Geopolymers Based on Mechanically Activated Fly Ash Blended with Dolomite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanical Activation
2.3. Synthesis of Geopolymers
2.4. Characterization Methods
3. Results and Discussion
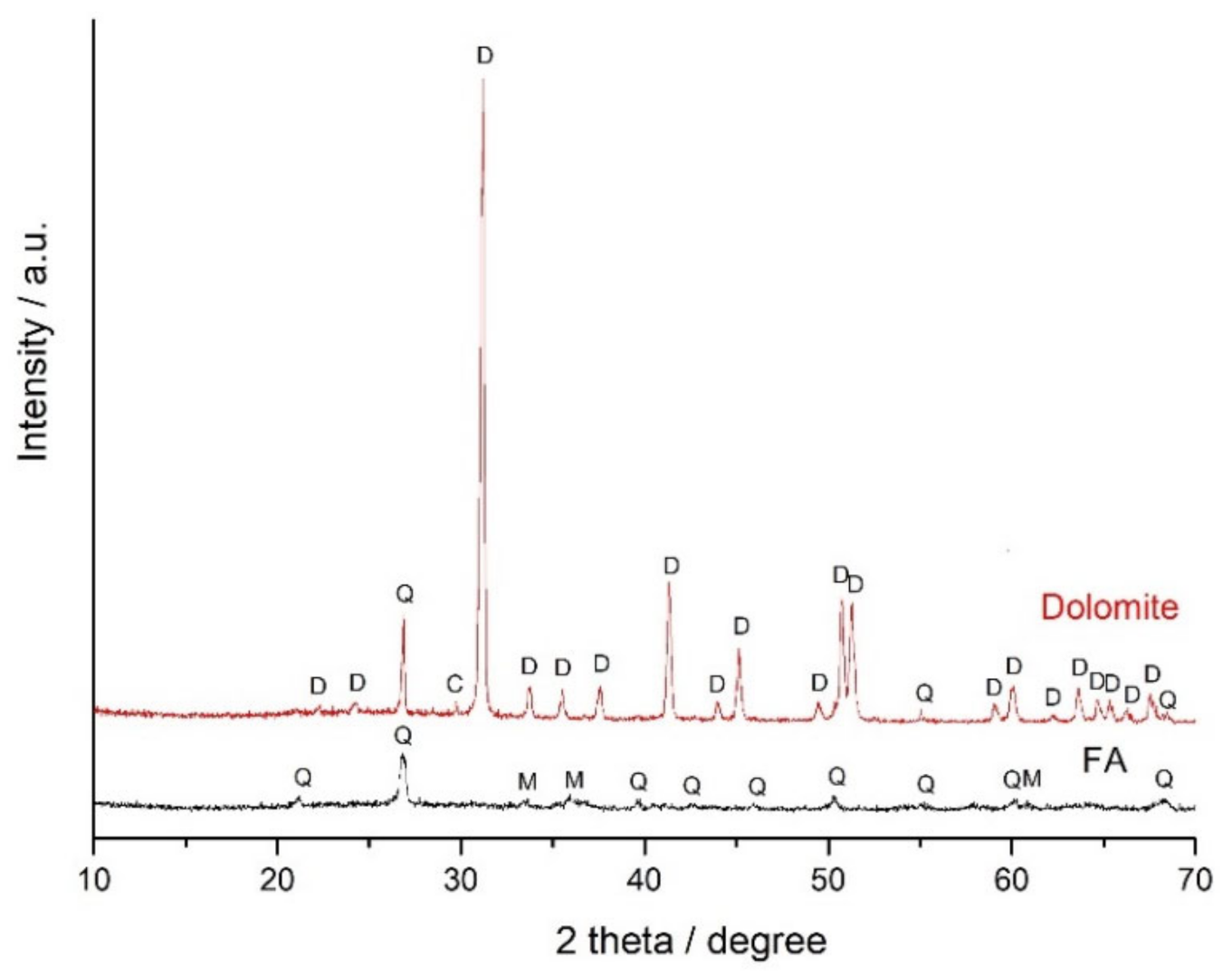
3.1. Effect of Mechanical Activation on the FA + Dolomite Blends
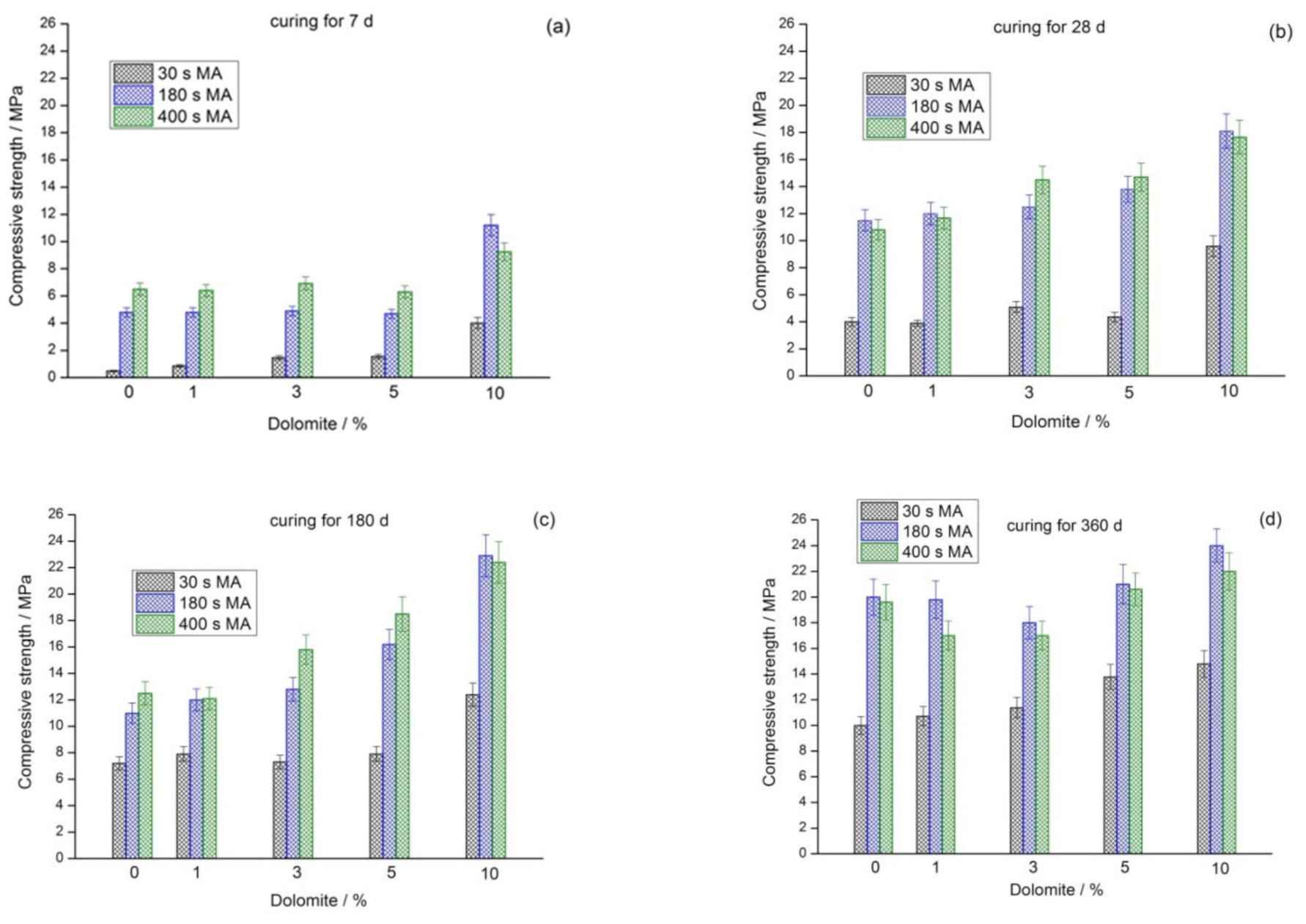
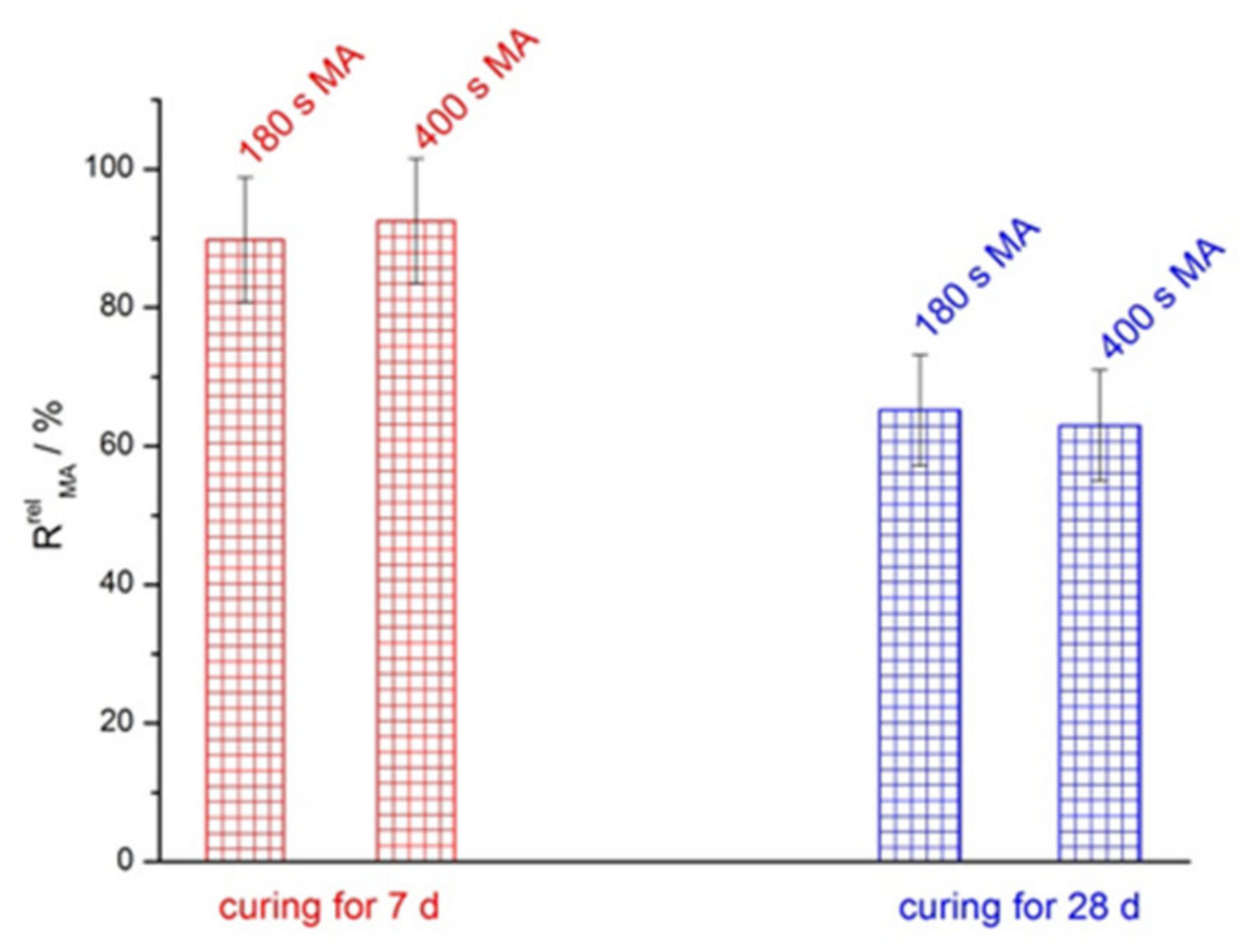
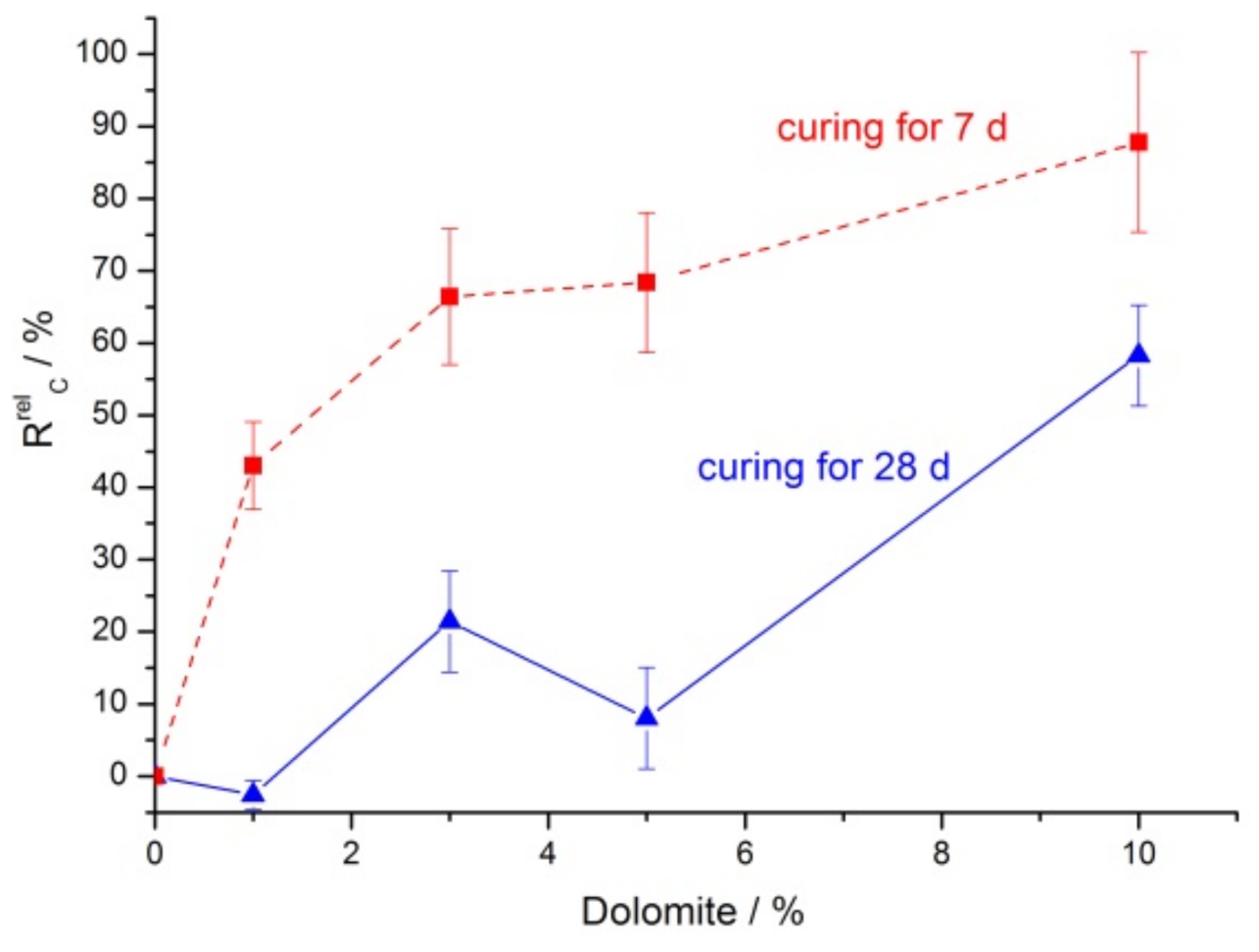
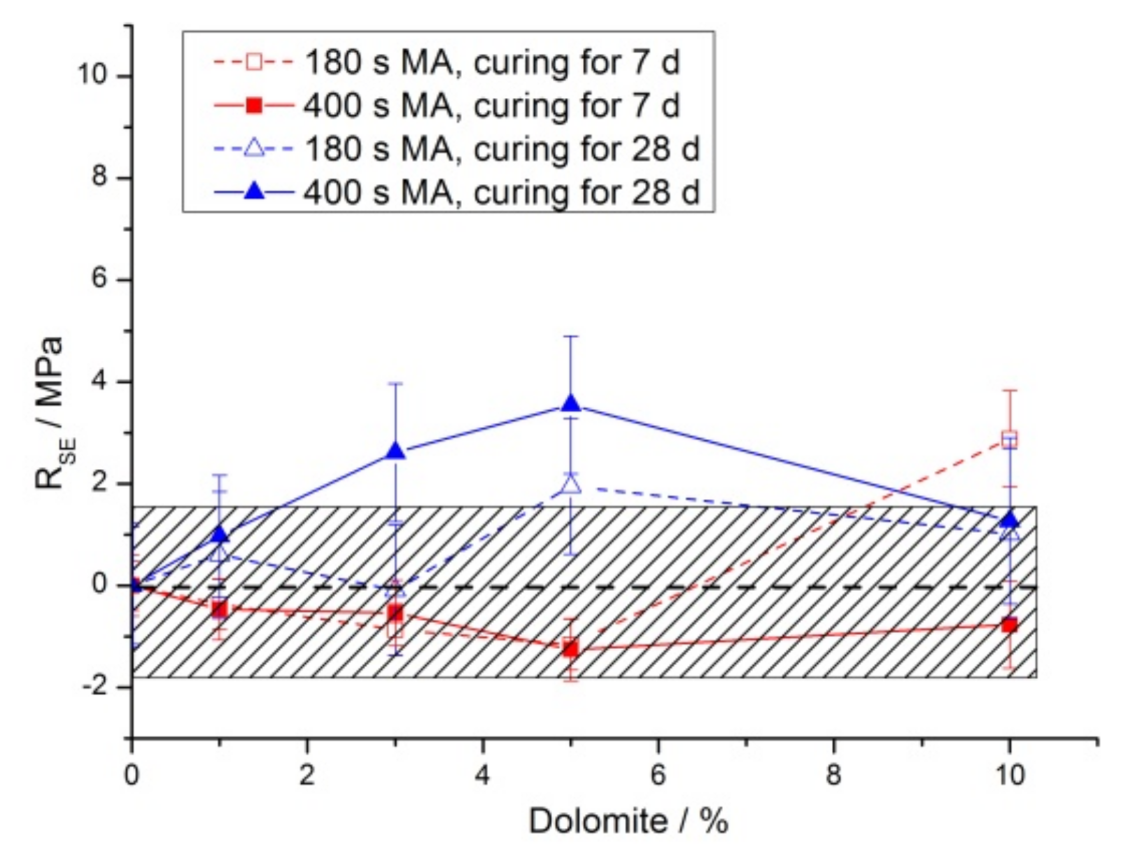
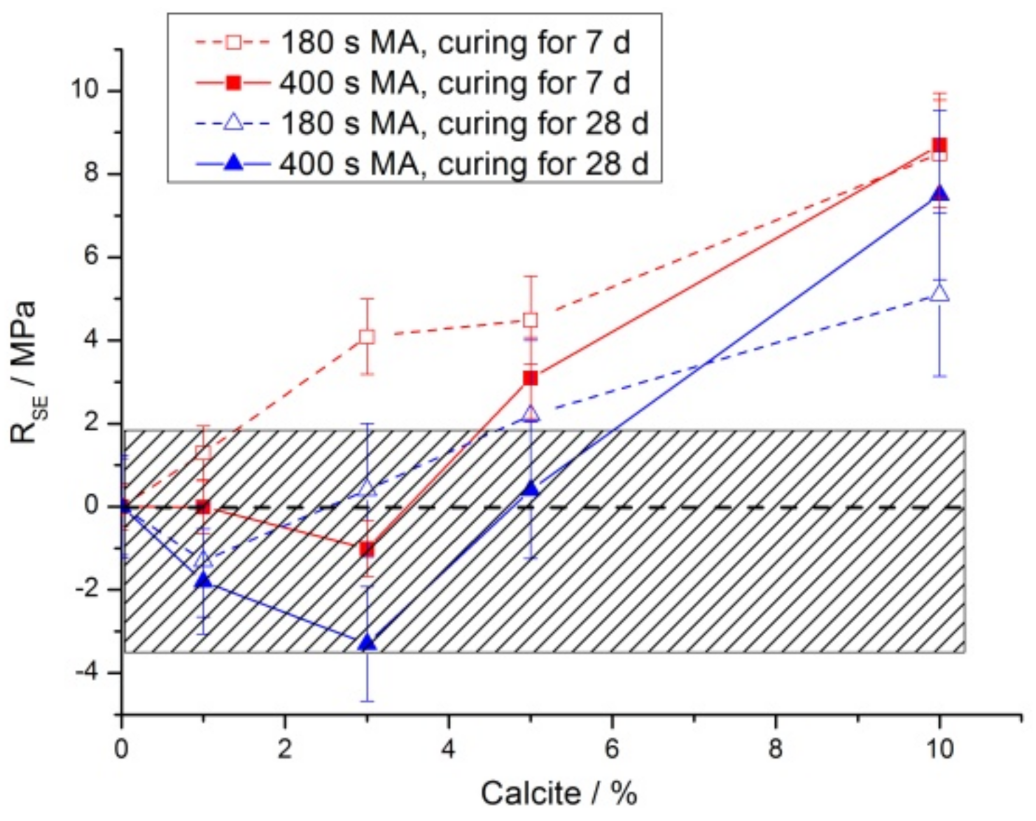
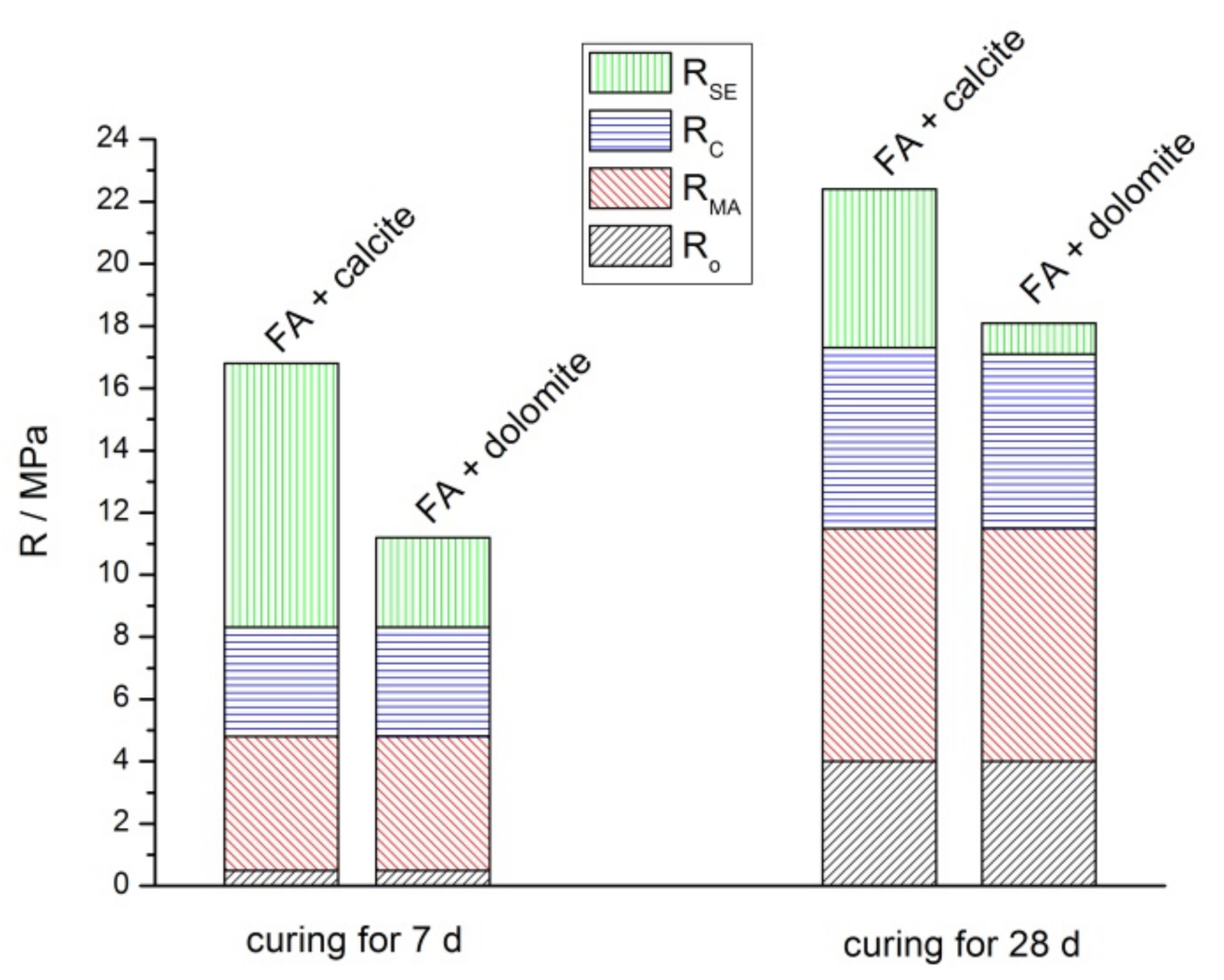
3.2. Mechanical Properties
- R(GXDY)—strength of the GXDY geopolymer at a certain age, MPaRo = R(G0D30)—strength of the G0D30 geopolymer at a certain age (reference point), MPa
- RMA—increment of MA in the geopolymer strength, MPa
- RrelMA—relative increment of MA in the geopolymer strength, %
- RC—increment of carbonate addition in the geopolymer strength, MPa
- RrelC—relative increment of carbonate addition in the geopolymer strength, %
- RSE—increment of the synergistic effect in the geopolymer strength, MPa
3.3. TG Analysis
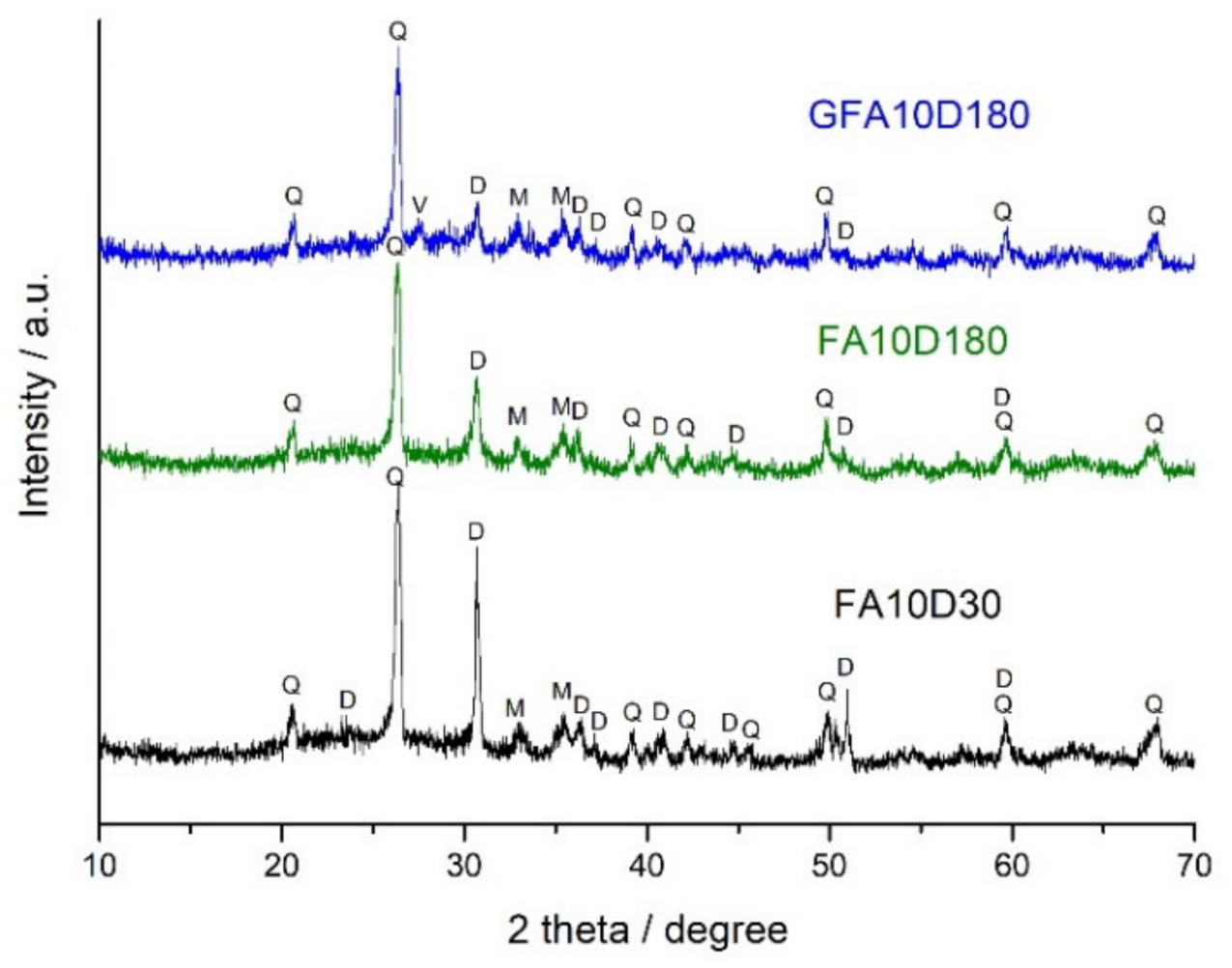
3.4. XRD and FTIR Spectroscopy Analysis
