Order-Disorder in the Structures of Lithium Aluminosilicate Minerals by XRD and Multinuclear NMR
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural Specimens and Synthetic Samples
2.2. Methods
2.2.1. Powder X-ray Diffraction (XRD)
2.2.2. Differential Thermal Analyses (DTA-TG)
2.2.3. Nuclear Magnetic Resonance (NMR)
2.2.4. Density Functional Theory (DFT)
3. Results
3.1. α-β Cristobalite s.s. in Li1−xAl1−xSi1+xO4 for 1.0 ≤ x ≤ 0.75
3.2. α-β Quartz s.s. in Li1−xAl1−xSi1+xO4 for 1.0 ≤ x ≤ 0.96
3.3. α-Quartz s.s. and β-Eucryptite s.s. in Li1−xAl1−xSi1+xO4 for 0.96 ≤ x ≤ 0.0
3.4. β-Eucryptite LiAlSiO4 End-Member
3.5. β-Spodumene s.s. Series in Li1−xAl1−xSi1+xO4
3.6. α-Spodumene LiAlSi2O6
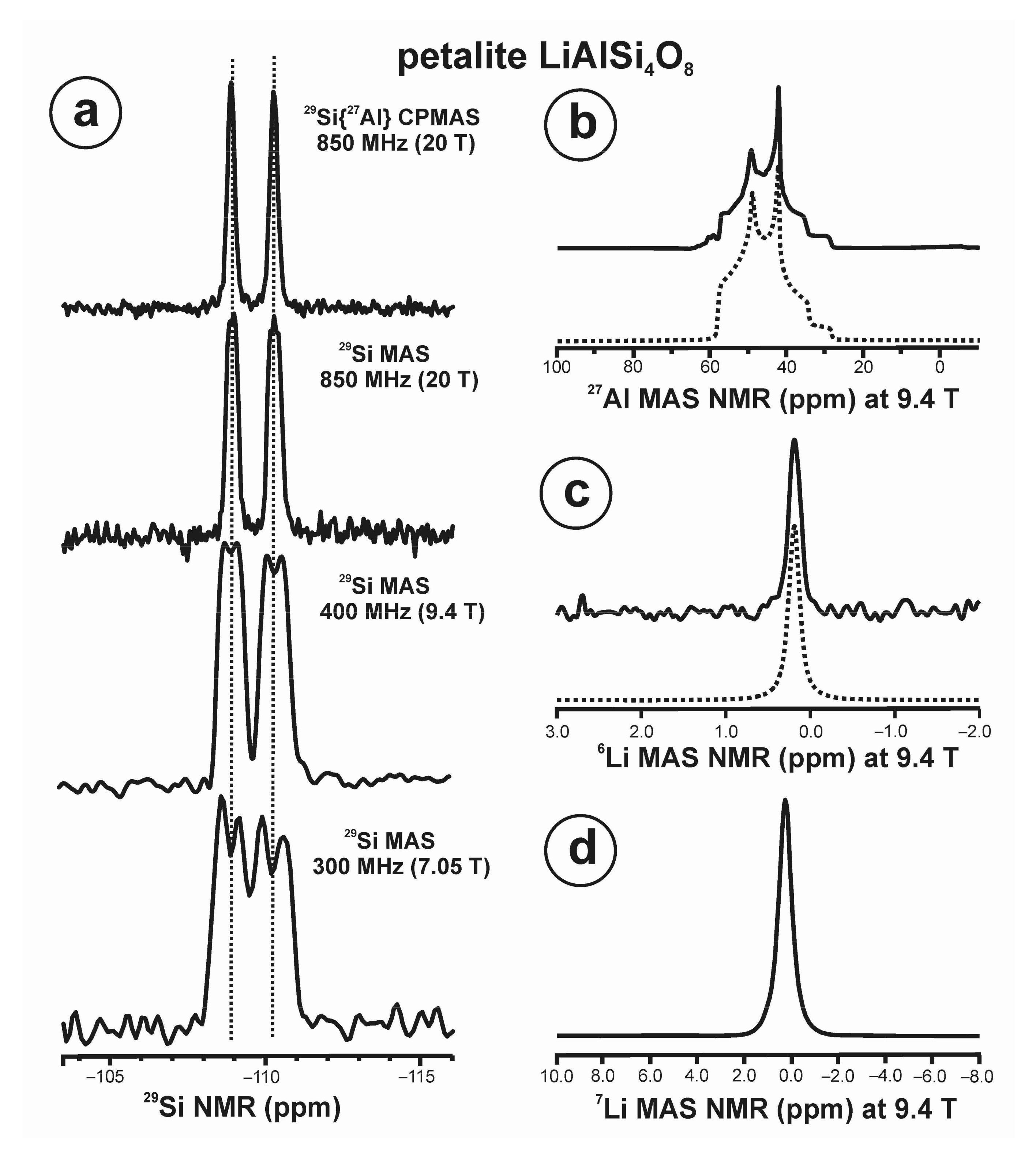
3.7. Petalite LiAlSi4O8
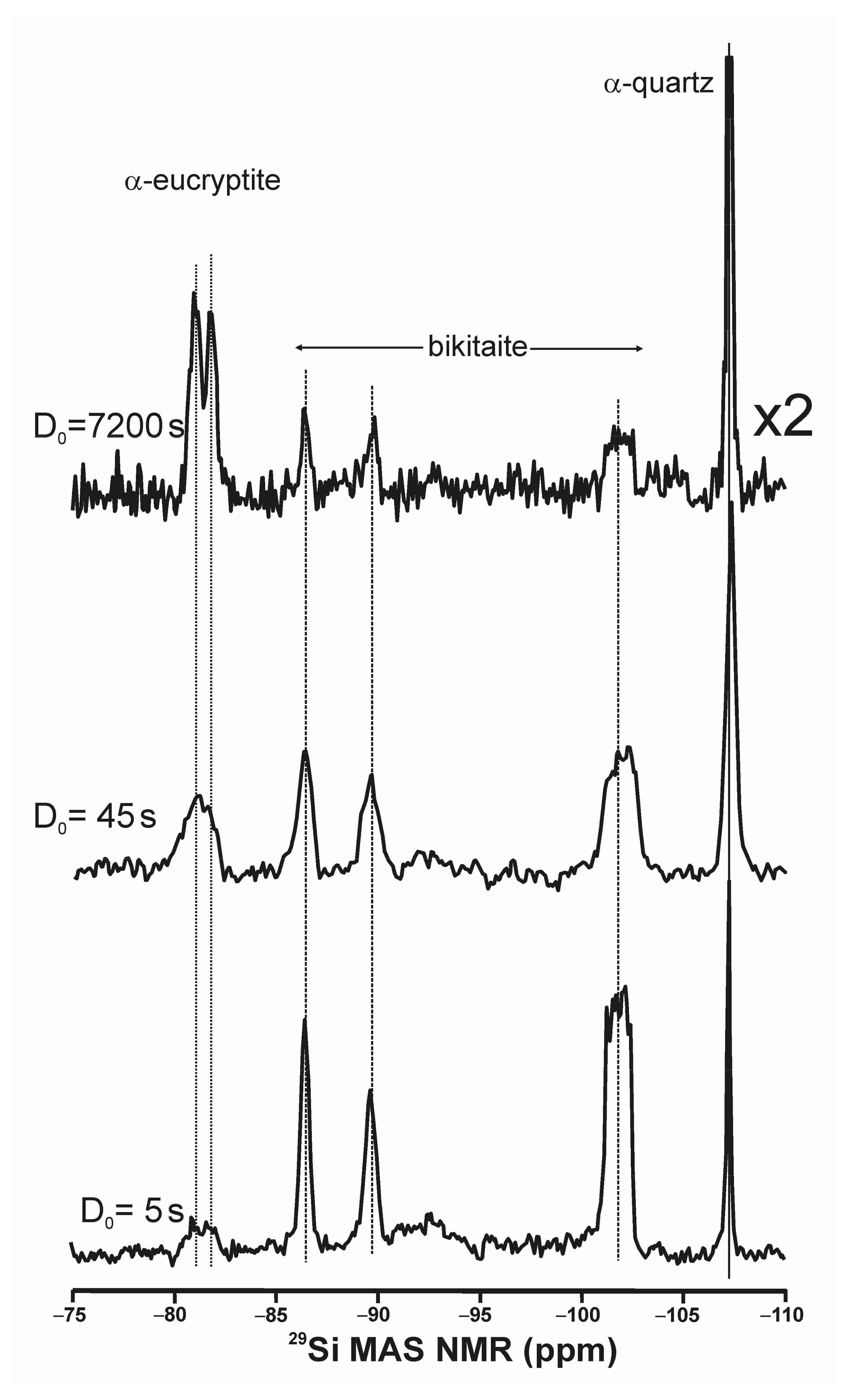
3.8. α-Eucryptite LiAlSiO4 and Bikitaite LiAlSi2O6·H2O
4. Discussion
- α-cristobalite SiO2 end-member (x = 1.00) and α-cristobalite s.s. for 0.75 ≤ x < 1.00 were derived from the crystallization of the β-phase at T ≥ 1000 °C, which suffers a displacive β → α transition on cooling. At room-temperature, the members of the solid solution are strictly pseudo-periodic, despite it is possible to describe their powder X-ray diffraction pattern with an average structure derived from the P42212 space group of α-cristobalite SiO2 end-member (Figure A8).
