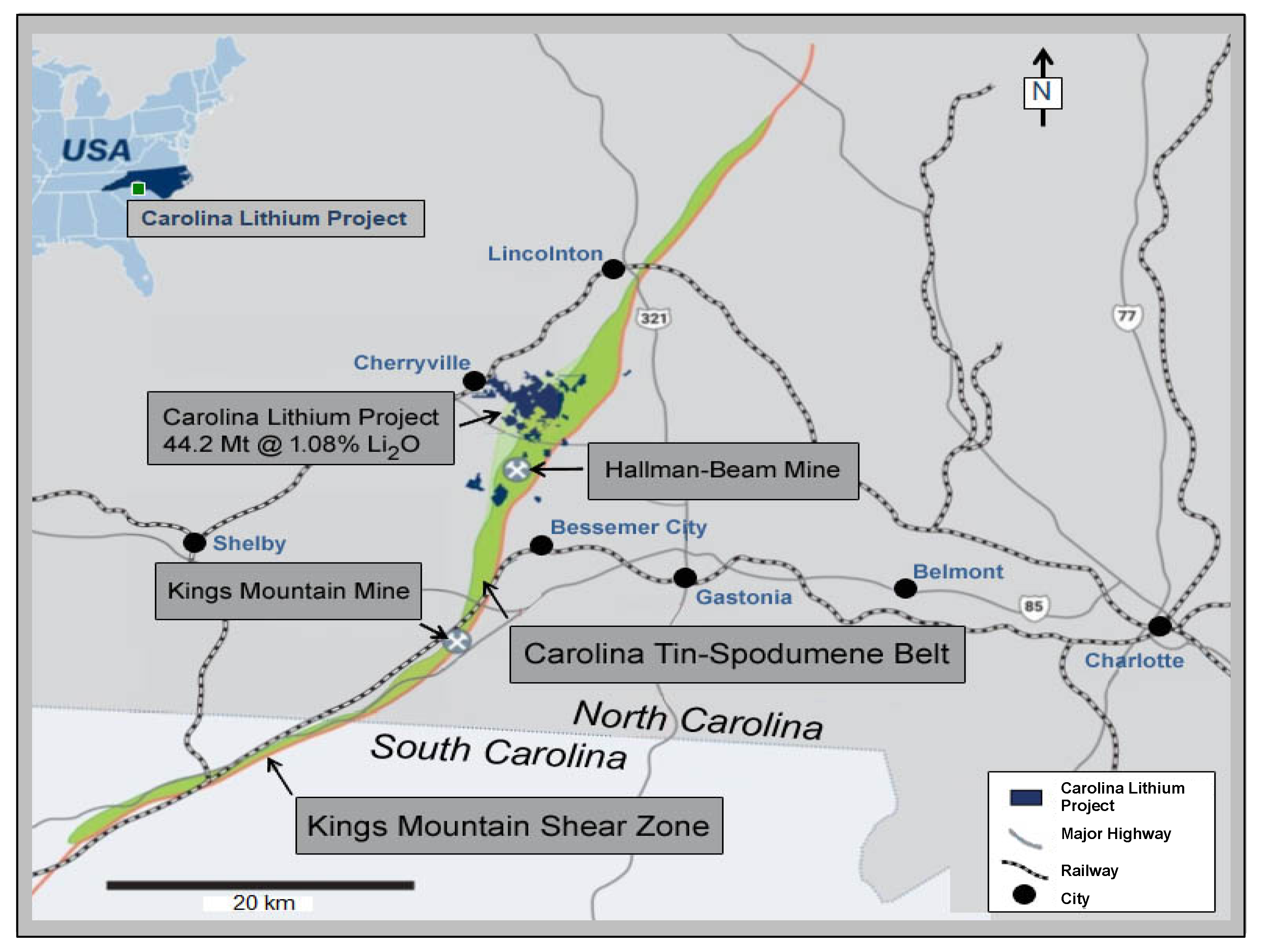
Exploration programs for rare-element granitic pegmatites typically utilize an integrated geological, mineralogical, and geochemical approach for identifying exposed and buried pegmatites of economic interest. Rock and mineral geochemistry has proven to be extremely effective in differentiating barren pegmatites lacking rare-element minerals from pegmatites that carry significant Be, Nb, Ta, Sn, or Li mineralization. The trace element content of pegmatite feldspars and micas have been proven to be useful markers for distinguishing chemically primitive pegmatites from moderately to highly evolved rare-element enriched pegmatites [
16]. In a pegmatite field or district, where tens to hundreds of mineralogically diverse pegmatite bodies may be present, the inexpensive and rapid analysis of Li, K, Rb, and Cs in feldspar and muscovite by handheld LIBS can be a unique tool for identifying prospective Li-enriched pegmatites in the field during an exploration program.
The wallrock of some LCT pegmatites may develop exomorphic halos enriched in Li, Rb, Cs, B, and Be via interaction with pegmatite-derived fluids [
64,
65]. The occurrence of exomorphic minerals, such as holmquistite, biotite, tourmaline, and emerald, in amphibolitic and schistose wallrock surrounding pegmatites attest to episodes of metasomatic alteration, which have been shown in some cases to be a useful tool in pegmatite exploration. LIBS analysis of alteration assemblages in wallrocks surrounding rare-element-enriched pegmatites has the potential to be an integral part of any pegmatite exploration program aimed at targeting Li-enriched pegmatites.
