Crystal Chemistry of Six Grossular Garnet Samples from Different Well-Known Localities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Electron-Probe Microanalysis (EPMA)
2.3. Synchrotron High-Resolution Powder X-ray Diffraction (HRPXRD)
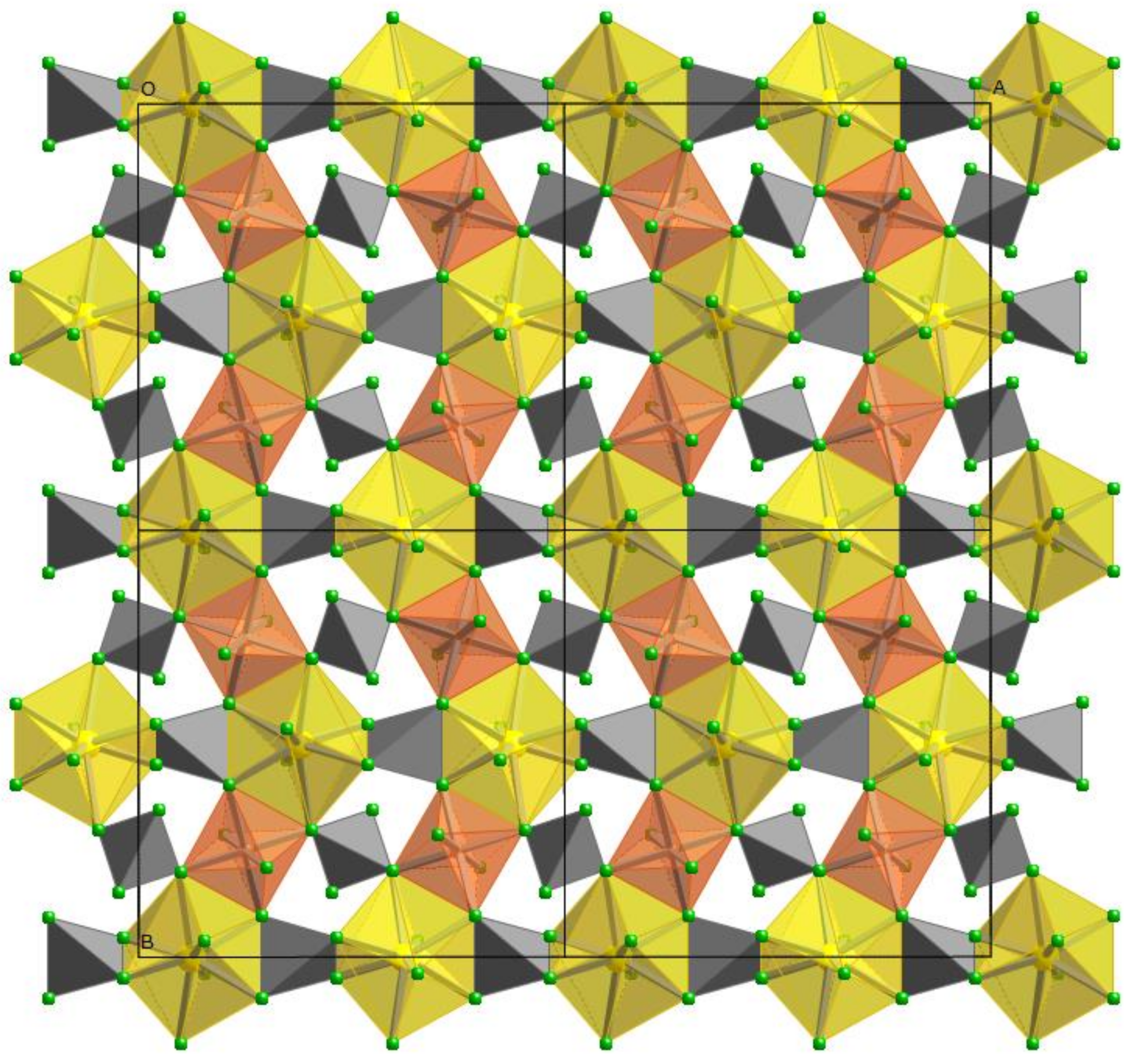
2.4. Rietveld Structural Refinement
3. Results
3.1. Chemical Analyses
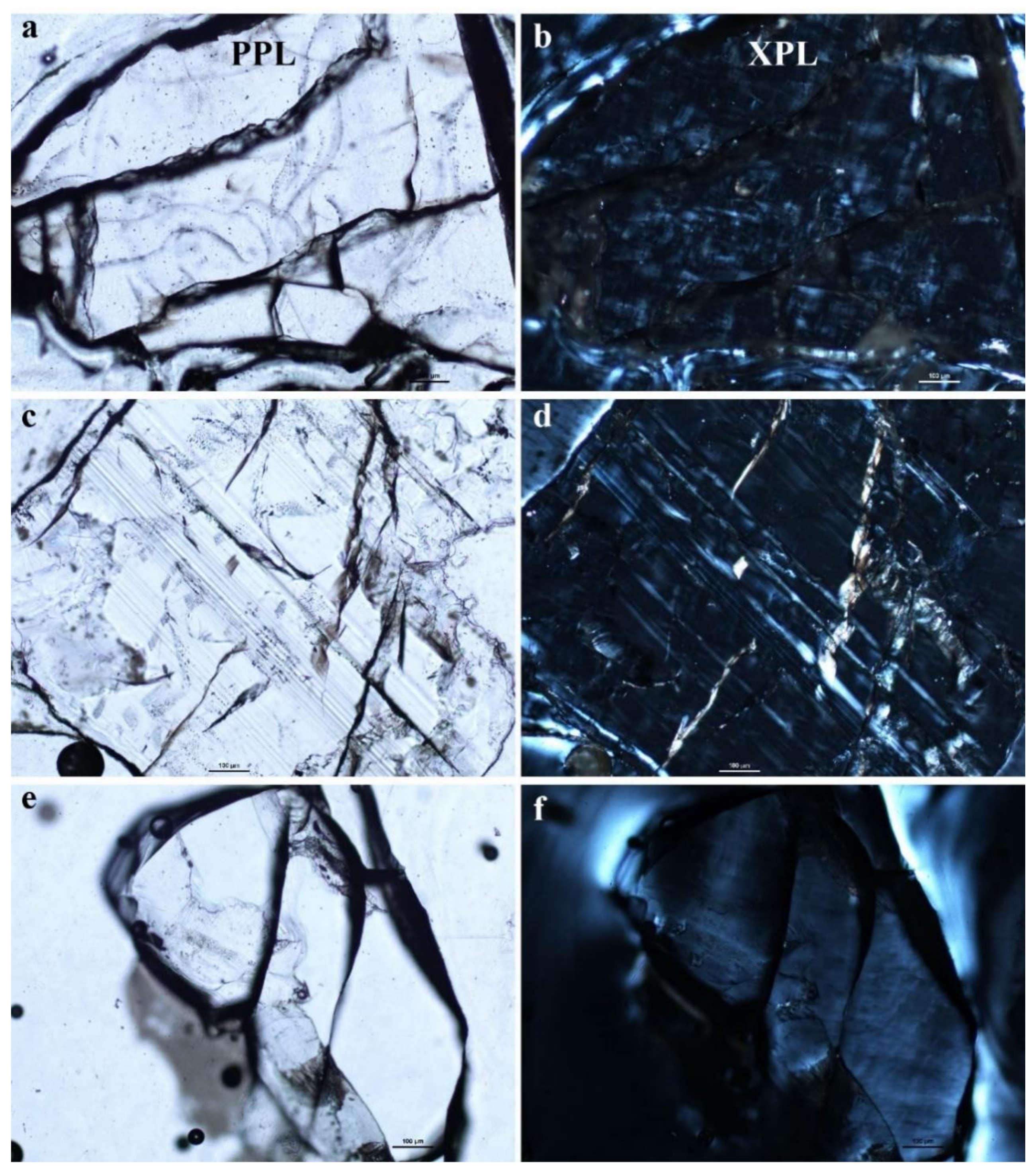
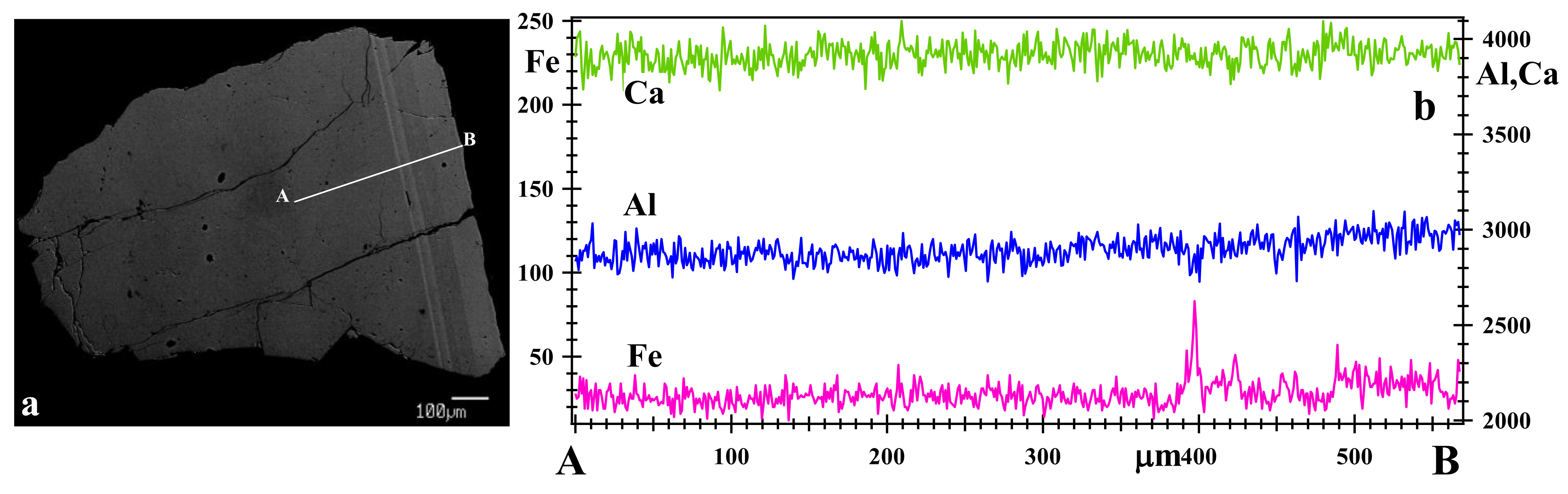
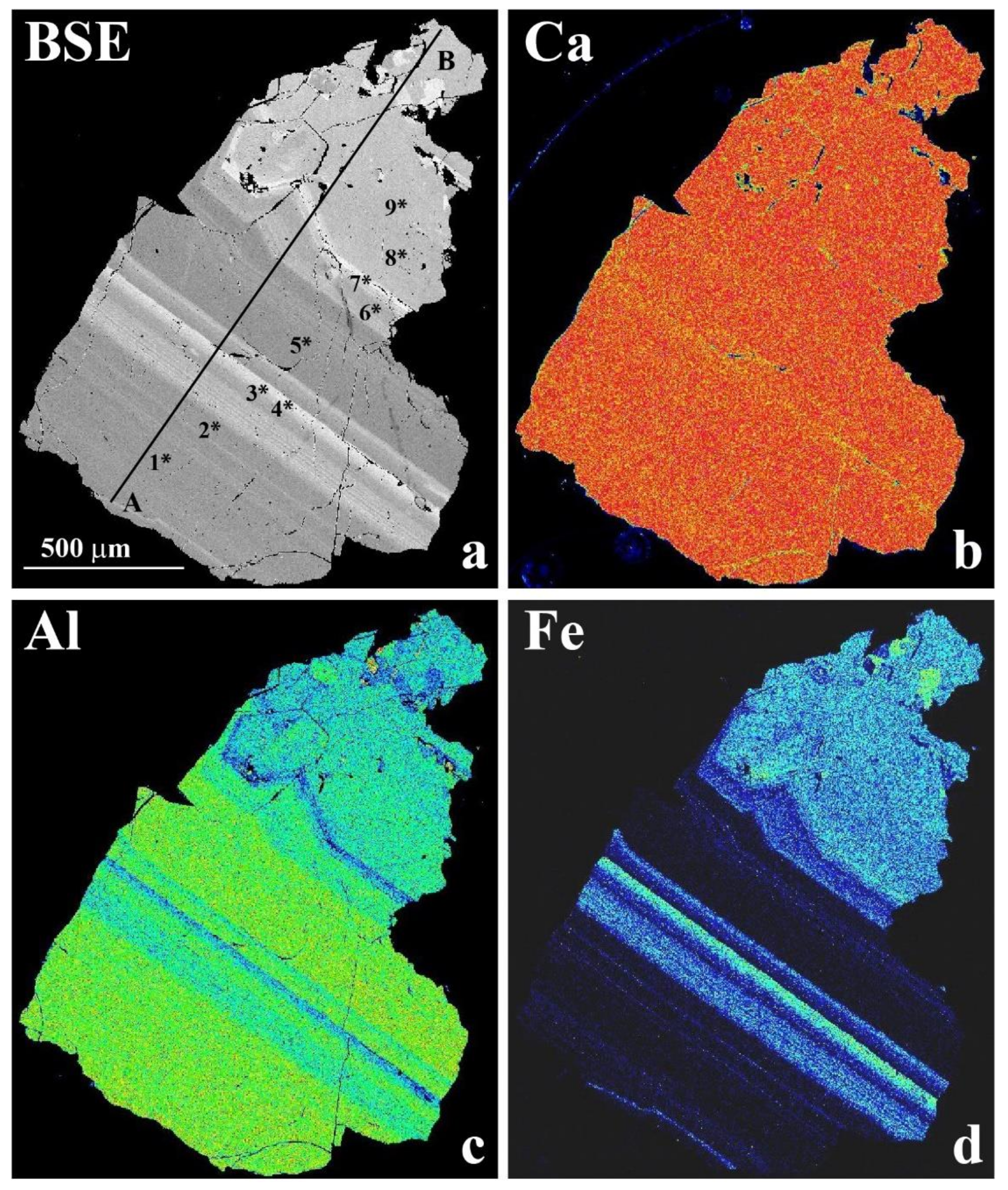
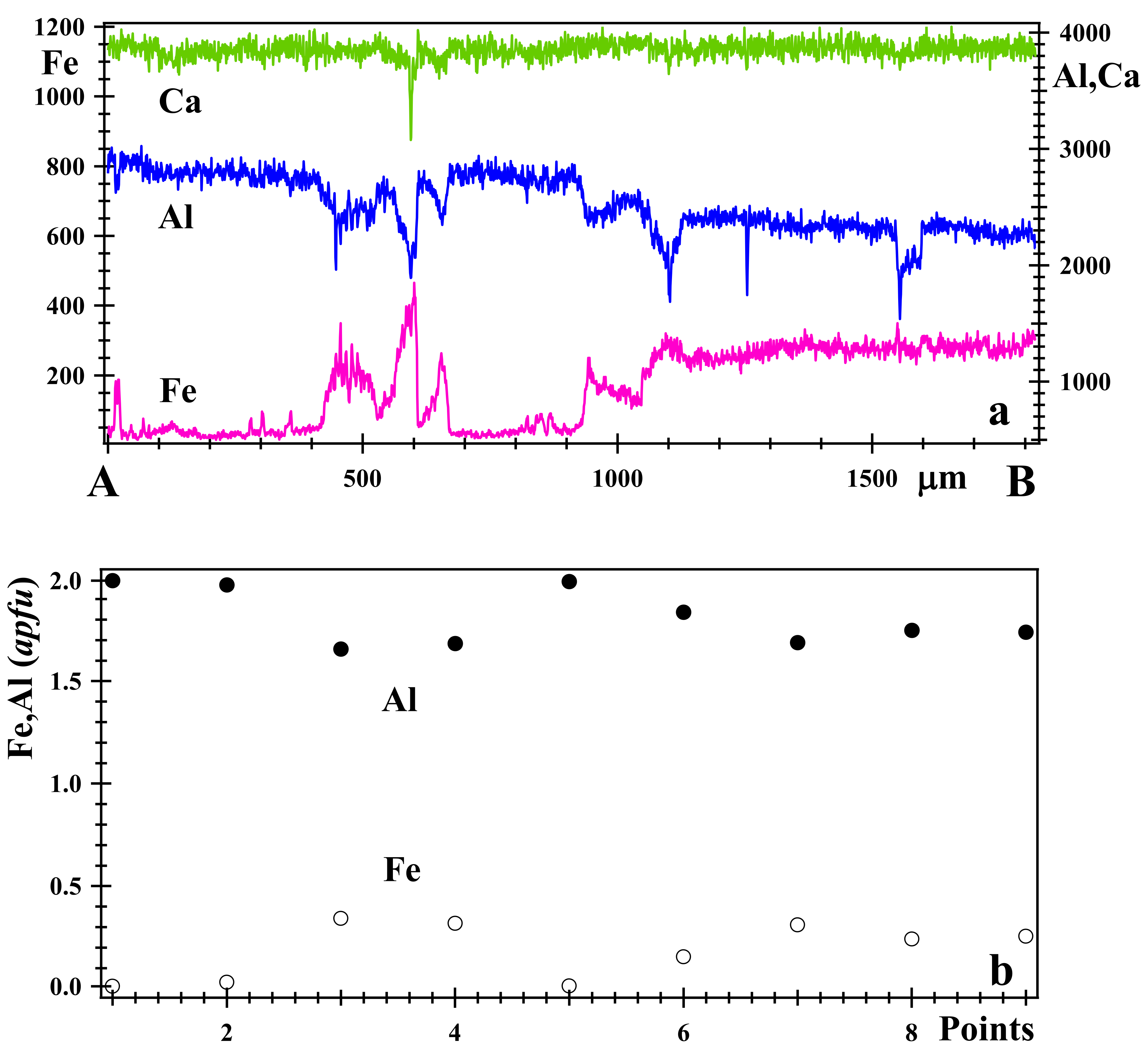
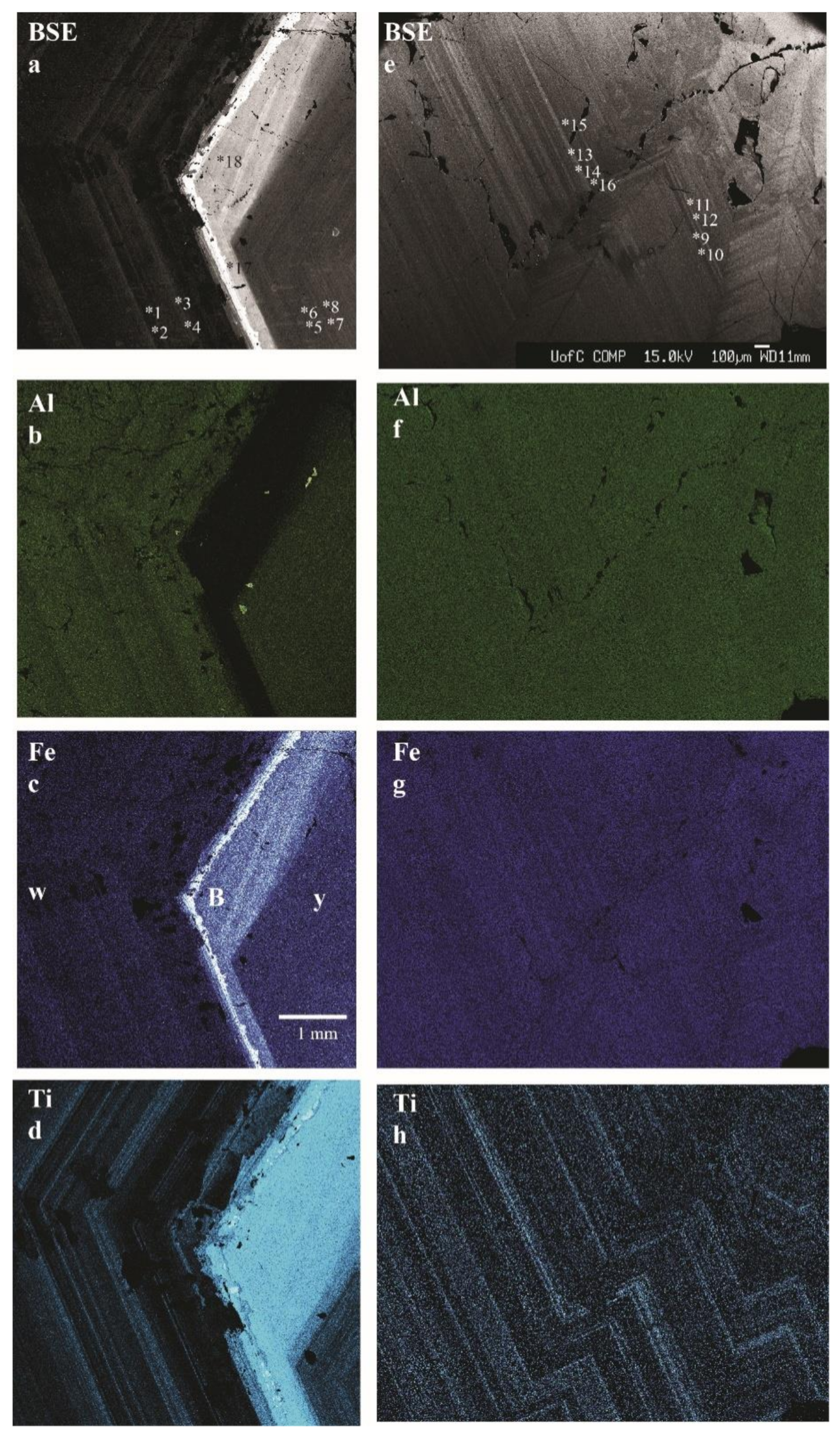
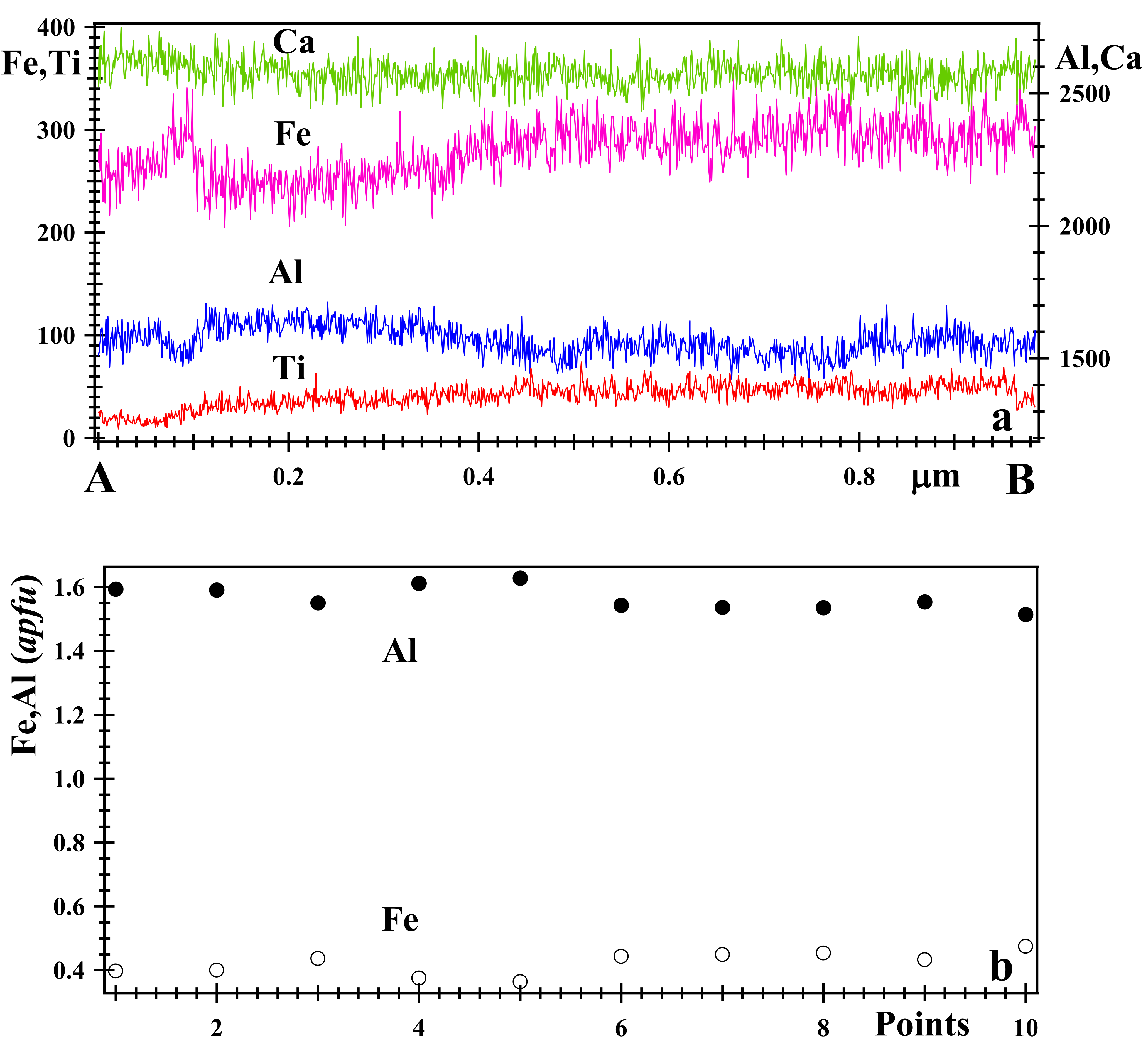
3.2. Optical Light Microscopy, Backscattered Electron (BSE) Images and X-ray Elemental Maps
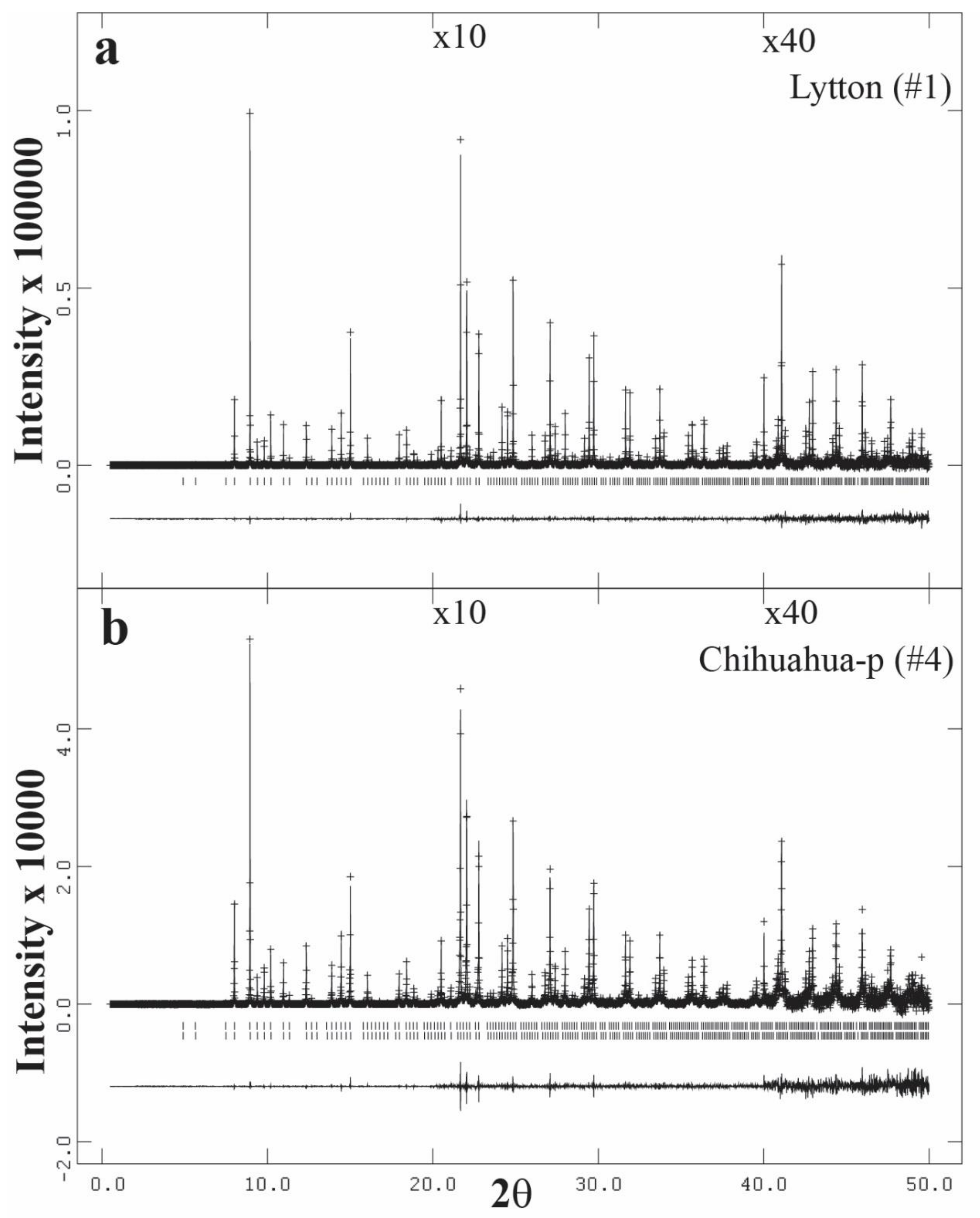
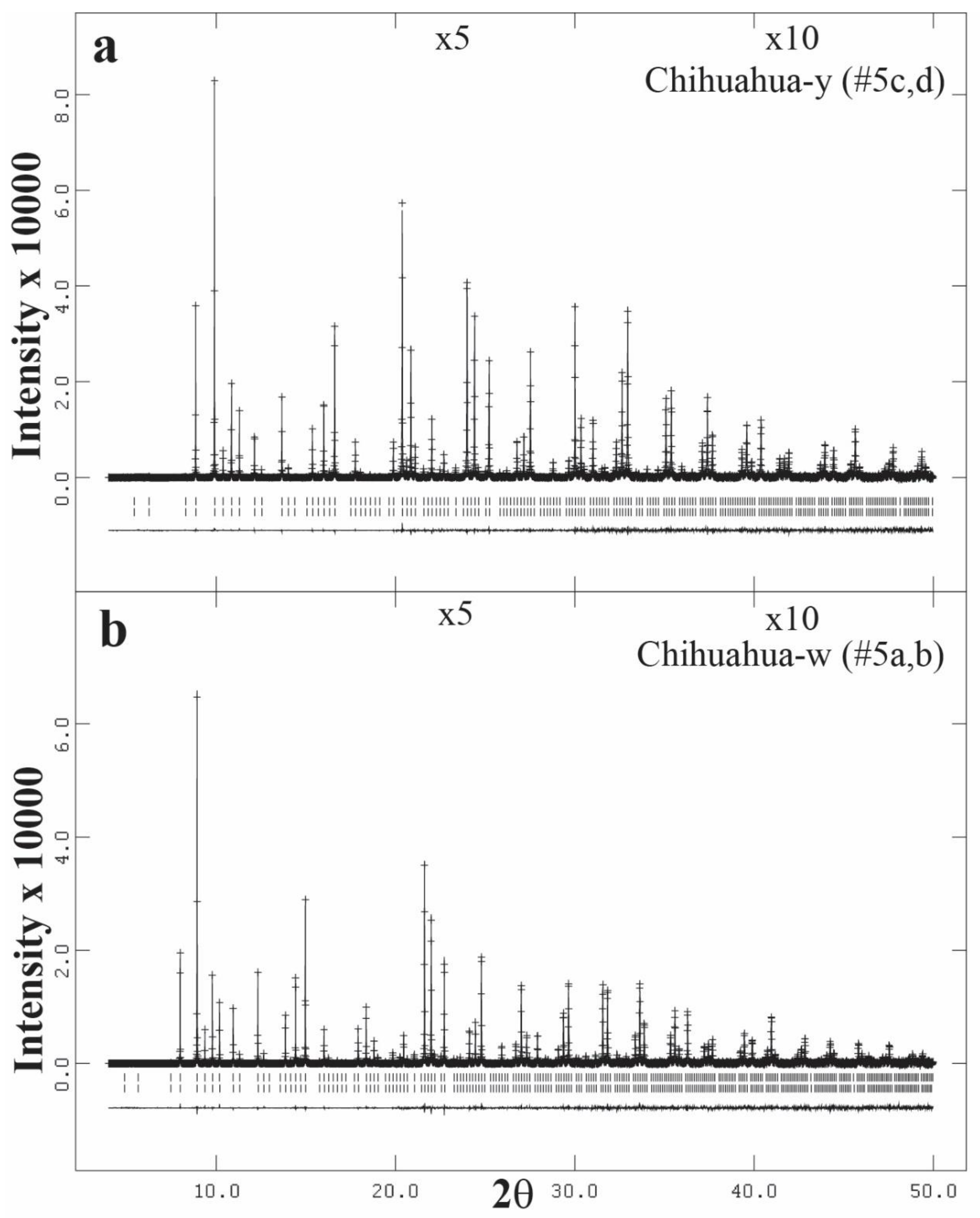
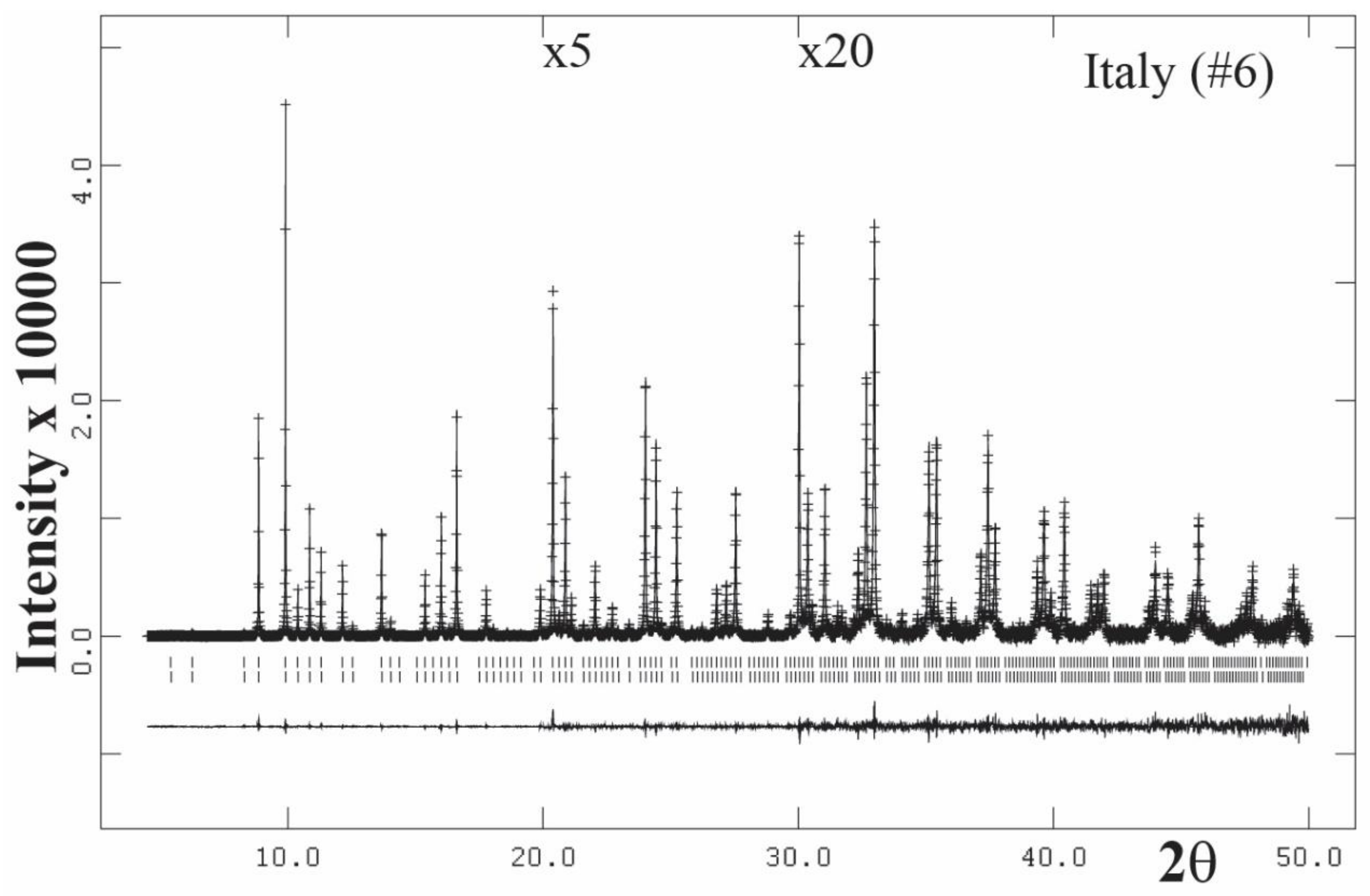
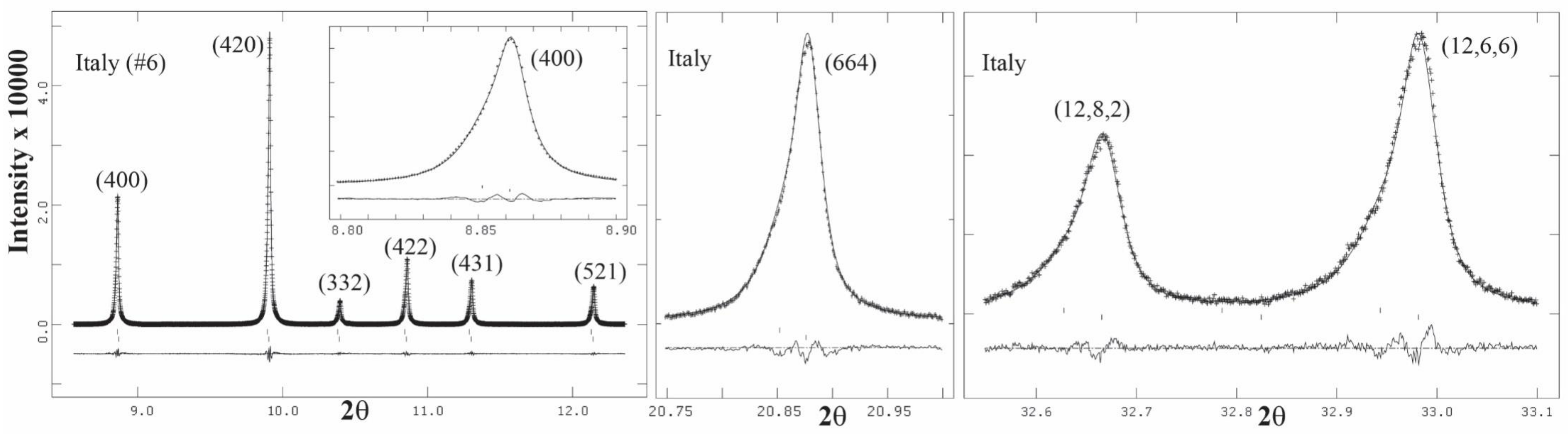
3.3. High-Resolution Powder X-ray Diffraction (HRPXRD) Traces
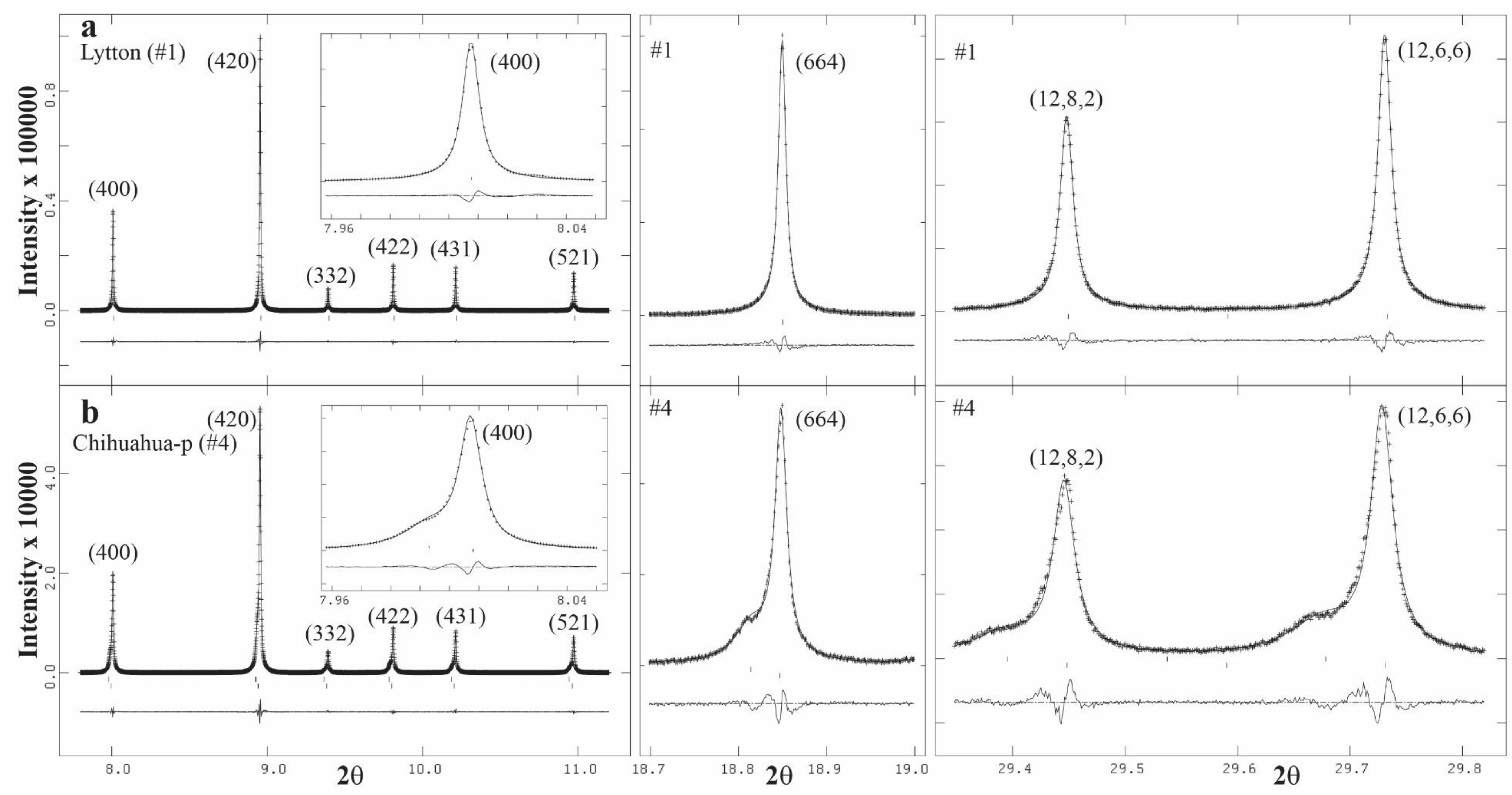
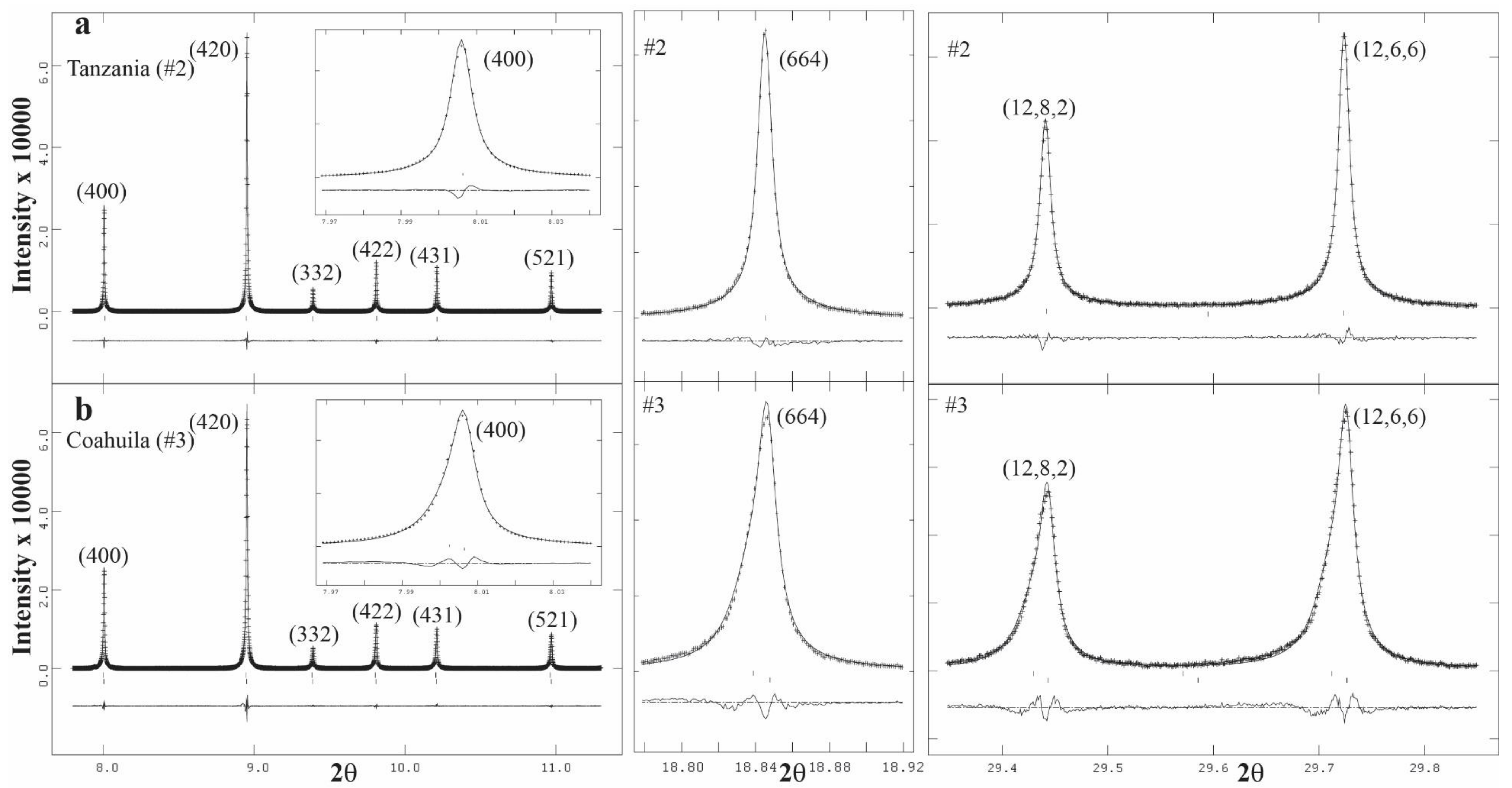
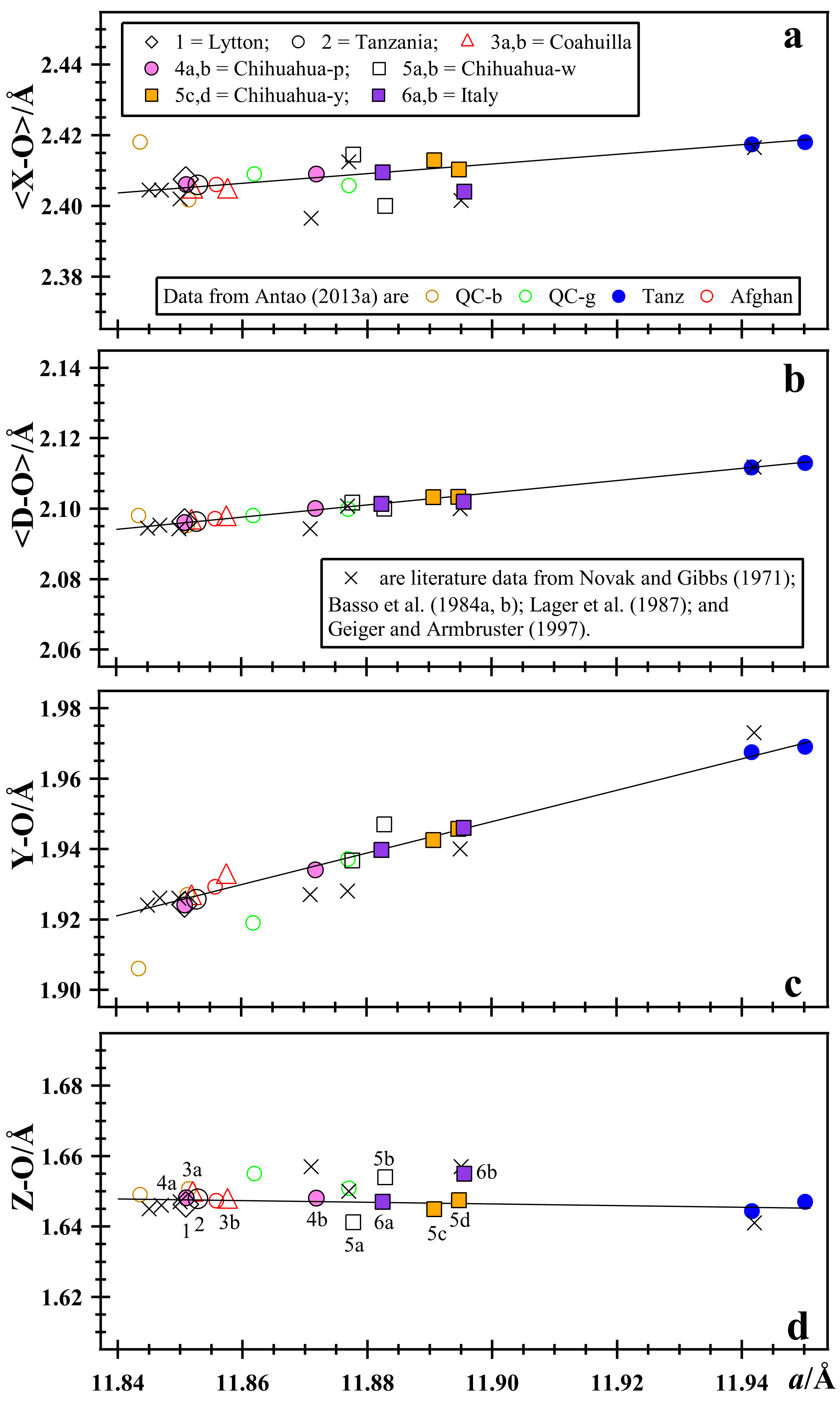
3.4. Rietveld Refinements and Structural Variations across Grossular–Andradite Solid Solutions
3.5. Is There a Miscibility Gap between Grossular and Andradite Solid Solutions?
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antao, S.M. Is near-endmember birefringent grossular non-cubic? New evidence from synchrotron diffraction. Can. Mineral. 2013, 51, 771–784. [Google Scholar] [CrossRef]
- Geiger, C.A.; Stahl, A.; Rossman, G.R. Raspberry-red grossular from Sierra de Cruces Range, Coahuila, Mexico. Eur. J. Mineral. 1999, 11, 1109–1113. [Google Scholar] [CrossRef]
- Antao, S.M. Three cubic phases intergrown in a birefringent andradite-grossular garnet and their implications. Phys. Chem. Miner. 2013, 40, 705–716. [Google Scholar] [CrossRef]
- Antao, S.M. The mystery of birefringent garnet: Is the symmetry lower than cubic? Powder Diffr. 2013, 28, 281–288. [Google Scholar] [CrossRef]
- Antao, S.M. Crystal structure of morimotoite from Ice River, Canada. Powder Diffr. 2014, 29, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Antao, S.M.; Klincker, A.M. Origin of birefringence in andradite from Arizona, Madagascar, and Iran. Phys. Chem. Miner. 2013, 40, 575–586. [Google Scholar] [CrossRef]
