Formation of Esseneite and Kushiroite in Tschermakite-Bearing Calc-Silicate Xenoliths Ejected in Alkali Basalt
Abstract
:1. Introduction
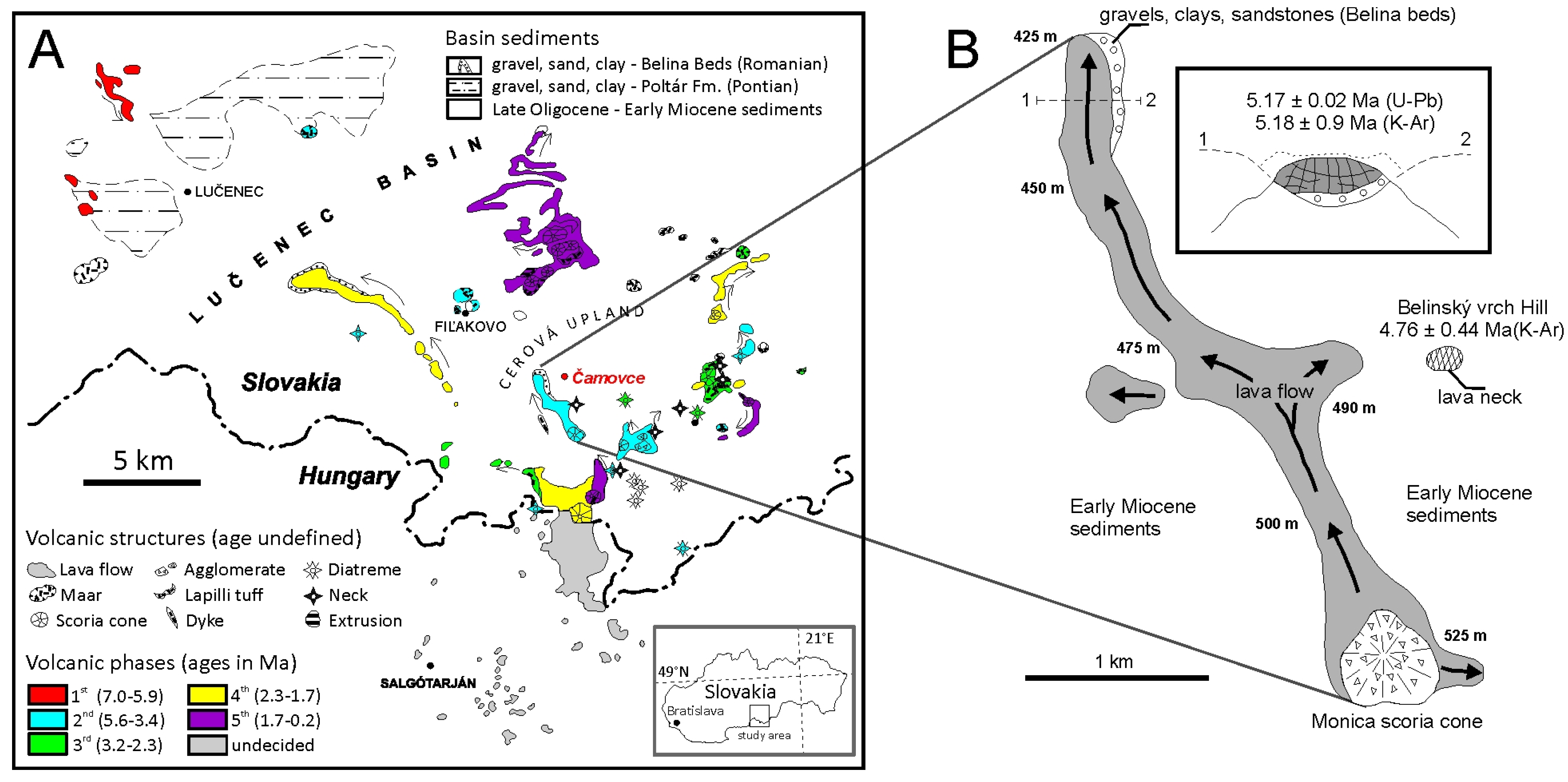
2. Geological Setting
3. Materials and Methods
4. Results
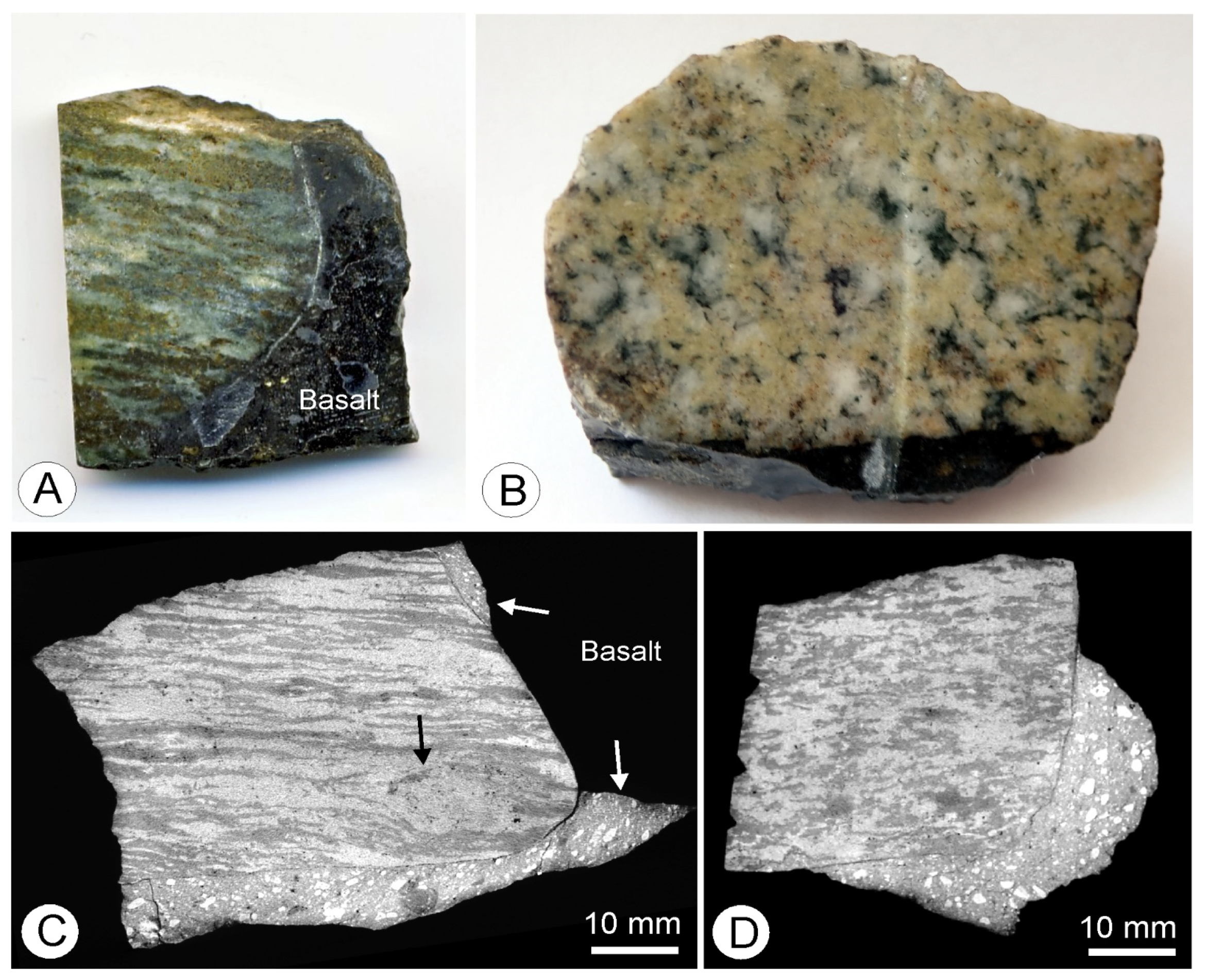
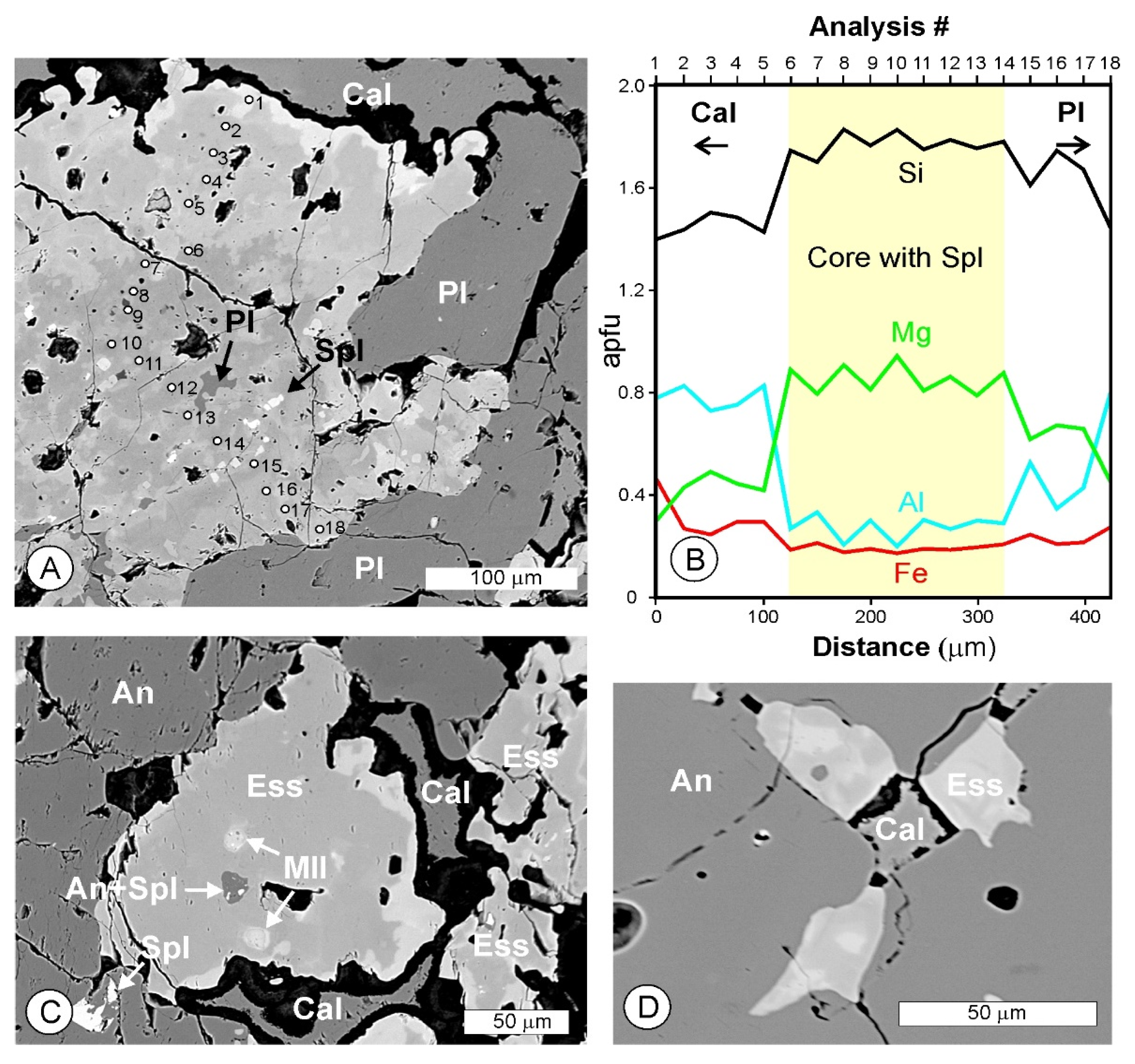
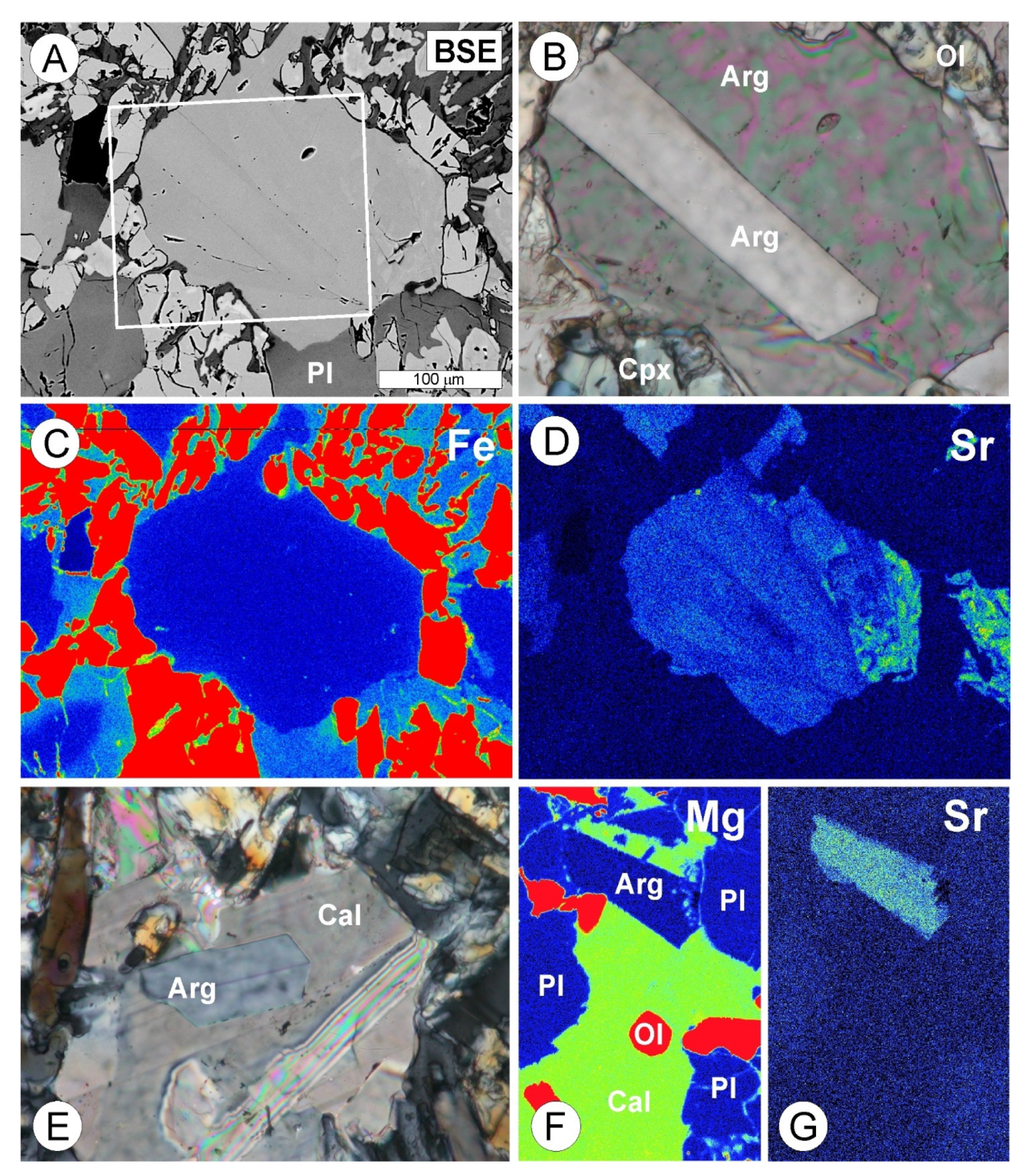
4.1. Petrography and Textures
4.2. Mineral Assemblages
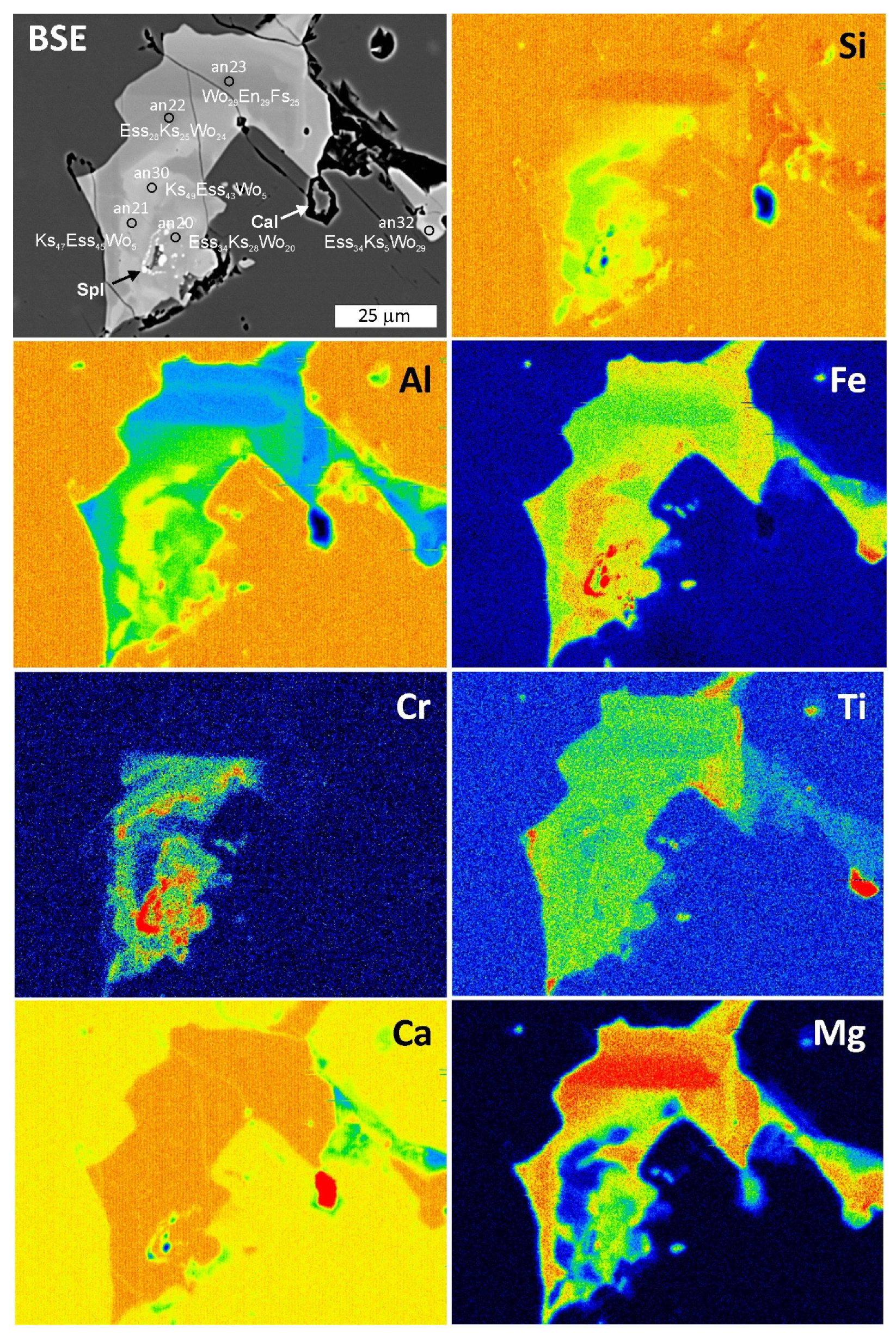
4.3. Mineral Compositions
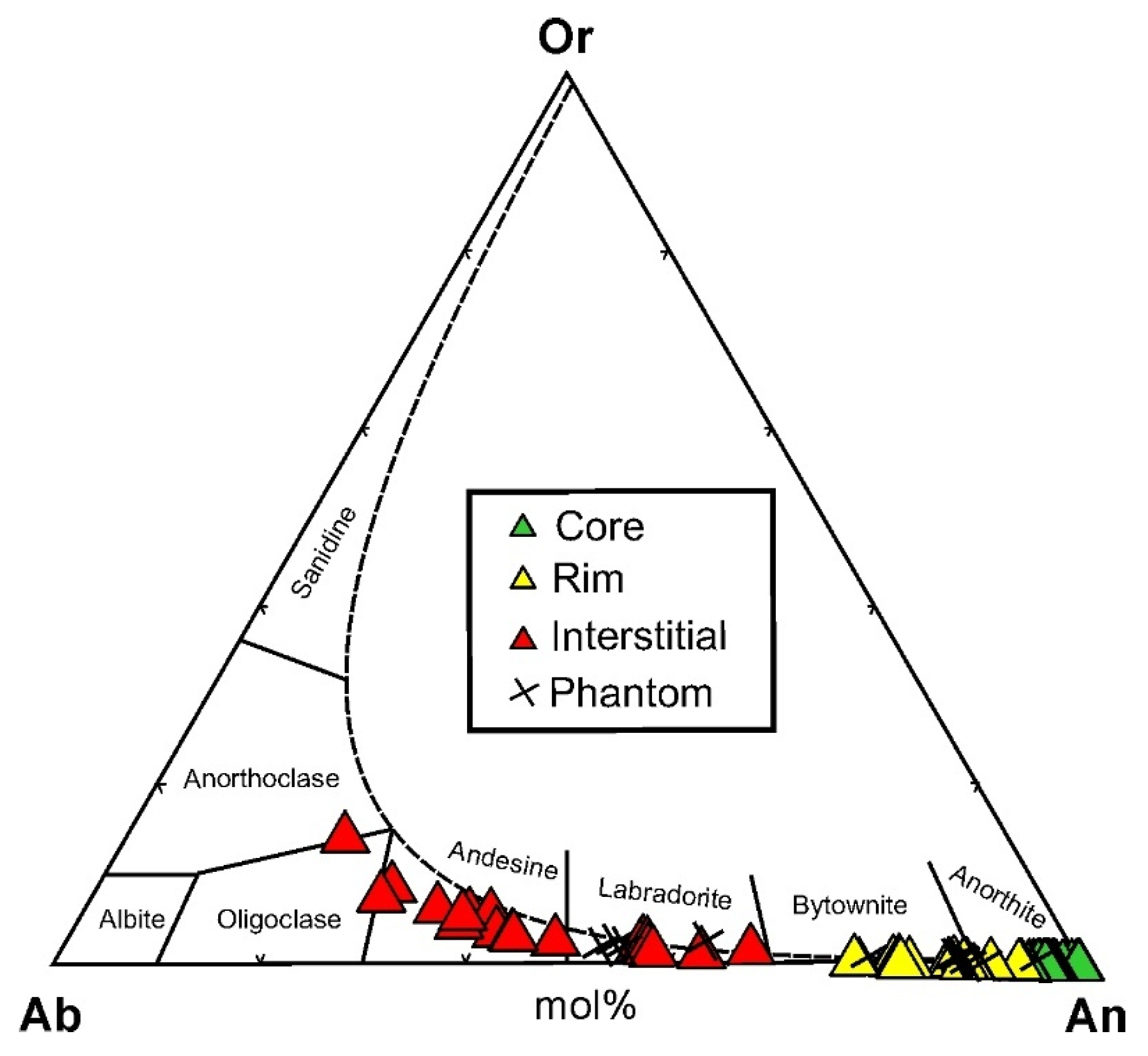
4.3.1. Plagioclase
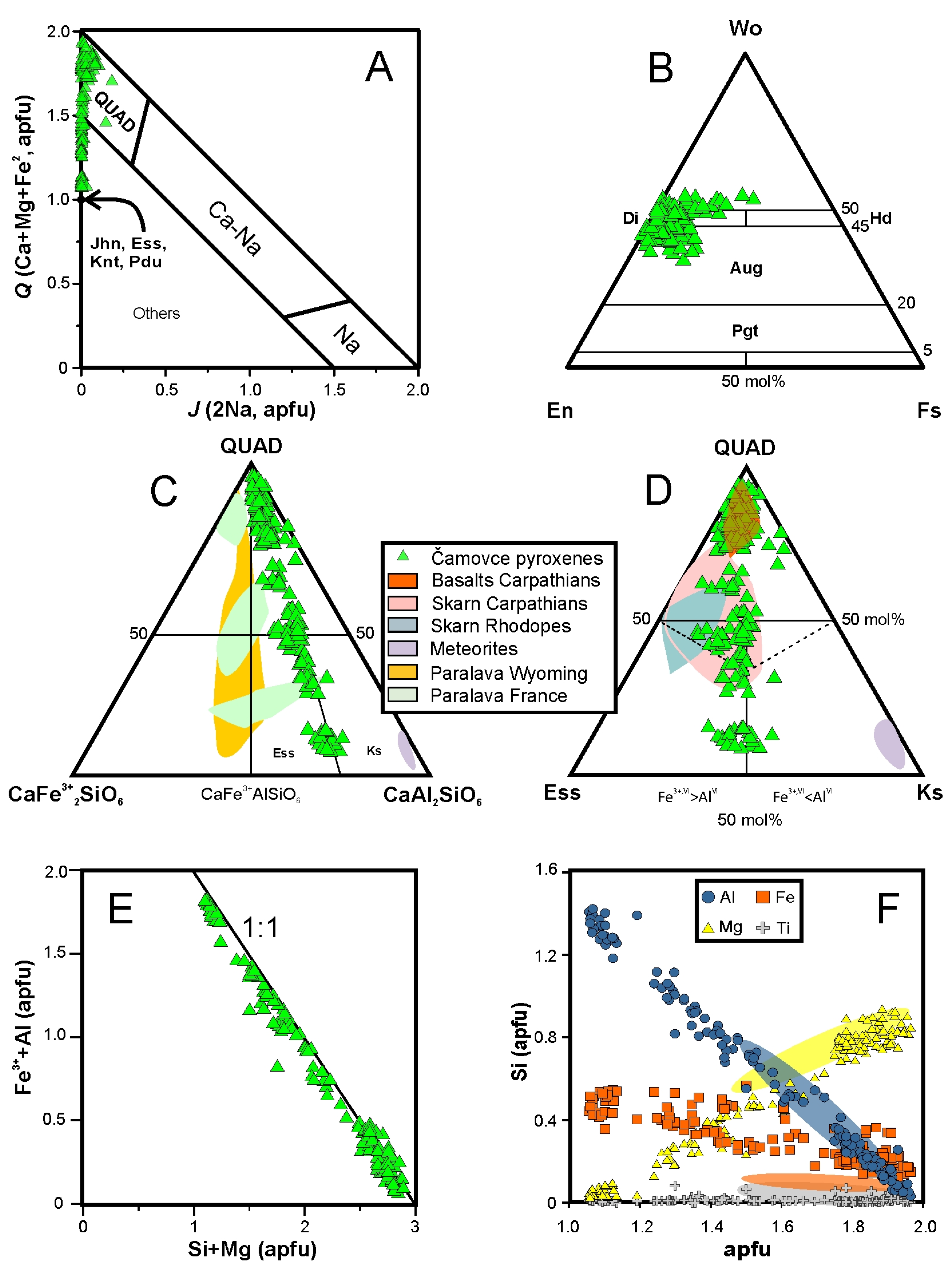
4.3.2. Pyroxene
4.3.3. Olivine
4.3.4. Amphibole
(AlIV1.80–1.86Si6.14–6.20)O22(OH)2
| EPMA (wt% Oxide) | Formula Based on 24 (O, OH, F, Cl) | |||||
|---|---|---|---|---|---|---|
| Analysis | 9 | 14 | 9 | 14 | ||
| SiO2 | 44.96 | 45.45 | T (8 apfu) | Si | 6.144 | 6.204 |
| TiO2 | 0.01 | 0.01 | Al | 1.856 | 1.796 | |
| Al2O3 | 23.62 | 24.21 | C (5 apfu) | Ti | 0.001 | 0.001 |
| Cr2O3 | b.d.l. | b.d.l. | Al | 1.948 | 2.100 | |
| NiO | 0.01 | 0.01 | Fe3+ | 0.187 | ||
| Fe2O3 * | 1.82 | 0.00 | Mn2+ | 0.009 | ||
| FeO | 2.22 | 3.65 | Fe2+ | 0.208 | 0.417 | |
