Exploratory Study of Separation of Sulphidised Chrome Spinels from Reduced Ilmenite
Abstract
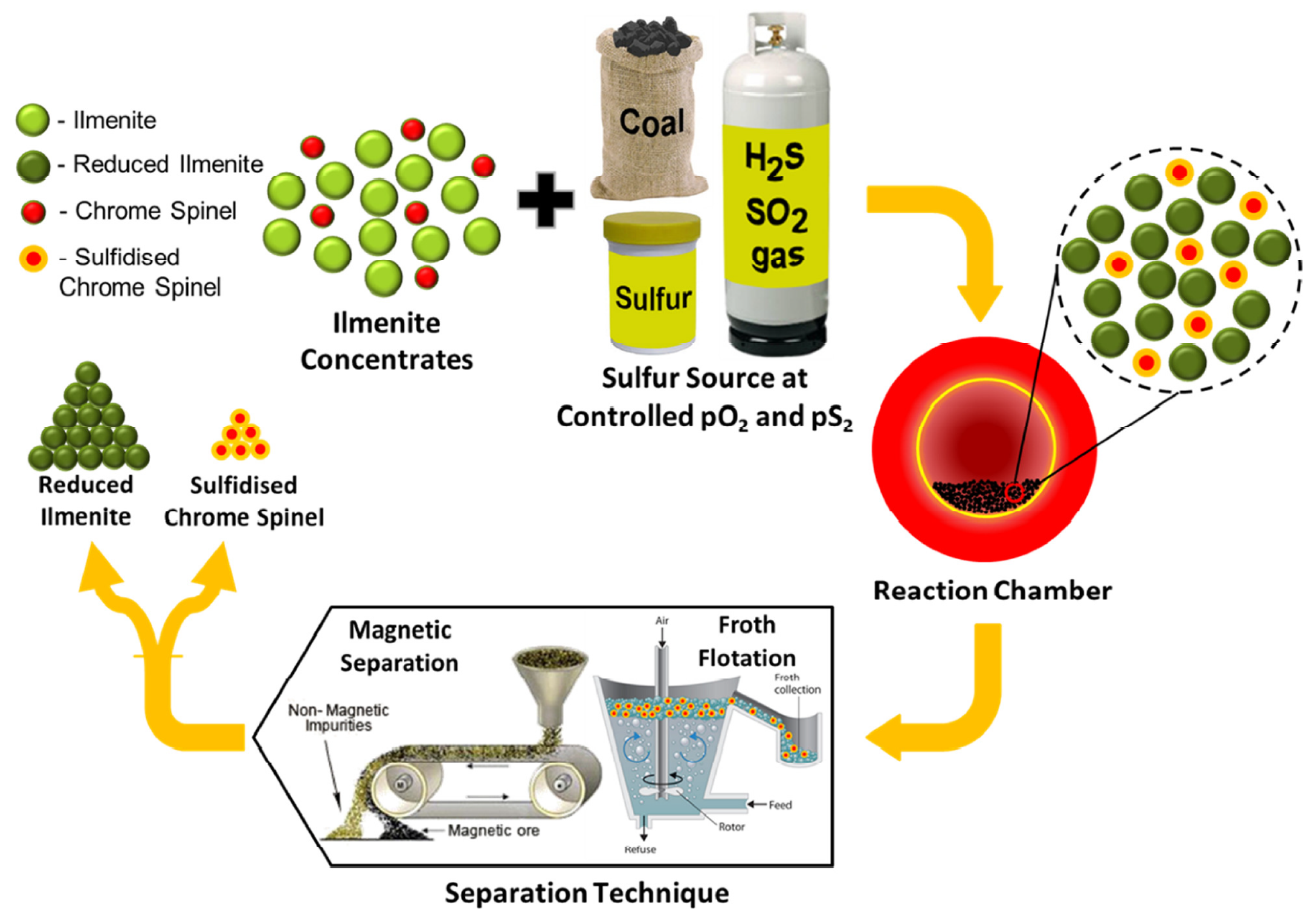
:1. Introduction
2. Experimental
2.1. Samples and Experimental Plan
2.2. Experimental Procedures
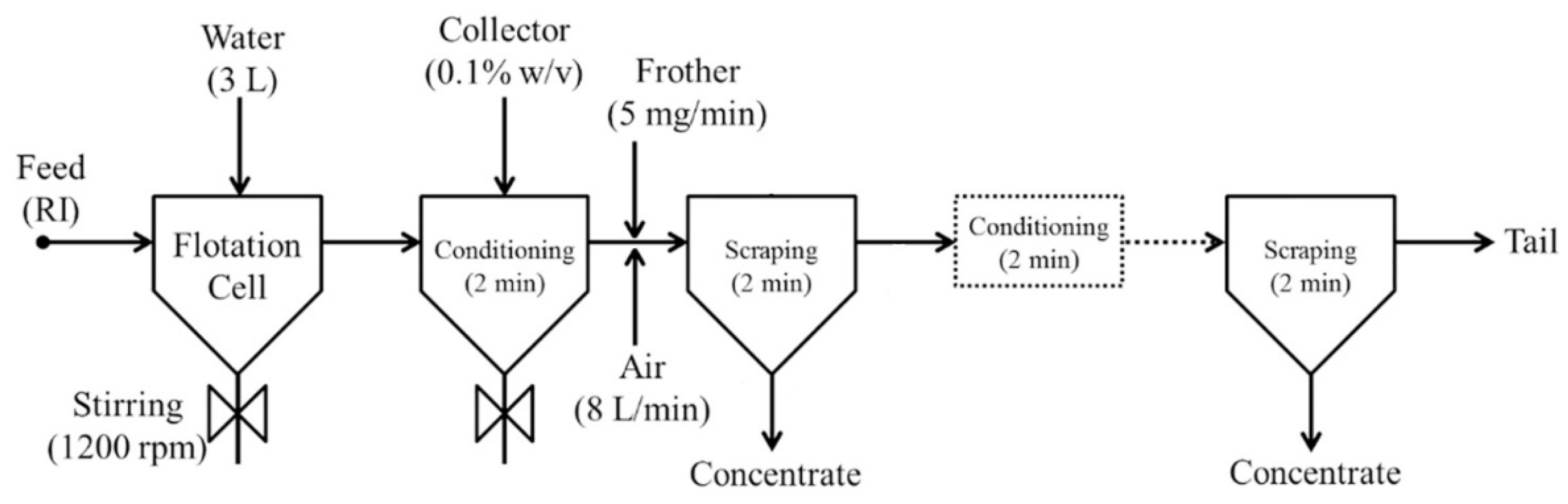
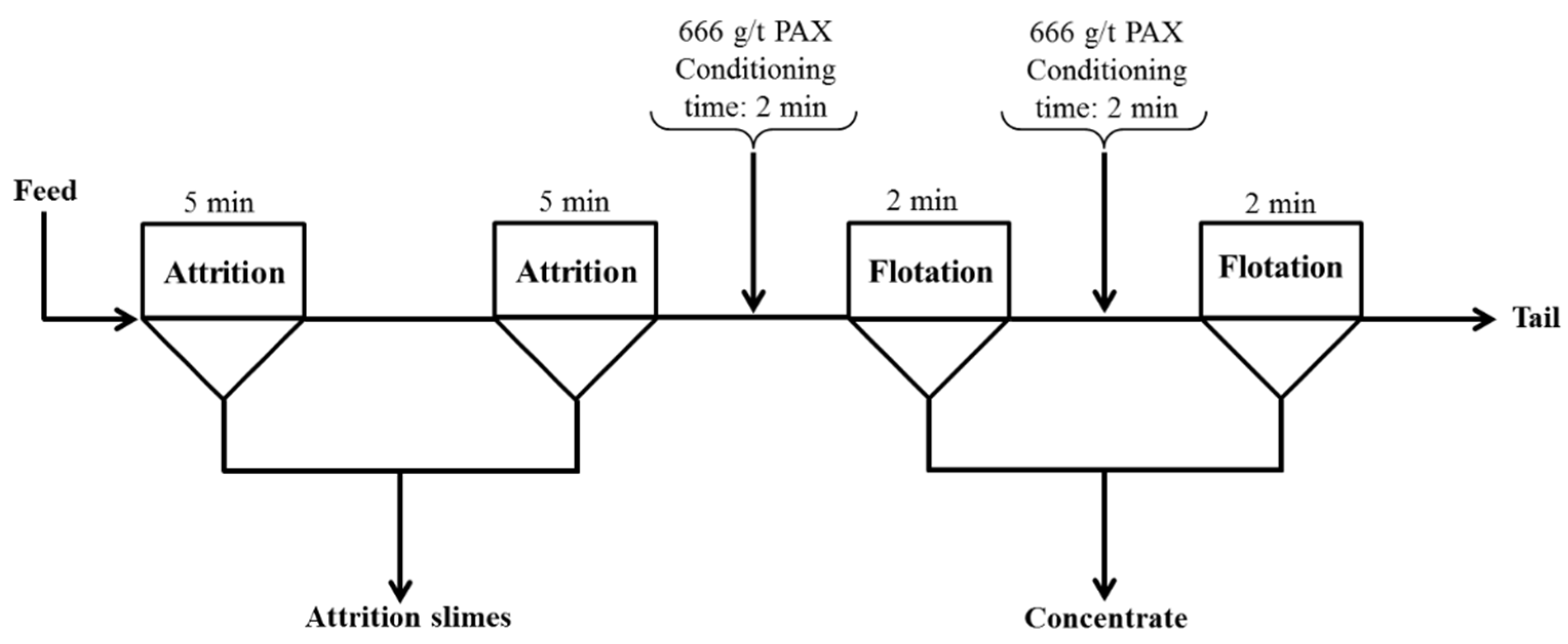
2.2.1. Froth Flotation
2.2.2. Attrition and Flotation
2.2.3. Grinding and Flotation
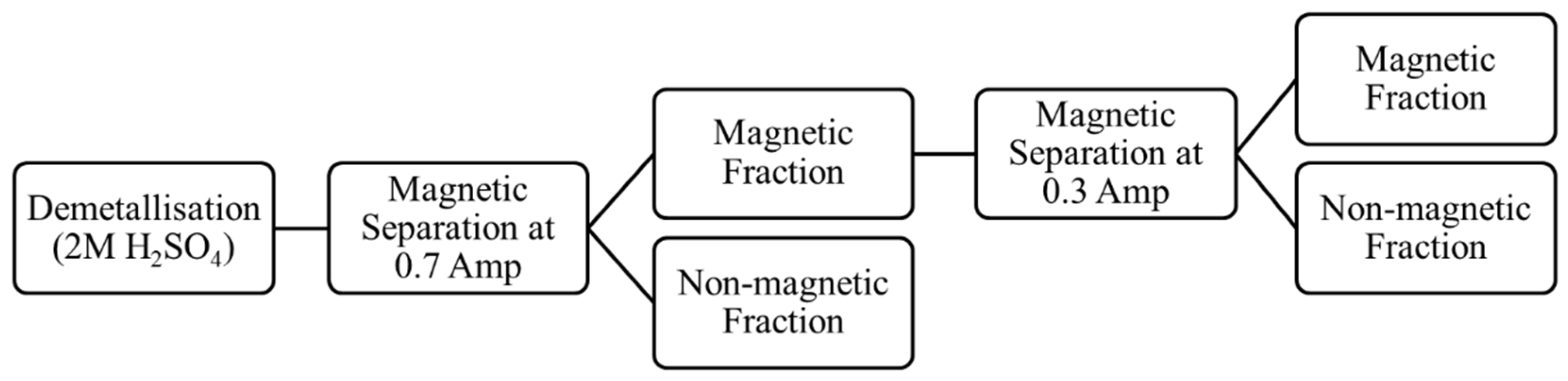
2.2.4. Demetallisation and Magnetic Separation
3. Results and Discussion
3.1. Flotation Tests
3.2. Demetallisation and Magnetic Separation Tests
4. Conclusions
- (1)
- The effect of residual char on the feed material during flotation was not apparent in this study. The results of Flotation Test-1 and Flotation Test-2 showed that the concentrates, tail weight splits and compositions were nearly the same in both cases. It is, however, recommended to remove the char from the feed sample prior to flotation to minimise the potential of any unwanted reagent adsorption (loss).
- (2)
- Changing the collector to a stronger xanthate collector (SEX to PAX) had a negligible impact on the chrome spinel recovery.
