A Study on the Possible Relationship between Physico-Chemical Properties of the Covering Soil and the Mobility of Radionuclides and Potentially Toxic Elements in a Recultivated Spoil Bank
Abstract
1. Introduction
- (1)
- Exchangeable and acid-soluble fractions;
- (2)
- Bound to reducible species (e.g., Fe and Mn oxides, oxyhydroxides);
- (3)
- Oxidizable forms bound to organic matter or sulfides;
- (4)
- Strong oxidative acid-soluble residual contents (aqua regia and/or H2O2/HNO3)
2. Materials and Methods
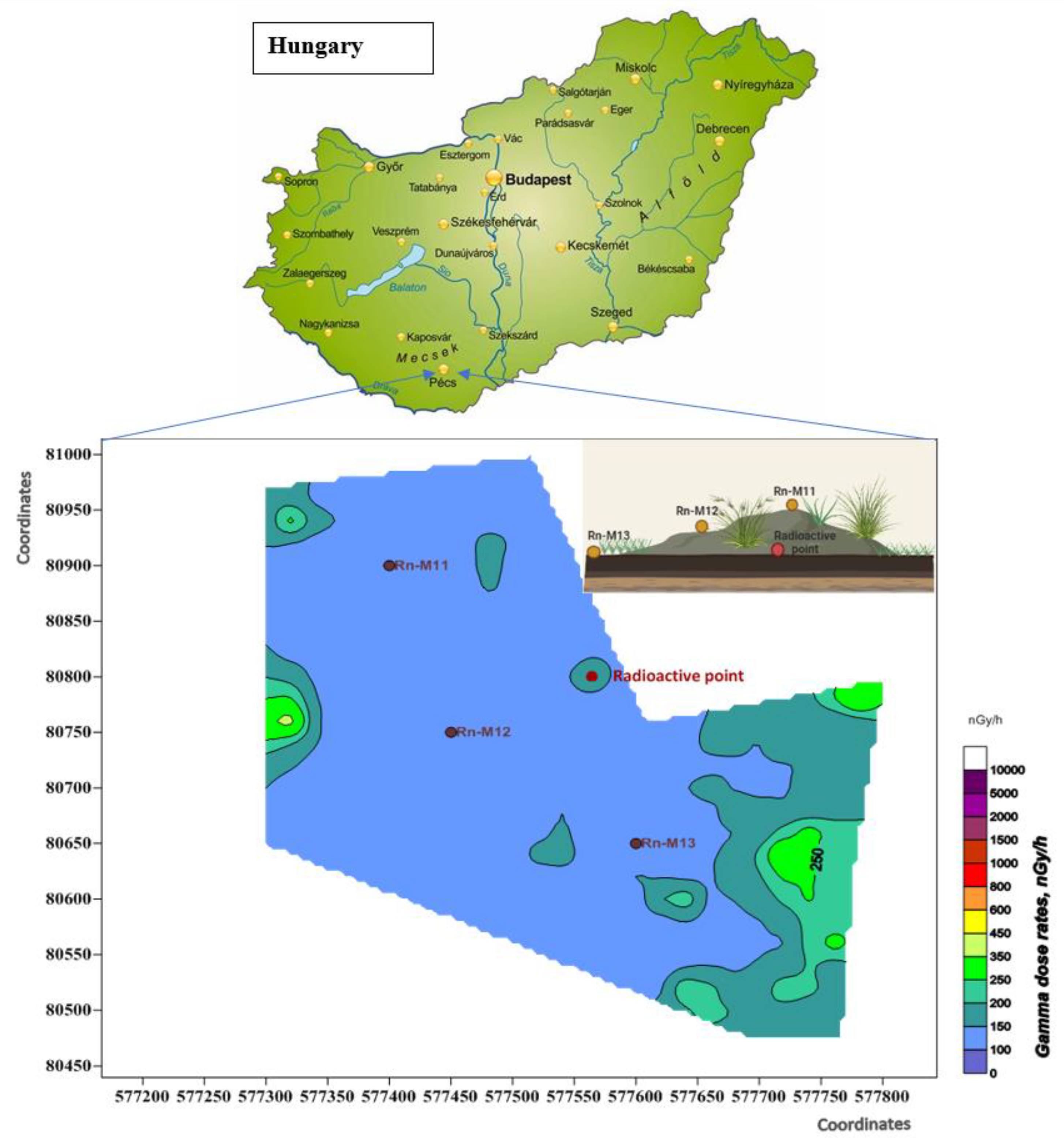
2.1. Geological Background
2.2. Sampling and Sample Preparation
2.2.1. Sampling
2.2.2. Soil Sampling and Sample Preparation
2.2.3. Plant Sampling and Sample Preparation
2.2.4. Water Sampling and Sample Preparation
2.3. Analytical Methods
2.3.1. Elemental Analysis
- Uranium ICP standard (UO2NO3)2 in HNO3 2–3% 10 mg/L—U;
- Yttrium standard solution (YNO3)3 in HNO3 0.5 mol/l – 1000 mg/L Y;
- Certified Elements Standard—Uranium; concentration: 1000 ± 3 µg/mL, 20 °C; matrix: 2.5% HNO3; density: 1.0152 g/mL, 20 °C
2.3.2. Radiochemical Analysis
2.3.3. Physico-Chemical Analysis
2.4. Gamma Spectroscopy
2.5. Calculations for the Soil/Plant Transfer Factor
or
TF = metal content in the plant (mg/kg) /metal content in soil (mg/kg)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Distribution of PTEs and Measured Elements along the Slope Position and Vertical Direction in the Covering Soil Layer, Their Mobility and Influencing Soil Characteristics, and Plant Transfer
- Pseudo-total PTE and other measured element content distribution vs. vertical position in the soil layer showed no significant trend.
- Pseudo-total PTE and other measured element content distribution vs. position on the slope showed an increasing tendency for certain PTEs along the top to bottom direction.
