Clay–Magnetite Co-Aggregates for Efficient Magnetic Removal of Organic and Inorganic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. XRD, FT-IR, and Zeta Potential
2.3. Adsorption Studies
3. Results and Discussion
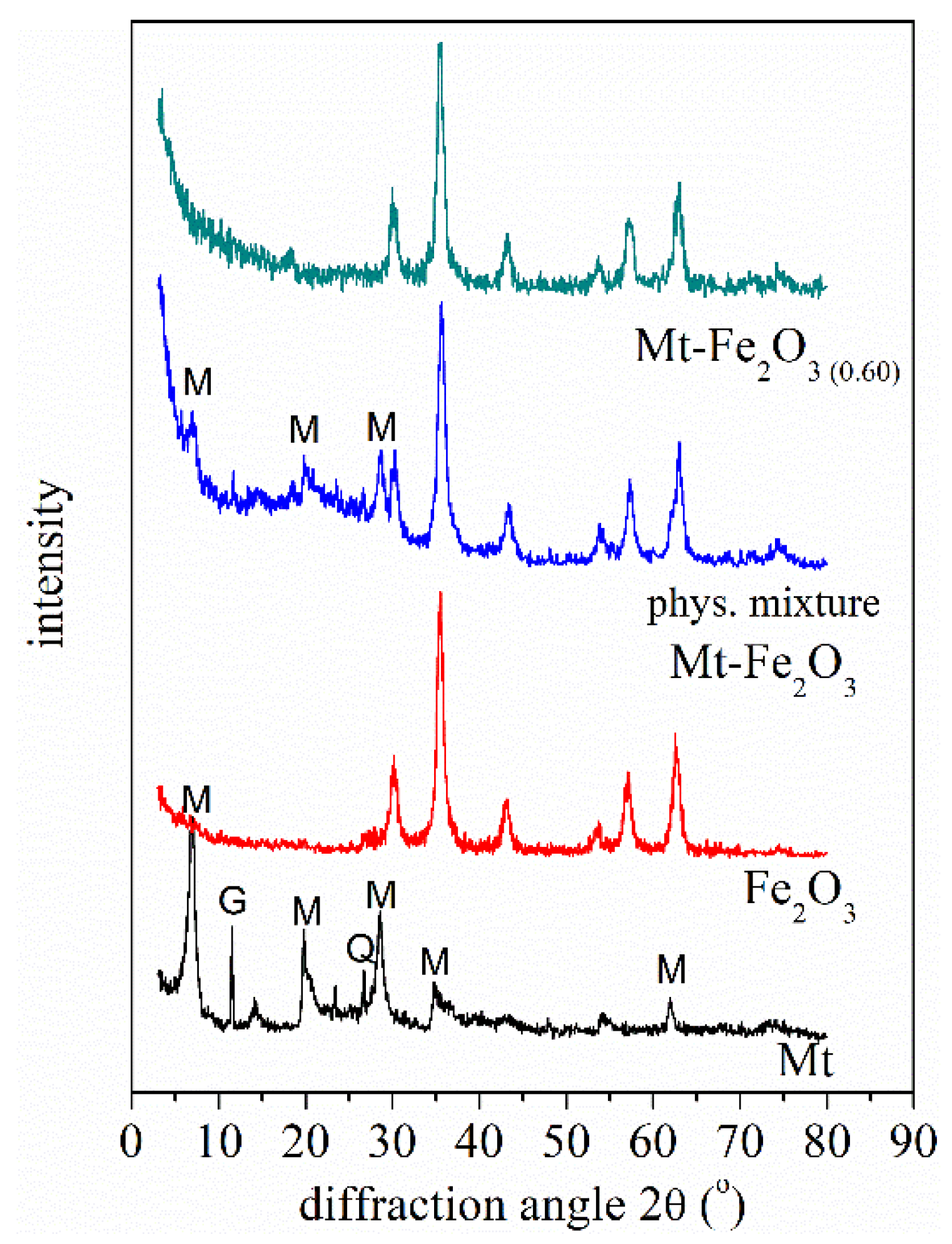
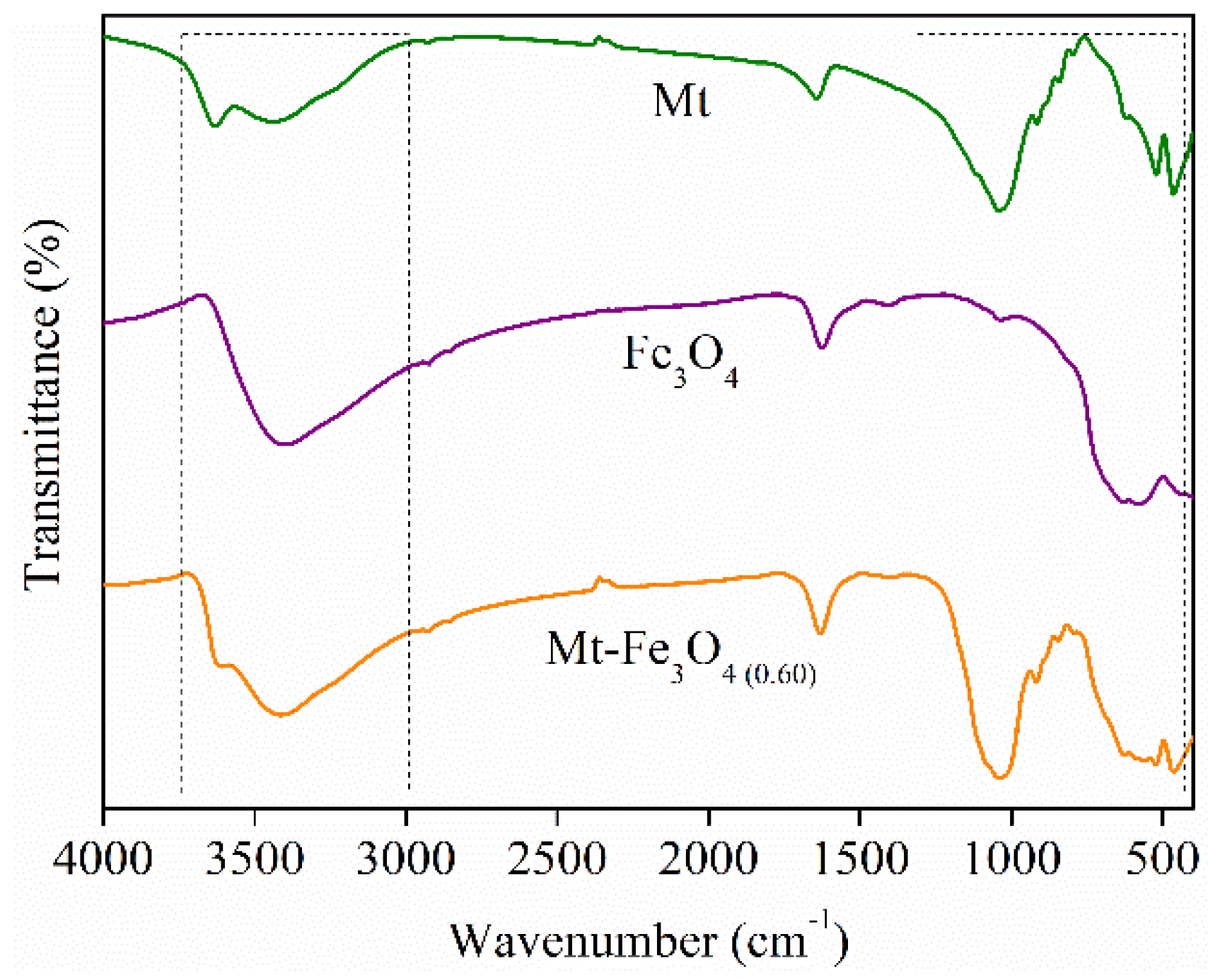
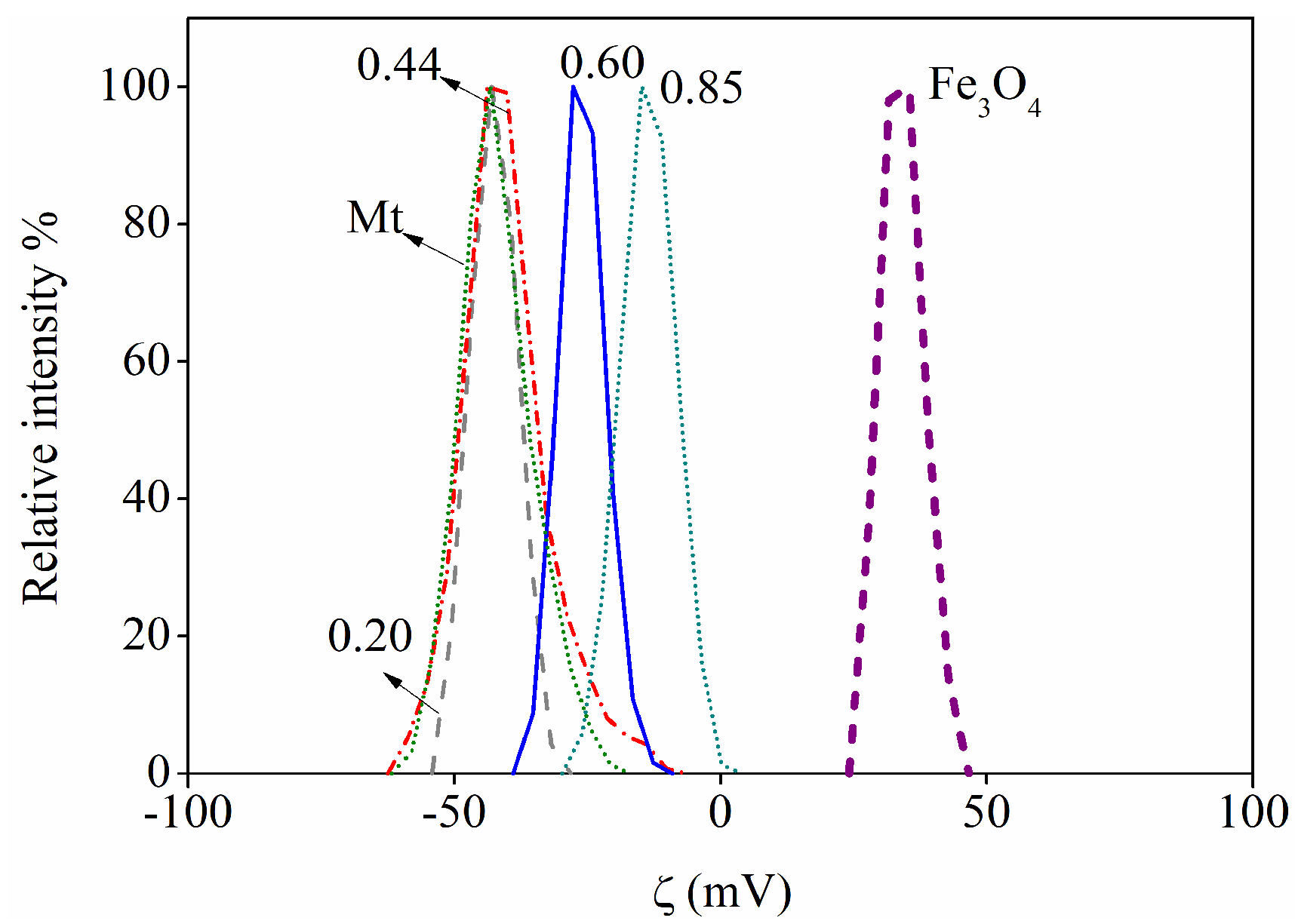
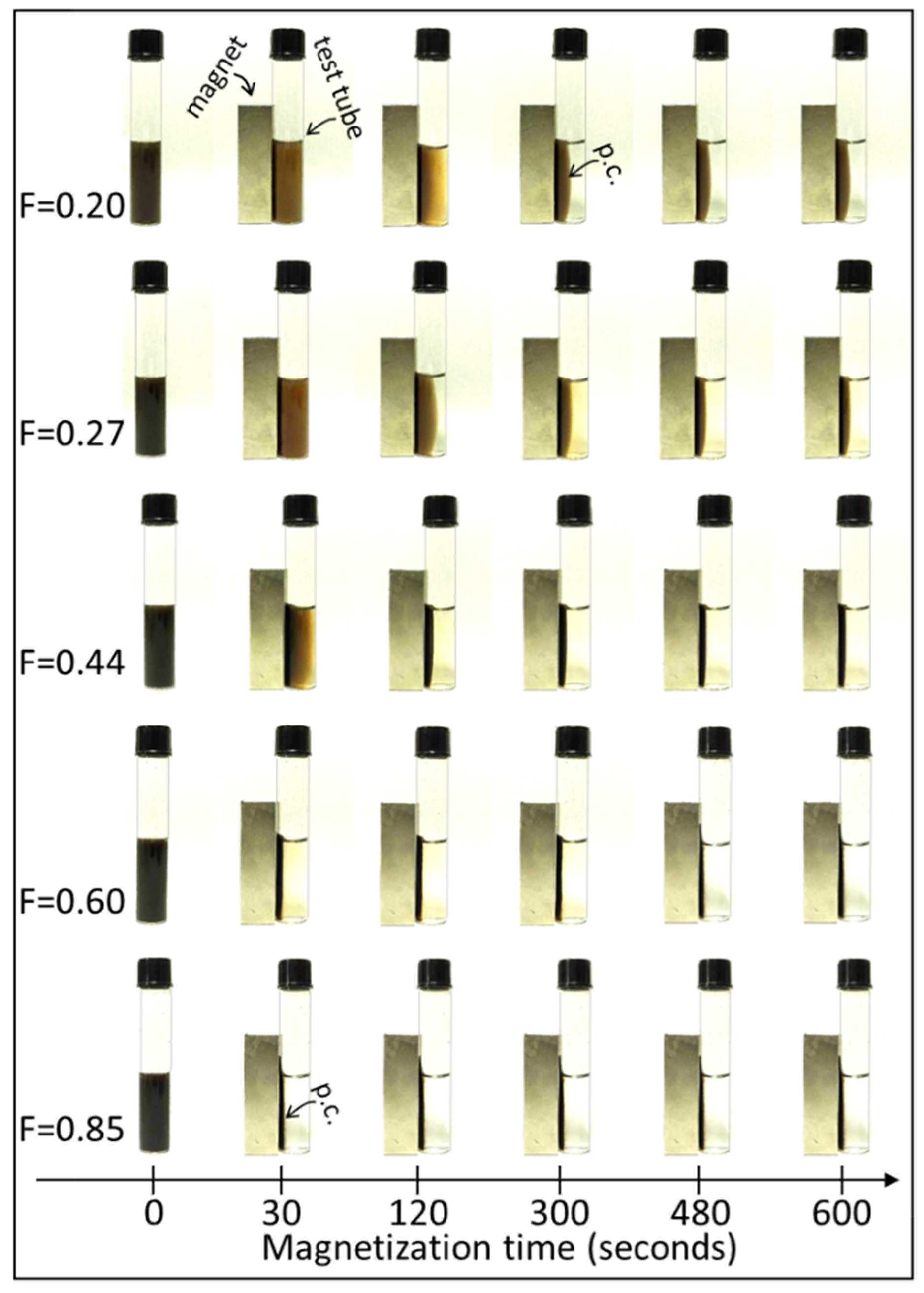
3.1. XRD, FT-IR, Zeta Potential, and Magnetic Remotion
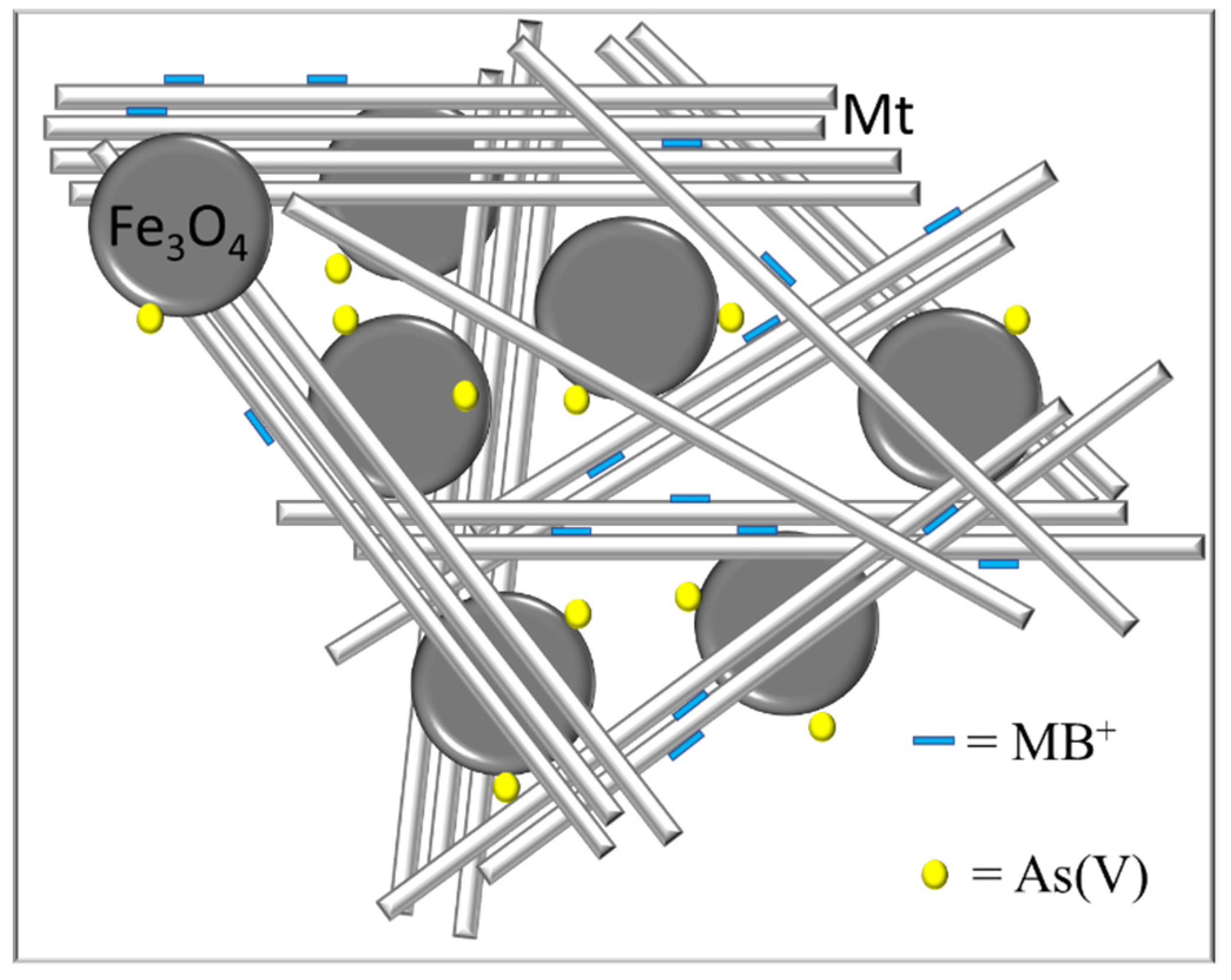
3.2. Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Filik, H.; Avan, A.A. Magnetic Nanostructures for Preconcentration, Speciation and Determination of Chromium Ions: A Review. Talanta 2019, 203, 168–177. [Google Scholar] [CrossRef]
- Karimi, M.A.; Mohadesi, A.; Hatefi-Mehrjardi, A.; Mohammadi, S.Z.; Yarahmadi, J.; Khayrkhah, A. Separation/Preconcentration and Speciation Analysis of Trace Amounts of Arsenate and Arsenite in Water Samples Using Modified Magnetite Nanoparticles. J. Chem. 2014, 2014. [Google Scholar] [CrossRef]
- Cao, Y.; Sheng, T.; Yang, Z.; Huang, D.; Sheng, L. Synthesis of Molecular-Imprinting Polymer Coated Magnetic Nanocomposites for Selective Capture and Fast Removal of Environmental Tricyclic Analogs. Chem. Eng. J. 2021, 426, 128678. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Foroutan, R.; Peighambardoust, S.H.; Khatooni, H.; Ramavandi, B. Decoration of Citrus Limon Wood Carbon with Fe3O4 to Enhanced Cd2+ Removal: A Reclaimable and Magnetic Nanocomposite. Chemosphere 2021, 282, 131088. [Google Scholar] [CrossRef]
- Saiz, S.; Nanozarah, P.; Kaedah, M.; Kimia, K. Size-Controlled Synthesis and Characterization of Fe3O4 Nanoparticles by Chemical Coprecipitation Method. Sains Malays. 2008, 37, 389–394. [Google Scholar]
- Jang, J.; Lee, D.S. Magnetite Nanoparticles Supported on Organically Modified Montmorillonite for Adsorptive Removal of Iodide from Aqueous Solution: Optimization Using Response Surface Methodology. Sci. Total Environ. 2018, 615, 549–557. [Google Scholar] [CrossRef]
- Shattar, S.F.A.; Zakaria, N.A.; Foo, K.Y. Utilization of Montmorillonite as a Refining Solution for the Treatment of Ametryn, a Second Generation of Pesticide. J. Environ. Chem. Eng. 2017, 5, 3235–3242. [Google Scholar] [CrossRef]
- Wang, G.; Hua, Y.; Su, X.; Komarneni, S.; Ma, S.; Wang, Y. Cr(VI) Adsorption by Montmorillonite Nanocomposites. Appl. Clay Sci. 2016, 124, 111–118. [Google Scholar] [CrossRef]
