Neptunium and Uranium Interactions with Environmentally and Industrially Relevant Iron Minerals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Synthesis
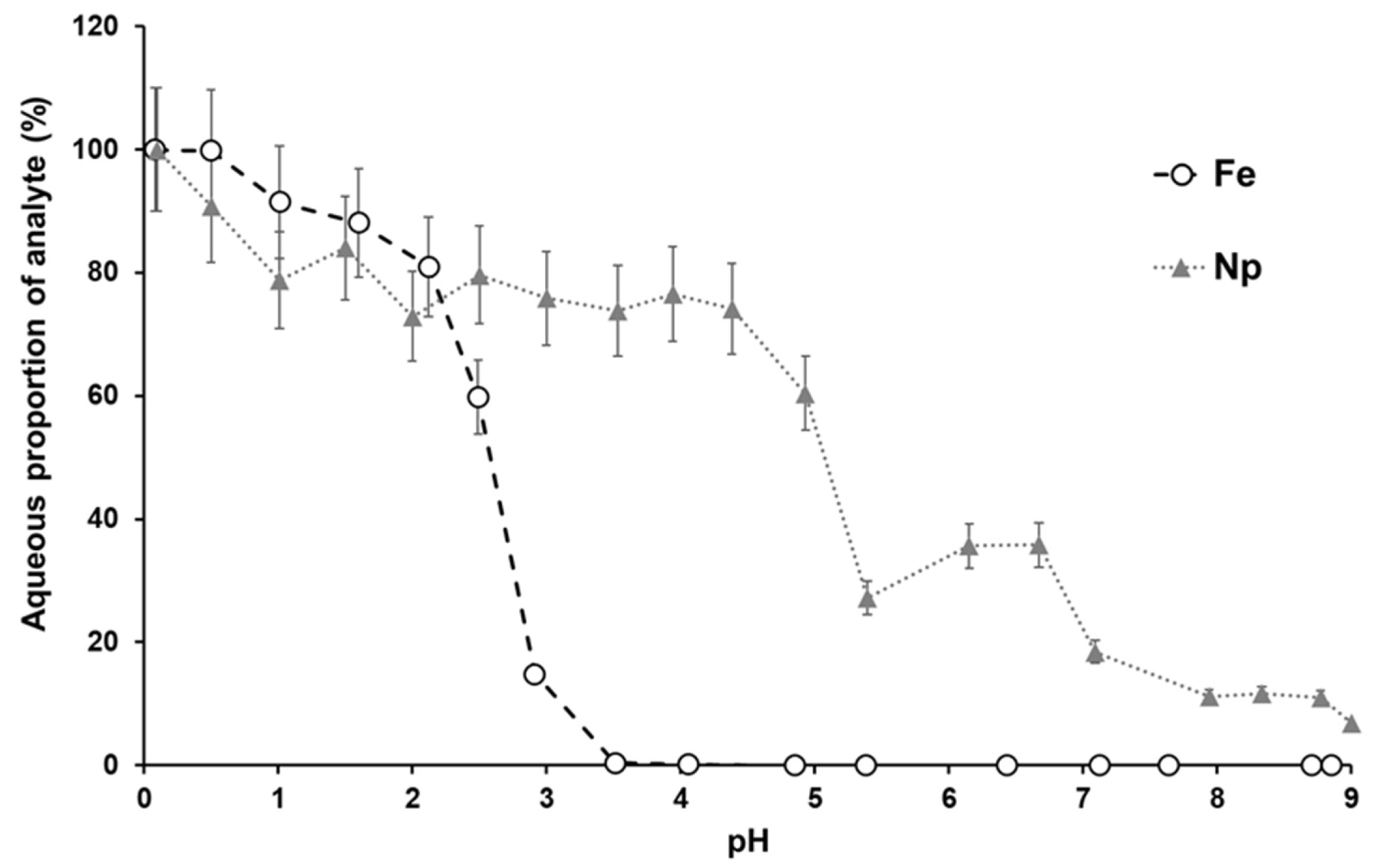
2.2. Adsorption Experiments
2.3. Np(V) and Fe(III) Co-Precipitation Experiment
2.4. X-ray Absorption Spectroscopy
3. Results and Discussion
3.1. Np(V) and Fe(III) Co-Precipitation Experiment Geochemical Data
3.2. LIII-Edge XAS Data
3.2.1. Np(V)-Neptunyl Sorbed to Ferrihydrite and Goethite
3.2.2. Np(V) and U(VI) with FeS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaszuba, J.P.; Runde, W.H. The Aqueous Geochemistry of Neptunium: Dynamic Control of Soluble Concentrations with Applications to Nuclear Waste Disposal. Environ. Sci. Technol. 1999, 33, 4427–4433. [Google Scholar] [CrossRef]
- Thompson, R.C. Neptunium: The Neglected Actinide: A Review of the Biological and Environmental Literature. Radiat. Res. 1982, 90, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Law, G.T.W.; Geissler, A.; Lloyd, J.R.; Livens, F.R.; Boothman, C.; Begg, J.D.C.; Denecke, M.A.; Rothe, J.; Dardenne, K.; Burke, I.T.; et al. Geomicrobiological Redox Cycling of the Transuranic Element Neptunium. Environ. Sci. Technol. 2010, 44, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Newsome, L.; Morris, K.; Lloyd, J.R. The Biogeochemistry and Bioremediation of Uranium and Other Priority Radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Thorpe, C.L.; Morris, K.; Lloyd, J.R.; Denecke, M.A.; Law, K.A.; Dardenne, K.; Boothman, C.; Bots, P.; Law, G.T.W. Neptunium and Manganese Biocycling in Nuclear Legacy Sediment Systems. Appl. Geochem. 2015, 63, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Nenot, J.C. Metabolism and Toxicity of Neptunium; No. AAEC-LIB/TRANS--780; Australian Atomic Energy Commission Research Establishment: Lucas Heights, Australia, 1983; p. 18. [Google Scholar]
- Lieser, K.; Mühlenweg, U. Neptunium in the Hydrosphere and in the Geosphere: I. Chemistry of Neptunium in the Hydrosphere and Sorption of Neptunium from Groundwaters on Sediments under Aerobic and Anaerobic Conditions. Radiochim. Acta 1988, 43, 27–36. [Google Scholar] [CrossRef]
- Wilson, P.D. The Nuclear Fuel Cycle from Ore to Wastes; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Smith, K.F.; Morris, K.; Law, G.T.W.; Winstanley, E.H.; Livens, F.R.; Weatherill, J.S.; Abrahamsen-Mills, L.G.; Bryan, N.D.; Mosselmans, J.F.W.; Cibin, G.; et al. Plutonium(Iv) Sorption During Ferrihydrite Nanoparticle Formation. ACS Earth Space Chem. 2019, 3, 2437–2442. [Google Scholar] [CrossRef] [Green Version]
- Winstanley, E.H.; Morris, K.; Abrahamsen-Mills, L.G.; Blackham, R.; Shaw, S. U(Vi) Sorption During Ferrihydrite Formation: Underpinning Radioactive Effluent Treatment. J. Hazard. Mater. 2019, 366, 98–104. [Google Scholar] [CrossRef]