- α-quartz SiO2 end-member and α-quartz s.s. for 0.75 ≤ x < 1.00 SiO2 were derived from the crystallization of the β-phase at T > 675 °C, which suffers a displacive β → α transition on cooling, and thus, were detected at room-temperature. The members of this solid solution are strictly pseudo-periodic, despite it being possible to describe its X-ray diffraction pattern with an average structure derived from the P3221 space group of α-quartz SiO2 end-member (Figure A4 and Figure A6). The solid solution is limited at high pressure but evident at 1 atm.
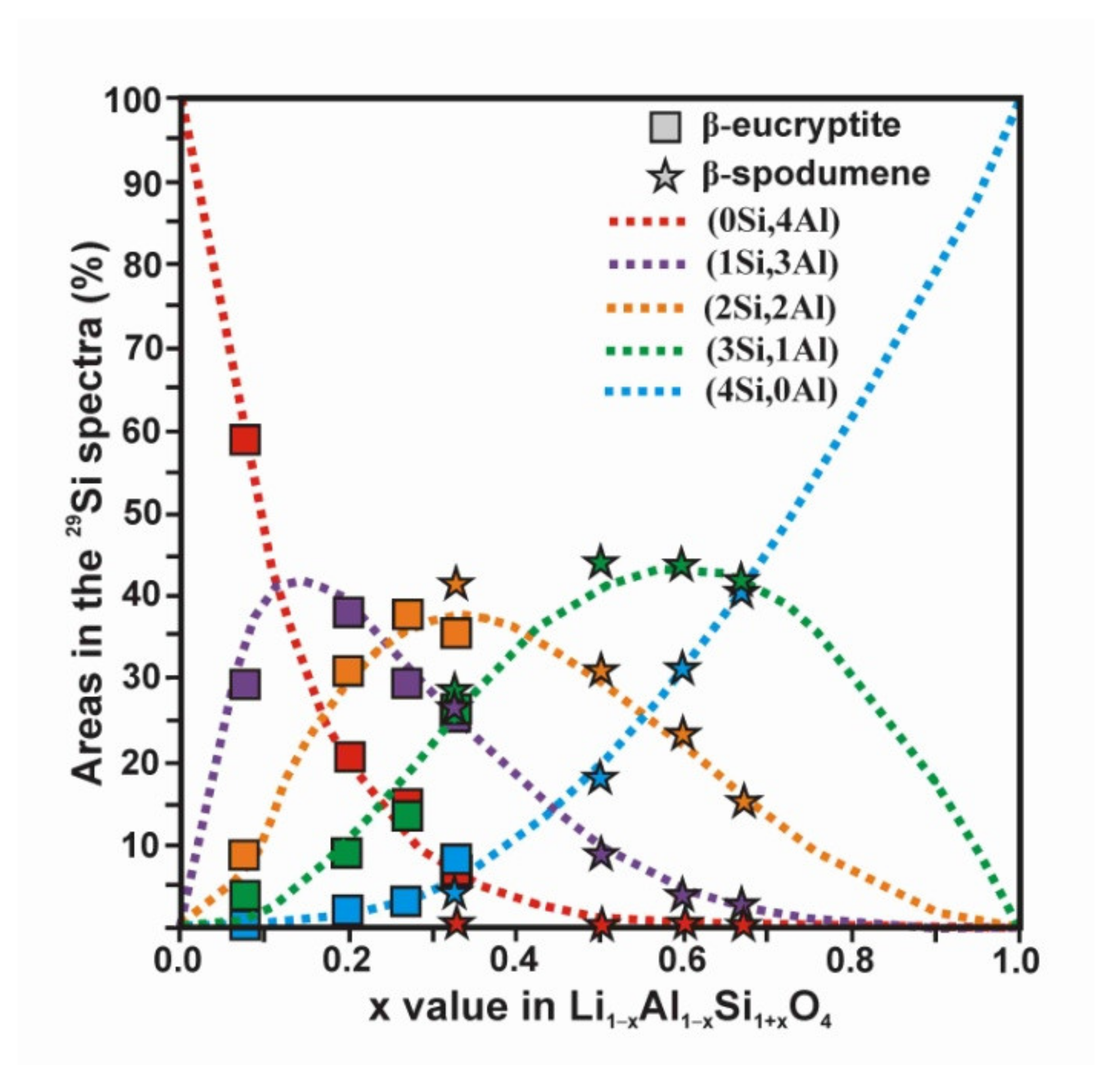
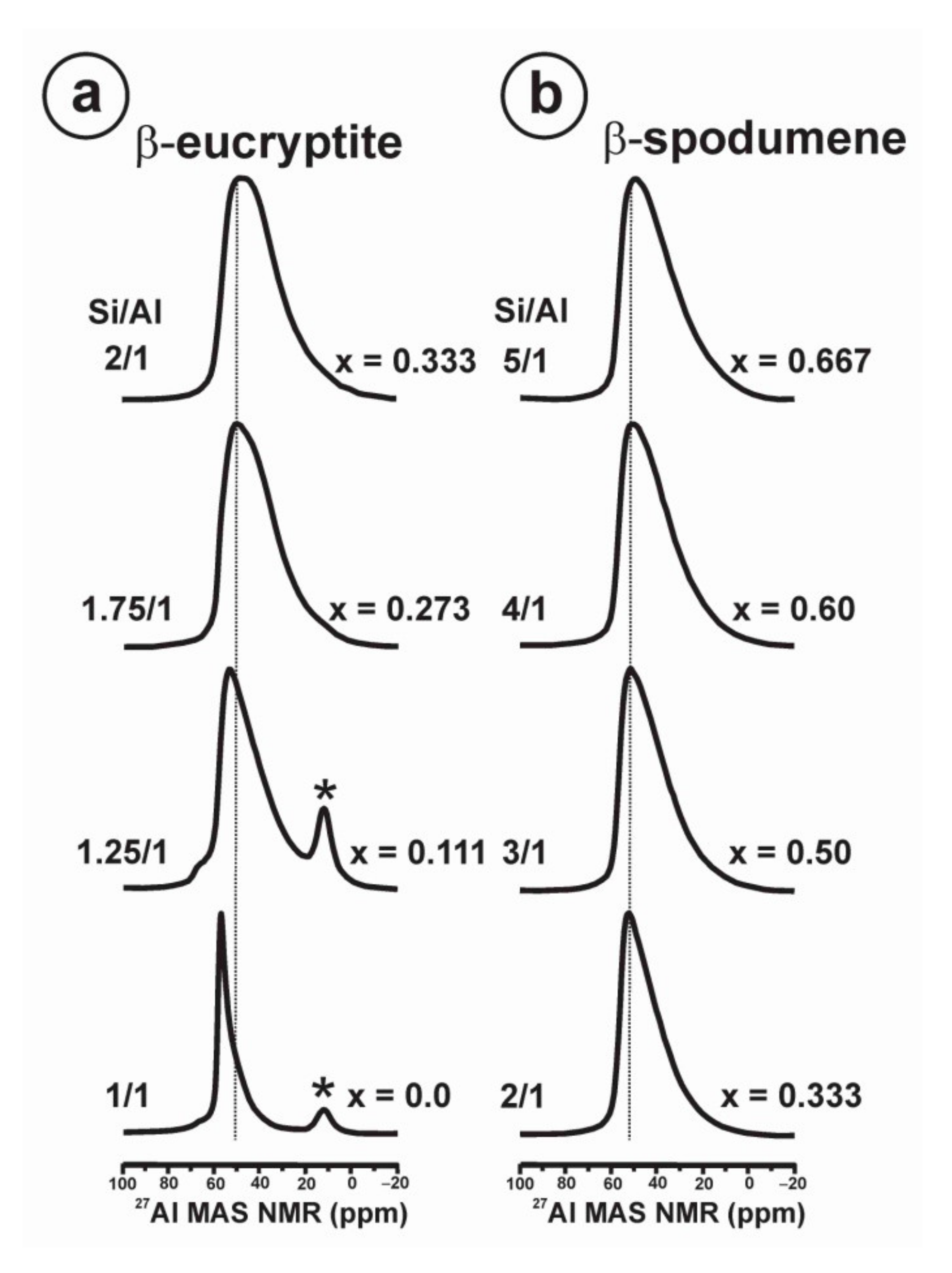
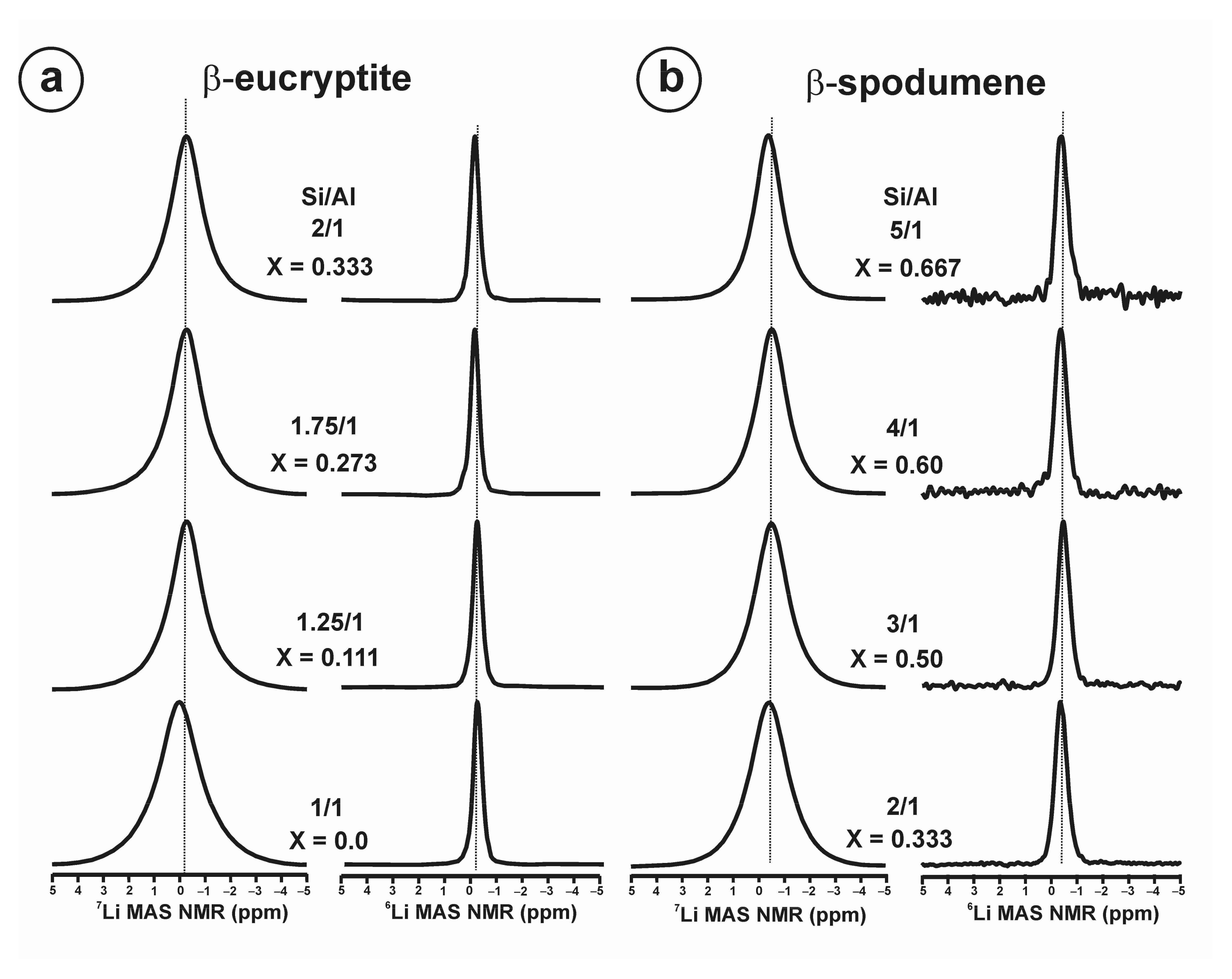
- β-eucryptite s.s. series for 0.0 ≤ x < 1.0 is formed at high temperatures, and it is observed at room temperature when the β → α transition is avoided on cooling. At room-temperature, the stuffed derivative of the β-quartz structure is evident mainly for x < 0.50 from XRD (Figure A5 and Figure A6), despite the members of the solid solution are strictly pseudoperiodic. An ordered state of the local scale is compatible with the Loewenstein’s rule. Only a single site distribution is resolved for Si and Al atoms and two site distributions are resolved for Li atoms by NMR.
- β-eucryptite LiAlSiO4 end-member (x = 0), crystallized at 1000 °C and 3 kbars, has a fully ordered local structure from NMR that is compatible with the long-range P6422 space group from XRD [12]. Hence, short-range order and long-range order are equivalent, because the local poly-atomic schemes are strictly periodic throughout the volume.
- β-spodumene s.s. series for 0.0 ≤ x ≤ 1.0 have strictly non-periodic structures but a Lowenstein order state at the local scale, and only a single site distribution is resolved for Si and Al atoms, whereas at least two sites for Li atoms are resolved by NMR. This short-range structure does not correspond with any available lattice model.
- Petalite LiAlSi4O10 mineral species have a fully ordered structure at the short-range scale from NMR, which is compatible with a long-range ordered structure with P2/a space group from XRD [30].
- α-spodumene LiAlSi2O6 mineral species has a fully ordered structure at the short-range scale from NMR, which is compatible with a long-range ordered structure with C2/c space group from XRD [34].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Powder XRD Patterns from Selected Minerals






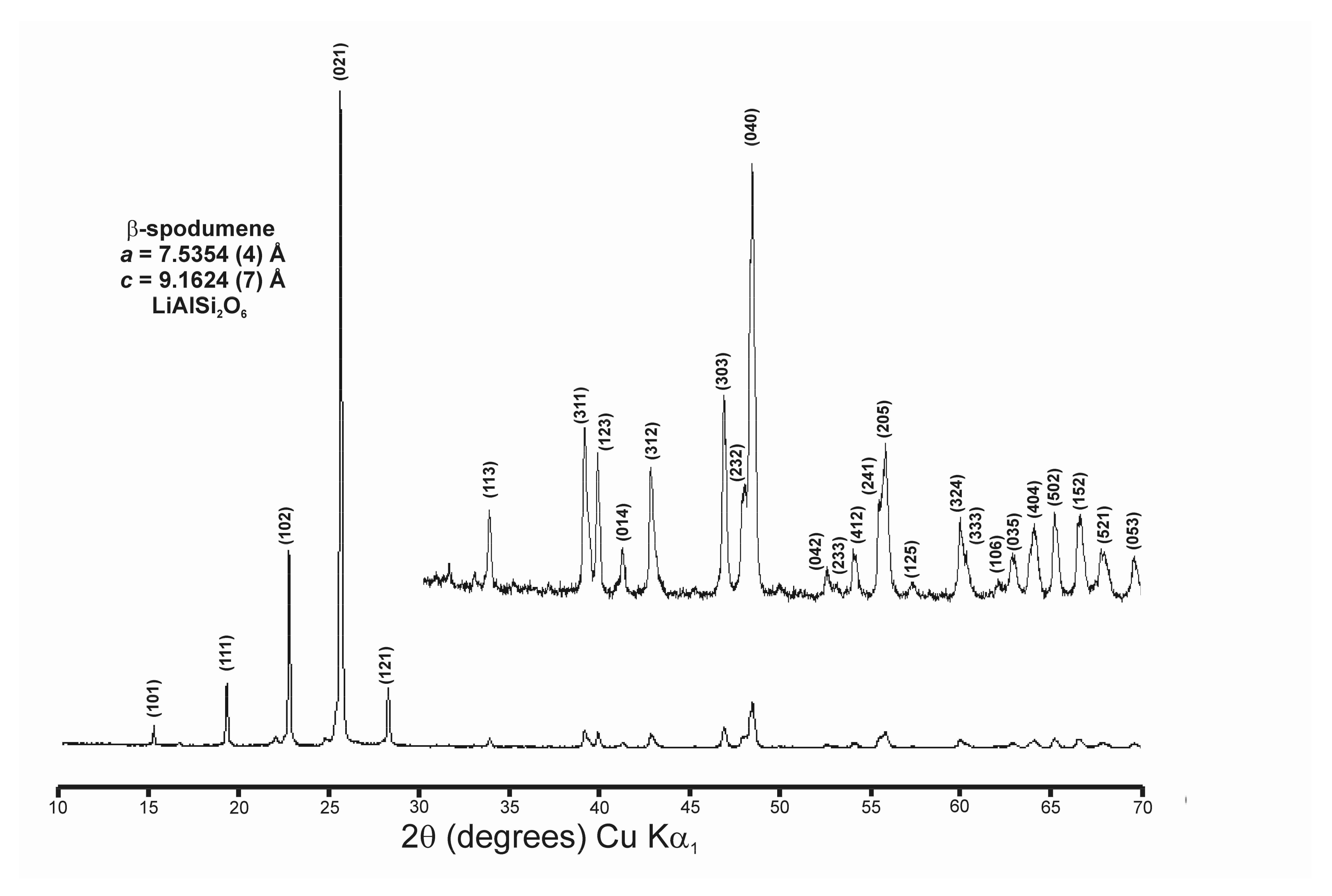

Appendix B. Rietveld Refinement of Cell Parameters from Powder XRD Patterns
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | - | - | - |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | 4.939 (4) | 5.433 (5) | 114.8 (2) |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | 4.987 (2) | 5.421 (3) | 116.7 (1) |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | 5.022 (1) | 5.433 (2) | 118.68 (7) |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 5.0667 (8) | 5.441 (1) | 120.96 (5) |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | 10.234 (4) | 10.906 (5) | 989.3 (8) |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 10.292 (2) | 10.917 (2) | 1001.5 (4) |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 10.368 (1) | 10.911 (2) | 1015.7 (4) |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | 10.4446 (7) | 10.9092 (9) | 1030.6 (2) |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 10.4678 (6) | 10.9210 (7) | 1036.3 (1) |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 10.506 (3) | 10.981 (4) | 1049.6 (8) |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | 10.4942 (6) | 10.9403 (8) | 1043.4 (1) |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | 10.517 (2) | 10.994 (3) | 1053.0 (5) |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | - | - | - |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | - | - | - |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | - | - | - |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | - | - | - |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | - | - | - |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | - | - | - |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 7.497 (1) | 9.056 (3) | 509.0 (3) |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 