Study on the Color-Influencing Factors of Blue Iolite
Abstract
1. Introduction
2. Materials and Methods
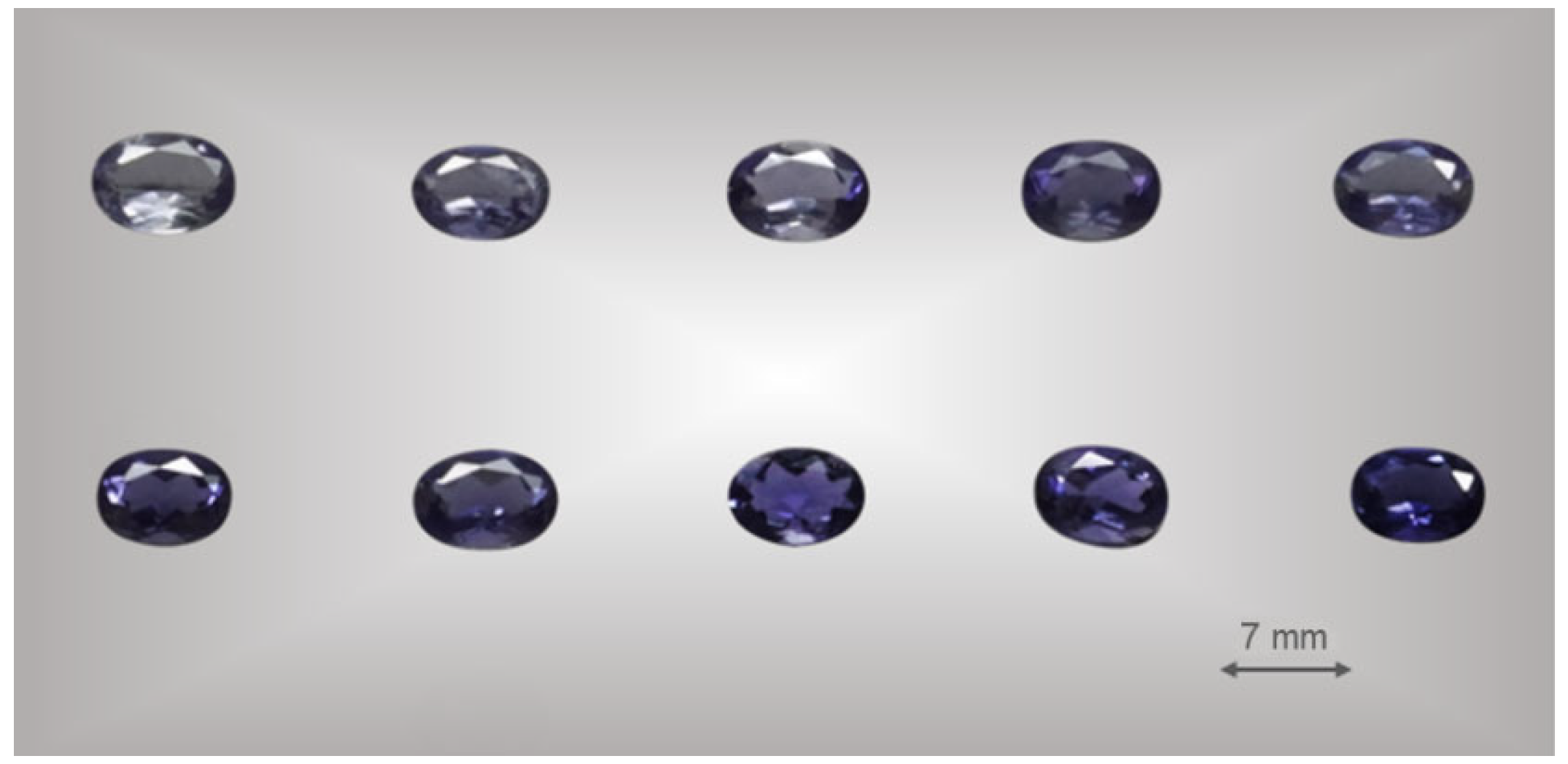
2.1. Materials
