3), Fe 0.065(3) | 0.0046(2) | 0.0046(2) | 0.0048(2) | 0 | 0 | 0.00229(7) | 0.0046(1) |
| O1 (12l) | 0.30832(10) | 0.23507(9) | 0 | O 1.0 | 0.0123(3) | 0.0094(3) | 0.0150(4) | 0 | 0 | 0.0081(3) | 0.0110(1) |
| O2 (24m) | 0.49776(6) | 0.14475(6) | 0.14511(6) | O 1.0 | 0.0096(2) | 0.0079(2) | 0.0073(3) | −0.0007(2) | −0.0024(2) | 0.0047(2) | 0.0081(1) |
| M (2b) | 0 | 0 | 0 | Na 0.050(7) | 0.021(8) | ||||||
| Ow (2a) | 0 | 0 | 1/4 | O 0.42(2) | 0.080(6) | ||||||
| H (12k) | 0.1226(13) | 0.0613(6) | 1/4 | H 0.18(5) | 0.080(2) | ||||||
| BS2G | |||||||||||
| Si (12l) | 0.38700(3) | 0.11538(3) | 0 | 1.0 | 0.0048(1) | 0.0043(1) | 0.0053(2) | 0 | 0 | 0.00238(7) | 0.00477(9) |
| Be (6f) | ½ | 0 | 1/4 | 1.0 | 0.0075(4) | 0.0053(5) | 0.0057(7) | 0 | 0 | 0.0027(2) | 0.0064(3) |
| Al (4c) | 2/3 | 1/3 | 1/4 | Al 0.965(3), Fe 0.035(3) | 0.0042(1) | 0.0042(1) | 0.0047(3) | 0 | 0 | 0.00212(7) | 0.0044(1) |
| O1 (12l) | 0.30882(9) | 0.23551(9) | 0 | 1.0 | 0.0118(3) | 0.0090(2) | 0.0149(4) | 0 | 0 | 0.0080(2) | 0.0106(1) |
| O2 (24m) | 0.49813(6) | 0.14500(6) | 0.14508(6) | 1.0 | 0.0089(2) | 0.0071(2) | 0.0076(3) | −0.0006(2) | −0.0024(2) | 0.0041(1) | 0.0078(1) |
| M (2b) | 0 | 0 | 0 | Na 0.047(9) | 0.05(2) | ||||||
| Ow (2a) | 0 | 0 | 1/4 | O 0.36(2) | 0.086(8) | ||||||
| H (12k) | 0.124(5) | 0.062(3) | 1/4 | H 0.21(5) | 0.086(2) | ||||||
| BS3G | |||||||||||
| Si (12l) | 0.38707(3) | 0.11541(3) | 0 | 1.0 | 0.00487(9) | 0.00428(9) | 0.0055(1) | 0 | 0 | 0.0024(1) | 0.00484(7) |
| Be (6f) | ½ | 0 | 1/4 | 1.0 | 0.0080(4) | 0.0062(5) | 0.0070(6) | 0 | 0 | 0.0031(2) | 0.0073(2) |
| Al (4c) | 2/3 | 1/3 | 1/4 | Al 0.961(3), Fe 0.039(3) | 0.0044(1) | 0.0044(1) | 0.0055(2) | 0 | 0 | 0.00219(6) | 0.0047(1) |
| O1 (12l) | 0.30867(8) | 0.23540(8) | 0 | 1.0 | 0.0118(2) | 0.0093(2) | 0.0151(3) | 0 | 0 | 0.0082(2) | 0.0108(1) |
| O2 (24m) | 0.49803(5) | 0.14498(5) | 0.14512(5) | 1.0 | 0.0091(1) | 0.0073(1) | 0.0076(2) | −0.0006(1) | −0.0024(1) | 0.0043(1) | 0.0079(1) |
| M (2b) | 0 | 0 | 0 | Na 0.051(6) | 0.022(7) | ||||||
| Ow (2a) | 0 | 0 | 1/4 | O 0.36(2) | 0.072(6) | ||||||
| H (12k) | 0.126(2) | 0.063(5) | 1/4 | H 0.28(4) | 0.072(2) | ||||||
| BN1R | |||||||||||
| Si (12l) | 0.38671(3) | 0.11503(3) | 0 | 1.0 | 0.0051(1) | 0.0046(1) | 0.0046(1) | 0 | 0 | 0.00252(7) | 0.00473(6) |
| Be (6f) | ½ | 0 | 1/4 | 1.0 | 0.0078(4) | 0.0059(5) | 0.0061(4) | 0 | 0 | 0.0029(2) | 0.0068(2) |
| Al (4c) | 2/3 | 1/3 | 1/4 | Al 0.936(3), Fe 0.064(3) | 0.0044(1) | 0.0044(1) | 0.0042(2) | 0 | 0 | 0.00221(6) | 0.00435(11) |
