Numerical Simulation of Gas–Liquid Two-Phase Flow CFD–PBM Model in a Micro–Nanobubble Generator
Abstract
1. Introduction
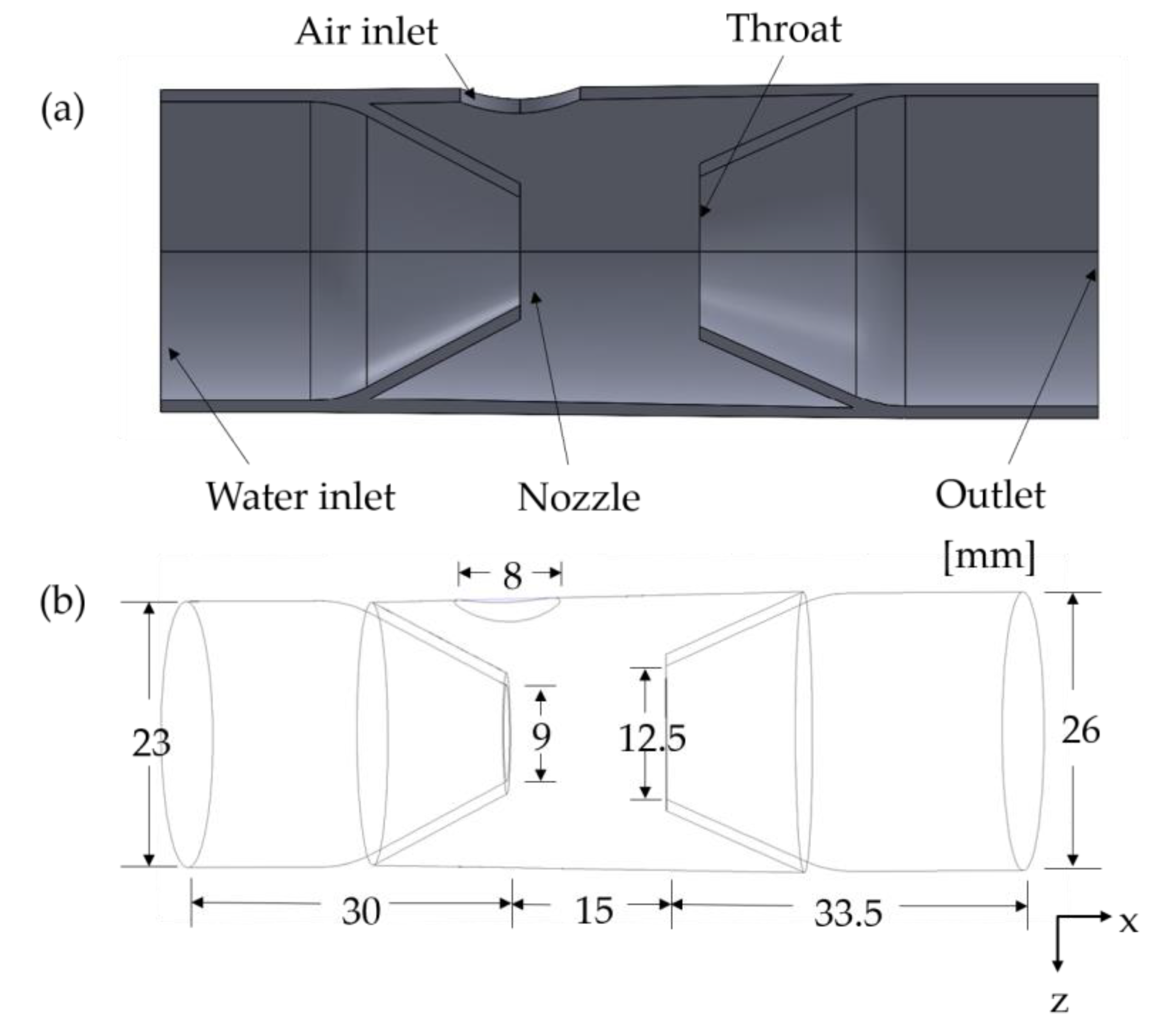
2. Materials and Methods
3. Mathematical Model
3.1. Gas–Liquid Two-Phase Flow Model
3.2. Interphase Force Model for Gas–Liquid Two-Phase Flow
3.2.1. Drag Force
3.2.2. Virtual Mass Force
3.2.3. Lift Force
3.3. Turbulence Model
3.4. Population Balance Model
3.4.1. Bubble Population Balance Model
3.4.2. Bubble Break-Up Model
3.4.3. Bubble coalescence model
4. Numerical Simulation of Gas–Liquid Two-Phase Flow in Micro–Nanobubble Generator
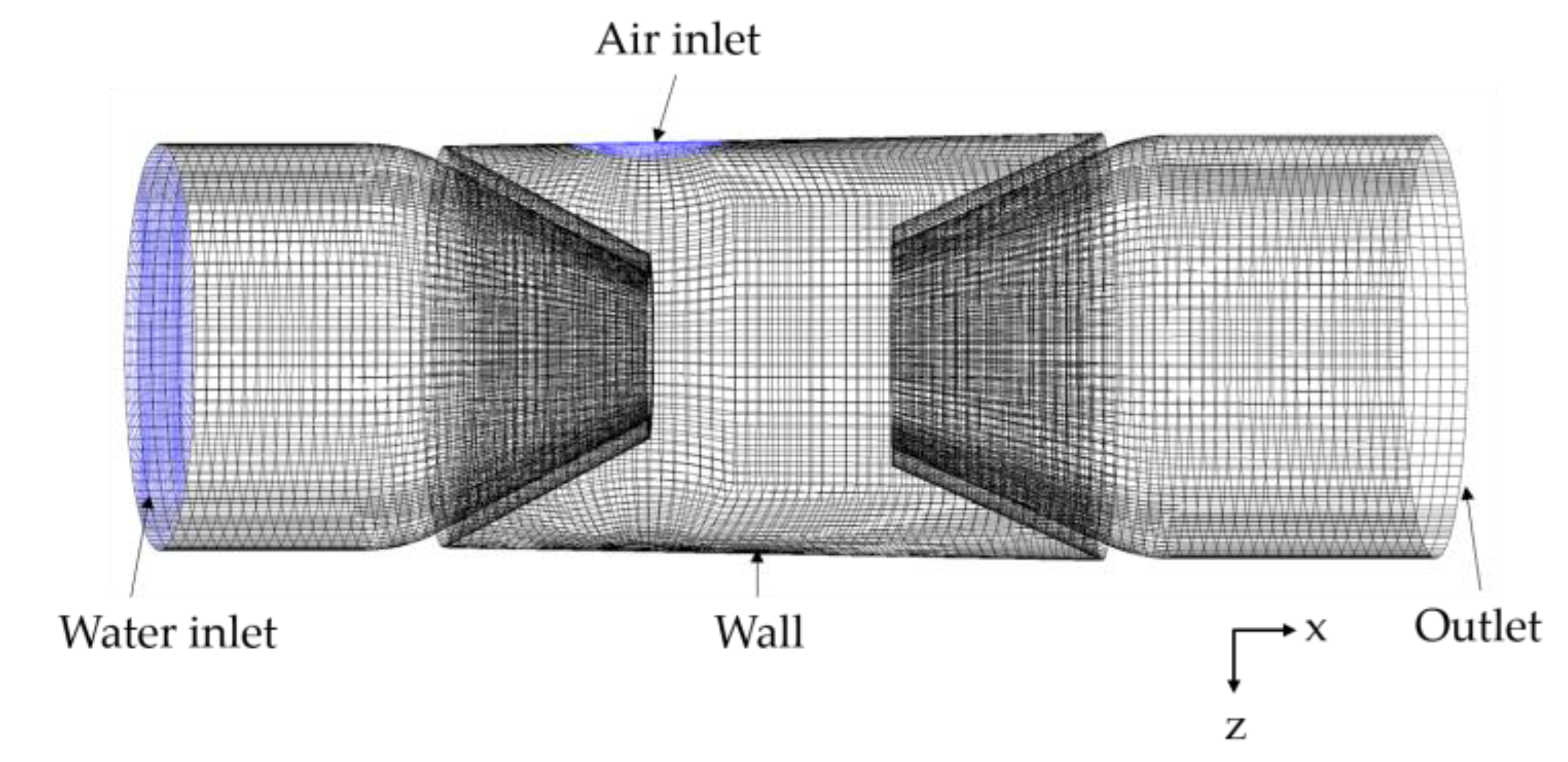
4.1. Meshing
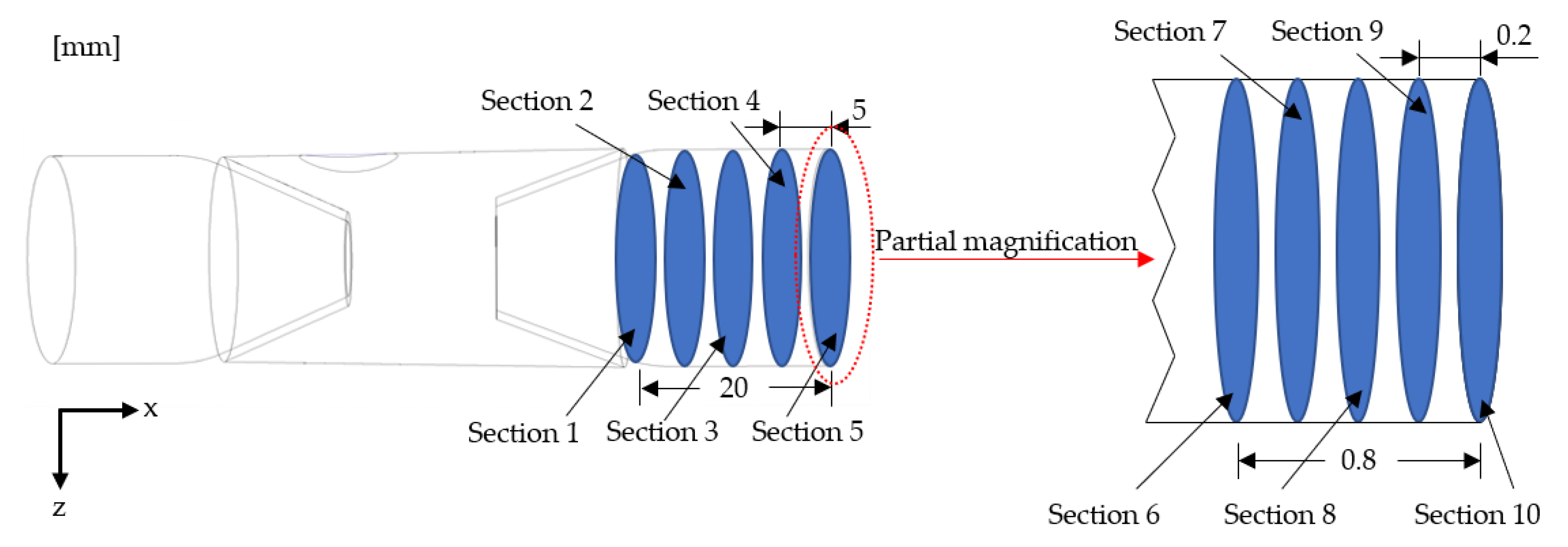
4.2. Two-Phase Flow Simulation Results and Analysis
5. Conclusions
- (1)
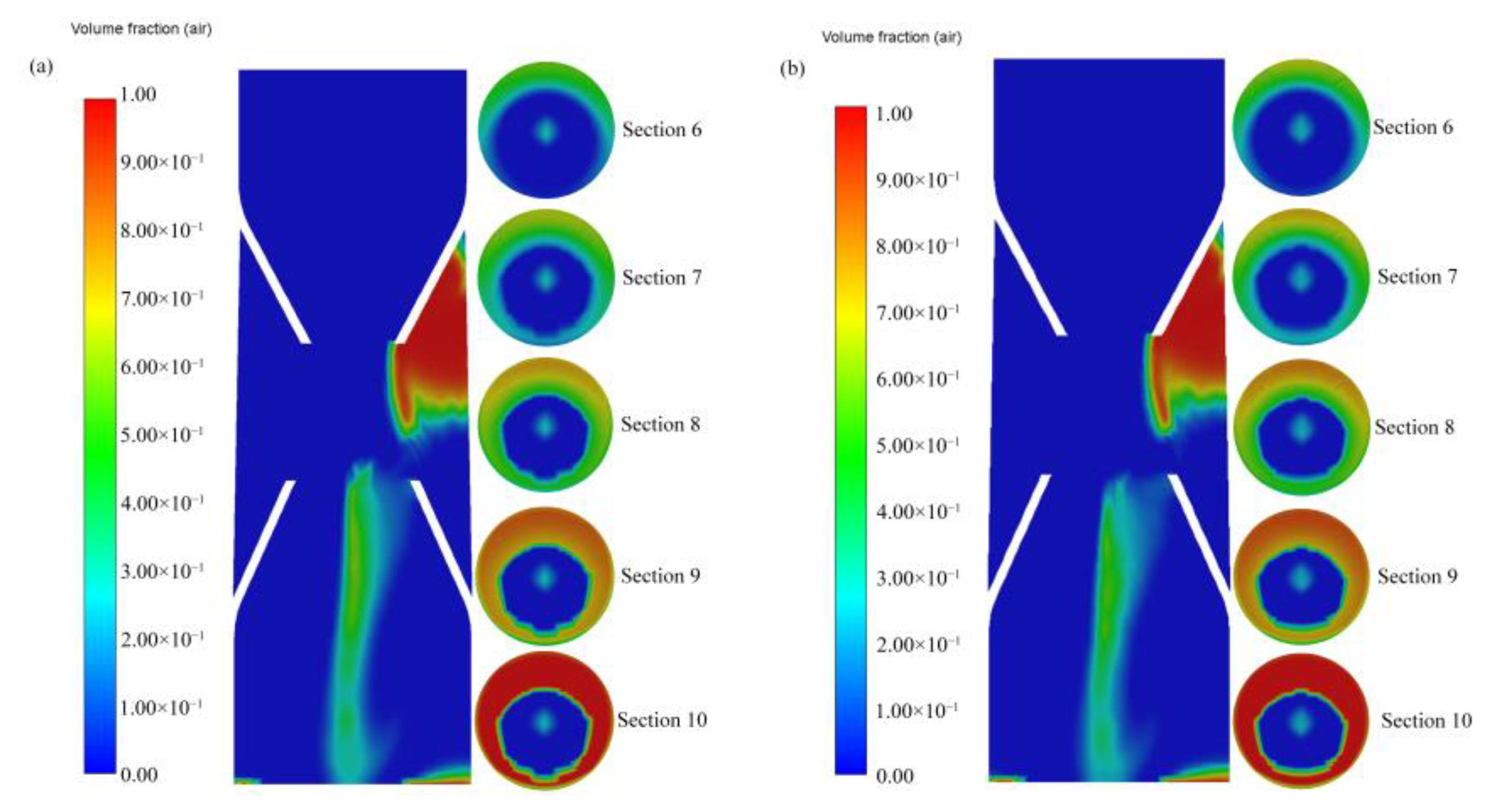
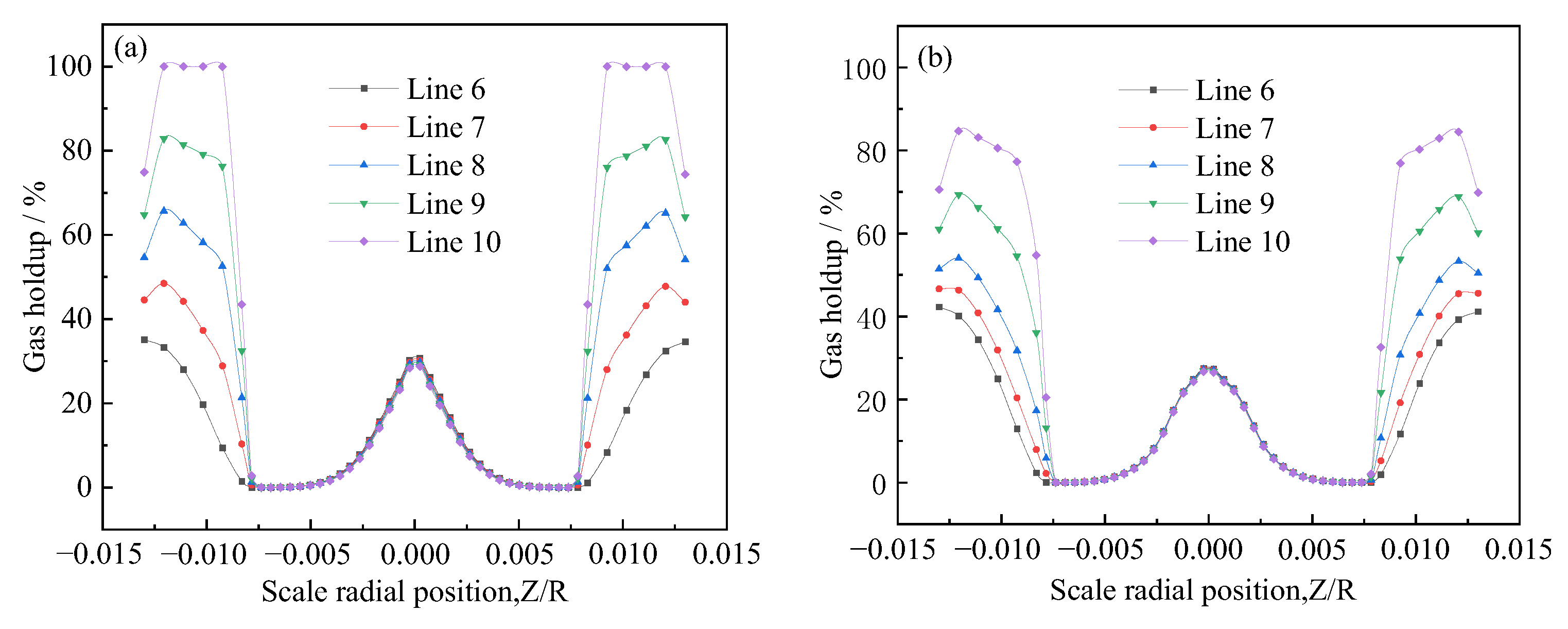
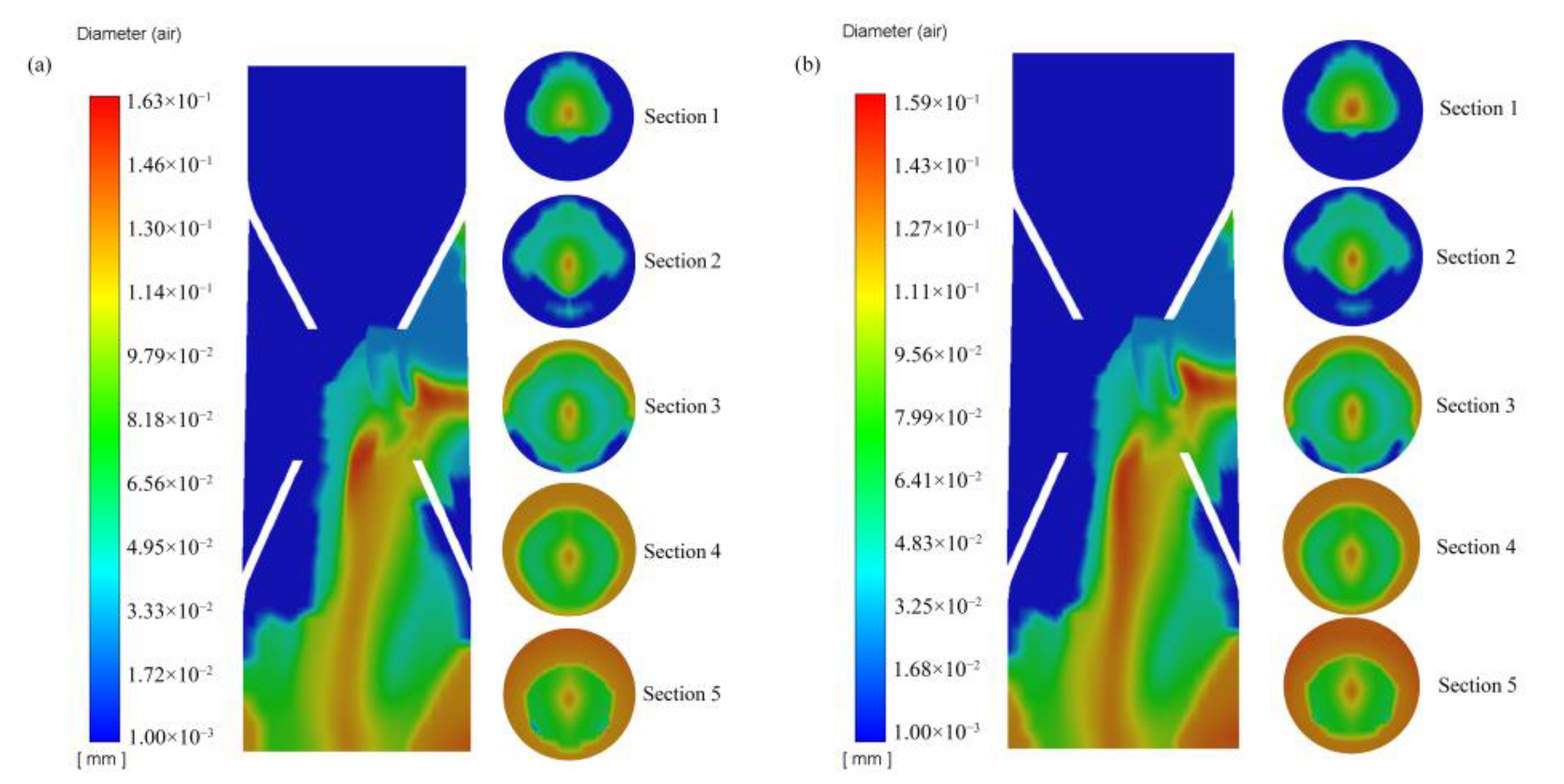
- After the gas entered the generator from the suction pipe, it mainly moved along the center of the pipe. After moving to the static mixing zone, most of the gas still gathered in the center of the pipe, and a small amount of gas slowly moved toward the wall. When approaching the outlet, a large amount of gas started to move toward the wall, meaning that the gas was mainly distributed on the wall at the outlet.
- (2)
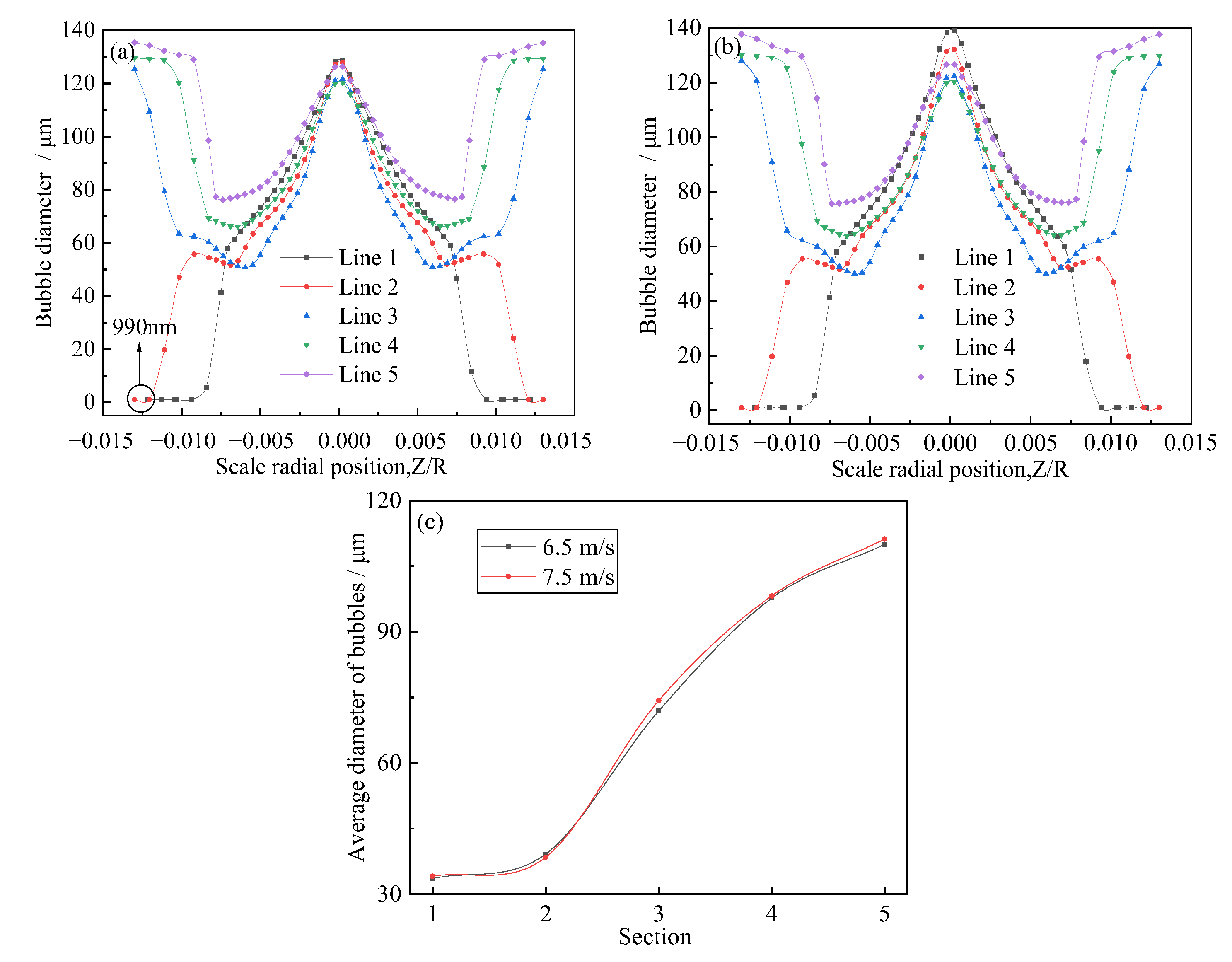
- Inhaled gas creates large-size bubbles at the center of the tube. By contrast, the bubble size at the pipe wall was smaller. As the bubbles moved to the outlet, the bubble size at the wall increased. The crushing efficiency was greater than the coalescence efficiency, and bubble breaking was the dominant process. The relatively large bubbles located in the center were broken into small ones and then