2.2. Methods
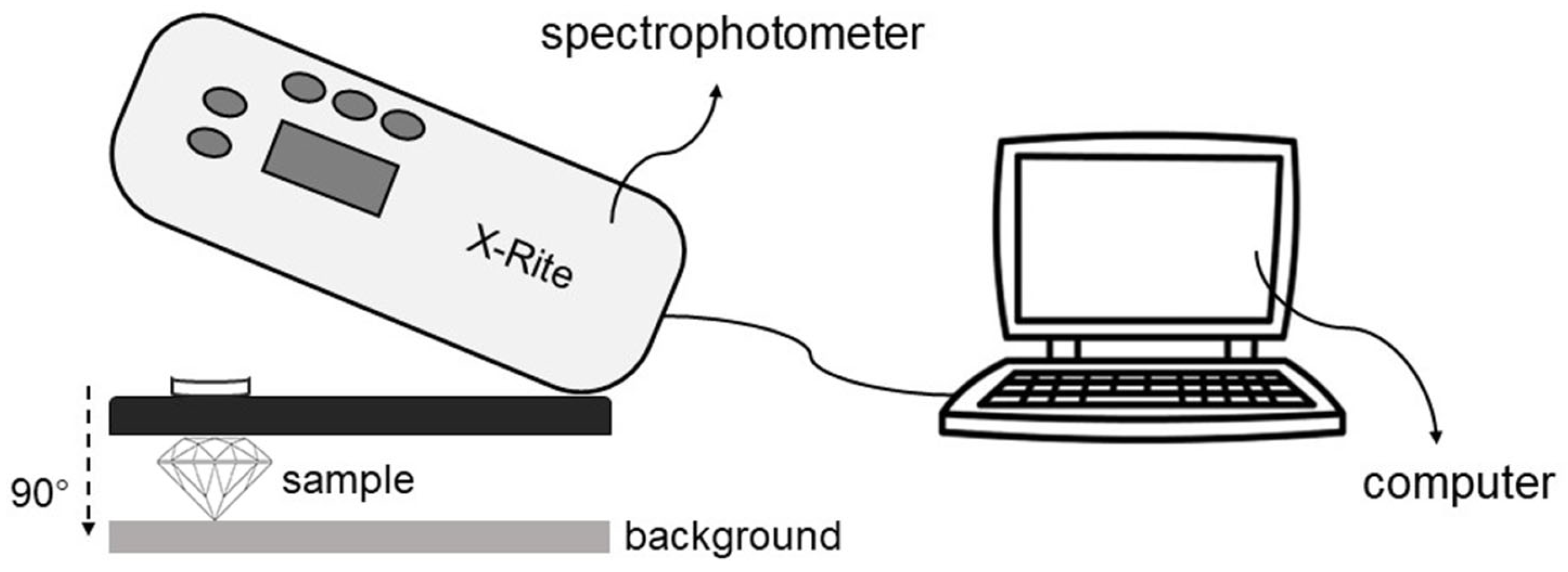
2.2.1. Colorimetric Analysis
2.2.2. ED-XRF
2.2.3. UV–Vis Spectroscopy
3. Results
3.1. Color Quantification