- Antao, S.M.; Klincker, A.M. Crystal structure of a birefringent andradite-grossular from Crowsnest Pass, Alberta, Canada. Powder Diffr. 2013, 29, 20–27. [Google Scholar] [CrossRef]
- Antao, S.M.; Round, S.A. Crystal chemistry of birefringent spessartine. Powder Diffr. 2014, 29, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, Q.; Duan, D.; Bao, X.; Liu, X. The study of crystal structure on grossular-andradite solid solution. Minerals 2019, 9, 691. [Google Scholar] [CrossRef] [Green Version]
- Brauns, R. Die Optischen Anomalien der Kristalle; Preisschr. Jablonowski Ges.: Leipzig, Germany, 1891. [Google Scholar]
- Brewster, D. On the optical figures produced by the disintegrated surfaces of crystals. Philos. Mag. Ser. 1853, 6, 16–30. [Google Scholar] [CrossRef]
- Mallard, E. Anomalies optiques. Ann. Mines. Mem. VII Ser. 1876, 10, 60. [Google Scholar]
- Allen, F.M.; Buseck, P.R. XRD, FTIR, and TEM studies of optically anisotropic grossular garnets. Am. Mineral. 1988, 73, 568–584. [Google Scholar]
- Brown, D.; Mason, R.A. An occurrence of sectored birefringence in almandine from the Gangon terrane, Labrador. Can. Mineral. 1994, 32, 105–110. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Orthosilicates; Longman Group Limited: New York, NY, USA, 1982; Volume 1A, 919p. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; John Wiley: New York, NY, USA, 1992. [Google Scholar]
- Rossman, G.R.; Aines, R.D. The hydrous components in garnets: Grossular-hydrogrossular. Am. Mineral. 1991, 76, 1153–1164. [Google Scholar]
- Akizuki, M. Origin of optical variations in grossular-andradite garnet. Am. Mineral. 1984, 66, 403–409. [Google Scholar]
- Akizuki, M.; Takéuchi, Y.; Terada, T.; Kudoh, Y. Sectoral texture of a cubo-dodecahedral garnet in grandite. Neues Jahrb. Mineral. Mon. 1998, 12, 565–576. [Google Scholar]
- Frank-Kamenetskaya, O.V.; Rozhdestvenskaya, L.V.; Shtukenberg, A.G.; Bannova, I.I.; Skalkina, Y.A. Dissymmetrization of crystal structures of grossular-andradite garnets Ca3(Al, Fe)2(SiO4)3. Struct. Chem. 2007, 18, 493–503. [Google Scholar] [CrossRef]
