Compositional Variations in Apatite and Petrogenetic Significance: Examples from Peraluminous Granites and Related Pegmatites and Hydrothermal Veins from the Central Iberian Zone (Spain and Portugal)
Abstract
1. Introduction
2. Geological Setting and Samples
2.1. S1 Granites
2.2. S2 Granites and Leucogranites
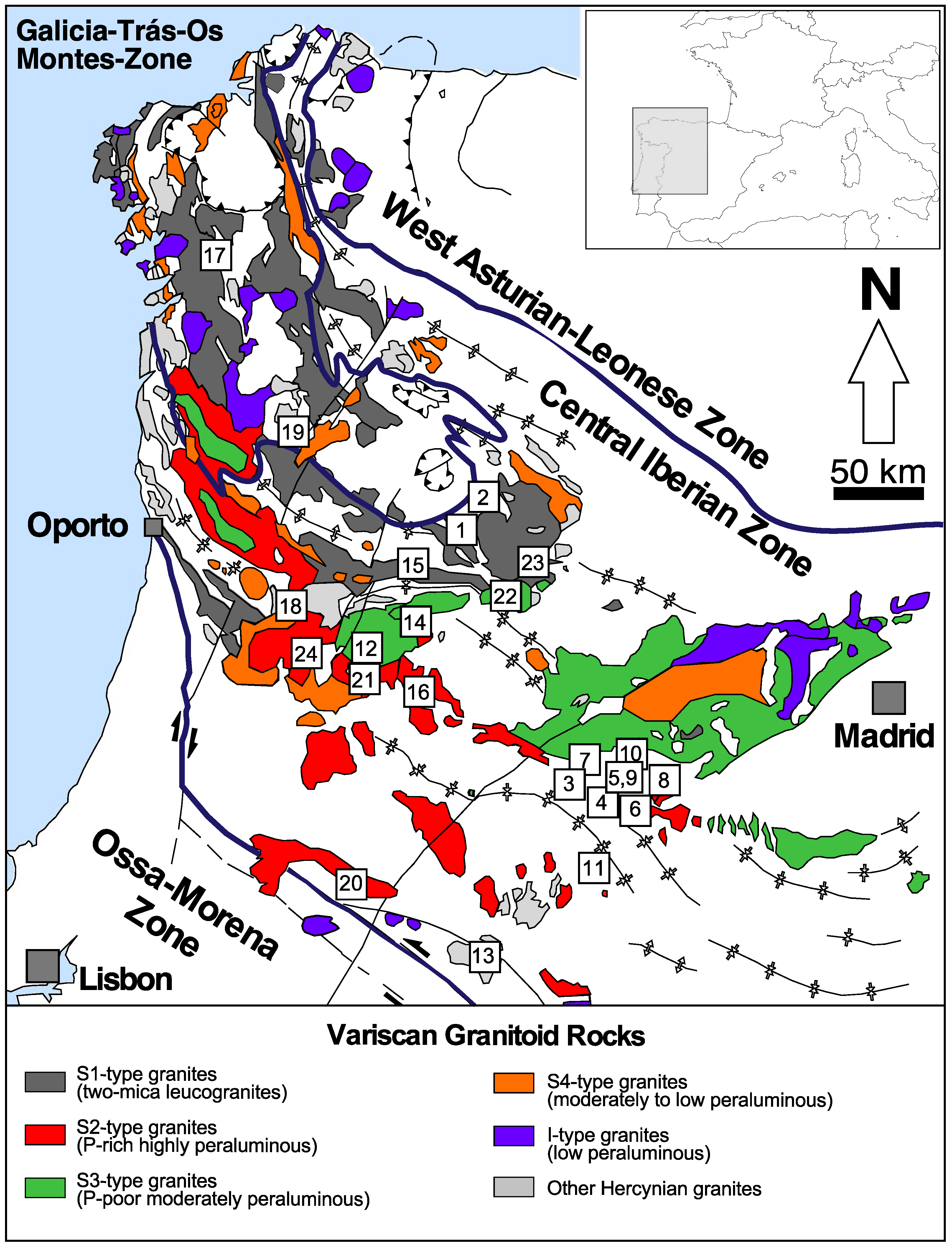
2.3. Barren Pegmatites
2.4. P-Rich Pegmatites
2.5. Li-Rich Pegmatites
2.6. Quartz Veins
2.7. P-Rich Quartz Veins
3. Data Collection and Analyses
| Locality | S1 Granites | S2 Granites | Leucogr. | B-Rich Leucogr. | Highly Fractionated Leucogr. | Barren Pegm. | P-Rich Pegm. | Li-Rich Pegm. | Qz-Veins | P-Rich qz Dyke | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Puentemocha | X | x | x | (1) | |||||||
| (2) Pinilla de Fermoselle | X | x | x | (1) | |||||||
| (3) Belvís de Monroy | x | x | x | x | (2)(3) | ||||||
| (4) Peraleda de San Román | x | x | (2) | ||||||||
| (5) Valdeverdeja | x | x | (2) | ||||||||
| (6) Villar del Pedroso | x | x | (2) | ||||||||
| (7) Navalmoral de la Mata | x | (2) | |||||||||
| (8 Aldeanueva de Barbarroya | x | (2) | |||||||||
| (9) Puente del Arzobispo | x | (2) | |||||||||
| (10) Oropesa | x | (2) | |||||||||
| (11) Logrosán | x | x | (4) | ||||||||
| (12) Guarda-Sabugal | x | (5) | |||||||||
| (13) Sierra–Bermeja | x | (6) | |||||||||
| (14) Castillejo de Dos Casas | x | x | X | (1) | |||||||
| (15) Fregeneda–Almendra | x | X | x | (1) | |||||||
| (16) Navasfrías | x | X | (7)(8) | ||||||||
| (17) Lalín–Forcarei | X | (9) | |||||||||
| (18) Queiriga | X | (1) | |||||||||
| (19) Barroso–Alvão | x | X | (10) | ||||||||
| (20) Tres Arroyos | x | X | (11) | ||||||||
| (21) Bendada | x | (1) | |||||||||
| (22) Cañada | x | (1) | |||||||||
| (23) Golpejas | x | (1) | |||||||||
| (24) Sitio do Castelo | x | (1) |
4. Results
4.1. Apatite Major Elements
| Anal. nº | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs. | * | * | * | ** | *** | **** | ** | ** | ** | * | * | * | * | * | ** |
| Locality | (1) | (1) | (2) | (7) | (12) | (13) | (3) | (5) | (5) | (14) | (14) | (14) | (14) | (14) | (3) |
| Lithology | S1 Granites | S2 Granites | Leucogranites | B-Rich Leucogranites | Highly Fractionates Leucogr. | ||||||||||
| SiO2 | b.d.l. | b.d.l. | - | b.d.l. | - | 0.03 | b.d.l. | b.d.l. | b.d.l. | 0.04 | 0.16 | 1.02 | b.d.l. | b.d.l. | 0.22 |
| TiO2 | - | - | - | b.d.l. | 0.01 | 0.02 | b.d.l. | b.d.l. | 0.02 | - | - | 0.41 | 0.16 | 0.03 | 0.04 |
| Al2O3 | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.09 | b.d.l. | b.d.l. | b.d.l. | 0.01 | b.d.l. | b.d.l. | 0.63 | 0.02 | 0.01 | 0.15 |
| FeO(t) | 0.74 | 0.64 | 0.41 | 0.49 | 0.38 | 0.43 | 0.75 | 1.14 | 0.14 | 1.19 | 0.07 | 1.52 | 0.31 | 0.88 | 3.54 |
| MnO | 0.97 | 1.23 | 0.51 | 0.03 | 0.45 | 0.77 | 2.84 | 1.38 | b.d.l. | 7.95 | 2.51 | 2.46 | 3.13 | 6.09 | 3.38 |
| MgO | b.d.l. | 0.04 | 0.04 | b.d.l. | 0.02 | 0.07 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | 0.05 | b.d.l. | 0.02 | 0.07 |
| ZnO | - | - | - | 0.04 | - | - | - | 0.02 | b.d.l. | - | - | 0.03 | b.d.l. | 0.08 | b.d.l. |
| CaO | 53.10 | 53.70 | 55.57 | 54.56 | 53.79 | 54.28 | 49.32 | 51.98 | 53.66 | 46.43 | 51.76 | 52.83 | 52.80 | 47.85 | 47.29 |
| Na2O | 0.02 | b.d.l. | b.d.l. | 0.12 | 0.06 | 0.05 | b.d.l. | 0.10 | b.d.l. | 0.04 | b.d.l. | b.d.l. | b.d.l. | 0.05 | 0.74 |
| K2O | - | - | b.d.l. | b.d.l. | 0.06 | 0.01 | b.d.l. | 0.01 | 0.01 | - | b.d.l. | 0.02 | 0.01 | 0.01 | 0.02 |
| BaO | - | - | - | - | 0.03 | - | - | - | - | - | - | 0.01 | b.d.l. | 0.09 | - |