| MnO | 0.07 | 0.08 | Mg | 2.656 | 2.464 | |
| MgO | 13.04 | 12.11 | C subtotal | 5.000 | 4.991 | |
| CaO | 10.78 | 11.01 | B (2 apfu) | Mn2+ | 0.008 | |
| Na2O | 1.65 | 1.80 | Fe2+ | 0.046 | ||
| K2O | 0.10 | 0.10 | Ca | 1.579 | 1.611 | |
| Cl | b.d.l. | b.d.l. | Na | 0.368 | 0.389 | |
| F | b.d.l. | b.d.l. | A (1 apfu) | Na | 0.070 | 0.088 |
| H2O+ | 1.69 | 1.69 | K | 0.017 | 0.017 | |
| Total | 99.96 | 100.10 | ▯ | 0.913 | 0.895 | |
| W (2 apfu) | OH | 2.000 | 2.000 | |||
4.3.5. Carbonates
4.3.6. Spinel-Group Minerals
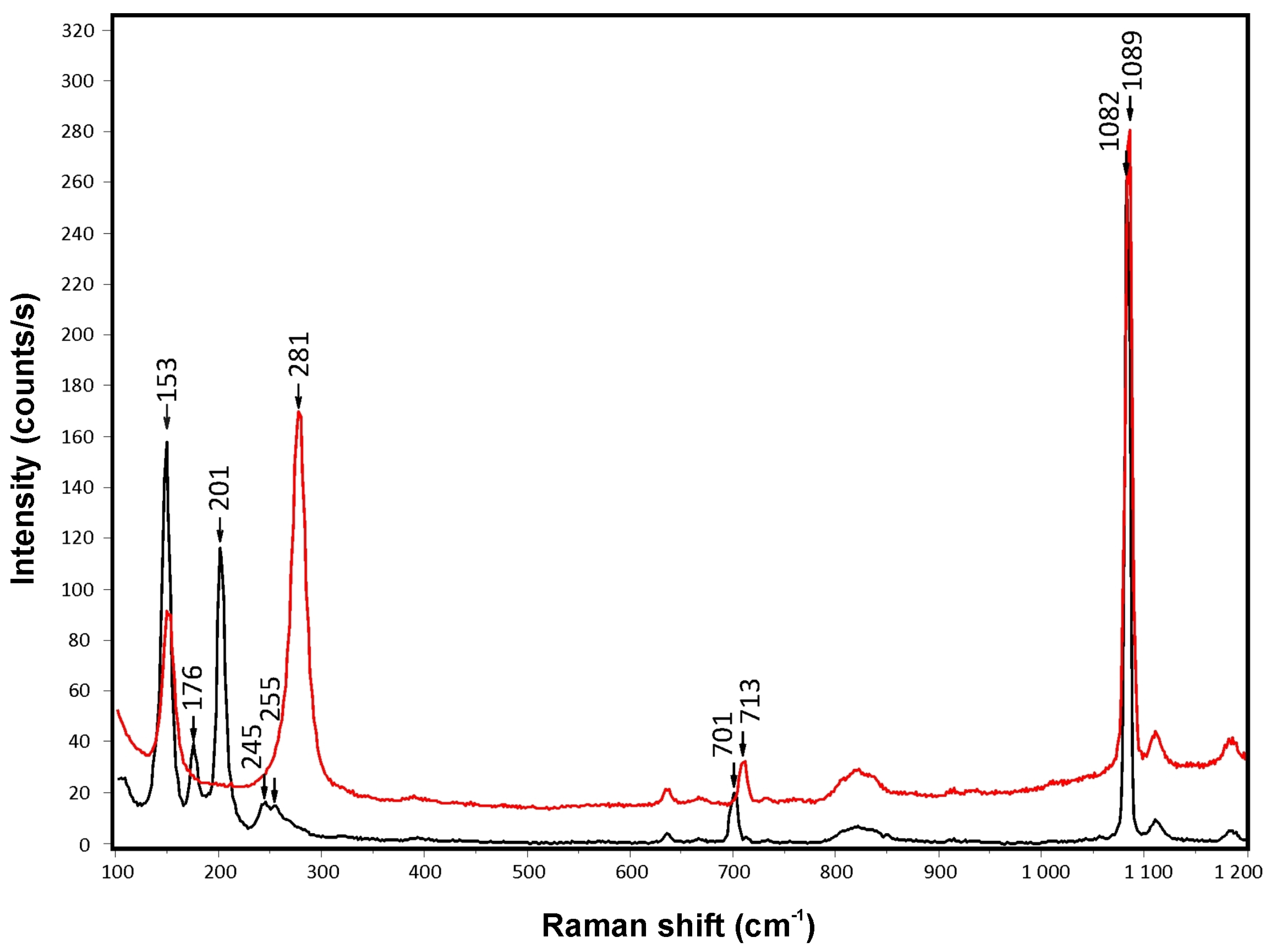
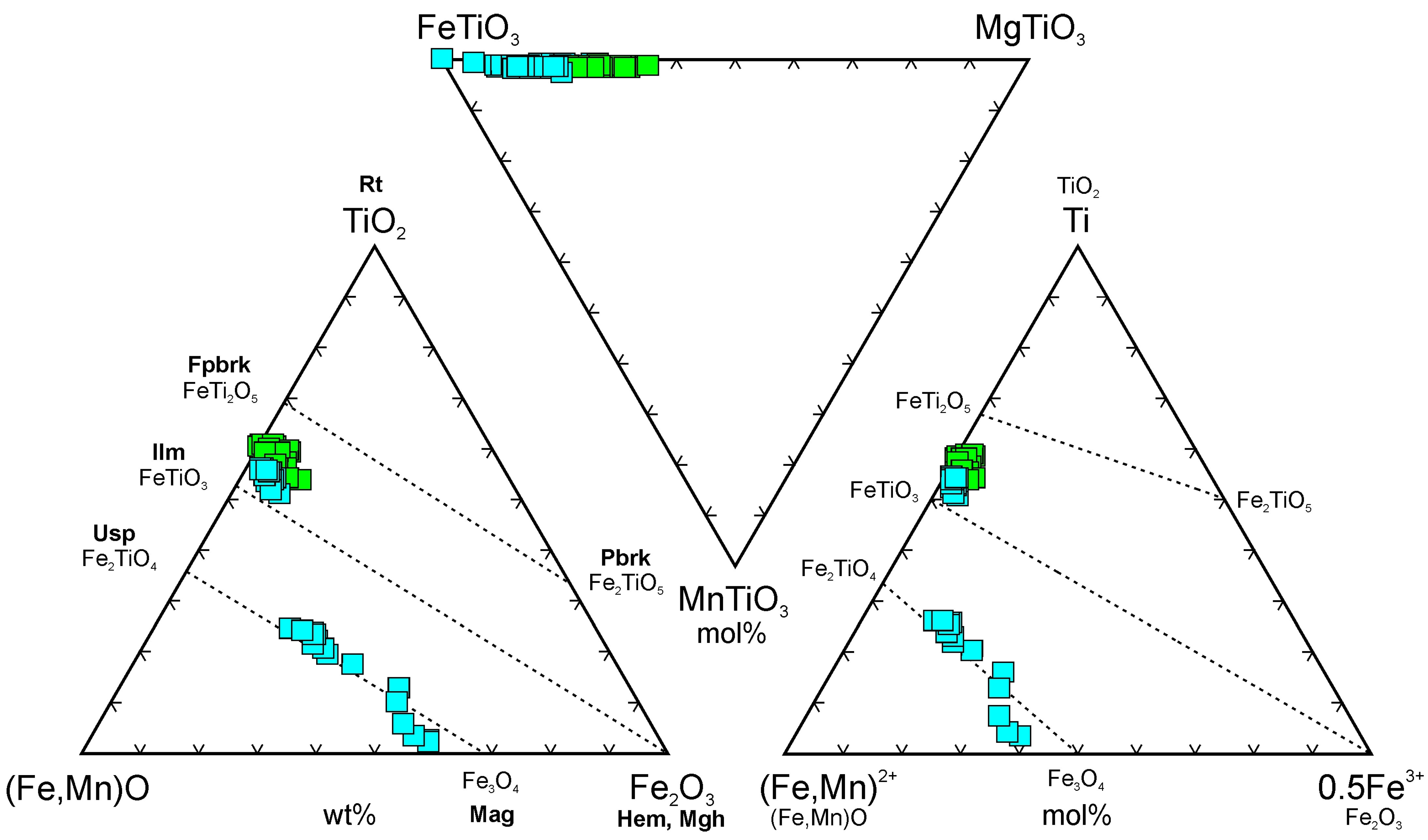
4.3.7. Rhombohedral Fe,Ti-Oxides
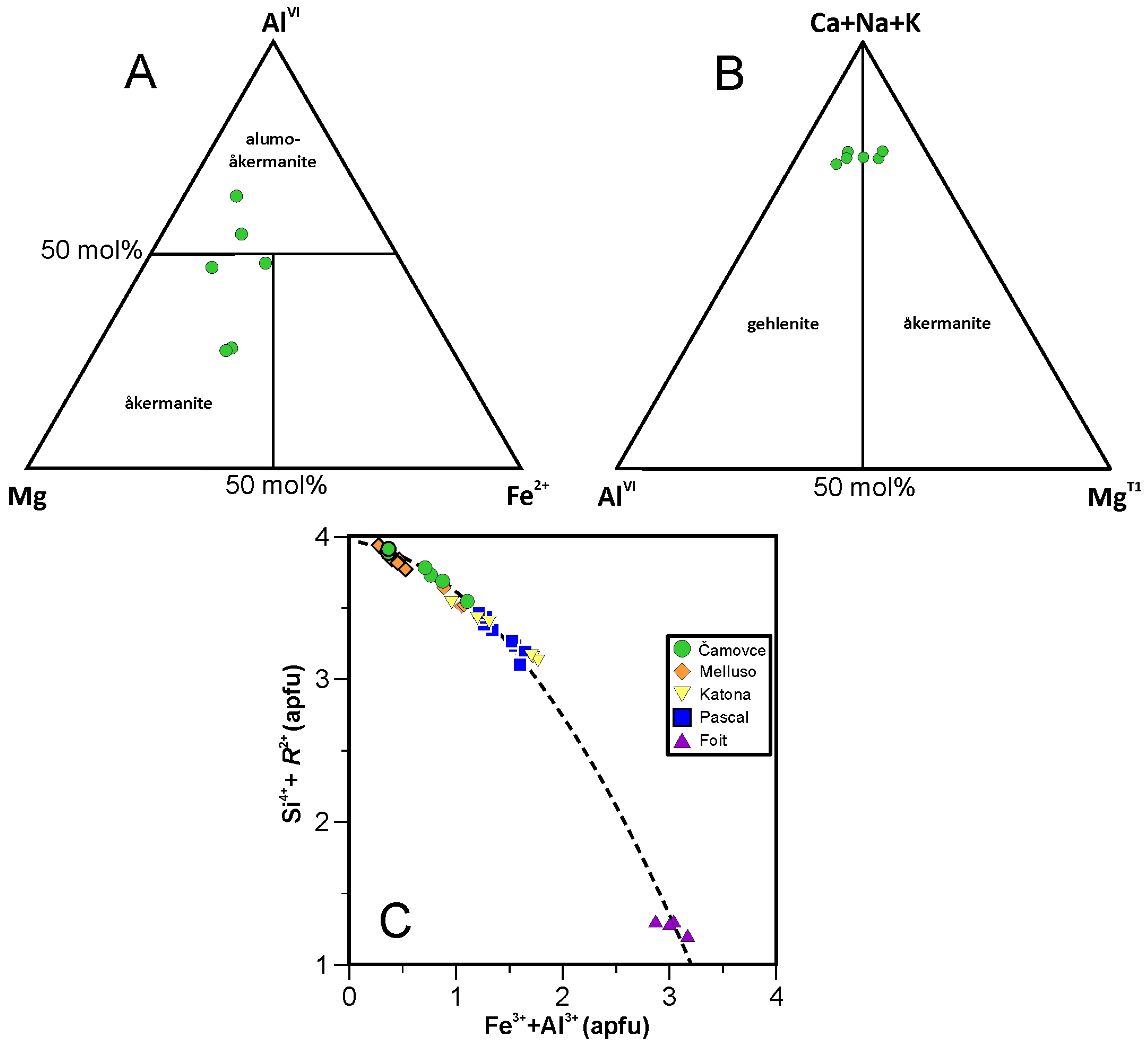
4.3.8. Melilite
| Sample | Ca-14 | Ca-14 | Ca-14 | Ca-14 | Ca-14 | Cam-14 |
|---|---|---|---|---|---|---|
| Analysis # | 49 | 56 | 57 | 61 | 86 | 10 |
| SiO2 (wt%) | 41.40 | 40.91 | 36.34 | 32.69 | 37.25 | 35.73 |
| TiO2 | 0.01 | b.d.l. | 0.02 | 0.02 | 0.02 | 0.04 |
| Al2O3 | 6.70 | 6.13 | 13.42 | 19.07 | 11.37 | 14.78 |
| Cr2O3 | 0.02 | 0.05 | 0.02 | 0.05 | 0.02 | 0.01 |
| FeO | 7.10 | 7.33 | 6.47 | 3.98 | 5.28 | 5.44 |