- Griffen, D.T.; Hatch, D.M.; Phillips, W.R.; Kulaksiz, S. Crystal chemistry and symmetry of a birefringent tetragonal pyralspite75-grandite25 garnet. Am. Mineral. 1992, 77, 399–406. [Google Scholar]
- Kingma, K.J.; Downs, J.W. Crystal-structure analysis of a birefringent andradite. Am. Mineral. 1989, 74, 1307–1316. [Google Scholar]
- Shtukenberg, A.G.; Popov, D.Y.; Punin, Y.O. Growth ordering and anomalous birefringence in ugrandite garnets. Mineral. Mag. 2005, 69, 537–550. [Google Scholar] [CrossRef]
- Htukenberg, A.G.; Punin, Y.O.; Frank-Kamenetskaya, O.V.; Kovalev, O.G.; Sokolov, P.B.; Shtukenberg, A.G.; Punin, Y.O.; Frank-Kamenetskaya, O.V.; Kovalev, O.G.; Sokolov, P.B. On the origin of anomalous birefringence in grandite garnets. Mineral. Mag. 2001, 65, 445–459. [Google Scholar] [CrossRef]
- Takéuchi, Y.; Haga, N.; Umizu, S.; Sato, G. The derivative structure of silicate garnets in grandite. Z. Krist. 1982, 158, 53–99. [Google Scholar] [CrossRef]
- Wildner, M.; Andrut, M. The crystal chemistry of birefringent natural uvarovites: Part II. Single-crystal X-ray structures. Am. Mineral. 2001, 86, 1231–1251. [Google Scholar] [CrossRef]
- Cesare, B.; Nestola, F.; Johnson, T.; Mugnaioli, E.; Della Ventura, G.; Peruzzo, L.; Bartoli, O.; Viti, C.; Erickson, T. Garnet, the archetypal cubic mineral, grows tetragonal. Sci. Rep. 2019, 9, 14672. [Google Scholar] [CrossRef] [Green Version]
- Tančić, P.; Kremenović, A.; Vulić, P. Structural dissymmetrization of optically anisotropic Grs64±1Adr36±1Sps2 grandite from Meka Presedla (Kopaonik Mt., Serbia). Powder Diffr. 2019, 35, 7–16. [Google Scholar] [CrossRef]
- McAloon, B.P.; Hofmeister, A.M. Single-crystal absorption and reflection infrared spectroscopy of birefringent grossular-andradite garnets. Am. Mineral. 1993, 78, 957–967. [Google Scholar]
- Krivovichev, S.V.; Panikorovskii, T.L.; Yakovenchuk, V.N.; Selivanova, E.A.; Ivanyuk, G.Y. Trigonal variation in the garnet supergroup: The crystal structure of nikmelnikovite, Ca12Fe2+Fe3+3Al3(SiO4)6(OH)20, from Kovdor massif, Kola Peninsula, Russia. Mineral. Mag. 2021, 1–22. [Google Scholar] [CrossRef]