7.515 (2) | 9.097 (3) | 513.8 (3) |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | - | - | - |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | - | - | - |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | - | - | - |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | - | - | - |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | - | - | - |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | 4.938 (1) | 5.412 (2) | 114.29 (7) |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | 4.9549 (4) | 5.4148 (6) | 115.12 (2) |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | 4.982 (1) | 5.427 (2) | 116.66 (7) |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | 5.0147 (5) | 5.4324 (7) | 118.31 (3) |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 5.0536 (4) | 5.4399 (6) | 120.32 (2) |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | 10.181 (2) | 10.893 (3) | 977.9 (4) |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 10.298 (17) | 10.899 (18) | 1000.9 (17) |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 10.373 (1) | 10.905 (2) | 1016.3 (3) |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | 10.4410 (6) | 10.9092 (7) | 1029.9 (1) |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 10.4693 (6) | 10.9153 (7) | 1036.1 (1) |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 10.520 (2) | 11.056 (3) | 1059.7 (5) |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | 10.497 (1) | 10.956 (1) | 1045.5 (3) |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | 10.522 (1) | 11.064 (2) | 1061.0 (4) |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | - | - | - |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | - | - | - |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | - | - | - |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | - | - | - |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 7.472 (4) | 9.013 (9) | 503.2 (7) |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | 7.4875 (5) | 9.0105 (8) | 505.1 (1) |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 7.493 (1) | 9.043 (2) | 507.8 (2) |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 7.513 (1) | 9.098 (1) | 513.6 (2) |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | 7.535 (4) | 9.152 (4) | 519.6 (5) |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 7.625 (3) | 9.124 (4) | 530.6 (5) |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 7.534 (2) | 9.122 (4) | 517.7 (4) |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | - | - | - |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | - | - | - |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | 4.9365 (4) | 5.4108 (7) | 114.19 (2) |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | 4.9553 (3) | 5.4152 (7) | 115.16 (2) |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | 4.9785 (6) | 5.4214 (9) | 116.37 (3) |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | 5.0104 (6) | 5.4307 (8) | 118.07 (3) |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 5.0450 (6) | 5.4375 (8) | 119.85 (3) |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | 10.180 (5) | 10.876 (9) | 976.1 (5) |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 10.279 (1) | 10.913 (3) | 998.6 (4) |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 10.348 (1) | 10.916 (2) | 1012.4 (3) |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | 10.4436 (5) | 10.9121 (7) | 1030.7 (1) |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 10.4715 (4) | 10.9251 (5) | 1037.5 (1) |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 10.517 (2) | 11.006 (3) | 1054.2 (5) |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | 10.495 (1) | 10.963 (2) | 1045.6 (3) |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | 10.516 (1) | 11.054 (2) | 1058.7 (4) |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | - | - | - |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | - | - | - |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | 7.364 (3) | 9.03 (3) | 489.7 (32) |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | 7.434 (7) | 9.01 (2) | 498.1 (12) |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 7.479 (2) | 8.994 (4) | 503.1 (3) |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | 7.4871 (5) | 9.0075 (8) | 504.9 (1) |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 7.4934 (5) | 9.0437 (8) | 507.8 (1) |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 7.5113 (7) | 9.094 (1) | 513.1 (1) |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | 7.5348 (9) | 9.157 (1) | 519.8 (1) |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 7.5405 (9) | 9.165 (1) | 521.1 (1) |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 7.5258 (8) | 9.124 (1) | 516.8 (1) |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | - | - | - |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | - | - | - |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | 4.935 (2) | 5.420 (3) | 114.4 (1) |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | 4.9581 (9) | 5.418 (1) | 115.34 (5) |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | 4.9815 (8) | 5.423 (1) | 116.55 (5) |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | 5.0095 (7) | 5.4286 (9) | 117.98 (4) |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 5.0506 (1) | 5.4397 (1) | 120.17 (3) |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | - | - | - |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | - | - | - |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | - | - | - |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | - | - | - |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 10.4801 (4) | 10,9361 (5) | 1040.2 (1) |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 10.502 (1) | 10.942 (1) | 1045.2 (2) |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | 10.5058 (6) | 10.9526 (7) | 1046.9 (1) |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | 10.5079 (7) | 11.091 (1) | 1060.6 (2) |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | a (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| 1/0 | 1.00 | Si2O4 | SiO2 | - | - | - |
| 100/1 | 0.98 | Li0.02Al0.02Si1.94O4 | LiAlSi100O202 | - | - | - |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | - | - | - |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | - | - | - |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | 7.385 (11) | 8.993 (26) | 490.5 (21) |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | 7.449 (4) | 8.998 (9) | 499.4 (8) |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 7.4803 (8) | 8.995 (1) | 503.3 (1) |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | 7.4876 (3) | 9.0100 (4) | 505.14 (4) |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 7.4938 (3) | 9.0511 (5) | 508.29 (5) |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 7.5076 (5) | 9.0936 (8) | 512.6 (9) |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | 7.5354 (4) | 9.1624 (6) | 520.3 (1) |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 7.5471 (5) | 9.1769 (7) | 522.7 (9) |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 7.5241 (7) | 9.124 (1) | 516.5 (1) |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | - | - | - |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | - | - | - |



References
- Venkateswaran, C.; Sreemoolanadhan, H.; Vaish, R. Lithium aluminosilicate (LAS) glass-ceramics: A review of recent progress. Int. Mater. Rev. 2021, 66, 1–38. [Google Scholar] [CrossRef]
- Van Tendeloo, G.; Van Landuyt, J.; Amelickx, S. The α-β phase transition in quartz and AlPO4 as studied by electron microscopy and diffraction. Phys. Status Solidi (a) 1976, 33, 723–735. [Google Scholar] [CrossRef]
- Dolino, G.; Bachheimer, J.P.; Berge, B.; Zeyen, C.M.E.; Van Tendeloo, G.; Van Landuyt, J.; Amelinckx, S. Incommensurate phase of quartz: III. Study of the coexistence state between the incommensurate and the α-phases by neutron scattering and electron microscopy. J. Phys. 1984, 45, 901–912. [Google Scholar] [CrossRef]
- Van Landuyt, J.; Van Tendeloo, G.; Amelickx, S.; Walker, M.B. Interpretation of Dauphiné-twin-domain configuration resulting from the a-b phase transition in quartz and aluminum phosphate. Phys. Rev. B 1985, 31, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Heaney, P.J.; Veblen, D.R. Observations on the α-β phase transition in quartz: A review of imaging and diffraction studies and some new results. Am. Mineral. 1991, 76, 1018–1032. [Google Scholar]