- Li, D.; Kaplan, D.I. Sorption Coefficients and Molecular Mechanisms of Pu, U, Np, Am and Tc to Fe (Hydr)Oxides: A Review. J. Hazard. Mater. 2012, 243, 1–18. [Google Scholar] [CrossRef]
- Bots, P.; Shaw, S.; Law, G.T.W.; Marshall, T.A.; Mosselmans, J.F.W.; Morris, K. Controls on the Fate and Speciation of Np(V) During Iron (Oxyhydr)Oxide Crystallization. Environ. Sci. Technol. 2016, 50, 3382–3390. [Google Scholar] [CrossRef] [Green Version]
- Bots, P.; van Veelen, A.; Mosselmans, F.W.J.; Muryn, C.; Wogelius, A.R.; Morris, K. Neptunium(V) and Uranium(Vi) Reactions at the Magnetite (111) Surface. Geosciences 2019, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Powell, B.A.; Zhang, S.; Rao, L. Surface Complexation Modeling of Neptunium (V) Sorption to Lepidocrocite (Γ-Feooh). Radiochim. Acta 2015, 103, 707–717. [Google Scholar] [CrossRef]
- Arai, Y.; Moran, P.; Honeyman, B.; Davis, J. In Situ Spectroscopic Evidence for Neptunium (V)-Carbonate Inner-Sphere and Outer-Sphere Ternary Surface Complexes on Hematite Surfaces. Environ. Sci. Technol. 2007, 41, 3940–3944. [Google Scholar] [CrossRef] [PubMed]
- Combes, J.M.; Chisholm-Brause, C.J.; Brown, G.E.; Parks, G.A.; Conradson, S.D.; Eller, P.G.; Triay, I.R.; Hobart, D.E.; Miejer, A. Exafs Spectroscopic Study of Neptunium(V) Sorption at the A-Feooh/Water Interface. Environ. Sci. Technol. 1992, 26, 376–382. [Google Scholar] [CrossRef]
- Livens, F.R.; Jones, M.J.; Hynes, A.J.; Charnock, J.M.; Mosselmans, J.F.W.; Hennig, C.; Steele, H.; Collison, D.; Vaughan, D.J.; Pattrick, R.A.D.; et al. X-ray Absorption Spectroscopy Studies of Reactions of Technetium, Uranium and Neptunium with Mackinawite. J. Environ. Radioact. 2004, 74, 211–219. [Google Scholar] [CrossRef]
- Moyes, L.N.; Jones, M.J.; Reed, W.A.; Livens, F.R.; Charnock, J.M.; Mosselmans, J.F.W.; Hennig, C.; Vaughan, D.J.; Pattrick, R.A.D. An X-ray Absorption Spectroscopy Study of Neptunium(V) Reactions with Mackinawite (Fes). Environ. Sci. Technol. 2002, 36, 179–183. [Google Scholar] [CrossRef]
- Müller, K.; Gröschel, A.; Rossberg, A.; Bok, F.; Franzen, C.; Brendler, V.; Foerstendorf, H. In Situ Spectroscopic Identification of Neptunium (V) Inner-Sphere Complexes on the Hematite–Water Interface. Environ. Sci. Technol. 2015, 49, 2560–2567. [Google Scholar] [CrossRef]
- Nakata, K.; Nagasaki, S.; Tanaka, S.; Sakamoto, Y.; Tanaka, T.; Ogawa, H. Reduction Rate of Neptunium (V) in Heterogeneous Solution with Magnetite. Radiochim. Acta 2004, 92, 145–150. [Google Scholar] [CrossRef]