- (3)
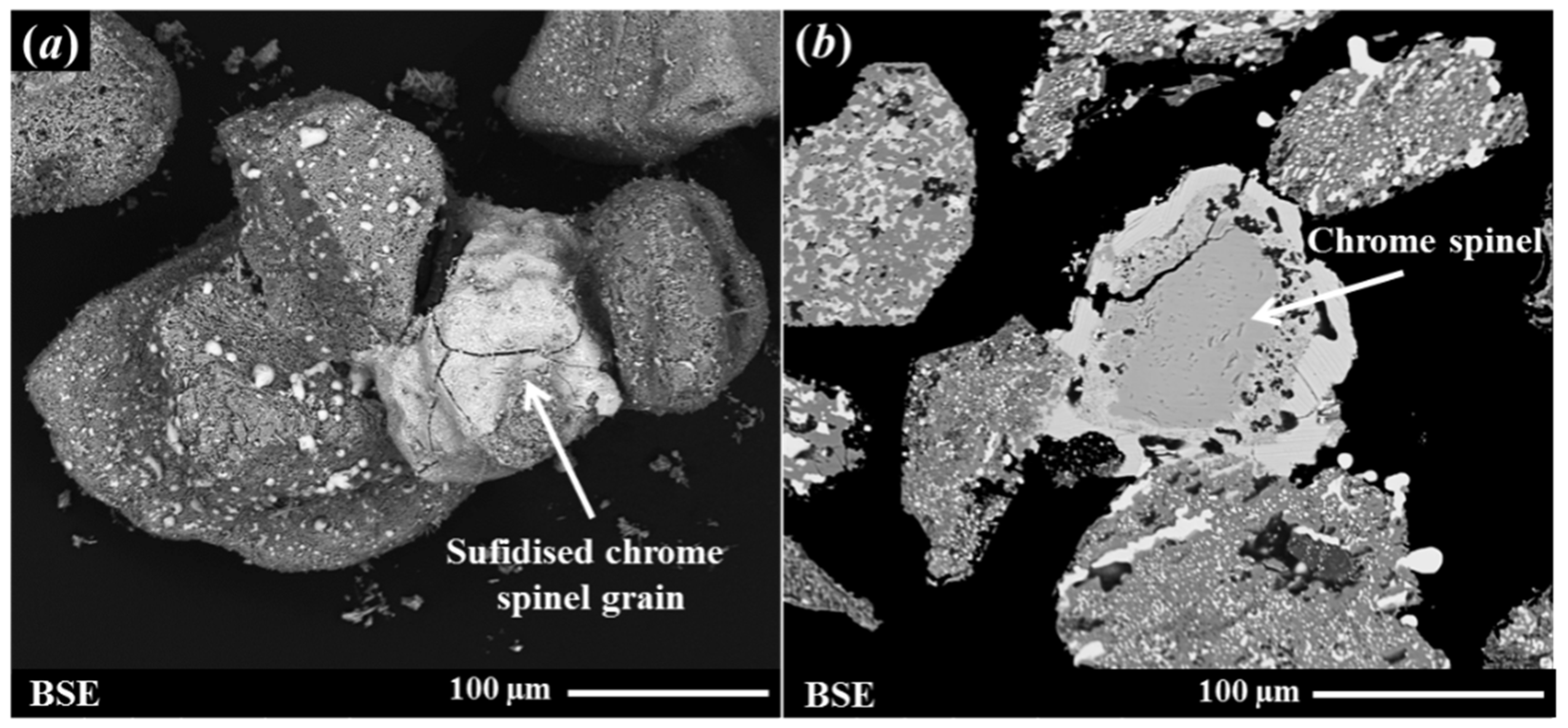
- Clustering or fusing of the reduced ilmenite and sulphidised chrome spinel grains produces a flotation feed containing composite particles, which limits the flotation effectiveness. A simple attrition process was ineffective in breaking up the clustered/fused particles; however, a light wet grind was more effective in breaking the fused grains. The latter process provided a good flotation feed size distribution, but it caused the sulphide rim to spall from chrome spinel surfaces, which also reduces the flotation efficiency. The bulk Cr2O3 level remained unchanged in this test. However, the size reduction led to a slightly more highly concentrated mass pull.
- (4)
- A definitive demonstration of flotation as a process for removing sulphidised chrome spinel was not proven effective under the conditions studied.
- (5)
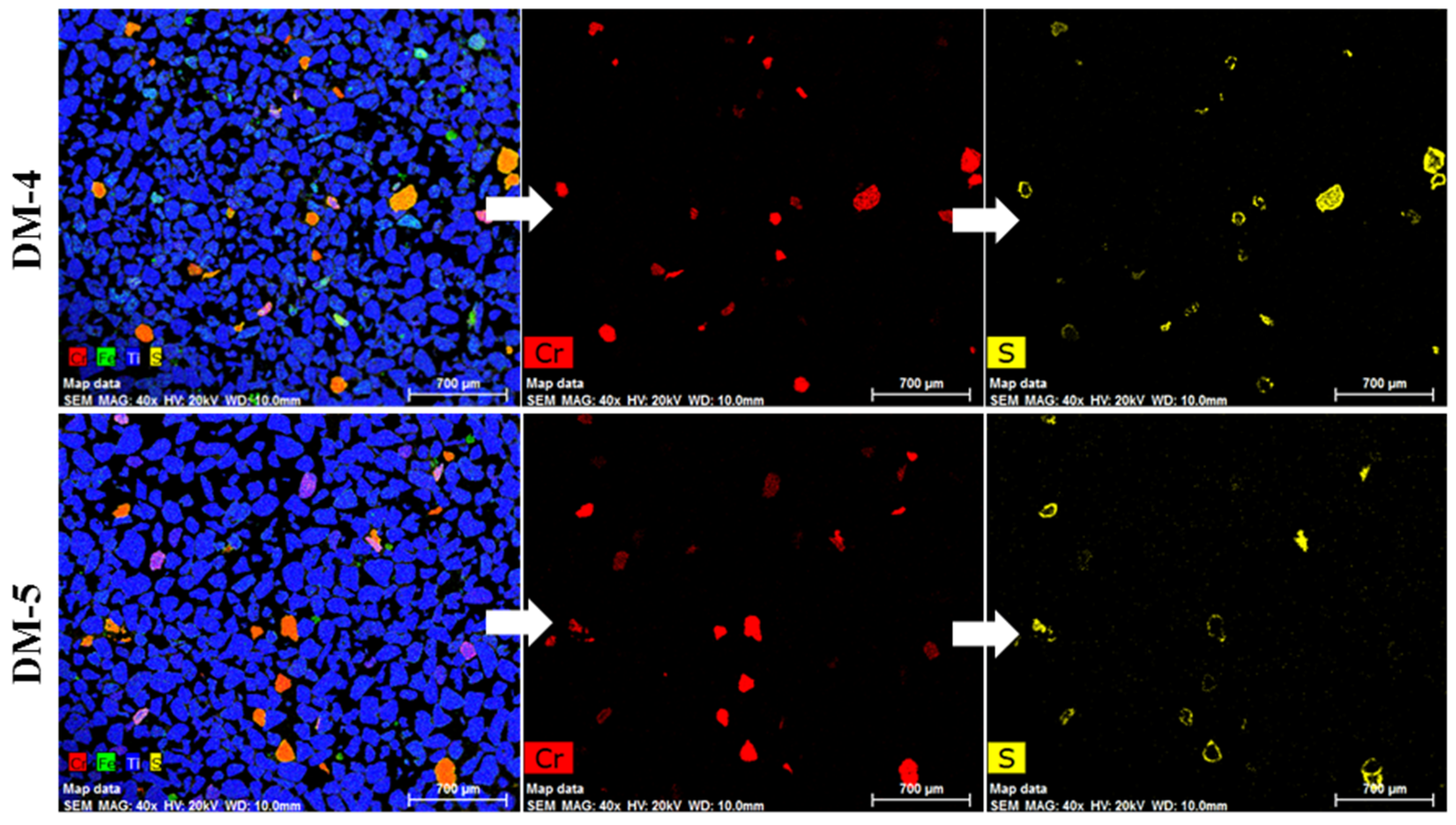
- The preliminary results obtained after a magnetic separation of a demetallised sample showed that the chrome spinel preferentially reported to the magnetic fraction (at 0.7 A). An additional magnetic separation of the non-magnetic fraction at 0.3 A improved the recovery of sulphidised chrome spinels.