3.2. Chemical Composition
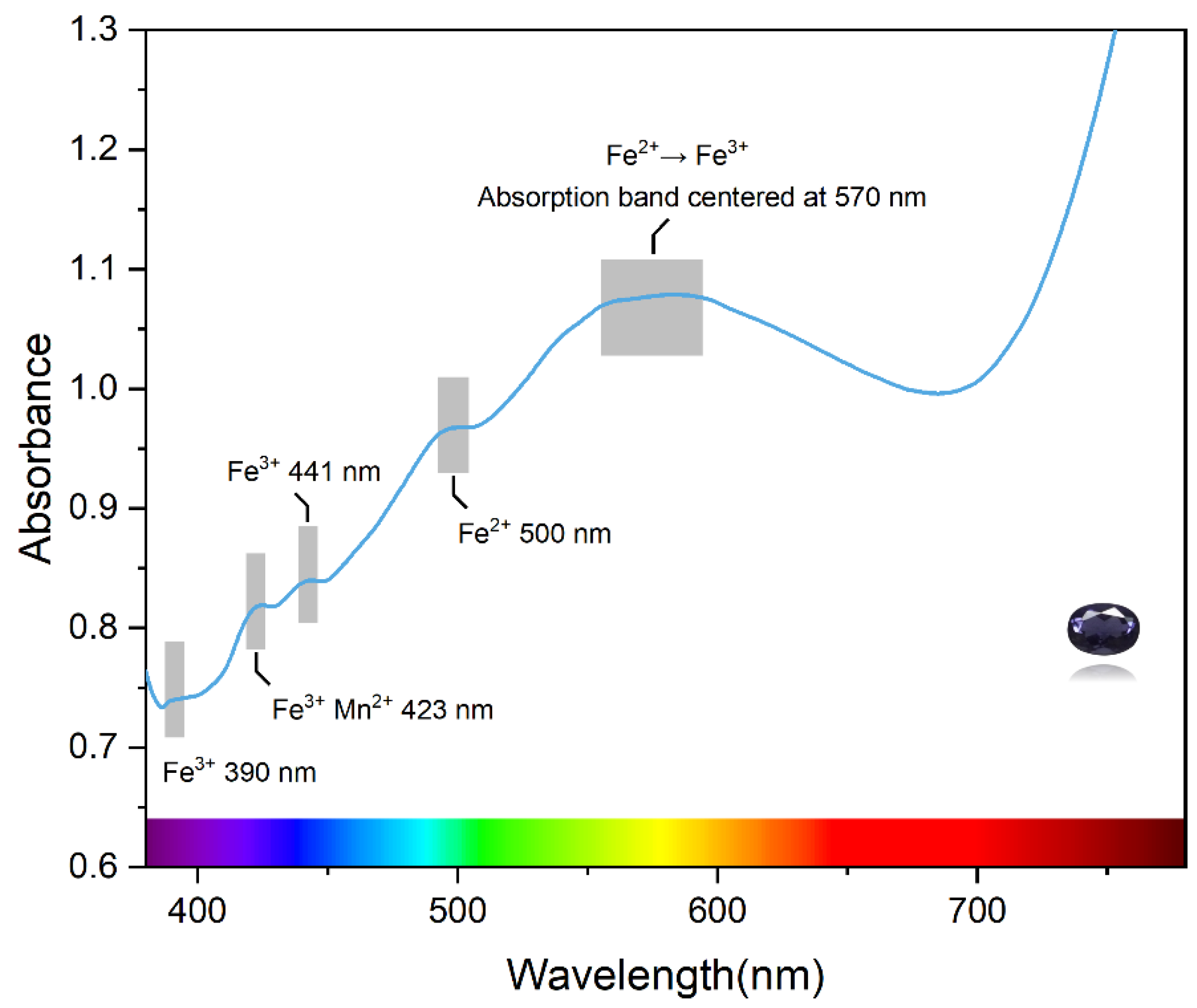
3.3. UV–Vis Spectroscopy Characteristics of Iolite
4. Discussion
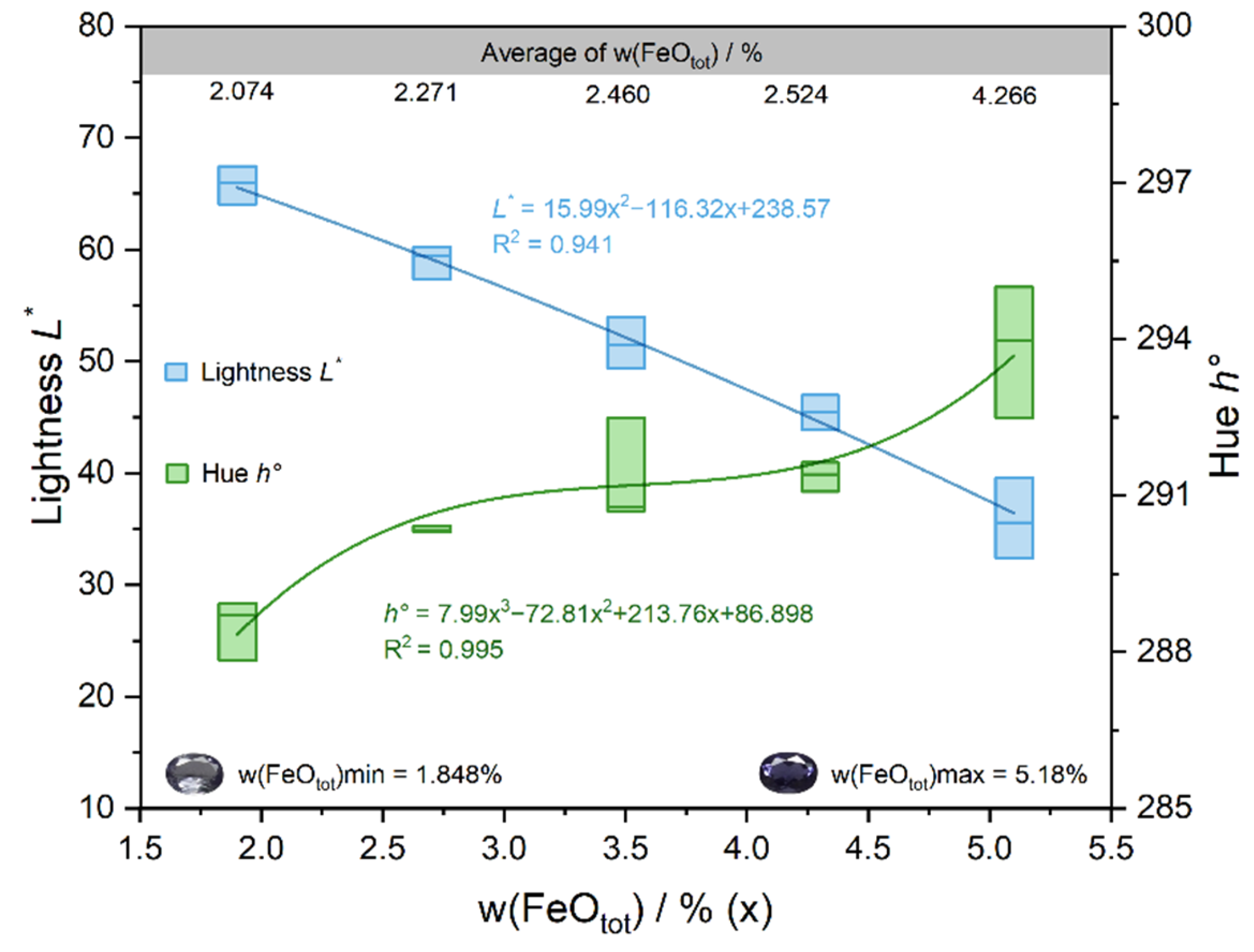