- Geroeeyan, A.; Niazi, A.; Konoz, E. Removal of Basic Orange 2 Dye and Ni2+from Aqueous Solutions Using Alkaline-Modified Nanoclay. Water Sci. Technol. 2021, 83, 2271–2286. [Google Scholar] [CrossRef]
- Jeganathan, M.; Dhakshinamoorthy, A.; Pitchumani, K. One-Pot Synthesis of 2-Substituted Quinoxalines Using K10-Montmorillonite as Heterogeneous Catalyst. Tetrahedron Lett. 2014, 55, 1616–1620. [Google Scholar] [CrossRef]
- Suresh, D.; Dhakshinamoorthy, A.; Pitchumani, K. A Green Route for the Synthesis of 2-Substituted Benzoxazole Derivatives Catalyzed by Al3+-Exchanged K10 Clay. Tetrahedron Lett. 2013, 54, 6415–6419. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Ponpandian, N.; Khan, M.A.; Siddiqui, M.R.; Ahmed, F.; Alsharaeh, E.H. Development of Adsorption and Electrosorption Techniques for Removal of Organic and Inorganic Pollutants from Wastewater Using Novel Magnetite/Porous Graphene-Based Nanocomposites. Sep. Purif. Technol. 2017, 188, 206–218. [Google Scholar] [CrossRef]
- Su, C. Environmental Implications and Applications of Engineered Nanoscale Magnetite and Its Hybrid Nanocomposites: A Review of Recent Literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef]
- Galindo-González, C.; de Vicente, J.; Ramos-Tejada, M.M.; López-López, M.T.; González-Caballero, F.; Durán, J.D.G. Preparation and Sedimentation Behavior in Magnetic Fields of Magnetite-Covered Clay Particles. Langmuir 2005, 21, 4410–4419. [Google Scholar] [CrossRef]