- (6)
- The demetallisation process followed by a magnetic separation provided insight into a potential route for the removal of chrome spinel from reduced ilmenite. However, further studies are required to optimise process parameters, such as the concentration of leachate, leaching time and different current settings for the magnetic separation.
- (7)
- The hot-acid leaching in 2M H2SO4 at 60 °C helped to reduce the Cr2O3 content from 1.67 to 0.55%, increase the TiO2 content up to 91.1% and reach a dissolution of 90.6% Fe2O3.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitehouse, J.; Roy, P.S.; Oakes, G.M. Mineral Sands Resource Potential of the Murray Basin; Geological Survey of New South Wales Report GS 1999/038; Geological Survey of NSW: Maitland, NSW, Australia, 1999. [Google Scholar]
- Roy, P.S.; Whitehouse, J.; Cowell, P.J.; Oakes, G.M. Mineral sands occurrences in the Murray Basin, Southeastern Australia. Econ. Geol. 2000, 95, 1107–1128. [Google Scholar] [CrossRef]
- Pownceby, M.I. Alteration and associated impurity element enrichment in detrital ilmenites from the Murray Basin, SE Australia: A product of multistage alteration. Aust. J. Earth Sci. 2010, 57, 243–258. [Google Scholar] [CrossRef]
- Ahmad, S.; Rhamdhani, M.A.; Pownceby, M.I.; Bruckard, W.J. Thermodynamic assessment and experimental study of sulphidation of ilmenite and chromite. Trans. Inst. Min. Metall. 2014, 123, 165–177. [Google Scholar] [CrossRef]