| NiO | 0.01 | b.d.l. | b.d.l. | 0.01 | b.d.l. | 0.01 |
| MnO | 0.15 | 0.21 | 0.26 | 0.09 | 0.14 | 0.11 |
| MgO | 6.61 | 6.55 | 3.85 | 3.65 | 5.43 | 4.13 |
| CaO | 36.39 | 37.04 | 37.56 | 38.03 | 36.55 | 36.77 |
| K2O | 0.03 | 0.03 | b.d.l. | 0.02 | 0.04 | 0.03 |
| Na2O | 1.89 | 1.87 | 1.72 | 1.51 | 2.17 | 2.14 |
| Total | 100.30 | 100.12 | 99.66 | 99.12 | 98.26 | 99.20 |
| Si (apfu) | 1.918 | 1.901 | 1.696 | 1.517 | 1.745 | 1.660 |
| Ti | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 |
| AlIV | 0.082 | 0.099 | 0.304 | 0.483 | 0.255 | 0.340 |
| AlVI | 0.284 | 0.237 | 0.434 | 0.560 | 0.373 | 0.470 |
| Cr | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.000 |
| Fe3+ * | 0.000 | 0.030 | 0.025 | 0.058 | 0.079 | 0.062 |
| Fe2+ | 0.275 | 0.255 | 0.228 | 0.096 | 0.128 | 0.150 |
| Ni | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Mn | 0.006 | 0.008 | 0.010 | 0.004 | 0.005 | 0.004 |
| Mg | 0.456 | 0.454 | 0.268 | 0.252 | 0.379 | 0.286 |
| Ca | 1.806 | 1.844 | 1.878 | 1.890 | 1.844 | 1.831 |
| K | 0.002 | 0.002 | 0.000 | 0.001 | 0.002 | 0.002 |
| Na | 0.169 | 0.168 | 0.156 | 0.136 | 0.197 | 0.193 |