6.1. Elemental Detection
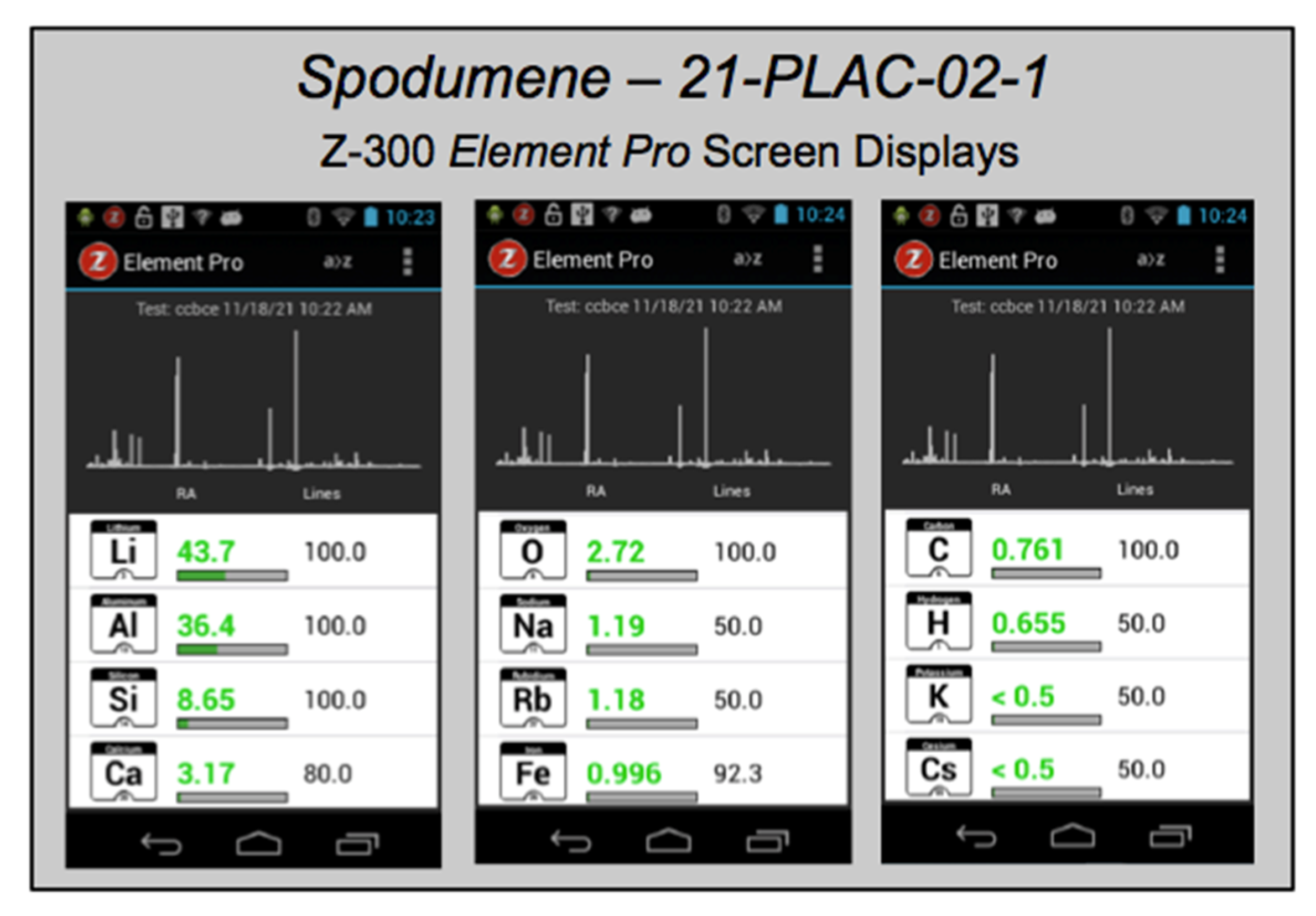
For rapid qualitative analysis, the Z-300 is used in the
Element Pro mode for element detection and identification. Relative emission strengths for each emission line in an acquired broadband LIBS spectrum are interrogated and compared with an onboard spectral library of selected elemental emission lines for the entire periodic table derived from the NIST atomic spectra database [
69]. After each analysis, the list of elements identified in the sample is displayed (
Figure 5), accompanied by a “likelihood” ranking that is a measure of the ratio of the number of elemental emission lines present in an acquired spectrum to the number of lines for each element in the spectral library and an estimated elemental “relative abundance” comparing how much of an element is present in the sample compared to other elements, with the caveat that there is no direct correlation between relative abundance and absolute element concentration. Used in this way, handheld LIBS analysis can be employed in the field to (i) detect the main elements present in a rock, mineral, or soil; (ii) rapidly distinguish between minerals of similar appearance; or (iii) identify an unknown mineral.
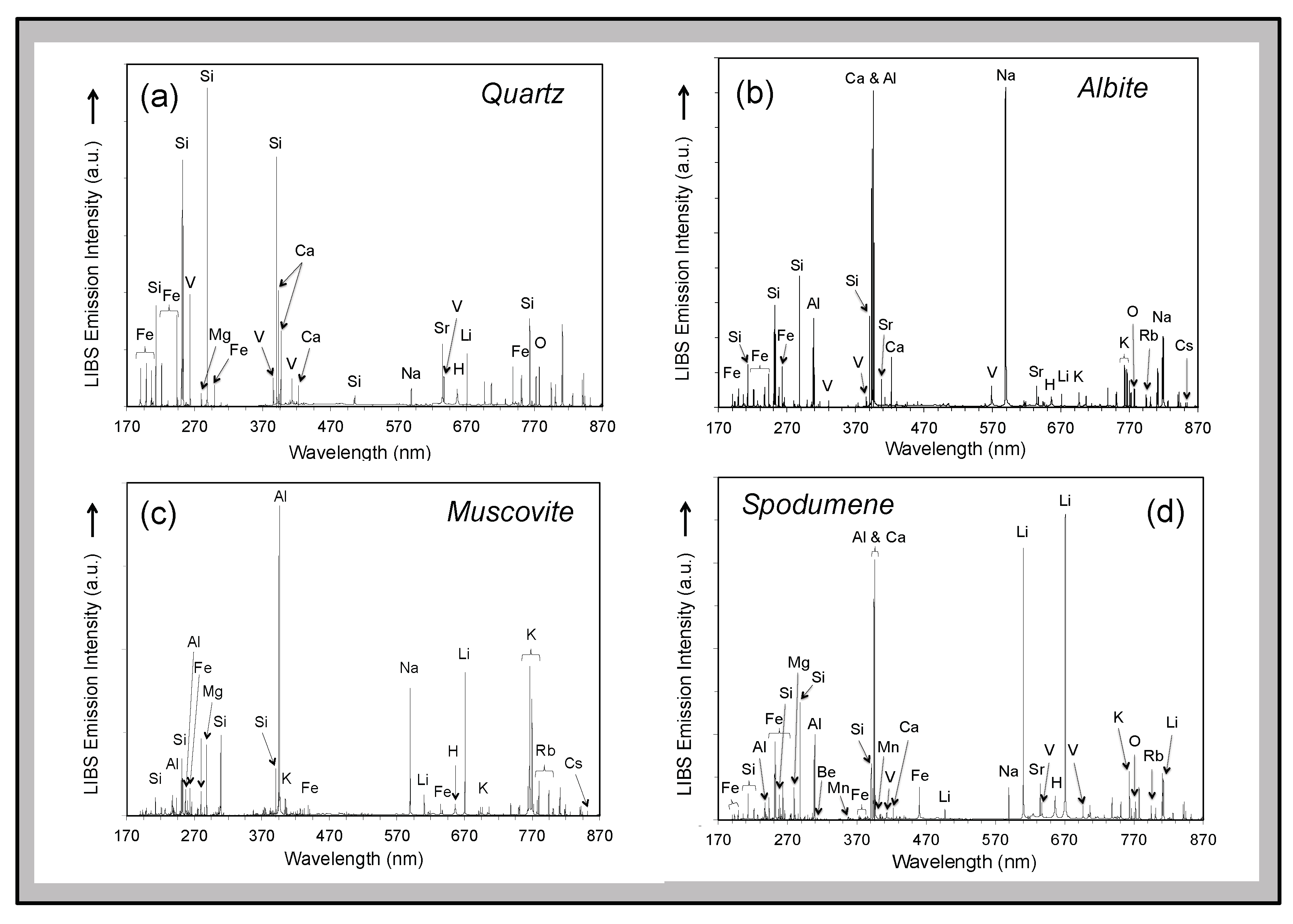
Analysis using the Z-300 handheld LIBS instrument identified 20 elements present in minerals of the pegmatite rock samples, drill cores, and outcrops analyzed across the CLP prospect above their different limits of detection (
Table 1)—Al, B, Ba, Be, Ca, Cs, Fe, H, K, La, Li, Mg, Mn, Na, O, P, Rb, Si, Sr, and V in some samples. Most elements are observed in the primary pegmatite minerals spodumene, quartz, feldspar, and/or muscovite; H and O are most pronounced in hydroxyl-bearing species; Be and La are only observed in the aluminosilicates; and P is only present in the phosphates (
Figure 6,
Figure 7,
Figure 8 and
Figure 9). This is essentially the same set of elements recorded by Fabre et al. [
63] in their handheld LIBS analysis of Li-bearing pegmatite minerals from the Fregenda–Almendra pegmatite field of the Iberian Peninsula.