- Roberts, H.E.; Morris, K.; Mosselmans, J.F.W.; Law, G.T.W.; Shaw, S. Neptunium Reactivity During Co-Precipitation and Oxidation of Fe(II)/Fe(III) (Oxyhydr)Oxides. Geosciences 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.D.; Demond, A.H. Permeability of Iron Sulfide (Fes)-Based Materials for Groundwater Remediation. Water Res. 2013, 47, 1267–1276. [Google Scholar] [CrossRef]
- Brookshaw, D.R.; Pattrick, R.A.; Bots, P.; Law, G.T.; Lloyd, J.R.; Mosselmans, J.F.W.; Vaughan, D.J.; Dardenne, K.; Morris, K. Redox Interactions of Tc (Vii), U (Vi), and Np (V) with Microbially Reduced Biotite and Chlorite. Environ. Sci. Technol. 2015, 49, 13139–13148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandratos, V.G.; Behrends, T.; Van Cappellen, P. Sulfidization of Lepidocrocite and Its Effect on Uranium Phase Distribution and Reduction. Geochim. Cosmochim. Acta 2014, 142, 570–586. [Google Scholar] [CrossRef]
- Alexandratos, V.G.; Behrends, T.; Van Cappellen, P. Fate of Adsorbed U(Vi) During Sulfidization of Lepidocrocite and Hematite. Environ. Sci. Technol. 2017, 51, 2140–2150. [Google Scholar] [CrossRef]
- Townsend, L.T.; Shaw, S.; Ofili, N.E.R.; Kaltsoyannis, N.; Walton, A.S.; Mosselmans, J.F.W.; Neill, T.S.; Lloyd, J.R.; Heath, S.; Hibberd, R.; et al. Formation of a U(Vi)–Persulfide Complex During Environmentally Relevant Sulfidation of Iron (Oxyhydr)Oxides. Environ. Sci. Technol. 2020, 54, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Lan, J.; Li, M.; Hu, W.; Liu, H.; Song, G.; Chen, D.; Shi, W.; Wang, X. Influence of Aqueous Sulfide on Speciation of U(Vi) Adsorbed to Nanomagnetite. Environ. Sci. Nano 2018, 5, 1981–1989. [Google Scholar] [CrossRef]
- Gallegos, T.J.; Fuller, C.C.; Webb, S.M.; Betterton, W. Uranium(Vi) Interactions with Mackinawite in the Presence and Absence of Bicarbonate and Oxygen. Environ. Sci. Technol. 2013, 47, 7357–7364. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cha, W.S.; Kim, J.-G.; Baik, M.H.; Jung, E.C.; Jeong, J.T.; Kim, K.; Chung, S.Y.; Lee, Y.J. Uranium(Iv) Remobilization under Sulfate Reducing Conditions. Chem. Geol. 2014, 370, 40–48. [Google Scholar] [CrossRef]
- Hyun, S.P.; Davis, J.A.; Sun, K.; Hayes, K.F. Uranium(Vi) Reduction by Iron(Ii) Monosulfide Mackinawite. Environ. Sci. Technol. 2012, 46, 3369–3376. [Google Scholar] [CrossRef]
- Veeramani, H.; Scheinost, A.C.; Monsegue, N.; Qafoku, N.P.; Kukkadapu, R.; Newville, M.; Lanzirotti, A.; Pruden, A.; Murayama, M.; Hochella, M.F. Abiotic Reductive Immobilization of U(Vi) by Biogenic Mackinawite. Environ. Sci. Technol. 2013, 47, 2361–2369. [Google Scholar] [CrossRef]
- Hua, B.; Deng, B. Reductive Immobilization of Uranium(Vi) by Amorphous Iron Sulfide. Environ. Sci. Technol. 2008, 42, 8703–8708. [Google Scholar] [CrossRef]