- Ahmad, S.; Rhamdhani, M.A.; Pownceby, M.I.; Bruckard, W.J. Selective Sulphidising Roasting for the Removal of Chrome Spinel Impurities from Weathered Ilmenite Ore. Int. J. Miner. Proc. 2016, 146, 29–37. [Google Scholar] [CrossRef]
- Rhamdhani, M.A.; Ahmad, S.; Pownceby, M.I.; Bruckard, W.J.; Harjanto, S. Selective Sulphidation of Impurities in Weathered Ilmenite. Part 1—Applicability to Different Ilmenite Deposits and Simulated Becher Kiln Conditions. Miner. Eng. 2018, 121, 55–65. [Google Scholar] [CrossRef]
- Pownceby, M.; MacRae, C.M.; Wilson, N.C. Electron microprobe mapping—A diagnostic tool for ilmenite characterisation. In Proceedings of the 3rd International Heavy Minerals Conference, Fremantle, WA, Australia, 18–19 June 2001; pp. 69–74. [Google Scholar]
- Pownceby, M.I.; Fisher-White, M.J. Chemical variability in chrome spinel grains from magnetically fractionated ilmenite concentrates: Implications for processing. Trans. Inst. Min. Metall. 2006, 115, 213–223. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Pownceby, M.I.; Smith, L.K.; Sparrow, G.J. Review and assessment of processing conditions for Murray Basin ilmenite concentrates. Trans. Inst. Min. Metall. 2015, 124, 47–63. [Google Scholar]
- Pownceby, M.I. Compositional and textural variation in detrital chrome-spinels from the Murray Basin, southeastern Australia. Mineral. Mag. 2005, 69, 191–204. [Google Scholar] [CrossRef]
- Becher, R.G.; Canning, R.G.; Goodheart, B.A.; Uusna, S. A new process for upgrading ilmenitic mineral sands. Aust. Inst. Min. Metall. Proc. 1965, 214, 21–44. [Google Scholar]