| SrO | b.d.l. | b.d.l. | 0.04 | - | 0.02 | - | - | - | - | b.d.l. | - | 0.20 | 0.02 | 0.03 | - |
| P2O5 | 42.65 | 43.28 | 43.05 | 41.41 | 42.55 | 41.33 | 42.34 | 42.01 | 42.88 | 42.16 | 42.10 | 38.28 | 39.73 | 38.67 | 39.03 |
| H2O* | 0.27 | 0.33 | 0.00 | 0.07 | 0.38 | 0.30 | 0.00 | 0.00 | 0.04 | 0.41 | 0.25 | 0.59 | 0.00 | 0.00 | 0.46 |
| F | 3.18 | 3.10 | 4.02 | 3.54 | 2.95 | 3.08 | 3.88 | 4.00 | 3.66 | 2.84 | 3.13 | 2.41 | 3.91 | 3.84 | 2.32 |
| Cl | b.d.l. | 0.04 | 0.04 | 0.04 | 0.01 | 0.02 | b.d.l. | 0.03 | b.d.l. | 0.00 | 0.12 | 0.01 | 0.21 | 0.62 | 0.49 |
| O=F,Cl | 1.34 | 1.31 | 1.70 | 1.50 | 1.24 | 1.30 | 1.63 | 1.69 | 1.54 | 1.20 | 1.35 | 1.02 | 1.69 | 1.75 | 1.09 |
| Total | 99.59 | 101.05 | 101.98 | 98.82 | 99.56 | 99.09 | 97.50 | 98.98 | 98.88 | 99.87 | 98.76 | 99.45 | 98.61 | 96.52 | 96.66 |
| Structural formulae on the basis of 26 (O,F,Cl,OH) | |||||||||||||||
| Si | b.d.l. | b.d.l. | - | b.d.l. | - | 0.005 | b.d.l. | b.d.l. | b.d.l. | 0.007 | 0.027 | 0.176 | b.d.l. | b.d.l. | 0.039 |
| Ti | - | - | - | b.d.l. | 0.001 | 0.003 | b.d.l. | b.d.l. | 0.003 | - | - | 0.053 | 0.021 | 0.004 | 0.005 |
| Al | b.d.l. | b.d.l. | b.d.l. | 0.004 | 0.018 | b.d.l. | b.d.l. | b.d.l. | 0.002 | b.d.l. | b.d.l. | 0.128 | 0.004 | 0.002 | 0.031 |
| Fe2+(t) | 0.104 | 0.089 | 0.056 | 0.070 | 0.053 | 0.061 | 0.108 | 0.162 | 0.020 | 0.169 | 0.010 | 0.219 | 0.045 | 0.131 | 0.526 |
| Mn2+ | 0.138 | 0.173 | 0.071 | 0.004 | 0.064 | 0.111 | 0.412 | 0.198 | b.d.l. | 1.147 | 0.361 | 0.359 | 0.459 | 0.919 | 0.509 |
| Mg | b.d.l. | 0.010 | 0.010 | b.d.l. | 0.005 | 0.018 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.003 | 0.013 | b.d.l. | 0.005 | 0.019 |
| Zn | - | - | - | 0.005 | - | - | b.d.l. | b.d.l. | - | - | 0.004 | b.d.l. | 0.011 | b.d.l. | |
| Ca | 9.570 | 9.540 | 9.803 | 9.961 | 9.688 | 9.898 | 9.061 | 9.452 | 9.684 | 8.470 | 9.426 | 9.760 | 9.790 | 9.137 | 9.003 |
| Na | 0.007 | b.d.l. | b.d.l. | 0.040 | 0.020 | 0.016 | b.d.l. | 0.033 | b.d.l. | 0.013 | b.d.l. | b.d.l. | b.d.l. | 0.017 | 0.255 |
| K | - | - | b.d.l. | b.d.l. | 0.013 | 0.002 | b.d.l. | 0.002 | 0.002 | - | b.d.l. | 0.004 | 0.002 | 0.002 | 0.005 |
| Ba | - | - | - | - | 0.002 | - | - | - | - | - | - | 0.001 | b.d.l. | 0.006 | - |
| Sr | b.d.l. | b.d.l. | 0.004 | - | 0.002 | - | - | - | - | b.d.l. | - | 0.020 | 0.002 | 0.003 | - |
| P | 6.074 | 6.076 | 6.001 | 5.974 | 6.056 | 5.955 | 6.147 | 6.036 | 6.115 | 6.077 | 6.058 | 5.589 | 5.821 | 5.835 | 5.871 |
| F | 1.692 | 1.625 | 2.093 | 1.908 | 1.568 | 1.658 | 2.104 | 2.147 | 1.950 | 1.529 | 1.682 | 1.314 | 2.140 | 2.164 | 1.304 |
| Cl | b.d.l. | 0.011 | 0.011 | 0.012 | 0.003 | 0.006 | b.d.l. | 0.009 | b.d.l. | 0.001 | 0.035 | 0.003 | 0.062 | 0.187 | 0.148 |
| OH | 0.308 | 0.363 | 0.000 | 0.081 | 0.429 | 0.337 | 0.000 | 0.000 | 0.050 | 0.470 | 0.282 | 0.683 | 0.000 | 0.000 | 0.549 |
| Anal. nº | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | (15) | (14) | (18) | (21) | (22) | (2) | (15) | (15) | (15) | (1) | (15) | (2) | (24) | (24) | (24) |
| Lithology | Li-Rich Pegmatites | P-Rich Pegmatites | Barren Pegmatites | Hydrothermal Qz Veins | P-Rich Quartz Dykes | ||||||||||
| SiO2 | - | 0.07 | - | - | - | - | - | - | - | b.d.l. | - | - | 0.01 | 0.01 | 0.02 |
| TiO2 | - | - | - | b.d.l. | b.d.l. | - | - | - | - | - | - | - | 0.02 | 0.03 | 0.03 |
| Al2O3 | - | b.d.l. | b.d.l. | 0.05 | b.d.l. | b.d.l. | - | - | - | 0.02 | - | b.d.l. | b.d.l. | 0.01 | 0.00 |
| FeO(t) | 0.01 | 0.29 | 0.21 | 0.85 | 0.83 | 3.51 | 0.56 | 0.21 | 1.24 | 0.14 | 0.06 | 0.22 | 0.34 | 1.20 | 1.26 |
| MnO | 3.98 | 6.52 | 5.09 | 4.70 | 7.19 | 5.72 | 2.66 | 1.79 | 4.87 | 0.41 | 0.18 | 0.74 | 3.65 | 5.56 | 5.62 |