4.1. Relationship between Fe, Mn and Color
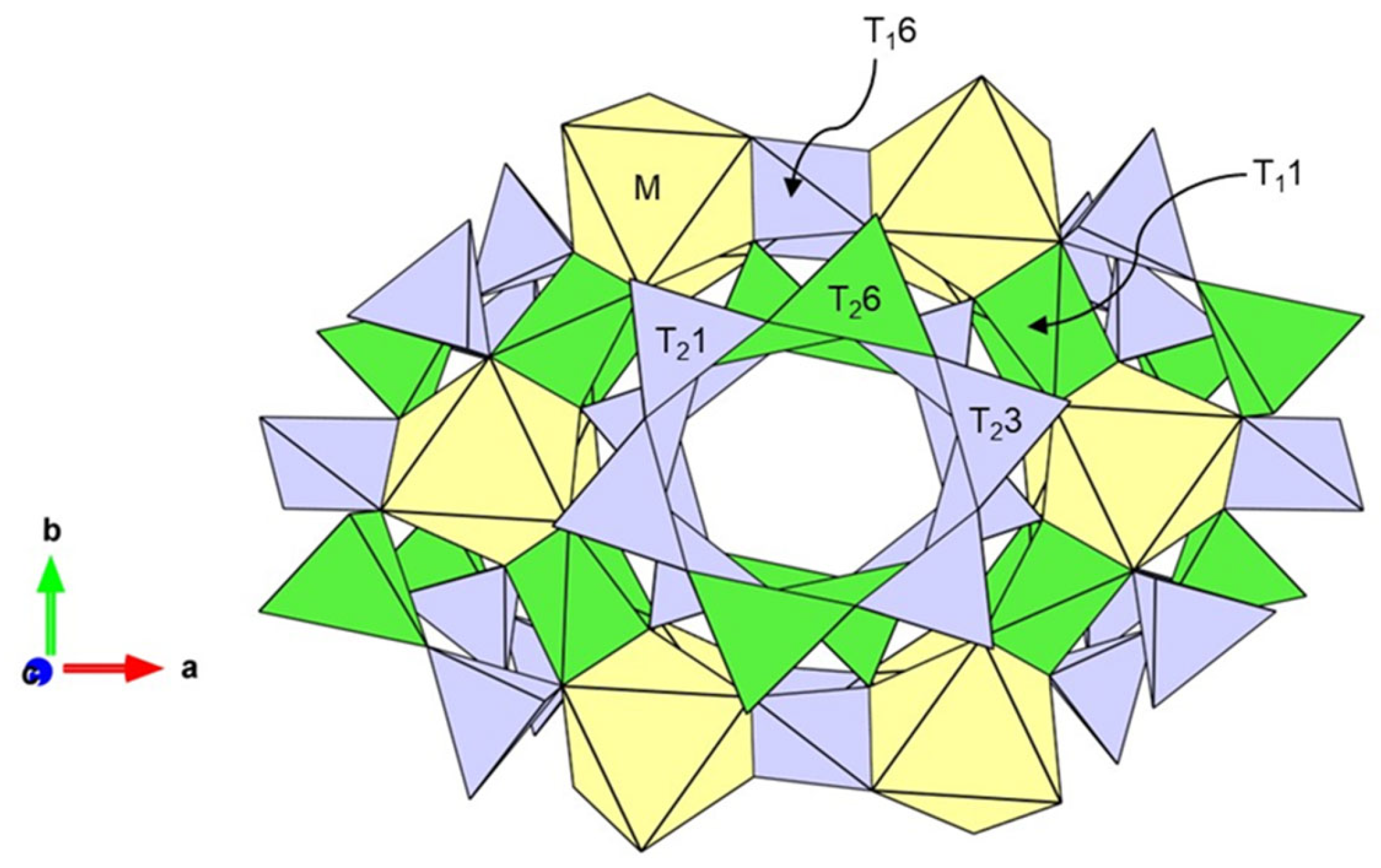
4.2. Color Causes of Blue Iolite
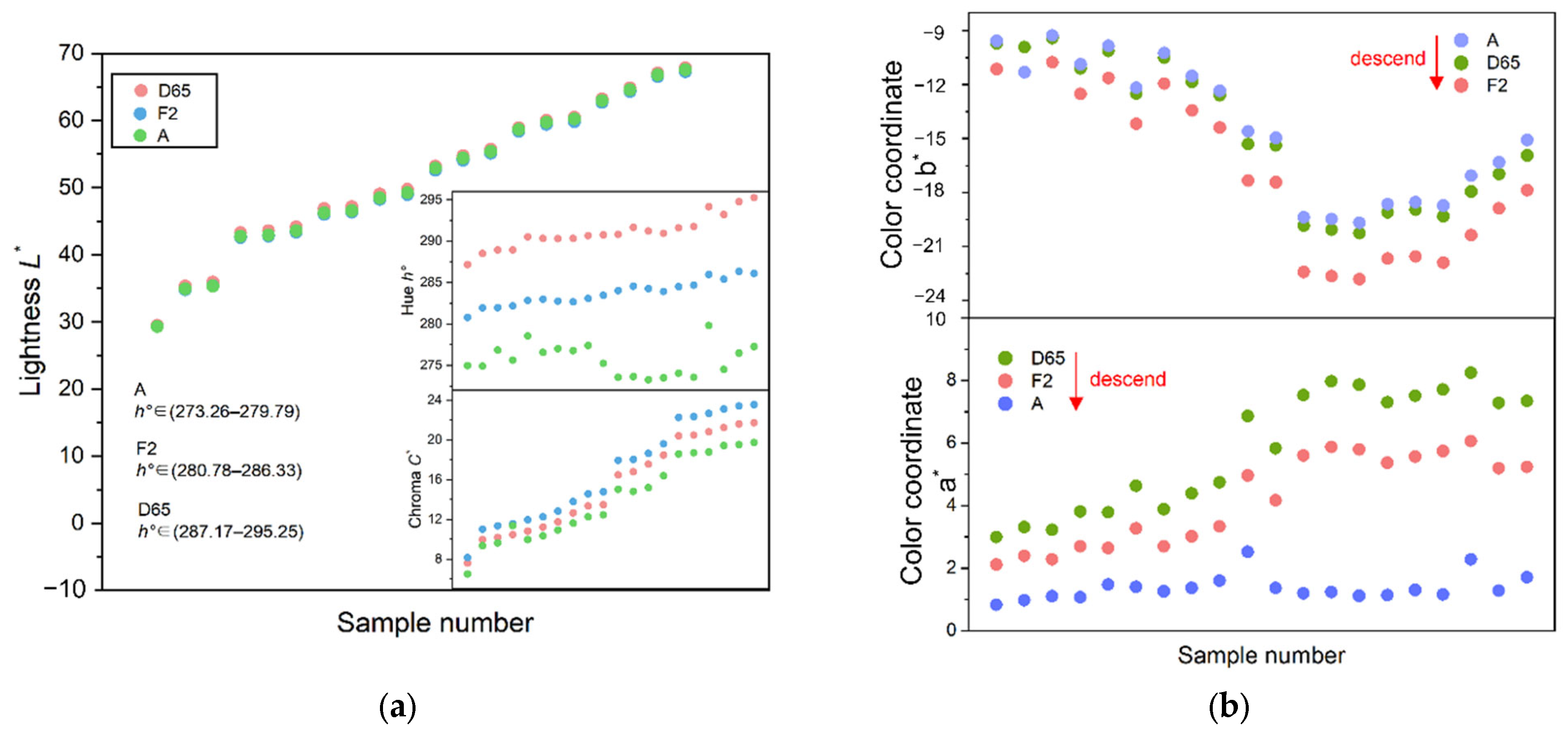
4.3. Influence of Standard Light Source on Color Appearance
5. Conclusions
- (1)
- The color coordinates a* and b* were the main factors affecting the variation of the chroma C*.