- Bank, H. Über grossular und hydrogrossular. Z. Dtsch. Gemmol. Ges. 1982, 31, 93–96. [Google Scholar]
- Ganguly, J.; Cheng, W.; O’Neill, H.S.C. Syntheses, volume, and structural changes of garnets in the pyrope-grossular join: Implications for stability and mixing properties. Am. Mineral. 1993, 78, 583–593. [Google Scholar]
- Hirai, H.; Nakazawa, H. Visualizing low symmetry of a grandite garnet on precession photographs. Am. Mineral. 1986, 71, 1210–1213. [Google Scholar]
- Hirai, H.; Nakazawa, H. Grandite garnet from Nevada: Confirmation of origin of iridescence by electron microscopy and interpretation of a moiré-like texture. Am. Mineral. 1986, 71, 123–126. [Google Scholar]
- Koritnig, S.; Rösch, H.; Schneider, A.; Seifert, F. Der Titan-zirkon-granat aus den Kalksilikatfels-Einschlüssen des Gabbro im Radautal, Harz, Bundesrepublik Deutschland. Tschermaks Mineral. Petrogr. Mitt. 1978, 25, 305–313. [Google Scholar] [CrossRef]
- Lager, G.A.; Armbruster, T.; Rotella, F.J.; Rossman, G.R. OH substitution in garnets: X-ray and neutron diffraction, infrared, and geometric-modeling studies. Am. Mineral. 1989, 74, 840–851. [Google Scholar]
- Manning, P.G.; Owens, D.R. Electron microprobe, X-ray diffraction, and spectral studies of South African and British Columbian “jades”. Can. Mineral. 1977, 15, 512–517. [Google Scholar]
- Zabinski, W. Hydrogarnets. Pr. Mineral. 1966, 3, 69. [Google Scholar]
- Schingaro, E.; Lacalamita, M.; Mesto, E.; Ventruti, G.; Pedrazzi, G.; Ottolini, L.; Scordari, F. Crystal chemistry and light elements analysis of Ti-rich garnets. Am. Mineral. 2016, 101, 371–384. [Google Scholar] [CrossRef]
- Bosi, F.; Hatert, F.; Hålenius, U.; Passero, M.; Miyawaki, R.; Mills, S.J. On the application of the IMA-CNMNC dominant-valency rule to complex mineral compositions. Mineral. Mag. 2019, 83, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Armbruster, T.; Birrer, J.; Libowitzky, E.; Beran, A. Crystal chemistry of Ti-bearing andradites. Eur. J. Mineral. 1998, 10, 907–921. [Google Scholar] [CrossRef]