- Spearing, D.R.; Stebbins, J.F. The 29Si NMR shielding tensor in low quartz. Am. Mineral. 1989, 74, 956–959. [Google Scholar]
- Spearing, D.R.; Farnan, I.; Stebbins, J.F. Dynamics of the α-β phase transitions in quartz and cristobalite as observed by in-situ high temperature 29Si and 17O NMR. Phys. Chem. Miner. 1992, 19, 307–321. [Google Scholar] [CrossRef]
- Martini, M.; Peleari, A.; Spinolo, G.; Vedda, A. Role of [AlO4]0 centers in the 380 nm thermoluminescence of quartz. Phys. Rev. B 1995, 52, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, L.; Correcher, V.; García-Guinea, J.; Delgado, A. Thermoluminescence of a lithium aluminum rich beta quartz for dosimetry purposes. Radiat. Protec. Dos. 1999, 84, 543–546. [Google Scholar] [CrossRef]
- Tscherry, V.; Schulz, H.; Laves, F. Average and superstructure of beta-eucryptite (LiAlSiO4). Part I. Average Structure. Zeit. Kristall. 1972, 135, 161–174. [Google Scholar]
- Tscherry, V.; Schulz, H.; Laves, F. Average and superstructure of beta-Eucryptite (LiAlSiO4). Part II. Superstructure. Zeit. Kristall. 1972, 135, 175–198. [Google Scholar]
- Pillars, W.W.; Peacor, D.R. The crystal structure of beta eucryptite as a function of temperature. Am. Mineral. 1973, 58, 681–690. [Google Scholar]
- Sartbaeva, A.; Redfern, S.A.T.; Lee, W.T. A neutron diffraction and Rietveld analysis of cooperative Li motion in beta-eucryptite. J. Phys. Cond. Mat. 2004, 16, 5267–5278. [Google Scholar] [CrossRef]
- Xu, H.; Heaney, P.J.; Beall, G.H. Phase transition induced by solid solution in stuffed derivatives of quartz: A power synchrotron XRD study of the LiAlSiO4-SiO2 join. Am. Mineral. 2000, 85, 971–979. [Google Scholar] [CrossRef]
- Xu, H.; Heaney, P.J.; Navrotsky, A. Thermal expansion and structural transformations of the stuffed derivatives of quartz along the LiAlSiO4-SiO2 join: A variable-temperature power synchrotron XRD study. Phys. Chem. Min. 2001, 28, 302–312. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H.; Heaney, P.J. Vibrational spectroscopy of beta-eucryptite (LiAlSiO4): Optical phonons and phase transitions(s). Phys. Chem. Min. 2003, 30, 457–462. [Google Scholar] [CrossRef]
- Phillips, B.L.; Xu, H.; Heaney, P.; Navrostsky, A. 29Si and 27Al MAS-NMR spectroscopy of β-eucryptite (LiAlSiO4): The enthalpy of Si,Al ordering. Am. Mineral. 2000, 85, 181–188. [Google Scholar] [CrossRef]
- Li, C.T. The crystal structure of LiAlSi2O6 III (high-quartz solid solution). Zeit. Krist. 1968, 127, 327–348. [Google Scholar]
- French, B.M. Virgilite: A new lithium aluminum silicate mineral from the Macusani glass, Peru. Am. Mineral. 1978, 63, 461–465. [Google Scholar]
- Shropshire, J.; Keat, P.; Vaughan, P.A. The crystal structure of keatite, a new form of silica. Zeit. Krist. 1959, 112, 409–413. [Google Scholar] [CrossRef]
- Demuth, T.; Jeanvoine, Y.; Hafner, J.; Angyan, J.G. Polymorphism in silica studied in the local density and generalized-gradient approximations. J. Phys. Cond. Matter 1999, 11, 3833–3874. [Google Scholar] [CrossRef]
- Skinner, B.J.; Evans, H.T. Crystal chemistry of β-spodumene solid solutions on the join Li2O·Al2O3-SiO2. Am. J. Sci. 1960, 258A, 312–324. [Google Scholar]
- Li, C.T.; Peacor, D.R. The crystal structure of LiAlSi2O6-II (beta-spodumene). Acta Cryst. 1968, B27, 1132–1140. [Google Scholar]
- Clarke, P.T.; Spink, J.M. The crystal structure of beta spodumene, LiAlSi2O6 II. Zeit. Krist. 1969, 130, 420–426. [Google Scholar] [CrossRef]
- Baumgartner, B.; Müller, G. Framework distortion by large ions in MAlSi2O6 aluminosilicates with keatite structure. Eur. J. Miner. 1990, 2, 155–162. [Google Scholar] [CrossRef]
- Nordmann, A.; Cheng, Y.-B.; Bastow, T.J.; Hill, A.J. Structural characterization of lithium aluminosilicate glass and glass ceramics derived from spodumene mineral. J. Phys. Cond. Matt. 1995, 7, 3115–3128. [Google Scholar] [CrossRef]
- Downs, R.T.; Palmer, D. The pressure behaviour of alpha-cristobalite. Am. Mineral. 1994, 79, 9–14. [Google Scholar]
- Wei, P.H. The structure of quartz. Zeit. Krist. 1935, 92, 355–362. [Google Scholar] [CrossRef]
- Tucker, M.G.; Keen, D.A.; Dove, M.T. A detailed structural characterization of quartz on heating through the alpha–beta transition. Mine. Mag. 2001, 65, 489–507. [Google Scholar] [CrossRef]