3.5. Microstructural Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao, Z.; Ji, X.; Sarker, P.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Jayaranjan, M.L.D.; Van Hullebusch, E.D.; Annachhatre, A.P. Reuse options for coal fired power plant bottom ash and fly ash. Rev. Environ. Sci. Bio/Technol. 2014, 13, 467–486. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Sun, X.; Zhao, Q.; Yang, Y.; Wang, P. Leachability and adverse effects of coal fly ash: A review. J. Hazard. Mater. 2020, 396, 122725. [Google Scholar] [CrossRef]
- Singh, A.K.; Masto, R.E.; Hazra, B.; Esterle, J.; Singh, P.K. Ash from Coal and Biomass Combustion; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 91–114. [Google Scholar]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020; pp. 23–208. [Google Scholar]
- Krivenko, P. Why alkaline activation—60 years of the theory and practice of alkali-activated materials. J. Ceram. Sci. Technol. 2017, 8, 323–334. [Google Scholar] [CrossRef]
- Singh, N.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. An overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Hu, Y.; Zhou, J.L.; Tam, V.W. Review on designs and properties of multifunctional alkali-activated materials (AAMs). Constr. Build. Mater. 2019, 200, 474–489. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 271–297. [Google Scholar] [CrossRef] [Green Version]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef] [PubMed]
- Vlachakis, C.; Perry, M.; Biondi, L. Self-Sensing Alkali-Activated Materials: A Review. Minerals 2020, 10, 885. [Google Scholar] [CrossRef]