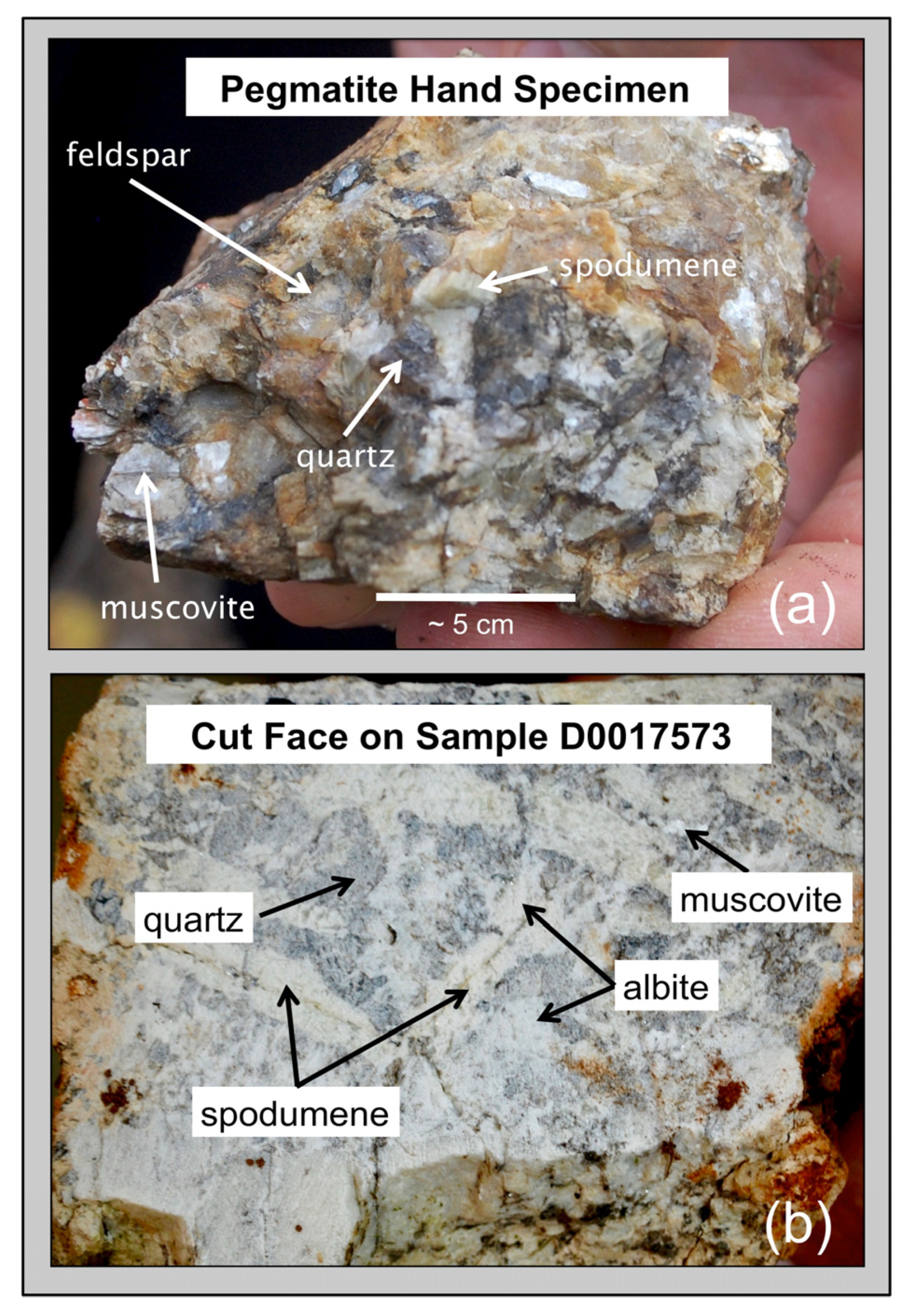
Visually distinguishing between feldspar and spodumene or the micas muscovite and lepidolite rapidly in outcrops during an exploration campaign can sometimes be difficult but is immediately obvious by comparison of LIBS spectra based on the presence of the primary Li emission peaks at 610.36 nm and 670.79 nm in the Li-rich minerals (
Figure 6). Similarly, phosphate minerals can be readily identified because the Z-300 analyzer records the ultraviolet region of the LIBS emission spectrum and, therefore, can observe the P emission lines at 213.62 nm and 214.95 nm (
Figure 7). Non-metal elements, such as F, are particularly difficult to analyze by spectroscopic techniques, so it is notable that the two prominent molecular bands for CaF between 529–543 nm and 590–606 nm are present in the LIBS spectra for tourmaline and fluorapatite shown in
Figure 7. Residual minerals in the regolith cover of the critical zone can be a useful guide to the presence of mineralized pegmatite at depth. For example, the presence of Li in detrital quartz in areas of deep soil cover lacking outcrop can be an important pathfinder to mineralization in the subsurface. Finally, under favorable circumstances, LIBS analysis can be helpful for rapid identification of accessory and uncommon minerals can be readily identified on site through a LIBS analysis (
Figure 7).
