Crystallographic Characteristics of Inorganic Mineral in Mammoth Ivory and Ivory
Abstract
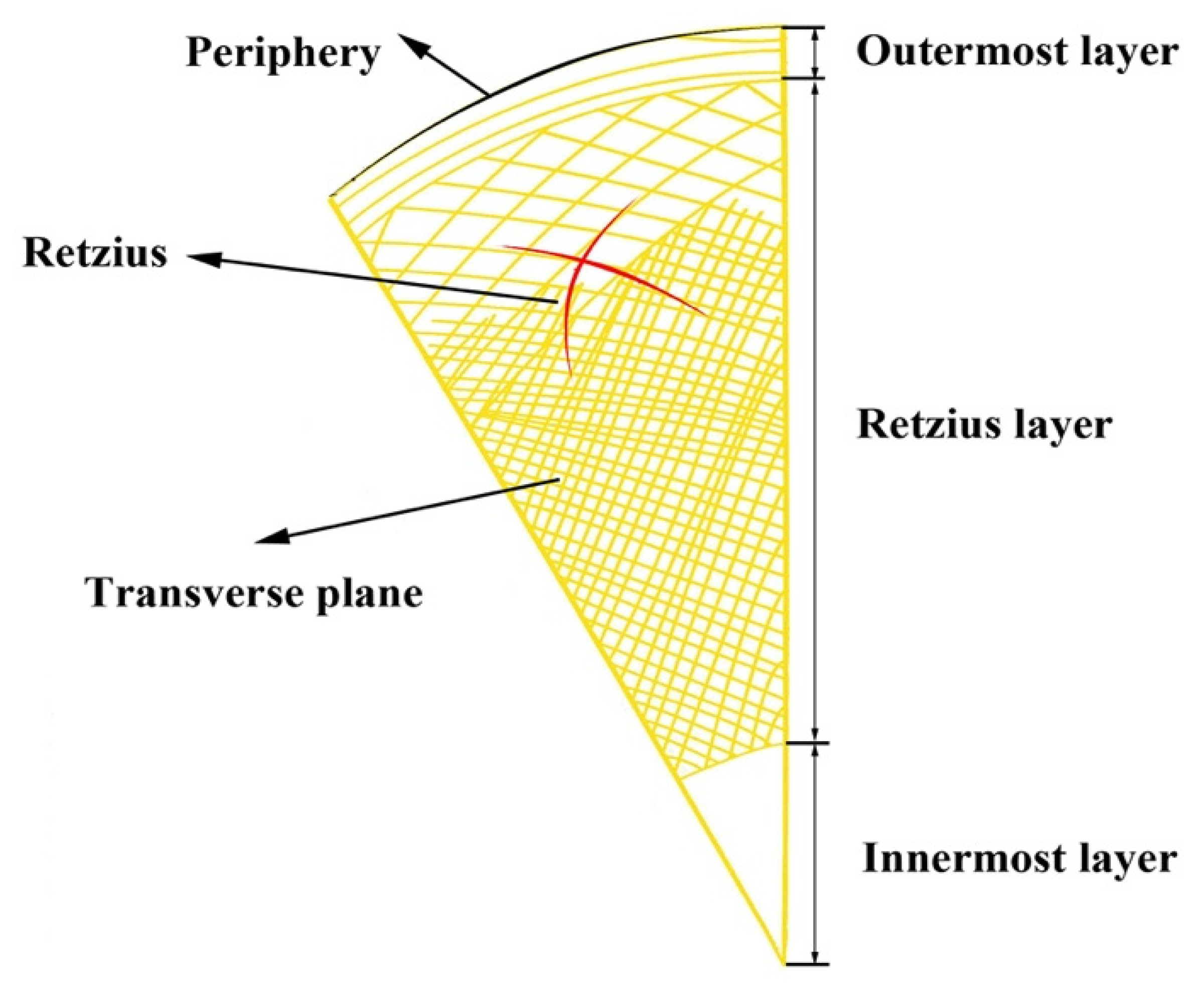
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. X-ray Diffraction
2.3. Scanning Electron Microscope and Energy Dispersive Spectroscopy
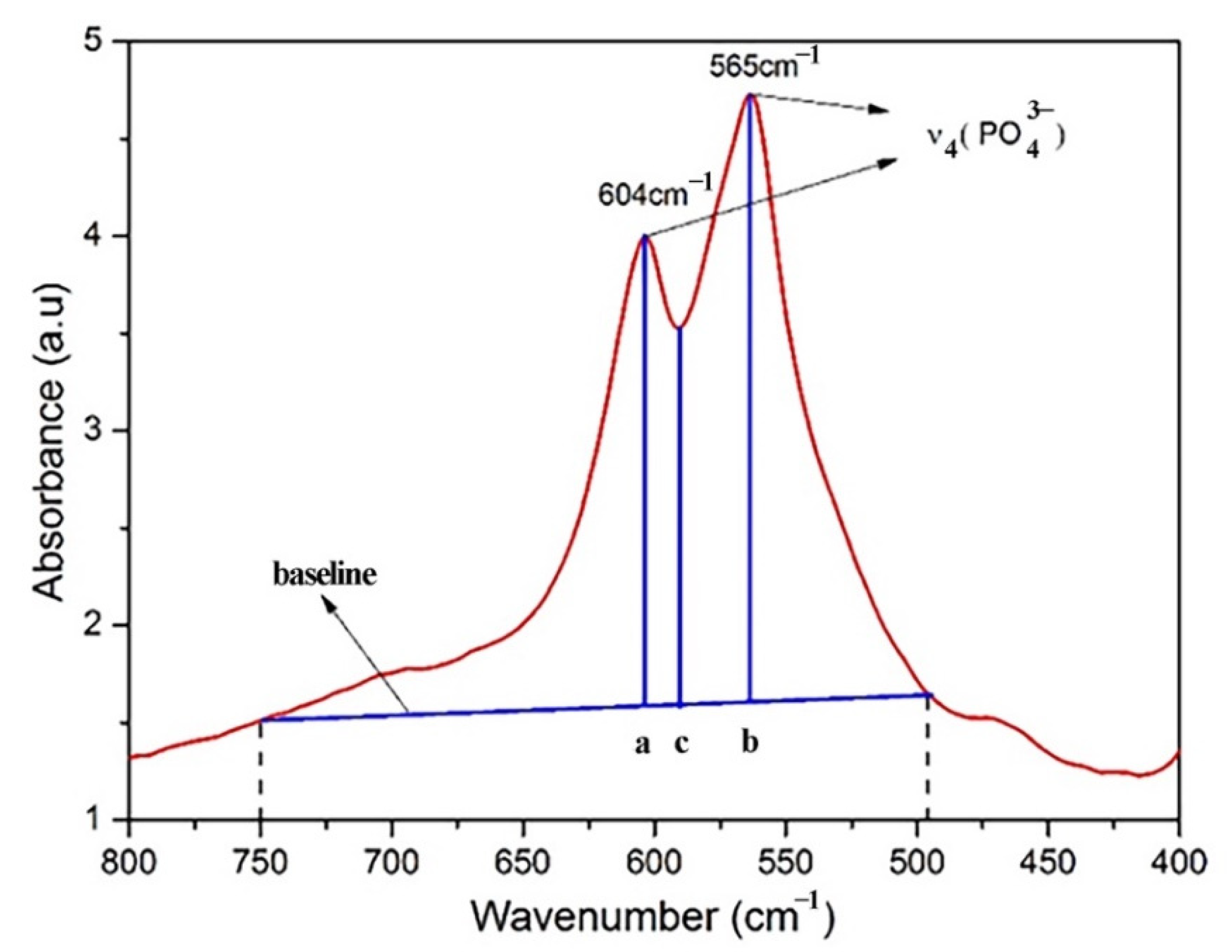
2.4. Infrared Spectrometry
3. Results and Discussion
3.1. Phase Identification
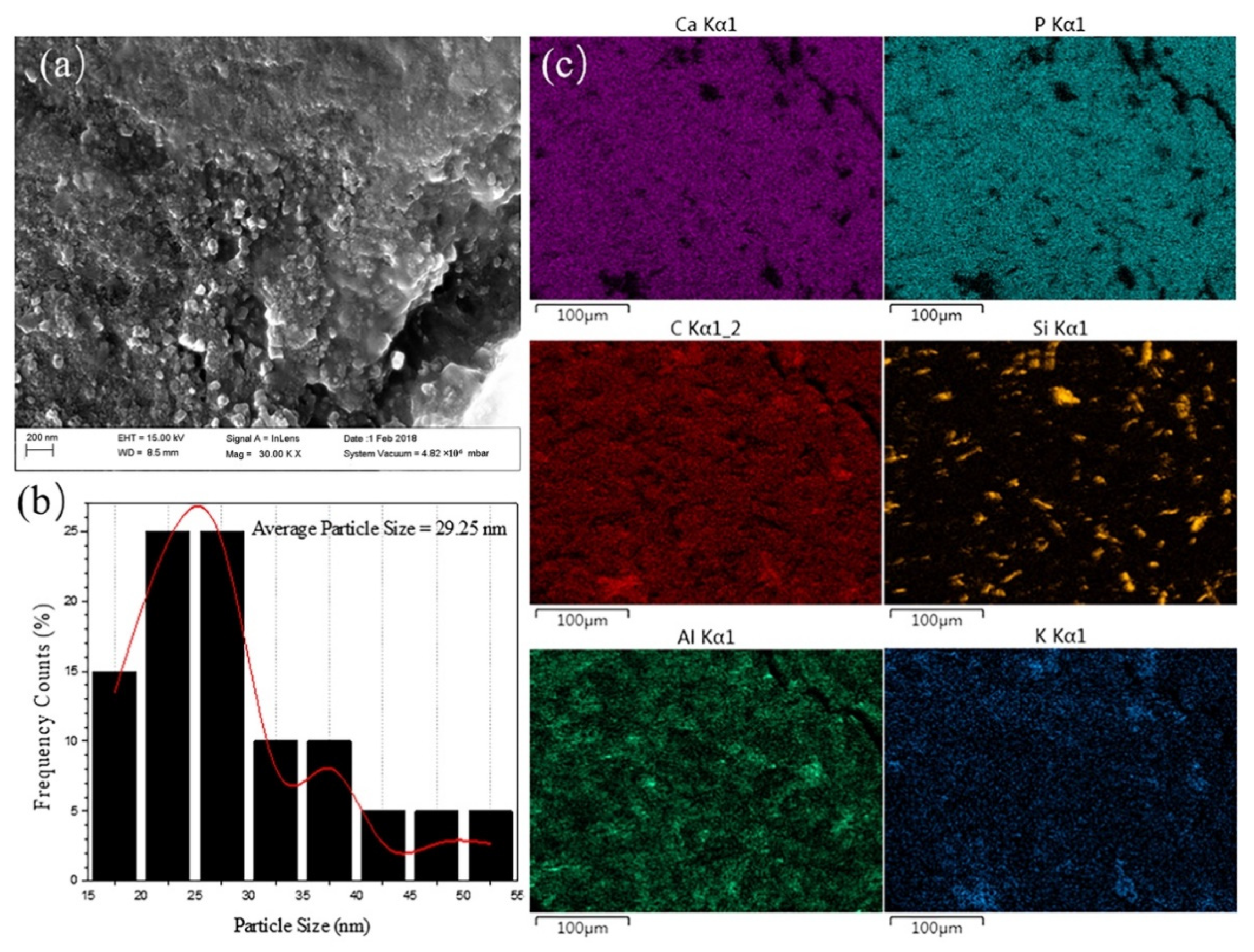
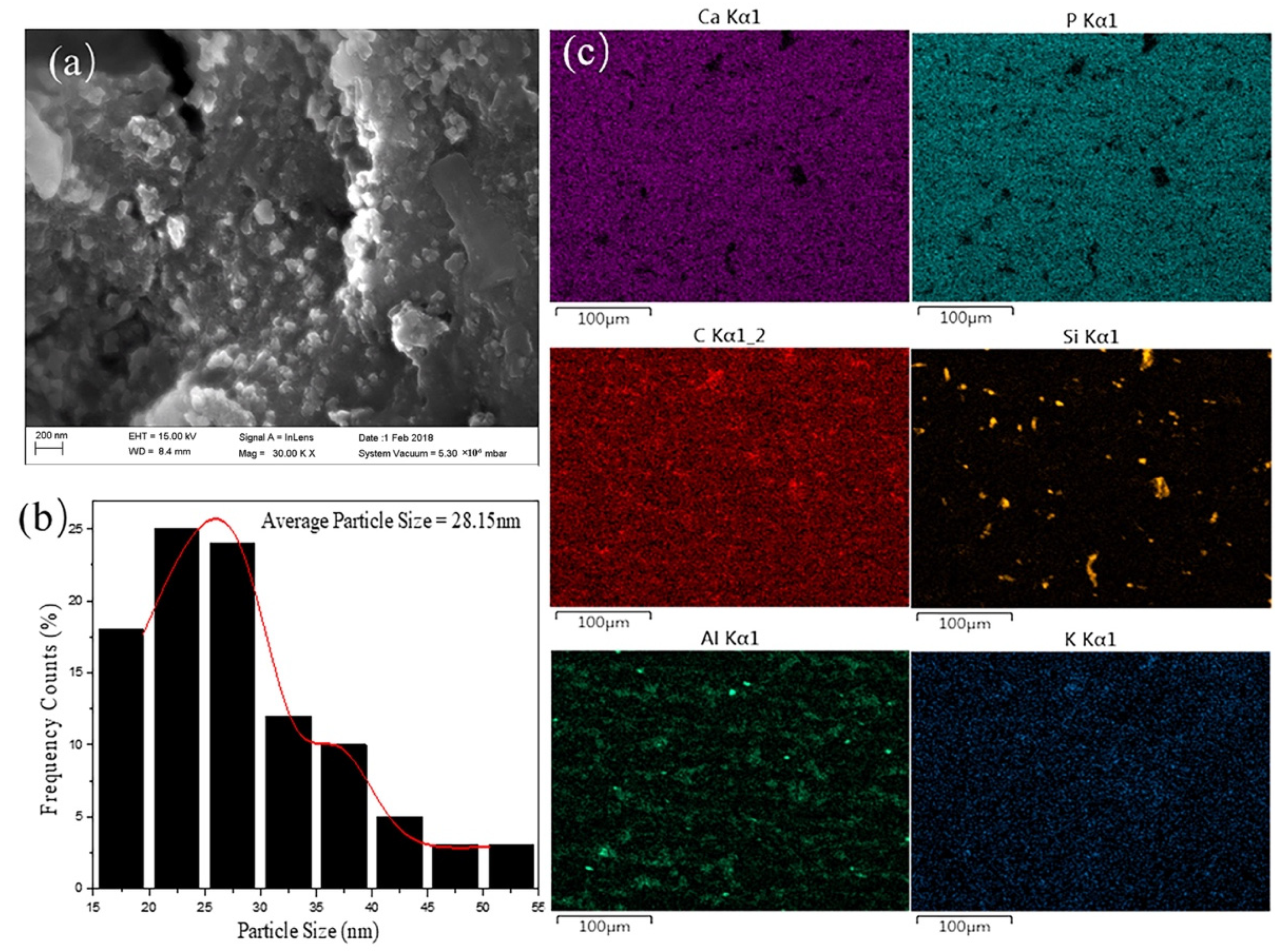
3.2. Elemental Analysis and Microstructure
3.3. Infrared Spectroscopy
3.4. Crystalline Characteristics
3.4.1. Crystal Size
3.4.2. Infrared Splitting Factor and Crystallinity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dove, P.M.; De Yoreo, J.J.; Weiner, S. Biomineralization; Mineralogical Society of America: Washington, DC, USA, 2003. [Google Scholar]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, 1st ed.; Oxford University Press: Oxford, UK, 2001; pp. 6–12, discussion 24–37. [Google Scholar]
- Cui, F.Z. Biomineralization, 1st ed.; Tsinghua University Press: Beijing, China, 2007. [Google Scholar]
- Bale, W.F. A Comparative Röntgen-Ray Diffraction Study of Several Natural Apatites and the Apatite-like Constituent of Bone and Tooth Substance. Am. J. Roentgenol. 1940, 43, 735–747. [Google Scholar]
