29Si Solid-State NMR Analysis of Opal-AG, Opal-AN and Opal-CT: Single Pulse Spectroscopy and Spin-Lattice T1 Relaxometry
Abstract
1. Introduction
2. Materials and Methods
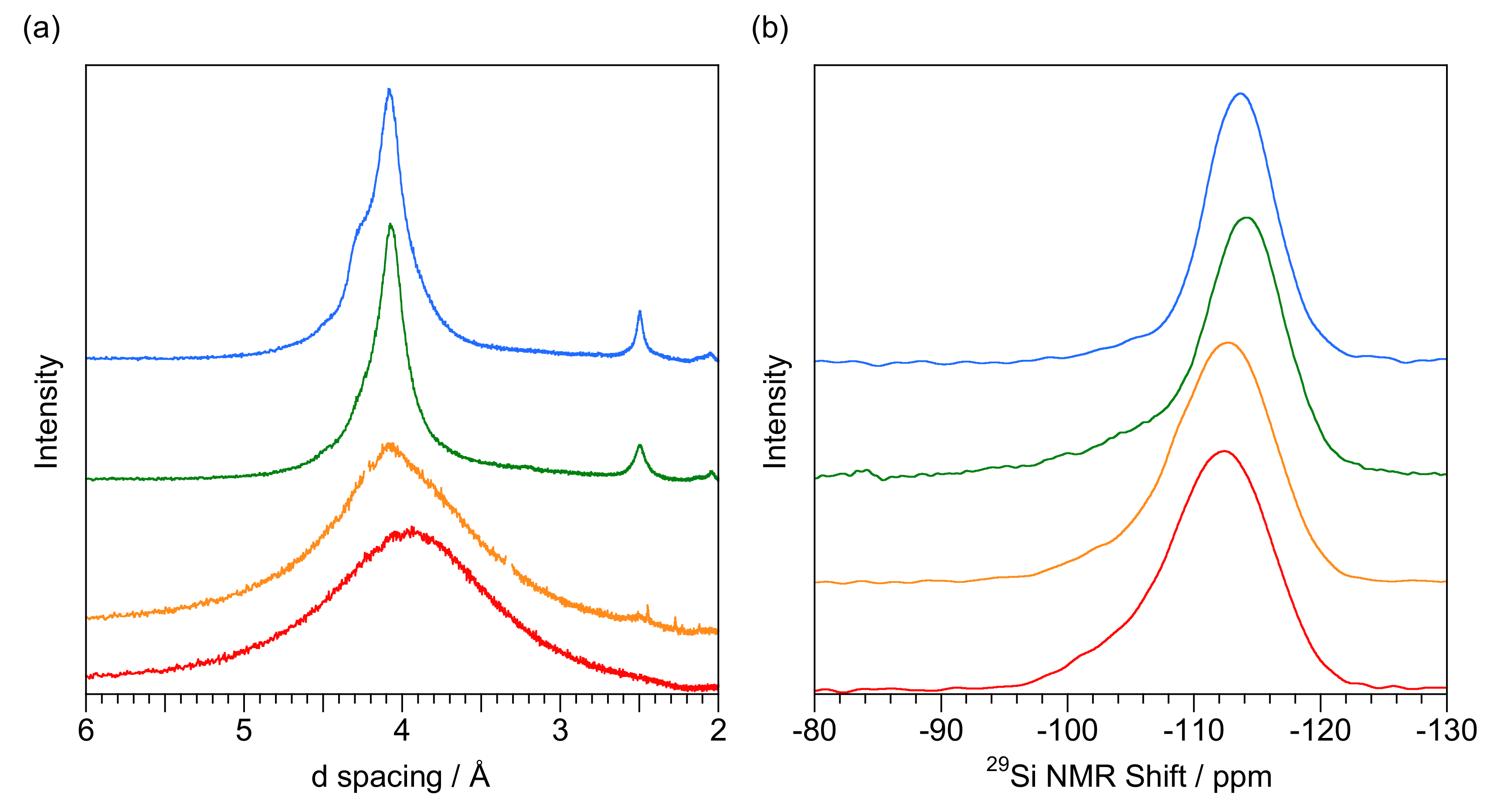
3. Results
4. Discussion
4.1. 29Si NMR Spectra of Opal
4.2. T1 Relaxation
4.3. Implications for the Formation and Characterisation of Opal-A
4.4. Implications for Characterisation of Opal-CT
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | Type | Location (Australia Unless Noted) | Description |
|---|---|---|---|
| E1937 | AN | Mt Cora, New South Wales | colorless glass |
| G1401 | AG | White Cliffs, New South Wales | translucent |
| G1421 | CT | Mt Cora, New South Wales | shiny black |
| G1442 | AG | William Creek, South Australia | glassy white with POC |
| G7107 | AN | Valeč, Czechia | colorless glass |
| G8608 | AG | White Cliffs, New South Wales | opaque white, POC |
| G8736 | AN | Springsure, Queensland | colorless glass |
| G8877 | AN | Squaretop near Dalby, Queensland | colorless glass |
| G9258 | AG | Andamooka, South Australia | translucent white POC |
| G7532 | AG | Queensland | white with POC on matrix |
| G7682 | AG | Mintabie, South Australia | glassy blue-grey |
| G9592 | AG | Cloncurry, Queensland | milky white with POC |
| G9811 | AG | Coober Pedy, South Australia | opaque white with POC |
| G9942 | CT | Angaston, South Australia | translucent glossy white |
| G13755 | CT | Ross, Tasmania | translucent white |
| G13761 | CT | Marvell Loch, Western Australia | translucent green |
| G13764 | AN | Dalby, Queensland | colorless glass |
| G13767 a | AG | Lightning Ridge, New South Wales, Australia | mixed samples |
| G14581 | AG-CT | Andamooka, South Australia | opaque brown with POC |
| G21471 | A | Rotorua, New Zealand | crumbly white |
| G32740 | AN | Valeč, Czechia | colorless glass |
| G32925 | CT | Zelinograd, Qazaqstan | glassy vermilion |
| G34475 b | AG | Dubnik, Slovakia | white opaque/translucent |
| G34996 a | AG | Coober Pedy, South Australia, Australia | opalised mussels |
| G34997 a | AG | Coober Pedy, South Australia, Australia | opalised belemnites |
| GNEW01 | AG | Caldes de Malavella, Catalonia, Spain | opaque cream |
| GNEW03 | CT | Mt Iyobo, Tanzania | opaque green |
| GNEW05 | CT | Bemia, Madagascar | transparent yellow |
| GNEW08 | CT | Ethiopia | glassy green and brown |
| GNEW09 | CT | Caldes de Malavella, Catalonia, Spain | translucent brown |
| GNEW19 | CT | Tanzania | orange glass |
| GNEW20 | CT | California, USA | translucent yellow |
| GNEW22 | AG | Lambina, South Australia | opaque white with POC |
| GNEW23 | AG | Caldes de Malavella, Catalonia, Spain | opaque grey-brown |
| GNEW24 | CT | Kutahuya, Turkey | translucent olive |
| GNEW27 | CT | Yozgat, Turkey | blue-green glass |
| GNEW28 | CT | Yozgat, Turkey | olive-green glass |
| GNEW30 | CT | East of Manzano Mts, New Mexico, USA | opaque white |
| GNEW31 | AG | Andamooka, South Australia, Australia | translucent white |
| M8736 | AN | Springsure, Queensland | colorless glass |
| M53441 | CT | Kremze, Czechia | opaque blue |
| MS-4 | AN | Mt Squaretop, Queensland | colorless glass |
| NMNH ETH S2 | CT | Mezezo, Ethiopia | brown bottle glass, POC |
| OOC3 | CT | Ménilmontant, France | opaque grey-brown |
| OOC5 | CT | Virgin Valley, Nevada, USA | opaque white |
| OOC10 | AN | Lesvos, Greece | colorless glass |
| SO3 | AG | Dubnik, Slovakia | translucent with POC |
| SO7 | AG | Dubnik, Slovakia | opaque white |
| SO17 | AG | Dubnik, Slovakia | opaque white |
| T1665 | A | Rotorua, New Zealand | crumbly white |
| T2233 | AG | Lightning Ridge, New South Wales | blue-grey glass |
| T4051 | CT | Charlies Swamp, South Australia | translucent grey |
| T18117 | AN | Mitchell Co. N. Carolina, USA | colorless glass |