- Mamedov, A.; Ostrander, J.; Aliev, F.; Kotov, N.A. Stratified Assemblies of Magnetite Nanoparticles and Montmorillonite Prepared by the Layer-by-Layer Assembly. Langmuir 2000, 16, 3941–3949. [Google Scholar] [CrossRef]
- Yuan, P.; Fan, M.; Yang, D.; He, H.; Liu, D.; Yuan, A.; Zhu, J.; Chen, T. Montmorillonite-Supported Magnetite Nanoparticles for the Removal of Hexavalent Chromium [Cr(VI)] from Aqueous Solutions. J. Hazard. Mater. 2009, 166, 821–829. [Google Scholar] [CrossRef]
- Larraza, I.; López-Gónzalez, M.; Corrales, T.; Marcelo, G. Hybrid Materials: Magnetite-Polyethylenimine-Montmorillonite, as Magnetic Adsorbents for Cr(VI) Water Treatment. J. Colloid Interface Sci. 2012, 385, 24–33. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Li, Y.; Chen, X.; Liu, Y. Enzymes Immobilized on Superparamagnetic Fe3O4@Clays Nanocomposites: Preparation, Characterization, and a New Strategy for the Regeneration of Supports. J. Phys. Chem. C 2011, 115, 6350–6359. [Google Scholar] [CrossRef]