- Lee, W.; van Deventer, J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Alghamdi, H.; Nair, S.A.; Neithalath, N. Insights into material design, extrusion rheology, and properties of 3D-printable alkali-activated fly ash-based binders. Mater. Des. 2019, 167, 107634. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Manguri, S.; Nassani, D.E.; Oleiwi, S.M. Effect of aggregate properties on the mechanical and absorption characteristics of geopolymer mortar. Eng. Sci. Technol. Int. J. 2017, 20, 1642–1652. [Google Scholar] [CrossRef]
- Embong, R.; Kusbiantoro, A.; Shafiq, N.; Nuruddin, M.F. Strength and microstructural properties of fly ash based geopolymer concrete containing high-calcium and water-absorptive aggregate. J. Clean. Prod. 2016, 112, 816–822. [Google Scholar] [CrossRef]
- Azimi, E.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials 2020, 13, 1015. [Google Scholar] [CrossRef] [Green Version]
- Aboulayt, A.; Riahi, M.; Touhami, M.O.; Hannache, H.; Gomina, M.; Moussa, R. Properties of metakaolin based geopolymer incorporating calcium carbonate. Adv. Powder Technol. 2017, 28, 2393–2401. [Google Scholar] [CrossRef]
- Yip, C.K.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Carbonate mineral addition to metakaolin-based geopolymers. Cem. Concr. Compos. 2008, 30, 979–985. [Google Scholar] [CrossRef]