- Hendricks, S.B.; Hill, W.L. The Nature of Bone and Phosphate Rock. Proc. Natl. Acad. Sci. USA 1950, 36, 731–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, K.E. Small But Extremely Tough. Science 2012, 336, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Currey, J. The design of mineralised hard tissues for their mechanical functions. J. Exp. Biol. 1999, 202, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A. An electron-microscopic study of the crystalline inorganic component of bone and its relationship to the organic matrix. J. Bone Joint Surg. 1952, 34, 389–435. [Google Scholar] [CrossRef] [PubMed]
- Johansen, E.; Parks, H.F. Electron Microscopic Observations on the Three-Dimensional Morphology of Apatite Crystallites of Human Dentine and Bone. J. Cell Biol. 1960, 7, 743–746. [Google Scholar] [CrossRef]
- Bocciarelli, D.S. Morphology of crystallites in bone. Calcif. Tissue Res. 1970, 5, 261–269. [Google Scholar] [CrossRef]
- Jackson, S.A.; Cartwright, A.G.; Lewis, D. The morphology of bone mineral crystals. Calcif. Tissue Res. 1978, 25, 217–222. [Google Scholar] [CrossRef]
- Weiner, S.; Price, P.A. Disaggregation of bone into crystals. Calcif. Tissue Int. 1986, 39, 365–375. [Google Scholar] [CrossRef]
- Landis, W.J.; Song, M.J.; Leith, A.L.; Mc Ewen, L.; Mc Ewen, B.F. Mineral and Organic Matrix Interaction in Normally Calcifying Tendon Visualized in Three Dimensions by High-Voltage Electron Microscopic Tomography and Graphic Image Reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Su, X.W.; Cui, F.Z. Hierarchical structure of ivory: From nanometer to centimeter. Mater. Sci. Eng. C 1999, 7, 19–29. [Google Scholar] [CrossRef]
- Locke, M. Structure of Ivory. J. Morphol. 2008, 269, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; He, M.Y.; Wu, J.L. Study of the Preferred Orientation of Hydroxyapatite in Ivory from Zimbabwe and Mammoth Ivory from Siberia. Crystals 2021, 11, 572. [Google Scholar] [CrossRef]
- Su, X.W.; Cui, F.Z. Direct Observations on Apatite Crystals in Ivory. J. Mater. Sci. Lett. 1997, 16, 1198–1200. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Dashti, A.; Nasiri, S.; Monshi, A.; Doustmohammadi, A.; Vilkauskas, A.; Janusas, G. X-ray Diffraction Analysis and Williamson-Hall Method in USDM Model for Estimating More Accurate Values of Stress-Strain of Unit Cell and Super Cells (2 × 2 × 2) of Hydroxyapatite, Confirmed by Ultrasonic Pulse-Echo Test. Materials 2021, 14, 2949. [Google Scholar] [CrossRef]