move to the periphery of the tube. Thereafter, bubble aggregation dominated, and small bubbles coalesced to form relatively large bubbles. The average diameter of the generated bubbles gradually increased from approximately 30 to 110 μm, and the growth rate of the bubbles from Sections 2 to 4 was particularly prominent. Additionally, the minimum diameter of the bubbles was about 0.99 μm.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Area | [m2] |
| a | Specific interfacial area | [m−1] |
| B | Birth rate | [m−3 s−1] |
| BB, V | The source term of the bubble broken to generate V | [–] |
| BC, V | The source terms combined into bubble V | [–] |
| c | Constant value | [–] |
| CD | Drag force coefficient | [–] |
| CT | Lift force coefficient | [–] |
| CV | Virtual mass force coefficient | [–] |
| DB, V | The source term of the bubble V broken | [–] |
| DC, V | The source term of the disappearance of V | [–] |
| db | Bubble diameter | [m] |
| fDz | The drag force on a single bubble | [N] |
| FDz | The drag force on the bubble group | [N] |
| FVz | Virtual mass force | [N] |
| FT | Lift force | [N] |
| g | Gravitational acceleration | [m/s2] |
| p | Pressure | [Pa] |
| PB | The bubble breakage frequency | [–] |
| PC | The bubble coalescence frequency | [–] |
| T | Temperature | [K] |
| t | Time | [s] |
| v | Velocity | [m/s] |
| xi | Representative volume for the ith size range | [m3] |
| Weij | Weber number | [–] |
| α | Volume fraction | [–] |
| β | Dimensionless daughter size distribution | [–] |
| ε | Turbulent energy dissipation rate | [m2/s3] |
| κ | Cell specific constant | [–] |
| λ | Power parameter | [–] |
| µ | Dynamic viscosity | [Pa s] |
| ν | Kinematic viscosity | [m2/s] |
| ρ | Density | [kg/m3] |