- Moyes, L.N.; Parkman, R.H.; Charnock, J.M.; Vaughan, D.J.; Livens, F.R.; Hughes, C.R.; Braithwaite, A. Uranium Uptake from Aqueous Solution by Interaction with Goethite, Lepidocrocite, Muscovite, and Mackinawite: An X-ray Absorption Spectroscopy Study. Environ. Sci. Technol. 2000, 34, 1062–1068. [Google Scholar] [CrossRef]
- Ofili, N.E.R.; Thetford, A.; Kaltsoyannis, N. Adsorption of U(Vi) on Stoichiometric and Oxidised Mackinawite: A Dft Study. Environ. Sci. Technol. 2020, 54, 6792–6799. [Google Scholar] [CrossRef] [PubMed]
- Weatherill, J.S.; Morris, K.; Bots, P.; Stawski, T.M.; Janssen, A.; Abrahamsen, L.; Blackham, R.; Shaw, S. Ferrihydrite Formation: The Role of Fe13 Keggin Clusters. Environ. Sci. Technol. 2016, 50, 9333–9342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waite, T.D.; Davis, J.A.; Payne, T.E.; Waychunas, G.A.; Xu, N. Uranium(Vi) Adsorption to Ferrihydrite: Application of a Surface Complexation Model. Geochim. Cosmochim. Acta 1994, 58, 5465–5478. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. Synthesis. In The Iron Oxides; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2004; pp. 525–540. [Google Scholar]
- Stookey, L.L. Ferrozine—A New Spectrophotometric Reagent for Iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; Van Cappellen, P. The Ferrozine Method Revisited: Fe(Ii)/Fe(Iii) Determination in Natural Waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Ohfuji, H.; Rickard, D. High Resolution Transmission Electron Microscopic Study of Synthetic Nanocrystalline Mackinawite. Earth Planet. Sci. Lett. 2006, 241, 227–233. [Google Scholar] [CrossRef]
- Fonselius, S.; Dyrssen, D.; Yhlen, B. Determination of Hydrogen Sulphide. In Methods of Seawater Analysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; pp. 91–100. [Google Scholar]
- Townsend, L.T.; Morris, K.; Harrison, R.; Schacherl, B.; Vitova, T.; Kovarik, L.; Pearce, C.I.; Mosselmans, J.F.W.; Shaw, S. Sulfidation of Magnetite with Incorporated Uranium. Chemosphere 2021, 276, 130117. [Google Scholar] [CrossRef]
- Diaz-Moreno, S.; Hayama, S.; Amboage, M.; Freeman, A.; Sutter, J.; Duller, G. I20; the Versatile X-ray Absorption Spectroscopy Beamline at Diamond Light Source. J. Phys. Conf. Ser. 2009, 190, 012038. [Google Scholar] [CrossRef]
- Dent, A.; Cibin, G.; Ramos, S.; Smith, A.; Scott, S.; Varandas, L.; Pearson, M.; Krumpa, N.; Jones, C.; Robbins, P. B18: A Core Xas Spectroscopy Beamline for Diamond. J. Phys. Conf. Ser. 2009, 190, 012039. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Athena, Artemis, Hephaestus: Data Analysis for X-ray Absorption Spectroscopy Using Ifeffit. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-Space Multiple-Scattering Calculation and Interpretation of X-ray-Absorption near-Edge Structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar] [CrossRef] [Green Version]
