Low-Temperature Flotation Separation of Diaspore from Kaolinite by Using a Mixed Collector
Abstract
:1. Introduction
2. Materials and Methods
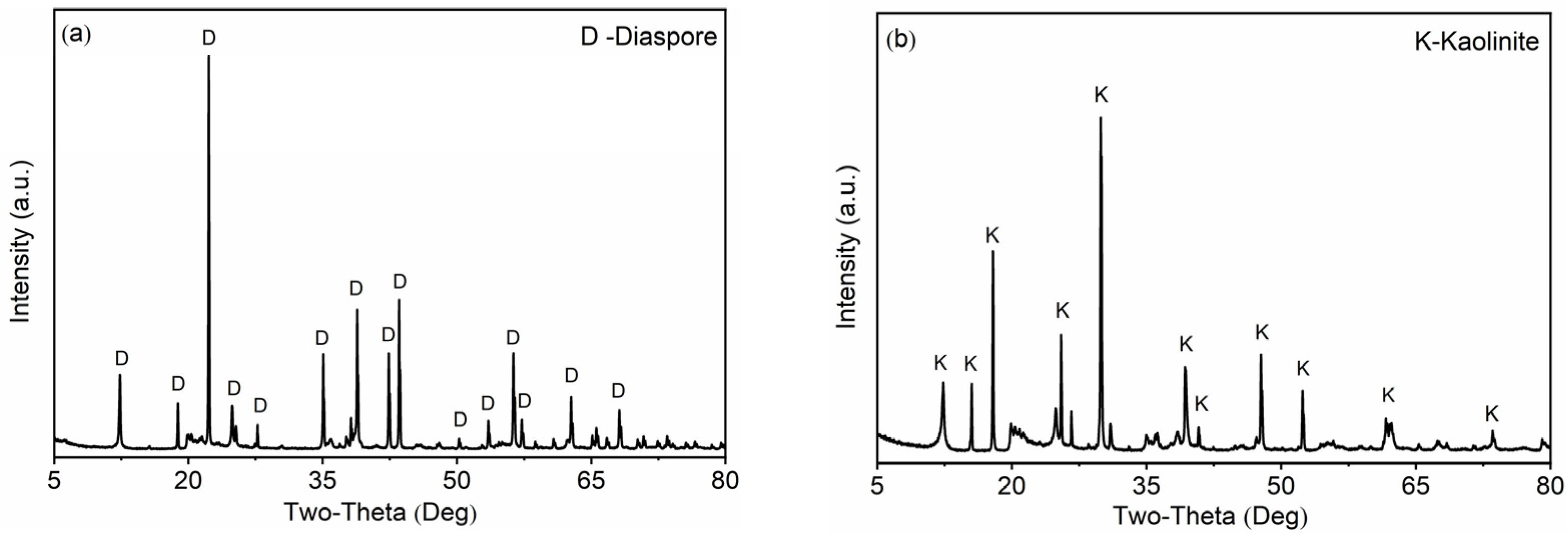
2.1. Materials
2.2. Reagents
2.3. Flotation Tests
2.4. Surface Tension Measurements
2.5. Zeta Potential Determinations
2.6. XPS Measurements
3. Results and Discussions
3.1. Micro-Flotation
3.2. Surface Tension Measurements
3.3. Zeta Potential Determinations
3.4. XPS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Z.; Sun, C.; Kou, J.; Fu, G.; Qi, X. Experimental and molecular dynamics simulation study on the effect of polyacrylamide on bauxite flotation. Miner. Eng. 2021, 164, 106810. [Google Scholar] [CrossRef]
- Zhang, N.; Ejtemaei, M.; Nguyen, A.V.; Zhou, C. XPS analysis of the surface chemistry of sulfuric acid-treated kaolinite and diaspore minerals with flotation reagents. Miner. Eng. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Liu, C.; Ansheng, F.; Zhenxu, G.; Xuefeng, C.; Yuehua, H. Flotation behavior of four dodecyl tertiary amines as collectors of diaspore and kaolinite. Min. Sci. Technol. 2011, 21, 249–253. [Google Scholar] [CrossRef]
- Yan, W.; Liu, C.; Ai, G.; Feng, Q.; Zhang, W. Flotation separation of scheelite from calcite using mixed collectors. Int. J. Miner. Process. 2017, 169, 106–110. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Lyu, F.; Sun, W.; Khoso, S.A.; Zhang, C.; Liu, R.; Wang, L.; Gao, J. Adsorption mechanism of propyl gallate as a flotation collector on scheelite: A combined experimental and computational study. Miner. Eng. 2019, 133, 19–26. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Yin, W.-Z.; Dong, X.-S.; Sun, C.-Y.; Yang, B.; Yao, J.; Li, H.-L.; Li, C.; Kim, H. New insights into the flotation responses of brucite and serpentine for different conditioning times: Surface dissolution behavior. Int. J. Miner. Metall. Mater. 2021, 28, 1898–1907. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, L.-H.; Hu, Y.-H.; Wang, D.-Z.; Li, C.-K.; Meng, W.; Wang, X.-J. Flotation and adsorption of quaternary ammonium cationic collectors on diaspore and kaolinite. Trans. Nonferrous Met. Soc. China 2011, 21, 2528–2534. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, M.; Xia, L.; Fu, W.; Zhou, W.; Yang, S. The utilization of citric acid as a depressant for the flotation separation of barite from fluorite. Miner. Eng. 2020, 156, 106491. [Google Scholar] [CrossRef]