| O1 (12l) | 0.30927(9) | 0.23565(8) | 0 | 1.0 | 0.0117(3) | 0.0092(2) | 0.0142(2) | 0 | 0 | 0.0078(2) | 0.01056(11) |
| O2 (24m) | 0.49795(5) | 0.14469(5) | 0.14517(5) | 1.0 | 0.0092(2) | 0.0072(2) | 0.0065(1) | −0.0006(1) | −0.0024(1) | 0.0042(1) | 0.00757(8) |
| M (2b) | 0 | 0 | 0 | K 0.021(6) | 0.07(3) | ||||||
| M’ (4e) | 0 | 0 | 0.174(11) | Cs 0.0011(8) | 0.023(5) | ||||||
| Ow (2a) | 0 | 0 | 1/4 | O 0.115(12) | 0.02(3) |
| BS1R | |||
|---|---|---|---|
| Si-O1 | 1.5964(7) | Ow-H | 0.98(1) |
| Si-O1′ | 1.5969(7) | M ↔ Ow | 2.29692(3) |
| Si-O2 (x 2) | 1.6184(6) | M ↔ O1 | 2.5738(6) |
| Be-O2 (x 4) | 1.6552(5) | ||
| Al-O2 (x 6) | 1.9164(5) | ||
| BS2G | |||
| Si-O1 | 1.5958(6) | Ow-H | 0.99(4) |
| Si-O1′ | 1.5966(7) | M ↔ Ow | 2.29817(5) |
| Si-O2 (x 2) | 1.6198(6) | M ↔ O1 | 2.5776(5) |
| Be-O2 (x 4) | 1.6559(5) | ||
| Al-O2 (x 6) | 1.9141(5) | ||
| BS3G | |||
| Si-O1 | 1.5959(6) | Ow-H | 1.00(4) |
| Si-O1′ | 1.5969(6) | M ↔ Ow | 2.29735(5) |
| Si-O2 (x 2) | 1.6189(5) | M ↔ O1 | 2.5760(6) |
| Be-O2 (x 4) | 1.6555(4) | ||
| Al-O2 (x 6) | 1.9139(4) | ||
| BN1R | |||
| Si-O1 | 1.5954(6) | M ↔ Ow | 2.29761(3) |
| Si-O1 | 1.5959(6) | M’ ↔ Ow | 0.69(10) |
| Si-O2 (x 2) | 1.6210(4) | M ↔ M’ | 2.99(10) |
| Be-O2 (x 4) | 1.6543(4) | M’ ↔ M | 1.60(10) |
| Al-O2 (x 6) | 1.9163(4) | M ↔ O1 | 2.5821(7) |
| M’ ↔ O1 | 3.04(5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatta, G.D.; Adamo, I.; Zullino, A.; Gagliardi, V.; Lorenzi, R.; Rotiroti, N.; Faldi, L.; Prosperi, L. A Multi-Methodological Investigation of Natural and Synthetic Red Beryl Gemstones. Minerals 2022, 12, 439. https://doi.org/10.3390/min12040439
Gatta GD, Adamo I, Zullino A, Gagliardi V, Lorenzi R, Rotiroti N, Faldi L, Prosperi L. A Multi-Methodological Investigation of Natural and Synthetic Red Beryl Gemstones. Minerals. 2022; 12(4):439. https://doi.org/10.3390/min12040439
Chicago/Turabian StyleGatta, Giacomo Diego, Ilaria Adamo, Andrea Zullino, Valentina Gagliardi, Roberto Lorenzi, Nicola Rotiroti, Ludovica Faldi, and Loredana Prosperi. 2022. "A Multi-Methodological Investigation of Natural and Synthetic Red Beryl Gemstones" Minerals 12, no. 4: 439. https://doi.org/10.3390/min12040439
APA StyleGatta, G. D., Adamo, I., Zullino, A., Gagliardi, V., Lorenzi, R., Rotiroti, N., Faldi, L., & Prosperi, L. (2022). A Multi-Methodological Investigation of Natural and Synthetic Red Beryl Gemstones. Minerals, 12(4), 439. https://doi.org/10.3390/min12040439






