Abstract
In order to provide important details concerning the adsorption reactions of Sr, batch reactions and a set of both ex situ and in situ Grazing Incidence X-ray Absorption Fine Structure (GIXAFS) adsorption experiments were completed on powdered TiO2 and on rutile(110), both reacted with either SrCl2 or SrCO3 solutions. TiO2 sorption capacity for strontium (Sr) ranges from 550 ppm (SrCl2 solutions, second order kinetics) to 1400 ppm (SrCO3 solutions, first order kinetics), respectively, and is rapid. Sr adsorption decreased as a function of chloride concentration but significantly increased as carbonate concentrations increased. In the presence of carbonate, the ability of TiO2 to remove Sr from the solution increases by a factor of ~4 due to rapid epitaxial surface precipitation of an SrCO3 thin film, which registers itself on the rutile(110) surface as a strontianite-like phase (d-spacing 2.8 Å). Extended X-ray Absorption Fine Structure (EXAFS) results suggest the initial attachment is via tetradental inner-sphere Sr adsorption. Moreover, adsorbates from concentrated SrCl2 solutions contain carbonate and hydroxyl species, which results in both inner- and outer-sphere adsorbates and explains the reduced Sr adsorption in these systems. These results not only provide new insights into Sr kinetics and adsorption on TiO2 but also provide valuable information concerning potential improvements in effluent water treatment models and are pertinent in developing treatment methods for rutile-coated structural materials within nuclear power plants.
1. Introduction
The production of nuclear energy and testing of nuclear weapons around the world has released radioactive strontium-90 (90Sr) into the environment, which has serious implications for environmental and human health. For example, in March 2011, a powerful earthquake damaged the Fukushima Daiichi nuclear reactor in Japan. The subsequent melt-down resulted in the contamination of large amounts of surface/sea water and land with 90Sr and cesium-137 (137Cs) [1,2,3,4]. The health implications for such a contaminant release are significant. Sr substitutes strongly for calcium (Ca) in bones and, therefore, stable Sr is used in the treatment of osteoporosis (a disorder that causes vertebral fractures) [5,6]. As such, the buildup of radioactive 90Sr isotope in bones is linked to bone cancer, bone marrow decay and leukemia [7,8,9]. These potential human impacts of 90Sr release therefore make it critical that wastewater from the Fukushima site (and surrounding fallout-affected area) is treated before being released into the environment.
Sr behaves similarly to Ca in the environment because they are both alkaline earths, and, thus, are chemically similar. In the environment Sr is solely present as divalent Sr2+, which is in general relatively mobile. However, pH, ionic strength of ground/seawaters, temperature and the presence of Mg2+/Ca2+ exchangeable substances will influence Sr mobility. Since calcium and carbonate are widely present in (sub)surface lithologies and in seawater, [10] Sr mobility is primarily controlled by surface adsorption and incorporation or, when available at high concentrations, co-precipitation with Ca-bearing mineral phases [11]. Alkaline earth impurities in Ca-minerals, such as high Mg2+ concentrations, may also influence Sr2+ incorporation in calcite because coupled substitution may relieve structural stress [12] caused by single element substitution. Furthermore, Sr2+, like Ca2+, has a relatively high hydration energy and, therefore, tends to adsorb to a wide range of negatively charged mineral surfaces via weakly bound outer-sphere complexes [13]. As such, solution pH will affect Sr availability when driving below or above the point of zero charge (PZC) of mineral surfaces controlling surface charge and reactivity [14]. Iron rich sediments and Fe(III)-oxyhydroxide minerals, for example, will place significant constraints on Sr mobility as a function of solution pH. In addition, clay minerals, such as illite, kaolinite and montmorillonite, all provide important adsorption surfaces for Sr, due to their relatively low PZC and permanent negative surface charge. Moreover, Zhao et al., (2014) [15] have shown that Sr adsorption below pH 9.5 is dominated by outer-sphere surface complexation or ion exchange. Under these conditions, Sr2+ sorption is reduced on natural materials when cations of smaller ionic radius, such as Ca2+ and Mg2+, are present. Sorption, however, is less affected by monovalent cations such as Na+ and K+. Oftentimes, monovalent cations dominate the fluid cation inventory at nuclear sites and both Na+ and K+ are abundant in seawater, which was used to cool Fukushima’s reactors during the fallout period [10,16,17]. In contrast, Chen et al. (2008) [18] observed a decrease in Sr adsorption with increasing NaClO4 concentrations. This was linked to the formation of an electrical double layer or caused by changes in activity coefficients. These are important factors to consider before designing treatment methods for contaminated seawater.
Several successful methods of treating Sr-containing wastewater have been developed, such as the use of active carbon [19] and carbon nanotubes [18], silica-based materials [20] and titanate-based materials [2,21,22]. Studies have shown that TiO2 surfaces are very efficient in removing Sr from wastewater solutions, nevertheless the chemistry of Sr adsorption on rutile is not well developed [23,24]. Titanate is currently being used as a component of the Advanced Liquid Processing System (ALPS) at Fukushima for the removal of Sr as it is relatively stable in alkaline salt solutions, and it has demonstrated the capacity to adsorb actinides such as uranium (U), plutonium (Pu) and neptunium (Np) as well as Sr [21,25] However, the potential of rutile (TiO2) for wastewater treatment has not been investigated in detail, despite its use in other remediation projects. Rutile has been used for degradation of molecules (catalysis) and for the purification of wastewaters and as a component in the immobilization of radioactive waste by transforming it into a ceramic form [22,26]. In addition, rutile is an important ingredient in paints [26] used at spent nuclear fuel cooling ponds. Due to its stable nature under various (geo)chemical conditions, rutile is an excellent component for coatings and as a substrate to be used in effluent treatment. Nevertheless, information concerning Sr adsorption onto rutile (TiO2) is not well developed, and adsorption information at the atomic scale on the specific orientations of rutile under conditions similar to Fukushima’s wastewater have not been investigated in great detail [27]. Information about surface specific active sites on mineral surfaces is important in order to predict how Sr may be adsorbed to TiO2. Moreover, identifying the important adsorption reactions that control mobility is vital in optimizing predictive models of Sr interacting with TiO2 and, potentially, could improve the methodology of wastewater treatment. Extracting important adsorption reaction information is challenging from powdered bulk experiments where the signal is averaged out over multiple surfaces with an unconstrained distribution of those surface types. To that end, in situ and ex situ Grazing Incidence EXAFS (GIXAFS) together with X-ray reflectivity and XPS experiments on single crystals of rutile were completed in order to constrain the adsorption configuration of Sr under different conditions onto the dominant and most reactive (110) orientation of rutile. Adsorption kinetics were also determined. Surface specific adsorption data yield accurate adsorption configuration information and will improve our predictive power for remediation purposes. Such data cannot typically be obtained from bulk adsorption experiments due to the convolved contributions to the spectra from multiple surfaces [28,29,30]. Bulk sorption experiments on rutile/anatase were completed in order to extract adsorption isotherms for Sr, CO32− and Cl− and also to provide a comparison to the in situ kinetics and adsorption results determined at the synchrotron.
In this study, we provide (1) advances in adsorption kinetics methodology in combination with GIXAFS, (2) detailed information of Sr adsorption kinetics and isotherm data on TiO2, and (3) local bonding configuration data for in situ and ex situ experiments of Sr adsorbed to rutile(110) in brackish water (high ionic strength) to simulate wastewater at Fukushima, Japan. We hypothesize an outer-sphere versus inner-sphere bonding configuration of Sr with and without carbonate abundantly present. Finally, we not only provide insights into Sr adsorption kinetics and behavior on TiO2 but also provide valuable information for the improvement of effluent water treatment prediction models and for the treatment of structural materials from nuclear power plants that have been coated with rutile-containing pigments.