| MgO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.02 | <0.01 | 0.01 |
| ZnO | - | - | - | - | - | - | - | - | - | - | - | - | b.d.l. | 0.05 | b.d.l. |
| CaO | 50.54 | 47.93 | 50.70 | 49.81 | 48.03 | 45.77 | 52.77 | 53.36 | 50.15 | 55.27 | 56.25 | 54.98 | 51.13 | 48.02 | 48.54 |
| Na2O | 0.04 | b.d.l. | 0.09 | 0.01 | 0.11 | 0.22 | 0.04 | b.d.l. | 0.12 | b.d.l. | 0.02 | b.d.l. | 0.01 | 0.03 | b.d.l. |
| K2O | b.d.l. | - | - | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | - | b.d.l. | b.d.l. | b.d.l. | 0.01 | 0.01 |
| BaO | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | 0.03 | 0.07 |
| SrO | 0.21 | 0.19 | - | 0.02 | - | b.d.l. | b.d.l. | 0.11 | b.d.l. | b.d.l. | b.d.l. | 0.07 | 0.09 | b.d.l. | 0.28 |
| P2O5 | 42.52 | 42.09 | 41.51 | 41.80 | 40.42 | 39.71 | 41.64 | 41.80 | 41.58 | 42.78 | 41.64 | 42.56 | 41.30 | 41.37 | 41.06 |
| H2O* | 0.45 | 0.54 | 0.23 | 0.23 | 0.46 | 0.65 | 0.36 | 0.63 | 0.10 | 0.30 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| F | 2.78 | 2.50 | 3.22 | 3.22 | 2.60 | - | 2.96 | 2.41 | 3.50 | 3.17 | 4.40 | 4.37 | 3.66 | 3.73 | 3.82 |
| Cl | b.d.l. | 0.13 | - | 0.02 | 0.14 | 4.10 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.02 | 0.10 | 0.06 |
| O=F,Cl | 1.17 | 1.08 | 1.36 | 1.36 | 1.13 | 0.93 | 1.25 | 1.01 | 1.47 | 1.33 | 1.85 | 1.84 | 1.54 | 1.59 | 1.62 |
| Total | 99.36 | 99.18 | 99.69 | 99.35 | 98.65 | 98.75 | 99.74 | 99.30 | 100.09 | 100.76 | 100.70 | 101.14 | 98.74 | 98.55 | 99.18 |
| Structural formulae on the basis of 26 (O,F,Cl,OH) | |||||||||||||||
| Si | - | 0.012 | - | - | - | - | - | - | - | b.d.l. | - | - | 0.001 | 0.001 | 0.004 |
| Ti | - | - | - | b.d.l. | b.d.l. | - | - | - | - | - | - | - | 0.002 | 0.004 | 0.004 |
| Al | - | b.d.l. | b.d.l. | 0.010 | b.d.l. | b.d.l. | - | - | - | 0.004 | - | b.d.l. | b.d.l. | 0.003 | 0.000 |
| Fe2+(t) | 0.001 | 0.041 | 0.030 | 0.121 | 0.121 | 0.520 | 0.079 | 0.030 | 0.176 | 0.019 | 0.008 | 0.031 | 0.049 | 0.173 | 0.181 |
| Mn2+ | 0.571 | 0.943 | 0.734 | 0.679 | 1.058 | 0.859 | 0.382 | 0.257 | 0.701 | 0.058 | 0.025 | 0.104 | 0.530 | 0.811 | 0.819 |
| Mg | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.005 | 0.005 | 0.001 | 0.003 |
| Zn | - | - | - | - | - | - | - | - | - | - | - | - | b.d.l. | 0.006 | b.d.l. |
| Ca | 9.166 | 8.766 | 9.254 | 9.098 | 8.940 | 8.690 | 9.587 | 9.697 | 9.137 | 9.852 | 10.072 | 9.768 | 9.398 | 8.866 | 8.947 |
| Na | 0.013 | b.d.l. | 0.030 | 0.003 | 0.037 | 0.076 | 0.013 | b.d.l. | 0.040 | 0.000 | 0.006 | b.d.l. | 0.005 | 0.009 | b.d.l. |
| K | b.d.l. | - | - | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | - | b.d.l. | b.d.l. | b.d.l. | 0.003 | 0.003 |
| Ba | - | - | - | - | - | - | - | - | - | - | - | - | 0.002 | 0.002 | 0.005 |
| Sr | 0.021 | 0.019 | - | 0.002 | - | b.d.l. | b.d.l. | 0.011 | b.d.l. | b.d.l. | b.d.l. | 0.007 | 0.009 | b.d.l. | 0.028 |
| P | 6.094 | 6.083 | 5.987 | 6.033 | 5.945 | 5.957 | 5.978 | 6.002 | 5.986 | 6.026 | 5.891 | 5.975 | 5.999 | 6.036 | 5.980 |
| F | 1.488 | 1.350 | 1.735 | 1.736 | 1.428 | - | 1.587 | 1.293 | 1.882 | 1.668 | 2.325 | 2.292 | 1.985 | 2.033 | 2.079 |
| Cl | b.d.l. | 0.036 | - | 0.006 | 0.041 | 1.231 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.006 | 0.004 | 0.030 | 0.019 |
| OH | 0.512 | 0.614 | 0.265 | 0.258 | 0.530 | 0.769 | 0.413 | 0.707 | 0.118 | 0.332 | 0.000 | 0.000 | 0.010 | 0.000 | 0.000 |
4.2. Apatite from the P-Rich Puentemocha Pegmatite
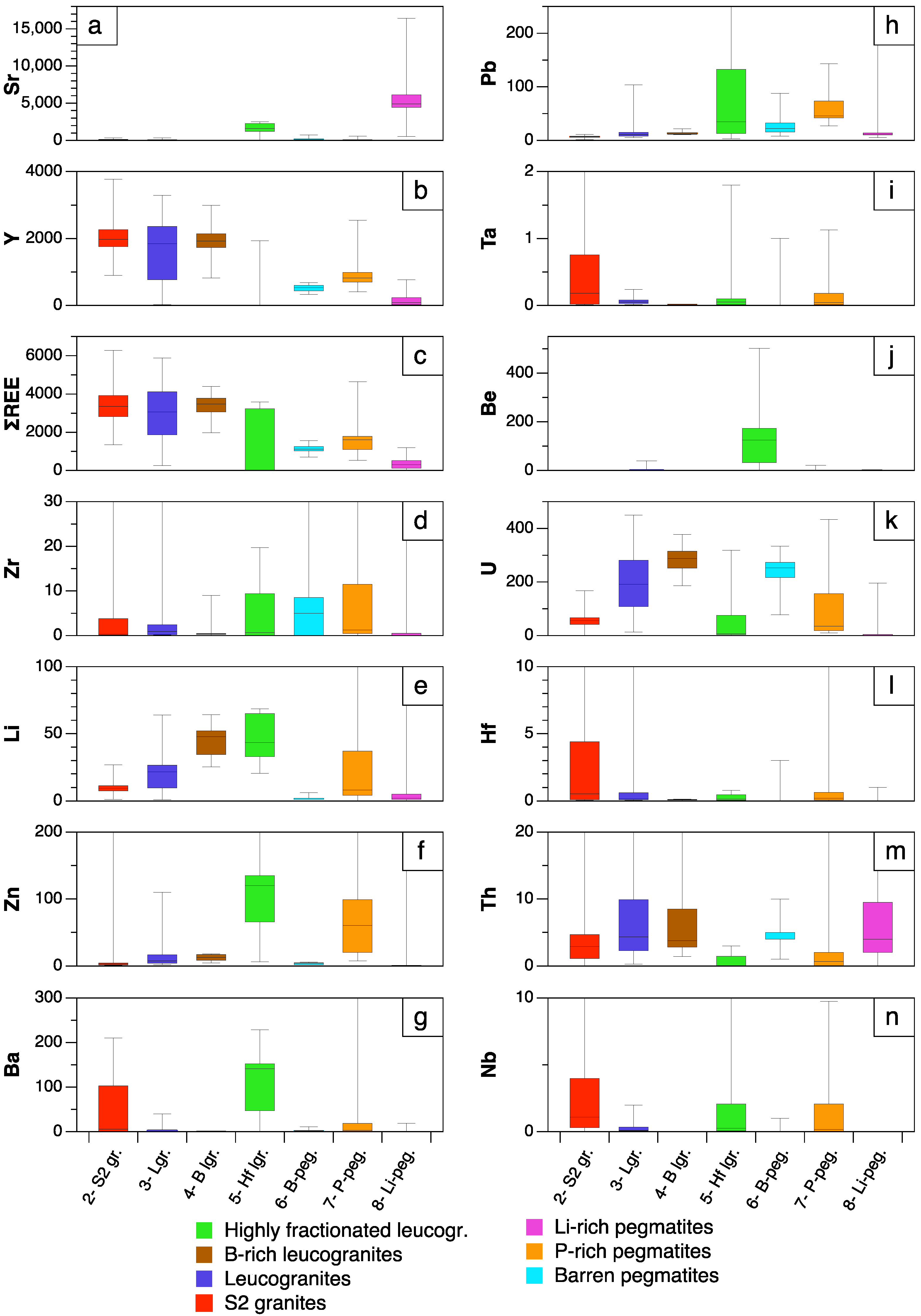
4.3. Apatite Trace Elements
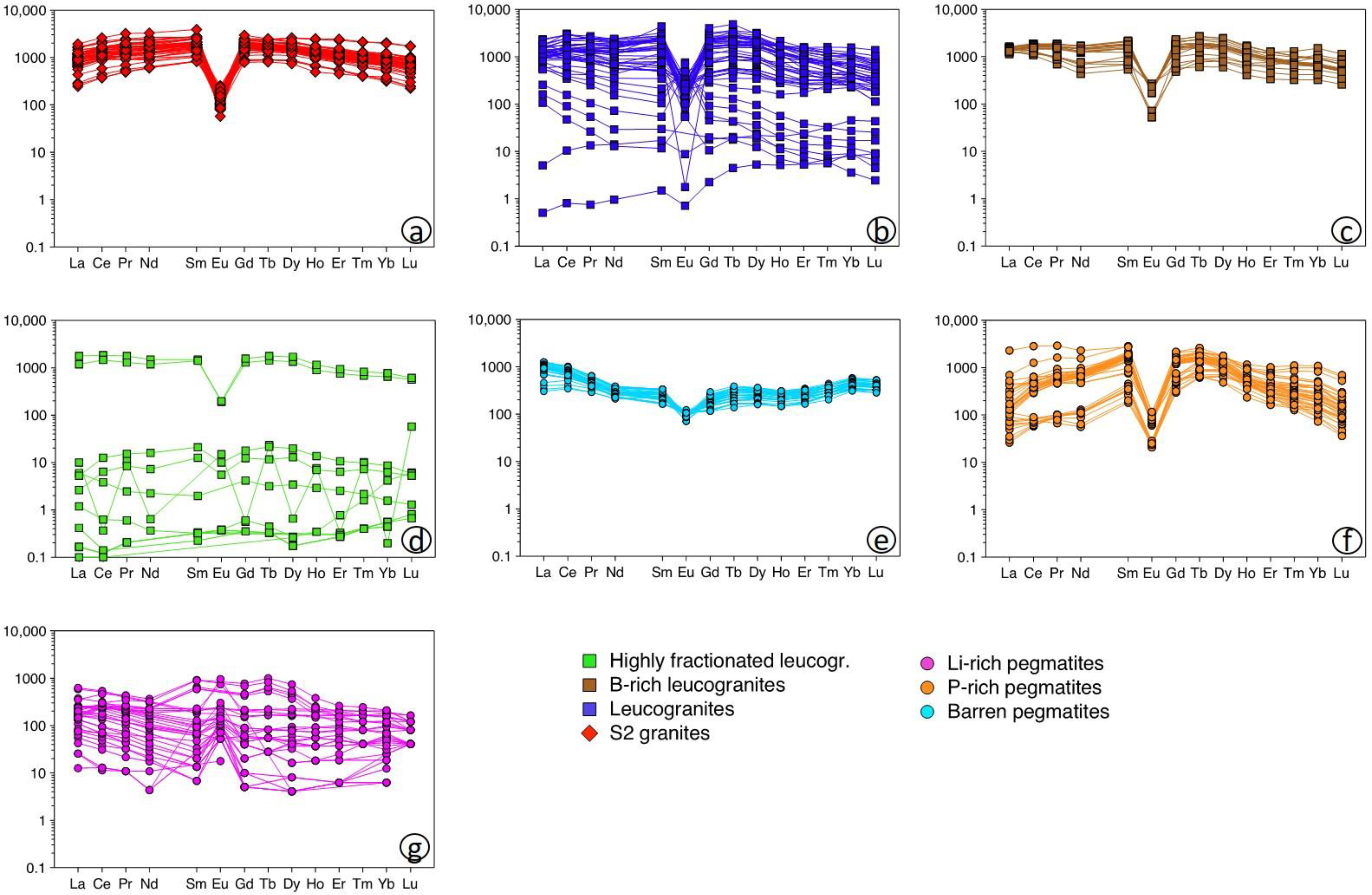
