Experimental Study on Mechanical Properties of Paste Backfill with Flue-Gas Desulphurisation Gypsum under Combined Action of Dry–Wet Cycles and Chloride Erosion
Abstract
:1. Introduction
2. Test Materials and Methodology
2.1. Specimen Preparation
2.2. Test Methodology
3. Mechanical Performance Tests
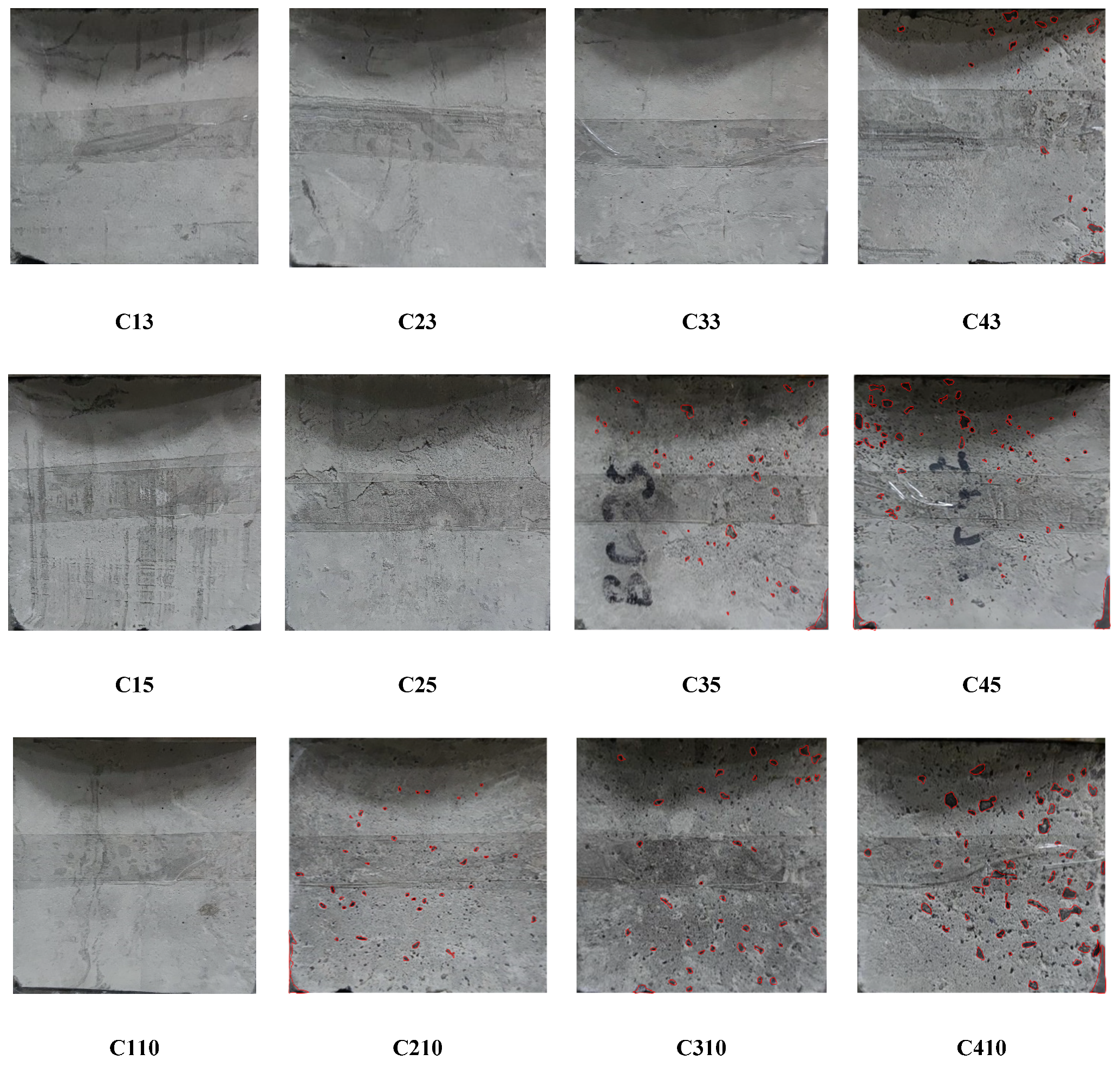
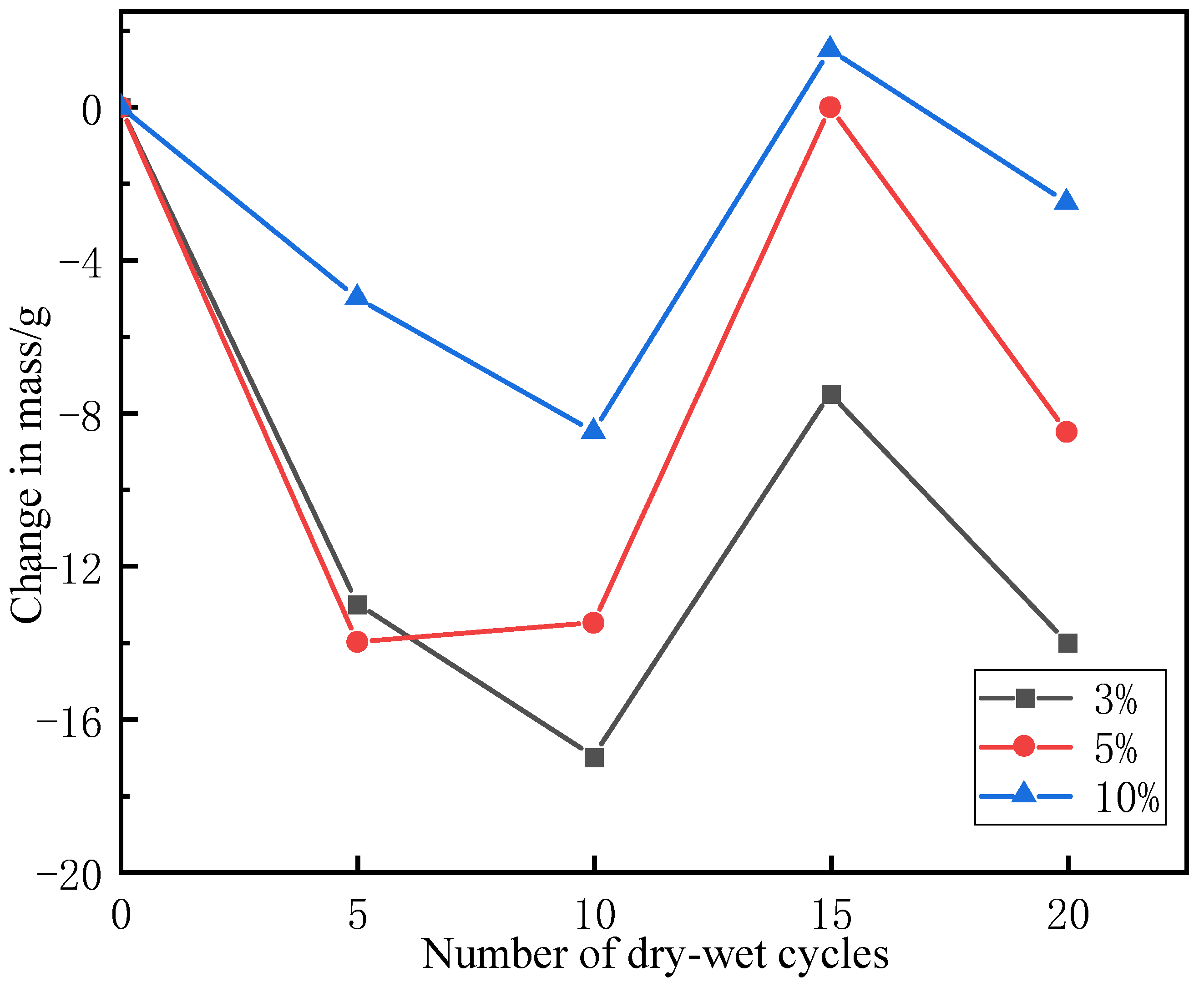
3.1. Effects of Dry–Wet Cycles Coupled with Chloride on Masses of Filled Specimens
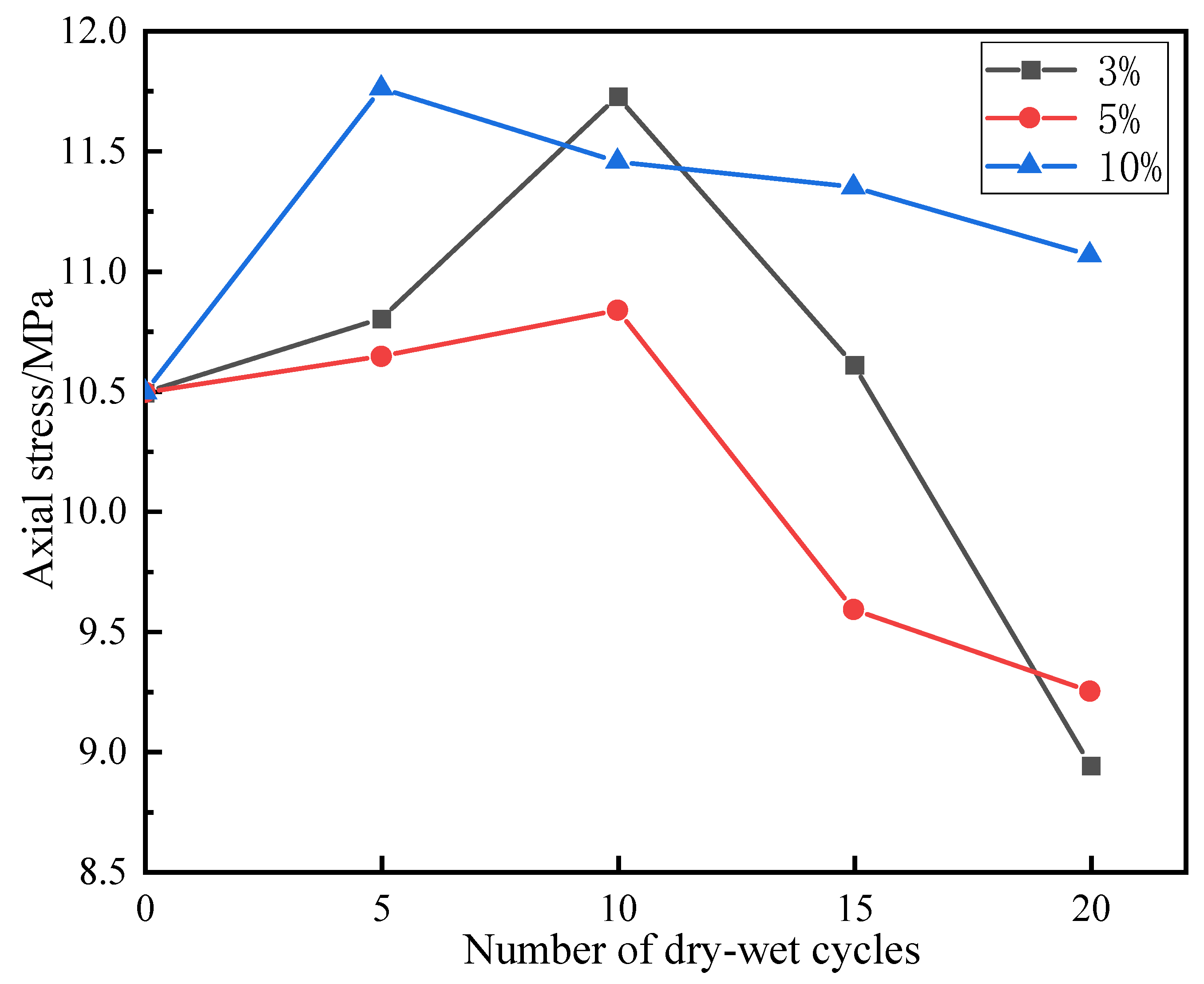
3.2. Effects of Dry–Wet Cycles Coupled with Chloride Salt on Uniaxial Compressive Strengths of Backfill Specimens
3.3. AE Characteristics
4. Mesoscopic Tests
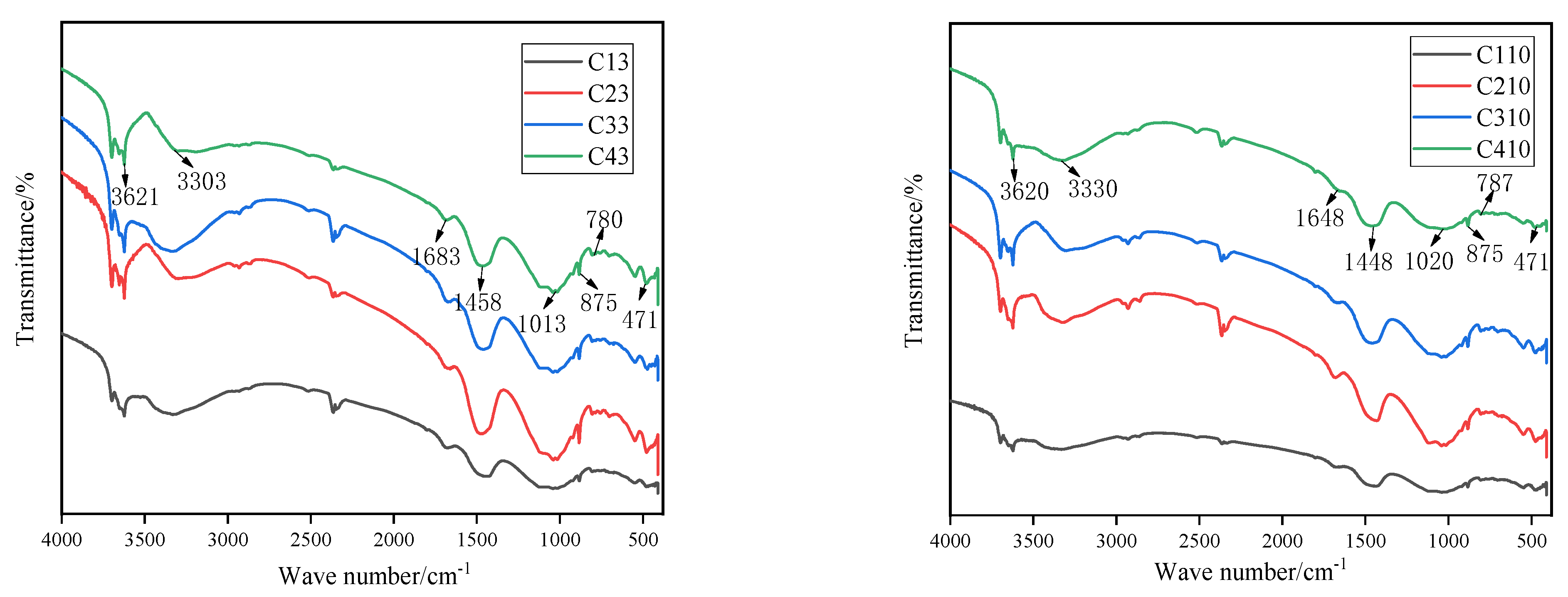
4.1. Analysis of Surface Functional Groups via FTIR Spectroscopy
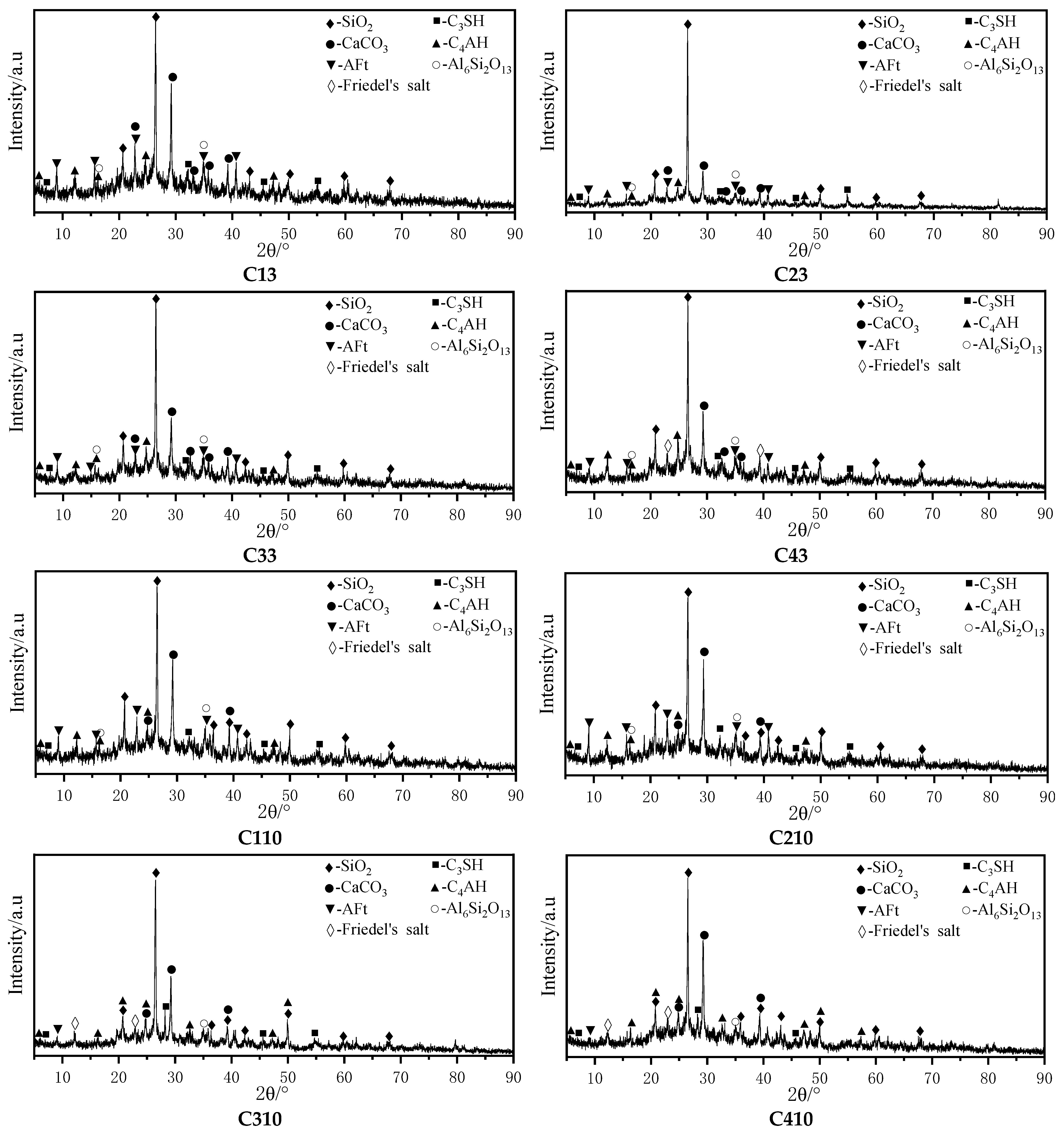