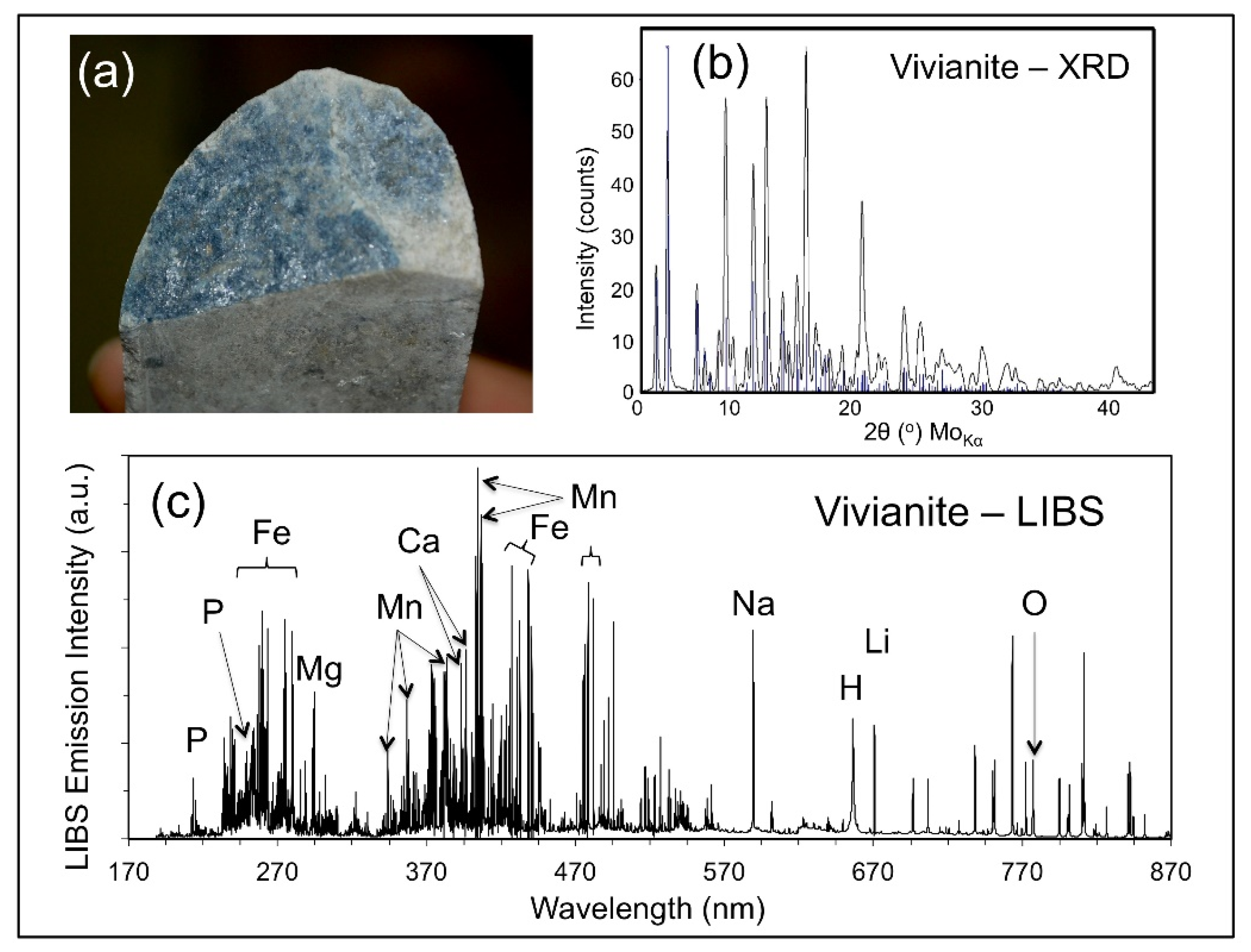
Late-stage mineralization is common along fissures and fractures throughout the strongly tectonized and deformed CTSB.
Figure 8 shows the occurrence of a blue-black, hypidiomorphic mineral along a fracture plane in a drill core (a) that was analyzed by X-ray diffraction analysis (b), and LIBS (c). LIBS analysis identified the presence of Fe, Li, and P, indicating that this mineral was either triphylite [LiFePO
4] or vivianite [Fe
3(PO
4)
2·8H
2O] produced from the alteration of triphylite, with the latter attribution subsequently confirmed by the X-ray diffraction analysis.
6.2. Element Spatial Distribution
Whole-rock lithogeochemistry of outcrop and drill core samples, together with microscale analysis of individual minerals, are two of the primary exploration tools for mapping the geochemical signature of ore systems. LIBS can be helpful in this context in two different ways. The Z-300 analyzer has a computer-controlled 3-D translational stage that permits rastering of the laser beam across the sample in the XY-direction at 12.5 μm steps over an area of up to 2 × 2 mm2, with the grid size and the number of laser shots fired at each point defined by the user. A user-selected number of non-analytical ‘cleaning’ shots can be performed prior to data collection. Therefore, compositional variation within a sample can be examined by the Z-300 analyzer at the ~100 μm spatial scale of the LIBS analysis through either the microscale mapping feature where the laser is rastered over a 2 mm area of the sample surface or by depth profiling in which successive laser shots are undertaken at the same spot to ablate a sample to progressively greater depths.