- PTE uptake by plants showed an increasing tendency in the top to bottom direction referring to migration along the slope.
- Soil pH and CEC had significant correlations with each other and decreased tendency vs. vertical position and top to bottom position.
- pH and CEC have shown different correlations with different mobility factors of elements determined by BCR fractionation of PTEs of 0–25 cm soil layer. The order of mobility was the following: U > Mn > Pb > Co > Cd > Ni > Cu > Cr > Zn > Fe.
- Co, Fe, and Ni mobility significantly decreased as the pH and CEC increased, while Cd, Cr, Pb, Cu, and U mobility increased with the decrease in pH and CEC.
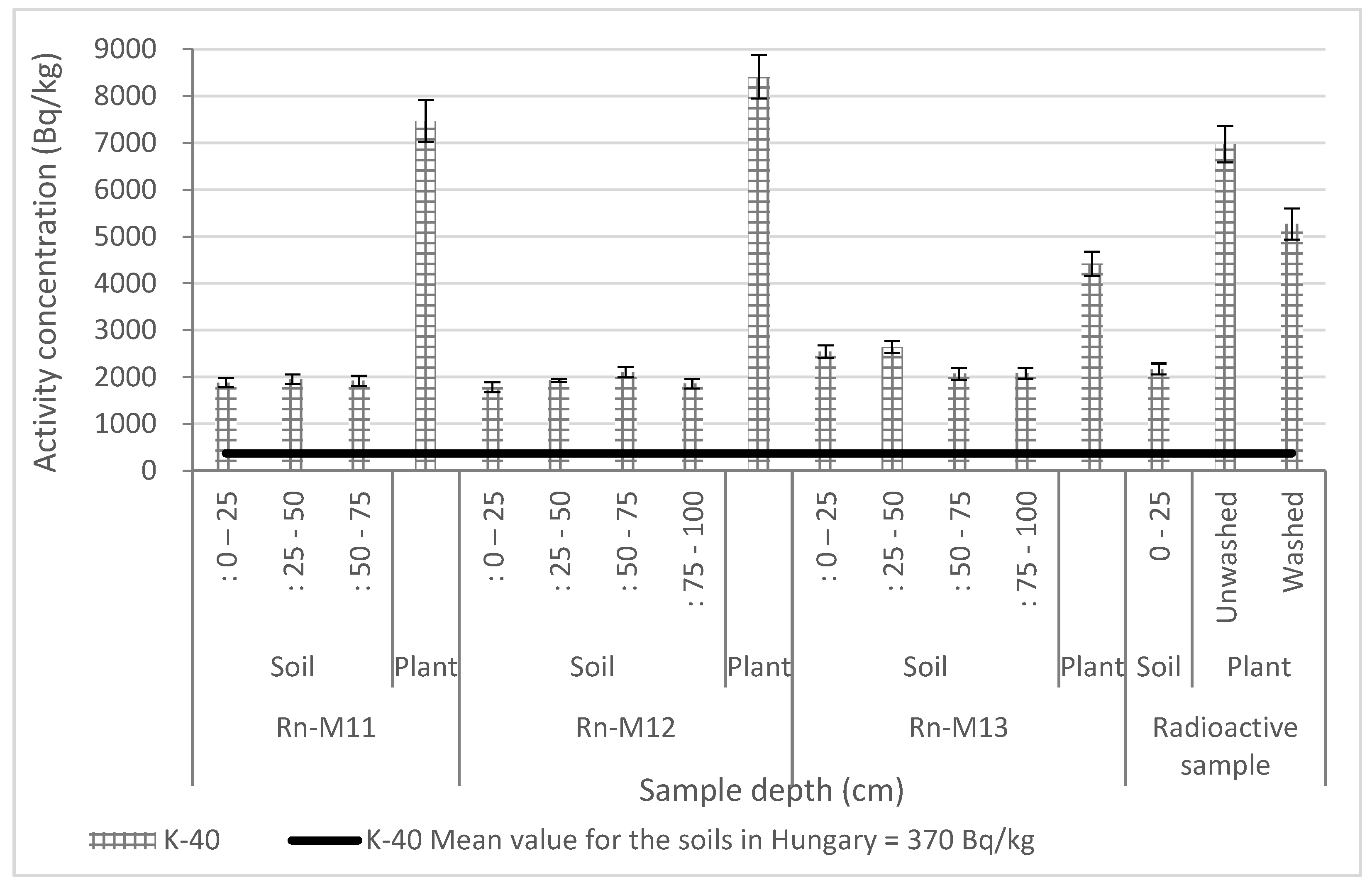
3.2. Radiochemical Results
3.2.1. Distribution of Radionuclides in Cover Soil in Vertical and Slope Position
3.2.2. Transfer Factors
- K-40: Rn-M12 > Rn-M11 > radioactive sample > Rn-M13;
- U-238 and U-235: Rn-M11 > Rn-M12 > Rn-M13 > radioactive sample
- Th-232: radioactive sample > Rn-M12 > Rn-M13 > Rn-M11
3.3. Uranium Concentration in Water
3.4. Statistical Analysis
3.4.1. Correlation Matrices and Comparison of Radionuclides, PTE Pseudo-Total, and Physicochemical Property Associations
- 1.
- U-238–U-235 (r = 1.00), U-238–Rn-222 (r = 0.73) and U-238–Cu (r = 0.97);
- 2.
- Th-232–Fe (r = 0.78), Th-232–P (r = 0.80), Th-232–Na (r = 0.79), and Th-232–Mn (r = 0.69);
- 3.
- U-235–Rn-222 (r = 0.73) and U-235–Cu (r = 0.97);
- 4.
- K-40–K (r = 0.84) and K-40–Co (r = 0.75), K-40–Mn (r = 0.60), K-40–Zn (r = 0.68), K-40–Fe (r = 0.57), and K-40–P (r = 0.54).