| Na-melilite ** (mol%) | 17.4 | 16.6 | 15.1 | 13.2 | 19.1 | 18.8 |
| Gehlenite | 1.5 | 0.0 | 24.7 | 42.2 | 13.5 | 24.2 |
| Åkermanite | 50.6 | 51.2 | 31.0 | 27.6 | 43.6 | 32.7 |
| Fe-åkermanite | 30.5 | 32.2 | 29.2 | 16.9 | 23.8 | 24.2 |
4.4. Thermobarometry
5. Discussion
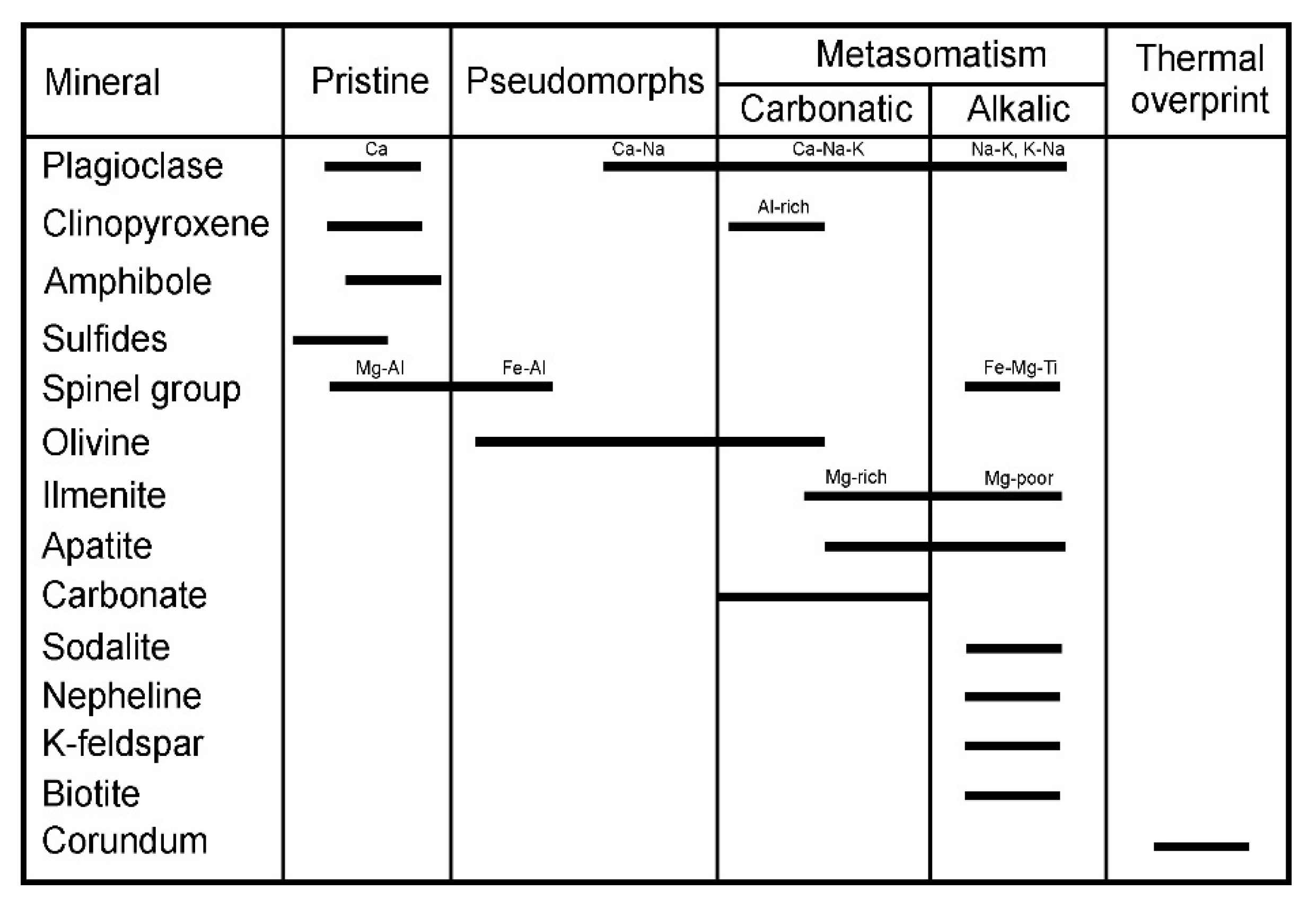
5.1. Protolith
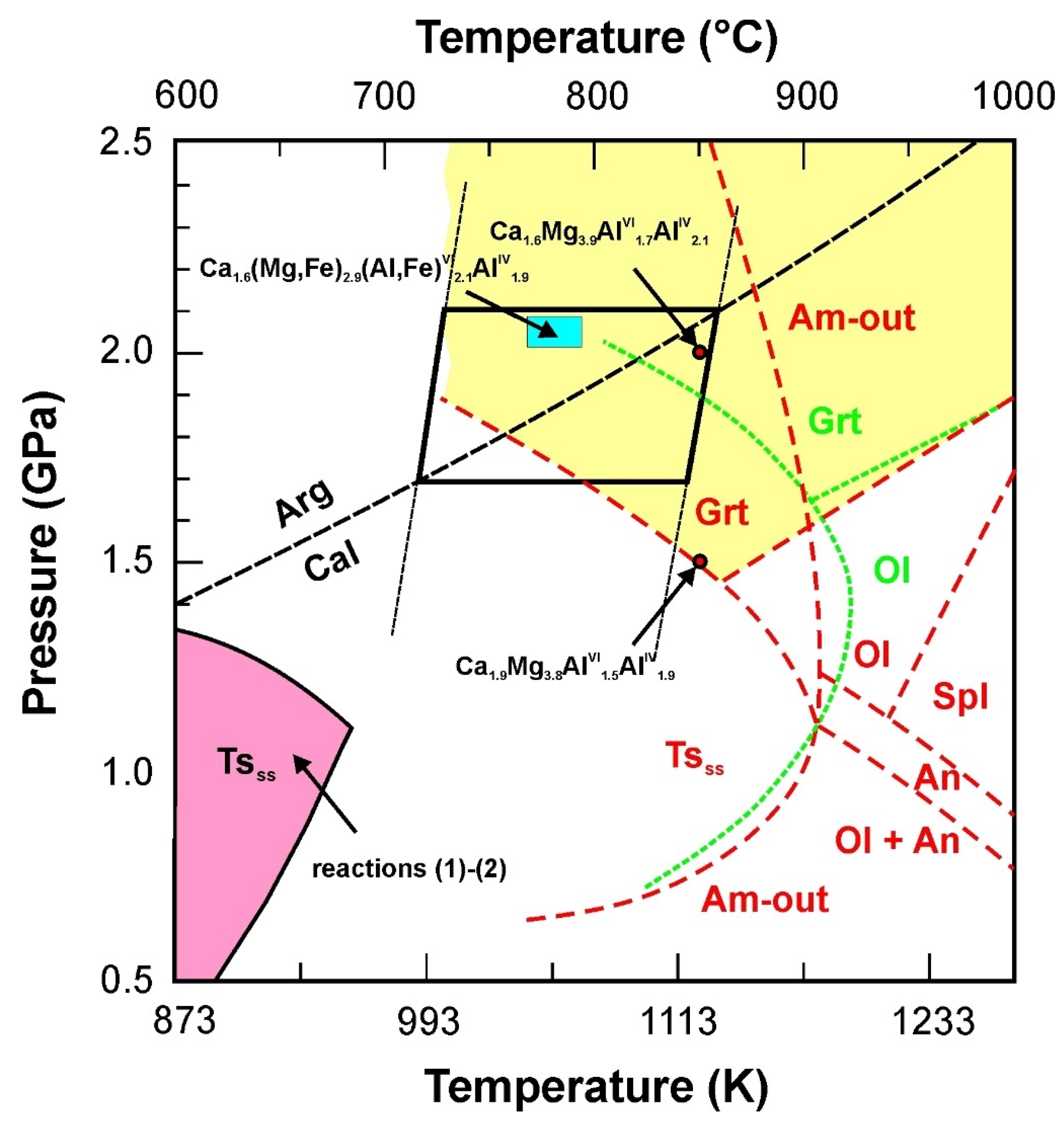
5.2. P-T Constraints on the Stability of Tschermakite, Kushiroite and Esseneite
Ca2Mg3Al4Si6O22(OH)2 + CaCO3 = 2CaMgSi2O6 + CaAl2Si2O8 + MgAl2O4 + H2O + CO2
Ca2Mg3Al4Si6O22(OH)2 + MgCO3 = Mg3Al2Si3O12 + CaMgSi2O6 + CaAl2Si2O8 + H2O + CO2
6. Conclusions
- The presence of Al,Fe3+-rich pyroxenes with melilite inclusions testifies a thermo-metamorphic interaction of xenoliths with strongly oxidizing carbonate-aluminosilicate melt with decreased aSiO2. Concentrations of the kushiroite endmember in some pyroxenes, up to 47.5 mol% (31 wt% Al2O3), are substantially higher than the highest natural terrestrial values known to the authors, i.e., 24 wt% in gehlenite-rich skarns from the Carpathians [7] and 22.3 wt% in exoskarn xenoliths ejected by the Merapi volcano, Indonesia [46].