- Moore, T. What’s new in minerals. Mineral. Rec. 1998, 29, 128. [Google Scholar]
- Ferrando, S.; Frezzotti, M.L.; Orione, P.; Conte, R.C.; Compagnoni, R. Late-Alpine rodingitization in the Bellecombe meta-ophiolites (Aosta Valley, Italian Western Alps): Evidence from mineral assemblages and serpentinization-derived H2-bearing brine. Int. Geol. Rev. 2010, 52, 1220–1243. [Google Scholar] [CrossRef]
- Locock, A.J. An excel spreadsheet to recast analyses of garnet into end-member components, and a synopsis of the crystal chemistry of natural silicate garnets. Comput. Geosci. 2008, 34, 1769–1780. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Wang, J.; Lee, P.L.; Toby, B.H. State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. [Google Scholar] [CrossRef]
- Lee, P.L.; Shu, D.; Ramanathan, M.; Preissner, C.; Wang, J.; Beno, M.A.; Von Dreele, R.B.; Ribaud, L.; Kurtz, C.; Antao, S.M.; et al. A twelve-analyzer detector system for high-resolution powder diffraction. J. Synchrotron Radiat. 2008, 15, 427–432. [Google Scholar] [CrossRef]
- Wang, J.; Toby, B.H.; Lee, P.L.; Ribaud, L.; Antao, S.M.; Kurtz, C.; Ramanathan, M.; Von Dreele, R.B.; Beno, M.A. A dedicated powder diffraction beamline at the advanced photon source: Commissioning and early operational results. Rev. Sci. Instrum. 2008, 79, 085105. [Google Scholar] [CrossRef]
- Antao, S.M.; Dhaliwal, I. Growth oscillatory zoning in erythrite, ideally Co3(AsO4)2·8H2O: Structural variations in vivianite-group minerals. Minerals 2017, 7, 136. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Crichton, W.A.; Parise, J.B. Effects of high pressure and temperature on cation ordering in magnesioferrite, MgFe2O4, using in situ synchrotron X-ray powder diffraction up to 1430 K and 6 GPa. Am. Mineral. 2005, 90, 1500–1505. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Mulder, W.H.; Lee, P.L. The R-3c→R-3m transition in nitratine, NaNO3, and implications for calcite, CaCO3. Phys. Chem. Miner. 2008, 35, 545–557. [Google Scholar] [CrossRef]