4.2. Crystal Calibration Analysis with XRD Diffraction
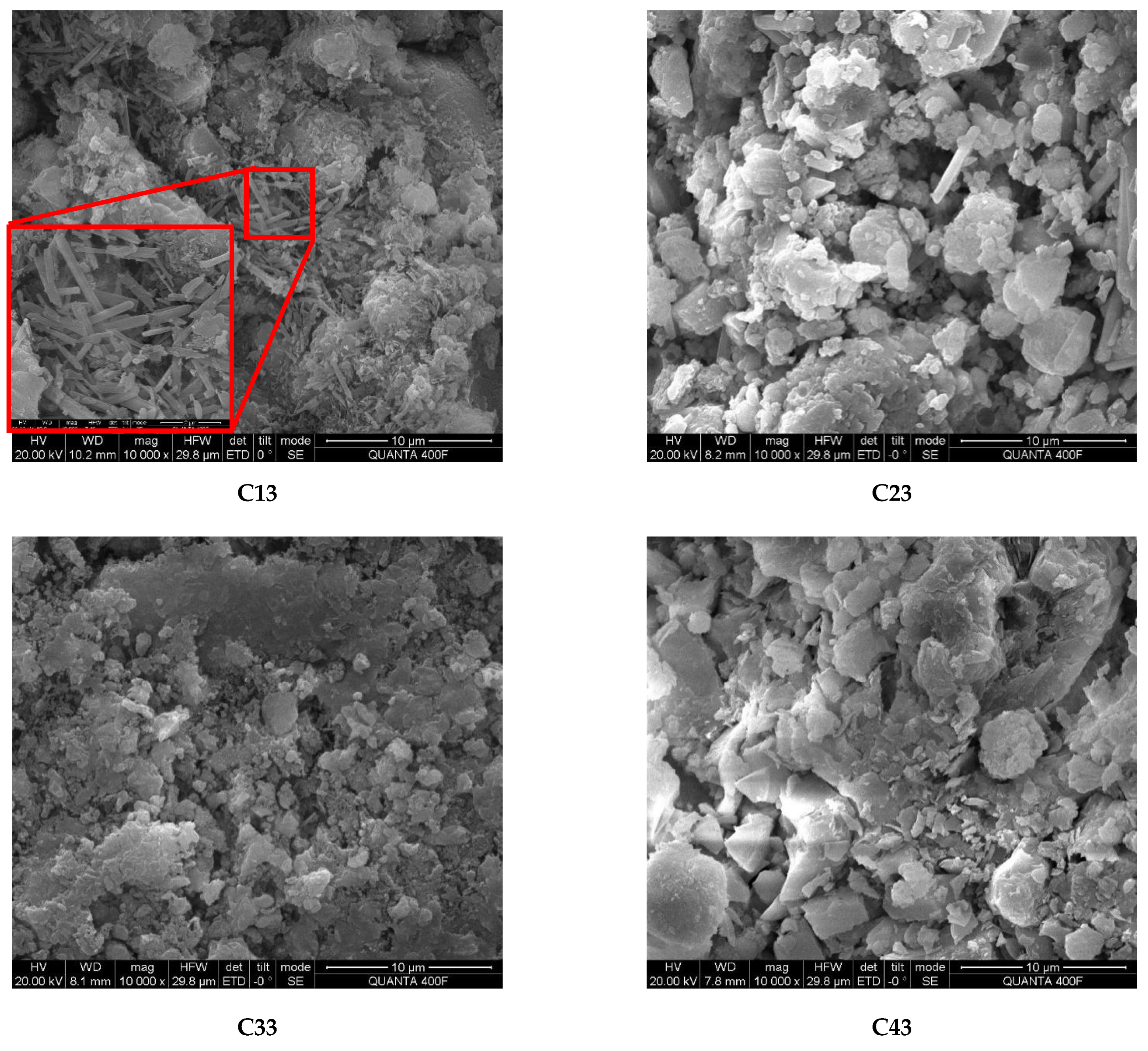
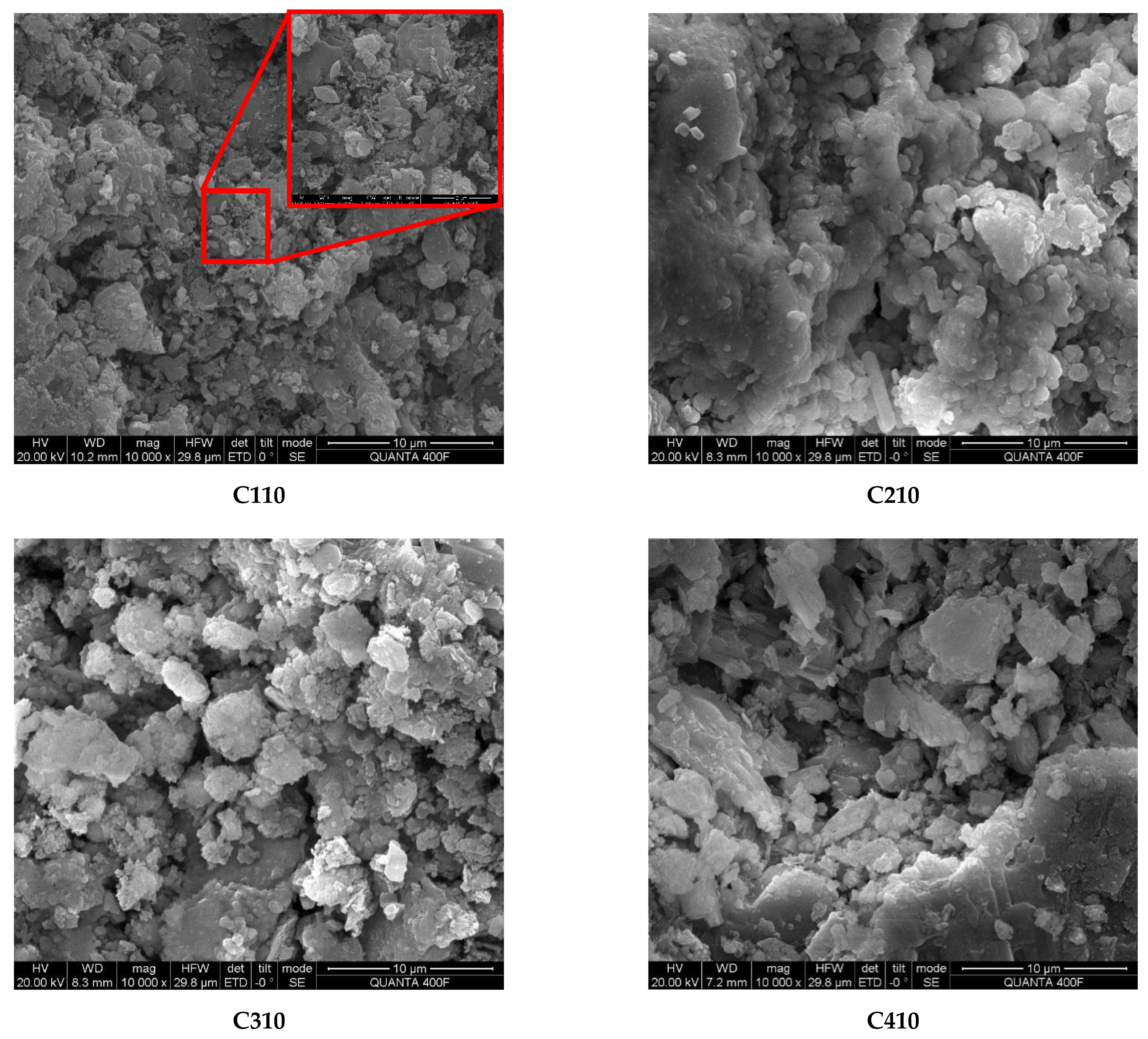
4.3. FE-SEM Mesostructure Analysis
5. Discussion of Mechanisms
- Chemical reactions of specimens after mixing
- 2.
- In the fifth and 10th dry–wet cycles, the masses, elastic moduli, and uniaxial strengths of the specimens were increased. Denudation did not obviously occur on the specimen surface. There were more AE signals. At this time, the specimen fractures were mainly vertical. This is because the hydration reaction inside the specimens was not yet complete, and the solution immersion provided additional time for this reaction. Simultaneously, Cl− fully reacted with the fly ash inside the specimen through diffusion and chemically activated the SiO2 and Al2O3 in the fly