- Effenberger, H. Petalit, LiAlSi4O10: Verfeinerung der Kristallstruktur, Diskussion der Raumgruppe und Infrarot-Messung. Tscher. Mineral. Petrog. Mitt. 1980, 27, 129–142. [Google Scholar] [CrossRef]
- Libeau, V.F. Untersuchungen an Schichtsilikaten des Formertyps Am(Si2O5)n. III. Zur Kristallstruktur von petalit, LiAlSiO4. Acta Cryst. 1961, 14, 399–406. [Google Scholar] [CrossRef]
- Hesse, K.F. Crystal structures of natural and synthetic alpha eucryptite, LiAlSiO4. Zeit. Krist. 1985, 172, 147–151. [Google Scholar] [CrossRef]
- Daniels, P.; Fyfe, C.A. Al, Si order in the crystal structure of alpha-eucryptite (LiAlSiO4). Amer. Mineral. 2001, 86, 279–283. [Google Scholar] [CrossRef]
- Tribaudino, M.; Nestola, F.; Prencipe, M.; Rundlof, H. A single-crystal neutron diffraction investigation of spodumene at 54 K. Can. Mineral. 2003, 41, 521–527. [Google Scholar] [CrossRef]
- Clark, J.R.; Appleman, D.E.; Papike, J.J. Crystal-chemical characterization of clinopyroxenes based on eight new structure refinements. Mineral. Soc. Am. Spec. Paper 1969, 2, 31–50. [Google Scholar]
- Cameron, M.; Papike, J.J. Crystal chemistry of silicate pyroxenes. Rev. Miner. Geochem. 1982, 7, 5–92. [Google Scholar]
- Bissert, G.; Liebau, F. The crystal structure of a triclinic bikitaite, LiAlSi2O6 (H2O) with ordered Al/Si distribution. Neues Jahr. Mineral. Monats. 1986, 6, 241–252. [Google Scholar]
- Ståhl, K.; Kvick, Å.; Ghose, S. One-dimensional water chain in the zeolite bikitaite: Neutron diffractiuon study at 13 and 295 K. Zeolites 1989, 9, 303–311. [Google Scholar] [CrossRef]
- Quartieri, S.; Sani, A.; Vezzalini, G.; Galli, E.; Fois, E.; Gamba, A.; Tabacchi, G. One-dimensional ice in bikitaite: Single-crystal X-ray diffraction, infra-red spectroscopy and ab-initio molecular dynamics studies. Microporous Mesoporous Mater. 1999, 30, 77–87. [Google Scholar] [CrossRef]
- Appleman, D.E. The crystal structure of bikitaite LiAlSi2O6H2O. Acta Cryst. 1960, 13, 1002. [Google Scholar]
- Kocman, V.; Gait, R.I.; Rucklidge, J. The crystal structure of bikitaite Li[AlSi2O6]·H2O. Am. Mineral. 1974, 59, 71–78. [Google Scholar]
- Arlt, T.; Angel, R.J. Displacive phase transitions in C-centred clinopyroxenes: Spodumene, LiScSi2O6 and ZnSiO3. Phys. Chem. Min. 2000, 27, 719–731. [Google Scholar] [CrossRef]
- Thompson, R.M.; Downs, R.T. Model pyroxenes II: Structural variation as a function of tetrahedral rotation. Am. Mineral. 2004, 89, 614–628. [Google Scholar] [CrossRef]
- Petch, H.E.; Cranna, N.G.; Volkoff, G.M. Second order nuclear quadrupole effects in single crystals: Part II. Experimental results for spodumene. Can. J. Phys. 1953, 31, 837–858. [Google Scholar] [CrossRef]
- Kirkpatrick, R.J. MAS NMR spectroscopy of minerals and glasses. Rev. Miner. Geochem. 1988, 18, 341–403. [Google Scholar]
- Tagai, T.; Ried, H.; Joswig, W.; Korekawa, M. Kristallographische Untersunchungen eines Petalits mittels Neutronenbeugung und Transmissions-elektronenmikroskopic. Zeit. Kristall. 1982, 160, 159–170. [Google Scholar] [CrossRef]
- Haussühl, E.; Schreuer, J.; Winkler, B.; Haussühl, S.; Bayarjargal, L.; Milman, V. Structure-property relations and thermodynamic properties of monoclinic petalite, LiAlSi4O10. J. Phys. Cond. Matt. 2012, 24, 345402. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.S. The temperature-dependence of the volume expansivity and the thermal expansion tensor of petalite between 4.2 K and 600 K. J. Mineral. Petrol. Sci. 2014, 109, 118–1242. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Haussühl, E.; Eichler, H.J.; Hanuza, J.; Mᶏczka, M.; Yoneda, H.; Shirakawa, A. Lithium silicate, LiAlSiO4 (petalite)-a novel monoclinic SRS-active crystal. Laser Phys. Lett. 2015, 12, 085002. [Google Scholar] [CrossRef]
- Hurlbut, C.S. Bikitaite, LiAlSi2O6·H2O, a new mineral from Southern Rhodesia. Am. Mineral. 1957, 32, 792–797. [Google Scholar]
- Hamilton, D.L.; Henderson, C.M.B. The preparation of silicate compositions by a gelling method. Min. Mag. 1968, 36, 832–838. [Google Scholar] [CrossRef]