2. Experimental Section
2.1. Powder Adsorption Rates and Isotherm Experiments
The adsorption kinetics and adsorption isotherms of Sr2+, CO32− and Cl− on powdered TiO2 were conducted in batch experiments. In all experiments, commercially bought TiO2 from Sigma Aldrich was a mixture of rutile (59.1%) and anatase (40.9%) (a rutile polymorph). The particle size was <100 nm and specific surface area 36.79 ± 0.1 m2 g−1 determined by BET. The powders were reacted with Sr in a solid–solution ratio of 1:40 and 1:80 in the SrCl2 and SrCO3 solutions, respectively (see Table S1). Although the substrate contains two polymorphs, both structures typically contain chains of distorted TiO6 octahedra. Their dominant surfaces are rutile(110) and anatase(101) and show subtle differences. Structurally, the difference between anatase and rutile is in terms of octahedral edge sharing, which might lead one to postulate subtle differences in adsorbate configuration. However, we expect these to be negligible in a powder experiment, which averages over many different attachment configurations. Finally, the point of zero charge (PZC) has been measured, and differences between rutile (pH 6) and anatase (pH 5.9) are determined to be minimal. As such, the fact that we are dealing with a polycrystalline mass of TiO2 that contains an unconstrained termination of different crystals, means that we expect the gross adsorption kinetics to be very similar in this two-phase system to what we would measure in a single-phase batch powder measurement. In any case, to avoid confusion when we discuss the data from these experiments, we will refer to “mix-TiO2” to make it clear that these experiments were with a mixed phase.
The adsorption capacity of Sr (qe, mg g−1) on TiO2 was calculated using the following formula
where C0 and Ce (mg L−1) are the initial and final Sr concentrations in solution, respectively, V (L) is the volume of the reaction solution and m (g) denotes the total mass of adsorbent.
Adsorption kinetics: Two sets of kinetics experiments with ionic strength (IS) of 0.25 M NaCl solution were prepared, one with 100 ppm Sr from SrCl2 6H2O and a lower concentration of 50 ppm Sr solution from SrCO3 in order to prevent precipitation during reaction. The calculated dominant species in the SrCl2 solutions were Sr2+ and SrCl+. Sr2+, SrCl+ and SrCO3 dominated in the SrCO3 solutions (PHREEQC [31], SIT database). Amounts of 0.5 g or 0.25 g mix-TiO2 was weighed out and reacted with the SrCl2 or SrCO3 solutions, respectively. Solutions were centrifuged, sampled and filtered (0.2 µm) at times t = 0, 15, 30, 60, 120, 240, 480, 1440 and 2880 min to be analyzed by ICP-AES (PerkinElmer Optima 5300DV, detection limit 1 ppb). The total adsorption kinetics were calculated by using the pseudo-first-order non-linear kinetics model described as
where qt and qe are the amounts of Sr adsorbed (mg g−1) at equilibrium and at time t (min), respectively, and K denotes the adsorption rate constant (min−1). In addition, a pseudo-second-order non-linear rate equation was used, described as
and its linear form as
where k2 (g mg−1 min−1) is the adsorption rate constant of the pseudo-second-order rate. Values of qe and k2 can be obtained from the slope and the intercept of the plot of the linearized form of each equation, respectively.
Adsorption isotherms: Three sets of adsorption isotherm experiments were conducted. The first experiment investigated the adsorption capacity of Sr by increasing [Sr2+] from 0 to 1000 ppm, dissolved in 20 mL Milli-Q and adsorbed on 0.5 g mix-TiO2. The second and third experiments focused on the effects of Cl− and CO32− anions on the Sr adsorption capacity. These anions are expected to have significant opposite effects with regards to the formation of an electrical double layer at the mineral interface. In the second experiment, we investigated the effect of CO32−; we added CO32− to 20 mL 100 ppm Sr solution (prepared from SrCl2) with 0.5 g mix-TiO2, and increased the [CO32−] stepwise from 0 to 5 mM CO32−. In the third experiment, the effect of Cl− on the Sr adsorption was studied in 20 mL 50 ppm Sr solution (prepared from SrCO3) and 0.25 g mix-TiO2. Here, [Cl−] was increased from 0 to 0.25 M Cl−. Samples were reacted for 24 h (±1 h), then sampled, filtered (0.2 µm) and analyzed by ICP-AES. In order to calculate the adsorption isotherm of Sr on TiO2, a Langmuir model was used, described as:
with Ce the final concentration of Sr (mg L−1), qe its corresponding adsorption capacity and qm and KL are constants, which are related to the adsorption capacity and net enthalpy of adsorption, respectively.
2.2. Ex Situ and In Situ Grazing Incidence EXAFS (GIXAFS) Adsorption Experiments
For both ex situ and in situ experiments in this study, commercially bought rutile(110) (dominant surface of TiO2) was used and reacted with Sr-bearing solutions. These oriented single crystals (10 mm × 10 mm × 0.5 mm, single side polished), were produced, cut and polished by Alineason Materials & Technology GmbH (Frankfurt am Main, Germany). The surfaces were prepared by chemo-mechanical polishing (CMP) to a root mean square (r.m.s.) surface roughness of 2–5 Å.
Before the experiments, two stock solutions were prepared: a 500 ppm SrCl2 (dissolved from SrCl2·6H2O) and a 50 ppm SrCO3 (dissolved from SrCO3) solution. In addition, 0.25 M NaCl was added to each solution to simulate the effects of brine on the bonding configuration of Sr on rutile. For both solutions, the pH was adjusted to 7 ± 0.2, using NaOH and HCl, respectively. The calculated solution species in the SrCl2 solution (PHREEQC [31], SIT database) are Sr2+ (98.8%), SrCl+ (1.1%) and 0.1% each of SrHCO3+, SrCO3 and SrOH+. In the SrCO3 solution, the dominant species are predicted to be mainly Sr2+ (>99.3%), with the remainder being SrHCO3+, SrCl+, SrCO3 and SrOH+ (together 0.7%).
2.2.1. Ex Situ Batch Experiments
These experiments were prepared by transferring 5 mL of each stock solution into a centrifuge tube along with the rutile(110) crystal. Two crystals were submerged in the solution for 48 h (±2 h) after which they were removed using Teflon coated tweezers. One crystal was left with solution on the crystal, while the second was wicked and dried under N2 gas (surface of interest was not touched), after which both crystals were transferred onto a domed ex situ sample holder suitable for GIXAFS. The sample holder was sealed using a single layer of Kapton tape and bagged.
2.2.2. In Situ Single Pass Flow-through Experiment
Samples were prepared by loading an unreacted rutile(110) crystal into a domed in situ sample flow-through cell designed for in situ GIXAFS (Figure S1). After loading the crystal, a sheet of (25 µm) Kapton foil was mounted underneath a metal bracket and secured with six bolts using a bolt-lock method. Teflon tubing was connected to the cell via two nylon nipples on either side of the cell to avoid spillage of reaction fluids. The cell was then mounted, together with a specifically designed Al spill tray, onto the stage so that any potential spillage would be contained.