- Tschermakite with high VIAl content (1.9–2.1 apfu) and very low occupancy of the A-site (<0.1 apfu), together with aragonite and calcite coexisting in carbonate pockets indicate high-pressure metamorphic conditions contradictory with other skarn xenoliths found in pyroclastic deposits [46,85,86] or layered intrusions [87] formed at pressures below 0.1 GPa. The olivine-ilmenite-aragonite-calcite and plagioclase-amphibole thermobarometers returned temperatures within the 770–860 °C range and pressures between 1.8 and 2.1 GPa, corresponding to depths of 60–70 km assuming lithostatic load. The presence of Al,Fe3+ pyroxenes, forsterite and Mg,Al-spinel at the expense of garnet is a consequence of the high Al content of the parental rock, low SiO2 activity, high CO2 partial pressure and strongly oxidizing conditions.
- The finding of relict tschermakite in spinel-plagioclase-forsterite pseudomorphs suggest a metamorphosed calc-silicate marble originating from a sedimentary protolith. On the other hand, a magmatic protolith, similar to layered gabbro-anorthosite complexes contaminated by calcic carbonatite melt [87] cannot be ruled out considering high Cr contents in spinels and pyroxenes, abundant Cu-sulfides, and high CaO contents, 0.3–1.0 wt% CaO, in forsterite.
- Interactions between xenoliths and basalt host were dimension-dependent, showing smaller xenoliths to undergo more intense alkalic metasomatic alteration recorded by the crystallization of interstitial biotite, sanidine, sodalite, nepheline, Mg-poor ilmenite and Fe-Ti magnetite. The thermal overprint of smaller xenoliths resulted in local exsolution of corundum from basic plagioclase.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosca, M.; Peacor, D. Chemistry and structure of esseneite (CaFe3+AlSiO6), a new pyroxene produced by pyrometamorphism. Am. Mineral. 1987, 72, 148–156. [Google Scholar]
- Foit, F.; Hooper, R.; Rosenberg, P. An unusual pyroxene, melilite, and iron oxide mineral assemblage in a coal-fire buchite from Buffalo, Wyoming. Am. Mineral. 1987, 72, 137–147. [Google Scholar]
- Kruszewski, L.; Gatel, P.; Thiéry, V.; Moszumanska, I.; Kusy, D. Crystallochemical behavior of slag minerals and the occurrence of potentially new mineral species from Lapanouse-de-Sévérac, France. In Coal and Peat Fires: A Global Perspective; Stracher, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 5, pp. 243–300. [Google Scholar]
- Chesnokov, B.V.; Shcherbakova, E.P. Mineralogiya gorelykh Otvalov Chelyabinskogo Ugolnogo Basseina (Opyt Mineralogii Tekhnogeneza) (Mineralogy of Burnt Dumps of the Chelyabinsk Coal Basin—Experience of Mineralogy of Technogenesis); Nauka: Moscow, Russia, 1991. [Google Scholar]
- Vapnik, Y.; Sharygin, V.; Sokol, E.V. Paralavas in a combustion metamorphic complex: Hatrurim Basin, Israel. Geol. Soc. Am. Rev. Eng. Geol. 2007, 18, 1–21. [Google Scholar]
- Yakubovich, O.V.; Zayakina, N.V.; Oleinikov, O.B.; Kostin, A.V. Esseneite from xenoliths in dacite lavas: Crystal structure and genesis. Geol. Ore Dep. 2019, 61, 689–695. [Google Scholar] [CrossRef]
- Pascal, M.; Katona, I.; Fonteilles, M.; Verkaeren, J. Relics of high-temperature clinopyroxene on the join Di–CaTs with up to 72 mol. % Ca(Al,Fe3+)AlSiO6 in the skarns of Ciclova and Magureaua Vatei, Carpathians, Romania. Can. Mineral. 2005, 43, 857–881. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; El Goresy, A.; Palme, H.; Zinner, E. Ca-, Al-rich inclusions in the unique chondrite ALH85085: Petrology, chemistry, and isotopic compositions. Geochim. Cosmochim. Acta 1993, 57, 2329–2359. [Google Scholar] [CrossRef]
- Kimura, M.; Mikouchi, T.; Suzuki, A.; Miyahara, M.; Ohtani, E.; Goresy, A. Kushiroite, CaAlAlSiO6: A new mineral of the pyroxene group from the ALH 85085 CH chondrite, and its genetic significance in refractory inclusions. Am. Mineral. 2009, 94, 1479–1482. [Google Scholar] [CrossRef]
- Ma, C.; Simon, S.; Rossman, G.; Grossman, L. Calcium Tschermak’s pyroxene, CaAlAlSiO6, from the Allende and Murray meteorites: EBSD and micro-Raman characterizations. Am. Mineral. 2009, 94, 1483–1486. [Google Scholar] [CrossRef]
- Hays, J.F. Stability and properties of the synthetic pyroxene CaAl2SiO6. Am. Mineral. 1966, 51, 1524–1529. [Google Scholar]
- Kimura, M.; El Goresy, A.; Mikouchi, T.; Suzuki, A.; Miyahara, M.; Ohtani, E. Kushiroite, CaAl2SiO6, a new mineral in carbonaceous chondrites: Its formation conditions and genetic significance in Ca-Al rich refractory inclusions. Meteor. Planet. Sci. 2009, 44, A110. [Google Scholar]
- Konečný, V.; Kováč, M.; Lexa, J.; Šefara, J. Neogene evolution of the Carpatho-Pannonian region: An interplay of subduction and back-arc diapiric uprise in the mantle. EGU Stephan Mueller Spec. Pub. Ser. 2002, 1, 105–123. [Google Scholar] [CrossRef]
- Downes, H.; Pantó, G.; Póka, T.; Mattey, D.; Greenwood, P. Calc-alkaline volcanics of the Inner Carpathian arc, Northern Hungary: New geochemical and oxygen isotopic results. Acta Vulcanol. 1995, 7, 29–41. [Google Scholar]
- Dobosi, G. Late-Cenozoic alkalic basalt magmatism in northern Hungary and Slovakia: Petrology, source compositions and relationship to tectonics. Acta Vulcanol. 1995, 7, 199–207. [Google Scholar]
- Konečný, V.; Lexa, J.; Balogh, K.; Konečný, P. Alkali basalt volcanism in Southern Slovakia: Volcanic forms and time evolution. Acta Vulcanol. 1995, 7, 167–172. [Google Scholar]
- Vass, D.; Elečko, M. (Eds.) Vysvetlivky ku Geologickej Mape Lučenskej Kotliny a Cerovej vrchoviny (Explanatory Notes to the Geological Map of Lučenská Kotlina Depression and Cerová Vrchovina Upland 1:50 000); Štátny Geologicky Ústav D. Štúra: Bratislava, Slovakia, 1992; 196p. (In Slovak) [Google Scholar]
- Huraiová, M.; Konečný, P.; Konečný, V.; Simon, K.; Hurai, V. Mafic and salic igneous xenoliths in late Tertiary alkaline basalts: Fluid inclusion and mineralogical evidence for a deep-crustal magmatic reservoir in the Western Carpathians. Eur. J. Mineral. 1996, 8, 901–916. [Google Scholar] [CrossRef] [Green Version]
- Huraiová, M.; Paquette, J.L.; Konečný, P.; Gannoun, A.M.; Hurai, V. Geochemistry, mineralogy, and zircon U–Pb–Hf isotopes in peraluminous A-type granite xenoliths in Pliocene–Pleistocene basalts of northern Pannonian Basin (Slovakia). Contrib. Mineral. Petrol. 2017, 172, 59. [Google Scholar] [CrossRef]
- Huraiová, M.; Konečný, P.; Hurai, V. Niobium Mineralogy of Pliocene A1-Type Granite of the Carpathian Back-Arc Basin, Central Europe. Minerals 2019, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Hurai, V.; Simon, K.; Wiechert, U.; Hoefs, J.; Konečný, P.; Huraiová, M.; Pironon, J.; Lipka, J. Immiscible separation of metalliferous Fe/Ti-oxide melts from fractionating alkali basalt: P-T-f O2 conditions and two-liquid elemental partitioning. Contrib. Mineral. Petrol. 1998, 133, 12–29. [Google Scholar] [CrossRef]
- Hurai, V.; Huraiová, M.; Konečny, P. REE minerals as geochemical proxies of Late-Tertiary alkalic silicate±carbonatite intrusions beneath Carpathian back-arc basin. Minerals 2021, 11, 369. [Google Scholar] [CrossRef]
- Konečný, V.; Lexa, J.; Konečný, P.; Balogh, K.; Elečko, M.; Hurai, V.; Huraiová, M.; Pristaš, J.; Sabol, M.; Vass, D. Guidebook to the Southern Slovakia Alkali Basalt Volcanic Field; Štátny Geologicky Ústav D. Štúra: Bratislava, Slovakia, 2004; 143p. [Google Scholar]
- Konečný, V. Paleogeografická Rekonštrukcia, Vulkanológia a Časová Evolúcia Cerovej Bazaltovej Formácie. In Geológia Lučenskej kotliny a Cerovej Vrchoviny; Vass, D., Elečko, M., Konečný, V., Eds.; Štátny Geologický Ústav D. Štúra: Bratislava, Slovakia, 2007; pp. 196–202. [Google Scholar]
- Merlet, C. An accurate computer correction program for quantitative electron probe microanalysis. Microchim. Acta 1994, 114, 363–376. [Google Scholar] [CrossRef]
- Åmli, R.; Griffin, W. Standards and correction factors for microprobe analysis of REE minerals. Am. Mineral. 1975, 60, 599–606. [Google Scholar]
- Konečný, P.; Siman, P.; Holický, I.; Janák, M.; Kollárová, V. Method of monazite dating by means of the microprobe. Miner. Slov. 2004, 36, 225–235. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, L.; Nimis, P.; Kuzmin, D.; Malkovets, V. Error sources in single-clinopyroxene thermobarometry and a mantle geotherm for the Novinka kimberlite, Yakutia. Am. Mineral. 2016, 101, 2222–2232. [Google Scholar] [CrossRef]