- Liu, C.; Ni, C.; Yao, J.; Chang, Z.; Wang, Z.; Zeng, G.; Luo, X.; Yang, L.; Ren, Z.; Shao, P.; et al. Hydroxypropyl amine surfactant: A novel flotation collector for efficient separation of scheelite from calcite. Miner. Eng. 2021, 167, 106898. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Gao, Y.; Hu, Y.; Sun, W. Flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Miner. Eng. 2016, 98, 261–263. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Huang, G.; Li, C. Low-temperature performance of cationic collector undecyl propyl ether amine for ilmenite flotation. Miner. Eng. 2017, 114, 50–56. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Liu, R. Interactions Between Sodium Oleate and Polyoxyethylene Ether and the Application in the Low-Temperature Flotation of Scheelite at 283 K. J. Surfactants Deterg. 2016, 19, 1289–1295. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, H.; Sun, W.; Hu, Y.; Qin, W.; Liu, R. Synergetic Effect of the Mixed Anionic/Non-Ionic Collectors in Low Temperature Flotation of Scheelite. Minerals 2017, 7, 87. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Gu, G.; Yan, W.; Wang, X.; Wang, C.; Xu, L. Flotation of fine pyrite by using N-dodecyl mercaptan as collector in natural pH pulp. J. Mater. Res. Technol. 2019, 8, 1571–1575. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Liu, C.; Soraya, D.A.D.; Li, C.; Li, H. Investigations on the synergistic effect of combined NaOl/SPA collector in ilmenite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127267. [Google Scholar] [CrossRef]
- Jiao, F.; Wu, J.; Qin, W.; Wang, X.; Liu, R. Interactions of tert dodecyl mercaptan with sphalerite and effects on its flotation behavior. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 104–113. [Google Scholar] [CrossRef]
- Yang, Z.; Teng, Q.; Liu, J.; Yang, W.; Hu, D.; Liu, S. Use of NaOL and CTAB mixture as collector in selective flotation separation of enstatite and magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 481–486. [Google Scholar] [CrossRef]
- Man, X.; Ou, L.; Wang, C.; Jin, S.; Ma, X. Flotation Separation of Diaspore and Kaolinite by Using a Mixed Collector of Sodium Oleate-Tert Dodecyl Mercaptan. Front. Chem. 2019, 7, 813. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Xiong, W.; Deng, J.; Chen, B.; Deng, S.; Wei, D. Flotation-magnetic separation for the beneficiation of rare earth ores. Miner. Eng. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- Jiang, Y.-R.; Li, W.; Feng, R. Preparation and performance of 4-alkyl-4,4-bis(hydroxycarbamoyl) carboxylic acid for flotation separation of diaspore against aluminosilicates. Miner. Eng. 2011, 24, 1571–1579. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Z.; Xu, L.; Hu, Y.; Huang, K.; Zhu, S. A comparison study of the flotation and adsorption behaviors of diaspore and kaolinite with quaternary ammonium collectors. Miner. Eng. 2014, 65, 124–129. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Zhong, H.; Liu, G. A novel surfactant 2-amino-6-decanamidohexanoic acid: Flotation performance and adsorption mechanism to diaspore. Miner. Eng. 2016, 93, 16–23. [Google Scholar] [CrossRef]