- Cabrera, M.; Maciel, J.C.; Quispe-Marcatoma, J.; Pandey, B.; Neri, D.F.M.; Soria, F.; Baggio-Saitovitch, E.; de Carvalho, L.B. Magnetic Composites from Minerals: Study of the Iron Phases in Clay and Diatomite Using Mössbauer Spectroscopy, Magnetic Measurements and XRD. Hyperfine Interact. 2014, 224, 197–204. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Sapag, K.; Garg, V.K.; Lago, R.M. Clay-Iron Oxide Magnetic Composites for the Adsorption of Contaminants in Water. Appl. Clay Sci. 2003, 22, 169–177. [Google Scholar] [CrossRef]
- Lombardi, B.; Baschini, M.; Torres Sánchez, R.M. Bentonite Deposits of Northern Patagonia. Appl. Clay Sci. 2003, 22, 309–312. [Google Scholar] [CrossRef]
- Pecini, E.M.; Springer, V.; Brigante, M.; Avena, M. Arsenate Interaction with the Surface of Nanomagnetic Particles. High Adsorption or Full Release. J. Environ. Chem. Eng. 2017, 5, 4917–4922. [Google Scholar] [CrossRef]
- Pecini, E.M.; Avena, M.J. Measuring the Isoelectric Point of the Edges of Clay Mineral Particles: The Case of Montmorillonite. Langmuir 2013, 29, 14926–14934. [Google Scholar] [CrossRef] [PubMed]
- Lenoble, V.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenite Oxidation and Arsenate Determination by the Molybdene Blue Method. Talanta 2003, 61, 267–276. [Google Scholar] [CrossRef]