The rutile crystal was reacted at the beamline with a 500 ppm SrCl2 solution for approximately 4 h using a syringe pump at a flow-rate of 2.5 mL h−1. During the reaction, total Sr fluorescence (see section GIXAFS analyses) was monitored in live time. After a plateau in the total fluorescence was reached, 0.4 mL Milli-Q water (2 cell volumes) at 2.5 mL h−1 was pumped through the cell, after which the cell was dried using flowing N2 until a stable (increased) baseline of Sr fluorescence was reached.
2.3. Sr K-Edge GIXAFS Analyses
The experiments were performed at beamline I18 at Diamond Light Source Ltd., Didcot, UK. This beamline is designed for imaging and spectroscopy experiments and provides the opportunity to perform GIXAFS experiments. A cryogenically cooled double Si (111) crystal monochromator was used to select the energy of the beam, which was calibrated against the first derivative of yttrium (Y), defined at 17,038 eV. The beam was collimated vertically and focused horizontally to a ca. 300 µm × 150 µm spot using an Rh-coated Si toroidal mirror and the horizontally focusing mirror of a pair of Rh-coated Kirkpatrick-Baez focusing mirrors. Fluorescence yield data were collected using a four element Si drift fluorescence detector, positioned 90° above the crystal surface.
Crystal alignment was performed by first completing a rocking curve measurement, by rocking the crystal horizontally in θc so that the crystal was positioned parallel to the incident beam and cutting half of the beam intensity. In addition, behind the sample, horizontal slits (250 μm) were placed to cut out any scattering produced by the crystal surface during alignment. Then, the angle of the crystal surface relative to the beam was set at ~11 millidegrees θc but kept below the critical angle for total external reflection so that the fluorescence signal for standing waves was maximized and undesirable elastic scattering was minimized.
The EXAFS, χ(k), were refined from the raw data by standard procedures, including background subtraction, normalization of absorption, conversion to R and k-space using Athena 0.9.23. For each sample ca. 8–12 spectra, depending on Sr loading, were averaged to maximize the signal-to-noise ratio. The spectra were then analyzed with Artemis 0.9.23 and the phase/amplitude functions were calculated by FEFF6L [32]. The theoretical fits were performed in R-space. The F-test and dimensional Hamilton test was performed to justify each path included in the fit [33]. Finally, shells were chosen that gave the best fit, e.g., split oxygen shell, and that were chemically reasonable based on the chemistry of each system (see Supplementary Material SS1) [28,29,30,34,35,36,37,38,39].
2.4. X-ray Reflectivity (XRR) and Grazing Incidence XRD GI-XRD
For the full experimental details and the equipment set-up, see van Veelen et al. (2016) [30]. In short, this technique was used to provide information concerning the surface adsorbed thin-films’ thickness (Z), density (ρ) and average roughness (σ) r.m.s., perpendicular to the mineral surface. The XRR scans were collected from 0–9° 2θ. A pipeline correction was applied on all spectra and then normalized in intensity. The spectra were finally analyzed using the Bruker software suite DIFFRAC Leptos 7.0 (Bruker AXS GmbH, Karlsruhe, Germany). In addition, afterwards, Grazing Incidence XRD (GI-XRD) was applied on all our surfaces in order to detect any signs of long-range order in the adsorbates.
2.5. X-ray Photoelectron Spectroscopy (XPS)
XPS analyses were conducted by a Kratos Axis Ultra spectrometer (Kratos Analytical Ltd., Shimadzu Corporation, Manchester, UK) using Al Kα monochromatic X-rays set at an energy of 1486.7 eV. The X-rays were calibrated using the C 1s peak, defined at 284.8 eV. For all analyses, the charge neutralizer was used and was fully achieved by monitoring the C 1s peak for adventitious carbon. The base pressure for the experiments was kept at ~3–4 × 10−9 bar. High resolution spectra were collected with a pass energy of 20 eV and a spot size of approximately 300 µm × 600 μm and analyzed using CasaXPS.
3. Results
3.1. Powder Adsorption Rate and Isotherm Experiments
The adsorption rates and isotherms of Sr, CO32− and Cl− in batch powder experiments are presented in Figure 1 and Table S2.
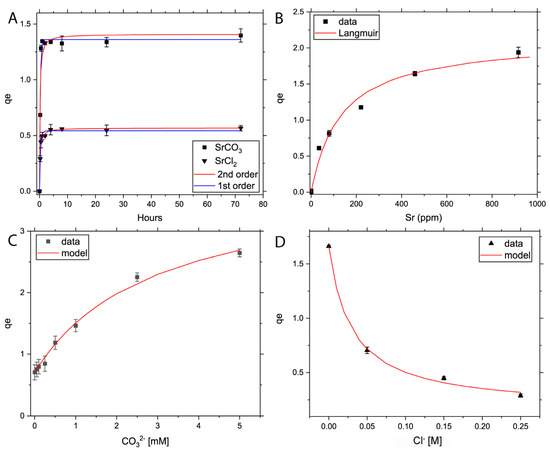
Figure 1.
Batch adsorption kinetics (A) and adsorption isotherm plots of Sr (B), carbonate effect isotherm (C) and chlorine effect isotherm (D) on adsorption of Sr to TiO2 powders. Panels (B–D) fitted the Langmuir isotherm best (line in red).
Adsorption rate experiments. Sr adsorption onto TiO2 powders reached equilibrium within 60 min for SrCl2 and 30 min for SrCO3 (Figure 1a). In the in situ experiment at the synchrotron, this was even quicker with equilibrium reached within 10 min (Figure 2). The difference in this reaction time can be explained by the significantly lower specific surface area of TiO2 (110) compared to powdered mix-TiO2. The sorption capacity of Sr with TiO2 in contact with SrCO3 is significantly higher (ca. 1.4 mg g−1) compared to the SrCl2 (ca. 0.57 mg g−1) systems. The adsorption kinetics of SrCl2 on powder and the in situ sorption experiments appear to follow a pseudo-second-order adsorption model with slightly higher r2 values relative to a first order model in all cases (Table S2). In contrast, SrCO3 adsorption in the powder experiments suggests a pseudo-first-order adsorption, which suggests rapid surface precipitation followed by slow chemisorption. However, the differences between the two models’ confidence levels are small and the sampling density in the powders between t = 0–1 h is relatively low for this high adsorption rate, suggesting that the in situ experiment is far more powerful and appropriate for this type of experiment.
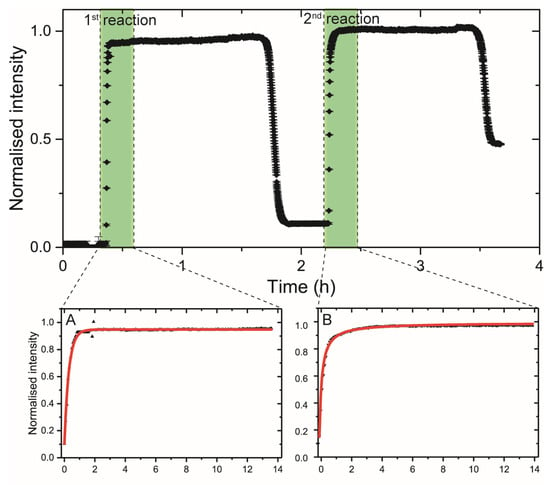
Figure 2.
Sr K-edge in situ adsorption kinetics measured (top panel) at the rutile(110) surface, using a 500 ppm SrCl2 solution, pH7. (A,B) are zoomed-in sections of the adsorption curves (green vertical bars) used to extract adsorption kinetics order and parameter information. First adsorption shows signs of rapid surface adsorption while the second adsorption shows signs of chemisorption on previous adsorbed Sr. Spectra collected at I18 at Diamond Light Source Ltd.