- (2)
- Fe is mainly present as Fe2+ and controls the lightness L* and the hue angle h°, while Mn does not have a significant effect on the color of iolite.
- (3)
- The intervalence charge transfer between Fe2+ and Fe3+ is responsible for the blue color of iolite.
- (4)
- The color parameters of iolite are affected by the change of light sources, and the hue angle h° is the most affected.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.K.; Mohanty, J.K. Characteristics of Cordierite (Iolite) of Bandarguha-Orabahala Area, Kalahandi District, Odisha, India. J. Geol. Geophys. 2017, 6, 1–8. [Google Scholar]
- Yu, X. Colored Gemmology, 2nd ed.; Geological Publishing House: Beijing, China, 2016. [Google Scholar]
- Government of India. Indian Minerals Yearbook; Indian Bureau of Mines: Nagpur, India, 2013.
- Faye, G.H.; Manning, P.G. The polarized optical absorption spectra of tourmaline, cordierite, chloritoid and vivianite: Ferrous-ferric electronic interaction as a source of pleochroism. Am. Miner. 1968, 53, 1174–1201. [Google Scholar]
- Pollak, H. Charge transfer in cordierite. Phys. Status Solidi 1976, 74, K31–K34. [Google Scholar] [CrossRef]
- Parkin, K.M.; Loeffler, B.M.; Burns, R.G. Mfssbauer spectra of Kyanite, Aquamarine and Cordierite showing intervalence charge transfer. Phys. Chem. Miner. 1977, 1, 301–311. [Google Scholar] [CrossRef]
- Pointer, M.R. A comparison of the CIE 1976 colour spaces. Color Res. Appl. 2009, 6, 108–118. [Google Scholar] [CrossRef]
- Kirillova, N.P.; Vodyanitskii, V.N.; Sileva, T.M. Conversion of soil color parameters from the Munsell system to the CIE-L*a*b* system. Eurasian Soil Sci. 2015, 48, 468–475. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Application of the CIE L*a*b* system to characterize soil color. Eurasian Soil Sci. 2016, 49, 1259–1268. [Google Scholar] [CrossRef]
- Mahajan, M.P.; Bandyopadhyay, S. Characterization and optimization of color attributes chroma (C*) and lightness (L*) in offset lithography halftone print on packaging boards. Color Res. Appl. 2020, 45, 325–335. [Google Scholar] [CrossRef]
- Mclaren, K. The development of the CIE 1976 (L*a*b*) uniform colour space and colour-difference formula. J. Soc. Dye. Colour 2008, 92, 338–341. [Google Scholar] [CrossRef]
- Ying, G.; Huan, W.; Xiang, L. Metamerism Appreciation of Jadeite-Jade Green under the Standard Light Sources D 65, A and CWF. Acta Geol. Sin. 2016, 90, 2097–2103. [Google Scholar]
- Ying, G.; Xiang, Z.; Ming, Q. Feasibility study on quality evaluation of Jadeite-jade color green based on GemDialogue color chip. Multimed. Tools. Appl. 2019, 78, 841–856. [Google Scholar]
- Xin, P.; Ying, G.; Liu, Z. Impact of different standard lighting sources on red jadeite and color quality grading. Earth Sci. Res. 2019, 23, 371–378. [Google Scholar]
- Liu, F.; Ying, G.; Lv, S.J.; Chen, G.G. Application of the entropy method and color difference formula to the evaluation of round brilliant cut diamond scintillation. Mathematics 2020, 8, 1489. [Google Scholar] [CrossRef]
- Han, J.; Ying, G.; Liu, S. Environmental issues on color quality evaluation of blue sapphire based on gemdialoguetm color comparison charts. Ekoloji 2018, 106, 1365–1376. [Google Scholar]