| T18511 | AN | Mt Gee, Arkaroola, South Australia | colorless glass |
| T22842 | CT | Missoula, Montana, USA | opaque white |
| WBO1 | AG | Winton, Queensland, Australia | bluish opaque, POC |
Appendix B
| 27Al | 31P | 49Ti | 51V | 55Mn | 57Fe | 59Co | 60Ni | 65Cu | 137Ba | 238U | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opal-AG | ||||||||||||
| G1401 | 6580 | 3.12 | 6.83 | 0.0701 | 9.17 | 485 | 0.0109 | 0.0694 | 0.0288 | 155 | 0.0265 | |
| G9811 | 5990 | 3.54 | 5.30 | 0.0896 | 5.50 | 393 | 0.0139 | 0.0566 | 0.0267 | 154 | 0.0265 | |
| T2233 | 6110 | 39.1 | 722 | 4.76 | 18.5 | 1040 | 50.0 | 17.3 | 159 | 144 | 2.36 | |
| G8608 | 4560 | 3.53 | 8.36 | 0.0895 | 5.30 | 271 | 0.0553 | 0.100 | 0.146 | 83.8 | 0.0572 | |
| Opal-AN | ||||||||||||
| E1937 | 5.19 | 3.29 | 0.128 | 0.347 | 0.0261 | 0.220 | 3.61 × 10−3 | 0.0236 | 9.03 × 10−3 | 0.122 | 0.298 | |
| MS-4 | 6.42 | 3.55 | 0.0207 | 0.495 | 0.0322 | — a | — a | 3.55 × 10−3 | — a | 2.27 × 10−4 | 0.135 | |
| G32740 | 8.10 | 8.43 | 0.633 | 0.458 | 0.122 | 6.30 | 0.0274 | 0.159 | 0.547 | 0.181 | 0.034 | |
| M8736 | 3.57 | 3.33 | 9.34 × 10−3 | 0.434 | 0.024 | — a | — a | 0.0192 | 7.57 × 10−3 | 4.38 × 10−3 | 0.116 | |
| OOC10 | 0.458 | 3.30 | 6.02 × 10−3 | 0.233 | 0.0247 | — a | 8.83 × 10−4 | 0.0217 | 0.0224 | 0.139 | 15.2 | |
| T18511 | 0.893 | 3.34 | 0.0109 | 0.0417 | 0.0272 | — a | — a | 3.43 × 10−3 | 2.43 × 10−3 | 0.0127 | 24.9 | |
| T18117 | 16.0 | 5.11 | 0.214 | 0.015 | 7.14 | 4.11 | 0.0260 | 0.0705 | 2.49 | 0.985 | 54.8 | |
| G7107 | 22.6 | 4.78 | 0.352 | 0.578 | 0.0526 | 2.31 | 7.50 × 10−4 | 0.0221 | 0.0376 | 0.0257 | 0.053 | |
| G8877 | 0.273 | 3.35 | 0.0654 | 0.775 | 0.0259 | — a | 1.77 × 10−3 | 0.0186 | 0.0277 | 0.0251 | 0.381 | |
| G13764 | 3.13 | 3.16 | 0.0403 | 0.0466 | 0.0328 | — a | — a | 0.0200 | 0.0281 | 2.44 × 10−3 | 0.295 | |
| Opal-CT | ||||||||||||
| G9942 | 29.8 | 3.93 | 2.70 | 0.348 | 0.267 | 54.3 | 0.0238 | 0.0951 | 0.124 | 0.06 | 0.0874 | |
| ETH S2 | 6760 | 2.86 | 46.8 | 5.36 × 10−4 | 17.1 | 724 | 0.0184 | 0.0226 | 0.0208 | 25.6 | 0.781 | |
| GNEW03 | 3640 | 4.30 | 0.330 | 0.804 | 2.43 | 17,500 | 1.14 | 9.62 | 8.85 | 1.96 | 0.212 | |
| OOC3 | 4320 | 16.6 | 743 | 57.4 | 1.51 | 1100 | 0.649 | 7.23 | 8.28 | 6.04 | 0.346 | |
| T22842 | 0.371 | 5.84 | 0.0829 | 0.224 | 0.137 | 201 | 4.22 × 10−3 | 0.0748 | 3.06 | 0.996 | 21.7 |
References
- Jones, J.B.; Sanders, J.V.; Segnit, E.R. Structure of opal. Nature 1964, 204, 990–991. [Google Scholar] [CrossRef]
- Jones, J.B.; Segnit, E.R. The Nature of Opal I. Nomenclature and Constituent Phases. J. Geol. Soc. Aust. 1971, 18, 57–68. [Google Scholar] [CrossRef]
- Smith, D.K. Opal, cristobalite and tridymite: Noncrystallinity versus crystallinity, nomenclature of the silica minerals and bibliography. Powder Diffr. 1998, 13, 2–19. [Google Scholar] [CrossRef]
- Elzea, J.M.; Rice, S.B. TEM and X-Ray diffraction evidence for cristobalite and tridymite stacking sequences in opal. Clays Clay Miner. 1996, 44, 492–500. [Google Scholar] [CrossRef]
- Ghisoli, C.; Caucia, F.; Marinoni, L. XRPD patterns of opals: A brief review and new results from recent studies. Powder Diffr. 2010, 25, 274–282. [Google Scholar] [CrossRef]
- Caucia, F.; Marinoni, L.; Bordini, V.; Ghisoli, C.; Adamo, I. Physical and chemical properties of some Italian opals. Period. Mineral. 2012, 81, 93–106. [Google Scholar]
- Eckert, J.; Gourdon, O.; Jacob, D.E.; Meral, C.; Monteiro, P.J.M.; Vogel, S.C.; Wirth, R.; Wenk, H.-R. Ordering of water in opals with different microstructures. Eur. J. Mineral. 2015, 27, 203–213. [Google Scholar] [CrossRef]
- Sodo, A.; Municchia, A.C.; Barucca, S.; Bellatreccia, F.; Ventura, G.D.; Butini, F.; Ricci, M.A. Raman, FT-IR and XRD investigation of natural opals. J. Raman Spectrosc. 2016, 47, 1444–1451. [Google Scholar] [CrossRef]
- Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. A Review of the Classification of Opals with Reference to Recent New Localities. Minerals 2019, 9, 299. [Google Scholar] [CrossRef]
- Segnit, E.R.; Stevens, T.J.; Jones, J.B. The Role of Water in Opals. J. Geol. Soc. Aust. 1965, 12, 211–226. [Google Scholar] [CrossRef]
- Sosnowska, I.; Buchenau, U.; Reichenauer, G.; Graetsch, H.; Ibel, K.; Frick, B. Structure and dynamics of the opal-water system. Phys. B 1997, 234, 455–457. [Google Scholar] [CrossRef]
- Jones, B. Siliceous sinters in thermal spring systems: Review of their mineralogy, diagenesis, and fabrics. Sediment. Geol. 2021, 413, 105820. [Google Scholar] [CrossRef]
- Day, R.; Jones, B. Variations in water content in opal-A and opal-CT from geyser discharge aprons. J. Sediment. Res. 2008, 78, 301–315. [Google Scholar] [CrossRef]
- McOrist, G.D.; Smallwood, A. Trace elements in precious and common opals using neutron activation analysis. J. Radioanal. Nucl. Chem. 1997, 223, 9–15. [Google Scholar] [CrossRef]
- Thomas, P.S.; Brown, L.D.; Ray, A.S.; Prince, K.E. A SIMS study of the transition elemental distribution beyween bands in banded sedimentary opal from the Lightning Ridge locality. Neues Jahrb. Minerol. Abh. 2006, 182, 193–999. [Google Scholar]
- Gaillou, E.; Delaunay, A.; Rondeau, B.; Bouhnik-le-Coz, M.; Fritsch, E.; Corren, G.; Monnier, C. The geochemistry of gem opals as evidence of their origin. Ore Geol. Rev. 2008, 34, 113–126. [Google Scholar] [CrossRef]
- Ansori, C. Model mineralisasi pembentukan opal banten. J. Geol. Indones. 2010, 5, 151–170. [Google Scholar] [CrossRef][Green Version]
- Simoni, M.; Caucia, F.; Adamo, I.; Galinetto, P. New occurence of fire opal from Bemia, Madagascar. Gems Gemol. 2010, 46, 114–121. [Google Scholar] [CrossRef]
- Rondeau, B.; Cenki-Tok, B.; Fritsch, E.; Mazzero, F.; Gauthier, J.-P.; Bodeur, Y.; Bekele, E.; Gaillou, E.; Ayalew, D. Geochemical and petrological characterizarion of gem opals from Wegel Tena, Wolo, Ethiopia: Opal formation in an Oligocene soil. Geochem. Explan. Environ. Anal. 2012, 12, 93–104. [Google Scholar] [CrossRef]
- Liesegang, M.; Milke, R. Australian sedimentary opal-A and its associated minerals: Implications for natural silica sphere formation. Am. Mineral. 2014, 99, 1488–1499. [Google Scholar] [CrossRef]
- Dutkiewicz, A.; Landgrebe, T.C.W.; Rey, P.F. Origin of silica and fingerprinting of Australian sedimentary opals. Gondwana Res. 2015, 27, 786–795. [Google Scholar] [CrossRef]
- McOrist, G.D.; Smallwood, A.; Fardy, J.J. Trace elements in Australian opals using neutron activation analysis. J. Radioanal. Nucl. Chem. Artic. 1994, 185, 293–303. [Google Scholar] [CrossRef]
- Bobon, M.; Christy, A.A.; Kluvanec, D.; Illasova, L. State of water molecules and silanol groups in opal minerals: A near infrared spectrscopic study of opals from Slovakia. Phys. Chem. Miner. 2011, 38, 809–818. [Google Scholar] [CrossRef]
- Chauviré, B.; Rondeau, B.; Mangold, N. Near infrared signature of opal and chalcedony as a proxy for their structure and formation conditions. Eur. J. Mineral. 2017, 29, 409–421. [Google Scholar] [CrossRef]
- Pineau, M.; Deit, L.L.; Chauviré, B.; Carter, J.; Rondeau, B.; Mangold, N. Toward the geological significance of hydrated silica detected by near infrared spectroscopy on Mars based on terrestrial reference samples. Icarus 2020, 348, 113706. [Google Scholar] [CrossRef]
- Wilson, M.J. The structure of opal-CT revisited. J. Non-Cryst. Solids 2014, 405, 68–75. [Google Scholar] [CrossRef]
- Fröhlich, F. The opal-CT nanostructure. J. Non-Cryst. Solids 2020, 533, 119938. [Google Scholar] [CrossRef]
- Rondeau, B.; Fritz, E.; Guiraud, M.; Renac, C. Opals from Slovakia (“Hungarian opals”): A reassesment of the conditions of formation. Eur. J. Mineral. 2004, 16, 789–799. [Google Scholar] [CrossRef]