Adsorption isotherms of SrCl2 follow a Langmuir isotherm (Figure 1B, Table S2). In addition, the adsorption capacity of TiO2 and KL-carbonate increased with increasing concentrations of HCO3−, suggesting a higher sorption affinity of Sr-TiO2. This is partly due to the rapid increase in SrHCO3+ species (Figure S2). By increasing the bicarbonate concentration, the adsorption capacity increased by a factor of 3.8. In contrast, Cl− concentrations showed a negative relationship with Sr-TiO2, with an 82.6% decrease in Sr adsorption at IS of 0.25 M. PHREEQC modeling showed an increase in SrCl+ (Figure S2), which suggests that the co-adsorption of Cl− inhibits Sr adsorption. Therefore, by comparing the K-values of Table 1 from both systems (K-value ratio KL-carbonate − KL-chlorine = 10), it can be noted that total dissolved carbonate affects Sr removal much stronger than chlorine, and, therefore, total dissolved carbonate (and indirectly pH) should be taken into account when determining total Sr uptake.

Table 1.
Shell-by-shell GIXAFS fit results of SrCO3, SrCl2 and Sr adsorbed on rutile(110).
3.2. X-ray Absorption near Edge Structure (XANES) Analyses
Rutile(110) was reacted in two ways, (1) ex situ mode and (2) in situ mode at the beamline. Sr K-edge extended XANES spectra (Figure 3) showing clear differences between the systems reacted under various conditions. The ex situ reacted rutile(110) surface exposed to a SrCl2-bearing solution resembles a two species adsorbate denoted as “hydrated” (wicked and dried under N2 gas) and “aqueous” (left with solution). The first adsorbate (denoted “aqueous”) resembles features of previously published aqueous Sr spectra, [8,40] while the second adsorbate (spectrum in red, denoted “hydrated”) shows strong similarities with the SrCl2·6H2O standard (see shift in white line position “a” and oscillation at feature “b”) and with hydrated Sr in hydroxyapatite [41] and is abbreviated as SrCl2 henceforth. An important detail here is that Sr2+ in this form is coordinated to nine water molecules, with chlorine further out at a distance of 4.909 Å from the Sr atom [42]. Therefore, the first low amplitude oscillation above the white line at approximately 16,125 eV (feature b), is a result of scattering features of organized clusters of water molecules around the Sr atom. These clusters will become significantly distorted when transformed into an aqueous species, resulting in various bond lengths and the disappearance of the oscillation at 16,125 eV [8]. This indicates that this second “hydrated” adsorbate of the ex situ samples is likely to be a precipitate. In contrast, the in situ SrCl2 experimental adsorbate solely resembles an aqueous-like Sr adsorbate (blue curve, feature type 2) but with subtle signs of SrCO3-like features (see feature type 1 at ~16,140 eV). This indicates that the in situ methodology would be more appropriate for interface chemistry as the results are consistent and uniform. Finally, the ex situ experiment with SrCO3 showed no changes from the initial reactant spectrum, indicating surface adsorption of a carbonate moiety.
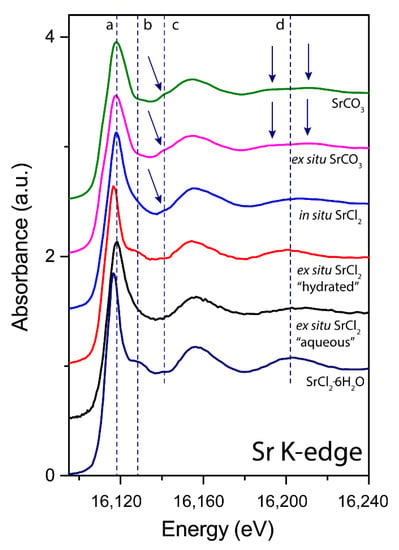
Figure 3.
Sr K-edge XANES of Sr adsorbed on rutile(110). There are spectral differences as denoted in the figure by a, b, c and d. The arrows highlight subtle changes between the spectra. Feature ‘a’ marks the top of the white line. Note that this varies depending on the species, e.g., SrCl2·6H2O is slightly lower than SrCO3. Features ‘b’ and ‘c’ show the subtle differences between the hydrated and carbonated species. Finally, feature ‘d’ shows the typical bifurcation of the first oscillation for SrCO3 around 16.2 keV. Note that for the ex situ experiment with SrCl2, two surface adsorbate species were identified, a hydrated species comparable to that of SrCl2·6H2O and an aqueous Sr species.
3.3. GIXAFS Analyses
In the EXAFS region (Figure 4) in the k-range of 4.2–5.3 Å−1, there is a shift in peak position towards higher k-space for the “aqueous” ex situ adsorbate (E) and for the in situ chloride sample (C) compared to the SrCl2 standard. In addition, the slight peak asymmetry for both adsorbates show a similarity to the SrCO3 standard. In contrast, the “hydrated” Sr species (D), has strong similarities with the SrCl2 standard, with a relatively narrow and symmetrical oscillation. Further out in higher k-space, from 6–7.2 Å−1, typically, a split positive antinode of SrCO3 can be expected [43]. These double peaks are indeed present in the SrCO3 bearing systems (A and B), but are lacking in both the in situ (C) and ex situ “aqueous” chloride adsorbates (E). Given that the oscillation and the peak positions of C and E are in sync with the SrCO3 standard and the comparable asymmetry in sample E, the spectra suggest that both samples produced from a chloride-dominated solution contain a considerable SrCO3 component.
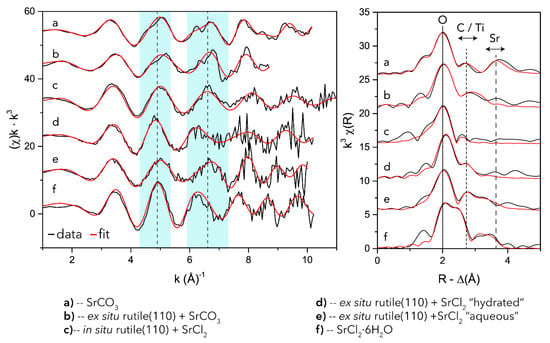
Figure 4.
Sr K-edge GIXAFS (left panel) and the Fourier back transformed EXAFS (right panel) on rutile(110), SrCO3 and SrCl2. The two cyan panels denote the maxima of the diagnostic k-space photoelectric wavenumbers for SrCO3 and SrCl2·6H2O.