- Warr, L. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Benisek, A.; Dachs, E.; Kroll, H. A ternary feldspar-mixing model based on calorimetric data: Development and application. Contrib. Mineral. Petrol. 2010, 160, 327–337. [Google Scholar] [CrossRef]
- Morimoto, N. Nomenclature of pyroxenes. Mineral. Petrol. 1988, 39, 55–76. [Google Scholar] [CrossRef]
- Tzvetanova, Y.; Tarassov, M.; Ganev, V.; Piroeva, I. Crystal chemistry of clinopyroxene with a high content of the Ca-Tschermak and esseneite components, Eastern Rhodopes, Bulgaria. In Proceedings of the Geonauki National Conference, Bulgarian Geological Society, Sofia, Bulgaria, 7–8 December 2016; pp. 35–36. [Google Scholar]
- Droop, G.A. general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral. Mag. 1987, 51, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Hattert, F.; Burke, E.A.J. The IMA-CNMNC dominant-constituent rule revisited and extended. Can. Mineral. 2008, 46, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Ejima, T.; Osanai, Y.; Akasaka, M.; Adachi, T.; Nakano, N.; Kon, Y.; Ohfuji, H.; Sereenen, J. Oxidation states of Fe in constituent minerals of a spinel lherzolite xenolith from Tariat Depression, Mongolia: The significance or Fe3+ in olivine. Minerals 2018, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Locock, A.J. An Excel spreadsheet to classify chemical analyses of amphiboles following the IMA 2012 recommendations. Comput. Geosci. 2014, 62, 1–11. [Google Scholar] [CrossRef]
- Bunch, T.E.; Okrusch, M. Al-rich pargasite. Am. Mineral. 1973, 58, 721–726. [Google Scholar]
- Leake, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of amphiboles: Report of the subcommittee on amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 1997, 35, 219–246. [Google Scholar]
- Stevens, R.E. Composition of some chromites of the western hemisphere. Am. Mineral. 1944, 29, 1–34. [Google Scholar]
- Haggerty, S.E. Oxide mineralogy of the upper mantle. Spinel mineral group. In Oxide Minerals: Petrologic and Magnetic Significance; Lindsley, D.H., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1991; Volume 25, pp. 355–416. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman: London, UK, 1992; 696p. [Google Scholar]
- Buddington, A.F.; Lindsley, D.H. Iron-titanium oxide minerals and synthetic equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Katona, I.; Pascal, M.L.; Fonteilles, M.; Verkaeren, J. The melilite (Gh50) skarns of Oravita, Banat, Romania: Transition to gehlenite (Gh85) and to vesuvianite. Can. Mineral. 2003, 41, 1255–1270. [Google Scholar] [CrossRef]
- Melluso, L.; Conticelli, S.; D’Antonio, M.; Mirco, N.P.; Saccani, E. Petrology and mineralogy of wollastonite-and melilite-bearing paralavas from the Central Apennines, Italy. Am. Mineral. 2003, 88, 1287–1299. [Google Scholar] [CrossRef]
- Whitley, S.; Halama, R.; Gertisser, R.; Preece, K.; Deegan, F.M.; Troll, V.R. Magmatic and metasomatic effects of magma-carbonate interaction recorded in calc-silicate xenoliths from Merapi volcano (Indonesia). J. Petrol. 2020, 61, egaa048. [Google Scholar] [CrossRef]
- Pascal, M.; Fonteilles, M.; Verkaeren, J.; Piret, R.; Marincea, S. The melilite-bearing high-temperature skarns of the Apuseni Mountains, Carpathians, Romania. Can. Mineral. 2001, 39, 1405–1434. [Google Scholar] [CrossRef] [Green Version]
- De Hoog, J.C.M.; Gall, L.; Cornell, D.H. Trace-element geochemistry if mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef] [Green Version]
- Bussweiler, Y.; Brey, G.P.; Pearson, D.G.; Stachel, T.; Stern, R.A.; Hardman, M.F.; Kjarsgaard, B.A.; Jackson, S.E. The aluminium-in-olivine thermometer for mantle peridotites—Experimental versus empirical calibration and potential applications. Lithos 2017, 272–273, 301–314. [Google Scholar] [CrossRef]
- Wan, Z.; Coogan, L.A.; Canil, D. Experimental calibration of aluminium partitioning between olivine and spinel as a geothermometer. Am. Mineral. 2008, 93, 1142–1147. [Google Scholar] [CrossRef]
- Coogan, L.A.; Saunders, A.D.; Wilson, R.N. Aluminium-in-olivine thermometry of primitive basalts: Evidence of an anomalously hot mantle source for large igneous provinces. Chem. Geol. 2014, 368, 1–10. [Google Scholar] [CrossRef]
- Köhler, T.P.; Brey, G.P. Calcium exchange between olivine and clinopyroxene calibrated as a geothermobarometer for natural peridotites from 2 to 60 kb with applications. Geochim. Cosmochim. Acta 1990, 54, 2375–2388. [Google Scholar] [CrossRef]
- Andersen, D.J.; Lindsley, D.H. The olivine-ilmenite thermometer. Lunar Planet. Sci. Conf. Proc. 1979, 10, 493–507. [Google Scholar]
- Andersen, D.J.; Lindsley, D.H. A valid Margules formulation for an asymmetric ternary solution: Revision of the olivine-ilmenite thermometer, with applications. Geochim. Cosmochim. Acta 1981, 45, 847–853. [Google Scholar] [CrossRef]
- Blundy, J.D.; Holland, T.J.B. Calcic amphibole equilibria and a new amphibole-plagioclase geothermometer. Contrib. Mineral. Petrol. 1990, 104, 208–224. [Google Scholar] [CrossRef]
- Holland, T.; Blundy, J. Non-ideal interactions in calcic amphiboles and their bearing on amphibole-plagioclase thermometry. Contrib. Mineral. Petrol. 1994, 116, 433–447. [Google Scholar] [CrossRef]
- Molina, J.F.; Cambeses, A.; Moreno, J.A.; Morales, I.; Montero, P.; Bea, F. A reassessment of the amphibole-plagioclase NaSi-CaAl Exchange thermometer with applications to igneous and high-grade metamorphic rocks. Am. Mineral. 2021, 106, 782–800. [Google Scholar] [CrossRef]
- Molina, J.F.; Moreno, J.A.; Castro, A.; Rodriguez, C.; Fershtater, G.B. Calcic amphibole thermobarometry in metamorphic and igneous rocks: New calibrations based on plagioclase/amphiboleAl-Si partitioning and amphibole-liquid Mg partitioning. Lithos 2015, 232, 286–305. [Google Scholar] [CrossRef]
- Hammarstrom, J.M.; Zen, E. Aluminium in hornblende: An empirical igneous geobarometer. Am. Mineral. 1986, 71, 1297–1313. [Google Scholar]
- Hollister, L.S.; Grissom, G.C.; Peters, E.K.; Stowell, H.H.; Sisson, V.B. Confirmation of the empirical correlation of Al in hornblende with pressure of solidication of calc-alkaline plutons. Am. Mineral. 1987, 72, 231–239. [Google Scholar]
- Schmidt, M.W. Amphibole composition in tonalite as a function of pressure: An experimental calibration of the Al-in-hornblende barometer. Contrib. Mineral. Petrol. 1992, 110, 304–310. [Google Scholar] [CrossRef]
- Johnson, M.C.; Rutherford, M.J. Experimental calibration of an aluminium-in-hornblende geobarometer applicable to Long Valley caldera (California) volcanic rocks. Geology 1989, 17, 837–841. [Google Scholar] [CrossRef]
- Ridolfi, F.; Renzulli, A. Calcic amphiboles in calc-alkaline and alkaline magmas: Thermobarometric and chemometric empirical equations valid up to 1,130 °C and 2.2 GPa. Contrib. Mineral. Petrol. 2012, 163, 877–895. [Google Scholar] [CrossRef]
- Prinz, M.; Dowty, E.; Keil, K.; Bunch, T.E. Spinel troctolite and anorthosite in Apollo 16 samples. Science 1973, 179, 74–76. [Google Scholar] [CrossRef]
- Bhandari, N.; Srivastava, N. Active Moon: Evidences from Chandrayaan-1 and the proposed Indian missions. Geosci. Lett. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Ashwal, L.D. Anorthosites; Springer: Berlin/Heidelberg, Germany, 1993; 422p. [Google Scholar]
- Kaufmann, F.E.D.; O’Driscoll, B.; Hecht, L. Lateral variations in the Unit 7-8 boundary zone of the Rum Eastern Layered Intrusion, NW Scotland: Implications for the origin and timing of Cr-spinel seam formation. Contrib. Mineral. Petrol. 2020, 175, 90. [Google Scholar] [CrossRef]
- Léger, A.; Ferry, J.M. Highly aluminous hornblende from low-pressure metacarbonates and a preliminary thermodynamic model for the Al content of calcic amphibole. Am. Mineral. 1991, 76, 1002–1017. [Google Scholar]
- Abdu, Y.A.; Hawthorne, F.C. Crystal structure and Mössbauer spectroscopy of tschermakite from the ruby locality at Fiskenaesset, Greenland. Can. Mineral. 2009, 47, 917–926. [Google Scholar] [CrossRef]
- Leake, B.E. On aluminous and edenitic hornblendes. Mineral. Mag. 1971, 38, 389–407. [Google Scholar] [CrossRef]