- Iluka Resources. 2022, Operations. Available online: https://www.iluka.com/operations-resource-development/operations (accessed on 26 August 2022).
- Heyes, G.; Bruckard, W.; Smith, L. Flotation of chromite—A review with applications to upgrading chromium contaminated ilmenite. In Proceedings of the International Heavy Minerals Conference, Fremantle, WA, Australia, 18–19 June 2001; The Australasian Institute of Mining and Metallurgy: Melbourne, VIC, Australia, 2001; pp. 137–142. [Google Scholar]
- Smith, L.K.; Bruckard, W.J. Flotation of Chrome Spinels from Ilmenite—Summary Report. 2002; unpublished data. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents, Chemistry, Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 175–207. [Google Scholar]
- Yosida, K. On the magnetic properties of pyrrhotite, chromium sulphide and α-hematite. Physica 1951, 17, 794–796. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; McLean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
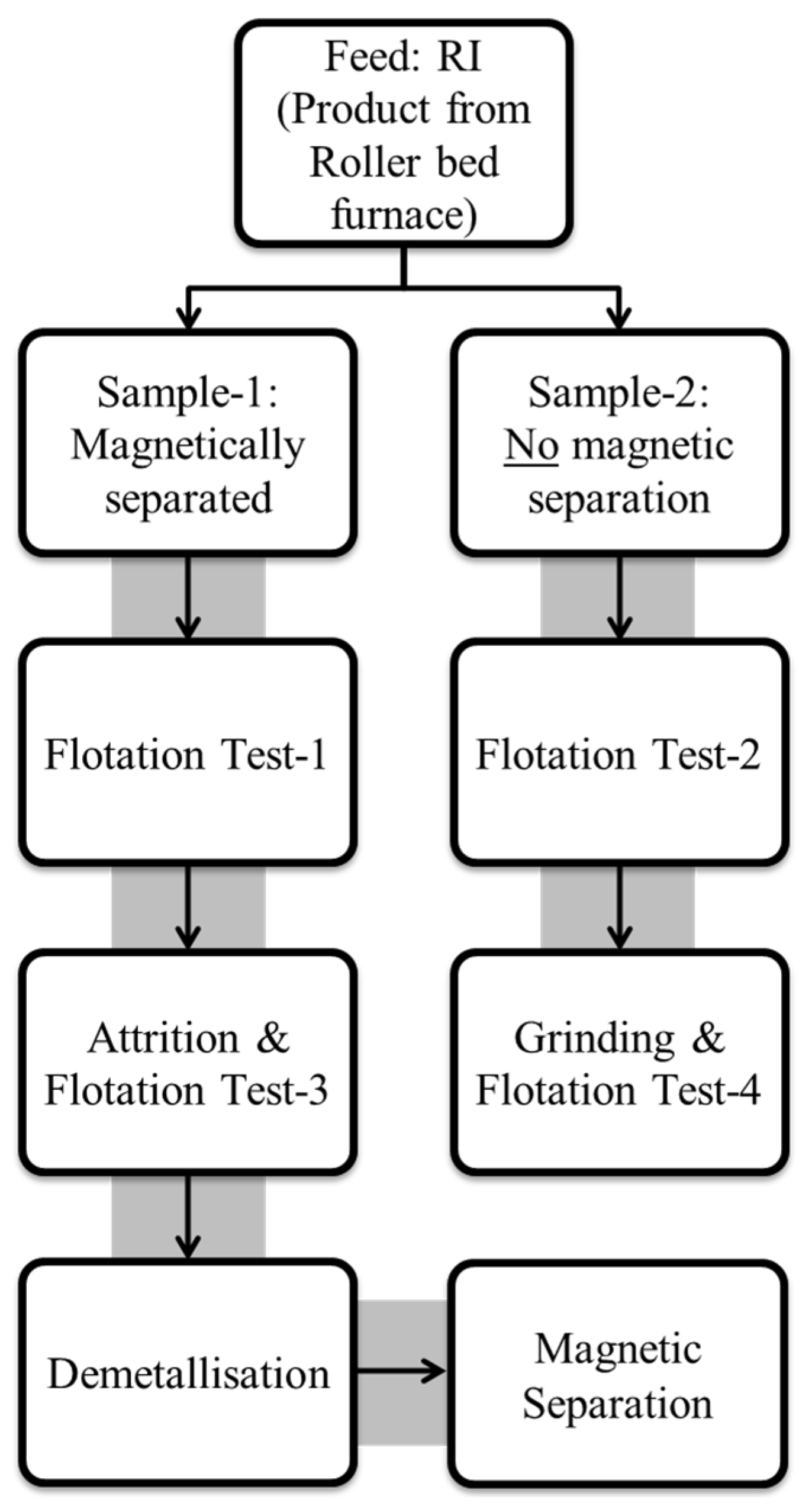

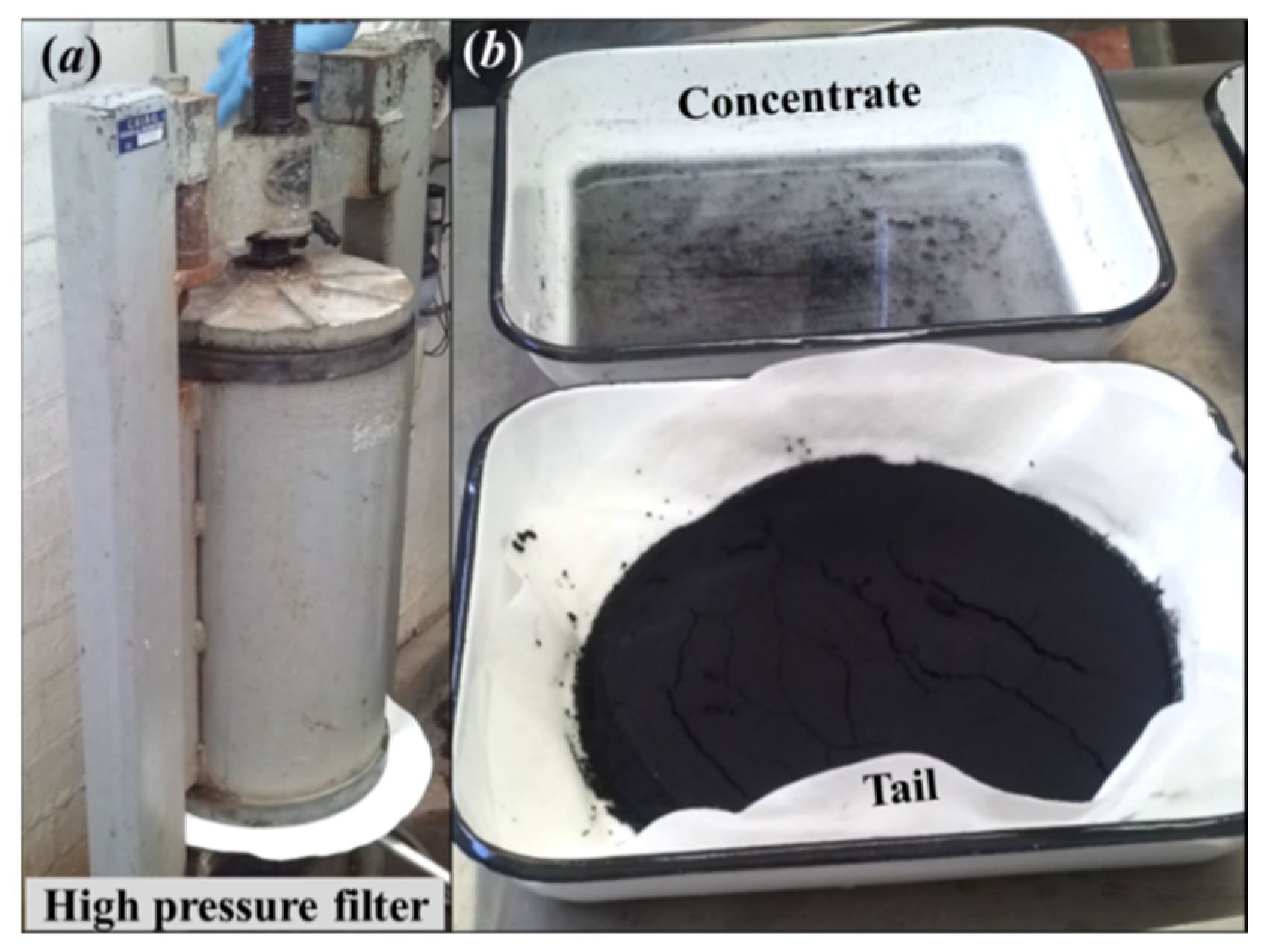
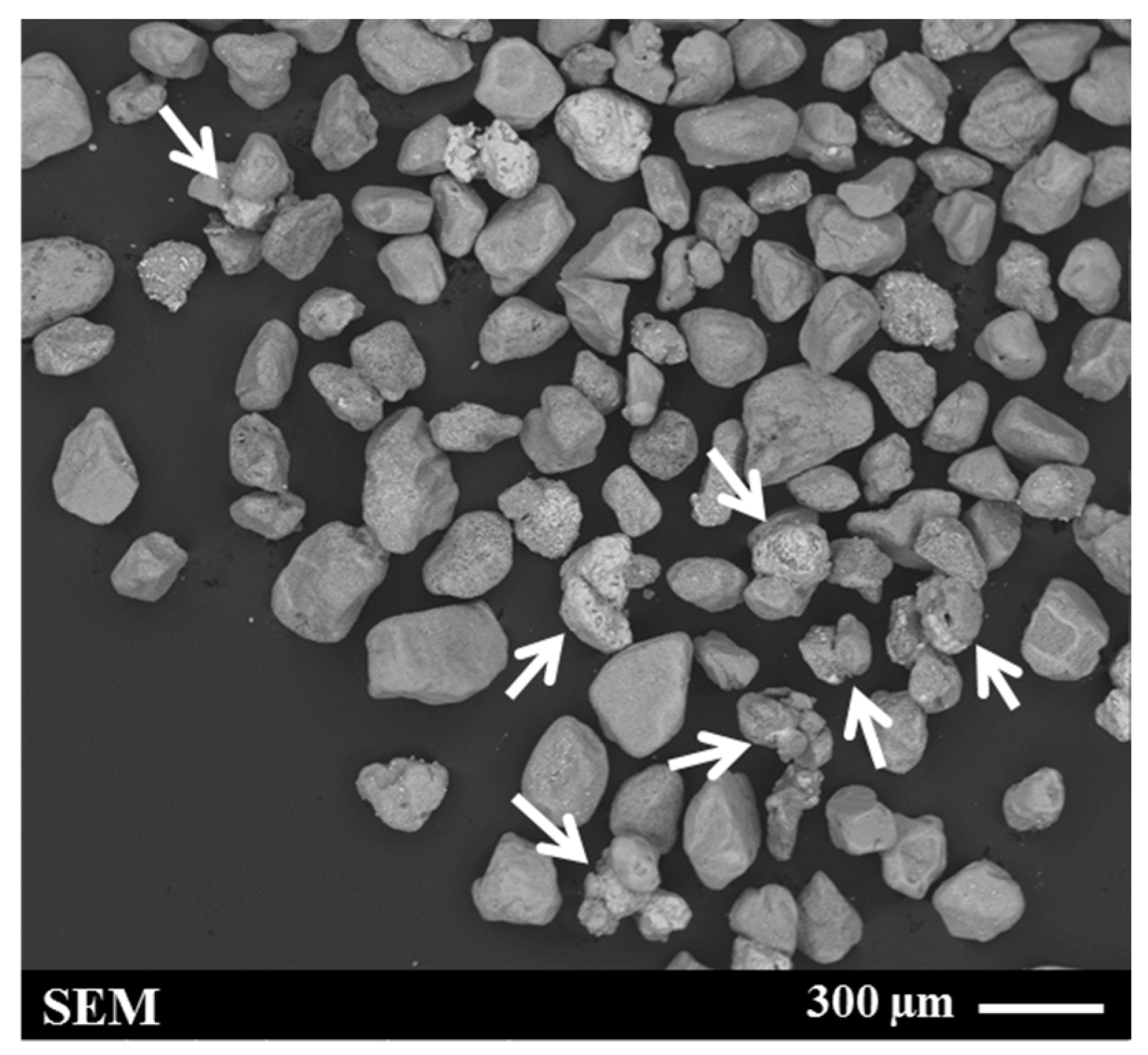
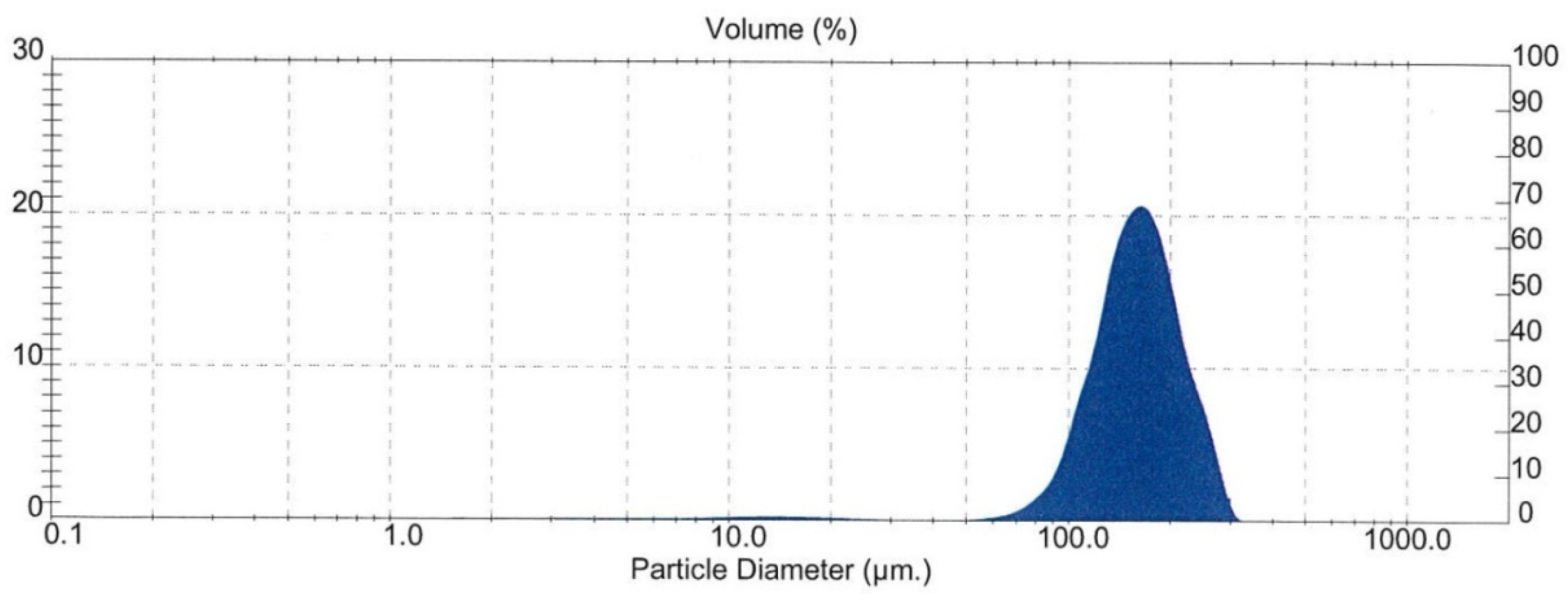
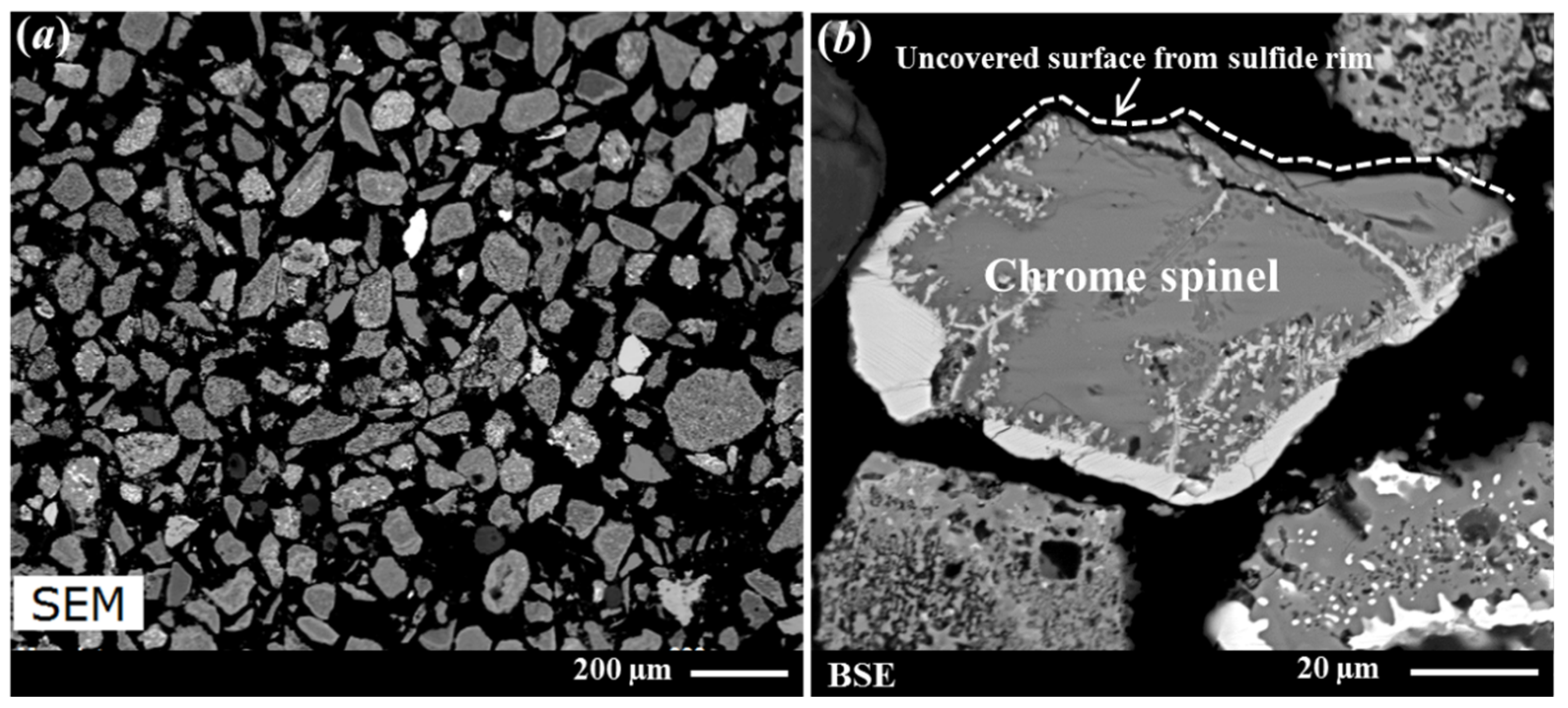


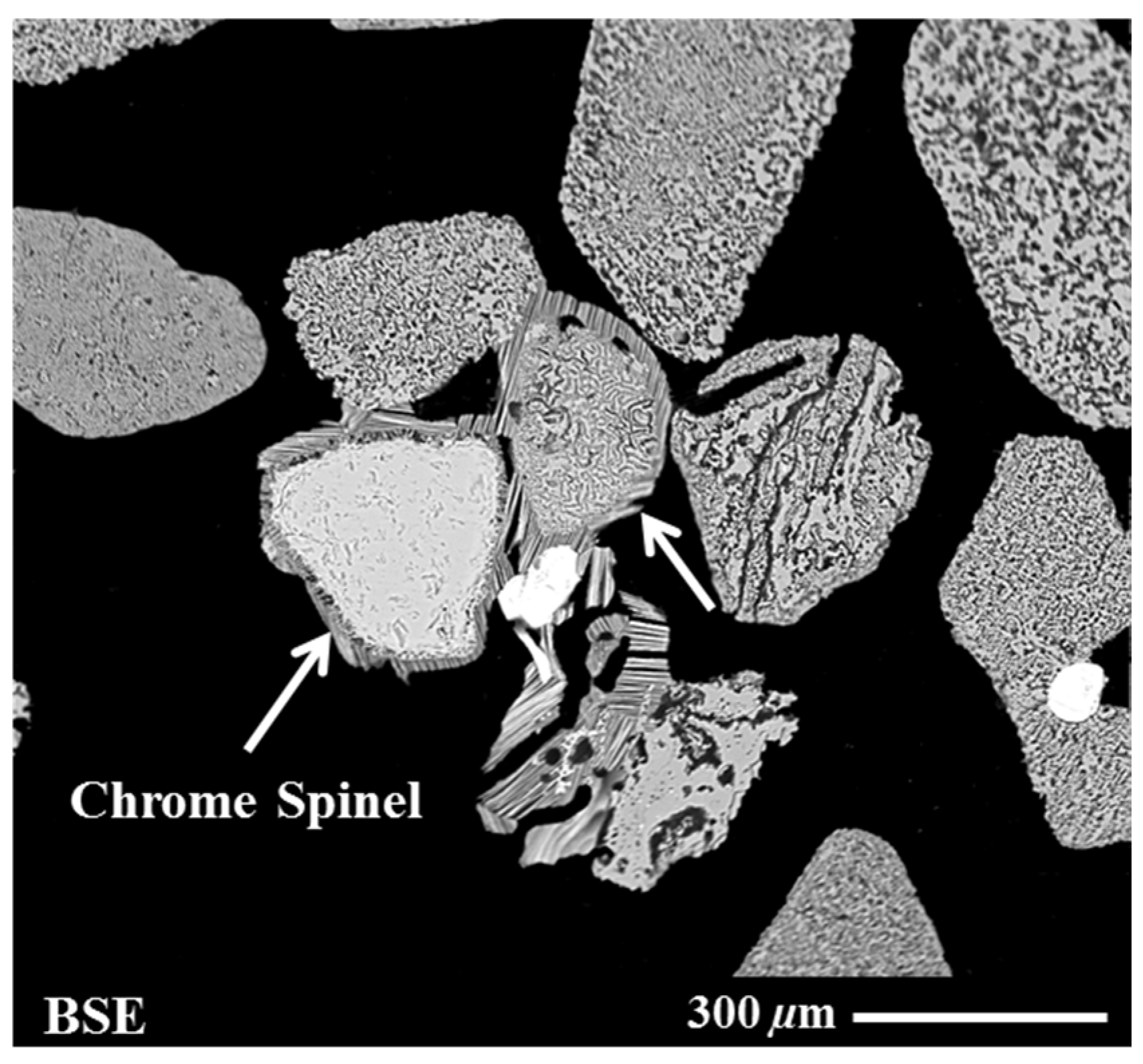

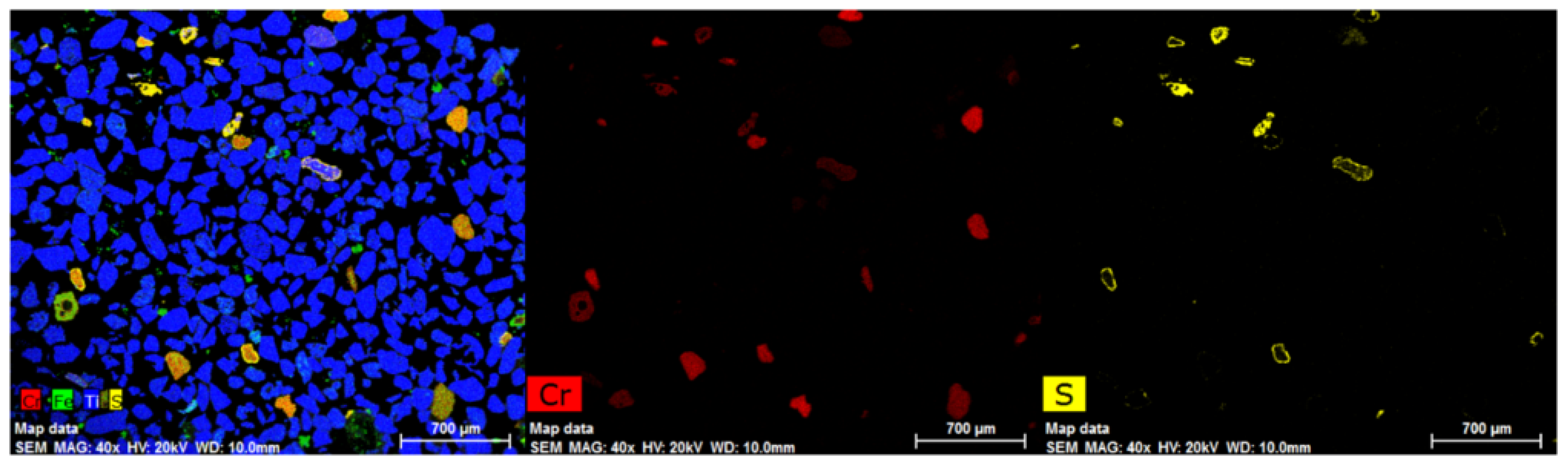

| Sample Origin | Sample ID | Experiment | Flotation Feed Material |
|---|---|---|---|
| RI (MBI-4) | Sample-1 | Flotation Test-1 | Magnetically separated RI |
| Sample-2 | Flotation Test-2 | RI with no magnetic separation | |
| Sample-3 | Flotation Test-3 | Tail sample from Flotation Test-1 | |