- Qian, J.; Song, M. Study on influence of limestone powder on the fresh and hardened properties of early age metakaolin based geopolymer. In Calcined Clays for Sustainable Concrete; RILEM Book series; Scrivener, K., Favier, A., Eds.; Springer: Lausanne, Switzerland, 2015; Volume 10, pp. 253–259. [Google Scholar]
- Cwirzen, A.; Provis, J.L.; Penttala, V.; Habermehl-Cwirzen, K. The effect of limestone on sodium hydroxide-activated metakaolin-based geopolymers. Constr. Build. Mater. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Perez-Cortes, P.; Escalante-Garcia, J.I. Alkali activated metakaolin with high limestone contents—Statistical modeling of strength and environmental and cost analyses. Cem. Concr. Compos. 2020, 106, 103450. [Google Scholar] [CrossRef]
- Bayiha, B.N.; Billong, N.; Yamb, E.; Kaze, R.C.; Nzengwa, R. Effect of limestone dosages on some properties of geopolymer from thermally activated halloysite. Constr. Build. Mater. 2019, 217, 28–35. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.; Morozov, V.P.; Gaifullin, A.R.; Potapova, L.I.; Gubaidullina, A.M.; Osin, Y. Marl-based geopolymers incorporated with limestone: A feasibility study. J. Non-Cryst. Solids 2018, 492, 1–10. [Google Scholar] [CrossRef]