- Spencer, R.J.; Levinson, A.A.; Koivula, J.L. Opals from Querétaro Mexico: Fluid inclusion study. Gems Gemmol. 1992, 28, 28–34. [Google Scholar] [CrossRef][Green Version]
- Chauviré, B.; Rondeau, B.; Alexandre, A.; Chamard-Boris, S.; La, C.; Mazzero, F. Pedogenic origin of precious opals from Wegel Tena (Ethiopia): Evidence from trace elements and oxygen isotopes. Appl. Geochem. 2019, 101, 127–139. [Google Scholar] [CrossRef]
- Floerke, O.W.; Jones, J.B.; Segnit, E.R. The genesis of hyalite. Neues Jahrb. Mineral. Mon. 1973, 2, 82–89. [Google Scholar] [CrossRef]
- Jones, J.B.; Segnit, E.R. The Occurence and Formation of Opal at Coober Pedy and Andamooka. Aust. J. Sci. 1966, 29, 129–133. [Google Scholar]
- Martin, E.; Gaillou, E. Insight on gem opal formation in volcanic ash deposits from a supereruption: A case study through oxygen and hydrogen isotopic composition of opals from Lake Tecopa, California, USA. Am. Mineral. 2018, 103, 803–811. [Google Scholar] [CrossRef]
- Williams, L.A.; Crerar, D.A. Silica diagenesis, II. General mechanisms. J. Sediment. Petrol. 1985, 55, 312–321. [Google Scholar]
- Liesegang, M.; Tomaschek, F. Tracing the continental diagenetic loop of the opal-A to opal-CT transformation with X-ray diffraction. Sediment. Geol. 2020, 398, 105603. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Campbell, K.A.; Moore, J.N.; Browne, P.R.L. Diagenesis of 1900-year-old siliceous sinter (opal-A to quartz) at Opal Mound, Roosevelt Hot Springs, Utah, U.S.A. Sediment. Geol. 2005, 179, 249–278. [Google Scholar] [CrossRef]
- Kano, K. Ordering of opal-CT in diagenesis. Geochem. J. 1983, 17, 87–93. [Google Scholar] [CrossRef]
- Rice, S.B.; Freund, H.; Huang, W.-L.; Clouse, J.A.; Isaacs, C.M. Application of Fourier transform infrared spectroscopy to silica diagenis: The opal-A to opal-CT transformation. J. Sediment. Res. 1995, A65, 639–647. [Google Scholar]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Microstructural changes accompanying the opal-A to opal-CT transition: New evidence from the siliceous sinters of Geysir, Haukadalur, Iceland. Sedimentology 2007, 54, 921–948. [Google Scholar] [CrossRef]
- Curtis, N.J.; Gascooke, J.R.; Pring, A. Silicon-oxygen region infra-red and Raman analysis of opals: The effect of sample preparation and measurement type. Minerals 2021, 11, 173. [Google Scholar] [CrossRef]
- de Jong, B.W.H.S.; van Hoek, J.; Veeeman, W.S.; Manson, D.V. X-ray diffraction and 29Si magic-angle-spinning NMR of opals: Incoherent long- and short-range order in opal-CT. Am. Mineral. 1987, 72, 1195–1203. [Google Scholar]
- Graetsch, H.; Mosset, A.; Gies, H. XRD and 29Si MAS-NMR study of some non-crystalline silica minerals. J. Non-Cryst. Solids 1990, 119, 173–190. [Google Scholar] [CrossRef]
- Adams, S.J.; Hawkes, G.E.; Curzon, E.H. A solid state 29Si nuclear magnetic resonance study of opal and other hydrous silicas. Am. Mineral. 1991, 76, 186371. [Google Scholar]
- Graetsch, H.; Gies, H.; Topalovic, I. NMR, XRD and IR study on microcrstalline opal. Phys. Chem. Miner. 1994, 21, 166–175. [Google Scholar] [CrossRef]
- Brown, L.D.; Ray, A.S.; Thomas, P.S. 29Si and 27Al NMR study of amorphous and paracrystalline opals from Australia. J. Non-Cryst. Solids 2003, 332, 242–248. [Google Scholar] [CrossRef]
- Paris, M.; Fritsch, E.; Aguilar-Reyes, B. 1H, 29Si and 27Al NMR study of the destabilization process of a paracrystalline opal from Mexico. J. Non-Cryst. Solids 2007, 353, 1650–1656. [Google Scholar] [CrossRef]
- Chemtob, S.M.; Rossman, G.R.; Stebbins, J.F. Natural hydrous amorphous silica: Quantitation of network speciation and hydroxyl content by 29Si MAS NMR and vibrational spectroscopy. Am. Mineral. 2012, 97, 203–211. [Google Scholar] [CrossRef]