EXAFS fits on the reference spectra of SrCO3 (A) are in good agreement with published data (Table 1) [43]. However, the SrCl2 standard (F) shows some signs of dehydration compared with the theoretical crystallography data, which we attribute to beam damage during the analyses of the powder or due to specimen heterogeneity. Nonetheless, the first shell oxygen atom distances are in good agreement with average Sr–O distances of 2.66 Å (versus 2.58 in SrCO3) published for SrCl2 [42]. EXAFS on nearly all of the Sr-reacted TiO2 surfaces, resolved [8,9] oxygen atoms in the first shell. The EXAFS fitting results for the ex situ sample (B) reacted with SrCO3 largely correspond to the SrCO3 standard, but the first shell of oxygen atoms is slightly further out (2.61 vs. 2.58), which is in very good agreement with previous published SrCO3 surface precipitates [43]. Moreover, sample B shows signs of a polarization effect. For example, the CN of the oxygen atoms is severely underestimated, which suggests surface species alignment in some way with the polarization vector (see Supplementary Material SS2), thus implying a well ordered SrCO3 surface precipitate. The fits for the ex situ aqueous (E) adsorbate, are in good agreement with published results for aqueous Sr [6]. However, fits for the chloride in situ adsorbate (C) were improved by introducing a split in the first shell while keeping the total oxygen coordination number equal to the best fit in the previous fitting routine. For both the in situ (C) and the ex situ “aqueous” (E) adsorbate, 1–2 Ti atoms could be fitted at 3.26 and 3.36 Å, respectively. This is consistent with tetradental adsorption. Carbon and chlorine were tried for these paths as well but did not improve the model and were therefore omitted. Finally, the “hydrated” Sr (D) species fits are in good agreement with the SrCl2 standard. We attempted to add Ti to the model, but since the data quality did not pass the Nyquist criteria, this addition was omitted. Nevertheless, the omitted Ti distance was modelled to be 3.41 Å, which would also suggest tetradental adsorption.
To conclude, the EXAFS data suggest that the adsorbates of both the in situ and ex situ samples reacted with SrCl2 solution show signs of inner-sphere adsorption to the rutile(110) surface, possibly by adopting flexible Sr–O bond lengths (distorted: longer and shorter bond lengths). The “hydrated” sorbate, partly because of its chlorinated nature, resembles an outer-sphere adsorbate, registering itself via its hydrated oxygen atoms onto the rutile(110) plane. In the experiment with carbonate, Sr appears to be in a slightly distorted SrCO3 surface precipitate film.
3.4. X-ray Reflectivity and GI-XRD
All analyzed surfaces showed signs of thin-film formation at the mineral surfaces (Figure 5, Table S3). Prior to the reaction with Sr, the mineral surfaces (r.m.s. roughness (σ) = 2.1 Å) showed a thin film of water on each surface with a thickness of 13.8 Å. Post reaction, the surfaces did not show signs of surface roughening. The average surface roughness, σ, measured was ca. 3 Å.
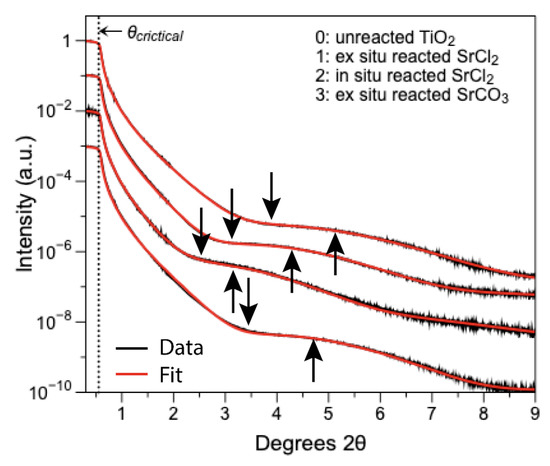
Figure 5.
X-ray reflectivity of Sr thin films on TiO2(110) surfaces. The in changes in angle positions of the downward facing arrows indicate different film thickness. In addition, all surfaces with Sr adsorbates show higher density film compared to the unreacted TiO2 surface denoted by the upward facing arrows.
All surfaces post reaction showed an increase in the amplitude of the Kiessig fringes, which indicates a factor 2–3 increase in the densities (ρ) of the surface film layers when compared to the thin film of water on the unreacted mineral surface (Figure 5 and Table S3). The densities of these adsorbates are consistent with mixed or hydrated phases of SrOHCl·4H2O (2.199 g cm−3), SrCl2·6H2O (1.957 g cm−3) and SrCO3 (3.844 g cm−3). In addition, GI-XRD revealed that on the ex situ reacted SrCO3 sample, a precipitate of SrCO3 was formed, which registers itself on the mineral surface along the (110) direction with d-spacing of 2.8 Å (Figure S3) [44]. The fact that a diffraction peak was detected means that this surface precipitate shows signs of long-range order and is, thus, an organized film with a preferred orientation layered adsorbate of approximately five sheets thick.
3.5. X-ray Photoelectron Spectroscopy (XPS)
XPS Ti 2p, Sr 3d, O 1s and C 1s (Figures S4 and S5, Table S4) were fitted using previous published peak positions [30,45,46,47,48]. The Ti 2p peak shifts approximately 0.3 eV to higher energy when the rutile surface is reacted with Sr compared to the pristine rutile surface. Sr 3d peak fitting and peak separation are consistent with those of Sr-oxides [45]. Specific differences between samples were only detected in the O 1s and C 1s spectra. Fitting results confirmed peak shifts consistent with both hydration and carbon coordination to Sr in the thin film and was only determined in the ex situ sample reacted with SrCl2. The in situ sample, in contrast, showed signs of SrO2/Sr(OH2) species and no carbonate species could be resolved. Moreover, the ex situ SrCO3 compared to the ex situ SrCl2, show high degrees of similarities with similar peak positions, indicating that the adsorbates are similar in composition. Finally, the Cl 2s peaks were detected on all crystals, but the intensity is significantly higher in the SrCl2 systems (Figure S6). This indicates that Cl likely interferes with Sr adsorption at the mineral interface, possibly by co-adsorption.
4. Discussion
The adsorption kinetics results show that Sr adsorption on powdered TiO2 and single crystal rutile is very rapid and efficient and, therefore, possibly a suitable substrate for the treatment of Sr-rich wastewater. Both the adsorption rates and in situ XAS experiment results are in good agreement with a second order adsorption rate model of Sr in the SrCl2 systems, which we link to electrostatic adsorption. Under these pH conditions, the surface of rutile(110) and anatase(101) is negatively charged (point of zero charge (PZC) rutile pH 6 and anatase pH 5.9 [49]), which allows for the adsorption of positively charged Sr complexes [50]. XRR suggests that the rutile(110) surfaces under all conditions do not change significantly in surface roughness, which indicates that the rutile surface is very stable. Moreover, the results of the in situ method suggest its superiority over conventional adsorption rate methods, giving high resolution kinetic information of a specific mineral orientation in combination with the molecular bonding configuration. The addition of Cl− decreases the adsorption of Sr, probably due to the increase in SrCl+ aqueous species and this, combined with the decrease in thickness of the electrical double layer due to the high IS, favors lower electrostatic adsorption. This would result in low d-plane potentials since the solution pH is close to the PZC of both rutile and anatase. This then forms a diffuse layer of Sr cations loosely attracted to the mineral surface. In contrast, the addition of carbonate increases both the adsorption capacity and adsorption rate, apparently changing from second order to first order adsorption kinetics. This is also consistent with rapid surface precipitation of Sr, and, when considering the adsorption isotherm of carbonate, this shows that carbonate is an important parameter in Sr removal. This feeds directly into the EXAFS results, where the spectra of surface films of SrCO3 are virtually identical to crystalline SrCO3.
EXAFS results are in good agreement with Sr adsorbed to rutile(110) as published in Zhang et al. (2004) [27] and Sverjensky (2006) [23]. The results (both ex situ and in situ SrCl2) suggest tetradentate inner-sphere adsorption of Sr (Figure 6), which is a stable adsorbate.
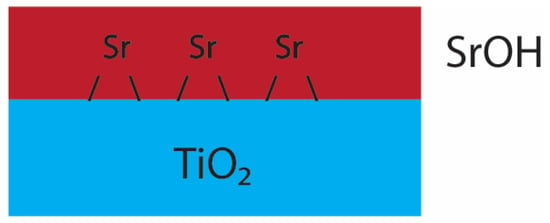
Figure 6.