- Ehm, L.; Michel, F.M.; Antao, S.M.; Martin, C.D.; Lee, P.L.; Shastri, S.D.; Chupas, P.J.; Parise, J.B. Structural changes in nanocrystalline mackinawaite (FeS) at high pressure. J. Appl. Crystallogr. 2009, 42, 15–21. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Hersi, A.A. Single-crystal XRD, TEM, and thermal studies of the satellite reflections in nepheline. Can. Mineral. 2003, 41, 759–783. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Parise, J.B. Haüyne: Phase transition and high-temperature structures obtained from synchrotron radiation and Rietveld refinements. Mineral. Mag. 2004, 68, 499–513. [Google Scholar] [CrossRef]
- Parise, J.B.; Antao, S.M.; Michel, F.M.; Martin, C.D.; Chupas, P.J.; Shastri, S.; Lee, P.L. Quantitative high-pressure pair distribution function analysis. J. Synchrotron Radiat. 2005, 12, 554–559. [Google Scholar] [CrossRef]
- Ehm, L.; Antao, S.M.; Chen, J.H.; Locke, D.R.; Michel, F.M.; Martin, C.D.; Yu, T.; Parise, J.B.; Lee, P.L.; Chupas, P.J.; et al. Studies of local and intermediate range structure in crystalline and amorphous materials at high pressure using high-energy X-rays. Powder Diffr. 2007, 22, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Skinner, L.B.; Benmore, C.J.; Antao, S.M.; Soignard, E.; Amin, S.A.; Bychkov, E.; Rissi, E.; Parise, J.B.; Yarger, J.L. Structural changes in vitreous GeSe4 under pressure. J. Phys. Chem. 2011, 116, 2212–2217. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Thermal analyses of sodalite, tugtupite, danalite, and helvite. Can. Mineral. 2002, 40, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.; Schubert, M.; Antao, S. Elevated radionuclide concentrations in heavy mineral-rich beach sands in the Cox’s Bazar region, Bangladesh and related possible radiological effects. Isot. Environ. Health Stud. 2012, 48, 512–525. [Google Scholar] [CrossRef]
- Kitamura, K.; Komatsu, H. Optical anisotropy associated with growth striation of yttrium garnet, Y3(Al,Fe)5O12. Krist. Technik. 1978, 13, 811–816. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report, LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000.