4.4. Rare Earth Element Patterns in Apatite
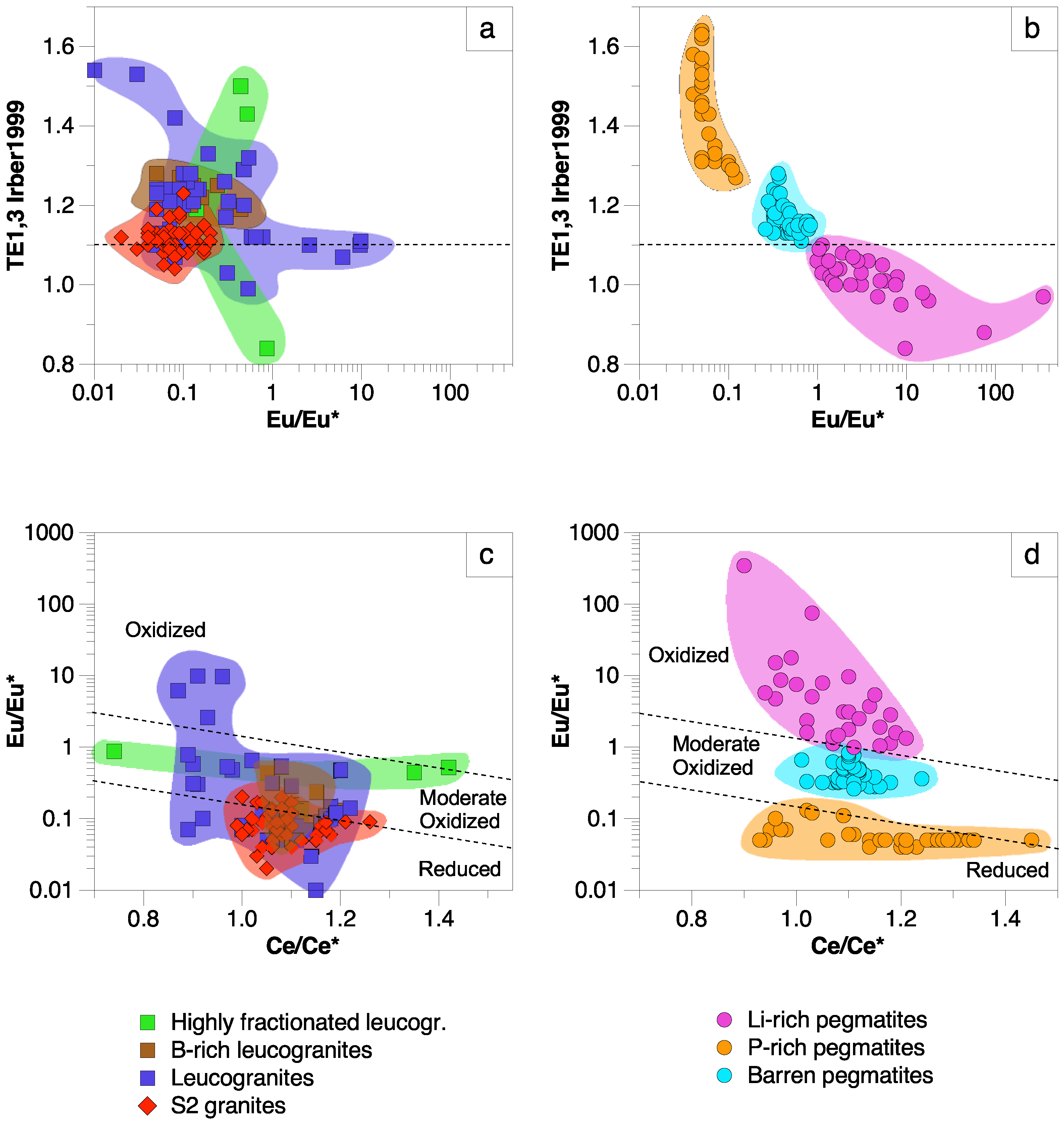
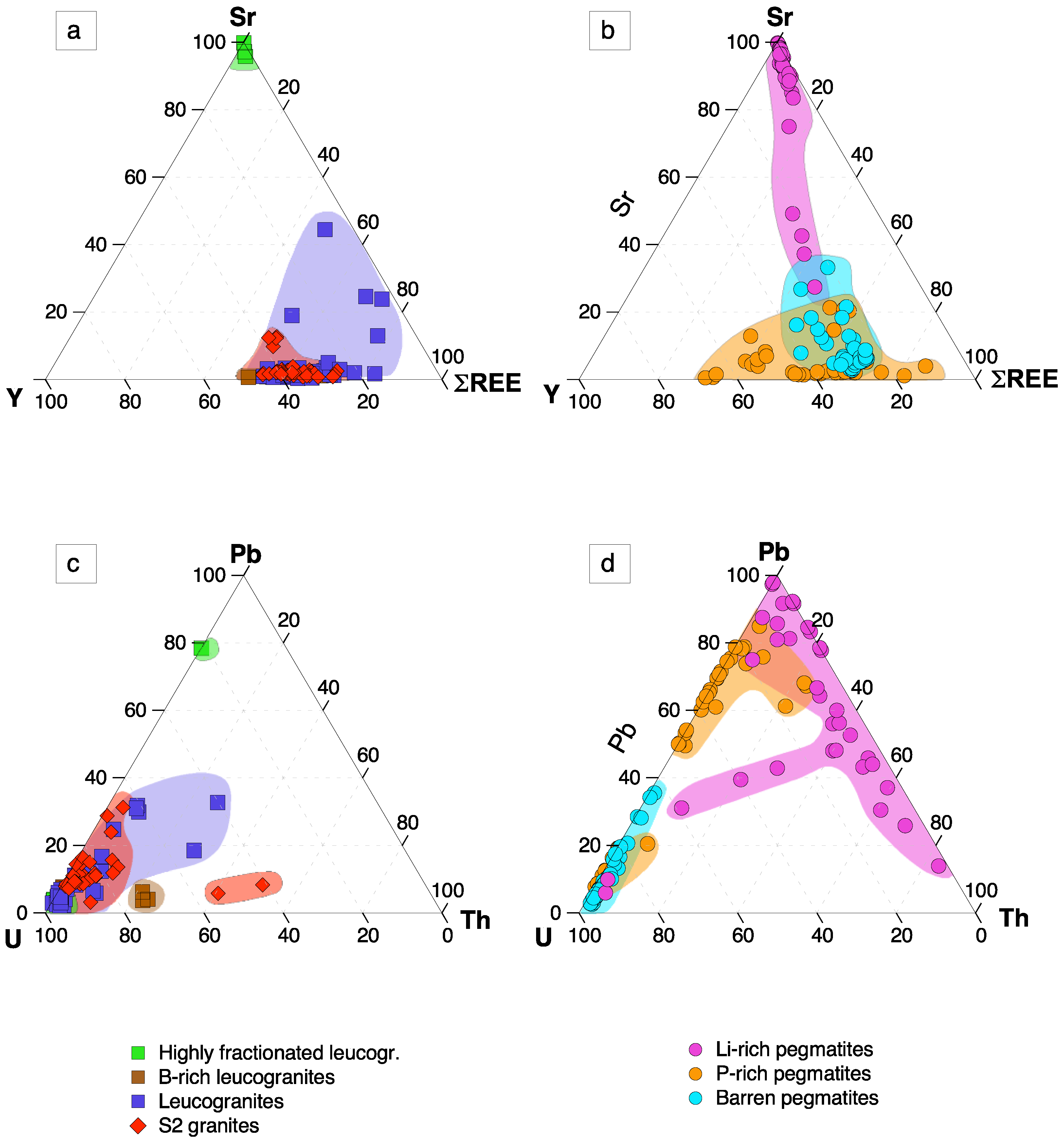
5. Discussion
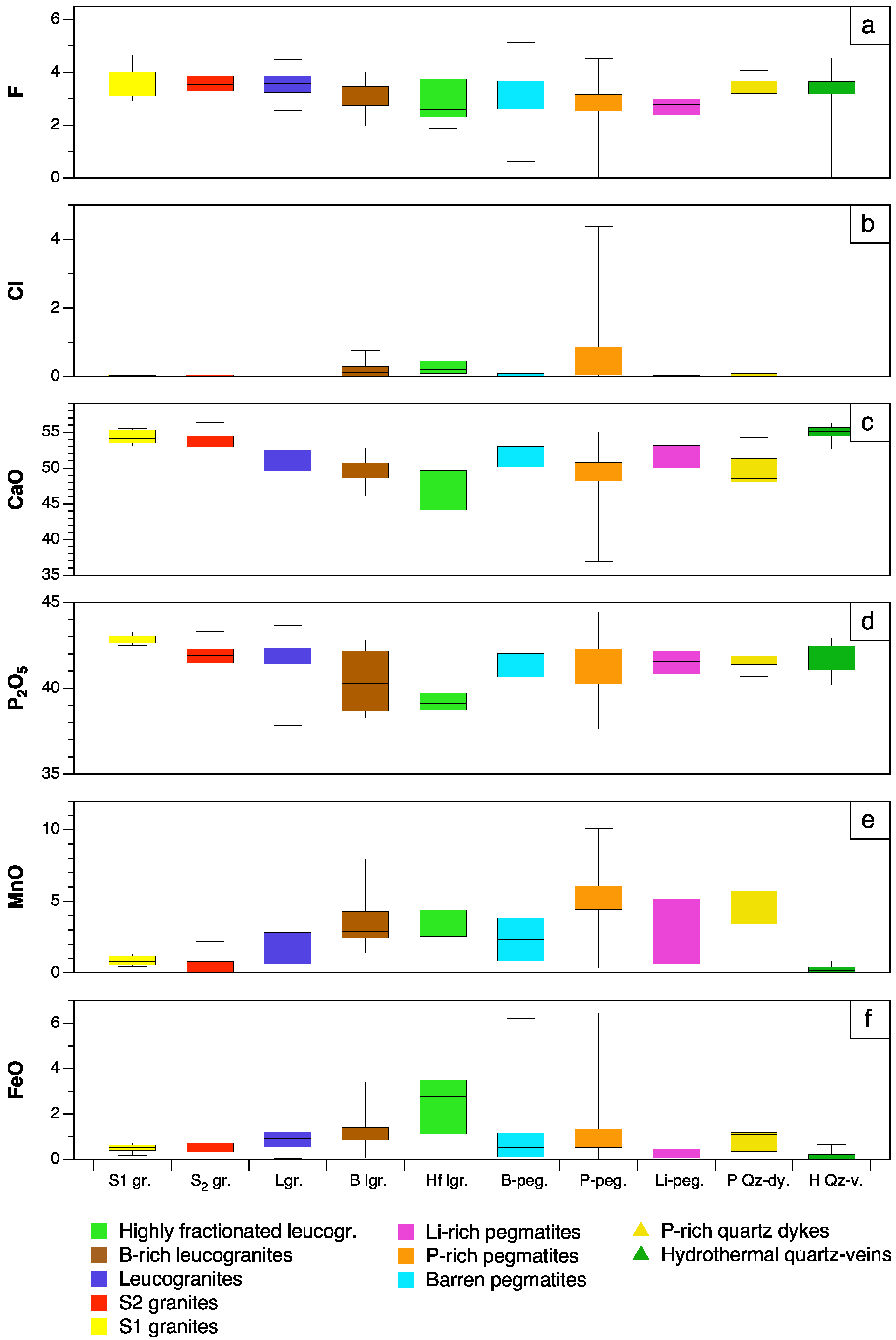
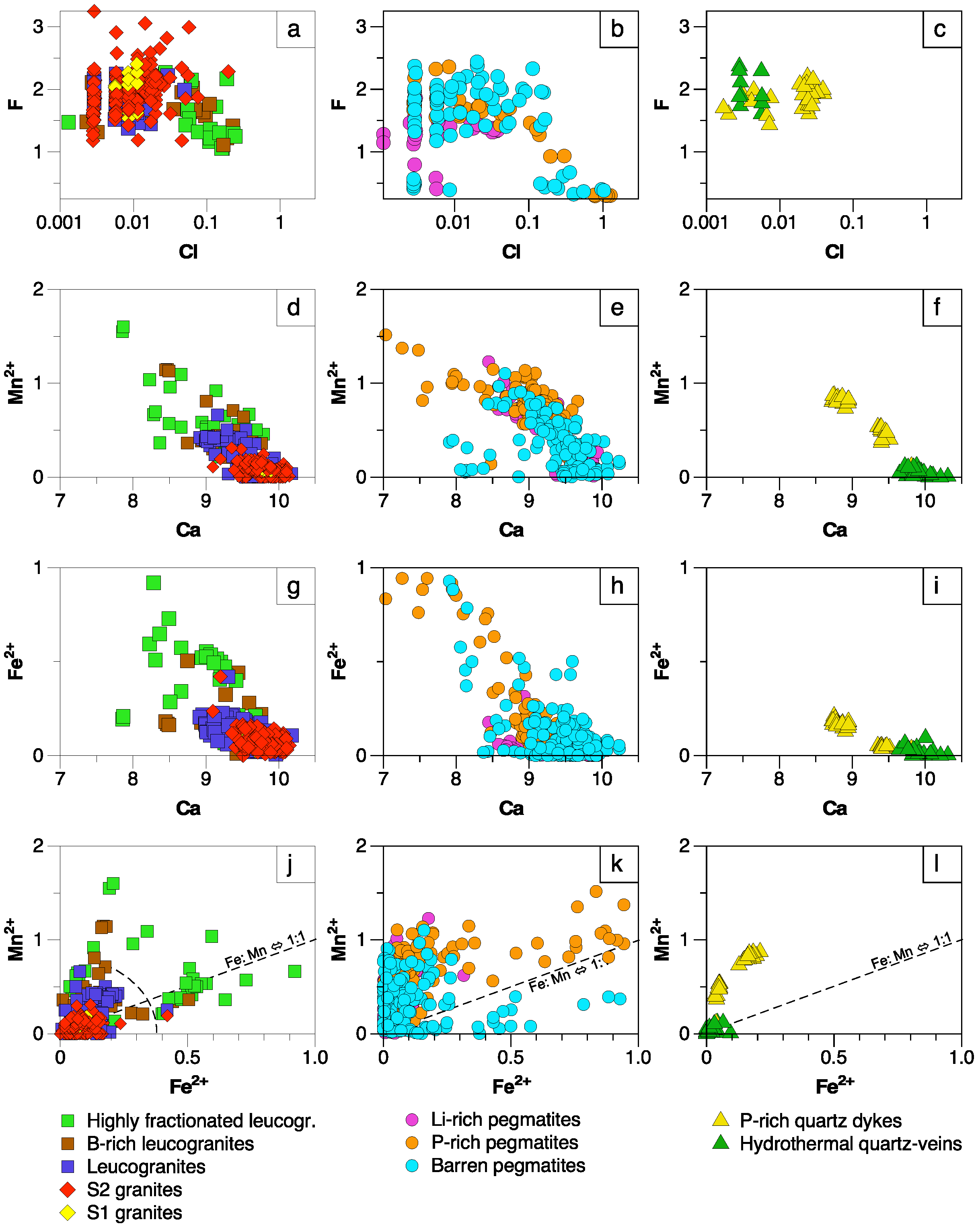
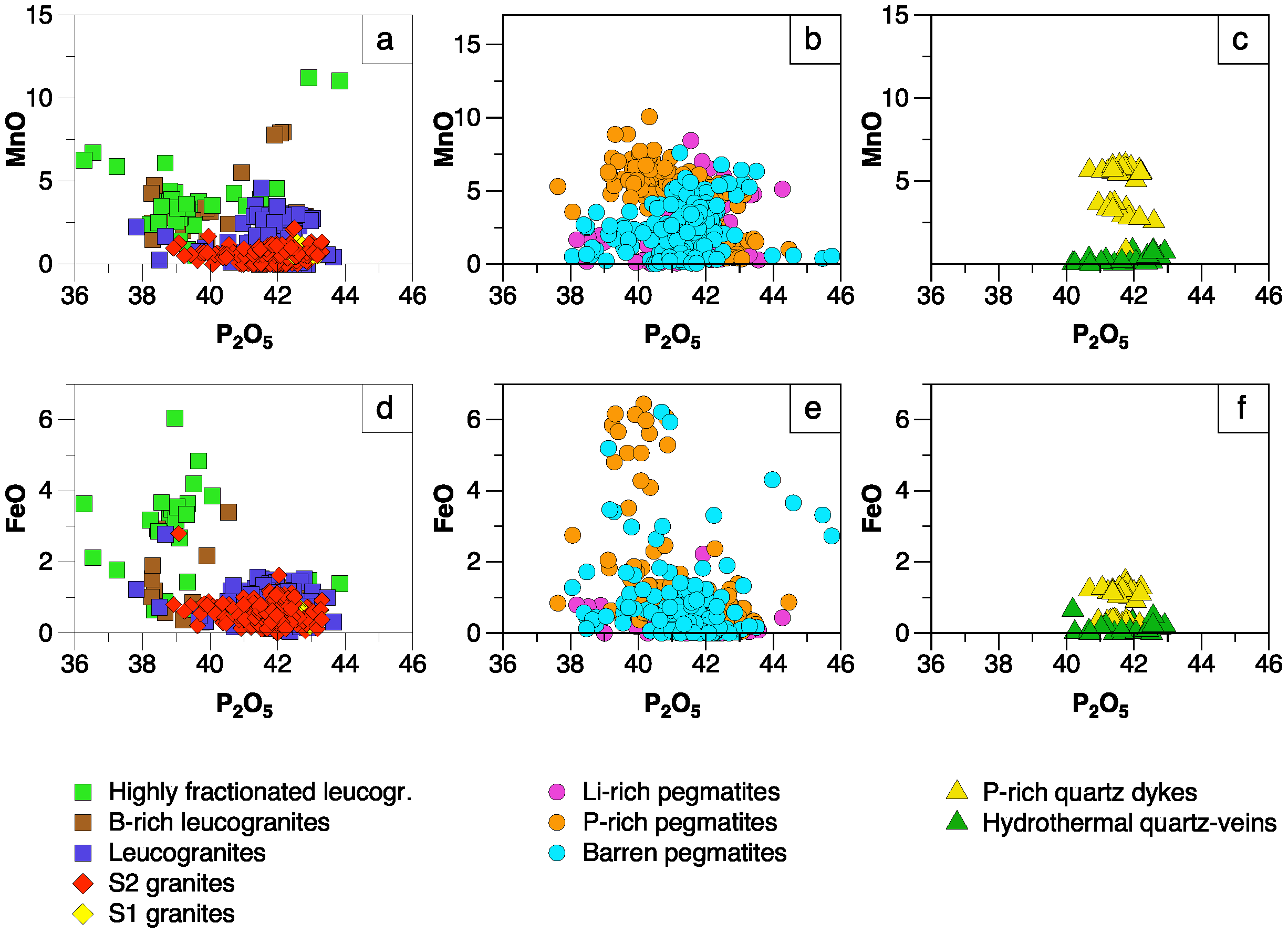
5.1. Significance of Major Element Variations
5.1.1. Manganese and Fe
5.1.2. F and Cl
5.2. Significance of Trace Element Variations
5.2.1. Eu and Ce Anomalies in Apatite
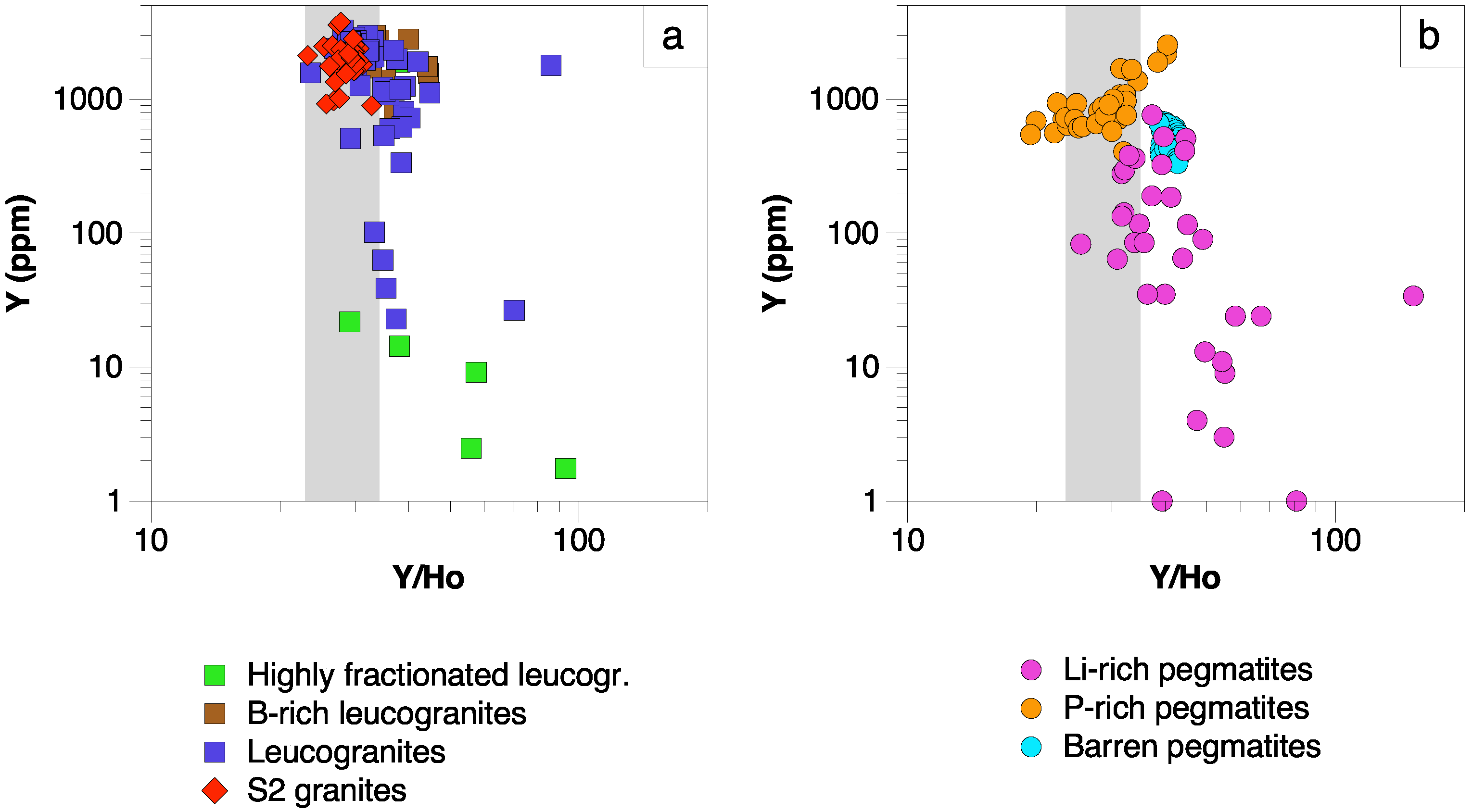
5.2.2. Strontium and Y Variations
5.2.3. Rare Earth Element Variations
5.3. Apatite as Archive of Processes in Granitic and Pegmatitic Systems
6. Summary and Conclusions
- (1)
- Apatite is a minor to accessory ubiquitous mineral in granitic and pegmatitic rocks. Here we present a systematic study on the composition of apatites associated with different Variscan granites, pegmatites and quartz veins from the CIZ.
- (2)
- Except for the minor occurrence of chlorapatite in P-rich pegmatites, most of the studied apatites correspond to fluorapatite. Important chemical variations have been found in apatite depending on the host lithology and evolution degree. Mn and Fe are the major elements whereas REE, Sr and Y are the main trace components in the apatite structure. While the highest Mn and Fe contents are found in the apatite from P-rich pegmatites, followed by the apatite from the P-rich quartz veins, the lowest values correspond to quartz veins and peraluminous granites. This may be attributed either to the low availability of Mn and Fe in late fluids exsolved from granitic and pegmatitic melts or to a high fO2.
- (3)
- Pegmatitic apatite with Fe/Mn > 1 may be indicative of the barren nature of its hosting pegmatite.
- (4)
- The Mn and Fe in granitic apatite increase with increasing evolution degree, probably due to a higher concentration of Mn in residual melts, together with an increase in SiO2 content and peraluminosity, rather than changes in fO2.
- (5)
- In the case of pegmatitic apatite, the fO2 and the polymerization degree of the melts seem not to have influenced the Mn and Fe content, but the higher availability of these transition elements and/or the lack of minerals competing for them.
- (6)
- The apatite from the P-rich pegmatites often appears together with Fe–Mn phosphates occurring in subrounded nodules, which probably crystallized from a P-rich fluid exsolved from the pegmatitic melt. If immiscibility took place, Mn, Fe and Cl would partition preferentially into the P-rich melt.
- (7)
- The apatite from the quartz-rich hydrothermal veins is the Mn- and Fe-poorest one, which may be attributed either to the low availability of these elements in late hydrothermal fluids exsolved from granitic and pegmatitic melts, or to a high fO2.
- (8)
- The REE contents in apatite decrease with the evolution degree of their hosting rocks, whereas the highest Sr and lowest Y contents are found in the most evolved lithologies (Li-rich pegmatites and B-P±F-rich leucogranites).
- (9)
- The REE patterns generally show strong tetrad effects, which do not seem to depend on the activity of fluids in the system. These, on the contrary, likely drive the non-CHARAC behavior of apatite from the most fractionated granites and pegmatites.
- (10)
- The strong Eu anomalies observed for most apatites associated with different granites, barren and P-rich pegmatites are interpreted to be related to low fO2 conditions. The positive Eu anomalies of some apatites from leucogranites and Li-rich pegmatites could reflect their early character, prior to the crystallization of feldspars.