Postulated adsorption model of Sr in the SrCl2 system. Red denotes the SrOH adsorbate film and blue the rutile(110) surface. In this system, there is a distorted oxygen shell with 4 oxygen atoms pointing away from the rutile surface.
This mode of adsorption is compensated by a distorted eight-fold oxygen coordination in strong agreement with Zhang et al. (2004), with four hydrated species pointing away from the mineral surface. This is, in addition, another reason why adsorption of Sr may be reduced in chloride rich solutions, since the high concentration of NaCl would interfere with SrOH+ at the mineral surface and, in turn, hinder inner-sphere surface adsorption. This would also create a diffuse layer, which would significantly decrease the surface potential and would explain the significant decrease in the adsorption capacity of TiO2 with increasing concentrations of chloride. Under these conditions (Figure 7), the proposed interference reaction would be:
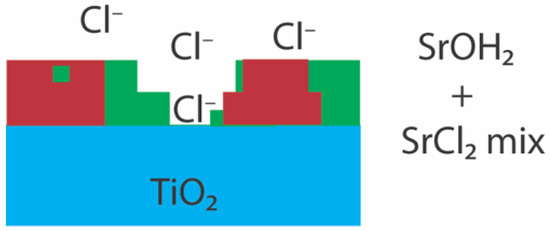
Figure 7.
Postulated adsorption model of SrCl2/SrOH mixture SrCl2 system. Red denotes the SrOH adsorbate film, green the SrCl2 adsorbate and blue the rutile(110) surface. This system is a mixture of two species, a species similar to Figure 6, and a species similar to the SrCl2 mineral structure. In the latter, it sits further away from the hydrate oxygen molecules situated around the Sr atom. It is also very likely to have free Cl− ions floating around, as seen in the XPS results (Figure S5).
This mode of adsorption describes an outer-sphere and weakly bonded Sr species. This is confirmed on the ex situ sample where two different species were detected: an aqueous Sr species and a SrCl2·6H2O-like species. The latter, as mentioned earlier, is fully hydrated in the first coordination shell. XPS and XRR further confirm the presence of hydrated components, showing significant –OH/Sr(OH)2 and Cl− peaks in XPS and adsorbate densities consistent with hydrated/chlorinated surface phases. The EXAFS spectra of the aqueous species, however, suggests that the adsorbed Sr (from SrCl2) also contains a component of carbonate and is corroborated by XPS peak position consistent with SrCO3/SrHCO3+. The difference between the SrCO3 and SrCl2 local structure allows SrCO3 to polymerize on the mineral surface.
In the SrCO3 system, EXAFS and GI-XRD indisputably suggest a crystalline surface precipitate, which registers itself along the rutile(110) surface (Figure 8). Under these conditions, SrHCO3+ would be the dominant species involved in surface adsorption, described as:
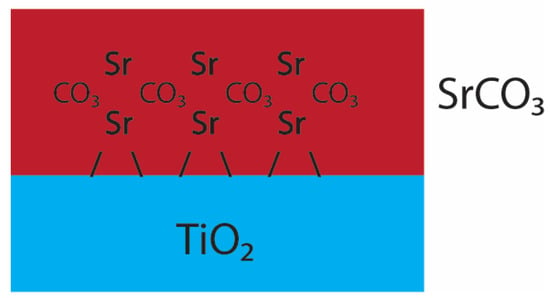
Figure 8.
Postulated adsorption model of Sr in the SrCl2 system. Red denotes the SrCO3 adsorbate film and blue the rutile(110) surface. Typical for this system is the SrCO3 precipitation onto the rutile(110) surface. The precipitate seems to have a highly organized texture as a d-spacing of 2.823 Å could be observed (Figure S2).
This system, with an abundance of SrCO3 present, would locally exceed the solubility limit of strontianite (SrCO3) at the mineral surface and result in surface precipitates. The adsorption kinetics of SrCO3, and both the EXAFS and XRD results, agree with a highly organized surface precipitate. In addition, XRR suggest minor compensations of the surface precipitate, with densities slightly lower than densities reported for crystalline SrCO3. Lastly, the fact that this adsorbate is highly organized and little chloride was detected, suggests a stable thin film. In contrast, significant lower adsorption in the SrCl2 systems has to be considered when treating salty wastewater as at Fukushima. Ultimately, the adsorption isotherm results show that this can be mitigated by increasing the carbonate concentration of the solutions. The results presented here might be of interest for the treatment of other radionuclides, such as Cs, U, Np and Pu, especially due to rutile’s stable nature under alkaline conditions and reactivity under high ionic strengths, which is usually the case at nuclear sites and in geological disposal facilities where Ti-bearing glasses are present as well.
5. Implications for Sr Wastewater Treatment
Constraining the mobility of Sr is critical for designing wastewater treatment processes and for the development of future remediation processes. The adsorption results in this contribution show that in situ GIXAFS is a powerful tool in determining the adsorption rate and adsorption kinetics order. Even though the substrate is likely to be applied in powdered form, and likely as a mixture of both rutile and anatase, it is important to understand how Sr interacts with the dominant and most reactive mineral surface. The fact that both isomorphs show similarities in the surface reactivity and protonation, suggests that we can use the rutile(110) as an analogue to understand Sr adsorption to these surfaces, which is otherwise difficult to do. Secondly, the adsorbates on rutile(110) reacted in situ are unambiguous compared to batch adsorption (ex situ) reactions, where the combination of signals from all surfaces simultaneously may obscure the details of the key attachment reactions of Sr onto titanium dioxides. The adsorbate kinetics of SrCl2 and SrCO3 apparently follow different orders, which are consistent with electrostatic adsorption and surface precipitation, respectively. In addition, chloride or carbonate profoundly affect the adsorption capacity and configuration, decreasing and increasing Sr adsorption, respectively. Under chlorinated and carbonated conditions, Sr tends to adsorb to rutile as an inner-sphere tetradentate adsorbate via distorted eight-fold coordinated oxygen atoms. It is expected that an increase in total carbonate in seawater would not only increase adsorption but also improve the adsorbate thin-film stability, organization and thin-film thickness as observed in our SrCO3 systems. These organized thin films are expected to be more stable than the adsorbates containing more SrCl+/SrCl2 species due to a diffuse layer in which Sr would adsorb weakly. Moreover, this study would be applicable for other settings where Sr concentrations are expected to be low, such as surface waters, where solid/liquid partitioning constants are expected to be constant (Kd ~ 10 L Kg−1). The implications of these findings will directly feed into the optimization of the molecular dynamics computational models for titanium dioxides in the use of saline wastewater treatment. Moreover, these results would be of great interest for the remediation and treatment of structural materials from spent nuclear cooling ponds, which are coated with titanium dioxide-containing pigments, and for radionuclide mobility in geological disposal facilities from Ti-bearing glasses or interaction with rutile in host-rock fractures, especially since rutile as a substrate is a stable mineral in the environment. Finally, due to its chemical stability, a titanium dioxide substrate could potentially be recycled post treatment and reused for wastewater treatment, which would have significant implications for the treatment costs.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/min11121386/s1, Figure S1: In situ flow-cell setup at the beamline; Figure S2: Sr Species modeling using PHREEQC; Figure S3: Grazing incidence XRD pattern of SrCO3 adsorbed to the rutile(110) surface; Figure S4: Ti 2p and Sr 3d measured XPS spectra; Figure S5: O 1s and C 1s measured XPS spectra; Figure S6: Cl 2s measured XPS spectra; Table S1: Full experimental conditions; Table S2: Batch bulk adsorption and synchrotron in situ adsorption fitting results; Table S3: X-ray reflectivity fitting results of adsorbed Sr on rutile(110); Table S4: XPS fitting results of Sr, O, Ti, and C under the four conditions, SS1: EXAFS fitting routine Sr EXAFS, SS2: Polarization dependence ex situ SrCO3.