- Holcomb, D.W.; Young, R.A. Thermal decomposition of human tooth enamel. Calcif. Tissue Int. 1980, 31, 189–201. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Apatites in biological systems. Prog. Cryst. Growth Charact. 1981, 4, 1–45. [Google Scholar] [CrossRef]
- Young, R.A.; Wiles, D.B. Application of the Rietveld Method for Structure Refinement with Powder Diffraction Data. Adv. X-ray Anal. 1980, 24, 1–23. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectrum of Minerals; Science Press: Beijing, China, 1982. [Google Scholar]
- Rehman, I.; Smith, R.; Hench, L.L.; Bonfield, W. Structural evaluation of human and sheep bone and comparison with synthetic hydroxyapatite by FT-Raman spectroscopy. J. Biomed. Mater. Res. 1995, 29, 1287–1294. [Google Scholar] [CrossRef]
- Vignoles, M.; Bonel, G.; Holcomb, D.W.; Young, R.A. Influence of preparation conditions on the composition of type B carbonated hydroxyapatite and on the localization of the carbonate ions. Calcif. Tissue Res. 1988, 43, 33–40. [Google Scholar] [CrossRef]
- Ito, A.; Aoki, H.; Akao, M.; Teraoka, K.; Tsutsumi, S.; Onuma, K.; Tateishi, T. Hydrothermal Growth of Carbonate-containing Hydroxyapatite Single Crystals. J. Cryst. Growth. 1996, 163, 1311–1317. [Google Scholar] [CrossRef]
- Michel, V.; Ildefonse, P.; Morin, G. Chemical and Structural Changes in Cervuselaphus Tooth Enamels during Fossilization (Lazaret Cave): A Combined IR and XRD Rietveld Analysis. Appl. Geochem. 1995, 10, 145–159. [Google Scholar] [CrossRef]
- Rey, C.; Renugopalakrishnan, V.; Shimizu, M.; Collins, B.; Glimcher, M.J. A Resolution-enhanced Fourier Transform Spectroscopic Study of the Environment of the CO32- ion in the Mineral Phase of Enamel during its Formation and Maturation. Calcif. Tissue Int. 1991, 49, 259–268. [Google Scholar] [CrossRef]
- Sillen, A.; Sealy, J.C. Diagenesis of Strontium in Fossil Bone: A Reconsideration of Nelson et al. (1986). J. Archaeol. Sci. 1995, 22, 313–320. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing Methods for Calculating Nano Crystal Size of Natural Hydroxyapatite Using X-Ray Diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Termine, J.D.; Posner, A.S. Infrared Analysis of Rat Bone: Age Dependency of Amorphous and Crystalline Mineral Fractions. Science 1966, 153, 1523–1525. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Tung, M.S. Chemical Stability of Carbonate- and Fluoride-Containing Apatites. Caries Res. 1983, 17, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the Near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Stutman, J.M.; Termine, J.D.; Posner, A.S. Vibrational spectra and structure of the phosphate ion in some calcium phosphates. Trans. N. Y. Acad. Sci. 1965, 27, 669–675. [Google Scholar] [CrossRef]
- Eanes, E.D.; Posner, A.S. Division of Biophysics Kinetics and Mechanism of Conversion of Noncrystalline Calcium Phosphate to Crystalline Hydroxyapatite. Trans. N. Y. Acad. Sci. 1965, 28, 233–241. [Google Scholar] [CrossRef]

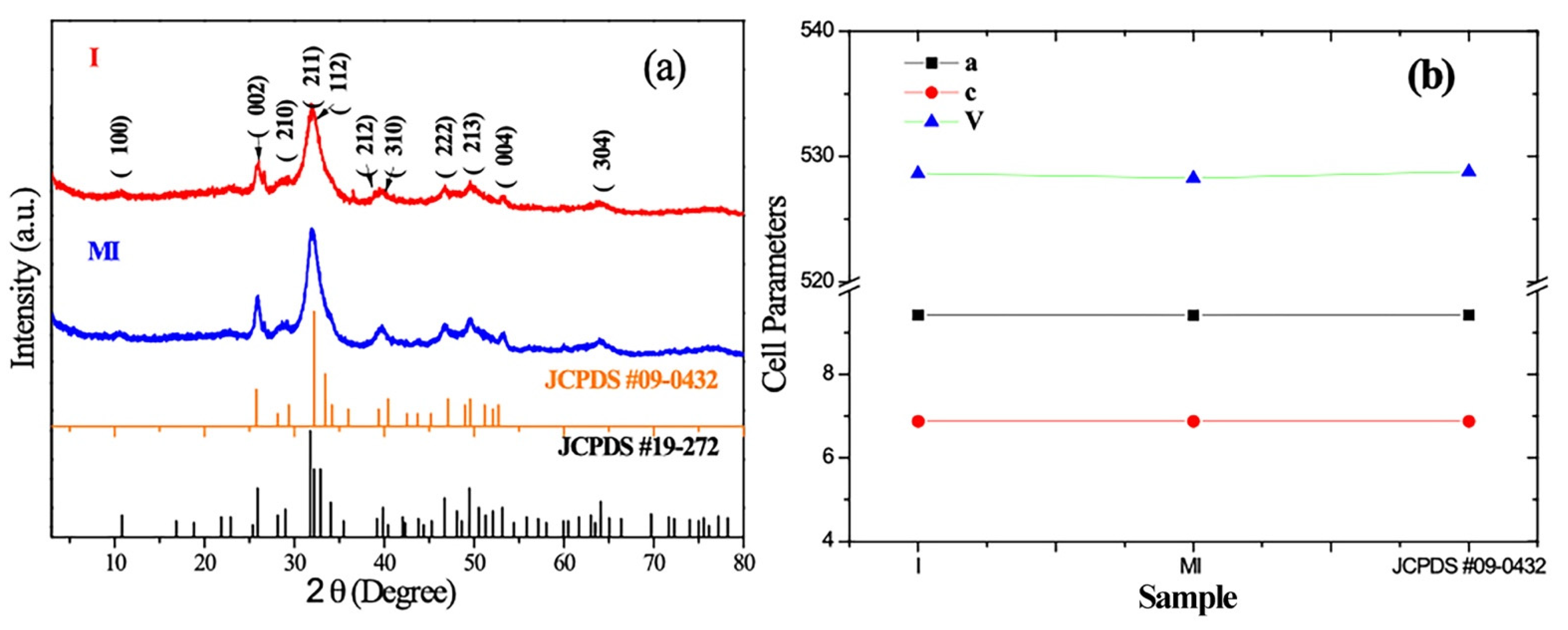


| a/Å | σa | c/Å | σc | V/Å3 | σV | |
|---|---|---|---|---|---|---|
| I | 9.4166 | 0.0195 | 6.8841 | 0.0006 | 528.65 | 1.0981 |
| MI | 9.4148 | 0.0178 | 6.8821 | 0.0006 | 528.29 | 0.9996 |
| JCPDS #09-0432 | 9.418 | 6.884 | 528.8 | |||
| Title | wB/% | Ca/P | Ca′/P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Ca | P | Mg | Na | ||||
| MI | OL | 36.9 | 37.4 | 13.8 | 7.8 | 1.0 | 0.4 | 1.37 | 1.60 |
| RL | 32.9 | 42.5 | 14.6 | 7.7 | 0.7 | 0.4 | 1.47 | 1.65 | |
| I | OL | 37.0 | 40.7 | 11.0 | 6.7 | 1.2 | 0.6 | 1.27 | 1.62 |