- Lippmaa, E.; Mági, M.; Samoson, A.; Engelhard, G.; Grimmer, A.-R. Structural Studies of Silicates by Solid-State High-Resolution 29Si NMR. J. Am. Chem. Soc. 1980, 102, 4889–4893. [Google Scholar] [CrossRef]
- Smith, J.V.; Blackwell, C.S. Nuclear magnetic resonance of silica polymorphs. Nature 1983, 303, 223–225. [Google Scholar] [CrossRef]
- Gladden, L.F.; Carpenter, T.A.; Elliot, S.R. 29Si MAS NMR studies of the spin-lattice relaxation time and bond-angle distribution in vitreous silica. Philos. Mag. B 1986, 53, L81–L87. [Google Scholar] [CrossRef]
- Liu, C.C.; Maciel, G.E. The Fumed Silica Surface: A Study by NMR. J. Am. Chem. Soc. 1996, 118, 5103–5119. [Google Scholar] [CrossRef]
- Léonardelli, S.; Facchini, L.; Fretigny, C.; Tougne, P.; Legrand, A.P. Silicon-29 Nuclear Magnetic Resonance Study of Silica. J. Am. Chem. Soc. 1992, 112, 6412–6418. [Google Scholar] [CrossRef]
- Malfait, W.J.; Halter, W.E.; Verel, R. 29Si NMR spectroscopy of silica glass: T1 relaxation and constraints on the Si–O–Si bond angle distribution. Chem. Geol. 2008, 256, 269–277. [Google Scholar] [CrossRef]
- Baccile, N.; Laurent, G.; Bonhomme, C.; Innocenzi, P.; Babonneau, F. Solid-State NMR Characterization of the Surfactant-Silica Interface in Templated Silicas: Acidic versus Basic Conditions. Chem. Mater. 2007, 19, 1343–1354. [Google Scholar] [CrossRef]
- Klinowski, J. Solid-State NMR Studies of Molecular Sieve Catalysts. Chem. Rev. 1991, 91, 1459–1479. [Google Scholar] [CrossRef]
- Smith, K.A.; Kirkpatrick, R.J.; Oldfield, E.; Henderson, D.M. High-resolution silicon-29 nuclear magnetic resonance spectroscopic study of rock-forming silicates. Am. Mineral. 1983, 68, 1206–1215. [Google Scholar]
- Li, J.; Hayakawa, S.; Shirosaki, Y.; Osaka, A. Revisiting structure of silica gels from water glass: An 1H and 29Si MAS and CP-MAS NMR study. J. Sol.-Gel Sci. Technol. 2013, 65, 135–142. [Google Scholar] [CrossRef]
- Myers, S.A.; Cygan, R.T.; Assink, R.A.; Boslough, M.B. 29Si MAS NMR relaxation study of shocked Coconino Sandstone from Meteor Crater, Arizona. Phys. Chem. Miner. 1998, 25, 313–317. [Google Scholar] [CrossRef]
- Barron, P.F.; Frost, R.L.; Skjemstad, J.O. 29Si Spin–lattice relaxation in aluminosilicates. J. Chem. Soc. Chem. Commun. 1983, 10, 581–583. [Google Scholar] [CrossRef]
- Narayanan, A.; Hartman, J.S.; Bain, A.O. Characterizing Nonexponential Spin-Lattice Relaxation in Solid-State NMR by Fitting to the Stretched Exponential. J. Magn. Reson. A 1995, 112, 58–65. [Google Scholar] [CrossRef]
- Peyron, M.; Pierens, G.K.; Lucas, A.J.; Hall, L.D.; Stewart, R.C. The Modified Stretched-Exponential Model for Characterization of NMR Relaxation in Porous Media. J. Magn. Reson. Ser. A 1996, 118, 214–220. [Google Scholar] [CrossRef]
- Alaimo, M.H.; Roberts, J.E. Effects of paramagnetic cations on the nonexponential spin-lattice relaxation of rare spin nuclei in solids. Solid State Nucl. Magn. Reson. 1997, 8, 214–250. [Google Scholar] [CrossRef]
- Leonova, E.; Grins, J.; Shariatgorji, M.; Ilag, L.L.; Edén, M. Solid-state NMR investigationsof Si-29 and N-15 enriched silicon nitride. Solid State Nucl. Magn. Reson. 2009, 36, 11–18. [Google Scholar] [CrossRef]
- Watanabe, T.; Shimizu, H.; Masuda, A.; Saito, H. Studies of 29Si spin-lattice relaxation times and paramagnetic impurities in clay minerals by magic-angle spinning 29Si-NMR and EPR. Chem. Lett. 1983, 12, 1293–1296. [Google Scholar] [CrossRef]
- Miró, J.; Martín-Martín, J.D.; Ibáñez, J.; Anadón, P.; Oms, O.; Tritlla, J.; Caja, M.A. Opaline chert nodules in maar lake sediments from Camp dels Ninots (La Selva Basin, NE Spain). Teo-Gemas 2016, 16, 387–390. [Google Scholar]
- Liesegang, M.; Milke, R. Silica colloid ordering in a dynamic sedimentary environment. Minerals 2018, 8, 12. [Google Scholar] [CrossRef]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A New Geochemical Database for Reference Materials and Isotopic Standards. Geostand. Geoanal. Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]