- Jun, T.; Ying, G.; Chang, X. Color effect of light sources on peridot based on CIE1976 L*a*b* color system and round RGB diagram system. Color Res. Appl. 2019, 44, 932–940. [Google Scholar]
- Jun, T.; Ying, G.; Chang, X. Metameric effects on peridot by changing background color. Josa A 2019, 36, 2030–2039. [Google Scholar]
- Cheng, R.; Ying, G. Study on the effect of heat treatment on amethyst color and the cause of coloration. Sci. Rep. 2020, 10, 14927. [Google Scholar] [CrossRef]
- Yan, L.; Shigley, J.; Halvorsen, A. Colour hue change of a gem tourmaline from the Umba Valley, Tanzania. J. Mater. Sci. 1999, 26, 386–396. [Google Scholar]
- Ying, G. Quality evaluation of tourmaline red based on uniform color space. Clust. Comput. 2017, 20, 3393–3408. [Google Scholar]
- Yan, L.; James, S.; Emmanuel, F.; Scott, H. The “alexandrite effect” in gemstones. Color Res. Appl. 1994, 19, 186–191. [Google Scholar]
- Krzemnicki, M.S. Colour change garnets from Madagascar: Comparison of colorimetric with chemical data. J. Gemmol. 2001, 27, 395–408. [Google Scholar] [CrossRef]
- Schmetzer, K. Colour-change garnets from Madagascar: Variation of chemical, spectroscopic and colorimetric properties. J. Gemmol. 2009, 31, 235–282. [Google Scholar] [CrossRef]
- Sun, Z.; Palke, A.; Renfro, N. Vanadium-and chromium-bearing pink pyrope garnet: Characterization and quantitative colorimetric analysis. Gems. Gemmol. 2016, 51, 348–369. [Google Scholar] [CrossRef]
- Sun, Z.; Palke, A.; Renfro, N.; Breitzmann, H.; Hand, D.; Muyal, J. Discovery of Color-Change Chrome Grossular Garnets from Ethiopia. Gems. Gemmol. 2018, 54, 233–236. [Google Scholar]
- Yan, L.; Shi, G.; Shen, W. Color phenomena of blue amber. Gems Gemmol. 2014, 50, 134–140. [Google Scholar]
- Wallace, J.H.; Wenk, H.R. Structure variations in low cordierites. Am. Miner. 1980, 65, 96–111. [Google Scholar]
- Meagher, E.P.; Gibbs, G.V. The polymorphism of cordierite: II, The crystal structure of Indialite. Can. Miner. 1977, 15, 43–49. [Google Scholar]
- Cohen, J.P.; Ross, F.K.; Gibbs, G.V. An X-ray and neutron diffraction study of hydrous low cordierite. Am. Miner. 1977, 62, 67–78. [Google Scholar]
- Armbruster, T. Fe-rich cordierites from acid volcanic rocks: An optical and X-ray single-crystal structure study. Contrib. Miner. Petrol. 1985, 91, 180–187. [Google Scholar] [CrossRef]
- Gibbs, G.V. The polymorphism of cordierite: I. The crystal structure of low cordierite. Am. Miner. 1966, 51, 1068–1087. [Google Scholar]
- Bertoldiab, C.; Proyerc, A.; Schonberga, D.G.; Behrensd, H.; Dachsb, E. Comprehensive chemical analyses of natural cordierites:implications for exchange mechanisms. Lithos 2004, 78, 389–409. [Google Scholar] [CrossRef]
- Charles, A.; Thomas, A.; Vladimir, K.; Simona, Q. Cordierite I: The coordination of Fe2+. Am. Miner. 2000, 85, 1255–1264. [Google Scholar]
- Charles, A.; Helmut, R.; Michael, C. Cordierite III: The site occupation and concentration of Fe3+. Contrib. Miner. Petrol. 2000, 140, 344–352. [Google Scholar]
- Guo, K.; Zhou, Z.; Qian, Z. A comparative study of element content and UV-Vis spectroscopy characteristics of rubies from Burma and Mozambique. Acta Petrol. Sin. 2018, 37, 1002–1010. [Google Scholar]
- Yuan, L.; Zhao, B.; Zhou, Z.; Xiang, C. Water irradiation dissociation and F-NIR spectral analysis of yellow beryl structure in Xinjiang. Acta Metall. Sin. 2012, 32, 103–105. [Google Scholar]