| Sample-4 | Flotation Test-4 | Tail sample from Flotation Test-2 |
| Sample Origin | Sample ID | Sample Description | Current (A) |
|---|---|---|---|
| Tail sample from Flotation Test-3 | DM-1 | Demetallised and magnetically separated | 0.7 |
| DM-2 | The magnetic fraction from DM-1 | 0.7 | |
| DM-3 | The non-magnetic fraction from DM-1 | 0.7 | |
| DM-4 | The magnetic fraction from DM-2 | 0.3 | |
| DM-5 | The non-magnetic fraction from DM-2 | 0.3 |
| Sample ID | pH | Flotation Time | Product | Weight (g) | Weight (%) |
|---|---|---|---|---|---|
| Flotation Test-1 | 9.1 | 10 min | Conc. | 0.18 | 0.06 |
| Tail | 311.34 | 99.94 | |||
| Flotation Test-2 | 9.3 | 10 min | Conc. | 0.15 | 0.05 |
| Tail | 306.68 | 99.95 | |||
| Flotation Test-3 | 8.3 | 10 min | Conc. | 1.30 | 0.45 |
| Tail | 290.20 | 99.55 | |||
| Flotation Test-4 | 8.5 | 10 min | Conc. | 3.66 | 1.29 |
| Tail | 280.34 | 98.71 |
| Samples | Cr2O3 | Fe2O3 * | TiO2 | MgO | Al2O3 | SiO2 | SO3 | MnO |
|---|---|---|---|---|---|---|---|---|
| MBI-4-RI | 1.50 | 35.4 | 65.6 | 1.31 | 1.61 | 1.43 | 2.87 | 0.94 |
| Flotation Test-1 | 1.41 | 34.2 | 63.9 | 1.26 | 1.53 | 1.42 | 3.82 | 0.90 |
| Flotation Test-2 | 1.59 | 34.3 | 63.1 | 1.28 | 1.54 | 1.53 | 3.04 | 0.91 |
| Flotation Test-3 | 1.67 | 33.3 | 63.0 | 1.28 | 1.58 | 1.40 | 4.18 | 0.92 |
| Flotation Test-4 | 1.42 | 33.5 | 61.0 | 1.23 | 1.50 | 1.56 | 3.70 | 0.88 |
| Sample | TiO2 | Fe2O3 * | Cr2O3 | MgO | Al2O3 | SiO2 | ZrO2 | SO3 | MnO |
|---|---|---|---|---|---|---|---|---|---|