- Oueslati, W.; Ben Rhaiem, H.; Lanson, B.; Ben Haj Amara, A. Selectivity of Na-Montmorillonite in Relation with the Concentration of Bivalent Cation (Cu2+, Ca2+, Ni2+) by Quantitative Analysis of XRD Patterns. Appl. Clay Sci. 2009, 43, 224–227. [Google Scholar] [CrossRef]
- Chen, G.; Han, B.; Yan, H. Interaction of Cationic Surfactants with Iron and SodiumMontmorillonite Suspensions. J. Colloid Interface Sci. 1998, 201, 158–163. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: Chantilly, VA, USA, 1974; p. 539. [Google Scholar]
- Costanzo, P.M. Baseline Studies of the Clay Minerals Society Source Clays: Introduction. Clays Clay Miner. 2001, 49, 372–373. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, P.; Zhu, J.; Chen, T.; Yuan, A.; He, H.; Chen, K.; Liu, D. Core-Shell Structured Iron Nanoparticles Well Dispersed on Montmorillonite. J. Magn. Magn. Mater. 2009, 321, 3515–3519. [Google Scholar] [CrossRef]
- Green-Pedersen, H.; Pind, N. Preparation, Characterization, and Sorption Properties for Ni(II) of Iron Oxyhydroxide-Montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2000, 168, 133–145. [Google Scholar] [CrossRef]
- D’Cruz, B.; Madkour, M.; Amin, M.O.; Al-Hetlani, E. Efficient and Recoverable Magnetic AC-Fe3O4 Nanocomposite for Rapid Removal of Promazine from Wastewater. Mater. Chem. Phys. 2020, 240, 122109. [Google Scholar] [CrossRef]