- Cohen, E.; Peled, A.; Bar-Nes, G. Dolomite-based quarry-dust as a substitute for fly-ash geopolymers and cement pastes. J. Clean. Prod. 2019, 235, 910–919. [Google Scholar] [CrossRef]
- Ortega-Zavala, D.E.; Santana-Carrillo, J.L.; Burciaga-Díaz, O.; Escalante-García, J.I. An initial study on alkali activated limestone binders. Cem. Concr. Res. 2019, 120, 267–278. [Google Scholar] [CrossRef]
- Aizat, E.A.; Al Bakri, A.M.M.; Liew, Y.M.; Heah, C.Y. Chemical composition and strength of dolomite geopolymer compo-sites. AIP Conf. Proc. 2017, 1885, 020192. [Google Scholar]
- Kumar, R.; Kumar, S.; Alex, T.C.; Singla, R. Mapping of calorimetric response for the geopolymerisation of mechanically activated fly ash. J. Therm. Anal. Calorim. 2018, 136, 1117–1133. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yokoyama, K.; Okura, K.; Murayama, N.; Ueda, M.; Naito, M. Synthesis of Geopolymers from Mechanically Activated Coal Fly Ash and Improvement of Their Mechanical Properties. Minerals 2019, 9, 791. [Google Scholar] [CrossRef] [Green Version]
- Temuujin, J.; Williams, R.; van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolic, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Hitomi, T.; Shirai, T. Surface modification of fly ash by mechano-chemical treatment. Ceram. Int. 2019, 45, 849–853. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Garcia-Lodeiro, I.; Maltseva, O.; Palomo, A. Mechanical-Chemical Activation of Coal Fly Ashes: An Effective Way for Recycling and Make Cementitious Materials. Front. Mater. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Mucsi, G. Mechanical activation of power station fly ash by grinding—A review. Epitoanyag J. Silic. Based Compos. Mater. 2016, 68, 56–61. [Google Scholar] [CrossRef]
- Nath, S.; Kumar, S. Role of particle fineness on engineering properties and microstructure of fly ash derived geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Hitomi, T.; Shirai, T. Fabrication of solidified bodies by utilizing mechanochemically modified fly ash powder. J. Ceram. Soc. Jpn. 2020, 128, 224–228. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Gurevich, B.I.; Myshenkov, M.S.; Chislov, M.V.; Kalinkina, E.V.; Zvereva, I.A.; Cherkezova-Zheleva, Z.; Paneva, D.; Petkova, V. Synthesis of Fly Ash-Based Geopolymers: Effect of Calcite Addition and Mechanical Activation. Minerals 2020, 10, 827. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 89th ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2008; 2736p. [Google Scholar]
- Schumann, W. Handbook of Rocks, Minerals, and Gemstones; Houghton Mifflin Company: New York, NY, USA, 1993; 380p. [Google Scholar]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Madai, F.; Debreczeni, Á. Control of geopolymer properties by grinding of land filled fly ash. Int. J. Miner. Process. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; Nicolas, R.S.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; Van Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.K.W.; Van Deventer, J.S.J. Use of Infrared Spectroscopy to Study Geopolymerization of Heterogeneous Amorphous Aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Díez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- White, W.B. The carbonate minerals. In The Infrared Spectra of Minerals; Farmer, W.C., Ed.; Mineralogical Society: London, UK, 1974; pp. 227–284. [Google Scholar]
- Provis, J.L.; Rose, V.; Bernal, S.A.; van Deventer, J.S.J. High-Resolution Nanoprobe X-ray Fluorescence Characterization of Heterogeneous Calcium and Heavy Metal Distributions in Alkali-Activated Fly Ash. Langmuir 2009, 25, 11897–11904. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; van Deventer, J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]


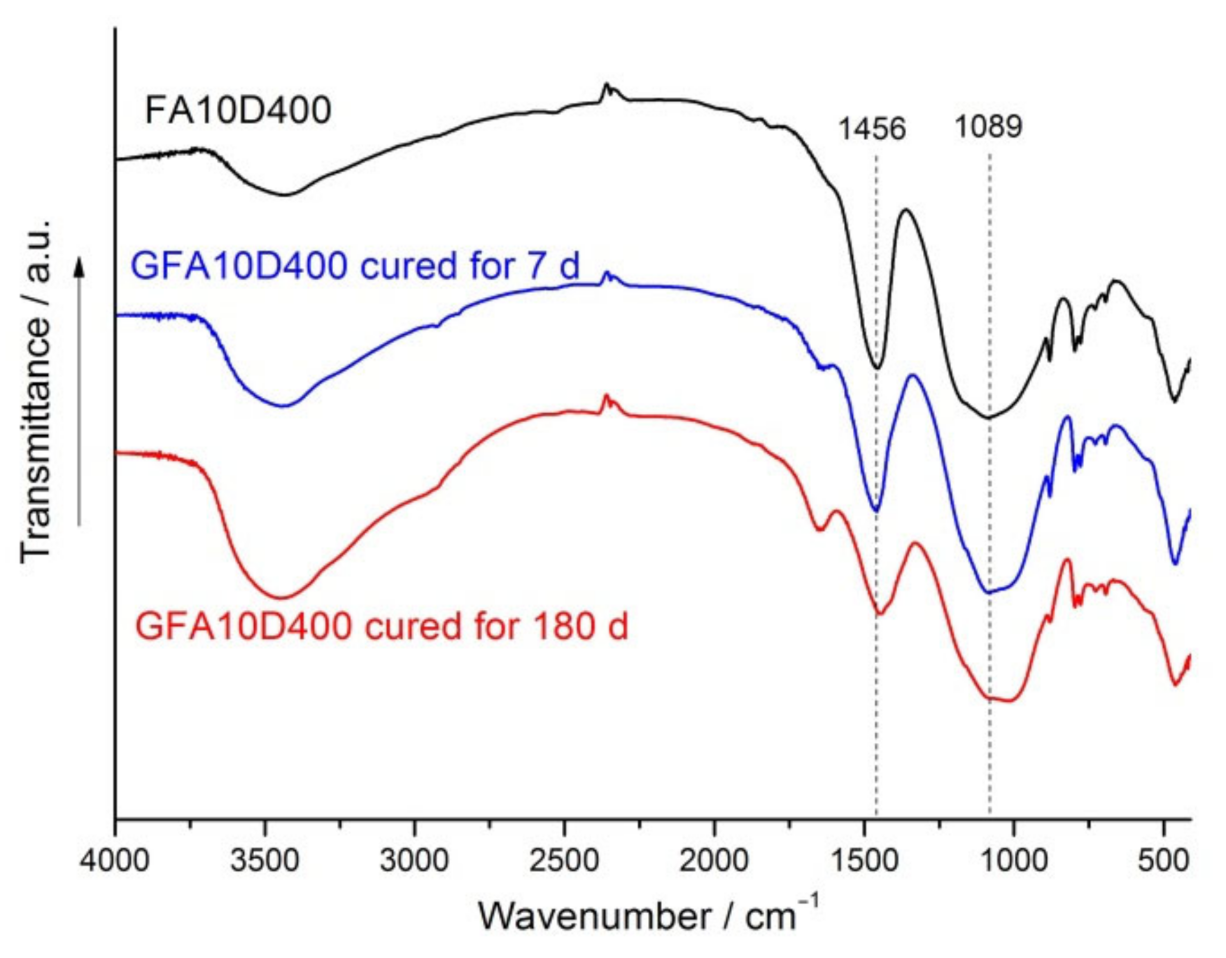
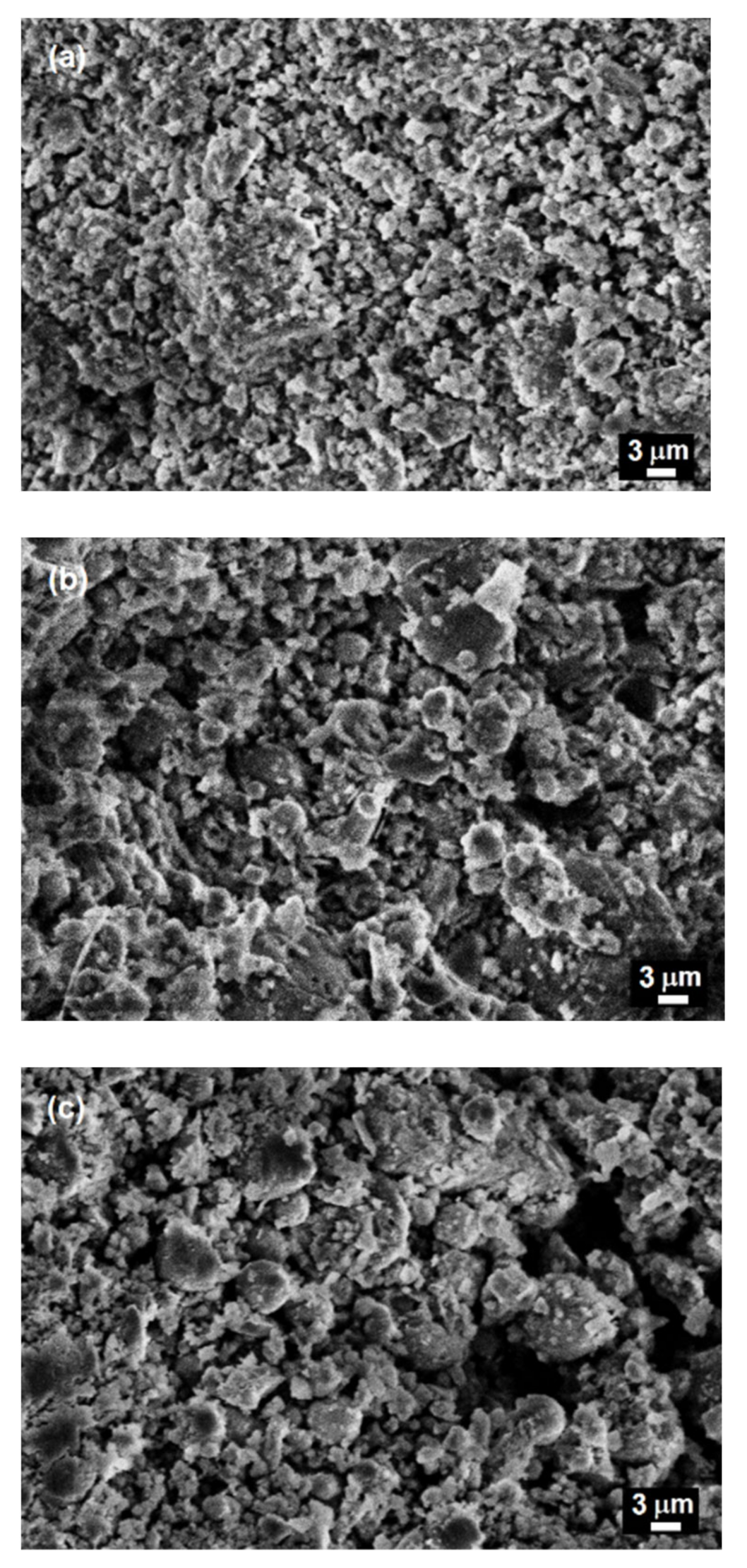
| Raw Materials | SiO2 | Al2O3 | Fe2O3 | FeO | CaO | MgO | SO3 | Na2O | K2O | C | P2O5 | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 56.26 | 18.39 | 8.58 | 0.69 | 2.14 | 2.60 | 0.18 | 4.04 | 1.32 | 0.88 | 0.32 | 1.13 | 2.28 |
| Dolomite | 4.76 | 0.18 | 0.23 | n/a | 30.66 | 21.96 | 0.01 | 0.12 | 0.09 | n/a | n/a | n/a | 44.35 |