Author Contributions
Conceptualization, A.v.V., R.A.W.; Methodology, A.v.V., R.A.W.; formal analysis, A.v.V., P.C.M.F., J.F.W.M., R.A.W.; writing—original draft preparation, A.v.V., R.A.W.; writing—review and editing, A.v.V., P.C.M.F., N.P.E., J.F.W.M., T.S., R.A.W.; visualization, A.v.V.; supervision, R.A.W., T.S.; funding acquisition, R.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge financial support for this research from the UK Nuclear Decommissioning Agency (RWMD) via EPSRC grant EP/I036389/1. Portions of this research were funded by Diamond Light Source Ltd. (SP 4940). Finally, we gratefully acknowledge the support of the U.S. Department of Energy through the LANL/LDRD Program and the G. T. Seaborg Institute to AvV. Los Alamos National Laboratory is operated by Triad National Security, LLC, for the National Nuclear Security Administration of U.S. Department of Energy (Contract No. 89233218CNA000001).
Data Availability Statement
Data used in this study may be made available by request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
References
- Koarai, K.; Kino, Y.; Takahashi, A.; Suzuki, T.; Shimizu, Y.; Chiba, M.; Osaka, K.; Sasaki, K.; Fukuda, T.; Isogai, E.; et al. 90Sr in teeth of cattle abandoned in evacuation zone: Record of pollution from the Fukushima-Daiichi Nuclear Power Plant accident. Sci. Rep. 2016, 6, 24077. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yonezawa, A.; Kumagai, K.; Sano, M.; Miyake, T. Cs and Sr removal over highly effective adsorbents ETS-1 and ETS-2. J. Mater. Chem. A 2015, 3, 1562–1568. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimada, A.; Hoshi, A.; Yasuda, M.; Ozawa, M.; Kameo, Y. Radiochemical analysis of rubble and trees collected from Fukushima Daiichi Nuclear Power Station. J. Nucl. Sci. Technol. 2014, 51, 1032–1043. [Google Scholar] [CrossRef]
- Kozai, N.; Ohnuki, T.; Arisaka, M.; Watanabe, M.; Sakamoto, F.; Yamasaki, S.; Jiang, M. Chemical states of fallout radioactive Cs in the soils deposited at Fukushima Daiichi Nuclear Power Plant accident. J. Nucl. Sci. Technol. 2012, 49, 473–478. [Google Scholar] [CrossRef]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata, J.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; et al. The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K.; Murata, H. Strontium Substitution in Bioactive Calcium Phosphates: A First-Principles Study. J. Phys. Chem. B 2009, 113, 3584–3589. [Google Scholar] [CrossRef]
- Rokita, E.; Hermes, C.; Nolting, H.-F.; Ryczek, J. Substitution of calcium by strontium within selected calcium phosphates. J. Cryst. Growth 1993, 130, 543–552. [Google Scholar] [CrossRef]
- D’Angelo, P.; Migliorati, V.; Sessa, F.; Mancini, G.; Persson, I. XANES Reveals the Flexible Nature of Hydrated Strontium in Aqueous Solution. J. Phys. Chem. B 2016, 120, 4114–4124. [Google Scholar] [CrossRef]
- McKinley, J.P.; Zachara, J.M.; Smith, S.C.; Liu, C. Cation exchange reactions controlling desorption of 90Sr2+ from coarse-grained contaminated sediments at the Hanford site, Washington. Geochim. Cosmochim. Acta 2007, 71, 305–325. [Google Scholar] [CrossRef] [Green Version]
- Ball, J.W.; Nordstrom, D.K. User’s Manual for WATEQ4F, with Revised Thermodynamic Data Base and Text Cases for Calculating Speciation of Major, Trace, and Redox Elements in Natural Waters; U.S. Geological Survey: Denver, CO, USA, 1991; pp. 91–183.
- Parkman, R.H.; Charnock, J.M.; Livens, F.R.; Vaughan, D.J. A study of the interaction of strontium ions in aqueous solution with the surfaces of calcite and kaolinite. Geochim. Cosmochim. Acta 1998, 62, 1481–1492. [Google Scholar] [CrossRef]
- Mucci, A.; Morse, J.W. The incorporation of Mg2+ and Sr2+ into calcite overgrowths: Influences of growth rate and solution composition. Geochim. Cosmochim. Acta 1983, 47, 217–233. [Google Scholar] [CrossRef]
- Chorover, J.; Choi, S.; Rotenberg, P.; Serne, R.J.; Rivera, N.; Strepka, C.; Thompson, A.; Mueller, K.T.; O’Day, P.A. Silicon control of strontium and cesium partitioning in hydroxide-weathered sediments. Geochim. Cosmochim. Acta 2008, 72, 2024–2047. [Google Scholar] [CrossRef]
- Chiang, P.N.; Wang, M.K.; Huang, P.M.; Wang, J.J.; Chiu, C.Y. Cesium and strontium sorption by selected tropical and subtropical soils around nuclear facilities. J. Environ. Radioact. 2010, 101, 472–481. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, Z.; Chen, C.; Hu, J.; Chen, H. Effect of environmental conditions on the adsorption behavior of Sr(II) by Na-rectorite. Appl. Clay Sci. 2014, 87, 1–6. [Google Scholar] [CrossRef]
- Marinin, D.V.; Brown, G.N. Studies of sorbent/ion-exchange materials for the removal of radioactive strontium from liquid radioactive waste and high hardness groundwaters. Waste Manag. 2000, 20, 545–553. [Google Scholar] [CrossRef]
- Zachara, J.M.; Ainsworth, C.C.; Brown, G.E., Jr.; Catalano, J.G.; McKinley, J.P.; Qafoku, O.; Smith, S.C.; Szecsody, J.E.; Traina, S.J.; Warner, J.A. Chromium speciation and mobility in a high level nuclear waste vadose zone plume. Geochim. Cosmochim. Acta 2004, 68, 13–30. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Hu, J.; Shao, D.; Li, J.; Wang, X. Adsorption behavior of multiwall carbon nanotube/iron oxide magnetic composites for Ni(II) and Sr(II). J. Hazard. Mater. 2009, 164, 923–928. [Google Scholar] [CrossRef]
- Chegrouche, S.; Mellah, A.; Barkat, M. Removal of strontium from aqueous solutions by adsorption onto activated carbon: Kinetic and thermodynamic studies. Desalination 2009, 235, 306–318. [Google Scholar] [CrossRef]
- Zhang, A.; Kuraoka, E.; Kumagai, M. Removal of Pd(II), Zr(IV), Sr(II), Fe(III), and Mo(VI) from simulated high level liquid waste by extraction chromatography utilizing the 17 icroporous silica-based polymeric materials. Sep. Purif. Technol. 2006, 50, 35–44. [Google Scholar] [CrossRef]
- Duff, M.C.; Hunter, D.B.; Hobbs, D.T.; Fink, S.D.; Dai, Z.; Bradley, J.P. Mechanisms of Strontium and Uranium Removal from High-Level Radioactive Waste Simulant Solutions by the Sorbent Monosodium Titanate. Environ. Sci. Technol. 2004, 38, 5201–5207. [Google Scholar] [CrossRef] [Green Version]