ash, generating new gel products (C–S–H, C–S–H, AFt, etc.) to fill the internal fractures and pores in the specimens. The reaction process is given by Equations (9) and (10). The FTIR and XRD spectra revealed that AFt was not converted to Friedel’s salt in large quantities, and AFt can provide higher strength to the specimens than Friedel’s salt. This led to increases in the masses and uniaxial strengths of the specimens at the beginning of the dry–wet cycles. As indicated by the FE-SEM images, the mesostructures of the specimens became more compact, which enhanced both the mass and the uniaxial strength. Additionally, AE signals were generated by the refracture of the closed original fractures within the specimens under load.Ca2+ + Al2O3 + 2OH− → CaO·Al2O3·H2OCa2+ + Al2O3 + Cl− + OH− → 3CaO·Al2O3·3CaCl2·10H2O
- 3.
- At the 15th and 20th dry–wet cycles, the specimens’ masses, elastic moduli, and uniaxial strengths were reduced. The denudation of the specimen surfaces was more severe, and the interlaced development of macroscopic vertical and horizontal fractures was observed. This is because, as the internal hydration reaction approached completion, the erosion failure of the specimens due to the chloride salt solution and the deterioration of the specimens due to the dry–wet cycles began to appear. First, the chloride salt entered the specimen’s interior through diffusion and reacted chemically with the previously formed gel products to produce Friedel’s salt. These products had certain swelling properties. The expansion and contraction of the gel products during the dry–wet cycles resulted in new fractures inside the specimens. The reaction process is given by Equation (11). Furthermore, the dry–wet cycles physically denudated the surfaces of the specimens through deterioration, and the internal structures of the specimens were also damaged. Under the coupled effects of these two factors and other related factors, the masses and uniaxial strengths of the specimens were reduced. The IR and XRD spectra revealed that Friedel’s salt started to appear in the late stage of the dry–wet cycles. Additionally, the FE-SEM images indicated that with the increase in the number of dry–wet cycles, the surfaces of the specimens started to deteriorate, and numerous pores and fractures appeared inside the specimen. As a result, the overall structure became loose.3CaO·Al2O3·CaSO4·12H2O + 2Cl− → 3CaO·Al2O3·CaCl2·10H2O + SO42− + 2H2O
- 4.