- Struppe, J.; Steuernagel, S.; Aussenacc, F.; Benevelli, F.; Gierth, P.; Wegner, S. Solid State NMR AVANCE Solids User Manual 003; Bruker Corporation: Billerica, MA, USA, 2016. [Google Scholar]
- Hayashi, S.; Hayamizu, K. Chemical Shift Standards in High-Resolution Solid-State NMR (1) 13C, 29Si and 1H Nuclei. Bull. Chem. Soc. Jpn. 1991, 64, 685–687. [Google Scholar] [CrossRef]
- Edwards, C.L.; Alemany, L.B.; Barron, A.R. Solid-State 29Si NMR Analysis of Cements: Comparing Different Methods of Relaxation Analysis for Determining Spin−Lattice Relaxation Times to Enable Determination of the C3S/C2S Ratio. Ind. Chem. Eng. Res. 2007, 46, 5122–5130. [Google Scholar] [CrossRef]
- Oster, J.L.; Kitajima, K.; Valley, J.; Rogers, B.; Maher, K. An evaluation of paired δ18O and (234U/238U)0 in opal as a tool for paleoclimate reconstruction in semi-arid environments. Chem. Geol. 2017, 449, 236–252. [Google Scholar] [CrossRef]
- Johnston, D.C. Stretched exponential relaxation arising from a continuous sum of exponential decays. Phys. Rev. B 2006, 74, 184430–184437. [Google Scholar] [CrossRef]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes The Art of Scientific Computing (Fortran Vesion); Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Graetsch, H.; Topalovic, I.; Gies, H. NMR spectra of moganite and chalcedony. Eur. J. Mineral. 1994, 6, 459–464. [Google Scholar] [CrossRef]
- Thomas, P.; Chauviré, B.; Flower-Donaldson, K.; Aldridge, L.; Smallwood, A.; Liu, B. FT-NIR and DSC characterisation of water in opal. Ceram. Int. 2020, 46, 29443–29450. [Google Scholar] [CrossRef]
- Brown, L.D.; Ray, A.S.; Thomas, P.S. Elemental Analysis of Australian amorphous banded opals by laser-ablation ICP-MS. Neues Jahrb. Mineral. Mon. 2004, 2004, 411–424. [Google Scholar] [CrossRef]
- Bartoli, F.; Rosa, D.B.; Doirisse, M.; Meyer, R.; Philippy, R.; Samama, J.-C. Role of aluminium in the structure of Brazilian opals. Eur. J. Mineral. 1990, 2, 611–619. [Google Scholar] [CrossRef]
- Smallwood, A. A new era for opal nomenclature. Aust. Gemmol. 1997, 19, 489–496. [Google Scholar]
- Megaw, P.K.M.; Fritsch, E.; Spano, T.L.; Gray, M. Geology and Mineralogy of Electric OpalTM: Green Daylight-Luminescing Hyalite Opal from Zacatecas, Mexico. Rocks Miner. 2018, 93, 404–413. [Google Scholar] [CrossRef]
- Gaillou, E. An Overview of Gem Opals: From the Geology to Color and Microstructure. In Proceedings of the Thirteenth Annual Sinkankas Symposium—Opal, Carlsbad, CA, USA, 18 April 2015. [Google Scholar]
- Vasil’ev, S.G.; Volkov, V.I.; Tatarinova, E.A.; Muzafarov, A.M. A Solid-State NMR Investigation of MQ Silicone Copolymers. Appl. Magn. Reson. 2003, 44, 1015–1025. [Google Scholar] [CrossRef]
- Bronnimann, C.E.; Zeigler, R.C.; Maciel, G.E. Proton NMR Study of Dehydration of the Silica Gel Surface. J. Am. Chem. Soc. 1988, 110, 2023–2026. [Google Scholar] [CrossRef]
- Lowe, I.J.; Tse, D. Nuclear Spin-Lattice Relaxation via Paramagnetic Centers. Phys. Rev. 1968, 166, 279–291. [Google Scholar] [CrossRef]
- Tse, D.; Hartmann, S.R. Nuclear spin-lattice relaxation via paramagentic centers without spin diffusion. Phys. Rev. Lett. 1968, 21, 511–514. [Google Scholar] [CrossRef]
- Maiti, B.; McGarvey, B.R. Spin-Lattice Relaxation Time of 13C in [Fe(phen)2(N13CS)2]. Nonexponential Decay. J. Magn. Reson. 1984, 58, 37–46. [Google Scholar] [CrossRef]
- Johnston, D.C.; Baek, S.-H.; Zong, X.; Borsa, F.; Schmalian, J.; Kondo, S. Dynamics of Magnetic Defects in Heavy Fermion LiV2O4 from Stretched Exponential 7Li NMR Relaxation. Phys. Rev. Lett. 2005, 95, 176408. [Google Scholar] [CrossRef]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef]