- Fe–P, Fe–Co, Fe–Mn, Fe–Na and Fe–Zn and Zn;
- K–Co and K–Mn;
- P–Co, P–Zn, P–Mn, and P–Na;
- Co–Mn;
- Mn–Na.
3.4.2. Principal Component Analysis for PTE Pseudo-Total, Radionuclides, and Soil Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample Description | Depth (cm) | Soil Moisture Content (%) | Soil pH | CEC (cmol(+)/kg) | SOM (%) | Plant Moisture Content (%) |
|---|---|---|---|---|---|---|
| Rn-M11 | 0–25 | 9.24 | 6.22 | 52.9 ± 6.71 | 1.61 | 33.1 |
| 25–50 | 7.44 | 6.12 | 20.2 ± 0.06 | 1.21 | ||
| 50–75 | 12.6 | 5.99 | 14.6 ± 4.55 | 2.56 | ||
| 75–100 | Hard rock (not sampled) | |||||
| Rn-M12 | 0–25 | 9.47 | 5.79 | 33.0 ± 0.69 | 0.75 | 31.2 |
| 25–50 | 8.99 | 5.50 | 8.23 ± 1.09 | 0.64 | ||
| 50–75 | 8.19 | 5.47 | 24.4 ± 1.31 | 0.96 | ||
| 75–100 | 8.43 | 5.38 | 33.0 ± 0.01 | 1.11 | ||
| Rn-M13 | 0–25 | 17.1 | 5.61 | 28.2 ± 0.44 | 5.27 | 25.0 |
| 25–50 | 14.3 | 5.27 | 31.8 ± 4.61 | 3.34 | ||
| 50–75 | 14.2 | 5.38 | 28.1 ± 2.67 | 1.92 | ||
| 75–100 | 14.2 | 5.36 | 36.6 ± 0.01 | 1.76 | ||
| Radioactive sample | 0–25 | 3.16 | 5.27 | 27.3 ± 0.01 | 1.31 | 60.3 |
| Pearsons’ (r) | U-238 | Th-232 | U-235 | K-40 | Rn-222 | Ca | Co | Cr | Cu | Fe | K | Mg | Mn | Na | Ni | P | Pb | U | Zn | CEC | pH | % * SM | % * SOM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U-238 | − | ||||||||||||||||||||||
| Th-232 | −0.65 | − | |||||||||||||||||||||
| U-235 | 1.00 | −0.65 | − | ||||||||||||||||||||
| K-40 | 0.13 | 0.31 | 0.13 | − | |||||||||||||||||||
| Rn-222 | 0.73 | −0.35 | 0.73 | 0.31 | − | ||||||||||||||||||
| Ca | −0.30 | −0.06 | −0.30 | −0.42 | −0.54 | − | |||||||||||||||||
| Co | 0.05 | 0.45 | 0.05 | 0.75 | 0.47 | −0.57 | − | ||||||||||||||||
| Cr | 0.69 | −0.43 | 0.69 | 0.16 | 0.62 | −0.51 | 0.33 | − | |||||||||||||||
| Cu | 0.97 | −0.54 | 0.97 | 0.29 | 0.74 | −0.30 | 0.18 | 0.67 | − | ||||||||||||||
| Fe | −0.55 | 0.78 | −0.55 | 0.57 | −0.05 | −0.22 | 0.79 | −0.23 | −0.42 | − | |||||||||||||
| K | 0.26 | 0.22 | 0.26 | 0.84 | 0.40 | −0.66 | 0.80 | 0.27 | 0.38 | 0.53 | − | ||||||||||||
| Mg | −0.19 | 0.32 | −0.19 | 0.34 | −0.15 | 0.56 | 0.30 | −0.34 | −0.08 | 0.48 | 0.17 | − | |||||||||||
| Mn | −0.33 | 0.69 | −0.33 | 0.60 | 0.18 | −0.57 | 0.86 | 0.05 | −0.22 | 0.88 | 0.62 | 0.10 | − | ||||||||||
| Na | −0.81 | 0.79 | −0.81 | 0.19 | −0.31 | 0.13 | 0.37 | −0.61 | −0.70 | 0.84 | 0.09 | 0.45 | 0.62 | − | |||||||||
| Ni | 0.39 | −0.22 | 0.39 | 0.12 | 0.45 | −0.50 | 0.39 | 0.94 | 0.38 | −0.04 | 0.20 | −0.35 | 0.23 | −0.39 | − | ||||||||
| P | −0.59 | 0.80 | −0.59 | 0.54 | −0.11 | −0.11 | 0.70 | −0.38 | −0.44 | 0.98 | 0.48 | 0.54 | 0.80 | 0.89 | −0.20 | − | |||||||
| Pb | 0.97 | −0.52 | 0.97 | 0.32 | 0.80 | −0.42 | 0.26 | 0.70 | 0.98 | −0.36 | 0.46 | −0.12 | −0.14 | −0.69 | 0.42 | −0.41 | − | ||||||
| U | 1.00 | −0.65 | 1.00 | 0.13 | 0.73 | −0.30 | 0.05 | 0.69 | 0.97 | −0.55 | 0.26 | −0.20 | −0.33 | −0.81 | 0.39 | −0.59 | 0.97 | − | |||||
| Zn | −0.20 | 0.49 | −0.20 | 0.68 | −0.12 | −0.05 | 0.50 | −0.19 | 0.04 | 0.60 | 0.58 | 0.50 | 0.47 | 0.43 | −0.16 | 0.66 | −0.03 | −0.20 | − | ||||
| CEC | −0.03 | 0.05 | −0.03 | 0.02 | 0.00 | −0.11 | −0.01 | −0.32 | −0.12 | 0.07 | 0.17 | 0.03 | −0.01 | 0.18 | −0.42 | 0.11 | −0.01 | −0.03 | −0.25 | − | |||
| pH | −0.28 | −0.05 | −0.28 | −0.44 | −0.60 | 0.11 | −0.71 | −0.39 | −0.37 | −0.41 | −0.36 | −0.45 | −0.46 | −0.13 | −0.38 | −0.35 | −0.38 | −0.28 | −0.26 | 0.28 | − | ||
| % *SM | −0.57 | 0.71 | −0.57 | 0.50 | −0.30 | −0.13 | 0.51 | −0.40 | −0.39 | 0.81 | 0.46 | 0.38 | 0.64 | 0.74 | −0.25 | 0.86 | −0.39 | −0.57 | 0.81 | 0.08 | −0.10 | − | |
| % *SOM | −0.11 | 0.38 | −0.11 | 0.78 | −0.15 | −0.27 | 0.50 | −0.12 | 0.07 | 0.53 | 0.78 | 0.33 | 0.46 | 0.26 | −0.13 | 0.55 | 0.07 | −0.11 | 0.86 | 0.08 | −0.04 | 0.75 | − |
References
- Juhasz, L.; Szerbin, P.; Lendvai, Z.; Csovari, M.; Benkovics, I. Planning for Uranium Mining and Milling Sites Environmental Restoration of in Central and Eastern Europe. In Proceedings of the Planning for Environmental Restoration of Uranium Mining and Milling Sites in Central and Eastern Europe: Proceedings of a Workshop Held under the Technical Co-Operation Project RER/9/022 on Environmental Restoration in Central and Eastern Europe; International Atomic Energy Agency: Vienna, Austria, 1996; pp. 102–116. [Google Scholar]
- Banik, J.; Csövári, M.; Németh, G. Uranium Ore Mining and the Remediation of the Site in Hungary. In Proceedings of the The Uranium Mining Remediation Exchange Group (UMREG); IAEA: Vienna, Austria, 2011; pp. 125–137. [Google Scholar]