| RL | 30.0 | 38.1 | 13.2 | 8.5 | 1.5 | 0.5 | 1.20 | 1.50 | |
| IR Absorption Bands | Positions (cm−1) | |
|---|---|---|
| MI | I | |
| ν(C=O) amide I | 1660 | 1661 |
| H2O | 1630 | 1631 |
| δ(N-H); ν(C-H) amide Ⅱ | 1557 | 1558 |
| ν3 (CO) | 1551 | 1552 |
| 1454 | 1456 | |
| 1412 | 1411 | |
| δ(N-H); ν(C-N) amide Ⅲ | 1239 | 1239 |
| ν3 (PO) | 1104 | 1106 |
| 1038 | 1036 | |
| ν1 (PO) | 960 | 959 |
| ν2 (CO) | 880 | 880 |
| 873 | 871 | |
| ν4 (PO) | 608 | 607 |
| 566 | 569 | |
| Bands | Mammoth Ivory | Ivory from an Elephant | |||
|---|---|---|---|---|---|
| cm−1 | Area | cm−1 | Area | ||
| ν3 (C-O) | A | 1551 | 6.35 | 1552 | 5.69 |
| B | 1412 | 5.01 | 1411 | 3.28 | |
| ν2 (C-O) | A | 880 | 27.72 | 880 | 19.88 |
| B | 873 | 8.56 | 872 | 11.53 | |
| ν4 (P-O) | 566 | 7.19 | 569 | 6.49 | |
| Area ratios | A1551/A566 | 0.883 | 0.877 | ||
| A1412/A566 | 0.697 | 0.505 | |||
| A880/A566 | 3.063 | 3.855 | |||
| A873/A566 | 1.776 | 1.191 | |||
| θ/(°) | FWHM ((002)) | β/rad | L(002)/nm | ||
|---|---|---|---|---|---|
| Ivory from an elephant | TP | 12.96 | 0.375 | 0.006545 | 21.50 |
| CP | 12.96 | 0.399 | 0.006964 | 20.20 | |
| RP | 12.96 | 0.426 | 0.007435 | 18.92 | |
| Mammoth ivory | TP | 12.94 | 0.348 | 0.006074 | 23.16 |