| Sample | Al2O3 | SiO2 | TiO2 | Fe2O3 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| Diaspore | 80.77 | 0.89 | 2.76 | 0.756 | 0.01 | 0.053 | 0.008 | 0.025 |
| Kaolinite | 33.68 | 45.44 | 0.97 | 0.88 | 0.24 | 0.077 | 0.89 | - |
| Surfactants | CMC(mol/L) | (mN/m) | (mol/m2) | ||
|---|---|---|---|---|---|
| NaOl | 2 × 10−3 | 25.87 | 8.84 × 10−7 | 1.88 | 14.62 |
| TDM | 8 × 10−3 | 45.61 | 2.01 × 10−6 | 0.83 | 11.36 |
| NaOl/TDM | 1 × 10−3 | 25.13 | 1.55 × 10−6 | 1.07 | 16.25 |
| Samples | Atomic Concentration of Elements (Atomic %) | ||||
|---|---|---|---|---|---|
| C | O | Al | Si | S | |
| Diaspore | 18.58 | 57.37 | 19.71 | 4.33 | - |
| Diaspore + NaOl | 47.51 | 36.22 | 12.96 | 3.31 | - |
| ∆a | 28.93 | −21.15 | −6.75 | −1.02 | - |
| Diaspore | 18.58 | 57.37 | 19.71 | 4.33 | - |
| Diaspore + NaOl/TDM | 53.42 | 32.52 | 11.45 | 2.45 | 0.17 |
| ∆a | 34.84 | −24.85 | −8.26 | −1.88 | - |
| Kaolinite | 10.14 | 61.38 | 13.21 | 15.27 | - |
| Kaolinite + NaOl | 21.26 | 53.16 | 12.16 | 13.42 | - |
| ∆a | 11.12 | −8.22 | −1.05 | −1.85 | - |
| Kaolinite | 10.14 | 61.38 | 13.21 | 15.27 | - |
| Kaolinite + NaOl/TDM | 21.29 | 52.83 | 12.34 | 13.47 | 0.08 |
| ∆a | 11.15 | −8.55 | −0.87 | −1.80 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, X.; Wang, C.; Yu, S.; Yang, X.; Liu, J.; Fu, Y.; Dong, Z.; Zhi, H.; Ou, L. Low-Temperature Flotation Separation of Diaspore from Kaolinite by Using a Mixed Collector. Minerals 2022, 12, 891. https://doi.org/10.3390/min12070891
Man X, Wang C, Yu S, Yang X, Liu J, Fu Y, Dong Z, Zhi H, Ou L. Low-Temperature Flotation Separation of Diaspore from Kaolinite by Using a Mixed Collector. Minerals. 2022; 12(7):891. https://doi.org/10.3390/min12070891
Chicago/Turabian StyleMan, Xiaofei, Chenliang Wang, Shichao Yu, Xiaofeng Yang, Jianjun Liu, Yafeng Fu, Zhenhai Dong, Hui Zhi, and Leming Ou. 2022. "Low-Temperature Flotation Separation of Diaspore from Kaolinite by Using a Mixed Collector" Minerals 12, no. 7: 891. https://doi.org/10.3390/min12070891
APA StyleMan, X., Wang, C., Yu, S., Yang, X., Liu, J., Fu, Y., Dong, Z., Zhi, H., & Ou, L. (2022). Low-Temperature Flotation Separation of Diaspore from Kaolinite by Using a Mixed Collector. Minerals, 12(7), 891. https://doi.org/10.3390/min12070891