- Wallner, A.; Stein, P. Uranium Mining in and for Europe; Austrian Institute of Ecology: Vienna, Austria, 2012. [Google Scholar]
- IAEA. Review of “Handbook of Parameter Values for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments”; International Atomic Energy Agency (IAEA) Technical Reports Series No. 472. IAEA: Vienna, Austria, 2011; Volume 102. [Google Scholar]
- Mecsekérc, Z. Uranium Monitoring. Available online: https://www.mecsekerc.hu/eng-uranipari-monitoring (accessed on 10 May 2020).
- Erdi-Krausz, G. Problems and Solutions for Water Treatment at the Closed Hungarian Uranium Industry. In Proceedings of the Recent Developments in Uranium Exploration, Production and Environmental Issues—IAEA-TECDOC-1463; IAEA in Cooperation with the OECD Nuclear Energy Agency and DIAMO State Owned Enterprise; IAEA: Staz, Czech Republic, 2005; p. 87. [Google Scholar]
- Skipperud, L.; Strømman, G.; Yunusov, M.; Stegnar, P.; Uralbekov, B.; Tilloboev, H.; Zjazjev, G.; Heier, L.S.; Rosseland, B.O.; Salbu, B. Environmental Impact Assessment of Radionuclide and Metal Contamination at the Former U Sites Taboshar and Digmai, Tajikistan. J. Environ. Radioact. 2013, 123, 50–62. [Google Scholar] [CrossRef]
- Fernández-Ondoño, E.; Bacchetta, G.; Lallena, A.M.; Navarro, F.B.; Ortiz, I.; Jiménez, M.N. Use of BCR Sequential Extraction Procedures for Soils and Plant Metal Transfer Predictions in Contaminated Mine Tailings in Sardinia. J. Geochemical Explor. 2017, 172, 133–141. [Google Scholar] [CrossRef]
- Waggitt, P. Uranium Mining Legacies Remediation and Renaissance Development: An International Overview. In Proceedings of the Uranium, Mining and Hydrogeology; Merkel, B.J., Hasche-berger, A., Eds.; Saxon State Ministry of Environment and Agriculture: Freiberg, Germany, 2008. [Google Scholar]
- Shiva Kumar, D.; Srikantaswamy, S. Factors Affecting on Mobility of Heavy Metals in Soil Environment. Int. J. Sci. Res. Dev. 2014, 2, 201–203. [Google Scholar]
- Sánchez-Donoso, R.; García Lorenzo, M.L.; Esbrí, J.M.; García-Noguero, E.M.; Higueras, P.; Crespo, E. Geochemical Characterization and Trace-Element Mobility Assessment for Metallic Mine Reclamation in Soils Affected by Mine Activities in the Iberian Pyrite Belt. Geosciences 2021, 11, 233. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Smičiklas, I.; Šljivić-Ivanović, M. Radioactive Contamination of the Soil: Assessments of Pollutants Mobility with Implication to Remediation Strategies. In Soil Contamination—Current Consequences and Further Solutions; Larramendy, M.L., Soloneski, S., Eds.; InTech: Rijeka, Croatia, 2016; pp. 268–276. ISBN 9789535128151. [Google Scholar]
- Salbu, B.; Lind, O.C.; Skipperud, L. Radionuclide Speciation and Its Relevance in Environmental Impact Assessments. J. Environ. Radioact. 2004, 74, 233–242. [Google Scholar] [CrossRef]
- Skipperud, L.; Salbu, B. Sequential Extraction as a Tool for Mobility Studies of Radionuclides and Metals in Soils and Sediments. Radiochim. Acta 2015, 103, 187–197. [Google Scholar] [CrossRef]
- Gupta, D.K.; Voronina, A. Remediation Measures for Radioactively Contaminated Areas; Springer International Publishing AG: Cham, Switzerland, 2019; ISBN 9783319733975. [Google Scholar]
- Igwe, J.C.; Nnorom, I.C.; Gbaruko, B.C. Kinetics of Radionuclides and Heavy Metals Behaviour in Soils: Implications for Plant Growth. Afr. J. Biotechnol. 2005, 4, 1541–1547. [Google Scholar] [CrossRef]
- Adesiji, N.E.; Ademola, J.A. Soil-to-Cassava Plant Transfer Factor of Natural Radionuclides on a Mining Impacted Soil in a Tropical Ecosystem of Nigeria. J. Environ. Radioact. 2019, 201, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ogundiran, M.B.; Osibanjo, O. Mobility and Speciation of Heavy Metals in Soils Impacted by Hazardous Waste. Chem. Speciat. Bioavailab. 2009, 21, 59–69. [Google Scholar] [CrossRef]
- Bielicka-Giełdoń, A.; Ryłko, E.; Zamojć, K. Distribution, Bioavailability and Fractionation of Metallic Elements in Allotment Garden Soils Using the BCR Sequential Extraction Procedure. Polish J. Environ. Stud. 2013, 22, 1013–1021. [Google Scholar]
- Ross, D.S.; Ketterings, Q. Recommended Methods for Determining Soil Cation Exchange Capacity. In Plant Growth Regulators to Manipulate Oat Stands; Rajala, A., Ed.; Springer: Berlin/Heidelerg, Germany, 2004; Volume 13, pp. 186–197. [Google Scholar]