- Konar, B.; Kim, D.G.; Jung, I.H. Critical thermodynamic optimization of the Li2O-Al2O3-SiO2 system and its application for the thermodynamic analysis of glass-ceramics. J. Eur. Ceram. Soc. 2018, 38, 3881–3904. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.O.; Bujoli, B.; Gan, Z.H.; Hoatson, G. Modelling one and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Zemann-Hedlik, A.; Zemann, J. Die Kristallstruktur von Petalit, LiAlSi4O10. Acta Cryst. 1955, 8, 781–787. [Google Scholar] [CrossRef]
- Lowenstein, W. The distribution of aluminum in the tetrahedral of silicat es and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Stumpf-Nothof, K.; Weiden, N. 29Si-MAS-NMR spectra of keatite-type aluminosilicates. Zeit. Kristall. 1992, 200, 265–274. [Google Scholar] [CrossRef]
- Warren, B.E.; Biscoe, J. The crystal structure of monoclinic pyroxenes. Zeit. Krist. 1931, 80, 391–401. [Google Scholar] [CrossRef]
- Ross, N.L.; Zhao, J.; Slebodnick, C.; Spencer, E.C.; Chakoumakos, B.C. Petalite under pressure: Elastic behaviour and phase stability. Am. Mineral. 2015, 100, 714–721. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, L.; Sanz, J.; Sobrados, J.; Gan, Z.H. Medium-range-order in disordered K-feldspars by multinuclear NMR. Am. Mineral. 2013, 98, 2115–2131. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Muñoz, L.; Sanz, J.; Sobrados, I.; Gan, Z.H.; Santos, J.I. Medium-range order in crystal structures of minerals. In Applications of NMR Spectroscopy in the Solid State; Biblioteca de Ciencias 47; Consejo Superior de Investigaciones Científicas (CSIC): Madrid, Spain, 2019; pp. 103–131. [Google Scholar]
- Klinowski, J.; Ramdas, S.; Thomas, J.M. A re-examination of Si, Al ordering in zeolite NaX and NaY. J. Chem. Soc. Faraday Trans. 1982, 78, 1025–1050. [Google Scholar] [CrossRef]
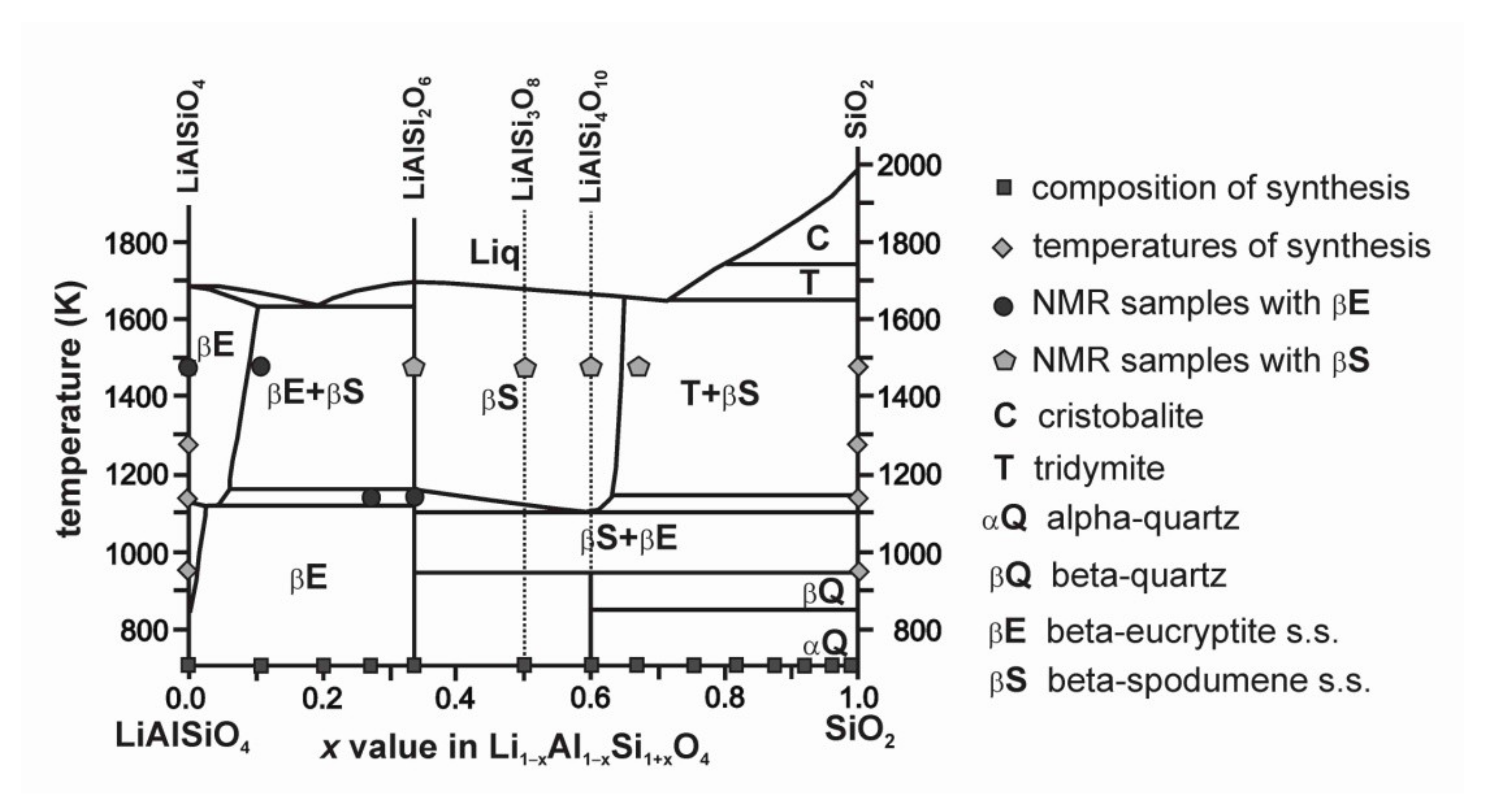
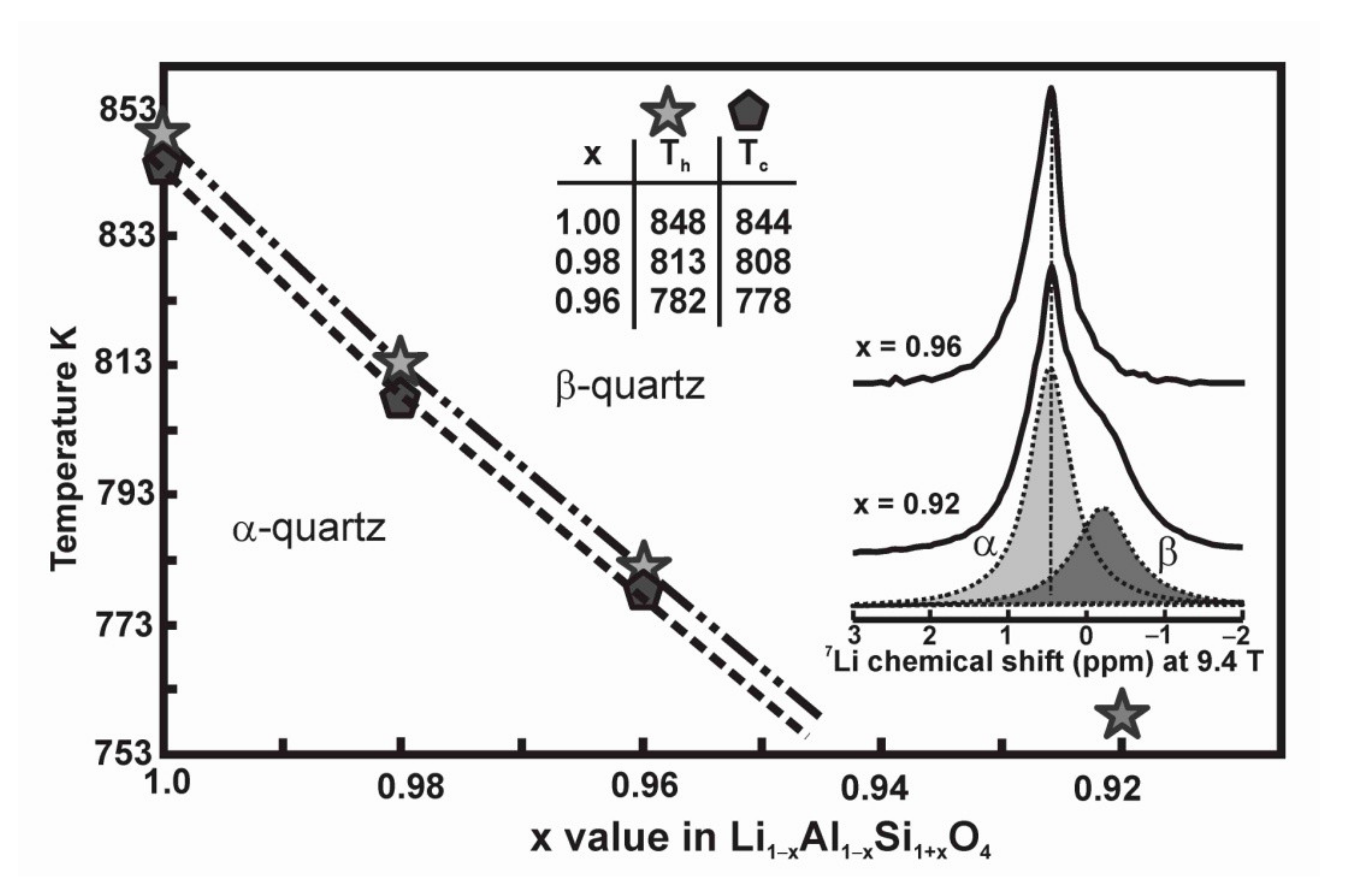
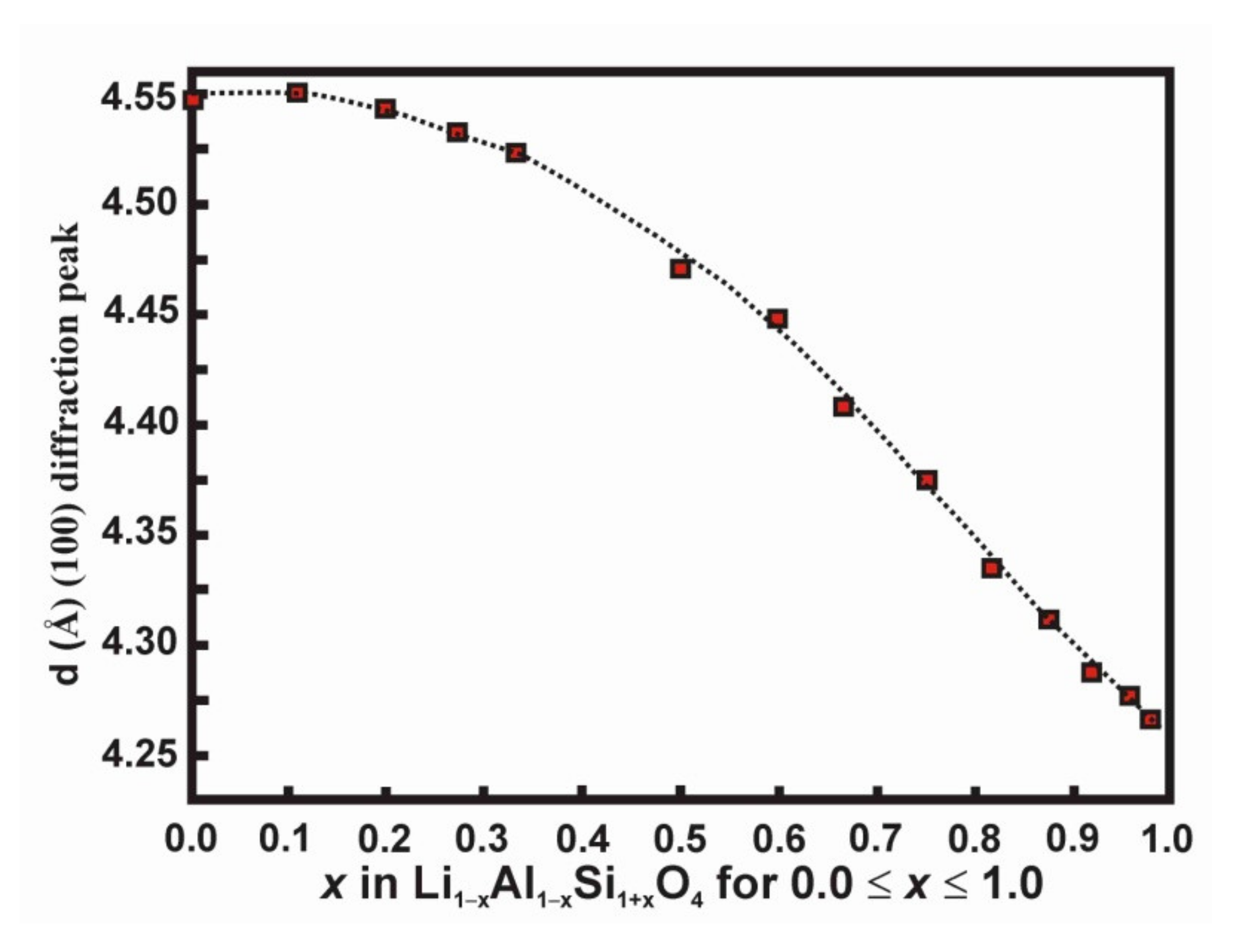




| Name | Formula | Space Group | Sites Si, Al, Li | Description | Ref. |
|---|---|---|---|---|---|
| α-cristobalite s.s. (*+) | SiO2 | P41212 | 1, 0, 0 | Natural and synthetic | [27] |
| α-quartz s.s. (*+) | SiO2 | P3221 | 1, 0, 0 | Natural and synthetic, low-temperature phase | [28] |
| β-quartz s.s. (*+) | SiO2 | P6422 | 1, 0, 0 | Natural and synthetic, high-temperature phase | [29] |
| P6222 | 1, 1, 1 | Synthetic, high-temperature phase, glass-ceramics, beta-quartz structure | [10] | ||
| β-eucryptite s.s. | SiO2-LiAlSiO4 | 2, 2, 3 | [12] | ||
| (+) | P6422 | 2, 2, 5 | [11] | ||
| 4, 4, 3 | [13] | ||||
| β-spodumene s.s. (+) | SiO2-LiAlSiO4 | P43212 | 2, 2, 1 | Synthetic, high-temperature phase, glass-ceramics, keatite structure | [23,24] |
| Petalite (*) | LiAlSi4O10 | P2/a | 2, 1, 1 | Typical of Li-rich granitic pegmatites | [30] [31] |
| Pa | 4, 1, 1 | ||||
| α-eucryptite (*) | LiAlSiO4 | R-3 | 2, 2, 1 2, 2, 2 | Typical of Li-rich granitic pegmatites with phenakite structure | [32] [33] |
| α-spodumene (*) | LiAlSi2O6 | C2/c C2 | 1, 1, 1 2, 2, 1 | Fibrous habit typical of Li-richgranitic pegmatites | [34] [35,36] |
| Bikitaite (*) | LiAlSi2O6·H2O | P1 P21 | 4, 2, 1 3, 2, 2 | Rare mineral from rare Li-richgranitic pegmatites | [37,38,39] [40,41] |
| Si/Al Ratio | x Value | Li1−xAl1−xSi1+xO4 | Formula | 675 °C, 100 h | 850 °C, 100 h | 1000 °C, 100 h | 1200 °C, 100 h |
|---|---|---|---|---|---|---|---|
| βS-βE(αQ)-C | βS-βE(αQ)-C | βS-βE(αQ)-C | βS-βE(αQ)-C | ||||
| 1/0 | 1.00 | Si2O4 | SiO2 | A | A | A | 0.0-0.0-100.0 |
| 100/1 | 0.98 | Li0.02Al0.02Si1.98O4 | LiAlSi100O202 | A | A + αQ | A + αQ | 0.0-1.3-98.7 |
| 50/1 | 0.96 | Li0.04Al0.04Si1.96O4 | LiAlSi50O102 | A | A + αQ | 0.0-92.9-7.1 + A | 0.0-12.3-87.7 |
| 25/1 | 0.92 | Li0.08Al0.08Si1.92O4 | LiAlSi25O52 | 0.0-100.0-0.0 | 0.0-100.0-0.0 | 9.3-81.3-9.4 | 8.3-30.9-60.8 |
| 15/1 | 0.875 | Li0.125Al0.125Si1.875O4 | LiAlSi15O32 | 0.0-100.0-0.0 | 0.0-100.0-0.0 | 1.4-64.7-33.9 | 3.9-33.6-62.5 |
| 10/1 | 0.818 | Li0.182Al0.182Si1.818O4 | LiAlSi10O22 | 0.0-100.0-0.0 | 0.0-100.0-0.0 | 3.3-67.2-29.6 | 11.6-45.1-43.3 |
| 7/1 | 0.75 | Li0.25Al0.25Si1.75O4 | LiAlSi7O16 | 0.0-100.0-0.0 | 5.9-75.7-18.4 | 8.1-74.2-17.7 | 35.7-46.8-17.6 |
| 5/1 | 0.667 | Li0.333Al0.333Si1.667O4 | LiAlSi5O12 | 0.0-100.0-0.0 | 74.1-23.9-2.1 | 91.0-9.0-0.0 | 100.0-0.0-0.0 |
| 4/1 | 0.60 | Li0.40Al0.40Si1.60O4 | LiAlSi4O10 | 35.4-64.4-0.0 | 56.8-8.9-34.3 | 81.5-17.5-0.0 | 100.0-0.0-0.0 |