- Yan, Q.; Ying, G. Explaining Colour Change in Pyrope-Spessartine Garnets. Minerals 2021, 11, 865. [Google Scholar]
- Rehman, H.U.; Martens, G.; Tsai, Y.L. An X-ray Absorption Near-Edge Structure (XANES) Study on the Oxidation State of Chromophores in Natural Kunzite Samples from Nuristan. Minerals 2020, 10, 463. [Google Scholar] [CrossRef]
- Fritsch, E.; Rossman, G.R. An update on color in gems. Part 2: Colors involving multiple atoms and color centers. Gems. Gemmol. 2018, 24, 3–15. [Google Scholar] [CrossRef]


| Properties | Results |
|---|---|
| Color | Light blue to blue–violet |
| Weight | 0.553–0.811 ct |
| Refractive indices | 1.532–1.551 |
| Specific gravity | 2.409–2.596 |
| Pleochroism | Dichroism visible to the naked eye Strong to medium blue or blue–violet; colorless to yellow |
| Microscopic inclusions | Needle-like or columnar gas–liquid inclusions Fingerprint-like inclusions |
| Sample | Iol-1 | Iol-4 | Iol-7 | Iol-5 | Iol-11 | Iol-12 | Iol-14 | Iol-18 | Iol-20 |
|---|---|---|---|---|---|---|---|---|---|
| Simulated color |  |  |  |  |  |  |  |  |  |
| L* | 67.07 | 64.85 | 60.44 | 58.92 | 55.72 | 49.06 | 46.85 | 35.89 | 29.47 |
| C* | 10.46 | 9.96 | 10.82 | 11.22 | 12.64 | 21.22 | 21.72 | 18.48 | 7.60 |
| h° | 288.50 | 288.93 | 290.51 | 290.29 | 290.33 | 290.81 | 291.21 | 293.21 | 295.25 |
| Oxides (wt%) | |||||||||
| SiO2 | 48.758 | 48.372 | 49.193 | 48.907 | 50.045 | 49.201 | 48.856 | 49.204 | 49.089 |
| Al2O3 | 34.727 | 34.585 | 34.065 | 34.835 | 34.462 | 34.510 | 35.117 | 34.787 | 35.012 |
| MgO | 13.186 | 14.031 | 13.342 | 12.982 | 11.623 | 12.544 | 12.615 | 11.433 | 9.249 |
| Fe2O3 | 2.026 | 1.848 | 2.432 | 2.393 | 2.643 | 2.259 | 2.493 | 3.556 | 5.180 |
| SO3 | 1.150 | 0.936 | 0.834 | 0.689 | 0.947 | 1.285 | 0.844 | 0.885 | 1.026 |
| MnO | 0.071 | 0.020 | 0.033 | 0.097 | 0.074 | 0.060 | 0.034 | 0.042 | 0.280 |
| Others | 0.082 | 0.208 | 0.101 | 0.097 | 0.206 | 0.141 | 0.041 | 0.093 | 0.164 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Oxides (wt.%) | L* | C* | h° | |
|---|---|---|---|---|
| FeOtot | r | −0.831 ** | 0.005 | 0.877 ** |
| sig | 0.000 | 0.984 | 0.000 | |
| MnO | r | −0.387 | −0.219 | 0.439 |
| sig | 0.092 | 0.354 | 0.053 | |
| FeOtot + MnO | r | −0.822 ** | −0.010 | 0.869 ** |
| sig | 0.000 | 0.967 | 0.000 |
| Color Parameters | df | Mean Square | F | p |
|---|---|---|---|---|
| L* | 2 | 1.557 | 0.020 | 0.980 |
| a* | 2 | 60.629 | 28.995 | 0.000 |
| b* | 2 | 19.542 | 0.924 | 0.406 |
| C* | 2 | 22.884 | 0.990 | 0.381 |
| h* | 2 | 771.901 | 379.442 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Guo, Y. Study on the Color-Influencing Factors of Blue Iolite. Minerals 2022, 12, 1356. https://doi.org/10.3390/min12111356
Liu X, Guo Y. Study on the Color-Influencing Factors of Blue Iolite. Minerals. 2022; 12(11):1356. https://doi.org/10.3390/min12111356
Chicago/Turabian StyleLiu, Xin, and Ying Guo. 2022. "Study on the Color-Influencing Factors of Blue Iolite" Minerals 12, no. 11: 1356. https://doi.org/10.3390/min12111356
APA StyleLiu, X., & Guo, Y. (2022). Study on the Color-Influencing Factors of Blue Iolite. Minerals, 12(11), 1356. https://doi.org/10.3390/min12111356