| Blend Code | FA (wt.%) | Dolomite (wt.%) | w/s Ratio (30 s MA) | w/s Ratio (180 s MA) | w/s Ratio (400 s MA) |
|---|---|---|---|---|---|
| FA0D | 100 | 0 | 0.23 | 0.25 | 0.28 |
| FA1D | 99 | 1 | 0.23 | 0.23 | 0.25 |
| FA3D | 97 | 3 | 0.24 | 0.24 | 0.25 |
| FA5D | 95 | 5 | 0.25 | 0.25 | 0.25 |
| FA10D | 90 | 10 | 0.25 | 0.25 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinkin, A.M.; Gurevich, B.I.; Kalinkina, E.V.; Chislov, M.V.; Zvereva, I.A. Geopolymers Based on Mechanically Activated Fly Ash Blended with Dolomite. Minerals 2021, 11, 700. https://doi.org/10.3390/min11070700
Kalinkin AM, Gurevich BI, Kalinkina EV, Chislov MV, Zvereva IA. Geopolymers Based on Mechanically Activated Fly Ash Blended with Dolomite. Minerals. 2021; 11(7):700. https://doi.org/10.3390/min11070700
Chicago/Turabian StyleKalinkin, Alexander M., Basya I. Gurevich, Elena V. Kalinkina, Mikhail V. Chislov, and Irina A. Zvereva. 2021. "Geopolymers Based on Mechanically Activated Fly Ash Blended with Dolomite" Minerals 11, no. 7: 700. https://doi.org/10.3390/min11070700
APA StyleKalinkin, A. M., Gurevich, B. I., Kalinkina, E. V., Chislov, M. V., & Zvereva, I. A. (2021). Geopolymers Based on Mechanically Activated Fly Ash Blended with Dolomite. Minerals, 11(7), 700. https://doi.org/10.3390/min11070700