- Tagawa, H.; Igarashi, K. Reaction of Strontium Carbonate with Anatase and Rutile. J. Am. Ceram. Soc. 1986, 69, 310–314. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Prediction of the speciation of alkaline earths adsorbed on mineral surfaces in salt solutions. Geochim. Cosmochim. Acta 2006, 70, 2427–2453. [Google Scholar] [CrossRef]
- Ridley, M.K.; Machesky, M.L.; Kubicki, J.D. Experimental Study of Strontium Adsorptio on Anatase Nanoparticles as a Function of Size with a Density Functional Theory and CD Model Interpretation. Langmuir 2015, 31, 703–713. [Google Scholar] [CrossRef]
- Behrens, E.A.; Sylvester, P.; Clearfield, A. Assessment of a Sodium Nonatitanate and Pharmacosiderite-Type Ion Exchangers for Strontium and Cesium Removal from DOE Waste Simulants. Environ. Sci. Technol. 1998, 32, 101–107. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Zhang, Z.; Fenter, P.; Cheng, L.; Sturchio, N.C.; Bedzyk, M.J.; Předota, M.; Bandura, A.; Kubicki, J.D.; Lvov, S.N.; Cummings, P.T.; et al. Ion Adsorption at the Rutile−Water Interface: Linking Molecular and Macroscopic Properties. Langmuir 2004, 20, 4954–4969. [Google Scholar] [CrossRef]
- van Veelen, A.; Copping, R.; Law, G.T.W.; Smith, A.J.; Bargar, J.R.; Rogers, J.; Shuh, D.K.; Wogelius, R.A. Uranium uptake onto Magnox sludge minerals studied using EXAFS. Mineral. Mag. 2012, 76, 3095–3104. [Google Scholar] [CrossRef]
- van Veelen, A.; Preedy, O.; Qi, J.; Law, G.T.W.; Morris, K.; Mosselmans, J.F.W.; Ryan, M.P.; Evans, N.D.M.; Wogelius, R.A. Uranium and technetium interactions with wüstite [Fe1–xO] and portlandite [Ca(OH)2] surfaces under geological disposal facility conditions. Mineral. Mag. 2014, 78, 1097–1113. [Google Scholar] [CrossRef]
- van Veelen, A.; Bargar, J.R.; Law, G.T.W.; Brown, G.E.; Wogelius, R.A. Uranium Immobilization and Nanofilm Formation on Magnesium-Rich Minerals. Environ. Sci. Technol. 2016, 50, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1999.
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Downward, L.; Booth, C.H.; Lukens, W.W.; Bridges, F. A Variation of the F-Test for Determining Statistical Relevance of Particular Parameters in EXAFS Fits. AIP Conf. Proc. 2007, 882, 129–131. [Google Scholar]
- Bots, P.; van Veelen, A.; Mosselmans, J.F.W.; Muryn, C.; Wogelius, R.A.; Morris, K. Neptunium (V) and Uranium (VI) Reactions at the Magnetite (111) Surface. Geosciences 2019, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Anné, J.; Wogelius, R.A.; Edwards, N.P.; van Veelen, A.; Buckley, M.; Sellers, W.I.; Bergmann, U.; Sokaras, D.; Alonso-Mori, R.; Harvey, V.L.; et al. Morphological and chemical evidence for cyclic bone growth in a fossil hyaena. J. Anal. At. Spectrom. 2018, 33, 2062–2069. [Google Scholar] [CrossRef]
- Anné, J.; Wogelius, R.A.; Edwards, N.P.; van Veelen, A.; Ignatyev, K.; Manning, P.L. Chemistry of bone remodelling preserved in extant and fossil Sirenia. Metallomics 2016, 8, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.A.; van Veelen, A.; Morris, K.; Mosselmans, J.F.W.; Wogelius, R.A.; Burton, N.A. Uranium (VI) Adsorbate Structures on Portlandite [Ca(OH)2] Type Surfaces Determined by Computational Modelling and X-ray Absorption Spectroscopy. Minerals 2021, 11, 1241. [Google Scholar] [CrossRef]
- Anné, J.; Edwards, N.P.; Wogelius, R.A.; Tumarkin-Deratzian, A.R.; Sellers, W.I.; van Veelen, A.; Bergmann, U.; Sokaras, D.; Alonso-Mori, R.; Ignatyev, K.; et al. Synchrotron imaging reveals bone healing and remodelling strategies in extinct and extant vertebrates. J. R. Soc. Interface 2014, 11, 20140277. [Google Scholar] [CrossRef] [Green Version]
- Edwards, N.P.; van Veelen, A.; Anne, J.; Manning, P.L.; Bergmann, U.; Sellers, W.I.; Egerton, V.M.; Sokaras, D.; Alonso-Mori, R.; Wakamatsu, K.; et al. Elemental characterization of melanin in feathers via synchrotron X-ray imaging and absorption spectroscopy. Sci. Rep. 2016, 6, 34002. [Google Scholar] [CrossRef]
- Thorpe, C.L.; Lloyd, J.R.; Law, G.T.W.; Burke, I.T.; Shaw, S.; Bryan, N.D.; Morris, K. Strontium sorption and precipitation behaviour during bioreduction in nitrate impacted sediments. Chem. Geol. 2012, 306, 114–122. [Google Scholar] [CrossRef]
- Anné, J.; Edwards, N.P.; van Veelen, A.; Egerton, V.M.; Manning, P.L.; Mosselmans, J. Fredrick, W.; Parry, S.; Sellers, W.I.; Buckley, M.; et al. Visualisation of developmental ossification using trace element mapping. J. Anal. At. Spectrom. 2017, 32, 967–974. [Google Scholar] [CrossRef]
- Agron, P.A.; Busing, W.R. Calcium and Strontium Dichloride Hexahydrates by Neutron Diffraction. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1986, 42, 141–143. [Google Scholar] [CrossRef]
- O’Day, P.A.; Newville, M.; Neuhoff, P.S.; Sahai, N.; Carroll, S.A. X-Ray Absorption Spectroscopy of Strontium(II) Coordination. J. Colloid Interface Sci. 2000, 222, 184–197. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, J.P.R. Crystal structures of aragonite, strontianite and witherite. Am. Mineral. 1971, 56, 758–767. [Google Scholar]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Stipp, S.L.; Hochella, M.F., Jr. Structure and bonding environments at the calcite surface as observed with X-ray photoelectron spectroscopy (XPS) and low energy electron diffraction (LEED). Geochim. Cosmochim. Acta 1991, 55, 1723–1736. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Liu, F.; Alim, M.A.; Chen, G. The origin of varistor property of SrTiO3-based ceramics. J. Mater. Sci. Mater. Electron. 2003, 14, 483–486. [Google Scholar] [CrossRef]
- Jung, W.; Tuller, H.L. Investigation of surface Sr segregation in model thin film solid oxide fuel cell perovskite electrodes. Energy Environ. Sci. 2012, 5, 5370–5378. [Google Scholar] [CrossRef] [Green Version]
- Kosmulski, M. pH-dependent surface charging and points of zero charge. IV. Update and new approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef]
- Kosmulski, M. The significance of the difference in the point of zero charge between rutile and anatase. Adv. Colloid Interface Sci. 2002, 99, 255–264. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).