| Τ | Reynolds stress tensor | [Pa] |
| χ | The dimensionless energy | [–] |
| χc | The critical dimensionless energy for breakup | [–] |
References
- Forbes, E. Shear, selective and temperature responsive flocculation: A comparison of fine particle flotation techniques. Int. J. Miner. Process. 2011, 99, 1–10. [Google Scholar] [CrossRef]
- Valderrama, L.; Rubio, J. High intensity conditioning and the carrier flotation of gold fine particles. Int. J. Miner. Process. 1998, 52, 273–285. [Google Scholar] [CrossRef]
- Hao, H.Q.; Li, L.X.; Somasundaran, P.; Yuan, Z.T. Adsorption of pregelatinized starch for selective flocculation and flotation of fine siderite. Langmuir 2019, 35, 6878–6887. [Google Scholar] [CrossRef] [PubMed]
- Chipakwe, V.; Sand, A.; Chelgani, S.C. Nanobubble assisted flotation separation of complex Pb–Cu–Zn sulfide ore–assessment of process readiness. Sep. Sci. Technol. 2022, 57, 1351–1358. [Google Scholar] [CrossRef]
- Parker, J.L.; Claesson, P.M.; Attard, P. Bubbles, cavities, and the long-ranged attraction between hydrophobic surfaces. J. Phys. Chem. 1994, 98, 8468–8480. [Google Scholar] [CrossRef]
- Lou, S.; Gao, J.; Xiao, X.; Li, X.; Li, G.; Zhang, Y.; Li, M.; Sun, J.; Li, X.; Hu, J. Studies of nanobubbles produced at liquid/solid interfaces. Mater. Charact. 2002, 48, 211–214. [Google Scholar] [CrossRef]
- Ducker, W.A. Contact angle and stability of interfacial nanobubbles. Langmuir 2009, 25, 8907–8910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X. Nanobubble stability induced by contact line pinning. J. Phys. Chem. 2013, 138, 014706. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Birkett, G.R.; Nguyen, A.V. Origin of interfacial nanoscopic gaseous domains and formation of dense gas layer at hydrophobic solid–water interface. Langmuir 2013, 29, 15266–15274. [Google Scholar] [CrossRef]