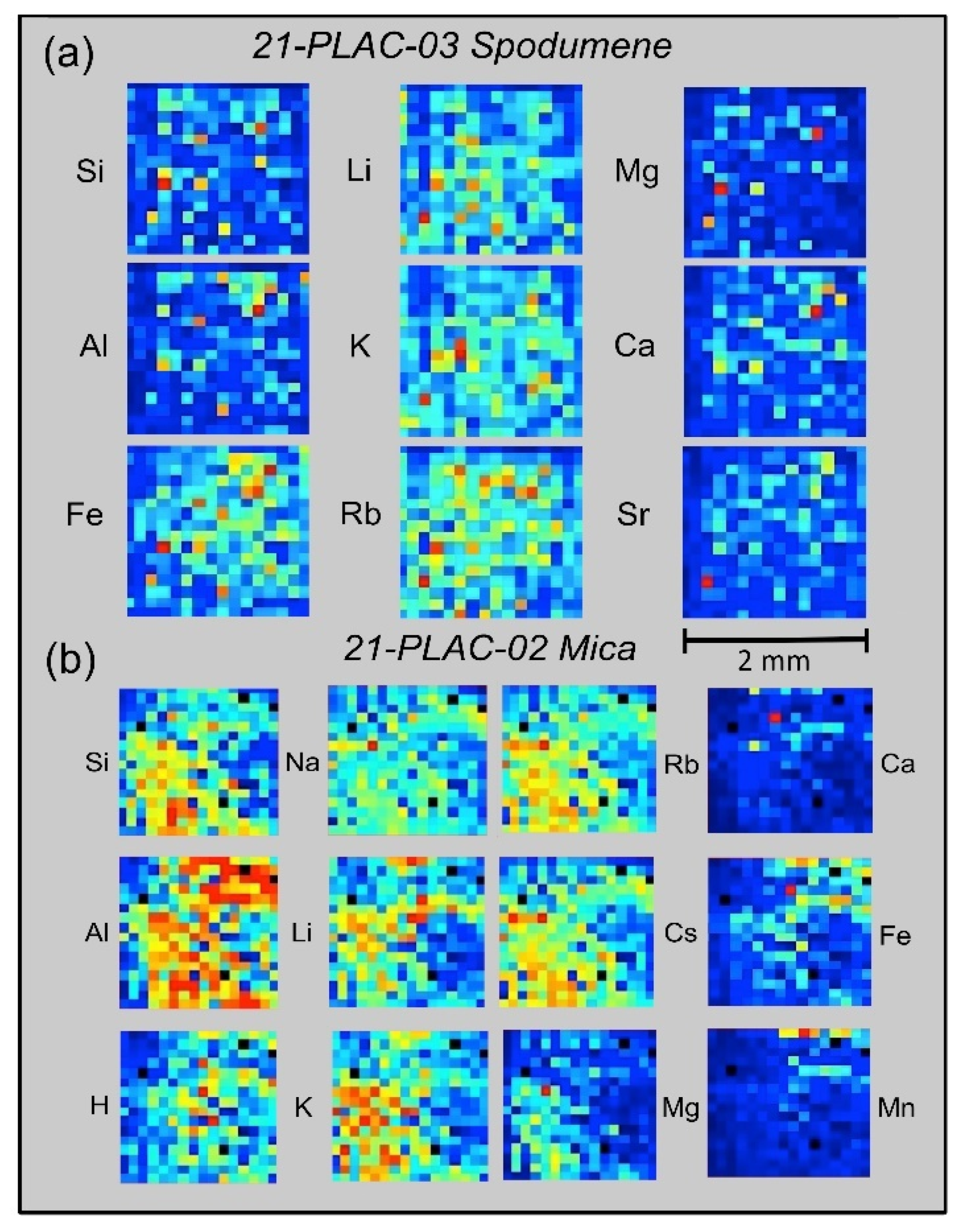
For microscale mapping, the
Geochem Pro mode of the Z-300 analyzer is used to identify spectral peaks and then generate relative concentration maps based on the recorded elemental intensities [
37,
46]. 2-D maps of relative emission intensity, commonly termed ‘heat maps’, are produced from individual laser shots spaced 12.5 μm across the surface of a sample over a 16 × 16 grid pattern. Examples from the CLP prospect are shown in
Figure 9 for spodumene and muscovite crystals from two outcrop samples, 21-PLAC-03 and 21-PLAC-02. Such information can reveal whether a mineral is homogeneous at the spatial scale of the LIBS analysis and also can be helpful in understanding the geochemical behavior of different elements at a small spatial scale, which can provide insight into the process of pegmatite formation.
The panels in
Figure 9a display the spatial distributions of variations in Si, Al, Fe, Li, K, Rb, Ca, Mg, and Sr abundances on the surface of a spodumene crystal in outcrop sample 21-PLAC-03, whereas those in
Figure 9b show the spatial distributions of variations in Si, Al, H, Na, Li, K, Rb, Cs, Ca, Fe, and Mn abundances across a 2-mm domain on the surface of a muscovite crystal in outcrop sample 21-PLAC-02. Two features are of particular note across the 2 × 2 mm
2 surface domains for the element distributions shown in
Figure 9. The first is the general compositional homogeneity of the spodumene compared to the muscovite at this spatial scale. By comparison to the nine ‘heat maps’ for 21-PLAC-03 spodumene, which are dominated by shades of dark and light blue, the individual ‘heat maps’ for 21-PLAC-02 muscovite exhibit the full range of color variation from almost entirely dark blue for Ca and Mn to domination by yellow and red colors for Al. The second is the coherent geochemical behavior of Li-K-Rb and Mg-Ca-Sr for the spodumene and Li-Na-K-Rb-Cs and Ca-Fe-Mn for the muscovite.