- Mereiter, K. The Crystal Structure of Liebigite, Ca2uo2(Co3)3·∼11h2o. Tschermaks Mineral. Petrogr. Mitt. 1982, 30, 277–288. [Google Scholar] [CrossRef]
- Carter, K.P.; Smith, K.F.; Tratnjek, T.; Shield, K.M.; Moreau, L.M.; Rees, J.A.; Booth, C.H.; Abergel, R.J. Spontaneous Chelation-Driven Reduction of the Neptunyl Cation in Aqueous Solution. Chem. Eur. J. 2020, 26, 2354–2359. [Google Scholar] [CrossRef]
- Sachs, S.; Schmeide, K.; Reich, T.; Brendler, V.; Heise, K.H.; Bernhard, G. Exafs Study on the Neptunium(V) Complexation by Various Humic Acids under Neutral Ph Conditions. Radiochim. Acta 2005, 93, 17. [Google Scholar] [CrossRef]
- Giandomenico, M.V.D.; Naour, C.L.; Simoni, E.; Guillaumont, D.; Moisy, P.; Hennig, C.; Conradson, S.D.; Auwer, C.D. Structure of Early Actinides(V) in Acidic Solutions. Radiochim. Acta 2009, 97, 347. [Google Scholar] [CrossRef]
- Gaona, X.; Tits, J.; Dardenne, K.; Liu, X.; Rothe, J.; Denecke, M.A.; Wieland, E.; Altmaier, M. Spectroscopic Investigations of Np(V/Vi) Redox Speciation in Hyperalkaline Tma-(Oh, Cl) Solutions. Radiochim. Acta 2012, 100, 759. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, D.R.; Amayri, S.; Drebert, J.; Grolimund, D.; Huth, J.; Kaplan, U.; Krause, J.; Reich, T. Speciation of Np(V) Uptake by Opalinus Clay Using Synchrotron Microbeam Techniques. Anal. Bioanal. Chem. 2012, 404, 2151–2162. [Google Scholar] [CrossRef]
- Gaona, X.; Wieland, E.; Tits, J.; Scheinost, A.C.; Dähn, R. Np(V/Vi) Redox Chemistry in Cementitious Systems: Xafs Investigations on the Speciation under Anoxic and Oxidizing Conditions. Appl. Geochem. 2013, 28, 109–118. [Google Scholar] [CrossRef]
- Maiwald, M.M.; Dardenne, K.; Rothe, J.; Skerencak-Frech, A.; Panak, P.J. Thermodynamics and Structure of Neptunium(V) Complexes with Formate. Spectroscopic and Theoretical Study. Inorg. Chem. 2020, 59, 6067–6077. [Google Scholar] [CrossRef]
- Denecke, M.A.; Dardenne, K.; Marquardt, C.M. Np(Iv)/Np(V) Valence Determinations from Np L3 Edge Xanes/Exafs. Talanta 2005, 65, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Allen, P.G.; Terminello, L.J.; Denecke, M.A.; Reich, T. Polarized X-ray-Absorption Spectroscopy of the Uranyl Ion: Comparison of Experiment and Theory. Phys. Rev. B 1996, 54, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, L.; Antonio, M.R.; Williams, C.; Wasserman, S.R. Xanes Spectroelectrochemistry: A New Method for Determining Formal Potentials. Anal. Chem. 1999, 71, 4622–4628. [Google Scholar] [CrossRef]
- Elo, O.; Müller, K.; Ikeda-Ohno, A.; Bok, F.; Scheinost, A.C.; Hölttä, P.; Huittinen, N. Batch Sorption and Spectroscopic Speciation Studies of Neptunium Uptake by Montmorillonite and Corundum. Geochim. Cosmochim. Acta 2017, 198, 168–181. [Google Scholar] [CrossRef]