| CP | 12.96 | 0.392 | 0.006842 | 20.56 | |
| RP | 12.96 | 0.395 | 0.006894 | 20.41 | |
| Sample | Sample Number | SF | C |
|---|---|---|---|
| Ivory from an elephant | MI-IL | 3.17 | 1.55 |
| MI-RL-1 * | 3.23 | 1.58 | |
| MI-RL-2 * | 3.26 | 1.59 | |
| MI-RL-3 * | 3.12 | 1.52 | |
| MI-RL-4 * | 3.16 | 1.54 | |
| Mammoth Ivory | I-IL | 1551 | 1.58 |
| I-RL-1 * | 1454 | 1.59 | |
| I-RL-2 * | 1412 | 1.52 | |
| I-RL-3 * | 1239 | 1.54 | |
| Fluorapatite | 20.5 | 10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; He, M.; Wu, J. Crystallographic Characteristics of Inorganic Mineral in Mammoth Ivory and Ivory. Minerals 2022, 12, 117. https://doi.org/10.3390/min12020117
Sun X, He M, Wu J. Crystallographic Characteristics of Inorganic Mineral in Mammoth Ivory and Ivory. Minerals. 2022; 12(2):117. https://doi.org/10.3390/min12020117
Chicago/Turabian StyleSun, Xueying, Mingyue He, and Jinlin Wu. 2022. "Crystallographic Characteristics of Inorganic Mineral in Mammoth Ivory and Ivory" Minerals 12, no. 2: 117. https://doi.org/10.3390/min12020117
APA StyleSun, X., He, M., & Wu, J. (2022). Crystallographic Characteristics of Inorganic Mineral in Mammoth Ivory and Ivory. Minerals, 12(2), 117. https://doi.org/10.3390/min12020117