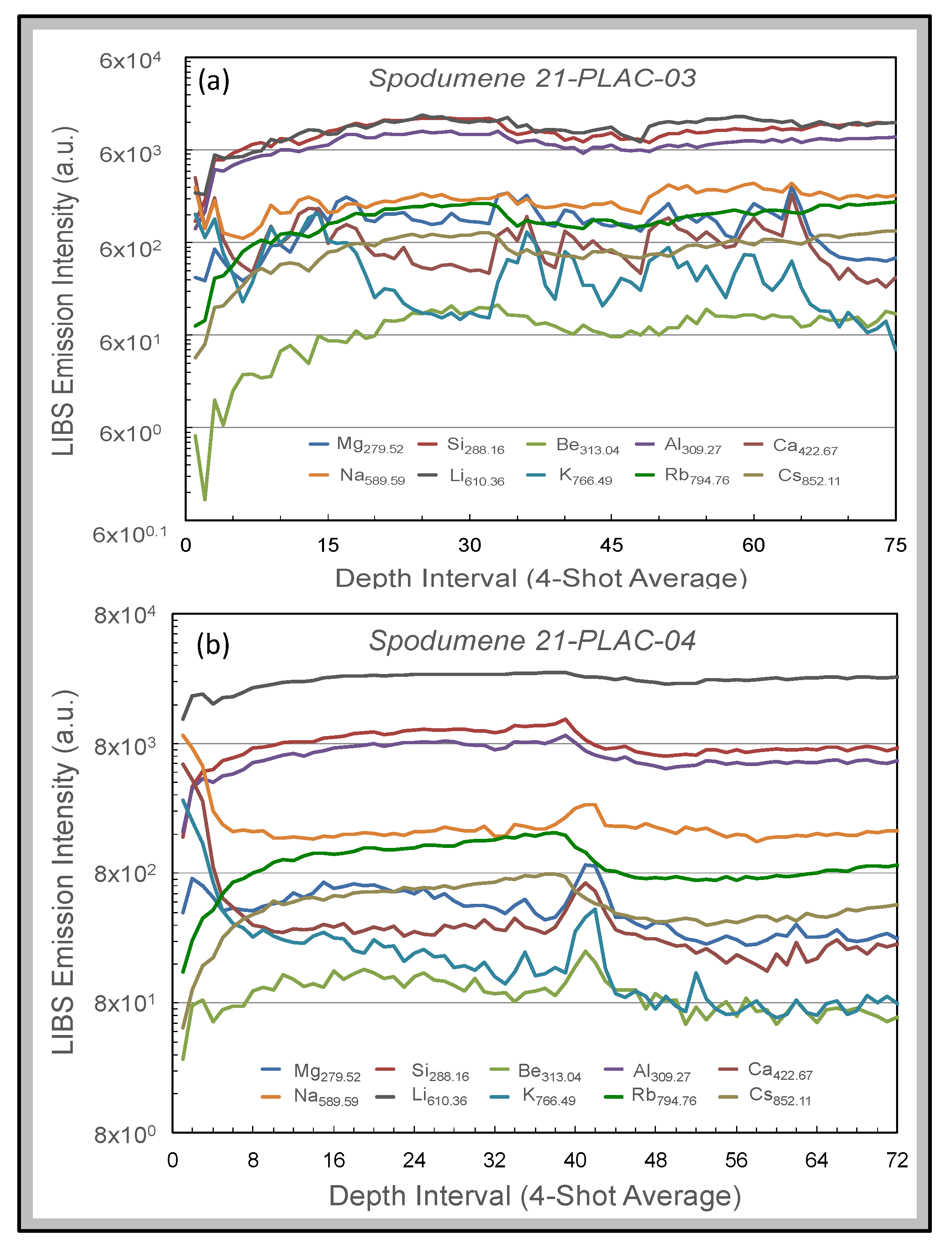
Figure 10 shows depth profiles for spodumenes in two outcrops. The profiles represent 4-shot averages of LIBS emission intensities of 10 spectral lines (Mg
279.52, Si
288.16, Be
313.04, Al
309.27, Ca
422.67, Na
589.59, Li
610.36, K
766.49, Rb
794.76, and Cs
852.11) for 300 successive laser shots at a single spot on sample 21-PLAC-03 and 288 successive laser shots at a single spot on sample 21-PLAC-04. These intensity variations are displayed on a logarithmic scale, as element intensities vary over five orders of magnitude for sample 21-PLAC-03 and four orders of magnitude for sample 21-PLAC-04. Thus, elements present in the samples at high abundance show subdued variation compared to elements of low concentration. Both depth profiles are characterized by significant variation over the first 5–6 depth intervals (i.e., 20–24 laser shots), which records decreases for some elements (e.g., Na, Mg, Ca) yet increases for others (e.g., Si, Al, Li, Rb, Cs). This behavior is interpreted to reflect the cumulative effect of surficial weathering of the spodumene that has caused elements of contrasting geochemical behavior being mobile to different extents. The other feature of note is the sharp increases in emission intensity for Na, Mg, Ca, K, and Be together with concomitant intensity decreases for Si, Al, Rb, and Cs observed for spodumene 21-PLAC-04 over the 40–45 laser shot depth interval. This compositional discontinuity likely reflects the encounter of the laser beam with an inclusion a few 10s of microns in size. Fabre et al. [
56] have described how such inclusions can be probed and compositionally interrogated using a laboratory LIBS system.
6.3. Quantification
LIBS can measure the elemental abundance by measuring the intensity of the light captured at specific spectral wavelengths because the intensity of the plasma emission is proportional to the concentration of an element in a material of interest. Quantitative analysis by LIBS can be straightforward if the material being analyzed is compositionally homogeneous, as is the general situation for metal and alloy analysis where LIBS is well established and has been widely applied for a variety of industrial applications [
33,
40,
70,
71,
72,
73,
74,
75]. This is not generally the case for geological materials, which are intrinsically variable in terms of composition, crystallinity, and texture. Both the chemical composition of the matrix being analyzed by LIBS and its physical characteristics affect the measured abundance of an analyte present in the plasma [
32] because these characteristics directly influence the excitation properties of the laser plasma produced by the ablation process [
76] and, therefore, the emission line intensity measured for any element. Chemical matrix effects arise when the presence of an element of low ionization potential in the plasma elevates the plasma density and thereby inhibits the emission of other elements to decrease their abundance in the plasma [
77]. Differences in material physical characteristics, such as crystallinity, hardness, opacity, grain size, coherence and texture, influence the degree of laser energy-material coupling so that elements of the same abundance in a dissimilar matrices will produce different emission intensities because of changes in the amount of sample ablated into the plasma with each laser pulse [
78,
79]. Further discussion of physical matrix effects is beyond the scope of this paper but has been described in detail in numerous previous studies, e.g., [
31,
36,
78,
80,
81,
82,
83,
84,
85,
86,
87,
88]. Chemical matrix efects are more readily ameliorated through optimization of the LIBS analytical system than physical matrix effects [
32] which, therefore, present the greatest challenge to, and impediment for, quantitative analysis of geomaterials by LIBS. Despite these complications, quantitative analysis is possible by LIBS, but to do so requires careful selection of emission lines and creation of univariate or multivariate calibration curves using physically and compositionally similar matrix-matched standards.
Quantitative analysis can be accomplished using
Geochem mode of the Z-300 analyzer developed beforehand from the analysis of a set of matrix-matched reference materials using either single-element or multivariate calibration procedures. Two general calibrations curves are installed on Z-300 analyzers purchased for geoscience applications; a general geochemistry calibration (“geochem”) based on >70 different geological materials and NIST standards and an iron ore calibration (“Fe ore”) based on a smaller number of OREAS 400 series standard reference materials. Additionally, users can create bespoke calibration curves using the Z-300
Profile Builder PC-based software package as described in Harmon et al. [
37].