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Cagliotti, G.; Paoletti, A.; Ricci, F.P. Choice of collimators for a crystal spectrometer for neutron diffraction. Nucl. Instrum. 1958, 3, 223–228. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye-Scherrer synchrotron X-ray data from alumina. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Antao, S.M.; Salvador, J.J. Crystal chemistry of birefringent uvarovite solid solutions. Minerals 2019, 9, 395. [Google Scholar] [CrossRef] [Green Version]
- Antao, S.M.; Mohib, S.; Zaman, M.; Marr, R.A. Ti-rich andradites: Chemistry, structure, multi-phases, optical anisotropy, and oscillatory zoning. Can. Mineral. 2015, 53, 133–158. [Google Scholar] [CrossRef]
- Antao, S.M.; Zaman, M.; Gontijo, V.L.; Camargo, E.S.; Marr, R.A. Optical anisotropy, zoning, and coexistence of two cubic phases in andradites from Quebec and New York. Contrib. Mineral. Petrol. 2015, 169, 10. [Google Scholar] [CrossRef]
- Antao, S.M.; Cruickshank, L.A. Two cubic phases in kimzeyite garnet from the type locality Magnet Cove, Arkansas. Acta Crystallogr. 2016, B72, 846–854. [Google Scholar] [CrossRef]
- Antao, S.M. Schorlomite and morimotoite: What’s in a name? Powder Diffr. 2014, 29, 346–351. [Google Scholar] [CrossRef]
- Antao, S.M.; Cruickshank, L.A. Crystal structure refinements of tetragonal (OH,F)-rich spessartine and henritermierite garnets. Acta Crystallogr. 2018, B74, 104–114. [Google Scholar] [CrossRef]
- Akizuki, M. Growth structure and crystal symmetry of grossular garnets from the Jeffrey mine, Asbestos, Quebec, Canada. Am. Mineral. 1989, 74, 859–864. [Google Scholar]
- Jamtveit, B. Oscillatory zonation patterns in hydrothermal grossular-andradite garnet: Nonlinear dynamics in regions of immiscibility. Am. Mineral. 1991, 76, 1319–1327. [Google Scholar]
- Pollok, K.; Jamtveit, B.; Putnis, A. Analytical transmission electron microscopy of oscillatory zoned grandite garnets. Contrib. Mineral. Petrol. 2001, 141, 358–366. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. A two-phase intergrowth of genthelvite from Mont Saint-Hilaire, Quebec. Can. Mineral. 2010, 48, 1217–1223. [Google Scholar] [CrossRef]
- Antao, S.M. Linear structural trends and multi-phase intergrowths in helvine-group minerals, (Zn,Fe,Mn)8[Be6Si6O24]S2. Minerals 2021, 11, 325. [Google Scholar] [CrossRef]
- Antao, S.M.; Hovis, G.L. Structural variations across the nepheline (NaAlSiO4)-kalsilite (KAlSiO4) series. Am. Mineral. 2021, 106, 801–811. [Google Scholar] [CrossRef]
- Antao, S.M.; Nicholls, J.W. Crystal chemistry of three volcanic K-rich nepheline samples from Oldoinyo Lengai, Tanzania and Mount Nyiragongo, Eastern Congo, Africa. Front. Earth Sci. 2018. [Google Scholar] [CrossRef]
- Zaman, M.M.; Antao, S.M. A possible radiation-induced transition from monazite-(Ce) to xenotime-(Y). Minerals 2021, 11, 16. [Google Scholar] [CrossRef]
- Ivanova, T.I.; Shtukenberg, A.G.; Punin, Y.O.; Frank-Kamenetskaya, O.V.; Sokolov, P.B. On the complex zonality in grandite garnets and implications. Mineral. Mag. 1998, 62, 857–868. [Google Scholar] [CrossRef]
- Baur, W.H.; Fischer, R.X. On the significance of small deviations from higher symmetry. Mineral. Mag. 2003, 67, 793–797. [Google Scholar] [CrossRef]
- Baur, W.H.; Tillmanns, E. How to avoid unnecessarily low symmetry in crystal structure determination. Acta Crystallogr. 1986, b42, 95–111. [Google Scholar] [CrossRef]
- Andrut, M.; Wildner, M. The crystal chemistry of birefringent natural uvarovites: Part I. Optical investigations and UV-VIS-IR absorption spectroscopy. Am. Mineral. 2001, 86, 1219–1230. [Google Scholar] [CrossRef]
- Antao, S.M. Crystal chemistry of birefringent hydrogrossular. Phys. Chem. Miner. 2015, 42, 455–474. [Google Scholar] [CrossRef]
- Armbruster, T. Structure refinement of hydrous andradite, Ca3Fe1.54Mn0.02Al0.26(SiO4)1.65(O4H4)1.35, from the Wessels mine, Kalahari manganese field, South Africa. Eur. J. Mineral. 1995, 7, 1221–1225. [Google Scholar] [CrossRef]
- Ferro, O.; Galli, E.; Papp, G.; Quartieri, S.; Szakall, S.; Vezzalini, G. A new occurrence of katoite and re-examination of the hydrogrossular group. Eur. J. Mineral. 2003, 15, 419–426. [Google Scholar] [CrossRef]
- Basso, R.; Cimmino, F.; Messiga, B. Crystal chemical and petrological study of hydrogarnets from a Fe-gabbro metarodingite (Gruppo Di Voltri, Western Liguria, Italy). Neues Jahrb. Mineral. Abh. 1984, 150, 247–258. [Google Scholar]
- Novak, G.A.; Gibbs, G.V. The crystal chemistry of the silicate garnets. Am. Mineral. 1971, 56, 1769–1780. [Google Scholar]
- Lager, G.A.; Rossman, G.R.; Rotella, F.J.; Schultz, A.J. Neutron-diffraction structure of a low-water grossular at 20 K. Am. Mineral. 1987, 72, 766–768. [Google Scholar]
- Geiger, C.A.; Armbruster, T. Mn3Al2Si3O12 spessartine and Ca3Al2Si3O12 grossular garnet: Structural dynamic and thermodynamic properties. Am. Mineral. 1997, 82, 740–747. [Google Scholar] [CrossRef]
- Basso, R.; Cimmino, F.; Messiga, B. Crystal chemistry of hydrogarnets from three different microstructural sites of a basaltic metarodingite from the Voltri Massif (Western Liguria, Italy). Neues Jahrb. Mineral. Abh. 1984, 148, 246–258. [Google Scholar]
- Becker, U.; Pollok, K. Molecular simulations of interfacial and thermodynamic mixing properties of grossular-andradite garnets. Phys. Chem. Miner. 2002, 29, 52–64. [Google Scholar] [CrossRef]
- Ganguly, J. The energetics of natural garnet solid solution II. Mixing of the calcium silicate end-members. Contrib. Mineral. Petrol. 1976, 55, 81–90. [Google Scholar] [CrossRef]
- Stowell, H.H.; Menard, T.; Ridgway, C.K. Ca-metasomatism and chemical zonation of garnet in contact metamorphic aureoles, Juneau Gold Belt, Southeastern Alaska. Can. Mineral. 1996, 34, 1195–1209. [Google Scholar]
- Evans, B.W.; Johannes, J.; Oterdoom, H.; Trommsdorff, V. Stability of chrysotile and antigorite in the serpentinite multisystem. Schweiz. Mineral. Petrogr. Mitt. 1976, 56, 79–93. [Google Scholar]





| Oxide (wt.%) | 1. Lytton | 2. Tanzania | 3. Coahuila | 4. Chihuahua-p † | |||
|---|---|---|---|---|---|---|---|
| Phase-3a | Phase-3b | Phase-4a | Phase-4b | ||||
| SiO2 | 39.70 | 40.02 | 40.25 | 40.44 | 39.48 | 39.26 | |
| TiO2 | 0.00 | 0.04 | 0.21 | 0.12 | 0.01 | 0.10 | |
| Al2O3 | 22.10 | 21.06 | 22.50 | 22.57 | 22.22 | 19.32 | |
| Cr2O3 | 0.01 | 0.00 | 0.00 | 0.02 | 0 | 0.00 | |
| Fe2O3 | 0.62 | 2.46 | 0.23 | 0.35 | 0.2 | 4.43 | |
| MnO | 0.02 | 0.11 | 1.07 | 1.25 | 1.63 | 0.16 | |
| MgO | 0.01 | 0.00 | 0.80 | 0.82 | 0.45 | 0.29 | |
| CaO | 36.98 | 37.25 | 35.61 | 35.61 | 34.92 | 36.12 | |
| ∑ | 99.44 | 100.94 | 100.66 | 101.18 | 98.9 | 99.68 | |
| Cations for 12 O atoms | |||||||
| Ca2+ | 2.994 | 2.992 | 2.844 | 2.831 | 2.843 | 2.957 | |
| Mn2+ | 0.001 | 0.007 | 0.067 | 0.078 | 0.105 | 0.010 | |
| Mg2+ | 0.001 | 0.000 | 0.089 | 0.091 | 0.051 | 0.033 | |
| ∑X | 2.996 | 2.999 | 3.000 | 3.000 | 2.999 | 3.000 | |
| Al3+ | 1.968 | 1.860 | 1.976 | 1.973 | 1.990 | 1.739 | |
| Fe3+ | 0.035 | 0.139 | 0.013 | 0.019 | 0.011 | 0.255 | |
| Ti4+ | 0.000 | 0.002 | 0.011 | 0.007 | 0.001 | 0.006 | |
| Cr3+ | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | |
| ∑Y | 2.004 | 2.001 | 2.000 | 2.000 | 2.001 | 2.000 | |
| Z = Si4+ | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | |
| End member mole % | |||||||
| Grossular (Grs) | 98.31 | 92.78 | 93.61 | 93.01 | 94.29 | 85.54 | |
| Andradite (Adr) | 1.45 | 6.93 | 0.64 | 0.96 | 0.46 | 12.73 | |
| Pyrope (Prp) | 0.04 | 0.00 | 2.96 | 3.04 | 1.7 | 1.1 | |
| Spessartine (Sps) | 0.04 | 0.23 | 2.24 | 2.61 | 3.49 | 0.34 | |
| Uvarovite (Uv) | 0.03 | 0.00 | 93.01 | 93.01 | 0.00 | 0.00 | |
| Schorlomite-Al | 0.00 | 0.01 | 0.96 | 0.96 | 0.00 | 0.00 | |
| Morimotoite-Mg | 0.00 | 0.00 | 3.04 | 3.04 | 0.00 | 0.00 | |
| Oxide (wt. %) | 5. Chihuahua-w ‡ | 5. Chihuahua-y ‡ | 5. Chihuahua-B ‡ | 6. Italy | |||
| Phase-5a | Phase-5b | Phase-5c | Phase-5d | Phase-5e | Phase-6a | Phase-6b | |
| SiO2 | 38.97 | 38.55 | 39.06 | 39.04 | 37.87 | 39.79 | 39.16 |
| TiO2 | 0.03 | 1.76 | 1.24 | 1.12 | 4.98 | 0.15 | 0.17 |
| Al2O3 | 19.37 | 17.13 | 17.10 | 17.10 | 8.70 | 18.33 | 16.77 |
| Cr2O3 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.03 |
| Fe2O3 | 4.20 | 5.54 | 6.55 | 6.71 | 14.91 | 6.42 | 8.24 |
| MnO | 0.14 | 0.10 | 0.17 | 0.20 | 0.12 | 0.16 | 0.56 |
| MgO | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.34 | 0.35 |
| CaO | 36.24 | 35.92 | 36.34 | 36.25 | 35.28 | 36.52 | 35.63 |
| ∑ | 98.95 | 99.00 | 100.47 | 100.43 | 101.86 | 101.71 | 100.91 |