| 3/1 | 0.50 | Li0.50Al0.50Si1.50O4 | LiAlSi3O8 | 27.7-72.3-0.0 | 47.2-46.4-6.4 | 68.6-31.4-0.0 | 100.0-0.0-0.0 |
| 2/1 | 0.333 | Li0.666Al0.666Si1.333O4 | LiAlSi2O6 | 0.0-100.0-0.0 | 7.1-92.9-0.0 | 24.9-75.1-0.0 | 100.0-0.0-0.0 |
| 1.75/1 (7/4) | 0.273 | Li0.727Al0.727Si1.273O4 | Li4Al4Si7O24 | 0.0-100.0-0.0 | 6.4-92.6-0.0 | 20.2-79.8-0.0 | 47.1-52.9-0.0 |
| 1.5/1 (3/2) | 0.20 | Li0.80Al0.80Si1.20O4 | Li2Al2Si3O10 | 30.2-18.9-27.3 | 7.3-92.7-0.0 | 48.8-51.6-0.0 | 37.3-62.7-0.0 |
| 1.25/1 (5/4) | 0.111 | Li0.889Al0.889Si1.111O4 | Li4Al4Si5O12 | 18.2-81.8-0.0 | 2.0-96.0-2.0 | 0.0-100.0-0.0 | 0.0-100.0-0.0 |
| 1/1 | 0 | LiAlSiO4 | LiAlSiO4 | 31.5–45.8–22.7 | 6.3-93.7-0.0 | 0.0-100.0-0.0 | 0.0-100.0-0.0 |
| Phase | x Value | % | δiso (ppm) | Δδiso (ppm) | G/L | CQ (kHz) | η |
|---|---|---|---|---|---|---|---|
| α-quartz s.s. | 0.92 | 70 30 | 0.5 −0.2 | 0.7 1.0 | 0.0 0.0 | 81.1 ± 0.37 26.6 ± 1.08 | 1.00 1.00 |
| 0.96 | 100 | 0.5 | 0.60 | 0.0 | 87.7 ± 0.38 | 0.61 ± 0.01 | |
| 0.98 | 100 | 0.5 | 0.37 | 0.0 | 93.1 ± 0.59 | 0.52 ± 0.02 | |
| β-eucryptite LiAlSiO4 | 0.00 | 100 | 0.0 | 2.38 | 0.43 | 95.4 ± 0.23 | 0.61 ± 0.01 |
| Phase | x Value | δ/% | (0Si,4Al) | (1Si,3Al) | (2Si,2Al) | (3Si,1Al) | (4Si,0Al) | Line Width | Barycentre |
|---|---|---|---|---|---|---|---|---|---|
| β-eucryptite | 0.000 (0.080) * | δ % | −90.9 59.42 | −96.5 28.14 | −103.1 8.37 | −108.4 4.06 | −114.9 0.00 | 3.6 ± 1.0 | −94.2 |
| 0.111 (0.200) * | δ % | −90.9 20.63 | −96.1 37.54 | −102.2 30.91 | −108.2 8.88 | −114.6 2.04 | 3.3 ± 0.8 | −98.3 | |
| 0.273 | δ % | −90.9 14.76 | −96.4 29.06 | −102.2 38.36 | −108.3 14.15 | −114.6 3.67 | 3.6 ± 1.0 | −100.2 | |
| 0.333 | δ % | −91.2 6.44 | −96.3 24.00 | −102.3 35.50 | −108.5 26.91 | −114.6 7.14 | 3.5 ± 1.0 | −102.7 | |
| β-spodumene | 0.333 | δ % | - 0.00 | −96.11 25.33 | −101.1 41.48 | −106.7 28.70 | −113.6 4.50 | 5.7 ± 0.7 | −102.1 |
| 0.500 | δ% | - 0.00 | −94.8 7.64 | −101.1 31.57 | −107.6 44.07 | −114.5 16.73 | 5.7 ± 0.1 | −105.7 | |
| 0.600 | δ% | - 0.00 | −95.4 3.80 | −101.6 23.44 | −107.9 42.61 | −114.5 30.15 | 5.5 ± 0.4 | −108.1 | |
| 0.667 | δ% | - 0.00 | −94.9 3.22 | −101.4 14.95 | −108.1 41.23 | −114.8 40.60 | 5.5 ± 0.1 | −109.4 |
| Phase | x Value | % | δiso (ppm) | Δδiso (ppm) | G/L | CQ (kHz) | η |
|---|---|---|---|---|---|---|---|
| β-eucryptite | 0.000 | 80.1 19.9 | 0.0 0.0 | 2.4 1.3 | 0.7 0.2 | 104.1 ± 0.4 20.7 ± 2.28 | 0.39 ± 0.00 0.60 ± 0.53 |
| 0.111 | 75.7 24.3 | −0.2 −0.2 | 2.9 1.2 | 0.6 0.9 | 90.9 ± 0.60 11.4 ± 0.24 | 0.50 ± 0.01 0.60 ± 0.68 | |
| 0.273 | 78.9 21.2 | −0.2 −0.2 | 2.5 1.0 | 0.6 1.0 | 91.5 ± 0.52 12.5 ± 2.54 | 0.48 ± 0.60 0.00 ± 0.40 | |
| 0.333 | 78.7 21.3 | −0.2 −0.2 | 2.4 1.1 | 0.6 0.9 | 88.6 ± 0.56 15.3 ± 0.92 | 0.49 ± 0.01 0.04 ± 1.15 | |
| β-spodumene | 0.333 | 88.6 11.4 | −0.3 −0.4 | 2.1 1.1 | 0.6 1.0 | 119.4 ± 0.43 21.0 ± 1.33 | 0.39 ± 0.4 0.60 ± 0.4 |
| 0.500 | 82.8 17.2 | −0.4 −0.4 | 1.7 1.0 | 0.6 0.0 | 118.8 ± 0.39 23.2 ± 2.95 | 0.38 ± 0.00 0.96 ± 0.61 | |
| 0.600 | 84.1 15.9 | −0.5 −0.5 | 1.6 0.9 | 0.6 0.3 | 116.5 ± 0.4 21.6 ± 1.35 | 0.41 ± 0.00 0.60 ± 0.25 | |
| 0.667 | 83.8 16.2 | −0.3 −0.3 | 1.5 0.9 | 0.6 0.2 | 115.4 ± 0.35 20.5 ± 1.32 | 0.42 ± 0.00 0.86 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Muñoz, L.; Sanz, J.; Florian, P.; Diez-Gómez, V.; Furio, M.; Sobrados, I. Order-Disorder in the Structures of Lithium Aluminosilicate Minerals by XRD and Multinuclear NMR. Minerals 2022, 12, 427. https://doi.org/10.3390/min12040427
Sánchez-Muñoz L, Sanz J, Florian P, Diez-Gómez V, Furio M, Sobrados I. Order-Disorder in the Structures of Lithium Aluminosilicate Minerals by XRD and Multinuclear NMR. Minerals. 2022; 12(4):427. https://doi.org/10.3390/min12040427
Chicago/Turabian StyleSánchez-Muñoz, Luis, Jesús Sanz, Pierre Florian, Virginia Diez-Gómez, Marta Furio, and Isabel Sobrados. 2022. "Order-Disorder in the Structures of Lithium Aluminosilicate Minerals by XRD and Multinuclear NMR" Minerals 12, no. 4: 427. https://doi.org/10.3390/min12040427
APA StyleSánchez-Muñoz, L., Sanz, J., Florian, P., Diez-Gómez, V., Furio, M., & Sobrados, I. (2022). Order-Disorder in the Structures of Lithium Aluminosilicate Minerals by XRD and Multinuclear NMR. Minerals, 12(4), 427. https://doi.org/10.3390/min12040427