- Agic, R.; Skopje, F.; Milenkovic, L.; Ilic, Z.S. Transfer Factor as Indicator. Fresenius Environ. Bull. 2015, 24, 4212–4219. [Google Scholar]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and Bioavailability of Heavy Metals and Metalloids in Soil Environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil-Plant Transfer of Trace Elements—An Environmental Issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Popic, J.M. Environmental Impact of Radionuclides and Trace Elements in the Thorium Rich Fen Area in Norway; Norwegian University of Life Sciences: Ås, Norway, 2014. [Google Scholar]
- Strok, M. Transfer of Natural Radionuclides from Hay and Silage to Cow’ s Milk in the Vicinity of a Former Uranium Mine. J. Environ. Radioact. 2012, 110, 64–68. [Google Scholar] [CrossRef]
- Opaluwa, O.D.; Aremu, M.O.; Ogbo, L.O.; Abiola, K.A.; Odiba, I.E.; Abubakar, M.M.; Nweze, N.O. Heavy Metal Concentrations in Soils, Plant Leaves and Crops Grown around Dump Sites in Lafia Metropolis, Nasarawa State, Nigeria. Adv. Appl. Sci. Res. 2012, 3, 780–784. [Google Scholar]
- Baran, A.; Tarnawski, M. Assessment of Heavy Metals Mobility and Toxicity in Contaminated Sediments by Sequential Extraction and a Battery of Bioassays. Ecotoxicology 2015, 24, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Rosado, D.; Usero, J.; Morillo, J. Ability of 3 Extraction Methods (BCR, Tessier and Protease K) to Estimate Bioavailable Metals in Sediments from Huelva Estuary (Southwestern Spain). Mar. Pollut. Bull. 2016, 102, 65–71. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Xiao, R.; Zhao, Q.; Jia, J.; Cui, B.; Liu, X. Heavy Metal Fractions and Ecological Risk Assessment in Sediments from Urban, Rural and Reclamation-Affected Rivers of the Pearl River Estuary, China. Chemosphere 2017, 184, 278–288. [Google Scholar] [CrossRef]
- Isimekhai, K.A.; Garelick, H.; Watt, J.; Purchase, D. Heavy Metals Distribution and Risk Assessment in Soil from an Informal E-Waste Recycling Site in Lagos State, Nigeria. Environ. Sci. Pollut. Res. 2017, 24, 17206–17219. [Google Scholar] [CrossRef]
- Stevanović, V.; Gulan, L.; Milenković, B.; Valjarević, A.; Zeremski, T.; Penjišević, I. Environmental Risk Assessment of Radioactivity and Heavy Metals in Soil of Toplica Region, South Serbia. Environ. Geochem. Health 2018, 40, 2101–2118. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.W.; Kim, D.H.; Kang, D.H.; Son, J.; Lee, S.Y.; Lee, C.K.; Lee, S.H.; Ji, W.H.; Baveye, P.C.; Yu, C. Effect of Farmland Type on the Transport and Spatial Distribution of Metal(Loid)s in Agricultural Lands near an Abandoned Gold Mine Site: Confirmation of Previous Observations. J. Geochem. Explor. 2017, 181, 129–137. [Google Scholar] [CrossRef]
- Sungur, A.; Soylak, M.; Ozcan, H. Investigation of Heavy Metal Mobility and Availability by the BCR Sequential Extraction Procedure: Relationship between Soil Properties and Heavy Metals Availability. Chem. Speciat. Bioavailab. 2014, 26, 219–230. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M. Geochemical Distribution of Co, Cu, Ni, and Zn in Soil Profiles of Fluvisols, Luvisols, Gleysols, and Calcisols Originating from Germany and Egypt. Geoderma 2017, 307, 122–138. [Google Scholar] [CrossRef]
- Soltani, N.; Keshavarzi, B.; Moore, F.; Sorooshian, A.; Ahmadi, M.R.; Sciences, A.; Ore, I.; Mining, G. Distribution of Potentially Toxic Elements (PTEs) in Tailings, Soils, and Plants around Gol-E-Gohar Iron Mine, a Case Study in Iran. Env. Sci. Pollut. Res. Int. 2017, 24, 18798–18816. [Google Scholar] [CrossRef]
- Korychenskyi, K.O.; Laptev, G.V.; Voitsekhovych, O.V.; Lavrova, T.V.; Dyvak, T.I. Speciation and mobility of uranium in tailings materials at the u-production legacy site in ukraine. Nucl. Phys. At. Energy 2018, 19, 270–279. [Google Scholar] [CrossRef]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of Heavy Metals by Modified BCR Sequential Extraction Procedure in Different Depths of Sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR Three Step Sequential Extraction Procedure Prior to the Certification of New Sediment and Soil Reference Materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Umoren, I.U.; Udoh, A.P.; Udousoro, I.I. Concentration and Chemical Speciation for the Determination of Cu, Zn, Ni, Pb and Cd from Refuse Dump Soils Using the Optimized BCR Sequential Extraction Procedure. Environmentalist 2007, 27, 241–252. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Shen, Z.; Niu, J.; Tang, Z. Distribution and Speciation of Heavy Metals in Sediments from the Mainstream, Tributaries, and Lakes of the Yangtze River Catchment of Wuhan, China. J. Hazard. Mater. 2009, 166, 1186–1194. [Google Scholar] [CrossRef]