| Cations for 12 O atoms | |||||||
| Ca2+ | 2.989 | 2.995 | 2.990 | 2.985 | 2.994 | 2.950 | 2.924 |
| Mn2+ | 0.009 | 0.007 | 0.011 | 0.013 | 0.000 | 0.010 | 0.036 |
| Mg2+ | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.039 | 0.040 |
| ∑X | 2.998 | 3.002 | 3.001 | 2.999 | 3.002 | 2.999 | 3.000 |
| Al3+ | 1.757 | 1.571 | 1.548 | 1.549 | 0.812 | 1.628 | 1.514 |
| Fe3+ | 0.243 | 0.324 | 0.379 | 0.388 | 0.889 | 0.364 | 0.475 |
| Ti4+ | 0.002 | 0.103 | 0.072 | 0.065 | 0.297 | 0.009 | 0.010 |
| Cr3+ | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.002 |
| ∑Y | 2.002 | 1.998 | 1.999 | 2.001 | 1.998 | 2.001 | 2.000 |
| Z = Si4+ | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 |
| End member mole % | |||||||
| Grossular (Grs) | 87.54 | 78.33 | 77.02 | 76.95 | 40.33 | 79.78 | 73.16 |
| Andradite (Adr) | 12.07 | 16.22 | 18.93 | 19.40 | 44.44 | 18.22 | 23.74 |
| Pyrope (Prp) | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 1.28 | 1.33 |
| Spessartine (Sps) | 0.30 | 0.22 | 0.37 | 0.43 | 0.27 | 0.34 | 1.20 |
| Uvarovite (Uv) | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| Schorlomite-Al | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 |
| Morimotoite-Mg | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | 0.00 | 0.00 |
| 1. Lytton | 2. Tanzania | 3. Coahuila | 4. Chihuahua-p | |||
|---|---|---|---|---|---|---|
| Single-Phase | Single-Phase | Phase-3a | Phase-3b | Phase-4a | Phase-4b | |
| wt.% | 100 | 100 | 56.8(1) | 43.2(1) | 72.5(1) | 27.5(1) |
| a (Å) | 11.85091(1) | 11.85286(1) | 11.85202(1) | 11.85760(8) | 11.85100(1) | 11.87186(5) |
| * Δa (Å) | - | - | - | −0.00558 | - | −0.02086 |
| † LY | 4.02 | 3.14 | 5.31 | 11.75 | 7.5 | 24.8 |
| ** Strain (%) | 0.07 | 0.05 | 0.09 | 0.21 | 0.13 | 0.43 |
| Reduced χ2 | 1.262 | 1.002 | 1.537 | 1.256 | ||
| ‡ R (F2) | 0.0343 | 0.0470 | 0.0455 | 0.0403 | ||
| wRp | 0.0520 | 0.0489 | 0.0656 | 0.0605 | ||
| Nobs | 678 | 646 | 1325 | 1363 | ||
| λ (Å) | 0.41374(2) | 0.41370(2) | 0.41370(2) | 0.41370(2) | ||
| Data points | 47992 | 47995 | 47995 | 47995 | ||
| 5. Chihuahua-w | 5. Chihuahua-y | 6. Italy | ||||
| Phase-5a | Phase-5b | Phase-5c | Phase-5d | Phase-6a | Phase-6b | |
| wt.% | 63.3(1) | 36.7(1) | 60.1(3) | 39.9(3) | 63.9(1) | 36.1(2) |
| a (Å) | 11.87775(1) | 11.88285(1) | 11.89069(1) | 11.89468(3) | 11.88242(2) | 11.89553(7) |
| * Δa (Å) | - | −0.00510 | - | −0.00399 | - | −0.01311 |
| † LY | 5.63 | 9.72 | 4.78 | 8.86 | 8.2 | 23.9 |
| ** Strain (%) | 0.10 | 0.17 | 0.08 | 0.15 | 0.14 | 0.42 |
| Reduced χ2 | 1.221 | 0.9869 | 1.185 | |||
| ‡ R (F2) | 0.0581 | 0.0243 | 0.0299 | |||
| wRp | 0.0521 | 0.0316 | 0.0412 | |||
| Nobs | 1296 | 1248 | 1007 | |||
| λ (Å) | 0.41370(2) | 0.45900(2) | 0.45900(2) | |||
| Data points | 47995 | 47994 | 47994 | |||
| 1. Lytton | 2. Tanzania | 3. Coahuila | 4. Chihuahua-p | ||||
|---|---|---|---|---|---|---|---|
| Single-Phase | Single-Phase | Phase-3a | Phase-3b | Phase-4a | Phase-4b | ||
| Ca(X) | U | 0.43(1) | 0.45(1) | 0.47(1) | 0.47(1) | 0.48(1) | 0.48(1) |
| Al(Y) | U | 0.32(1) | 0.32(1) | 0.36(1) | 0.36(1) | 0.33(1) | 0.33(1) |
| Si(Z) | U | 0.26(1) | 0.29(1) | 0.37(1) | 0.37(1) | 0.30(1) | 0.30(1) |
| O | x | 0.03835(3) | 0.03820(3) | 0.03791(7) | 0.0382(1) | 0.03818(4) | 0.0385(1) |
| y | 0.04505(2) | 0.04526(3) | 0.04525(7) | 0.0457(1) | 0.04516(4) | 0.0456(1) | |
| z | 0.65121(3) | 0.65129(3) | 0.65144(9) | 0.6517(1) | 0.65120(5) | 0.6517(1) | |
| U | 0.76(1) | 0.79(1) | 0.88(1) | 0.88(1) | 0.82(1) | 0.82(1) | |
| Ca(X) | sof | 0.944(1) | 0.952(1) | 0.949(2) | 0.928(3) | 0.947(1) | 0.946(3) |
| Al(Y) | sof | 0.950(1) | 0.993(1) | 0.947(3) | 0.984(3) | 0.951(2) | 0.972(3) |
| Fe(Y) | sof | 0.050(1) | 0.007(1) | 0.053(3) | 0.016(3) | 0.049(2) | 0.028(3) |
| Si(Z) | sof | 0.937(1) | 0.938(1) | 0.932(2) | 0.950(3) | 0.937(1) | 0.932(3) |
| Ca(X) | EPMA | 0.999 | 1.000 | 0.994 | 0.994 | 1.002 | 0.996 |
| Al(Y) | EPMA | 0.984 | 0.930 | 0.988 | 0.987 | 0.995 | 0.870 |
| Fe(Y) | EPMA | 0.018 | 0.070 | 0.007 | 0.010 | 0.006 | 0.128 |
| Si(Z) | EPMA | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Ca(X) | † Δ(sof) | −0.055 | −0.048 | −0.045 | −0.066 | −0.055 | −0.050 |
| Al(Y) | Δ(sof) | −0.034 | 0.063 | −0.041 | −0.003 | −0.044 | 0.103 |
| Fe(Y) | Δ(sof) | 0.033 | −0.063 | 0.047 | 0.007 | 0.044 | −0.100 |
| Si(Z) | Δ(sof) | −0.063 | −0.062 | −0.068 | −0.050 | −0.063 | −0.068 |
| Ca(X) | ‡ Δe | −1.1 | −1.0 | −0.9 | −1.3 | −1.1 | −1.0 |
| Al(Y) | Δe | −0.4 | 0.8 | −0.5 | 0.0 | −0.6 | 1.3 |
| Fe(Y) | Δe | 0.8 | −1.6 | 1.2 | 0.2 | 1.1 | −2.6 |
| Si(Z) | Δe | −0.9 | −0.9 | −1.0 | −0.7 | −0.9 | −1.0 |
| 5. Chihuahua-w | 5. Chihuahua-y | 6. Italy | |||||
| Phase-5a | Phase-5a | Phase-5c | Phase-5d | Phase-6a | Phase-6b | ||
| Ca(X) | U | 0.45(1) | 0.45(1) | 0.461(4) | 0.461(4) | 0.490(6) | 0.490(6) |
| Al(Y) | U | 0.37(1) | 0.37(1) | 0.330(5) | 0.330(5) | 0.326(6) | 0.326(6) |
| Si(Z) | U | 0.37(1) | 0.37(1) | 0.375(6) | 0.375(6) | 0.335(9) | 0.335(9) |
| O | x | 0.03883(5) | 0.03785(8) | 0.03870(6) | 0.03839(5) | 0.03839(5) | 0.03758(8) |
| y | 0.04503(5) | 0.04714(8) | 0.04565(6) | 0.04602(5) | 0.04574(4) | 0.04643(8) | |
| z | 0.65182(6) | 0.65225(9) | 0.65200(6) | 0.65221(5) | 0.65192(5) | 0.65225(9) | |
| U | 0.89(1) | 0.89(1) | 0.945(7) | 0.945(7) | 0.997(9) | 0.997(9) | |
| Ca(X) | sof | 0.937(2) | 0.940(2) | 0.951(2) | 0.934(1) | 0.939(1) | 0.953(2) |
| Al(Y) | sof | 0.970(2) | 0.927(3) | 0.889(2) | 0.872(1) | 0.909(1) | 0.834(2) |
| Fe(Y) | sof | 0.030(2) | 0.073(3) | 0.111(2) | 0.128(1) | 0.091(1) | 0.166(2) |
| Si(Z) | sof | 0.933(2) | 0.935(2) | 0.938(2) | 0.943(1) | 0.926(1) | 0.919(2) |
| Ca(X) | EPMA sof | 1.000 | 1.001 | 1.001 | 1.001 | 0.995 | 0.998 |
| Al(Y) | EPMA sof | 0.879 | 0.786 | 0.774 | 0.775 | 0.814 | 0.757 |
| Fe(Y) | EPMA sof | 0.122 | 0.162 | 0.190 | 0.194 | 0.182 | 0.238 |
| Si(Z) | EPMA sof | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Ca(X) | † Δ(sof) | −0.063 | −0.061 | −0.050 | −0.067 | −0.056 | −0.045 |
| Al(Y) | Δ(sof) | 0.092 | 0.142 | 0.115 | 0.098 | 0.095 | 0.077 |
| Fe(Y) | Δ(sof) | −0.092 | −0.089 | −0.079 | −0.066 | −0.091 | −0.072 |
| Si(Z) | Δ(sof) | −0.067 | −0.065 | −0.062 | −0.057 | −0.074 | −0.081 |
| Ca(X) | ‡ Δe | −1.3 | −1.2 | −1.0 | −1.3 | −1.1 | −0.9 |
| Al(Y) | Δe | 1.2 | 1.8 | 1.5 | 1.3 | 1.2 | 1.0 |
| Fe(Y) | Δe | −2.4 | −2.3 | −2.0 | −1.7 | −2.4 | −1.9 |
| Si(Z) | Δe | −0.9 | −0.9 | −0.9 | −0.8 | −1.0 | −1.1 |
| 1. Lytton | 2. Tanzania | 3. Coahuila | 4. Chihuahua-p | ||||
|---|---|---|---|---|---|---|---|
| Phase-1 | Phase-2 | Phase-3a | Phase-3b | Phase-4a | Phase-4b | ||
| Z-O | x4 | 1.6463(3) | 1.6478(4) | 1.649(1) | 1.647(1) | 1.6481(5) | 1.648(1) |
| Y-O | x6 | 1.9242(3) | 1.9257(4) | 1.926(1) | 1.932(2) | 1.9240(6) | 1.934(2) |
| X-O | x4 | 2.3244(3) | 2.3235(3) | 2.320(1) | 2.323(1) | 2.3232(5) | 2.328(1) |
| X′-O | x4 | 2.4905(3) | 2.4882(3) | 2.488(1) | 2.485(1) | 2.4888(5) | 2.489(1) |
| <X-O> | [8] | 2.4075 | 2.4059 | 2.404 | 2.404 | 2.4060 | 2.409 |
| * <D-O> | [4] | 2.0964 | 2.0963 | 2.096 | 2.097 | 2.0960 | 2.100 |
| ∠Y-O-Z | x1 | 136.03(2) | 135.83(2) | 135.70(6) | 135.50(8) | 135.90(3) | 135.62(7) |
| 5. Chihuahua-w | 5. Chihuahua-y | 6. Italy | |||||
| Phase-5a | Phase-5b | Phase-5c | Phase-5d | Phase-6a | Phase-6b | ||
| Z-O | x4 | 1.6412(7) | 1.654(1) | 1.6449(7) | 1.6475(6) | 1.6470(6) | 1.655(1) |
| Y-O | x6 | 1.9367(7) | 1.947(1) | 1.9425(7) | 1.9457(6) | 1.9397(6) | 1.946(1) |
| X-O | x4 | 2.3308(6) | 2.326(1) | 2.3326(6) | 2.3301(5) | 2.3287(5) | 2.323(1) |
| X′-O | x4 | 2.4982(6) | 2.474(1) | 2.4938(7) | 2.4900(6) | 2.4903(5) | 2.484(1) |
| <X-O> | [8] | 2.4145 | 2.400 | 2.4129 | 2.4103 | 2.4095 | 2.404 |
| * <D-O> | [4] | 2.1017 | 2.100 | 2.1033 | 2.1034 | 2.1014 | 2.102 |
| ∠Y-O-Z | x1 | 136.06(4) | 134.42(6) | 135.62(4) | 135.26(3) | 135.484(34) | 134.72(6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antao, S.M. Crystal Chemistry of Six Grossular Garnet Samples from Different Well-Known Localities. Minerals 2021, 11, 767. https://doi.org/10.3390/min11070767
Antao SM. Crystal Chemistry of Six Grossular Garnet Samples from Different Well-Known Localities. Minerals. 2021; 11(7):767. https://doi.org/10.3390/min11070767
Chicago/Turabian StyleAntao, Sytle M. 2021. "Crystal Chemistry of Six Grossular Garnet Samples from Different Well-Known Localities" Minerals 11, no. 7: 767. https://doi.org/10.3390/min11070767
APA StyleAntao, S. M. (2021). Crystal Chemistry of Six Grossular Garnet Samples from Different Well-Known Localities. Minerals, 11(7), 767. https://doi.org/10.3390/min11070767