- Forbes, T.Z.; Burns, P.C.; Skanthakumar, S.; Soderholm, L. Synthesis, Structure, and Magnetism of Np2o5. J. Am. Chem. Soc. 2007, 129, 2760–2761. [Google Scholar] [CrossRef]
- Krawczyk-Bärsch, E.; Scheinost, A.C.; Rossberg, A.; Müller, K.; Bok, F.; Hallbeck, L.; Lehrich, J.; Schmeide, K. Uranium and Neptunium Retention Mechanisms in Gallionella Ferruginea/Ferrihydrite Systems for Remediation Purposes. Environ. Sci. Pollut. Res. 2021, 28, 18342–18353. [Google Scholar] [CrossRef]
- Ulrich, K.-U.; Rossberg, A.; Foerstendorf, H.; Zänker, H.; Scheinost, A.C. Molecular Characterization of Uranium(Vi) Sorption Complexes on Iron(Iii)-Rich Acid Mine Water Colloids. Geochim. Cosmochim. Acta 2006, 70, 5469–5487. [Google Scholar] [CrossRef]
- Rossberg, A.; Ulrich, K.-U.; Weiss, S.; Tsushima, S.; Hiemstra, T.; Scheinost, A.C. Identification of Uranyl Surface Complexes on Ferrihydrite: Advanced Exafs Data Analysis and Cd-Music Modeling. Environ. Sci. Technol. 2009, 43, 1400–1406. [Google Scholar] [CrossRef]
- Dodge, C.J.; Francis, A.J.; Gillow, J.B.; Halada, G.P.; Eng, C.; Clayton, C.R. Association of Uranium with Iron Oxides Typically Formed on Corroding Steel Surfaces. Environ. Sci. Technol. 2002, 36, 3504–3511. [Google Scholar] [CrossRef]
- Sherman, D.M.; Peacock, C.L.; Hubbard, C.G. Surface Complexation of U(Vi) on Goethite (A-Feooh). Geochim. Cosmochim. Acta 2008, 72, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.L.; Conradson, S.D.; Ekberg, S.A.; Hess, N.J.; Neu, M.P.; Palmer, P.D.; Runde, W.; Tait, C.D. Exafs Studies of Pentavalent Neptunium Carbonato Complexes. Structural Elucidation of the Principal Constituents of Neptunium in Groundwater Environments. J. Am. Chem. Soc. 1996, 118, 2089–2090. [Google Scholar] [CrossRef]
- Gagliardi, L.; Roos, B.O. Coordination of the Neptunyl Ion with Carbonate Ions and Water: A Theoretical Study. Inorg. Chem. 2002, 41, 1315–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girvin, D.C.; Ames, L.L.; Schwab, A.P.; McGarrah, J.E. Neptunium Adsorption on Synthetic Amorphous Iron Oxyhydroxide. J. Colloid Interface Sci. 1991, 141, 67–78. [Google Scholar] [CrossRef]
- McBriarty, M.E.; Kerisit, S.; Bylaska, E.J.; Shaw, S.; Morris, K.; Ilton, E.S. Iron Vacancies Accommodate Uranyl Incorporation into Hematite. Environ. Sci. Technol. 2018, 52, 6282–6290. [Google Scholar] [CrossRef] [PubMed]
- Burgos, W.D.; McDonough, J.T.; Senko, J.M.; Zhang, G.; Dohnalkova, A.C.; Kelly, S.D.; Gorby, Y.; Kemner, K.M. Characterization of Uraninite Nanoparticles Produced by Shewanella Oneidensis Mr-1. Geochim. Cosmochim. Acta 2008, 72, 4901–4915. [Google Scholar] [CrossRef]
- Husar, R.; Hübner, R.; Hennig, C.; Martin, P.M.; Chollet, M.; Weiss, S.; Stumpf, T.; Zänker, H.; Ikeda-Ohno, A. Intrinsic Formation of Nanocrystalline Neptunium Dioxide under Neutral Aqueous Conditions Relevant to Deep Geological Repositories. Chem. Commun. 2015, 51, 1301–1304. [Google Scholar] [CrossRef] [Green Version]