- Weijs, J.H.; Lohse, D. Why surface nanobubbles live for hours. Phys. Rev. Lett. 2013, 110, 054501. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bao, S.; Liu, C.; Yuan, D.; Huang, W. An analytical model of the growth of invisible bubbles on solid surfaces in a supersaturated solution. Chem. Eng. Sci. 2020, 215, 114968. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B.; Fan, M. Effects of nanobubble and hydrodynamic parameters on coarse quartz flotation. Int. J. Min. Sci. Technol. 2019, 29, 289–295. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. Nanobubble column flotation of fine coal particles and associated fundamentals. Int. J. Miner. Process. 2013, 124, 109–116. [Google Scholar] [CrossRef]
- Cui, R. Study on the Improvement of Low-Rank Coal and Its Semi-Coke Adsorption on Congo Red Dye by Micro-Nano Bubbles. Master’s. Thesis, China University of Mining and Technology, Xuzhou, China, 2020. [Google Scholar]
- Ma, F.; Zhang, P.; Tao, D. Surface nanobubble characterization and its enhancement mechanisms for fine-particle flotation: A review. Int. J. Miner. Metall. Mater. 2022, 29, 727–738. [Google Scholar] [CrossRef]
- Ahmadi, R.; Khodadadi, D.A.; Abdollahy, M.; Fan, M. Nano–microbubble flotation of fine and ultrafine chalcopyrite particles. Int. J. Min. Sci. Technol. 2014, 24, 559–566. [Google Scholar] [CrossRef]
- Mitra, S.; Hoque, M.M.; Evans, G.; Nguyen, A.V. Direct visualisation of bubble-particle interactions in presence of cavitation bubbles in an ultrasonic flotation cell. Miner. Eng. 2021, 174, 107258. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z. Nanobubble generation and its applications in froth flotation (part II): Fundamental study and theoretical analysis. Min. Sci. Technol. 2010, 20, 159–177. [Google Scholar] [CrossRef]