- Saleem, M.; Iqbal, J.; Akhter, G.; Shah, M.H. Fractionation, Bioavailability, Contamination and Environmental Risk of Heavy Metals in the Sediments from a Freshwater Reservoir, Pakistan. J. Geochem. Explor. 2018, 184, 199–208. [Google Scholar] [CrossRef]
- Khumalo, L.; Heltai, G.; Horváth, M. The Migration of Potentially Toxic Elements during the Recultivation of the Uranium Mining Deposit in Mecsek. Acta Hydrol. Slovaca 2020, 20, 210–217. [Google Scholar] [CrossRef]
- Khumalo, L.; Heltai, G.; Várhegyi, A.; Horváth, M. Mobility of Potentially Toxic Elements from the Abandoned Uranium Mine’s Spoil Bank. Ecol. Chem. Eng. S 2021, 28, 241–258. [Google Scholar] [CrossRef]
- Heltai, G.; Győri, Z.; Fekete, I.; Halász, G.; Kovács, K.; Takács, A.; Khumalo, L.; Horváth, M. Application of Flexible Multi-Elemental ICP-OES Detection in Fractionation of Potentially Toxic Element Content of Solid Environmental Samples by a Sequential Extraction Procedure. Microchem. J. 2019, 149, 104029. [Google Scholar] [CrossRef]
- Juhasz, L.; Erdi-Krausz, G. Consequences of the hungarian uranium mining and milling. In Proceedings of a workshop held within the Technical Co-operation Project on Environmental Restoration in Central and Eastern Europe in Budapest, Hungary, 4–8 October 1993; Planning for Environmental Restoration of Radioactiviely Contaminated Sites in Cent; International Atomic Energy Agency: Vienna, Austria, 1993; pp. 151–156. [Google Scholar]
- René, M. History of Uranium Mining in Central Europe. In Uranium—Safety, Resources, Separation and Thermodynamic Calculation; Awwad, N.S., Ed.; IntechOpen: London, UK, 2018; Volume 1, pp. 1–20. ISBN 9781626239777. [Google Scholar]
- OrangeSmile.com. Maps of Hngary. Available online: http://www.https//www.orangesmile.com/travelguide/hungary/country-maps.htm (accessed on 3 April 2021).
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. The Certification of the Extractable Contents (Mass Fractions) of Cd, Cr, Cu, Ni. Pb and Zn in Freshwater Sediment Following a Sequential Extraction Procedure—BCR-701; Belgium. 2001. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/additional-certification-pb-mass-fraction-bcr-320r-channel-sediment (accessed on 17 April 2020).
- Khumalo, L.H.N.; Heltai, G.; Horváth, M. Mobility of Radionuclides from the Spoil Deposit No. 1 of the Abandoned Uranium Mine in Pécs, Hungary. In Proceedings of the 5th International Conference on Environmental Radioactivity ENVIRA 2019: Variations of Environmental Radioniclides; Světlík, I., Povinec, P.P., Pachnerová Brabcová, K., Eds.; Czech Technical University in Prague: Prague, Czech, 2019; p. 159. [Google Scholar]
- MSZ 1484-3:2006 Testing of Waters. Part 3: Determination of Dissolved, Suspended and Total Metals in Water by AAS and ICP-OES. Available online: http://www.mszt.hu/web/guest/home (accessed on 1 November 2020).
- Bernard, B.B.; Bernard, H.; Brooks, J.M. Determination of Total Carbon, Total Organic Carbon and Inorganic Carbon in Sediments. Available online: https://www.tdi-bi.com/analytical_services/environmental/NOAA_methods/TOC.pdf (accessed on 24 April 2021).
- Júnior, J.A.S.; Cardoso, J.J.R.F.; Silva, C.M.; Silveira, S.V.; Amaral, R.S. Determination of Radionuclides in the Environment Using Gamma-Spectrometry. J. Radioanal. Nucl. Chem. 2006, 269, 451–455. [Google Scholar] [CrossRef]
- Gerzabek, M.H.; Strebl, F.; Temmel, B. Plant Uptake of Radionuclides in Lysimeter Experiments. Environ. Pollut. 1998, 99, 93–103. [Google Scholar] [CrossRef]
- Intawongse, M.; Dean, J.R. Uptake of Heavy Metals by Vegetable Plants Grown on Contaminated Soil and Their Bioavailability in the Human Gastrointestinal Tract. Food Addit. Contam. 2007, 23, 36–48. [Google Scholar] [CrossRef]
- Laţo, A.; Radulov, I.; Berbecea, A.; Laţo, K.; Crista, F. The Transfer Factor of Metals in Soil-Plant System Mp Ms. Res. J. Agric. Sci. 2012, 44, 67–72. [Google Scholar]
- Statstutor Pearson’ s Correlation. 2015. Available online: http://www.statstutor.ac.uk/resources/uploaded/pearsons.pdf (accessed on 30 November 2020).