Creating Z-300 calibrations utilizes a concentration versus intensity ratio approach that depends on two considerations: the number spectral lines for an element of interest and the presence of distinct emission lines that are not affected by overlap with lines from other elements present in the sample. Any LIBS calibration curve will perform best when developed for a specific matrix of interest. First, intensity values for elements are calculated after performing a Savitsky-Golay smoothing [
89] on the LIBS spectrum, followed by a background subtraction, and finally integration of measured emission intensities across the defined spectral region of interest (ROI) to obtain a summed area under the peak value. Intensity ratios are then calculated by combining one or more summed peak intensity values for the analyte element of interest in the numerator of the ratio and the denominator consisting either of the emission intensity for a single element or the sum of emission intensities of multiple elements for complex matrices. Ideally, elements used in the denominator comprise the bulk of the sample composition and remain relatively constant from sample to sample. Whilst concentration values are required for each of the target elements for which the calibration is being developed, they are not required for the elements in the denominator as elements of approximately constant composition (e.g., Al, Si and other major elements in silicate minerals) are used for this spectral intensity normalization. Once a set of calibration curves has been constructed, subsequent LIBS analysis using the Z-300 in the
Geochem mode will calculate and display elemental concentrations for a test sample in real time.
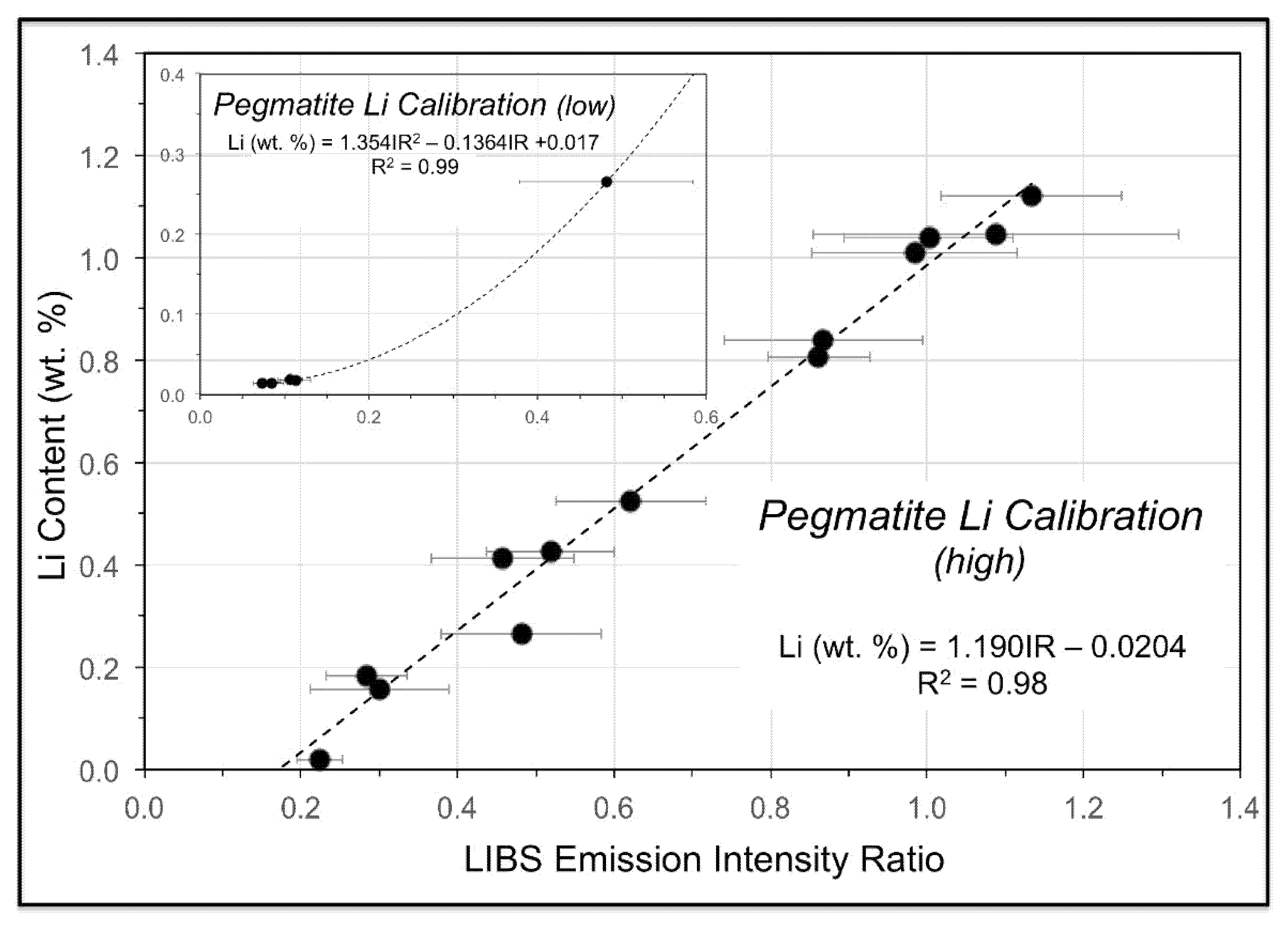
As noted above, two provisional calibration curves have been developed on the Z-300 instrument from our initial work at the CLP prospect to illustrate this capability. These calibrations will be refined and enhanced, and new calibrations for other minerals developed, as our study continues and more samples of known composition are acquired. The first calibration (
Figure 11) is for a set of mica samples with Li contents ranging from 0.014 to 2.59 wt. % from the collection of the U.S. Smithsonian Institution National Museum of Natural History that represent different LCT pegmatite subtypes and encompass a broad range of lithologies and geologic settings [
90]. The second calibration (
Figure 12) is for a suite of pressed pellets from 17 pulverized pegmatite core samples representing 1-m intervals in three drill holes on the CLP prospect previously analyzed by X-ray fluorescence (XRF) and inductively coupled plasma mass spectrometry (ICPMS). Low- and high-range calibration curves were developed for these samples, which ranged in Li content from 0.015 to 1.12 wt. %. Li contents measured for minerals, drill core, and soil from across the CLP prospect with the Z-300 handheld LIBS analyzer using these calibrations ranged from 0.005–2.672 wt. % (
Table A3).
The calibration spectra were acquired with the same laser raster and pulse settings of 12 locations with two cleaning pulses and four data acquisition pulses per location. The laser was pulsed at a rate of 50 Hz and detector gating was used to avoid the collection of continuum light emitted early in the plasma lifetime, thus producing sharper spectra with lower background. All 48 data pulses were averaged to produce a single spectrum for calibration use. Each sample was analyzed five times in this way and resulting intensity ratios were averaged. For the mica calibration, the Li intensity ratio consisted of the Li peak at 610.36 nm in the numerator and the sum of the peaks for Al at 394.40 nm, Ca at 396.85 nm, and Na at 819.48 nm in the denominator. The pegmatite powder calibration for our work at the CLP prospect used the intensity of Li nm peak at 610.36 nm in the numerator that was normalized to a combination of the intensities for the Al peak at 394.40 nm and the Fe peak at 438.35 nm.