- Tao, D.; Wu, Z.; Sobhy, A. Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms. Powder Technol. 2021, 379, 12–25. [Google Scholar] [CrossRef]
- Tussupbayev, N.K.; Rulyov, N.N.; Kravtchenco, O.V. Microbubble augmented flotation of ultrafine chalcopyrite from quartz mixtures. Miner. Process. Extr. Metall. 2016, 125, 5–9. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Filippov, L.O.; Kravchenko, O.V. Combined microflotation of glass beads. Colloids. Surf. 2020, 598, 124810. [Google Scholar] [CrossRef]
- Etchepare, R.; Azevedo, A.; Calgaroto, S.; Rubio, J. Removal of ferric hydroxide by flotation with micro and nanobubbles. Sep. Purif. Technol. 2017, 184, 347–353. [Google Scholar] [CrossRef]
- Tsave, P.K.; Kostoglou, M.; Karapantsios, T.D.; Lazaridis, N.K. A Hybrid Device for Enhancing Flotation of Fine Particles by Combining Micro-Bubbles with Conventional Bubbles. Minerals 2021, 11, 561. [Google Scholar] [CrossRef]
- Taghavi, F.; Noaparast, M.; Pourkarimi, Z.; Nakhaei, F. Comparison of mechanical and column flotation performances on recovery of phosphate slimes in presence of nano-microbubbles. J. Cent. South Univ. 2022, 29, 102–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Zhang, Y. Role of nanobubbles in the flotation of fine rutile particles. Miner. Eng. 2021, 172, 107140. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Yao, W.; Xu, Z.; Fan, R. Effective Separation of High-Ash Fine Coal Using Water Containing Positively Charged Nanobubbles and Polyaluminum Chloride. ACS Omega. 2022, 7, 2210–2216. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Yao, W.; Xu, Z.; Fan, R. Enhanced kaolinite flotation using amine coated nanobubbles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128296. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Meng, H. CFD–PBM numerical simulation on the break-up and coalescence process of dispersed phase droplet in Kenics static mixer. Chin. J. Process. Eng. 2021, 21, 935–943. [Google Scholar]
- Zhang, W. Numerical Simulation of Gas–Liquld (Solid) Flow in Terephthalic Acid Reactor Using CFD–PBM Coupled Model. Master’s Thesis, Beijing Institute of Petrochemical Technology, Beijing, China, 2021. [Google Scholar]
- Gao, S.; Xu, Y.; Li, J.; Ye, S.; Huang, W. Simulation study of microbubbles break-up and coalescence in centrifugal pump based on TFM-PBM coupling model. CIESC J. 2021, 72, 5082–5093. [Google Scholar]
- Duan, W.; Wang, C.; Zheng, Q. CFD–PBM simulation of three-phase flow in the mixing zone of a centrifugal contactor. J. Fluid Mech. 2021, 49, 25–32. [Google Scholar]
- Xing, C.; Wang, T.; Wang, J. Experimental study and numerical simulation with a coupled CFD–PBM model of the effect of liquid viscosity in a bubble column. Chem. Eng. Sci. 2013, 95, 313–322. [Google Scholar] [CrossRef]
- Jia, X. CFD–PBM Coupled Simulation of Two Phase Flow in Coal Liquefaction Bubble Column Reactor. Master’s Thesis, East China University of Science and Technology, Shanghai, China, 2020. [Google Scholar]
- Li, D.; Buffo, A.; Podgórska, W.; Marchisio, D.L.; Gao, Z. Investigation of droplet breakup in liquid–liquid dispersions by CFD–PBM simulations: The influence of the surfactant type. Chin. J. Chem. Eng. 2017, 25, 1369–1380. [Google Scholar] [CrossRef]
- Wang, S.; Bu, Q.; Luan, D.; Zhang, Y.; Li, L.; Wang, Z.; Shi, W. Study on gas–liquid flow characteristics in stirred tank with dual-impeller based on CFD-PBM coupled model. Chin. J. Chem. Eng. 2021, 38, 63–75. [Google Scholar] [CrossRef]
