Production Technologies of Ancient Bricks from Padua, Italy: Changing Colors and Resistance over Time
Abstract
:1. Introduction
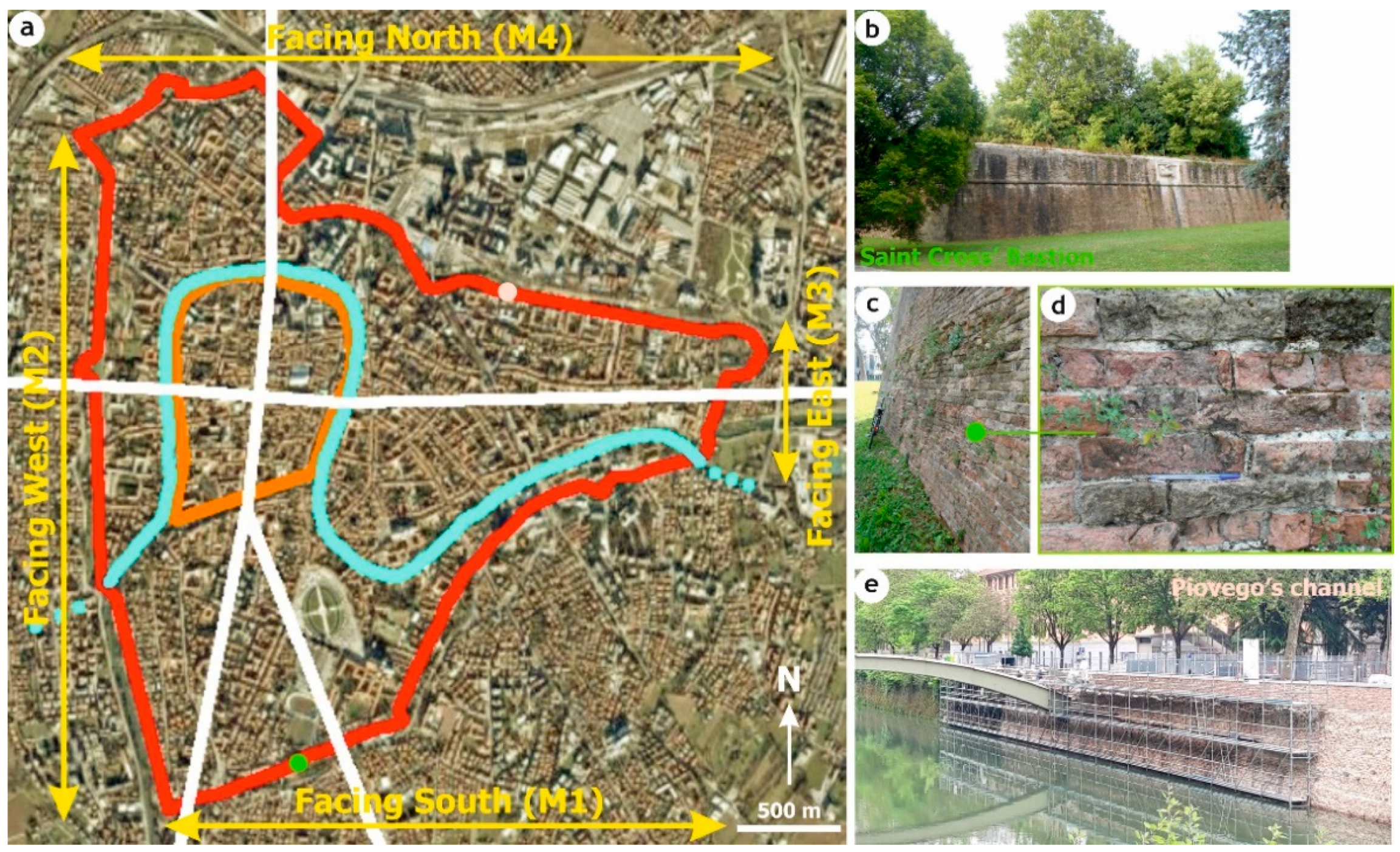
The Renaissance Walls of Padua: Construction and Environs
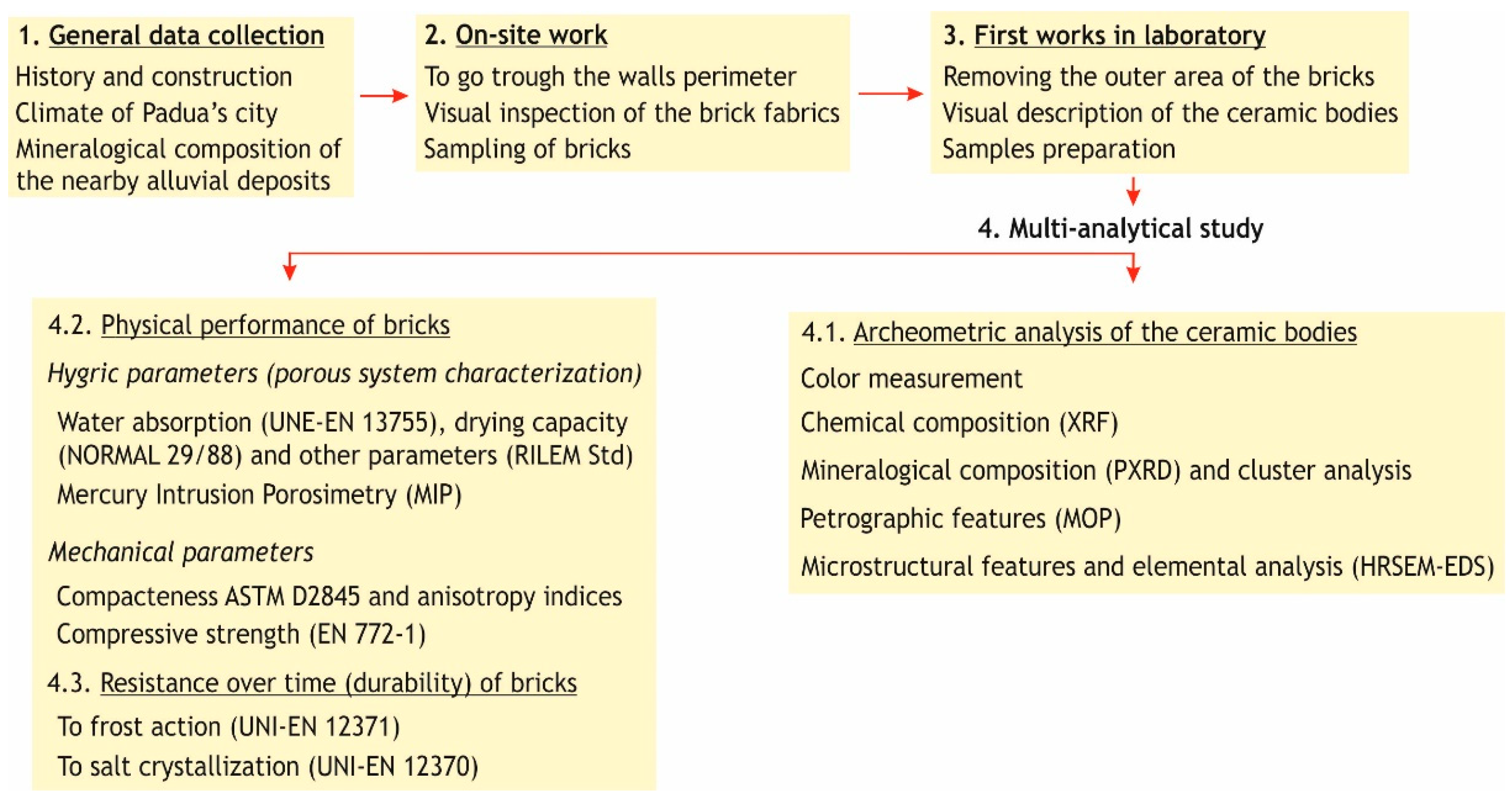
2. Materials and Methods