- (11)
- The increase in Sr in apatite from most Li-rich pegmatites and B-P±F-rich leucogranites is the result of the limitation of albite for hosting appreciable amounts of Sr in its structure, thus being incorporated into apatite
- (12)
- The triangular ΣREE-Sr-Y and U-Th-Pb plots, as well as the Eu anomaly versus TE1,3 diagram, are potentially good discriminant tools, mainly for pegmatites from the CIZ.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoskin, P.W.O.; Kinny, P.D.; Wyborn, D.; Chappell, B.W. Identifying accessory mineral saturation during differentiation in granitoid magmas: An integrated approach. J. Petrol. 2000, 41, 1365–1396. [Google Scholar] [CrossRef]
- Bea, F.; Fershtater, G.B.; Corretgé, L.G. The geochemistry of phosphorus in granite rocks and the effect of aluminium. Lithos 1992, 29, 43–56. [Google Scholar] [CrossRef]
- Pichavant, M.; Montel, J.M.; Richard, L.R. Apatite solubility in peraluminous liquids: Experimental data and an extension of the Harrison-Watson model. Geochim. Cosmochim. Acta 1992, 56, 3855–3861. [Google Scholar] [CrossRef]
- Miles, A.J.; Graham, C.M.; Hawkesworth, C.J.; Gillespie, M.R.; Hinton, R.W. Evidence for distinct stages of magma history recorded by the compositions of accessory apatite and zircon. Contrib. Mineral. Petrol. 2013, 166, 1–19. [Google Scholar] [CrossRef]
- Lisowiec, K.; Slaby, E.; Götze, J. Cathodoluminescence (CL) of apatite as an insight into magma mixing in the granitoid pluton of Karkonosze, Poland. In Proceedings of the Conference on Raman and Luminescence Spectroscopy in the Earth Sciences, Wien, Austria, 3–6 July 2013. [Google Scholar]
- Azadbakht, Z.; Lentz, D.; McFarlane, C. Apatite Chemical Compositions from Acadian-Related Granitoids of New Brunswick, Canada: Implications for Petrogenesis and Metallogenesis. Minerals 2018, 8, 598. [Google Scholar] [CrossRef]
- Chu, M.-F.; Wang, K.-L.; Griffin, W.L.; Chung, S.-L.; O’Reilly, S.Y.; Pearson, N.J.; Iizuka, Y. Apatite Composition: Tracing Petrogenetic Processes in Transhimalayan Granitoids. J. Petrol. 2009, 50, 1829–1855. [Google Scholar] [CrossRef]
- Piccoli, P.; Candela, P. Apatite in Igneous Systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Rev. Mineral. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Belousova, E.A.; Griffin, W.L.; O’Reilly, S.Y.; Fisher, N.I. Apatite as an indicator mineral for mineral exploration: Trace-element compositions and their relationship to host rock type. J. Geochem. Explor. 2002, 76, 45–69. [Google Scholar] [CrossRef]
- Cao, M.; Li, G.; Qin, K.; Seitmuratova, E.Y.; Liu, Y. Major and trace element characteristics of apatites in granitoids from central Kazakhstan: Implications for petrogenesis and mineralization. Resour. Geol. 2012, 62, 63–83. [Google Scholar] [CrossRef]
- Teiber, H.; Marks, M.A.W.; Arzamastsev, A.A.; Wenzel, T.; Markl, G. Compositional variation in apatite from various host rocks: Clues with regards to source composition and crystallization conditions. Neues Jahrb. Fur Mineral. Abh. 2015, 192, 151–167. [Google Scholar] [CrossRef]
- Teiber, H.; Marks, M.A.W.; Wenzel, T.; Siebel, W.; Altherr, R.; Markl, G. The distribution of halogens (F, Cl, Br) in granitoid rocks. Chem. Geol. 2014, 374–375, 92–109. [Google Scholar] [CrossRef]
- Bruand, E.; Fowler, M.; Storey, C.; Darling, J. Apatite trace element and isotope applications to petrogenesis and provenance. Am. Mineral. 2017, 102, 75–84. [Google Scholar] [CrossRef]
- Bromiley, G.D. Do concentrations of Mn, Eu and Ce in apatite reliably record oxygen fugacity in magmas? Lithos 2021, 384–385, 105900. [Google Scholar] [CrossRef]
- Cao, M.-J.; Zhou, Q.-F.; Qin, K.-Z.; Tang, D.-M.; Evans, N.J. The tetrad effect and geochemistry of apatite from the Altay Koktokay No. 3 pegmatite, Xinjiang, China: Implications for pegmatite petrogenesis. Mineral. Petrol. 2013, 107, 985–1005. [Google Scholar] [CrossRef]
- Mao, M.; Rukhlov, A.S.; Rowins, S.M.; Spence, J.; Coogan, L.A. Apatite Trace Element Compositions: A Robust New Tool for Mineral Exploration. Econ. Geol. 2016, 111, 1187–1222. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Scharrer, M.; Ladenburger, S.; Markl, G. Comment on “Apatite: A new redox proxy for silicic magmas?” [Geochimica et Cosmochimica Acta 2014, 132, 101–119]. Geochim. Cosmochim. Acta 2016, 183, 267–270. [Google Scholar] [CrossRef]
- Xie, F.; Tang, J.; Chen, Y.; Lang, X. Apatite and zircon geochemistry of Jurassic porphyries in the Xiongcun district, southern Gangdese porphyry copper belt: Implications for petrogenesis and mineralization. Ore Geol. Rev. 2018, 96, 98–114. [Google Scholar] [CrossRef]
- Stokes, T.N.; Bromiley, G.D.; Potts, N.J.; Saunders, K.E.; Miles, A.J. The effect of melt composition and oxygen fugacity on manganese partitioning between apatite and silicate melt. Chem. Geol. 2019, 506, 162–174. [Google Scholar] [CrossRef]
- Sun, S.-J.; Zhang, R.-Q.; Cong, Y.-N.; Zhang, L.-P.; Sun, W.-D.; Li, C.-Y.; Ding, X. Analogous diagenetic conditions of dark enclave and its host granite derived by magma mixing: Evidence for a post-mixing magmatic process. Lithos 2020, 356-357, 105373. [Google Scholar] [CrossRef]
- Belousova, E.A.; Walters, S.; Griffin, W.L.; O’Reilly, S.Y. Trace-element signatures of apatites in granitoids from the Mt Isa Inlier, northwestern Queensland. Aust. J. Earth Sci. 2001, 48, 603–619. [Google Scholar] [CrossRef]
- Decrée, S.; Boulvais, P.; Tack, L.; André, L.; Baele, J.M. Fluorapatite in carbonatite-related phosphate deposits: The case of the Matongo carbonatite (Burundi). Mineral. Deposita 2016, 51, 453–466. [Google Scholar] [CrossRef]
- Duan, D.-F.; Jiang, S.-Y. Using apatite to discriminate synchronous ore-associated and barren granitoid rocks: A case study from the Edong metallogenic district, South China. Lithos 2018, 310–311, 369–380. [Google Scholar] [CrossRef]
- Imai, A. Variation of Cl and SO3 Contents of Microphenocrystic Apatite in Intermediate to Silicic Igneous Rocks of Cenozoic Japanese Island Arcs: Implications for Porphyry Cu Metallogenesis in the Western Pacific Island Arcs. Resour. Geol. 2008, 54, 357–372. [Google Scholar] [CrossRef]
- Wang, H.; Cai, K.; Sun, M.; Xia, X.-P.; Lai, C.-K.; Li, P.; Wan, B.; Zhang, Z. Apatite as a magma redox indicator and its application in metallogenic research. Lithos 2022, 422–423, 106749. [Google Scholar] [CrossRef]
- Villaseca, C. On the origin of granite types in the Central Iberian Zone: Contribution from integrated U-Pb and Hf isotope studies of zircon. In Proceedings of the VIII Congresso Ibérico de Geoquímica, Castelo Branco, Portugal, 24–28 September 2011; pp. 29–34. [Google Scholar]
- Roda-Robles, E.; Villaseca, C.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I. Petrogenetic relationships between Variscan granitoids and Li-(F-P)-rich aplite-pegmatites in the Central Iberian Zone: Geological and geochemical constraints and implications for other regions from the European Variscides. Ore Geol. Rev. 2018, 95, 408–430. [Google Scholar] [CrossRef]
- Martín-Izard, A.; Reguilón, R.; Palero, F. Las mineralizaciones litiníferas del oeste de Salamanca y Zamora. Estud. Geológicos 1992, 48, 19–30. [Google Scholar] [CrossRef]
- Tornos, F.; Delgado, A.; Casquet, C.; Galindo, C. 300 Million years of episodic hydrothermal activity: Stable isotope evidence from hydrothermal rocks of the Eastern Iberian Central System. Mineral. Depos. 2000, 35, 551–569. [Google Scholar] [CrossRef]
- Neiva, A.M.R. Portuguese granites associated with Sn-W and Au mineralizations. Bull. Geol. Soc. Finl. 2002, 74, 79–101. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I.; Martins, T.; Torres-Ruiz, J. Geology and mineralogy of Li mineralization in the Central Iberian Zone (Spain and Portugal). Mineral. Mag. 2016, 80, 103–126. [Google Scholar] [CrossRef]
- Llorens, T.; Moro, M.C. Fe-Mn phosphate associations as indicators of the magmatic-hydrothermal and supergene evolution of the Jálama batholith in the Navasfrías Sn-W District, Salamanca, Spain. Mineral. Mag. 2012, 76, 1–24. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A.; Errandonea-Martin, J. The Tres Arroyos Granitic Aplite-Pegmatite Field (Central Iberian Zone, Spain): Petrogenetic Constraints from Evolution of Nb-Ta-Sn Oxides, Whole-Rock Geochemistry and U-Pb Geochronology. Minerals 2020, 10, 1008. [Google Scholar] [CrossRef]
- Breiter, K.; Ackerman, L.; Svojtka, M.; Müller, A. Behavior of trace elements in quartz from plutons of different geochemical signature: A case study from the Bohemian Massif, Czech Republic. Lithos 2013, 175, 54–67. [Google Scholar] [CrossRef]
- Černý, P.; Burt, D.M. Paragenesis, crystallochemical characteristics, and geochemical evolution of micas in granitic pegmatites. In Micas; Bailey, S.W., Ed.; Reviews in Mineralogy; Mineralogical Society of America: Chantilly, Virginia, 1984; Volume 13, pp. 257–298. [Google Scholar]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. Mica and feldspar as indicators of the evolution of a highly evolved granite-pegmatite system in the Tres Arroyos area (Central Iberian Zone, Spain). J. Iberian Geol. 2018, 44, 375–403. [Google Scholar] [CrossRef]
- Marchal, K.L.; Simmons, W.B.; Falster, A.U.; Webber, K.L.; Roda-Robles, E. Geochemistry, mineralogy, and evolution of Li-Al micas and feldspars from the Mount Mica pegmatite, Maine, USA. Can. Mineral. 2014, 52, 221–233. [Google Scholar] [CrossRef]
- Oyarzabal, J.; Galliski, M.A.; Perino, E. Geochemistry of K-feldspar and Muscovite in Rare-element Pegmatites and Granites from the Totoral Pegmatite Field, San Luis, Argentina. Resour. Geol. 2009, 59, 315–329. [Google Scholar] [CrossRef]
- Roda, E.; Keller, P.; Pesquera, A.; Fontan, F. Micas of the muscovite–lepidolite series from Karibib pegmatites, Namibia. Mineral. Mag. 2007, 71, 41–62. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Vieira, R.; Pesquera, A.; Lima, A. Chemical variations and significance of phosphates from the Fregeneda-Almendra pegmatite field, Central Iberian Zone (Spain and Portugal). Mineral. Petrol. 2010, 100, 23–34. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, J. Occurrence, paragenesis and compositional evolution of tourmaline from the Tormes Dome Area, Central Iberian Zone, Spain. Can. Mineral. 2011, 49, 207–224. [Google Scholar] [CrossRef]
- Fransolet, A.M.; Keller, P.; Fontan, F. The phoshate mineral associations of the Tsaobismund pegmatite, Namibia. Contrib. Mineral. Petrol. 1986, 92, 502–517. [Google Scholar] [CrossRef]
- Ginsburg, A.I. Specific geochemical features of the pegmatitic process. In Proceedings of the 21st International Geological Congress Session Norden Report, Copenhagen, Denmark, 1960; Volume 17. [Google Scholar]
- Keller, P.; Fontan, F.; Fransolet, A.-M. Intercrystalline cation partitioning between minerals of the triplite-zwieselite-magniotriplite and the triphylite-lithiophilite series in granitic pegmatites. Contrib. Mineral. Petrol. 1994, 118, 239–248. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; García de Madinabeitia, S.; Gil-Ibarguchi, J.I.; Nizamoff, J.; Simmons, W.; Falster, A.; Galliski, M.A. On the geochemical character of primary Fe-Mn phosphates belonging to the triphylite-lithiophilite, graftonite-beusite, and triplite-zwieselite series: First results and implications for pegmatite petrogenesis. Can. Mineral. 2014, 52, 321–335. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.; Torres-Ruiz, J. From granite to highly evolved pegmatite: A case study of the Pinilla de Fermoselle granite–pegmatite system (Zamora, Spain). Lithos 2012, 153, 192–207. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, J. The Puentemocha Beryl-Phosphate Granitic Pegmatite, Salamanca, Spain: Internal Structure, Petrography and Mineralogy. Can. Mineral. 2012, 50, 1573–1587. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Simmons, W.; Gil-Crespo, P.P.; Webber, K.; Nizamoff, J.; Falster, A. Paragenetic relationships, geochemistry and petrogenetic significance of primary Fe Mn phosphates from pegmatites: The case study of Cañada (Salamanca, Spain) and Palermo (New Hampshire, USA) pegmatites. Lithos 2020, 374–375, 105710. [Google Scholar] [CrossRef]