- A higher-concentration solution can enter the specimen interior and enhance the uniaxial strength of the specimen faster owing to the higher concentration gradient. The strength deterioration rates of the specimens in the higher-concentration solution were lower than those for the low-concentration solution, but the surface denudation was significant. When the concentration of chloride salt in the solution is higher, the erosion on the surface of the specimen is more severe, and the chloride salt is more likely to invade the specimen interior and chemically react with the interior components, which has a positive effect on the specimen’s strength. In a large amount, a solution with a high concentration of chloride salt can react with the internal components of the specimens or precipitate during the dry–wet cycles. This partially fills the fractures and pores inside the specimens and has a buffering effect on the strength deterioration of the specimens under the coupled effects of dry–wet cycles and chloride erosion. Moreover, a high concentration of Cl− can maintain Friedel’s salt; conversely, a lower concentration of Cl− leads to a looser internal structure because it cannot maintain Friedel’s salt, as shown in Equations (12) and (13). These factors led to the considerable strength deterioration of the specimens subjected to dry–wet cycles in the low-concentration solution. The IR and XRD spectra indicated that Friedel’s salt started to appear late in the dry–wet cycles, and the FE-SEM images indicated that the specimens in the high-concentration solution had fewer pores and fractures than those in the low-concentration solution at the same number of dry–wet cycles. The specimens in the high-concentration solution had better integrity than those in the low-concentration solution.CaCl2 + 3Ca(OH)2 + 12H2O → CaCl2·3Ca(OH)2·12H2O3CaO·Al2O3·CaCl2·10H2O (s) → 4Ca2+ + 2Al(OH)4− +2Cl− + 4OH− + 4H2O
6. Conclusions
- At the early stage of dry–wet cycling in a chloride solution, the strengths of paste specimens were improved owing to the continued hydration reaction and chloride catalysis.
- With increasing drying and wetting cycles of paste specimens in a chlorine salt solution, the erosion effect of chlorine salts and the deterioration effect of the dry–wet cycles began to appear, and the strengths of the paste specimens began to decline.
- Compared with the low-concentration chlorine salt solution, the high-concentration solution can improve the strength of the paste faster and have a buffering effect on the strength deterioration of the paste.
- The changes in the mechanical properties of desulphurised gypsum backfill samples under the coupled effects of chloride salt erosion and dry–wet cycles were investigated, and it was determined that the desulphurised gypsum backfill could maintain a certain strength in this special environment. The results provide a laboratory reference and theoretical basis for the field application of desulphurised gypsum backfill.
- In this study, backfill samples were taken as the research objects. There are size differences between the samples and field backfill. Therefore, it is necessary to study the mechanical properties of backfill under the coupled effects of chloride erosion and dry–wet cycles at the field scale.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTIR | Fourier-Transform Infrared Spectroscopy |
| XRD | X-Ray Diffraction Analysis |
| FE-SEM | Field-Emission Scanning Electron Microscopy |
| XRF | X-Ray Fluorescence |
| Aft | Ettringite |
| C–A–H | xCaO·Al2O3·yH2O |
| AE | Acoustic Emission |
| RA | The Ratio of Rise Time to Amplitude |
| PDS | Pressure Density Stage |
| LES | Linear Elastic Deformation Stage |
| PPYS | Pre-Peak Yielding Stage |
| PPDS | Post-Peak Damage Stage |
| C–S–H | xCaO·SiO2·yH2O |
| C3SH | 3CaO·SiO2·yH2O |
| C4AH | 4CaO·Al2O3·H2O |
| C3A | 3CaO·Al2O3 |
| C4AF | 3CaO·Al2O3·Fe2O3 |
References
- Wang, F.; Jiang, B.; Chen, S.; Ren, M. Surface collapse control under thick unconsolidated layers by backfilling strip mining in coal mines. Int. J. Rock Mech. Min. Sci. 2019, 113, 268–277. [Google Scholar] [CrossRef]
- Li, L.; Yang, P. A numerical evaluation of continuous backfilling in cemented paste backfilled stope through an application of wick drains. Int. J. Min. Sci. Technol. 2015, 25, 897–904. [Google Scholar] [CrossRef]
- Choudhary, B.; Kumar, S. Underground void filling by cemented mill tailings. Int. J. Min. Sci. Technol. 2013, 23, 893–900. [Google Scholar] [CrossRef]
- Feng, G.; Jia, X.; Guo, Y.; Qi, T.; Li, D.; Li, Z.; Feng, J.; Liu, G.; Song, K.; Kang, L. Influence of the wasted concrete coarse aggregate on the performance of cemented paste backfill. J. China Coal Soc. 2015, 40, 1320–1325. [Google Scholar]
- Chen, S.; Du, Z.; Zhang, Z.; Yin, D.; Feng, F.; Ma, J. Effects of red mud additions on gangue-cemented paste backfill properties. Powder Technol. 2020, 367, 833–840. [Google Scholar] [CrossRef]
- Li, F.; Yin, D.; Zhu, C.; Wang, F.; Jiang, N.; Zhang, Z. Effects of Kaolin Addition on Mechanical Properties for Cemented Coal Gangue-Fly Ash Backfill under Uniaxial Loading. Energies 2021, 14, 3693. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Wu, S.-C.; Zhang, X.-Q.; Wu, A.-X. Effect of particle gradation characteristics on yield stress of cemented paste backfill. Int. J. Miner. Met. Mater. 2020, 27, 10–17. [Google Scholar] [CrossRef]