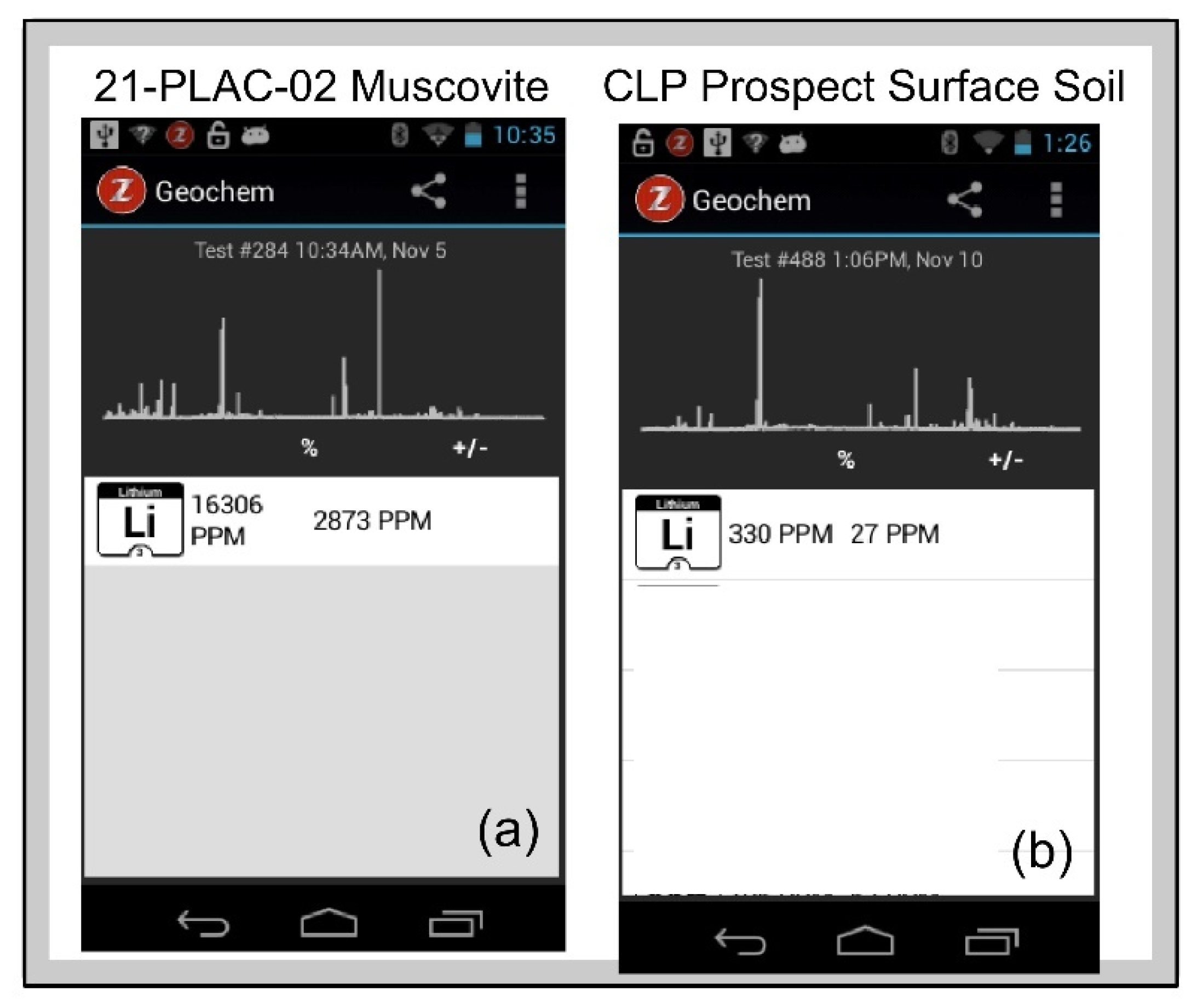
Figure 13a shows the Z-300
Geochem mode screen display of LIBS spectra and sample Li composition of a muscovite from pegmatite outcrop sample 21-PLAC-02 and a surface exposure of the Cecil soil on the CLP prospect determined by comparison of an analyzed sample against the calibration that is provided to the analyst in real time.
The pegmatite powder calibration (
Figure 12) was validated by analysis of a set of 14 pelletized powdered pegmatite samples from the Kleiber Oy Li deposit in Kaustinen-Kokkola area of central Ostrabothnia in western Finland, where Paleoproterozoic albite-spodumene pegmatites crosscut the Pohjanmaa schist belt situated between the Central Finland Granite and Vaasa Migmatite Complex of the Svecofennian Orogen [
91,
92]. Li contents for this validation suite range from 0.01–1.12 wt. % and, as shown in
Table 2, analysis of the Kleiber Oy pegmatite powders against the calibration for the CLP pegmatite powders yielded Li contents very close to the assay values (Li
LIBS = 0.941Li
assay, R
2 = 0.97).
This example shows that calibrations for quantification can be developed using handheld LIBS in situations of appropriate matrix matching between standards and samples. But what if that isn’t possible? We considered this through analysis of a dozen samples from soil core 20-BD-359 from the CLP prospect drilled through the Cecil soil into saprolite. The Cecil soil is a well drained and moderately permeable soil derived from the deep weathering of felsic, igneous and high-grade metamorphic rocks on uplands throughout the Piedmont region of North Carolina [
93] that is comprised primarily of Al and Fe oxyhydroxide minerals [
94] rather than the aluminosilicate matrix on which the pegmatite powder calibration is based. The soil core samples, which were prepared as pressed pellets in exactly the same way as the pulverized pegmatites, were analyzed using both our pegmatite powder calibration and the SciAps general geochemistry calibration. Neither calibration produced the robust results shown in
Table 2 for the Kleiber Oy pegmatite powders, but the latter yielded a more statistically significant relationship between Li assay values and LIBS abundance estimates than the former: Li
LIBS = 0.828Li
assay, R
2 = 0.18 using the pegmatite powder calibration versus Li
LIBS = 0.368Li
assay, R
2 = 0.52 using the Z-300 general geochemistry calibration. As illustrated in
Table 3, soil Li abundances are closer to actual values for this general geochemistry calibration, which is based on >70 different geological materials that include a variety of soils, than for the calibration derived solely from the pegmatite powder which has an aluminosilicate matrix. A similar situation is observed when the suite of micas used to develop the mica calibration is analysed using the pegmatite powder calibration (
Table 4), with Li abundances lower using the pegmatite powder calibration than with the mica calibration. These two examples highlight the importance of matrix matching for quantitative LIBS.