- Martínez Catalán, J.R.; Schulmann, K.; Ghienne, J.-F. The Mid-Variscan Allochthon: Keys from correlation, partial retrodeformation and plate-tectonic reconstruction to unlock the geometry of a non-cylindrical belt. Earth Sci. Rev. 2021, 220, 103700. [Google Scholar] [CrossRef]
- Ferreira, N.; Iglesias, M.; Noronha, F.; Pereira, E.; Ribeiro, A.; Ribeiro, M.L. Granitoides da zona Centro-Iberica e seu enquadramento geodinámico. In Geología de los Granitoides y Rocas Asociadas del Macizo Hespérico. Libro homenaje a L. C. García de Figuerola; Bea, F., Carnicero, A., Gonzalo, J.C., López Plaza, M., Rodríguez Alonso, M.D., Eds.; Rueda: Madrid, Spain, 1987; pp. 37–53. [Google Scholar]
- López-Plaza, M.; Martínez-Catalán, J.R. Síntesis estructural de los granitoides del Macizo Hespérico. In Geología de los Granitoides y Rocas Asociadas del Macizo Hespérico; Bea, F., Carnicero, A., Gonzalo, J.C., López-Plaza, M., Rodríguez Alonso, M.D., Eds.; Rueda: Madrid, Spain, 1987; pp. 125–210. [Google Scholar]
- Bea, F.; Montero, P.; Zinger, T. The nature, origin, and thermal influence of the granite source layer of Central Iberia. J. Geol. 2003, 111, 579–595. [Google Scholar] [CrossRef]
- Dias, G.; Leterrier, J.; Mendes, A.; Simões, P.P.; Bertrand, J.M. U-Pb zircon and monazite geochronology of post-collisional Hercynian granitoids from the Central Iberian Zone (Northern Portugal). Lithos 1998, 45, 349–369. [Google Scholar] [CrossRef]
- Fernández-Suárez, J.; Dunning, G.R.; Jenner, G.A.; Gutierrez-Alonso, G. Variscan collisional magmatism and deformation in NW Iberia: Constraints from U-Pb geochronology of granitoids. J. Geol. Soc. 2000, 157, 565–576. [Google Scholar] [CrossRef]
- Gutiérrez-Alonso, G.; Fernández-Suárez, J.; Jeffries, T.E.; Johnston, S.T.; Pastor-Galán, D.; Murphy, J.B.; Franco, M.P.; Gonzalo, J.C. Diachronous post-orogenic magmatism within a developing orocline in Iberia, European Variscides. Tectonics 2011, 30, 17. [Google Scholar] [CrossRef]
- Bea, F.; Montero, P.; Molina, J.F. Mafic precursors, peraluminous granitoids, and late lamprophyres in the Avila batholith: A model for the generation of Variscan batholiths in Iberia. J. Geol. 1999, 107, 399–419. [Google Scholar] [CrossRef]
- Dias, G.; Simões, P.P.; Ferreira, N.; Leterrier, J. Mantle and Crustal Sources in the Genesis of Late-Hercynian Granitoids (NW Portugal): Geochemical and Sr-Nd Isotopic Constraints. Gondwana Res. 2002, 5, 287–305. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Antunes, I.M.H.R.; Neiva, A.M.R.; Farinha Ramos, J.M.; Silva, P.B.; Silva, M.M.V.G.; Corfu, F. Petrogenetic links between lepidolite-subtype aplite-pegmatite, aplite veins and associated granites at Segura (central Portugal). Chem. Erde Geochem. 2013, 73, 323–341. [Google Scholar] [CrossRef]
- Gallego Garrido, M. Las Mineralizaciones de Li Asociadas a Magmatismo Acido en Extremadura y su Encuadre en la Zona Centro-Ibérica. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1992. [Google Scholar]
- Garate-Olave, I.; Müller, A.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. Extreme fractionation in a granite–pegmatite system documented by quartz chemistry: The case study of Tres Arroyos (Central Iberian Zone, Spain). Lithos 2017, 286–287, 162–164. [Google Scholar] [CrossRef]
- Martins, T.; Roda-Robles, E.; Lima, A.; De Parseval, P. Geochemistry and evolution of micas in the Barroso-Alvão pegmatite field, Northern Portugal. Can. Mineral. 2012, 50, 1117–1129. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera Pérez, A.; Velasco Roldan, F.; Fontan, F. The granitic pegmatites of the Fregeneda area (Salamanca, Spain): Characteristics and petrogenesis. Mineral. Mag. 1999, 63, 535–558. [Google Scholar] [CrossRef]
- Vieira, R.; Roda-Robles, E.; Pesquera, A.; Lima, A. Chemical variation and significance of micas from the Fregeneda-Almendra pegmatitic field (Central-Iberian Zone, Spain and Portugal). Am. Mineral. 2011, 96, 637–645. [Google Scholar] [CrossRef]
- Lima, A. Estrutura, Mineralogia e Génese dos Filões Aplitopegmatíticos com Espodumena da Região do Barroso-Alvão (Norte de Portugal). Ph.D. Thesis, Univ. Porto, Porto, Portugal, Institut National Polytechnique de Lorraine, Nancy, France, 2000; 270p. [Google Scholar]
- Neiva, A.M.R.; Ramos, J.M.F. Geochemistry of granitic aplite-pegmatite sills and petrogenetic links with granites, Guarda-Belmonte area, central Portugal. Eur. J. Mineral. 2010, 22, 837–854. [Google Scholar] [CrossRef]
- Pesquera, A.; Roda-Robles, E.; Gil-Crespo, P.P.; Valls, D.; Ruiz, J.T. The metasomatic enrichment of Li in psammopelitic units at San José-Valdeflórez, Central Iberian Zone, Spain: A new type of lithium deposit. Sci. Rep. 2020, 10, 10828. [Google Scholar] [CrossRef]
- Ferrerira, N.; Vieira, G. Guia Geológico e Geomorfológico do Parque Natural da Serra da Estrela—Locais de Interesse Geológico e Geomorfológico; Instituto da Conservação da Naturaleza—Instituto Geológico e Mineiro: Alfragide, Portugal, 1999; 111p. [Google Scholar]
- Garate-Olave, I.; Roda Robles, E.; Gil-crespo, P.P.; Müller, A.; Pesquera, A. El Sistema Granito-Pegmatita de Tres Arroyos (Alburquerque, Badajoz): Petrografía, Mineralogía y Modelo Petrogenético. Macla 2016, 21, 41–43. [Google Scholar]
- Capdevila, R.; Corretgé, L.G.; Floor, P. Les granitoides Varisques de la Meseta Ibérique. Bull. Soc. Géol. Fr. 1973, S7-XV, 209–228. [Google Scholar] [CrossRef]
- Gomes, M.E.P.; Teixeira, R.J.S.; Neiva, A.M.R.; Corfu, F. Geochemistry and geochronology of granitoids from Bemposta-Picote region, Northeastern Portugal. Comun. Geol. 2014, 101, 115–118. [Google Scholar]
- López-Moro, F.J.; López-Plaza, M.; Romer, R.L. Generation and emplacement of shear-related highly mobile crustal melts: The synkinematic leucogranites from the Variscan Tormes Dome, Western Spain. Int. J. Earth Sci. 2012, 101, 1273–1298. [Google Scholar] [CrossRef]
- Teixeira, R.J.S.; Neiva, A.M.R.; Gomes, M.E.P.; Corfu, F.; Cuesta, A.; Croudace, I.W. The role of fractional crystallization in the genesis of early syn-D3, tin-mineralized Variscan two-mica granites from the Carrazeda de Ansiães area, northern Portugal. Lithos 2012, 153, 177–191. [Google Scholar] [CrossRef]
- Cuesta, A.; Gallastegui, G. Galicia Occidental. In Geología de España; Vera, J.A., Ed.; SGE-IGME: Madrid, Spain, 2004; pp. 96–100. [Google Scholar]
- Ortega, L. Estudio petrogenético del granito sincinemático de dos micas de A Espenuca (A Coruña). Lab. Xeolóxico Laxe Ser. Nova Terra 1998, 14, 377. [Google Scholar]
- Almeida, A.; Leterrier, J.; Noronha, F.; Bertrand, J.M. U-Pb zircon and monazite geochronology of the hercynian two-mica granite composite pluton of Cabeceiras de Basto (Northern Portugal). Comptes Rendus L’académie Sci. Ser. IIA—Earth Planet. Sci. 1998, 326, 779–785. [Google Scholar] [CrossRef]
- Antunes, I.M.H.R.; Neiva, A.M.R.; Silva, M.M.V.G.; Corfu, F. Geochemistry of S-type granitic rocks from the reversely zoned Castelo Branco pluton (central Portugal). Lithos 2008, 103, 445–465. [Google Scholar] [CrossRef]
- Chicharro, E.; Villaseca, C.; Valverde-Vaquero, P.; Belousova, E.; López-Garcia, J.A. Zircon U-Pb and Hf isotopic constraints on the genesis of a post-kinematic S-type Variscan tin granite: The Logrosan cupola (Central Iberian Zone). J. Iberian Geol. 2014, 40, 451–470. [Google Scholar] [CrossRef]
- Merino Martínez, E.; Villaseca, C.; Orejana, D.; Pérez-Soba, C.; Belousova, E.; Andersen, T. Tracing magma sources of three different S-type peraluminous granitoid series by in situ U–Pb geochronology and Hf isotope zircon composition: The Variscan Montes de Toledo batholith (central Spain). Lithos 2014, 200–201, 273–298. [Google Scholar] [CrossRef]
- Ramírez, J.A.; Menéndez, L. A geochemical study of two peraluminous granites from south-central Iberia: The Nisa-Albuquerque and Jalama batholiths. Mineral. Mag. 1999, 63, 85–104. [Google Scholar] [CrossRef]
- Errandonea-Martin, J.; Sarrionandia, F.; Janousek, V.; Carracedo-Sánchez, M.; Gil-Ibarguchi, J.I. Origin of cordierite-bearing monzogranites from the southern Central Iberian Zone—Inferences from the zoned Sierra Bermeja Pluton (Extremadura, Spain). Lithos 2019, 342–343, 440–462. [Google Scholar] [CrossRef]
- Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, J.; Roda-Robles, E. Insights into petrogenesis of the Jálama pluton (Central Iberian Zone, western Spain). Int. Geol. Rev. 2018, 60, 157–187. [Google Scholar] [CrossRef]
- González-Menéndez, L.; Azor, A.; Rubio-Ordóñez, Á.; Sánchez-Almazo, I. The metamorphic aureole of the Nisa-Alburquerque batholith (SW Iberia): Implications for deep structure and emplacement mode. Int. J. Earth Sci. 2011, 100, 1533–1550. [Google Scholar] [CrossRef]
- Merino, E.; Villaseca, C.; Orejana, D.; Jeffries, T. Gahnite, chrysoberyl and beryl co-occurrence as accessory minerals in a highly evolved peraluminous pluton: The Belvís de Monroy leucogranite (Cáceres, Spain). Lithos 2013, 179, 137–156. [Google Scholar] [CrossRef]
- Merino Martínez, E. Geochemistry, U–Pb Geochronology and Hf-Isotope Zircon Composition of Variscan Granitoids from the Montes de Toledo Batholith. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Vindel, E.; Chicharro, E.; Villaseca, C.; López-García, J.Á.; Sánchez, V. Hydrothermal phosphate vein-type ores from the southern Central Iberian Zone, Spain: Evidence for their relationship to granites and Neoproterozoic metasedimentary rocks. Ore Geol. Rev. 2014, 62, 143–155. [Google Scholar] [CrossRef]
- Neiva, A.M.R.; Silva, P.B.; Ramos, J.M.F. Geochemistry of granitic aplite-pegmatite veins and sills and their minerals from Cabeço dos Poupos, Sabugal, Central Portugal. Asoc. Geológica Argent. Ser. D Publicación Espec. 2011, 143, 141–143. [Google Scholar] [CrossRef]
- Martínez-Catalán, J.R.; Martínez Poyatos, D.; Bea, F. Zona Centroibérica: Introducción. In Geología de España; Vera, J.A., Ed.; SGE-IGME: Madrid, Spain, 2004; pp. 68–69. [Google Scholar]
- Garate-Olave, I. Petrography, Mineralogy and Origin of the Rare Elements Granitic Aplopegmatites from Tres Arroyos (Badajoz, Spain). Ph.D. Thesis, Universidad del País Vasco (UPV/EHU), Leioa, Spain, 2018. [Google Scholar]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. The phosphate mineral associations from the Tres Arroyos aplite-pegmatites (Badajoz, Spain): Petrography, mineral chemistry and petrogenetic implications. Can. Mineral. 2020, 58, 747–765. [Google Scholar] [CrossRef]
- Martins, T. Multidisciplinary Study of Pegmatites and Associated Li and SN-Nb-Ta Mineralisation from the Barroso-Alvão Region. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2009. [Google Scholar]
- Llorens, T. Las Mineralizaciones Magmático-Hidrotermales de Sn-W(Nb-Ta) del Distrito de Navasfrías (SO de Salamanca). Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2011. [Google Scholar]
- Roda, E.; Pesquera, A.; Fontan, F.; Keller, P. Phosphate mineral associations in the Cañada pegmatite (Salamanca, Spain): Paragenetic relationships, chemical compositions, and implications for pegmatite evolution. Am. Mineral. 2004, 89, 110–125. [Google Scholar] [CrossRef]