- Althouse, A.D.; Soman, P. Understanding the True Significance of a P Value. J. Nucl. Cardiol. 2017, 24, 191–194. [Google Scholar] [CrossRef]
- 6/2009. (IV. 14.) KvVM-EüM-FVM Common Order about the Standard Limits and Measurement of Contamination for the Protection of underground Water and Geological Medium; Budapest, Hungary. 2009. Available online: https://net.jogtar.hu/jogszabaly?docid=a0900006.kvv (accessed on 3 March 2021).
- Rékási, M.; Filep, T. Fractions and Background Concentrations of Potentially Toxic Elements in Hungarian Surface Soils. Environ. Monit. Assess. 2012, 184, 7461–7471. [Google Scholar] [CrossRef]
- Van Herreweghe, S.; Swennen, R.; Vandecasteele, C.; Cappuyns, V. Solid Phase Speciation of Arsenic by Sequential Extraction in Standard Reference Materials and Industrially Contaminated Soil Samples. Environ. Pollut. 2003, 122, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D.; Pavlović, M.; Čakmak, D.; Kostić, O.; Jarić, S.; Sakan, S.; Đorđević, D.; Mitrović, M.; Gržetić, I.; Pavlović, P. Fractionation, Mobility, and Contamination Assessment of Potentially Toxic Metals in Urban Soils in Four Industrial Serbian Cities. Arch. Environ. Contam. Toxicol. 2018, 75, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, S.M.; Gazquez, M.J.; Perez-Lopez, R.; Bolivar, J.P. Validation of the BCR Sequential Extraction Procedure for Natural Radionuclides. Chemosphere 2018, 198, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, P.S.; Dzhenloda, R.K.; Dampilova, B.V.; Doroshkevich, S.G.; Karandashev, V.K. Unexpected Behavior of Zn, Cd, Cu, and Pb in Soils Contaminated by Ore Processing after 70 Years of Burial. Environ. Chem. Lett. 2018, 16, 637–645. [Google Scholar] [CrossRef]
- IAEA. Handbook of Parameter Values for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments; Elsevier Ltd.: Vienna, Austria, 2010; Volume 472, (accessed on 1 February 2021). [Google Scholar] [CrossRef]
- United Nations. Sources and Effects of Ionizing Radiation—United Nations Scientific Committee on the Effects of Atomic Radiation; United Nations Publication: New York, NY, USA, 2000; pp. 1–659. ISBN 9211422388.
- Valkovic, V. Determination of Radionuclides In Environmental Samples. In Environmental Analysis: Techniques, Applications and Quality Assurance; Barcelo, D., Ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1993; pp. 311–356. ISBN 9781402095986. [Google Scholar]
- Almeida, G.M.; Campos, S.S.S.; Gennari, R.F.; Souza, S.O. Determination of the concentration of radionuclides in soil and water next the uranium mine of caetité-ba. In Proceedings of the International Nuclear Atlantic Conference; Nuclear Energy: New Jobs for a Better Life, Belo Horizonte, Brazil, 24–28 October 2011. [Google Scholar]
- Manigandan, P.K. Activity Concentration of Radionuclides in Plants in the Environment of Western Ghats. Iran J. of Radiat. Res. 2009, 7, 85–90. [Google Scholar]
- Banik, J.; Baudu-Picquet, I.; Benkovics, I.; Csövari, M.; Csicsak, J.; Edwards, C.; Guler, H.; Hideg, J.; Holden, P.; Jarrell, J.; et al. IAEA Safety Report Series No.35—Surveillance and Monitoring of Near Surface Disposal Facilities for Radioactive Waste; IAEA: Vienna, Austria, 2004; pp. 1–65. ISBN 978-9201069191. [Google Scholar]
- Bell, M.J.; Dayal, R.; Gera, F.; Green, T.H.; Han, K.W.; Holub, J.; Hubbell, J.; Hunter, G.; Kontic, B.; Kuèar-Dragièevic, C.; et al. IAEA Treatment of Liquid Effluent from Uranium Mines and Mills—Report of a Co-Ordinated Research Project 1996–2000; IAEA: Vienna, Austria, 2004; pp. 145–167. ISBN 92-0-112304-3. [Google Scholar]
- Erőss, A.; Csondor, K.; Izsák, B.; Vargha, M.; Horváth, Á.; Pándics, T. Uranium in Groundwater—The Importance of Hydraulic Regime and Groundwater Flow System’s Understanding. J. Environ. Radioact. 2018, 195, 90–96. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Drinking Water Quality, Volume 1. Recommendations, 3rd ed.; WHO: Geneva, Switzerland, 2008; Volume 1, ISBN 9789241547611. [Google Scholar]
- Ministerial Decree, No. 15/2001 (VI. 6.) KöM Environment on Radioactive Discharges to the Atmosphere and into Waters during the Use of Atomic Energy and on Monitoring of the Discharge; Budapest, Hungary. 2001. Available online: https://net.jogtar.hu/jogszabaly?docid=a0100015.kom (accessed on 19 March 2021).
- Abiye, T.; Shaduka, I. Radioactive Seepage through Groundwater Flow from the Uranium Mines, Namibia. Hydrology 2017, 4, 11. [Google Scholar] [CrossRef]
- Gulan, L.; Milenkovic, B.; Zeremski, T.; Milic, G.; Vuckovic, B. Persistent Organic Pollutants, Heavy Metals and Radioactivity in the Urban Soil of Priština City, Kosovo and Metohija. Chemosphere 2017, 171, 415–426. [Google Scholar] [CrossRef]
- Bai, H.; Hu, B.; Wang, C.; Bao, S.; Sai, G.; Xu, X.; Zhang, S.; Li, Y. Assessment of Radioactive Materials and Heavy Metals in the Surface Soil around the Bayanwula Prospective Uranium Mining Area in China. Int. J. Environ. Res. Public Health 2017, 14, 300. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Othman, R.; Ismail, M.R. Applying Limestone or Basalt in Combination with Bio-Fertilizer to Sustain Rice Production on an Acid Sulfate Soil in Malaysia. Sustainability 2016, 8, 700. [Google Scholar] [CrossRef]
- OECD/NEA. Managing Environmental and Health Impacts of Uranium Mining; Organisation for Economic Co-Operation and Development: Paris, France; OECD/NEA Publishing: Paris, France, 2014.