- Fall, M.; Pokharel, M. Coupled effects of sulphate and temperature on the strength development of cemented tailings backfills: Portland cement-paste backfill. Cem. Concr. Compos. 2010, 32, 819–828. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhou, N.; Sheng, L.; Zhang, L.; Zhu, L.; Wang, L. Study of the leaching and solidification mechanism of heavy metals from gangue-based cemented paste backfilling materials. J. China Univ. Min. Technol. 2021, 50, 523–531. [Google Scholar]
- Zhang, J.; Tu, S.; Cao, Y.; Tan, Y.; Xin, H.; Pang, J. Coal gangue intelligent separation and backfilling technology and its engi-neering application in underground coal mine. J. China Univ. Min. Technol. 2021, 50, 523–531. [Google Scholar]
- Chen, S.; Jiang, T.; Zhang, H.; Kong, K.; Bie, L. Emission reduction process for dechlorinating flue-gas desulfurization gypsum and reducing wastewater effluents: Application prospects from laboratory-scale studies. Energy Sci. Eng. 2020, 8, 2662–2679. [Google Scholar] [CrossRef]
- Chandara, C.; Azizli, K.A.M.; Ahmad, Z.A.; Sakai, E. Use of waste gypsum to replace natural gypsum as set retarders in portland cement. Waste Manag. 2009, 29, 1675–1679. [Google Scholar] [CrossRef]
- Hou, K. Flue gas desulfurization slag from power plant replaces natural gypsum as cement retarder. Cement 2005, 10, 21–23. [Google Scholar]
- Tzouvalas, G.; Rantis, G.; Tsimas, S. Alternative calcium-sulfate-bearing materials as cement retarders: Part II. FGD gypsum. Cem. Concr. Res. 2004, 34, 2119–2125. [Google Scholar] [CrossRef]
- Colak, A. The long-term durability performance of gypsum–Portland cement–natural pozzolan blends. Cem. Concr. Res. 2002, 32, 109–115. [Google Scholar] [CrossRef]
- Guo, X.L.; Shi, H.S. Thermal treatment and utilization of flue gas desulphurization gypsum as an admixture in cement and concrete. Constr. Build. Mater. 2008, 22, 1471–1476. [Google Scholar] [CrossRef]
- Guo, X.-L.; Shi, H.-S.; Liu, H.-Y. Effects of a combined admixture of slag powder and thermally treated flue gas desulphurization (FGD) gypsum on the compressive strength and durability of concrete. Mater. Struct. 2008, 42, 263–270. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, L.; Yang, L.; Zhang, L.; Zhao, F. Study on water resistant gypsum blocks from desulfurization gypsum. Fly Ash Compr. Utilizat. 2009, 2, 27–30. [Google Scholar]
- Qiao, X.; Poon, C.; Cheeseman, C. Use of flue gas desulphurization (FGD) waste and rejected fly ash in waste stabilization systems. Waste Manag. 2006, 26, 141–149. [Google Scholar] [CrossRef]
- Li, B.; Yin, H.; Mao, X.; Li, Y.; Zhang, L.; Liu, R.; Qiu, P. Macroscopic and microscopic fracture features of concrete used in coal mine under chlorine salt erosion. Int. J. Min. Sci. Technol. 2016, 26, 455–459. [Google Scholar] [CrossRef]
- Zhang, H. Effect of Chloride on Early Mechanical Properties of Cement Paste Filling Body. In Proceedings of the Technical Forum on Wastewater Treatment and Zero Discharge of High-salt Wastewater, Hangzhou, China, 25 October 2019. [Google Scholar]
- Zhang, J.; Yao, Y.; Wang, X.; Guo, S.; Dong, G. Study on the combination of Ettringite and chloride ion in different chloride solutions. J. Build. Mater. 2021, 6, 1–9. [Google Scholar]
- Ekolu, S.; Thomas, M.; Hooton, R.D. Pessimum effect of externally applied chlorides on expansion due to delayed ettringite formation: Proposed mechanism. Cem. Concr. Res. 2006, 36, 688–696. [Google Scholar] [CrossRef]
- Du, Z.; Chen, S.; Yin, D.; Yao, D.; Zhang, Z. Experimental study of stability of paste backfill under chloride erosion environment. J. China Univ. Min. Technol. 2021, 50, 532–538, 547. [Google Scholar]
- Zhu, Q.; Jiang, L.; Chen, Y.; Xu, J.; Mo, L. Effect of chloride salt type on chloride binding behavior of concrete. Constr. Build. Mater. 2012, 37, 512–517. [Google Scholar] [CrossRef]
- Farnam, Y.; Washington, T.; Weiss, W. The Influence of Calcium Chloride Salt Solution on the Transport Properties, of Cementitious Materials. Adv. Civ. Eng. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Chen, S.; Du, Z.; Zhang, Z.; Zhang, H.; Xia, Z.; Feng, F. Effects of chloride on the early mechanical properties and microstructure of gangue-cemented paste backfill. Constr. Build. Mater. 2020, 235, 117504. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; He, F.; Yuan, Q.; Wang, D.; Huang, Y.; Li, Q. Chloride binding and its effects on microstructure of cement-based materials. J. Chin. Ceram. Society 2013, 41, 187–198. [Google Scholar]
- Chen, S.; Jiang, T.; Wang, H.; Feng, F.; Yin, D.; Li, X. Influence of cyclic wetting-drying on the mechanical strength characteristics of coal samples: A laboratory-scale study. Energy Sci. Eng. 2019, 7, 3020–3037. [Google Scholar] [CrossRef]
- Li, Y.; Ba, M.; Liu, J.; He, Z. Resistance to chloride erosion of cement matrix composite materials under dry-wet cycling and their micro-structural changes. Acta Mater. Comp. Sin. 2017, 34, 2856–2865. [Google Scholar]
- Chen, Y.; Gao, J.; Tang, L.; Li, X. Resistance of concrete against combined attack of chloride and sulfate under drying–wetting cycles. Constr. Build. Mater. 2016, 106, 650–658. [Google Scholar] [CrossRef]
- Aldaood, A.; Bouasker, M.; Al-Mukhtar, M. Impact of wetting–drying cycles on the microstructure and mechanical properties of lime-stabilized gypseous soils. Eng. Geol. 2014, 174, 11–21. [Google Scholar] [CrossRef]
- Kalkan, E. Impact of wetting–drying cycles on swelling behavior of clayey soils modified by silica fume. Appl. Clay Sci. 2011, 52, 345–352. [Google Scholar] [CrossRef]