- London, D.; Wolf, M.B.; Morgan, G.B.; Gallego-Garrido, M. Experimental Silicate–Phosphate Equilibria in Peraluminous Granitic Magmas, with a Case Study of the Alburquerque Batholith at Tres Arroyos, Badajoz, Spain. J. Petrol. 1999, 40, 215–240. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Garate-Olave, I.; Torres-Ruiz, J. The Li-Rich Aplopegmatite from Castillejo de Dos Casas (Salamanca, Spain): Example of a Highly Fractionated Granite-Pegmatite System. In Proceedings of the 13th Biennial SGA Meeting, Nancy, France, 24–27 August 2015; Volume 2, pp. 835–838. [Google Scholar]
- Fuertes-Fuente, M. Las Pegmatitas del Area de Lalín-Forcarey (Galicia) y las Mineralizaciones de Elementos Escasos Asociadas. Ph.D. Thesis, Universidad de Oviedo, Oviedo, Spain, 1996. [Google Scholar]
- Carvalho, J.M.F.; Farinha, J.A.L.B. Lithium potentialities in Northern Portugal. In Proceedings of the 17th Industrial Minerals International Congress, Barcelona, Spain, 28–31 March 2004; pp. 1–10. [Google Scholar]
- Cardoso-Fernandes, J.; Santos, D.; Lima, A.; Teodoro, A.C.; Perrotta, E.; Roda-Robles, E. Validation of Remote Sensing Techniques in Greenfield Exploration Areas for Lithium (LI) in Central Portugal: A Study Case. In Proceedings of the 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS, Brussels, Belgium, 11–16 July 2021; pp. 6622–6625. [Google Scholar]
- Garate-Olave, I.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera-Pérez, A.; Vieira, R.; Lima, A. Estudio textural y Mineralógico del Dique de Cuarzo con Fosfatos de Folgosinho (Guarda, Portugal). Macla 2012, 16, 220–221. [Google Scholar]
- Pouchou, J.L.; Pichoir, F. “PAP” φ(ρZ) procedure for improved quantitative microanalysis. In Microbean Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Pérez-Soba, C.; Villaseca, C.; Fernández, A. Magmatic graphite inclusions in Mn-Fe-rich fluorapatite of perphosphorus granites (the Belvís pluton, Variscan Iberian Belt). Am. Mineral. 2017, 102, 728–742. [Google Scholar] [CrossRef]
- Errandonea-Martin, J. Petrology of Cordierite-Bearing Monzogranites and Related Mesocratic Rocks from the Sierra Bermeja Pluton (Southern Iberian Massif). Ph.D. Thesis, Universidad del País Vasco (UPV/EHU), Leioa, Spain, 2019. [Google Scholar]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Paul, B.; Paton, C.; Norris, A.; Woodhead, J.; Hellstrom, J.; Hergt, J.; Greig, A. CellSpace: A module for creating spatially registered laser ablation images within the Iolite freeware environment. J. Anal. At. Spectrom. 2012, 27, 700–706. [Google Scholar] [CrossRef]
- McDonough, W.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 67, 1050–1056. [Google Scholar] [CrossRef]
- Irber, W. The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu∗, Sr/Eu, Y/Ho, and Zr/Hf of evolving peraluminous granite suites. Geochim. Cosmochim. Acta 1999, 63, 489–508. [Google Scholar] [CrossRef]
- Pieczka, A. Beusite and an unusual Mn-rich apatite from the Szklary granitic pegmatite, Lower Silesia, Southwestern Poland. Can. Mineral. 2007, 45, 901–914. [Google Scholar] [CrossRef]
- Miles, A.J.; Graham, C.M.; Hawkesworth, C.J.; Gillespie, M.R.; Hinton, R.W.; Bromiley, G.D. Apatite: A new redox proxy for silicic magmas? Geochim. Cosmochim. Acta 2014, 132, 101–119. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Chew, D.; Kenny, G.; Henrichs, I.; Mulligan, D. The trace element composition of apatite and its application to detrital provenance studies. Earth Sci. Rev. 2020, 201, 103044. [Google Scholar] [CrossRef]
- Stokes, T.N. The Crystal Chemistry of Accessory Minerals as a Probe of Magmatic Oxygen Fugacity: An Experimental Study; University of Edinburgh: Edinburgh, Scotland, 2018. [Google Scholar]
- Villaseca, C.; Ruiz-Martinez, C.C.; Péraz-Soba, C. Magnetic susceptibility of Variscan granite-types of the Spanish Central System and the redox state of magma. Geol. Acta 2017, 15, 379–394. [Google Scholar] [CrossRef]
- Pichavant, M.; Villaros, A.; Deveaud, S.; Scaillet, B.; Lahlafi, M. The Influence of Redox State on Mica Crystallization in Leucogranitic and Pegmatitic Liquids. Can. Mineral. 2016, 54, 559–581. [Google Scholar] [CrossRef]
- Puziewicz, J.; Johannes, W. Experimental study of a biotite bearing granitic system under water saturated and water undersaturated conditions. Contrib. Mineral. Petrol. 1990, 104, 397–406. [Google Scholar] [CrossRef]
- Wones, D.R.; Eugster, H.P. Stability of biotite: Experiment, Theory, and Application. Am. Mineral. 1965, 50, 1228–1272. [Google Scholar]
- Harrison, T.M.; Watson, E.B. The behavior of apatite during crustal anatexis: Equilibrium and kinetic considerations. Geochim. Cosmochim. Acta 1984, 48, 1467–1477. [Google Scholar] [CrossRef]
- Mysen, B.; Richet, P. Silicate Glasses and Melts; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Keppler, H. Partitioning of phosphorus between melt and fluid in the system haplogranite-H2O-P2O5. Chem. Geol. 1994, 117, 345–353. [Google Scholar] [CrossRef]
- London, D. Internal differentiation of rare-element pegmatites: Effects of boron, phosphorus, and fluorine. Geochim. Cosmochim. Acta 1987, 51, 403–420. [Google Scholar] [CrossRef]
- Roda, E.; Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, J.; Fontan, F. Origin and internal evolution of the Li-F-Be-B-P-bearing Pinilla de Fermoselle pegmatite (Central Iberian Zone, Zamora, Spain). Am. Mineral. 2005, 90, 1887–1899. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Fontan, F.; Pesquera, A.; Keller, P. The Fe-Mn phosphate associations from the Pinilla de Fermoselle pegmatite, Zamora, Spain: Occurrence of kryzhanovskite and natrodufrenite. Eur. J. Mineral. 1998, 10, 155–167. [Google Scholar] [CrossRef]
- Rutherford, M.J.; Hess, P.C.; Daniel, G.H. Experimental liquid line of descent and liquid immiscibility for basalt 70017. In Proceedings of the Lunar and Planetary Science Conference Proceedings, Houston, TX, USA, 18–22 March 1974; Volume 1, pp. 569–583. [Google Scholar]
- James, P.F.; Mcmillan, P.W. Quantitative measurements of phase separation in glasses using transmission electron microscopy. Part 2. A study of lithia-silica glasses and the influence of phosphorus pentoxide. Phys. Chem. Glasses 1970, 11, 64–70. [Google Scholar]
- Prowatke, S.; Klemme, S. Trace element partitioning between apatite and silicate melts. Geochim. Cosmochim. Acta 2006, 70, 4513–4527. [Google Scholar] [CrossRef]
- Hughes, J.M.; Rakovan, J. Structurally Robust, Chemically Diverse: Apatite and Apatite Supergroup Minerals. Elements 2015, 11, 165–170. [Google Scholar] [CrossRef]
- Schettler, G.; Gottschalk, M.; Harlov, D.E. A new semi-micro wet chemical method for apatite analysis and its application to the crystal chemistry of fluorapatite-chlorapatite solid solutions. Am. Mineral. 2011, 96, 138–152. [Google Scholar] [CrossRef]
- Zhu, C.; Sverjensky, D.A. Partitioning of F-Cl-OH between minerals and hydrothermal fluids. Geochim. Cosmochim. Acta 1991, 55, 1837–1858. [Google Scholar] [CrossRef]
- Ding, T.; Ma, D.; Lu, J.; Zhang, R. Apatite in granitoids related to polymetallic mineral deposits in southeastern Hunan Province, Shi–Hang zone, China: Implications for petrogenesis and metallogenesis. Ore Geol. Rev. 2015, 69, 104–117. [Google Scholar] [CrossRef]
- Webster, J.D.; Piccoli, P.M. Magmatic Apatite: A Powerful, Yet Deceptive, Mineral. Elements 2015, 11, 177–182. [Google Scholar] [CrossRef]
- Burnham, A.D.; Berry, A.J. The effect of oxygen fugacity, melt composition, temperature and pressure on the oxidation state of cerium in silicate melts. Chem. Geol. 2014, 366, 52–60. [Google Scholar] [CrossRef]
- Burnham, A.D.; Berry, A.J.; Halse, H.R.; Schofield, P.F.; Cibin, G.; Mosselmans, J.F.W. The oxidation state of europium in silicate melts as a function of oxygen fugacity, composition and temperature. Chem. Geol. 2015, 411, 248–259. [Google Scholar] [CrossRef]
- Sha, L.K.; Chappell, B.W. Apatite chemical composition, determined by electron microprobe and laser-ablation inductively coupled plasma mass spectrometry, as a probe into granite petrogenesis. Geochim. Cosmochim. Acta 1999, 63, 3861–3881. [Google Scholar] [CrossRef]
- Duan, X.-X.; Chen, B.; Sun, K.-K.; Wang, Z.-Q.; Yan, X.; Zhang, Z. Accessory mineral chemistry as a monitor of petrogenetic and metallogenetic processes: A comparative study of zircon and apatite from Wushan Cu- and Zhuxiling W(Mo)-mineralization-related granitoids. Ore Geol. Rev. 2019, 111, 102940. [Google Scholar] [CrossRef]
- Pan, L.-C.; Hu, R.-Z.; Wang, X.-S.; Bi, X.-W.; Zhu, J.-J.; Li, C. Apatite trace element and halogen compositions as petrogenetic-metallogenic indicators: Examples from four granite plutons in the Sanjiang region, SW China. Lithos 2016, 254–255, 118–130. [Google Scholar] [CrossRef]
- Kontak, D.J.; Martin, R.F. Alkali feldspar in the peraluminous South Mountain Batholith, Nova Scotia: Trace-element data. Can. Mineral. 1997, 35, 959–977. [Google Scholar]
- Abdullin, F.; Solé, J.; Solari, L.; Shchepetilnikova, V.; Meneses-Rocha, J.J.; Pavlinova, N.; Rodríguez-Trejo, A. Single-grain apatite geochemistry of Permian–Triassic granitoids and Mesozoic and Eocene sandstones from Chiapas, southeast Mexico: Implications for sediment provenance. Int. Geol. Rev. 2016, 58, 1132–1157. [Google Scholar] [CrossRef]
- Ballard, J.R.; Palin, M.J.; Campbell, I.H. Relative oxidation states of magmas inferred from Ce(IV)/Ce(III) in zircon: Application to porphyry copper deposits of northern Chile. Contrib. Mineral. Petrol. 2002, 144, 347–364. [Google Scholar] [CrossRef]
- Drake, M.J.; Weill, D.F. Partition of Sr, Ba, Ca, Y, Eu2+, Eu3+, and other REE between plagioclase feldspar and magmatic liquid: An experimental study. Geochim. Cosmochim. Acta 1975, 39, 689–712. [Google Scholar] [CrossRef]
- Abramov, S.S. Modeling of REE fractionation in the acid melt-fluoride-chloride fluid system. Dokl. Earth Sci. 2001, 377, 198–200. [Google Scholar]
- Muecke, G.K.; Clarke, D.B. Geochemical evolution of the South Mountain Batholith, Nova Scotia; rare-earth-element evidence. Can. Mineral. 1981, 19, 133–145. [Google Scholar]
- Candela, P.A. Theoretical constraints on the chemistry of the magmatic aqueous phase. In Ore-Bearing Granite Systems, Petrogenesis and Mineralizaing Processes; Stein, H.J., Hannah, J.L., Eds.; Geological Society of America: Boulder, CO, USA, 1990; Volume 246, pp. 11–19. [Google Scholar]
- Yu, M.; Xia, Q.-X.; Zheng, Y.-F.; Zhao, Z.-F.; Chen, Y.-X.; Chen, R.-X.; Luo, X.; Li, W.-C.; Xu, H. The composition of garnet in granite and pegmatite from the Gangdese orogen in southeastern Tibet: Constraints on pegmatite petrogenesis. Am. Mineral. 2021, 106, 265–281. [Google Scholar] [CrossRef]
- Watson, E.B.; Green, T.H. Apatite/liquid partition coefficients for the rare earth elements and strontium. Earth Planet. Sci. Lett. 1981, 56, 405–421. [Google Scholar] [CrossRef]
- RØnsbo, J.G. Coupled substitutions involving REEs and Na and Si in apatites in alkaline rocks from the Ilimaussaq Intrusion, South Greenland, and the petrological implications. Am. Mineral. 1989, 74, 896–901. [Google Scholar]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Dolejs, D.; Stemprok, M. Magmatic and hydrothermal evolution of Li-F granites: Cinovec and Krasno intrusions, Krusne hory batholith, Czech Republic. Bull. Geosci. 2001, 76, 77–99. [Google Scholar]