- Kemp, A.J.; Leake, B.E. Two hydrous-rich aluminous hornblendes. Mineral. Mag. 1975, 40, 308–311. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Mineral. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Oba, T. Phase relationship of Ca2Mg3Al2Si6Al2O22(OH)2—Ca2Mg3Fe3+2Si6Al2O22(OH)2 join at high pressure—The stability of tschermakite. J. Fac. Sci. Hokkaido Univ. 1978, 18, 339–350. [Google Scholar]
- Connolly, J. Multivariable phase diagrams; an algorithm based on generalized thermodynamics. Am. J. Sci. 1990, 290, 666–718. [Google Scholar] [CrossRef]
- Connolly, J.A.D.; Kerrick, D.M. An algorithm and computer program for calculating composition phase diagrams. CALPHAD 1987, 11, 1–55. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R.A. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Petrol. 1998, 16, 309–343. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R.A. Compensated-Redlich-Kwong (CORK) equation for volumes and fugacities of CO2 and H2O in the range 1 bar to 50 kbar and 100–1600 °C. Contrib. Mineral. Petrol. 1991, 109, 265–273. [Google Scholar] [CrossRef]
- Evans, B.; Trommsdorff, V. On elongate olivine of metamorphic origin. Geology 1974, 2, 131–132. [Google Scholar] [CrossRef]
- Ohasi, H.; Hariya, Y. Decomposition of CaFe3+AlSiO6 pyroxene at high pressure and low oxygen partial pressure. J. Jap. Assoc. Mineral. Petrol. Econ. Geol. 1975, 70, 347–351. [Google Scholar] [CrossRef]
- Ohasi, H.; Hariya, Y. Phase relation of CaFeAlSiO6 pyroxene at high pressures and temperatures. J. Jap. Assoc. Mineral. Petrol. Econ. Geol. 1975, 70, 93–95. [Google Scholar] [CrossRef]
- Onuma, K. Effect of oxygen fugacity on fassaitic pyroxene. J. Fac. Sci. Hokkaido Univ. 1983, 20, 185–194. [Google Scholar]
- Onuma, K.; Akasaka, M.; Yagi, K. The bearing of the system CaMgSi2O6-CaAl2SiO6-CaFeAlSiO6 on fassaitic pyroxene. Lithos 1981, 14, 173–182. [Google Scholar] [CrossRef]
- Lesnov, F.P.; Korolyuk, V.N. Pervye dannye o razpredelenii izomorfnoi primesi zheleza v plagioklazach bazit-giperbazitovych plutonov skladchatych oblastei SSSR (First results of the distribution of isomorphic admixtures of iron in plagioclases of basic-hyperbasic plutons in folded areas of USSR). Dokl. Akad Nauk SSSR 1977, 234, 922–924. [Google Scholar]
- Tegner, C.; Cawthorn, R.G. Iron in plagioclase in the Bushveld and Skaergaard intrusions: Implications for iron contents in evolving basic magmas. Contrib. Mineral. Petrol. 2010, 159, 719–730. [Google Scholar] [CrossRef]
- Jolis, E.M.; Troll, V.R.; Harris, C.; Freda, C.; Gaeta, M.; Orsi, G.; Siebe, C. Skarn xenolith record crustal CO2 liberation during Pompeii and Pollena eruptions, Vesuvius volcanic system, central Italy. Chem. Geol. 2015, 415, 17–36. [Google Scholar] [CrossRef]
- Matthews, S.J.; Marquillas, R.A.; Kemp, A.J.; Grange, F.K.; Gardeweg, M.C. Active skarn formation beneath Lascar Volcano, northern Chile: A petrographic and geochemical study of xenoliths in eruption products. J. Metamorph. Geol. 1996, 14, 509–530. [Google Scholar] [CrossRef]
- Wenzel, T.; Baumgartner, L.P.; Brügmann, G.E.; Konnikov, E.G.; Kislov, E.V. Partial melting and assimilation of dolomitic xenoliths by mafic magma: The Ioko-Dovyren intrusion (North Baikal region, Russia). J. Petrol. 2002, 43, 2049–2074. [Google Scholar] [CrossRef]






| Sample | Cam-14 | Ca-14 | Ca-14 ◊ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis # | 15 | 20 | 22 | 23 | 21 | 30 | 32 | 69 | 11 | 52 | 64 | 90 | 1 | 10 | 18 |
| SiO2 (wt%) | 32.95 | 35.06 | 37.76 | 44.03 | 27.44 | 27.77 | 38.20 | 28.02 | 27.55 | 36.06 | 33.07 | 28.79 | 36.54 | 50.49 | 38.78 |
| TiO2 | 2.76 | 0.47 | 0.39 | 0.32 | 0.36 | 0.22 | 2.21 | 0.21 | 0.30 | 0.28 | 0.36 | b.d.l. | 0.65 | 0.12 | 0.31 |
| Al2O3 | 17.60 | 20.64 | 18.42 | 11.83 | 31.01 | 31.41 | 11.94 | 27.35 | 31.12 | 21.48 | 21.47 | 27.06 | 17.28 | 4.66 | 18.83 |
| Fe2O3 * | 14.58 | 11.44 | 9.49 | 8.95 | 15.31 | 14.75 | 10.49 | 16.04 | 14.41 | 10.89 | 13.73 | 15.41 | 12.55 | 4.78 | 10.37 |
| Cr2O3 | b.d.l. | 0.99 | 1.14 | b.d.l. | 0.63 | 0.22 | b.d.l. | 0.19 | 0.32 | 0.23 | 0.71 | 0.19 | 0.18 | 0.06 | 0.02 |
| V2O3 | 2.09 | 0.02 | 0.01 | 0.09 | b.d.l. | b.d.l. | 1.28 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| FeO | 2.98 | 1.62 | 0.53 | 0.38 | 0.05 | 0.00 | 7.98 | 1.31 | 1.14 | 0.71 | 1.28 | 2.51 | 2.92 | 1.41 | 0.00 |
| NiO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.03 | b.d.l. | 0.01 | 0.01 | b.d.l. | 0.02 | 0.02 | 0.01 | b.d.l. | 0.01 |
| MnO | 0.27 | 0.05 | 0.01 | 0.10 | 0.03 | b.d.l. | 0.53 | 0.06 | 0.01 | 0.04 | 0.05 | 0.09 | 0.18 | 0.18 | 0.05 |
| MgO | 3.19 | 5.15 | 7.43 | 10.60 | 0.85 | 0.78 | 4.03 | 0.58 | 0.55 | 6.13 | 3.90 | 0.61 | 5.32 | 17.48 | 8.03 |
| CaO | 23.74 | 24.46 | 24.75 | 24.95 | 24.60 | 24.97 | 24.16 | 24.89 | 24.63 | 24.95 | 24.97 | 24.38 | 24.48 | 20.96 | 25.37 |
| K2O | b.d.l. | 0.01 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | b.d.l. |
| Na2O | 0.11 | 0.03 | b.d.l. | 0.32 | b.d.l. | b.d.l. | 0.08 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.06 | 0.18 | 0.02 |
| Total | 100.28 | 99.94 | 99.94 | 101.58 | 100.29 | 100.15 | 100.89 | 98.69 | 100.04 | 100.78 | 99.57 | 99.06 | 100.18 | 100.32 | 101.79 |
| Si (apfu) | 1.298 | 1.342 | 1.427 | 1.622 | 1.056 | 1.066 | 1.498 | 1.108 | 1.064 | 1.356 | 1.283 | 1.135 | 1.407 | 1.837 | 1.433 |
| AlIV | 0.702 | 0.658 | 0.573 | 0.378 | 0.944 | 0.934 | 0.502 | 0.892 | 0.938 | 0.646 | 0.718 | 0.866 | 0.593 | 0.163 | 0.567 |
| AlVI | 0.115 | 0.274 | 0.247 | 0.136 | 0.462 | 0.488 | 0.050 | 0.383 | 0.477 | 0.304 | 0.263 | 0.391 | 0.191 | 0.037 | 0.253 |
| Ti | 0.082 | 0.013 | 0.011 | 0.009 | 0.011 | 0.006 | 0.065 | 0.006 | 0.009 | 0.008 | 0.010 | 0.000 | 0.019 | 0.003 | 0.009 |
| Fe3+ | 0.432 | 0.330 | 0.270 | 0.248 | 0.443 | 0.426 | 0.328 | 0.477 | 0.419 | 0.308 | 0.401 | 0.457 | 0.364 | 0.131 | 0.288 |
| Cr | 0.000 | 0.030 | 0.034 | 0.000 | 0.019 | 0.007 | 0.000 | 0.006 | 0.010 | 0.007 | 0.022 | 0.006 | 0.005 | 0.002 | 0.001 |
| V | 0.066 | 0.001 | 0.000 | 0.003 | 0.040 | ||||||||||
| Fe2+ | 0.098 | 0.052 | 0.017 | 0.012 | 0.002 | 0.000 | 0.244 | 0.043 | 0.037 | 0.022 | 0.042 | 0.083 | 0.094 | 0.043 | 0.000 |
| Ni | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | ||||||||||
| Mn | 0.009 | 0.002 | 0.000 | 0.003 | 0.001 | 0.000 | 0.018 | 0.002 | 0.001 | 0.006 | 0.006 | 0.002 | |||
| Mg | 0.187 | 0.294 | 0.418 | 0.582 | 0.049 | 0.045 | 0.235 | 0.034 | 0.032 | 0.343 | 0.226 | 0.036 | 0.305 | 0.948 | 0.442 |
| CaM1 | 0.076 | 0.005 | 0.002 | 0.008 | 0.014 | 0.027 | 0.021 | 0.054 | 0.019 | 0.005 | 0.038 | 0.029 | 0.015 | 0.000 | 0.006 |
| CaM2 | 0.926 | 0.998 | 1.000 | 0.977 | 1.000 | 1.000 | 0.994 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.995 | 0.817 | 0.998 |
| K | 0.000 | 0.000 | 0.000 | ||||||||||||
| Na | 0.008 | 0.002 | 0.000 | 0.023 | 0.000 | 0.000 | 0.000 | 0.005 | 0.013 | 0.002 | |||||
| Total | 3.999 | 4.001 | 3.999 | 4.001 | 4.002 | 4.000 | 3.995 | 4.007 | 4.005 | 4.003 | 4.005 | 4.006 | 3.999 | 4.000 | 4.001 |
| Q | 1.29 | 1.35 | 1.44 | 1.58 | 1.06 | 1.07 | 1.49 | 1.13 | 1.09 | 1.37 | 1.31 | 1.15 | 1.41 | 1.81 | 1.45 |
| J | 0.02 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.0 |
| Jd | 0.9 | 0.2 | 0.0 | 2.3 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.5 | 1.3 | 0.2 |
| Ess | 44.7 | 33.5 | 27.5 | 24.6 | 44.8 | 42.8 | 33.6 | 48.7 | 43.3 | 31.8 | 41.4 | 46.7 | 36.5 | 13.1 | 28.9 |
| CaTi-Ts | 8.4 | 1.4 | 1.1 | 0.9 | 1.1 | 0.6 | 6.7 | 0.6 | 0.9 | 0.8 | 1.0 | 0.0 | 1.9 | 0.3 | 0.9 |
| Ks | 11.9 | 27.8 | 25.2 | 13.4 | 46.6 | 49.0 | 5.1 | 38.1 | 47.5 | 33.0 | 26.4 | 38.8 | 19.2 | 3.7 | 25.3 |
| Wo | 19.3 | 19.6 | 24.1 | 29.4 | 5.0 | 5.4 | 29.4 | 8.7 | 4.9 | 17.3 | 17.7 | 8.3 | 21.9 | 32.2 | 22.7 |
| En | 9.7 | 14.9 | 21.3 | 28.9 | 2.5 | 2.2 | 12.1 | 1.7 | 1.6 | 17.1 | 11.3 | 1.8 | 15.3 | 47.3 | 22.1 |
| Fs | 5.1 | 2.6 | 0.9 | 0.6 | 0.1 | 0.0 | 12.5 | 2.2 | 1.9 | 0.0 | 2.2 | 4.2 | 4.7 | 2.1 | 0.0 |
| QUAD | 34.1 | 37.2 | 46.2 | 58.8 | 7.5 | 7.6 | 54.0 | 12.6 | 8.4 | 34.4 | 31.1 | 14.4 | 41.9 | 81.7 | 44.8 |
| Name ** | Ess | Ess | Ess | Wo | Ks | Ks | Ess | Ess | Ks | Ks | Ess | Ess | Ess | En | Ess |
| Sample | Cam-13 | Cam-15 | Ca-22 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | 1 | 12 | 15 | 17 | 19 | 61 | 37 | 39 | 41 | 43 | 45 | 6 | 10 | 2 | 4 | 6 | 10 | 14 |
| SiO2 | b.d.l. | 0.33 | 0.08 | 0.08 | b.d.l. | 0.07 | 0.03 | 0.07 | 0.02 | 0.05 | 0.05 | 0.51 | 0.04 | 0.09 | 0.11 | 0.07 | 0.46 | 0.11 |