- Zhou, S.; Lv, H.; Wu, Y. Degradation behavior of concrete under corrosive coal mine environment. Int. J. Min. Sci. Technol. 2019, 29, 307–312. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, R.; Jian, W. Study on water conversion law and solidification mechanism of cement-slag-fly ash solidified silt. J. Eng. Geol. 2021, 6, 1–11. [Google Scholar]
- Jiang, T. Experimental Study on the Effect of Flue Gas Desulfurization Gypsum Dechlorinating on the Properties of Paste Back-Filling Materials. Master’s Thesis, Shandong University of Science and Technology, Qingdao, China, 2021. [Google Scholar]
- Ma, J.; Jiang, N.; Wang, X.; Jia, X.; Yao, D. Numerical Study of the Strength and Characteristics of Sandstone Samples with Combined Double Hole and Double Fissure Defects. Sustainability 2021, 13, 7090. [Google Scholar] [CrossRef]
- Wang, Z. Effect of dry-wet cycles on mechanical properties of fly ash high-moisture materials. Fly Ash Compr. Utilizat. 2018, 3, 40–42. [Google Scholar]
- Ammasi, A.K. Ragul Strength and Durability of High Volume Fly Ash in Engineered Cementitious Composites. Mater. Today Proc. 2018, 5, 24050–24058. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J. Preparation of Ca-Al hydrotalcite-like compounds and their desilication in sodium aluminate solution. Sci. Sin. Chim. 2010, 40, 577–584. [Google Scholar] [CrossRef]
- Sun, R.; Hu, X.; Ling, Y.; Zuo, Z.; Zhuang, P.; Wang, F. Chloride diffusion behavior of engineered cementitious composite under dry-wet cycles. Constr. Build. Mater. 2020, 260, 119943. [Google Scholar] [CrossRef]
- Hirao, H.; Yamada, K.; Takahashi, H.; Zibara, H. Chloride Binding of Cement Estimated by Binding Isotherms of Hydrates. J. Adv. Concr. Technol. 2005, 3, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Suryavanshi, A.; Narayan, S. Stability of Friedel’s salt in carbonated concrete structural elements. Cem. Concr. Res. 1996, 26, 729–741. [Google Scholar] [CrossRef]
- Wang, X.; Wang, E.; Liu, X.; Jia, H.; Li, D.; Wang, H. Time-frequency-space response of damage evolution for concrete based on AE-UT joint monitoring under step-loading. Int. J. Min. Sci. Technol. 2019, 48, 268–277. [Google Scholar]
- Shiotani, T.; Ohtsu, M.; Ikeda, K. Detection and evaluation of AE waves due to rock deformation. Constr. Build. Mater. 2001, 15, 235–246. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, X.; Fu, S.; Li, Y. Effect of desulphurization gypsum on the strength and microstructure of the sludge ash ce-mentitious system. J. Zhengzhou Univ. (Eng. Sci.) 2021, 6, 1–6. [Google Scholar]




| Raw Materials | Chemical Composition and Content (%) | |||||
|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | SO3 | Fe2O3 | |
| Cement | 22.30 | 4.82 | 62.47 | 2.34 | 1.82 | 3.83 |
| Fly ash | 56.30 | 18.05 | 11.84 | 3.01 | 1.19 | 4.93 |
| Coal gangue | 59.83 | 20.10 | 4.89 | 1.22 | - | 6.52 |
| Blast furnace slag | 35.83 | 12.73 | 38.09 | 7.86 | - | 2.79 |
| Desulphurised gypsum | 24.40 | 2.62 | 32.25 | 1.33 | 37.58 | 0.73 |
| Solution Concentration | Uniaxial Compressive Strength (MPa) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Dry–Wet Cycles | 3% | Average | 5% | Average | 10% | Average | |||||||
| 0 | 10.77 | 10.74 | 10.14 | 10.49 | - | - | - | - | - | - | - | - | |
| 5 | 9.41 | 10.97 | 12.02 | 10.80 | 10.47 | 10.61 | 10.86 | 10.64 | 10.20 | 12.19 | 12.90 | 11.76 | |
| 10 | 12.72 | 11.81 | 10.65 | 11.73 | 10.81 | 11.73 | 9.97 | 10.84 | 10.96 | 13.15 | 10.26 | 11.46 | |
| 15 | 11.20 | 11.04 | 9.59 | 10.61 | 9.75 | 10.37 | 8.65 | 9.59 | 10.13 | 11.31 | 12.61 | 11.35 | |
| 20 | 8.95 | 7.94 | 9.94 | 8.94 | 7.90 | 9.59 | 10.25 | 9.25 | 9.14 | 11.98 | 12.08 | 11.07 | |
| Solution Concentration | Elastic Modulus (MPa) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Dry–Wet Cycles | 3% | Average | 5% | Average | 10% | Average | |||||||
| 0 | 835.21 | 764.58 | 833.17 | 810.99 | - | - | - | - | - | - | - | - | |
| 5 | 725.60 | 1019.87 | 902.74 | 882.74 | 945.18 | 819.49 | 912.33 | 892.33 | 756.21 | 1025.90 | 921.05 | 901.05 | |
| 10 | 1044.36 | 931.05 | 1017.71 | 997.71 | 755.93 | 916.95 | 866.44 | 846.44 | 819.87 | 1030.47 | 955.17 | 935.17 | |
| 15 | 942.89 | 886.97 | 944.93 | 924.93 | 884.44 | 888.26 | 916.35 | 896.35 | 627.94 | 1004.25 | 846.10 | 826.10 | |
| 20 | 603.23 | 520.11 | 591.67 | 571.67 | 647.76 | 733.23 | 720.49 | 700.49 | 646.37 | 1096.05 | 901.21 | 881.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, F.; Yin, D.; Jiang, T.; Zhang, Z. Experimental Study on Mechanical Properties of Paste Backfill with Flue-Gas Desulphurisation Gypsum under Combined Action of Dry–Wet Cycles and Chloride Erosion. Minerals 2021, 11, 882. https://doi.org/10.3390/min11080882
Wang S, Wang F, Yin D, Jiang T, Zhang Z. Experimental Study on Mechanical Properties of Paste Backfill with Flue-Gas Desulphurisation Gypsum under Combined Action of Dry–Wet Cycles and Chloride Erosion. Minerals. 2021; 11(8):882. https://doi.org/10.3390/min11080882
Chicago/Turabian StyleWang, Sheng, Feng Wang, Dawei Yin, Tianqi Jiang, and Zhen Zhang. 2021. "Experimental Study on Mechanical Properties of Paste Backfill with Flue-Gas Desulphurisation Gypsum under Combined Action of Dry–Wet Cycles and Chloride Erosion" Minerals 11, no. 8: 882. https://doi.org/10.3390/min11080882
APA StyleWang, S., Wang, F., Yin, D., Jiang, T., & Zhang, Z. (2021). Experimental Study on Mechanical Properties of Paste Backfill with Flue-Gas Desulphurisation Gypsum under Combined Action of Dry–Wet Cycles and Chloride Erosion. Minerals, 11(8), 882. https://doi.org/10.3390/min11080882