- Golia, E.E.; Tsiropoulos, G.N.; Füleky, G.; Floras, S.; Vleioras, S. Pollution Assessment of Potentially Toxic Elements in Soils of Different Taxonomy Orders in Central Greece. Environ. Monit. Assess. 2019, 191, 106. [Google Scholar] [CrossRef] [PubMed]



| Depth (cm) | Rn-M11 | Rn-M12 | Rn-M13 | Radioactive Sample |
|---|---|---|---|---|
| 0–25 | 2.3.1: 1 and 3; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1 and 3; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1 and 3; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1 and 3; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 |
| 25–50 | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | Not enough soil cover |
| 50–75 | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | |
| 75–100 | Hard rock | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 | 2.3.1: 1; 2.3.2: 1 and 2; 2.3.3: 1, 2, 3, and 4 |
| Radionuclides | Rn-M11 | Rn-M12 | Rn-M13 |
|---|---|---|---|
| Pb-212 (238.6 keV) | n.d. | 1.25 ± 4.5 | n.d. |
| Pb-214 (295.2 keV) | 1.97 ± 4.9 | 3.27 ± 4.5 | 1.16 ± 2.0 |
| Ac-228 (338.3 keV) | n.d. | 1.56 ± 4.9 | 2.35 ± 2.1 |
| Pb-214 (352 keV) | 0.16 ± 4.4 | 2.57 ± 4.4 | 0.88 ± 2.0 |
| Tl-208 (583.1 keV) Bi-214 (609.3 keV) Ac-228 (911.6 keV) Ac-228 (969.1 keV) | 1.34 ± 4.8 | 6.40 ± 4.3 | 0.22 ± 2.2 |
| 3.17 ± 4.4 | 1.72 ± 4.5 | 1.11 ± 2.0 | |
| 7.95 ± 4.4 | 1.68 ± 4.2 | 0.68 ± 2.4 | |
| n.d. | n.d. | n.d. | |
| Bi-214 (1120.3 keV) | 2.30 ± 4.5 | 0.85 ± 4.7 | 2.44 ± 2.0 |
| K-40 | 3.97 ± 4.5 | 4.73 ± 4.3 | 1.74 ± 1.9 |
| U-238 | 2.06 ± 4.5 | 1.71 ± 4.7 | 1.55 ± 2.0 |
| Th-232 | 0.16 ± 2.6 | 1.67 ± 4.5 | 0.86 ± 2.1 |
| TF values for grasses [65] | |||
| Mean | Minimum | Maximum | |
| Pb | 0.31 | 0.11 | 1.0 |
| K (in pasture grasses) | 0.73 | - | - |
| U | 0.017 | 0.00020 | 5.5 |
| Th | 0.042 | 0.00074 | 0.65 |
| Results from the Current Study | Results from the IAEA Study [71] | |||
|---|---|---|---|---|
| Sample ID | Sample Description | U Concentration (mg/L) | Sample Description | U Concentration (mg/L) |
| PK-33/1 | Groundwater (No. I) | 6.06 ± 0.03 | groundwater | 0.01–0.04 |
| PK-44/3 | Groundwater (No. I) | 0.23 ± 0.001 | pond water | 0.03 |
| PK-29/1 | Groundwater (No. I) | 1.87 ± 0.01 | process water | <0.5 |
| 1504/1 | Groundwater (No. I) | 2.78 ± 0.01 | seepage water | 2–5 |
| P-2/5 | Groundwater (No. II) | 1.90 ± 0.06 | ||
| P-2/6 | Groundwater (No. II) | 0.52 ± 0.003 | ||
| Elfolyó | treated mine water | 0.32 ± 0.001 | ||
| 6/11 Szint Északi-táró | mine water from the spoil deposit No. I | 2.46 ± 0.01 | ||
| mixed water: mine water from the waste deposit No. III and leaking water from precipitation | 6.72 ± 0.04 | |||
| IIIM. Gyűjtő | seepage water from the waste deposit No. III | 6.99 ± 0.02 | ||
| Cs-0 | seepage water from the waste rock pile No. II | 0.84 ± 0.003 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, M.; Heltai, G.; Várhegyi, A.; Mbokazi, L. A Study on the Possible Relationship between Physico-Chemical Properties of the Covering Soil and the Mobility of Radionuclides and Potentially Toxic Elements in a Recultivated Spoil Bank. Minerals 2022, 12, 1534. https://doi.org/10.3390/min12121534
Horváth M, Heltai G, Várhegyi A, Mbokazi L. A Study on the Possible Relationship between Physico-Chemical Properties of the Covering Soil and the Mobility of Radionuclides and Potentially Toxic Elements in a Recultivated Spoil Bank. Minerals. 2022; 12(12):1534. https://doi.org/10.3390/min12121534
Chicago/Turabian StyleHorváth, Márk, György Heltai, András Várhegyi, and Lamlile Mbokazi. 2022. "A Study on the Possible Relationship between Physico-Chemical Properties of the Covering Soil and the Mobility of Radionuclides and Potentially Toxic Elements in a Recultivated Spoil Bank" Minerals 12, no. 12: 1534. https://doi.org/10.3390/min12121534
APA StyleHorváth, M., Heltai, G., Várhegyi, A., & Mbokazi, L. (2022). A Study on the Possible Relationship between Physico-Chemical Properties of the Covering Soil and the Mobility of Radionuclides and Potentially Toxic Elements in a Recultivated Spoil Bank. Minerals, 12(12), 1534. https://doi.org/10.3390/min12121534