| TiO2 | 53.80 | 55.01 | 55.31 | 54.51 | 54.68 | 53.15 | 54.53 | 54.38 | 55.30 | 55.26 | 54.70 | 53.09 | 53.50 | 53.73 | 53.74 | 51.61 | 51.45 | 52.58 |
| Al2O3 | 0.11 | 0.20 | 0.04 | 0.04 | 0.13 | 0.04 | 0.05 | 0.03 | 0.07 | 0.09 | 0.04 | 0.07 | 0.07 | 0.06 | 0.05 | 0.05 | 0.26 | 0.06 |
| Cr2O3 | b.d.l. | 0.04 | 0.01 | b.d.l. | b.d.l. | 0.05 | 0.02 | b.d.l. | 0.05 | 0.02 | 0.02 | 0.05 | b.d.l. | 0.39 | 0.07 | 0.03 | 0.01 | 0.00 |
| V2O3 | 0.07 | 0.10 | 0.28 | 0.16 | 0.13 | 0.08 | 0.07 | 0.21 | 0.27 | 0.26 | 0.22 | 0.06 | 0.08 | 0.10 | 0.17 | 0.01 | 0.14 | 0.16 |
| Fe2O3 | 3.77 | b.d.l. | b.d.l. | 1.73 | 2.55 | 4.07 | 32.56 | 35.49 | 35.66 | 34.62 | 32.79 | 30.81 | 30.81 | 32.53 | 34.71 | 33.60 | 34.55 | 34.63 |
| FeO | 33.13 | 34.57 | 35.27 | 34.63 | 34.08 | 34.16 | 2.51 | 1.19 | 0.00 | 0.44 | 2.17 | 5.11 | 4.59 | 3.46 | 1.59 | 6.22 | 4.69 | 4.20 |
| NiO | n.a. | n.a. | n.a. | n.a. | n.a. | b.d.l. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.01 | 0.02 | 0.02 | 0.07 | b.d.l. | b.d.l. | 0.02 |
| ZnO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.04 | 0.02 | 0.02 | 0.03 | b.d.l. | 0.01 | b.d.l. | 0.01 | 0.02 | 0.03 | 0.05 | 0.02 | 0.00 |
| MnO | 0.54 | 0.53 | 0.69 | 0.55 | 0.60 | 0.72 | 0.57 | 0.59 | 0.49 | 0.60 | 0.49 | 0.52 | 0.63 | 0.76 | 0.74 | 0.73 | 0.64 | 0.73 |
| MgO | 7.53 | 7.74 | 7.20 | 7.24 | 7.46 | 6.89 | 8.30 | 6.68 | 7.37 | 7.95 | 8.54 | 9.12 | 9.31 | 8.22 | 7.13 | 6.76 | 6.43 | 6.68 |
| CaO | 1.04 | 0.35 | 0.26 | 0.82 | 0.95 | 0.57 | 0.91 | 0.78 | 0.29 | 0.30 | 0.61 | 0.65 | 0.12 | 0.38 | 0.16 | 0.15 | 0.18 | 0.13 |
| Total | 100.00 | 98.88 | 99.13 | 99.75 | 100.57 | 99.84 | 99.57 | 99.44 | 99.55 | 99.58 | 99.64 | 100.00 | 99.18 | 99.77 | 98.58 | 99.28 | 98.83 | 99.30 |
| Ilmenite Formula Based on 3 O and 2 Cations | ||||||||||||||||||
| Si (apfu) | 0.000 | 0.008 | 0.002 | 0.002 | 0.000 | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.012 | 0.001 | 0.002 | 0.003 | 0.002 | 0.011 | 0.003 |
| Ti | 0.964 | 0.993 | 1.002 | 0.980 | 0.974 | 0.959 | 0.975 | 0.985 | 0.996 | 0.991 | 0.976 | 0.940 | 0.956 | 0.961 | 0.980 | 0.939 | 0.940 | 0.956 |
| Al | 0.003 | 0.006 | 0.001 | 0.001 | 0.004 | 0.001 | 0.001 | 0.001 | 0.002 | 0.003 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 | 0.008 | 0.002 |
| Cr | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.007 | 0.001 | 0.001 | 0.000 | 0.000 |
| V | 0.001 | 0.002 | 0.005 | 0.003 | 0.002 | 0.001 | 0.001 | 0.004 | 0.005 | 0.005 | 0.004 | 0.001 | 0.001 | 0.002 | 0.003 | 0.000 | 0.003 | 0.003 |
| Fe3+ | 0.068 | 0.000 | 0.000 | 0.031 | 0.046 | 0.074 | 0.045 | 0.022 | 0.000 | 0.008 | 0.039 | 0.091 | 0.082 | 0.062 | 0.029 | 0.113 | 0.086 | 0.076 |
| Fe2+ | 0.660 | 0.694 | 0.710 | 0.692 | 0.675 | 0.685 | 0.647 | 0.715 | 0.714 | 0.690 | 0.651 | 0.606 | 0.612 | 0.647 | 0.703 | 0.680 | 0.702 | 0.700 |
| Ni | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 |
| Zn | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 |
| Mn | 0.011 | 0.011 | 0.014 | 0.011 | 0.012 | 0.015 | 0.011 | 0.012 | 0.010 | 0.012 | 0.010 | 0.010 | 0.013 | 0.015 | 0.015 | 0.015 | 0.013 | 0.015 |
| Mg | 0.267 | 0.277 | 0.259 | 0.258 | 0.263 | 0.246 | 0.294 | 0.240 | 0.263 | 0.282 | 0.302 | 0.320 | 0.330 | 0.291 | 0.258 | 0.244 | 0.233 | 0.241 |
| Ca | 0.026 | 0.009 | 0.007 | 0.021 | 0.024 | 0.015 | 0.023 | 0.020 | 0.007 | 0.008 | 0.016 | 0.017 | 0.003 | 0.010 | 0.004 | 0.004 | 0.005 | 0.003 |
| Ilmenite Endmember Mole Fractions * | ||||||||||||||||||
| Ilmenite | 0.663 | 0.715 | 0.733 | 0.706 | 0.686 | 0.682 | 0.656 | 0.732 | 0.731 | 0.704 | 0.656 | 0.596 | 0.598 | 0.647 | 0.710 | 0.656 | 0.688 | 0.688 |
| Geikielite | 0.269 | 0.285 | 0.267 | 0.263 | 0.268 | 0.245 | 0.298 | 0.246 | 0.269 | 0.288 | 0.305 | 0.315 | 0.322 | 0.291 | 0.260 | 0.235 | 0.228 | 0.237 |
| Sample | Cam-13 | Cam-15 | Ca-22 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | 2 | 11 | 16 | 18 | 20 | 60 | 38 | 40 | 42 | 44 | 46 | 5 | 9 | 1 | 3 | 5 | 9 | 13 |
| SiO2 | 38.37 | 38.56 | 38.77 | 38.21 | 38.67 | 38.51 | 39.36 | 38.75 | 38.88 | 38.74 | 38.85 | 39.04 | 39.40 | 39.11 | 38.78 | 38.32 | 38.77 | 38.02 |
| TiO2 | 0.06 | n.a. | n.a. | n.a. | n.a. | 0.07 | 0.01 | 0.06 | 0.11 | 0.04 | 0.11 | 0.06 | 0.02 | 0.02 | 0.02 | 0.02 | 0.06 | 0.01 |
| Al2O3 | 0.03 | 0.10 | 0.01 | 0.01 | 0.02 | 0.04 | 0.00 | 0.01 | 0.03 | 0.02 | 0.02 | b.d.l. | 0.02 | 0.02 | 0.08 | 0.10 | 0.08 | 0.03 |
| Cr2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | b.d.l. | b.d.l. | 0.01 | 0.01 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | 0.01 |
| Fe2O3 | 1.82 | 2.16 | 1.50 | 1.37 | 1.46 | 1.57 | 0.92 | 0.91 | 1.13 | 1.75 | 2.25 | 1.21 | 1.48 | 1.26 | 0.28 | 0.64 | 1.14 | 2.32 |
| FeO | 18.43 | 17.91 | 18.10 | 18.91 | 19.09 | 20.23 | 17.91 | 19.08 | 18.22 | 17.66 | 15.99 | 16.95 | 15.92 | 18.05 | 19.31 | 19.10 | 18.81 | 18.75 |
| NiO | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.02 | 0.05 | 0.01 | 0.05 | 0.05 | 0.06 | 0.06 | 0.10 | 0.08 | 0.09 | 0.07 | 0.08 |
| MnO | 0.34 | 0.30 | 0.33 | 0.32 | 0.35 | 0.47 | 0.29 | 0.32 | 0.27 | 0.28 | 0.25 | 0.30 | 0.29 | 0.34 | 0.33 | 0.33 | 0.29 | 0.31 |
| MgO | 41.11 | 41.24 | 41.71 | 40.48 | 40.97 | 40.23 | 42.52 | 41.09 | 41.90 | 42.15 | 43.32 | 42.76 | 43.87 | 42.07 | 40.70 | 40.34 | 41.16 | 40.37 |
| CaO | 0.42 | 0.95 | 0.40 | 0.39 | 0.46 | 0.35 | 0.31 | 0.34 | 0.29 | 0.30 | 0.32 | 0.34 | 0.32 | 0.30 | 0.38 | 0.36 | 0.52 | 0.39 |
| Total | 100.62 | 101.28 | 100.88 | 99.74 | 101.07 | 101.53 | 101.35 | 100.62 | 100.83 | 101.01 | 101.18 | 100.73 | 101.38 | 101.28 | 99.96 | 99.33 | 100.89 | 100.30 |
| Olivine Formula Based on 4 O and 3 Cations | ||||||||||||||||||
| Si (apfu) | 0.981 | 0.979 | 0.986 | 0.987 | 0.986 | 0.983 | 0.992 | 0.990 | 0.988 | 0.982 | 0.977 | 0.988 | 0.986 | 0.989 | 0.997 | 0.992 | 0.988 | 0.978 |
| Ti | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Al | 0.001 | 0.003 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.003 | 0.003 | 0.002 | 0.001 |
| Cr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Fe3+ | 0.035 | 0.041 | 0.029 | 0.027 | 0.028 | 0.030 | 0.017 | 0.018 | 0.022 | 0.033 | 0.043 | 0.023 | 0.028 | 0.024 | 0.005 | 0.013 | 0.022 | 0.045 |
| Fe2+ | 0.394 | 0.380 | 0.385 | 0.408 | 0.407 | 0.432 | 0.377 | 0.408 | 0.387 | 0.374 | 0.336 | 0.359 | 0.333 | 0.382 | 0.415 | 0.414 | 0.401 | 0.404 |
| Ni | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| Mn | 0.007 | 0.007 | 0.007 | 0.007 | 0.008 | 0.010 | 0.006 | 0.007 | 0.006 | 0.006 | 0.005 | 0.006 | 0.006 | 0.007 | 0.007 | 0.007 | 0.006 | 0.007 |
| Mg | 1.567 | 1.561 | 1.581 | 1.559 | 1.557 | 1.531 | 1.597 | 1.566 | 1.586 | 1.593 | 1.624 | 1.612 | 1.636 | 1.585 | 1.559 | 1.557 | 1.563 | 1.549 |
| Ca | 0.011 | 0.026 | 0.011 | 0.011 | 0.013 | 0.010 | 0.008 | 0.009 | 0.008 | 0.008 | 0.009 | 0.009 | 0.009 | 0.008 | 0.011 | 0.010 | 0.014 | 0.011 |
| Olivine Endmember Mole Fraction | ||||||||||||||||||
| Fayalite * | 0.215 | 0.213 | 0.207 | 0.218 | 0.218 | 0.232 | 0.198 | 0.214 | 0.205 | 0.204 | 0.189 | 0.191 | 0.181 | 0.204 | 0.212 | 0.215 | 0.213 | 0.225 |
| T (°C) at 1 GPa | 780 | 775 | 705 | 754 | 775 | 771 | 785 | 695 | 703 | 757 | 767 | 819 | 798 | 796 | 728 | 724 | 695 | 737 |
| T (°C) at 2 GPa | 815 | 822 | 748 | 794 | 813 | 805 | 824 | 733 | 747 | 801 | 807 | 855 | 834 | 833 | 767 | 754 | 725 | 769 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reato, L.; Huraiová, M.; Konečný, P.; Marko, F.; Hurai, V. Formation of Esseneite and Kushiroite in Tschermakite-Bearing Calc-Silicate Xenoliths Ejected in Alkali Basalt. Minerals 2022, 12, 156. https://doi.org/10.3390/min12020156
Reato L, Huraiová M, Konečný P, Marko F, Hurai V. Formation of Esseneite and Kushiroite in Tschermakite-Bearing Calc-Silicate Xenoliths Ejected in Alkali Basalt. Minerals. 2022; 12(2):156. https://doi.org/10.3390/min12020156
Chicago/Turabian StyleReato, Luca, Monika Huraiová, Patrik Konečný, František Marko, and Vratislav Hurai. 2022. "Formation of Esseneite and Kushiroite in Tschermakite-Bearing Calc-Silicate Xenoliths Ejected in Alkali Basalt" Minerals 12, no. 2: 156. https://doi.org/10.3390/min12020156
APA StyleReato, L., Huraiová, M., Konečný, P., Marko, F., & Hurai, V. (2022). Formation of Esseneite and Kushiroite in Tschermakite-Bearing Calc-Silicate Xenoliths Ejected in Alkali Basalt. Minerals, 12(2), 156. https://doi.org/10.3390/min12020156