- Kawabe, I. Tetrad effects and fine structures of REE abundance patterns of granitic and rhyolitic rocks: ICP-AES determinations of REE and Y in eight GSJ reference rocks. Geochem. J. 1995, 29, 213–230. [Google Scholar] [CrossRef]
- Monecke, T.; Kempe, U.; Monecke, J.; Sala, M.; Wolf, D. Tetrad effect in rare earth element distribution patterns: A method of quantification with application to rock and mineral samples from granite-related rare metal deposits. Geochim. Cosmochim. Acta 2002, 66, 1185–1196. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiong, L.; Hen, X.D.; Wang, Y.X.; Wang, Q.; Bao, Z.; Jahn, B. Controls on the REE tetrad effect in granites: Evidence from the Qianlishan and Baerzhe granites, China. Geochem. J. 2002, 36, 527–543. [Google Scholar]
- Cardoso-Fernandes, J.; Lima, A.; Roda-Robles, E.; Ribeiro, M.D.A.; Teodoro, A.C. Vectoring Lithium (Li) Mineralizations: A First Approach to Pegmatite Geochemical Halo Definition in the Fregeneda-Almendra Area. In Proceedings of the Goldschmidt, Lyon, France, 4–9 July 2021. [Google Scholar]
- Anders, E.; Grevesse, N. Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Savina, E.A. Tetrad effects in the rare earth element patterns of granitoid rocks as an indicator of fluoride-silicate liquid immiscibility in magmatic systems. Petrology 2010, 18, 514–543. [Google Scholar] [CrossRef]
- Bea, F. Residence of REE, Y, Th and U in granites and crustal protoliths; Implications for the chemistry of crustal melts. J. Petrol. 1996, 37, 521–552. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Z.; Di, H.; Zhang, Z.; Mao, C.; Tan, H.; Huang, J.; Zhou, F.; Zhang, L.; Chen, J.; et al. Apatite U-Pb Dating and Composition Constraints for Magmatic–Hydrothermal Evolution in the Giant Renli Nb-Ta Deposit, South China. Minerals 2022, 12, 344. [Google Scholar] [CrossRef]
- Pan, Y.; Breaks, F.W. Rare-earth elements in fluorapatite, separation lake area, Ontario: Evidence for S-type granite—Rare-element pegmatite linkage. Can. Mineral. 1997, 35, 659–671. [Google Scholar]
- Boswell, J.T. Porphyry system fertility discrimination and mineralization vectoring using igneous apatite substitutions to derive pre-exsolution melt mineralization component concentrations. Master’s Thesis, The University of Utah, Salt Lake City, UT, USA, 2014. [Google Scholar]
- Imai, A. Metallogenesis of Porphyry Cu Deposits of the Western Luzon Arc, Philippines: K-Ar ages, SO3 Contents of Microphenocrystic Apatite and Significance of Intrusive Rocks. Resour. Geol. 2002, 52, 147–161. [Google Scholar] [CrossRef]



| Anal. nº | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lithology | Granite | Wall Zone | Intermediate Zone | Phosphate Nodule | Greissen Masses | ||||||||||
| SiO2 | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | b.d.l. | b.d.l. |
| Al2O3 | b.d.l. | 0.02 | 0.01 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | b.d.l. | b.d.l. | b.d.l. | 0.22 | 0.02 | b.d.l. |
| FeO(t) | 0.64 | 0.74 | 0.56 | 0.45 | 1.05 | 0.24 | 0.23 | 0.34 | 0.24 | 6.14 | 4.09 | 5.98 | 0.65 | 0.14 | 0.30 |
| MnO | 1.23 | 1.33 | 1.21 | 1.30 | 1.58 | 0.46 | 1.61 | 1.72 | 1.54 | 6.60 | 6.45 | 6.76 | 0.04 | 0.41 | 0.85 |
| MgO | 0.04 | 0.01 | 0.04 | 0.02 | 0.05 | b.d.l. | b.d.l. | b.d.l. | 0.01 | 0.04 | 0.00 | 0.06 | b.d.l. | b.d.l. | b.d.l. |
| CaO | 53.70 | 53.47 | 53.55 | 53.45 | 53.08 | 54.83 | 55.02 | 54.59 | 54.70 | 41.53 | 43.99 | 41.86 | 53.80 | 55.27 | 54.89 |
| Na2O | b.d.l. | b.d.l. | 0.05 | 0.06 | 0.09 | 0.04 | b.d.l. | 0.03 | 0.04 | 0.34 | 0.08 | 0.31 | 0.34 | b.d.l. | 0.01 |
| SrO | b.d.l. | 0.03 | 0.03 | 0.06 | b.d.l. | 0.04 | b.d.l. | 0.17 | 0.10 | b.d.l. | b.d.l. | b.d.l. | 0.02 | b.d.l. | 0.26 |
| P2O5 | 43.28 | 42.58 | 42.72 | 42.98 | 42.67 | 42.82 | 43.24 | 43.42 | 43.48 | 39.91 | 40.36 | 40.23 | 40.19 | 42.78 | 41.96 |
| H2O* | 0.33 | 0.34 | 0.41 | 0.29 | 0.35 | 0.33 | 0.19 | 0.31 | 0.40 | 0.57 | 0.64 | 0.63 | 0.23 | 0.30 | 0.13 |
| F | 3.10 | 3.04 | 2.90 | 3.17 | 3.03 | 3.09 | 3.43 | 3.19 | 3.00 | b.d.l. | b.d.l. | b.d.l. | 3.15 | 3.17 | 3.48 |
| Cl | 0.04 | 0.02 | 0.03 | 0.01 | 0.04 | b.d.l. | 0.01 | b.d.l. | 0.02 | 4.37 | 4.18 | 4.19 | 0.01 | b.d.l. | b.d.l. |
| O=F,Cl | 1.31 | 1.28 | 1.23 | 1.33 | 1.29 | 1.30 | 1.45 | 1.34 | 1.27 | 0.99 | 0.94 | 0.95 | 1.33 | 1.33 | 1.47 |
| Total | 99.36 | 99.18 | 99.69 | 99.35 | 98.65 | 98.75 | 99.74 | 99.30 | 100.09 | 100.76 | 100.70 | 101.14 | 98.74 | 98.55 | 99.18 |
| Structural formulae on the basis of 26 (O,F,Cl,OH) | |||||||||||||||
| Si | b.d.l. | b.d.l. | b.d.l. | 0.003 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.002 | b.d.l. | b.d.l. |
| Al | b.d.l. | 0.004 | 0.002 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.045 | 0.004 | b.d.l. |
| Fe2+(t) | 0.089 | 0.104 | 0.078 | 0.063 | 0.147 | 0.033 | 0.032 | 0.047 | 0.033 | 0.916 | 0.604 | 0.886 | 0.094 | 0.019 | 0.042 |
| Mn2+ | 0.173 | 0.189 | 0.171 | 0.184 | 0.224 | 0.065 | 0.224 | 0.239 | 0.214 | 0.998 | 0.965 | 1.014 | 0.006 | 0.058 | 0.121 |
| Mg | 0.010 | 0.002 | 0.010 | 0.005 | 0.012 | 0.000 | b.d.l. | b.d.l. | 0.002 | 0.011 | b.d.l. | 0.016 | b.d.l. | b.d.l. | b.d.l. |
| Ca | 9.540 | 9.598 | 9.599 | 9.552 | 9.506 | 9.789 | 9.694 | 9.603 | 9.622 | 7.941 | 8.326 | 7.946 | 10.005 | 9.852 | 9.884 |
| Na | b.d.l. | b.d.l. | 0.016 | 0.019 | 0.029 | 0.013 | b.d.l. | 0.010 | 0.013 | 0.118 | 0.027 | 0.106 | 0.114 | b.d.l. | 0.003 |
| Sr | b.d.l. | 0.003 | 0.003 | 0.006 | 0.000 | 0.004 | b.d.l. | 0.016 | 0.010 | b.d.l. | b.d.l. | b.d.l. | 0.002 | b.d.l. | 0.025 |
| P | 6.076 | 6.039 | 6.051 | 6.070 | 6.039 | 6.041 | 6.020 | 6.036 | 6.044 | 6.030 | 6.036 | 6.034 | 5.906 | 6.026 | 5.970 |
| F | 1.625 | 1.611 | 1.534 | 1.672 | 1.602 | 1.628 | 1.784 | 1.656 | 1.558 | b.d.l. | b.d.l. | b.d.l. | 1.729 | 1.668 | 1.850 |
| Cl | 0.011 | 0.006 | 0.009 | 0.003 | 0.011 | b.d.l. | 0.003 | b.d.l. | 0.006 | 1.322 | 1.252 | 1.258 | 0.003 | b.d.l. | b.d.l. |
| OH | 0.363 | 0.384 | 0.457 | 0.325 | 0.387 | 0.372 | 0.213 | 0.344 | 0.437 | 0.678 | 0.748 | 0.742 | 0.268 | 0.332 | 0.150 |
| Anal. nº | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs. | *** | ** | **** | ** | * | * | ** | ** | * | * | * | * | * | * |
| Locality | (13) | (7) | (3) | (3) | (14) | (14) | (3) | (3) | (15) | (15) | (22) | (22) | (15) | (14) |
| Lithology | S2 Granites | Leucogranites | B-Rich Leucogranites | Highly Fractionates Leucogr. | Barren Pegmatites | P-Rich Pegmatites | Li-Rich Pegmatites | |||||||
| Li | 11.5 | 11.3 | 33.0 | 31.0 | 65.1 | 20.7 | 1.0 | 1.0 | 6.6 | 43.5 | 2.0 | 11.0 | ||
| Be | 1.0 | 0.0 | 139.0 | 173.0 | 1.0 | 0.0 | 0.0 | 1.0 | ||||||
| Sc | 0.8 | 1.4 | 15.0 | 18.0 | 16.9 | 10.0 | 3.0 | 4.7 | 42.4 | 1.0 | 0.0 | |||
| V | 0.0 | 0.0 | 0.7 | 1.0 | 0.7 | 43.0 | 0.0 | 1.0 | ||||||
| Cr | 2.0 | 2.0 | 2.0 | 1.0 | 1.1 | 12.7 | 1.0 | 1.0 | ||||||
| Co | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 1.0 | 1.8 | 0.0 | 0.0 | |||||
| Ni | 2.0 | 2.0 | 2.0 | 2.0 | 1.5 | 8.3 | 2.0 | 2.0 | ||||||
| Cu | 1.0 | 0.0 | 0.0 | 0.3 | 11.9 | 0.0 | 0.0 | |||||||
| Zn | 1.0 | 8.0 | 13.0 | 65.8 | 135.0 | 2.0 | 2.0 | 68.0 | 95.0 | 1.0 | 1.0 | |||
| Rb | 5.9 | 1.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.7 | 38.0 | 0.0 | 9.0 | ||||
| Sr | 138.5 | 97.4 | 73.0 | 65.6 | 36.0 | 32.0 | 2250.0 | 1290.0 | 136.0 | 733.0 | 66.9 | 217.0 | 7610.0 | 4366.0 |
| Y | 2480.0 | 2520.0 | 2900.0 | 535.0 | 2817.0 | 1758.0 | 9.1 | 21.8 | 599.0 | 456.0 | 940.0 | 577.0 | 24.0 | 134.0 |
| Zr | 0.3 | 0.3 | 0.0 | 9.0 | 0.7 | 9.4 | 5.0 | 1.0 | 1.2 | 184.0 | 0.0 | 2.0 | ||
| Nb | 0.6 | 0.1 | 0.0 | 0.0 | 2.1 | 0.0 | 0.0 | 0.2 | 4.1 | 0.0 | 0.0 | |||
| Sn | 55.0 | 44.0 | 3.0 | 1.0 | 1.0 | 3.0 | ||||||||
| Cs | 0.5 | 0.0 | 0.0 | 0.0 | 2.0 | |||||||||
| Ba | 6.9 | 2.0 | 2.0 | 142.0 | 199.0 | 3.0 | 1.0 | 34.0 | 183.0 | 1.0 | 7.0 | |||
| La | 247.0 | 263.0 | 538.0 | 253.0 | 344.0 | 367.0 | 1.4 | 1.2 | 249.0 | 201.0 | 33.0 | 540.0 | 26.0 | 56.0 |
| Ce | 1013.0 | 918.0 | 1782.0 | 638.0 | 793.0 | 802.0 | 2.4 | 7.8 | 472.0 | 399.0 | 230.0 | 1740.0 | 42.0 | 171.0 |
| Pr | 153.0 | 162.0 | 254.0 | 80.6 | 88.0 | 82.0 | 0.2 | 1.4 | 50.0 | 38.0 | 63.9 | 271.0 | 4.0 | 21.0 |
| Nd | 770.0 | 813.0 | 1073.0 | 245.0 | 305.0 | 258.0 | 1.0 | 7.3 | 158.0 | 125.0 | 382.0 | 1050.0 | 13.0 | 87.0 |
| Sm | 315.0 | 320.0 | 401.0 | 75.9 | 152.0 | 100.0 | 0.3 | 3.2 | 44.0 | 33.0 | 319.0 | 405.0 | 2.0 | 19.0 |
| Eu | 10.7 | 7.5 | 15.2 | 13.4 | 3.0 | 3.0 | 0.6 | 5.0 | 6.0 | 4.9 | 6.5 | 8.0 | 8.0 | |
| Gd | 392.0 | 423.0 | 480.0 | 76.2 | 217.0 | 116.0 | 0.8 | 3.5 | 46.0 | 34.0 | 432.0 | 289.0 | 1.0 | 18.0 |
| Tb | 73.7 | 75.9 | 102.0 | 17.0 | 61.0 | 32.0 | 0.1 | 0.8 | 11.0 | 8.0 | 94.2 | 48.9 | 0.0 | 3.0 |
| Dy | 524.0 | 497.0 | 636.0 | 108.0 | 428.0 | 228.0 | 0.8 | 4.9 | 76.0 | 55.0 | 436.0 | 211.0 | 2.0 | 19.0 |
| Ho | 98.0 | 95.1 | 97.0 | 15.3 | 71.0 | 40.0 | 0.2 | 0.8 | 14.0 | 11.0 | 42.0 | 19.2 | 0.0 | 4.0 |
| Er | 242.0 | 237.0 | 218.0 | 35.4 | 199.0 | 121.0 | 0.4 | 1.7 | 46.0 | 36.0 | 70.0 | 33.7 | 1.0 | 12.0 |
| Tm | 30.6 | 32.9 | 29.0 | 5.1 | 32.0 | 22.0 | 0.1 | 0.2 | 9.0 | 7.0 | 7.3 | 4.0 | 0.0 | 2.0 |
| Yb | 175.0 | 194.0 | 185.0 | 35.9 | 241.0 | 176.0 | 0.3 | 1.4 | 75.0 | 57.0 | 34.9 | 22.4 | 3.0 | 11.0 |
| Lu | 20.7 | 24.4 | 19.0 | 4.6 | 28.0 | 21.0 | 0.0 | 0.1 | 10.0 | 8.0 | 2.8 | 2.2 | 0.0 | 1.0 |
| Hf | 0.5 | 0.2 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.4 | 4.8 | 0.0 | 0.0 | |||
| Ta | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.5 | 0.0 | 0.0 | ||
| Pb | 9.0 | 11.2 | 16.0 | 11.0 | 133.0 | 4.9 | 22.0 | 56.0 | 103.0 | 76.7 | 19.0 | 11.0 | ||
| Th | 2.9 | 3.5 | 8.3 | 55.0 | 66.0 | 0.1 | 5.0 | 2.0 | 1.2 | 26.5 | 22.0 | 13.0 | ||
| U | 72.0 | 81.4 | 431.0 | 258.0 | 186.0 | 214.0 | 1.6 | 124.0 | 187.0 | 229.0 | 67.0 | 272.0 | 3.0 | 1.0 |
| B | 22.3 | 5.6 | ||||||||||||
| ΣLREE | 2498.0 | 2476.0 | 4048.0 | 1292.5 | 1681.6 | 1609.1 | 5.3 | 20.9 | 972.2 | 795.6 | 1027.9 | 4006.0 | 86.9 | 353.0 |
| ΣHREE | 1566.7 | 1586.8 | 1781.2 | 310.9 | 1280.4 | 759.1 | 2.7 | 13.9 | 291.7 | 222.4 | 1124.1 | 636.9 | 17.1 | 77.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A.; Lima, A.; Garate-Olave, I.; Merino-Martínez, E.; Cardoso-Fernandes, J.; Errandonea-Martin, J. Compositional Variations in Apatite and Petrogenetic Significance: Examples from Peraluminous Granites and Related Pegmatites and Hydrothermal Veins from the Central Iberian Zone (Spain and Portugal). Minerals 2022, 12, 1401. https://doi.org/10.3390/min12111401
Roda-Robles E, Gil-Crespo PP, Pesquera A, Lima A, Garate-Olave I, Merino-Martínez E, Cardoso-Fernandes J, Errandonea-Martin J. Compositional Variations in Apatite and Petrogenetic Significance: Examples from Peraluminous Granites and Related Pegmatites and Hydrothermal Veins from the Central Iberian Zone (Spain and Portugal). Minerals. 2022; 12(11):1401. https://doi.org/10.3390/min12111401
Chicago/Turabian StyleRoda-Robles, Encarnación, Pedro Pablo Gil-Crespo, Alfonso Pesquera, Alexandre Lima, Idoia Garate-Olave, Enrique Merino-Martínez, Joana Cardoso-Fernandes, and Jon Errandonea-Martin. 2022. "Compositional Variations in Apatite and Petrogenetic Significance: Examples from Peraluminous Granites and Related Pegmatites and Hydrothermal Veins from the Central Iberian Zone (Spain and Portugal)" Minerals 12, no. 11: 1401. https://doi.org/10.3390/min12111401
APA StyleRoda-Robles, E., Gil-Crespo, P. P., Pesquera, A., Lima, A., Garate-Olave, I., Merino-Martínez, E., Cardoso-Fernandes, J., & Errandonea-Martin, J. (2022). Compositional Variations in Apatite and Petrogenetic Significance: Examples from Peraluminous Granites and Related Pegmatites and Hydrothermal Veins from the Central Iberian Zone (Spain and Portugal). Minerals, 12(11), 1401. https://doi.org/10.3390/min12111401