- Pewkliang, B.; Pring, A.; Brugger, J. The formation of precious opal: Clues from the opalization of bone. Can. Mineral. 2008, 46, 139–149. [Google Scholar] [CrossRef]
- Rey, P.F. Opalisation of the Great Artesian Basin (Central Australia): An Australian story with a Martian twist. Aust. J. Earth Sci. 2013, 60, 219–314. [Google Scholar] [CrossRef]
- Gaillou, E.; Fritsch, E.; Aguilar-Reyes, B.; Rondeau, B.; Post, J.; Barreau, A.; Ostroumov, M. Common gem opal: An investigation of micro- to nano-structure. Am. Mineral. 2008, 93, 1865–1873. [Google Scholar] [CrossRef]
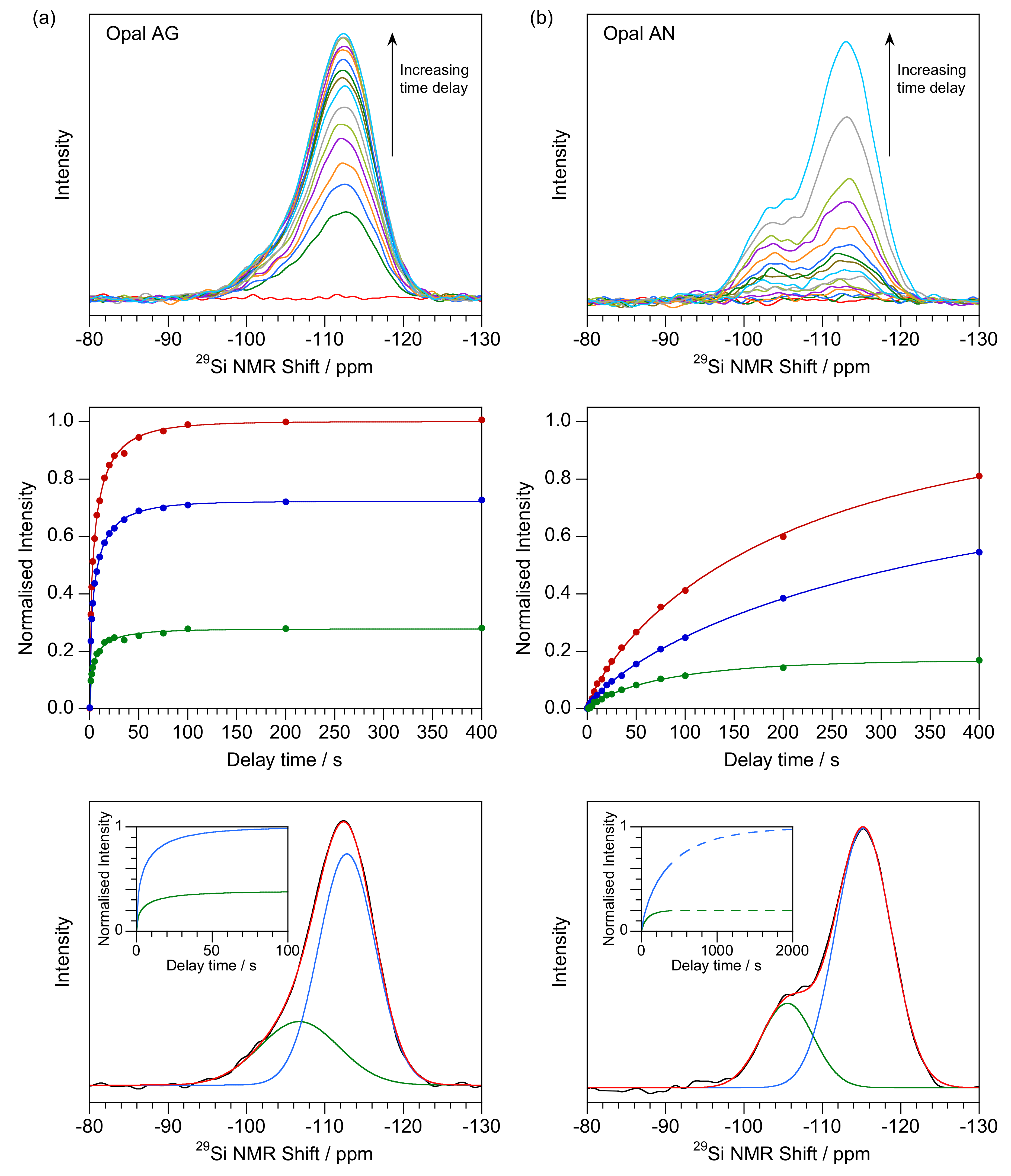

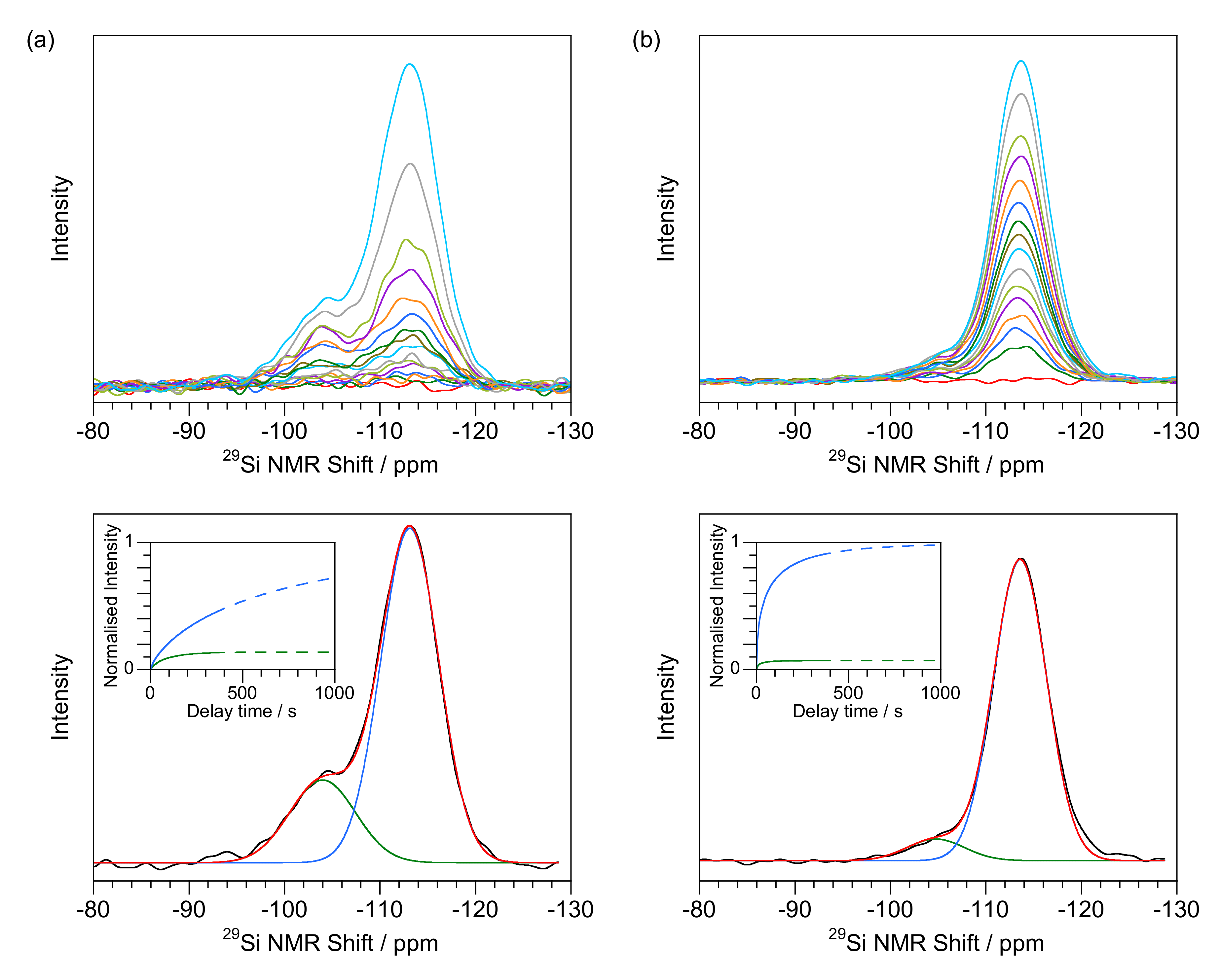
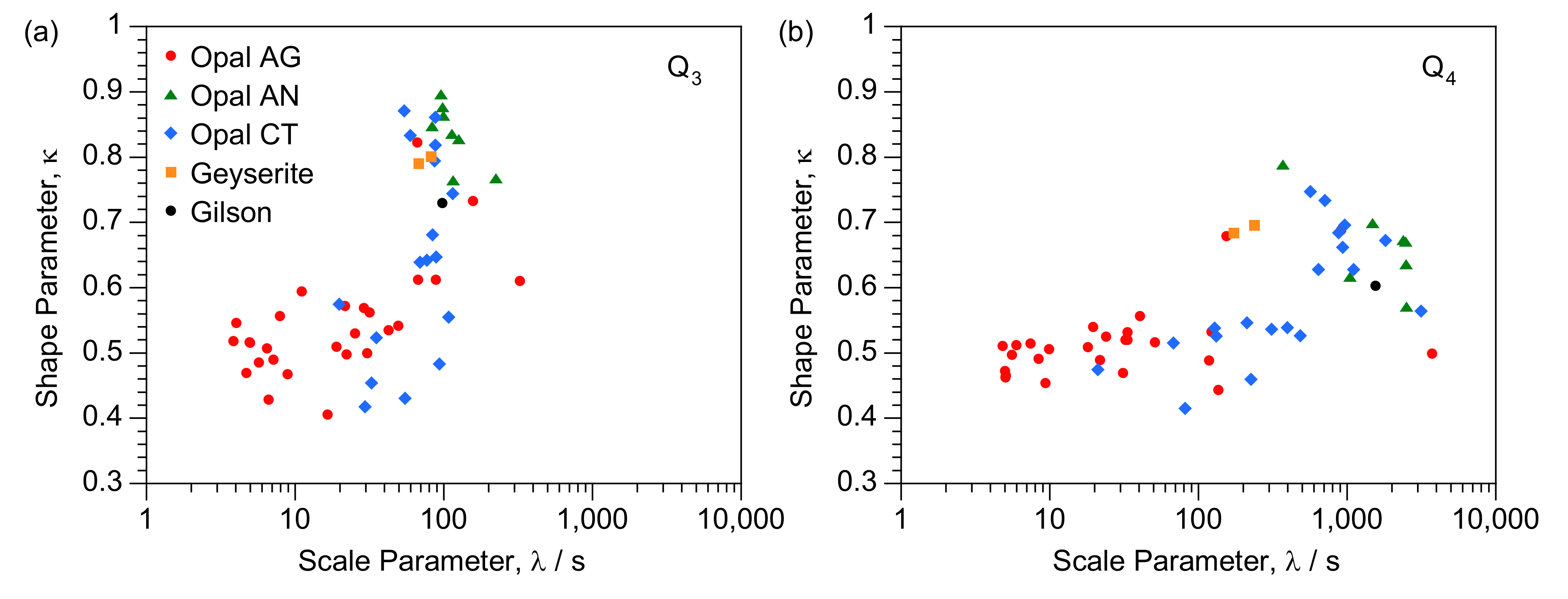
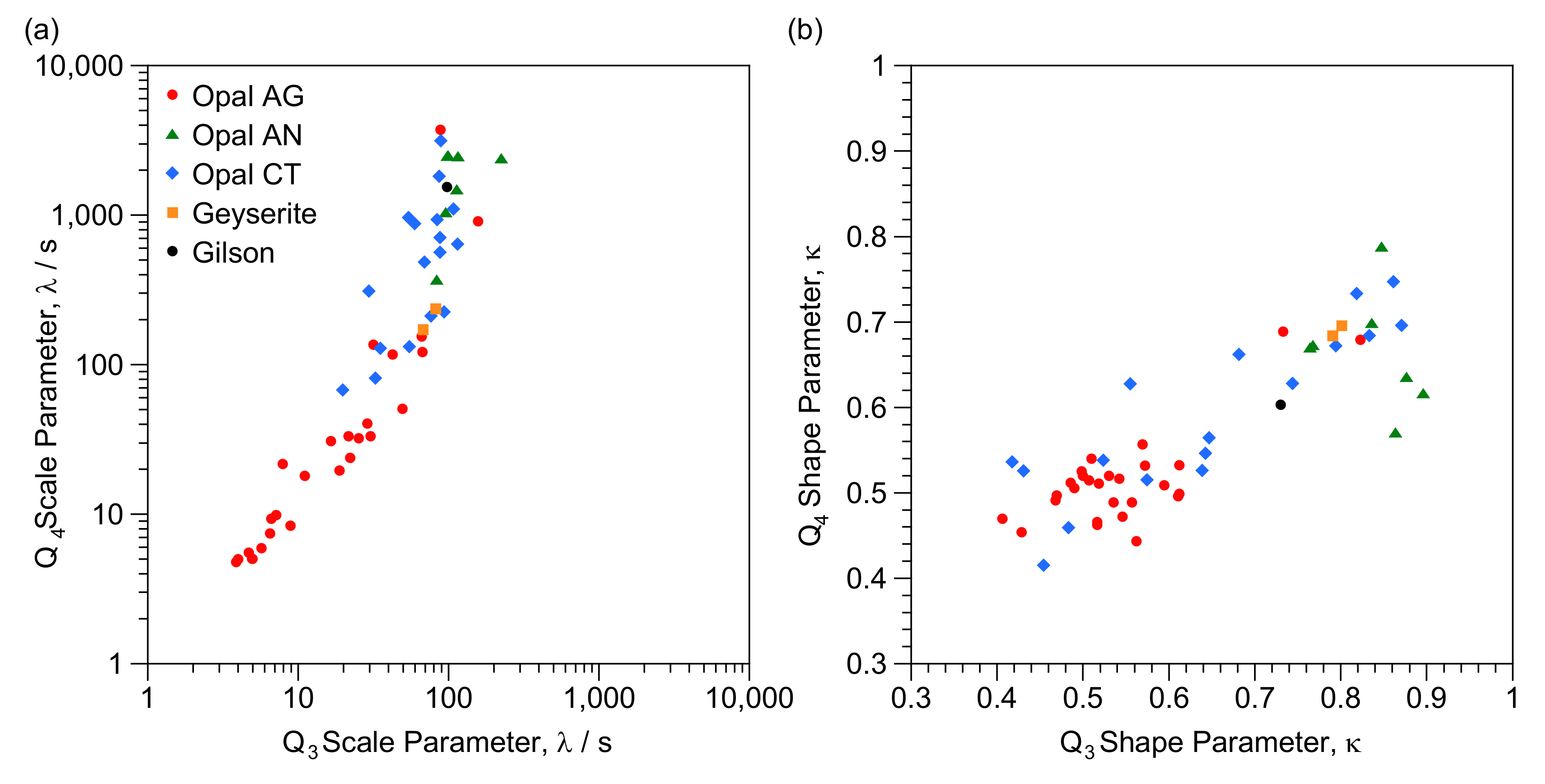

| Q3 λ (s) | Q3 κ | % Q3 Relaxation after 400 s | Q4 λ (s) | Q4 κ | % Q4 Relaxation after 400 s | Q3/Q4 Ratio | |
|---|---|---|---|---|---|---|---|
| Australian seam opal | |||||||
| G1401 | 6.48 ± 0.05 | 0.51 ± 0.01 | >99 | 7.43 ± 0.01 | 0.51 ± 0.01 | >99 | 0.24 ± 0.01 |
| G1442 | 5.71 ± 0.06 | 0.49 ± 0.01 | >99 | 5.94 ± 0.02 | 0.51 ± 0.01 | >99 | 0.38 ± 0.01 |
| G7682 | 6.64 ± 0.13 | 0.43 ± 0.01 | >99 | 9.37 ± 0.1 | 0.45 ± 0.01 | >99 | 0.18 ± 0.01 |
| G8608 | 11.1 ± 0.1 | 0.59 ± 0.01 | >99 | 18.1 ± 0.1 | 0.51 ± 0.01 | 99 | 0.33 ± 0.01 |
| G9258 | 8.88 ± 0.12 | 0.47 ± 0.01 | >99 | 8.40 ± 0.02 | 0.49 ± 0.01 | >99 | 0.28 ± 0.01 |
| G9592 | 21.6 ± 0.3 | 0.57 ± 0.01 | >99 | 33.3 ± 0.1 | 0.53 ± 0.01 | 98 | 0.21 ± 0.01 |
| G9811 | 7.15 ± 0.05 | 0.49 ± 0.01 | >99 | 9.92 ± 0.03 | 0.51 ± 0.01 | >99 | 0.49 ± 0.01 |
| G13767 a,b | 3.85 ± 0.05 | 0.52 ± 0.01 | >99 | 4.80 ± 0.01 | 0.51 ± 0.01 | >99 | 0.24 ± 0.01 |
| G13767 a,c | 4.69 ± 0.06 | 0.47 ± 0.01 | >99 | 5.54 ± 0.02 | 0.50 ± 0.01 | >99 | 0.28 ± 0.01 |
| GNEW22 | 5.05 ± 0.06 | 0.47 ± 0.01 | >99 | 4.95 ± 0.01 | 0.52 ± 0.01 | >99 | 0.32 ± 0.01 |
| GNEW31 | 4.00 ± 0.04 | 0.55 ± 0.01 | >99 | 5.00 ± 0.01 | 0.47 ± 0.01 | >99 | 0.18 ± 0.01 |
| T2233 | 5.04 ± 0.06 | 0.46 ± 0.02 | >99 | 4.92 ± 0.01 | 0.52 ± 0.10 | >99 | 0.32 ± 0.01 |
| G1401 d | 11.4 ± 0.3 | 0.38 ± 0.01 | >99 | 7.17 ± 0.02 | 0.50 ± 0.01 | >99 | 0.18 ± 0.01 |
| G8608 d | 14.1 ± 0.2 | 0.55 ± 0.01 | >99 | 18.9 ± 0.4 | 0.50 ± 0.01 | 99 | 0.26 ± 0.01 |
| Queensland boulder opal | |||||||
| G7532 | 66.9 ± 1.5 | 0.61 ± 0.01 | 95 | 122 ± 1 | 0.53 ± 0.01 | 85 | 0.20 ± 0.01 |
| WBO1 | 157 ± 6 | 0.73 ± 0.01 | 86 | 910 ± 36 | 0.69 ± 0.01 | 43 | 0.12 ± 0.01 |
| Coober Pedy (Australia) opalised molluscs | |||||||
| G34996 a (1) | 22.2 ± 0.3 | 0.50 ± 0.01 | >99 | 23.8 ± 0.1 | 0.53 ± 0.01 | >99 | 0.26 ± 0.01 |
| G34996 a (2) | 18.9 ± 0.3 | 0.51 ± 0.01 | >99 | 19.6 ± 0.01 | 0.54 ± 0.01 | >99 | 0.27 ± 0.01 |
| G34997 a (1) | 49.3 ± 1.2 | 0.54 ± 0.01 | 96 | 50.9 ± 0.2 | 0.52 ± 0.01 | 95 | 0.18 ± 0.01 |
| G34997 a (2) | 30.4 ± 0.6 | 0.50 ± 0.01 | 97 | 33.2 ± 0.1 | 0.52 ± 0.01 | 97 | 0.23 ± 0.01 |
| G34997 a (3) | 25.2 ± 0.4 | 0.53 ± 0.01 | 99 | 32.2 ± 0.1 | 0.52 ± 0.01 | 98 | 0.28 ± 0.01 |
| Slovakian opals | |||||||
| SO3 | 31.7 ± 0.5 | 0.56 ± 0.01 | 99 | 136 ± 2 | 0.44 ± 0.01 | 80 | 0.24 ± 0.01 |
| SO7 | 28.9 ± 0.3 | 0.57 ± 0.01 | 97 | 40.4 ± 0.1 | 0.56 ± 0.01 | 96 | 0.20 ± 0.01 |
| SO17 | 16.5 ± 0.7 | 0.41 ± 0.01 | 99 | 31.0 ± 0.2 | 0.47 ± 0.02 | 85 | 0.15 ± 0.01 |
| G34280 | 66.2 ± 0.5 | 0.82 ± 0.01 | 99 | 154 ± 2 | 0.68 ± 0.01 | 85 | 0.76 ± 0.01 |
| Spanish menilites (Camp del Ninots) | |||||||
| GNEW01 | 88.4 ± 4.1 | 0.61 ± 0.01 | 92 | 3730 ± 590 | 0.50 ± 0.01 | 28 | 0.15 ± 0.01 |
| GNEW23 | 325 ± 38 | 0.61 ± 0.01 | 62 | >10,000 g | 0.50 ± 0.01 | - g | - g |
| Hydrophane (Slovakia) | |||||||
| G34475 e | 7.89 ± 0.09 | 0.56 ± 0.01 | >99 | 21.7 ± 0.1 | 0.49 ± 0.01 | 98 | 0.21 ± 0.01 |
| G34475 f | 42.5 ± 1.0 | 0.54 ± 0.01 | 96 | 118 ± 1 | 0.49 ± 0.02 | 84 | 0.18 ± 0.01 |
| Opal-AN (worldwide) | |||||||
| E1937 | 113 ± 2 | 0.84 ± 0.01 | 94 | 1480 ± 60 | 0.70 ± 0.01 | 33 | 0.09 ± 0.01 |