- Catalano, J.G.; Brown, G.E. Uranyl Adsorption onto Montmorillonite: Evaluation of Binding Sites and Carbonate Complexation. Geochim. Cosmochim. Acta 2005, 69, 2995–3005. [Google Scholar] [CrossRef]

| Experiment | Path | N | σ2 (Å2) | R (Å) | S02 | ΔE0 | R-Factor | k-Range | R-Range |
|---|---|---|---|---|---|---|---|---|---|
| Np(V) on ferrihydrite | Oax | 2 | 0.002 (1) | 1.86 (1) | 0.9 | −2.2 (21) | 0.0192 | 3–13.5 | 1.25–2.7 |
| Oeq1 | 5 | 0.008 (2) | 2.49 (3) | ||||||
| Np(V) co-precipitated with ferrihydrite | Oax | 2 | 0.001 (0) | 1.86 (1) | 0.9 | −5.7 (19) | 0.0168 | 3–12 | 1.1–2.6 |
| Oeq1 | 5 | 0.009 (2) | 2.46 (2) | ||||||
| Np(V) on goethite | Oax | 2 | 0.001 (1) | 1.85 (1) | 1.0 | −3.5 (20) | 0.0248 | 3–13.5 | 1.25–2.7 |
| Oeq1 | 5 | 0.007 (2) | 2.49 (3) | ||||||
| Np(V) with FeS | O1 | 4 | 0.004 (1) | 2.25 (1) | 0.9 | 5.7 (1) | 0.0145 | 3–11.5 | 1–4 |
| O2 | 4 | 0.005 (2) | 2.40 (1) | ||||||
| Np1 | 4 | 0.009 (2) | 3.83 (2) | ||||||
| O MS1 | 24 | 0.009 # | 3.67 # | ||||||
| O MS2 | 24 | 0.009 # | 4.23 # | ||||||
| U(VI) with FeS | O1 | 4 | 0.004 (2) | 2.31 (1) | 0.9 | 6.2 (10) | 0.0178 | 3–12.5 | 1.4–4.6 |
| O2 | 2.5 | 0.006 (4) | 2.45 (3) | ||||||
| U1 | 6 | 0.006 (1) | 3.86 (1) | ||||||
| O | 8 | 0.012 (9) | 4.37 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Townsend, L.T.; Smith, K.F.; Winstanley, E.H.; Morris, K.; Stagg, O.; Mosselmans, J.F.W.; Livens, F.R.; Abrahamsen-Mills, L.; Blackham, R.; Shaw, S. Neptunium and Uranium Interactions with Environmentally and Industrially Relevant Iron Minerals. Minerals 2022, 12, 165. https://doi.org/10.3390/min12020165
Townsend LT, Smith KF, Winstanley EH, Morris K, Stagg O, Mosselmans JFW, Livens FR, Abrahamsen-Mills L, Blackham R, Shaw S. Neptunium and Uranium Interactions with Environmentally and Industrially Relevant Iron Minerals. Minerals. 2022; 12(2):165. https://doi.org/10.3390/min12020165
Chicago/Turabian StyleTownsend, Luke T., Kurt F. Smith, Ellen H. Winstanley, Katherine Morris, Olwen Stagg, J. Frederick W. Mosselmans, Francis R. Livens, Liam Abrahamsen-Mills, Richard Blackham, and Samuel Shaw. 2022. "Neptunium and Uranium Interactions with Environmentally and Industrially Relevant Iron Minerals" Minerals 12, no. 2: 165. https://doi.org/10.3390/min12020165
APA StyleTownsend, L. T., Smith, K. F., Winstanley, E. H., Morris, K., Stagg, O., Mosselmans, J. F. W., Livens, F. R., Abrahamsen-Mills, L., Blackham, R., & Shaw, S. (2022). Neptunium and Uranium Interactions with Environmentally and Industrially Relevant Iron Minerals. Minerals, 12(2), 165. https://doi.org/10.3390/min12020165