- Zhang, B.; Kong, L.; Jin, H.; He, G.; Yang, S.; Guo, X. CFD simulation of gas–liquid flow in a high-pressure bubble column with a modified population balance model. Chin. J. Chem. Eng. 2018, 26, 1350–1358. [Google Scholar] [CrossRef]
- Alam, H.S.; Sutikno, P.; Fauzi Soelaiman, T.A.; Sugiarto, A.T. CFD-PBM Coupled modeling of bubble size distribution in a swirling-flow nanobubble generator. Eng. Appl. Comp. Fluid 2022, 16, 677–693. [Google Scholar] [CrossRef]
- Ren, F.; Noda, N.A.; Ueda, T.; Sano, Y.; Takase, Y.; Umekage, T.; Tanaka, H. CFD-PBM approach for the gas-liquid flow in a nanobubble generator with honeycomb structure. J. Disper. Sci. Technol. 2019, 40, 306–317. [Google Scholar] [CrossRef]
- Liu, Y. Measurement and Numerical Simulation of Gas–Liquid Two Phase Flow in Jet Micro–Bubble Generator. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2018. [Google Scholar]
- Wu, M.; Song, H.Y.; Liang, X.; Huang, N.; Li, X.B. Generation of micro-nano bubbles by self-developed swirl-type micro-nano bubble generator. Chem. Eng. Process. 2022, 181, 109136. [Google Scholar] [CrossRef]
- Wu, M.; Yuan, S.Y.; Song, H.Y.; Li, X.B. Micro-nano bubbles production using a swirling-type venturi bubble generator. Chem. Eng. Process. 2022, 170, 108697. [Google Scholar] [CrossRef]
- Deng, X.W.; Lv, B.; Cheng, G.; Lu, Y. Mechanism of micro/nano-bubble formation and cavitation effect on bubbles size distribution in flotation. Physicochem. Probl. Miner. Process. 2020, 56, 504–512. [Google Scholar] [CrossRef]
- Thakre, S.S.; Joshi, J.B. CFD simulation of bubble column reactors: Importance of drag force formulation. Chem. Eng. Sci. 1999, 54, 5055–5060. [Google Scholar] [CrossRef]