| G8877 | 224 ± 16 | 0.77 ± 0.01 | 79 | 2390 ± 160 | 0.67 ± 0.01 | 26 | 0.07 ± 0.01 |
| G32740 | 98.2 ± 1.7 | 0.88 ± 0.01 | 97 | 2490 ± 130 | 0.63 ± 0.01 | 27 | 0.07 ± 0.01 |
| M8736 | 126 ± 3 | 0.83 ± 0.01 | 93 | >10,000 g | 0.65 ± 0.01 | - g | - g |
| MS-4 | 105 ± 3 | 0.76 ± 0.01 | 92 | 2470 ± 130 | 0.67 ± 0.01 | 26 | 0.06 ± 0.01 |
| OOC10 | 99.6 ± 3.0 | 0.86 ± 0.01 | 96 | 2510 ± 160 | 0.57 ± 0.01 | 30 | 0.05 ± 0.01 |
| T18117 | 83.4 ± 0.8 | 0.85 ± 0.01 | 98 | 371 ± 5 | 0.79 ± 0.01 | 65 | 0.20 ± 0.01 |
| T18511 | 96.0 ± 4.0 | 0.90 ± 0.02 | 97 | 1050 ± 80 | 0.62 ± 0.01 | 42 | 0.06 ± 0.01 |
| Geyserites (New Zealand) | |||||||
| G21471 | 81.8 ± 0.9 | 0.80 ± 0.01 | 97 | 237 ± 2 | 0.70 ± 0.01 | 76 | 0.22 ± 0.01 |
| T1665 | 67.6 ± 0.5 | 0.79 ± 0.05 | 98 | 172 ± 2 | 0.72 ± 0.01 | 83 | 0.63 ± 0.01 |
| Synthetic opal | |||||||
| Gilson | 97.6 ± 3.2 | 0.73 ± 0.01 | 94 | 1550 ± 60 | 0.60 ± 0.01 | 36 | 0.06 ± 0.01 |
| Q3 | Q4 | ||||||
|---|---|---|---|---|---|---|---|
| λ (s) | κ | % Relaxation after 400 s | λ (s) | κ | % Relaxation after 400 s | Q3/Q4 Ratio | |
| G1421 | 83.9 ± 4.4 | 0.68 ± 0.01 | 94 | 936 ± 50 | 0.66 ± 0.02 | 43 | 0.07 ± 0.01 |
| G9942 | 89.1 ± 4.8 | 0.65 ± 0.01 | 93 | >3000 | 0.56 ± 0.01 | 27 | 0.04 ± 0.01 |
| G13755 | 29.5 ± 3.6 | 0.42 ± 0.02 | 95 | 311 ± 7 | 0.54 ± 0.01 | 68 | 0.06 ± 0.01 |
| G13761 | 35.2 ± 2.5 | 0.52 ± 0.02 | 97 | 129 ± 1 | 0.54 ± 0.01 | 84 | 0.05 ± 0.01 |
| G32925 | 87.8 ± 1.5 | 0.82 ± 0.01 | 97 | 710 ± 23 | 0.73 ± 0.01 | 48 | 0.14 ± 0.01 |
| GNEW05 | - a | - a | - a | 21.1 ± 0.2 | 0.47 ± 0.01 | 98 | - a |
| GNEW08 | 93.2 ± 15.7 | 0.48 ± 0.02 | 87 | 226 ± 5 | 0.46 ± 0.01 | 73 | 0.05 ± 0.01 |
| GNEW09 | 87.6 ± 1.7 | 0.86 ± 0.01 | 98 | 566 ± 17 | 0.75 ± 0.01 | 54 | 0.13 ± 0.01 |
| GNEW19 | 86.8 ± 3.1 | 0.79 ± 0.01 | 96 | 1820 ± 130 | 0.67 ± 0.01 | 30 | 0.06 ± 0.01 |
| GNEW20 | 69.2 ± 2.3 | 0.64 ± 0.01 | 95 | 485 ± 11 | 0.53 ± 0.01 | 59 | 0.08 ± 0.01 |
| GNEW24 | 19.8 ± 0.7 | 0.58 ± 0.01 | >99 | 67.8 ± 0.5 | 0.52 ± 0.01 | 92 | 0.07 ± 0.01 |
| GNEW27 | - a | - a | - a | 398 ± 12 | 0.54 ± 0.01 | 63 | - a |
| GNEW28 | 32.6 ± 0.2 | 0.45 ± 0.01 | 96 | 81.3 ± 13.8 | 0.42 ± 0.01 | 86 | 0.09 ± 0.01 |
| GNEW30 | 54.3 ± 1.3 | 0.87 ± 0.01 | >99 | 962 ± 44 | 0.70 ± 0.01 | 42 | 0.06 ± 0.01 |
| M53441 | 59.7 ± 1.8 | 0.83 ± 0.01 | 99 | 879 ± 44 | 0.68 ± 0.01 | 44 | 0.06 ± 0.01 |
| NMNH Eth S2 | 91.0 ± 1.9 | 0.61 ± 0.01 | 92 | 371 ± 9 | 0.62 ± 0.01 | 65 | 0.45 ± 0.01 |
| OOC3 | 76.8 ± 4.6 | 0.64 ± 0.01 | 94 | 212 ± 5 | 0.55 ± 0.01 | 76 | 0.09 ± 0.01 |
| OOC5 | 108 ± 7 | 0.55 ± 0.01 | 87 | 1100 ± 50 | 0.63 ± 0.01 | 41 | 0.09 ± 0.01 |
| T4051 | 115 ± 3 | 0.74 ± 0.01 | 92 | 642 ± 19 | 0.63 ± 0.01 | 52 | 0.15 ± 0.01 |
| T22842 | 54.8 ± 8.8 | 0.43 ± 0.02 | 92 | 109 ± 1 | 0.50 ± 0.01 | 52 | 0.06 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. 29Si Solid-State NMR Analysis of Opal-AG, Opal-AN and Opal-CT: Single Pulse Spectroscopy and Spin-Lattice T1 Relaxometry. Minerals 2022, 12, 323. https://doi.org/10.3390/min12030323
Curtis NJ, Gascooke JR, Johnston MR, Pring A. 29Si Solid-State NMR Analysis of Opal-AG, Opal-AN and Opal-CT: Single Pulse Spectroscopy and Spin-Lattice T1 Relaxometry. Minerals. 2022; 12(3):323. https://doi.org/10.3390/min12030323
Chicago/Turabian StyleCurtis, Neville J., Jason R. Gascooke, Martin R. Johnston, and Allan Pring. 2022. "29Si Solid-State NMR Analysis of Opal-AG, Opal-AN and Opal-CT: Single Pulse Spectroscopy and Spin-Lattice T1 Relaxometry" Minerals 12, no. 3: 323. https://doi.org/10.3390/min12030323
APA StyleCurtis, N. J., Gascooke, J. R., Johnston, M. R., & Pring, A. (2022). 29Si Solid-State NMR Analysis of Opal-AG, Opal-AN and Opal-CT: Single Pulse Spectroscopy and Spin-Lattice T1 Relaxometry. Minerals, 12(3), 323. https://doi.org/10.3390/min12030323