- Sondi, I.; Biscan, J.; Pravdic, V. Electrokinetics of Pure Clay Minerals Revisited. J. Colloid Interface Sci. 1996, 178, 514–522. [Google Scholar] [CrossRef]
- Guz, L.; Curutchet, G.; Torres Sánchez, R.M.; Candal, R. Adsorption of Crystal Violet on Montmorillonite (or Iron Modified Montmorillonite) Followed by Degradation through Fenton or Photo-Fenton Type Reactions. J. Environ. Chem. Eng. 2014, 2, 2344–2351. [Google Scholar] [CrossRef]
- Miller, S.; Low, P. Characterization of the Electrical Double Layer of Montmorillonite. Langmuir 1990, 11, 572–578. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The Effect of Humic Acid Adsorption on pH-Dependent Surface Charging and Aggregation of Magnetite Nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Favela-Camacho, S.E.; Samaniego-Benítez, E.J.; Godínez-García, A.; Avilés-Arellano, L.M.; Pérez-Robles, J.F. How to Decrease the Agglomeration of Magnetite Nanoparticles and Increase Their Stability Using Surface Properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Luengo, C.; Puccia, V.; Avena, M. Arsenate Adsorption and Desorption Kinetics on a Fe(III)-Modified Montmorillonite. J. Hazard. Mater. 2011, 186, 1713–1719. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Z.; Luo, H.; Hu, B.; Dang, Z.; Yang, C.; Li, L. Adsorption of Arsenic on Modified Montmorillonite. Appl. Clay Sci. 2014, 97, 17–23. [Google Scholar] [CrossRef]