- Tomiyama, A.; Tamai, H.; Zun, I.; Hosokawa, S. Transverse migration of single bubbles in simple shear flows. Chem. Eng. Sci. 2002, 57, 1849–1858. [Google Scholar] [CrossRef]
- Luo, H. Coalescence, Breakup and Liquid Circulation in Bubble Column Reactors. Ph.D. Thesis, The University of Trondheim, Trondheim, Norway, 1993. [Google Scholar]
- Luo, H.; Svendsen, H.F. Theoretical model for drop and bubble breakup in turbulent dispersions. AIChE J. 1996, 42, 1225–1233. [Google Scholar] [CrossRef]
| Bubble Group | Diameter/mm |
|---|---|
| Bin-0 | 1.024 |
| Bin-1 | 0.512 |
| Bin-2 | 0.256 |
| Bin-3 | 0.128 |
| Bin-4 | 0.064 |
| Bin-5 | 0.032 |
| Bin-6 | 0.016 |
| Bin-7 | 0.008 |
| Bin-8 | 0.004 |
| Bin-9 | 0.002 |
| Bin-10 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, W.; Wang, J.; Song, Y.; Wu, B.; Wen, J.; Li, K.; Li, B. Numerical Simulation of Gas–Liquid Two-Phase Flow CFD–PBM Model in a Micro–Nanobubble Generator. Minerals 2022, 12, 1270. https://doi.org/10.3390/min12101270
Xu W, Li W, Wang J, Song Y, Wu B, Wen J, Li K, Li B. Numerical Simulation of Gas–Liquid Two-Phase Flow CFD–PBM Model in a Micro–Nanobubble Generator. Minerals. 2022; 12(10):1270. https://doi.org/10.3390/min12101270
Chicago/Turabian StyleXu, Weiguang, Wenjuan Li, Jianwei Wang, Yongsheng Song, Biao Wu, Jiankang Wen, Kaiguo Li, and Bin Li. 2022. "Numerical Simulation of Gas–Liquid Two-Phase Flow CFD–PBM Model in a Micro–Nanobubble Generator" Minerals 12, no. 10: 1270. https://doi.org/10.3390/min12101270
APA StyleXu, W., Li, W., Wang, J., Song, Y., Wu, B., Wen, J., Li, K., & Li, B. (2022). Numerical Simulation of Gas–Liquid Two-Phase Flow CFD–PBM Model in a Micro–Nanobubble Generator. Minerals, 12(10), 1270. https://doi.org/10.3390/min12101270