| Tail of Float Test-3 | 63.0 | 33.3 | 1.67 | 1.28 | 1.58 | 1.40 | - | 4.18 | 0.92 |
| DM--1 | 89.6 | 3.13 | 1.67 | 1.79 | 1.98 | 1.61 | 0.53 | 0.37 | 0.69 |
| DM-2 | 88.8 | 3.86 | 1.86 | 1.86 | 2.14 | 1.64 | 0.20 | 0.53 | 0.75 |
| DM-3 | 91.1 | 1.26 | 0.55 | 0.52 | 1.82 | 3.85 | 1.02 | 0.19 | 0.31 |
| DM-4 | 85.4 | 4.97 | 2.29 | 2.71 | 2.22 | 1.70 | 0.25 | 0.46 | 0.98 |
| DM-5 | 93.0 | 1.76 | 1.41 | 0.91 | 1.87 | 1.18 | 0.18 | 0.37 | 0.47 |
| Sample DM-1 | Magnetic Fraction (DM-2) | Non-Magnetic Fraction (DM-3) |
| 30.72 g | 9.64 g |
| Sample DM-2 | Magnetic Fraction (DM-4) | Non-Magnetic Fraction (DM-5) |
| 16.32 g | 14.32 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Rhamdhani, M.A.; Pownceby, M.I.; Bruckard, W.J. Exploratory Study of Separation of Sulphidised Chrome Spinels from Reduced Ilmenite. Minerals 2022, 12, 1252. https://doi.org/10.3390/min12101252
Ahmad S, Rhamdhani MA, Pownceby MI, Bruckard WJ. Exploratory Study of Separation of Sulphidised Chrome Spinels from Reduced Ilmenite. Minerals. 2022; 12(10):1252. https://doi.org/10.3390/min12101252
Chicago/Turabian StyleAhmad, Sazzad, Muhammad A. Rhamdhani, Mark I. Pownceby, and Warren J. Bruckard. 2022. "Exploratory Study of Separation of Sulphidised Chrome Spinels from Reduced Ilmenite" Minerals 12, no. 10: 1252. https://doi.org/10.3390/min12101252
APA StyleAhmad, S., Rhamdhani, M. A., Pownceby, M. I., & Bruckard, W. J. (2022). Exploratory Study of Separation of Sulphidised Chrome Spinels from Reduced Ilmenite. Minerals, 12(10), 1252. https://doi.org/10.3390/min12101252