- Akin, I.; Arslan, G.; Tor, A.; Ersoz, M.; Cengeloglu, Y. Arsenic(V) Removal from Underground Water by Magnetic Nanoparticles Synthesized from Waste Red Mud. J. Hazard. Mater. 2012, 235, 62–68. [Google Scholar] [CrossRef]

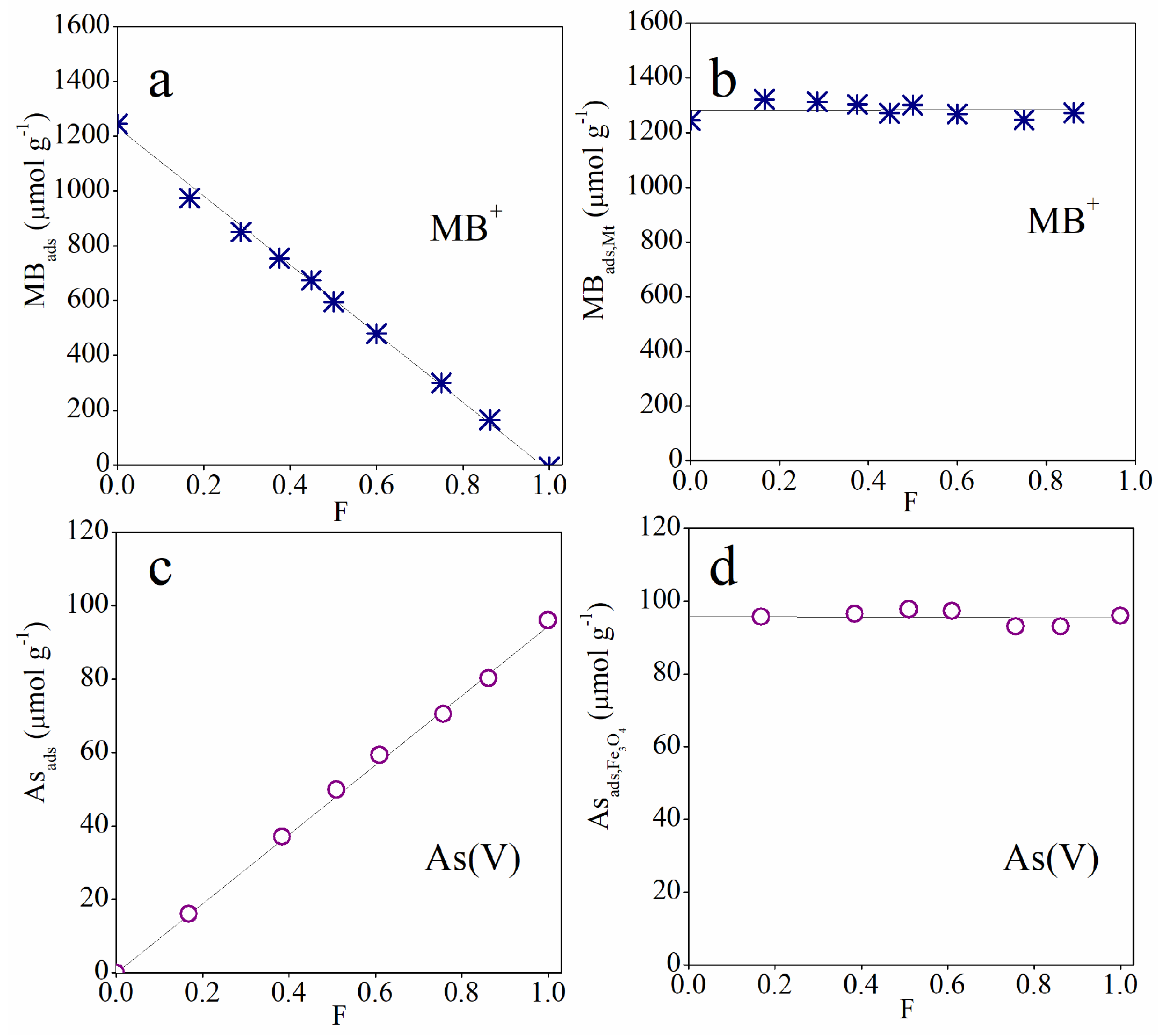

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecini, E.; Avena, M. Clay–Magnetite Co-Aggregates for Efficient Magnetic Removal of Organic and Inorganic Pollutants. Minerals 2021, 11, 927. https://doi.org/10.3390/min11090927
Pecini E, Avena M. Clay–Magnetite Co-Aggregates for Efficient Magnetic Removal of Organic and Inorganic Pollutants. Minerals. 2021; 11(9):927. https://doi.org/10.3390/min11090927
Chicago/Turabian StylePecini, Eliana, and Marcelo Avena. 2021. "Clay–Magnetite Co-Aggregates for Efficient Magnetic Removal of Organic and Inorganic Pollutants" Minerals 11, no. 9: 927. https://doi.org/10.3390/min11090927
APA StylePecini, E., & Avena, M. (2021). Clay–Magnetite Co-Aggregates for Efficient Magnetic Removal of Organic and Inorganic Pollutants. Minerals, 11(9), 927. https://doi.org/10.3390/min11090927