2.1. Ceramic Bricks from the Renaissance Walls
2.2. Analytical Techniques
2.2.1. Color, Composition and Texture
2.2.2. The Porous System
2.2.3. Compactness, Anisotropy Indices and Compressive Strength
2.2.4. Durability
3. Results and Discussion
3.1. Bricks Manufacturing Technologies
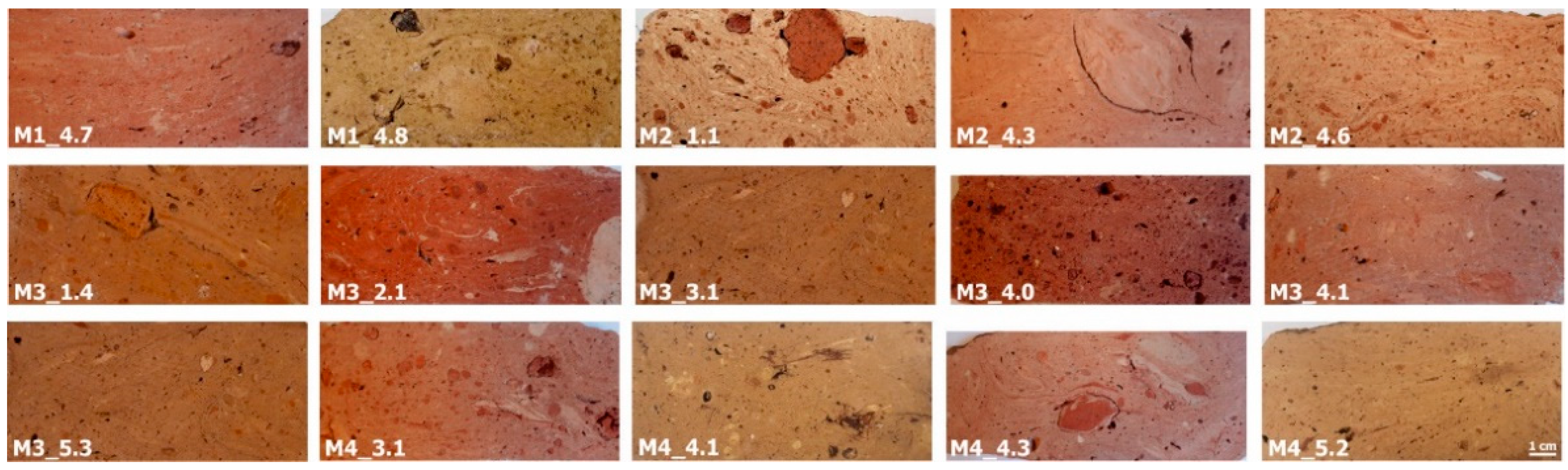
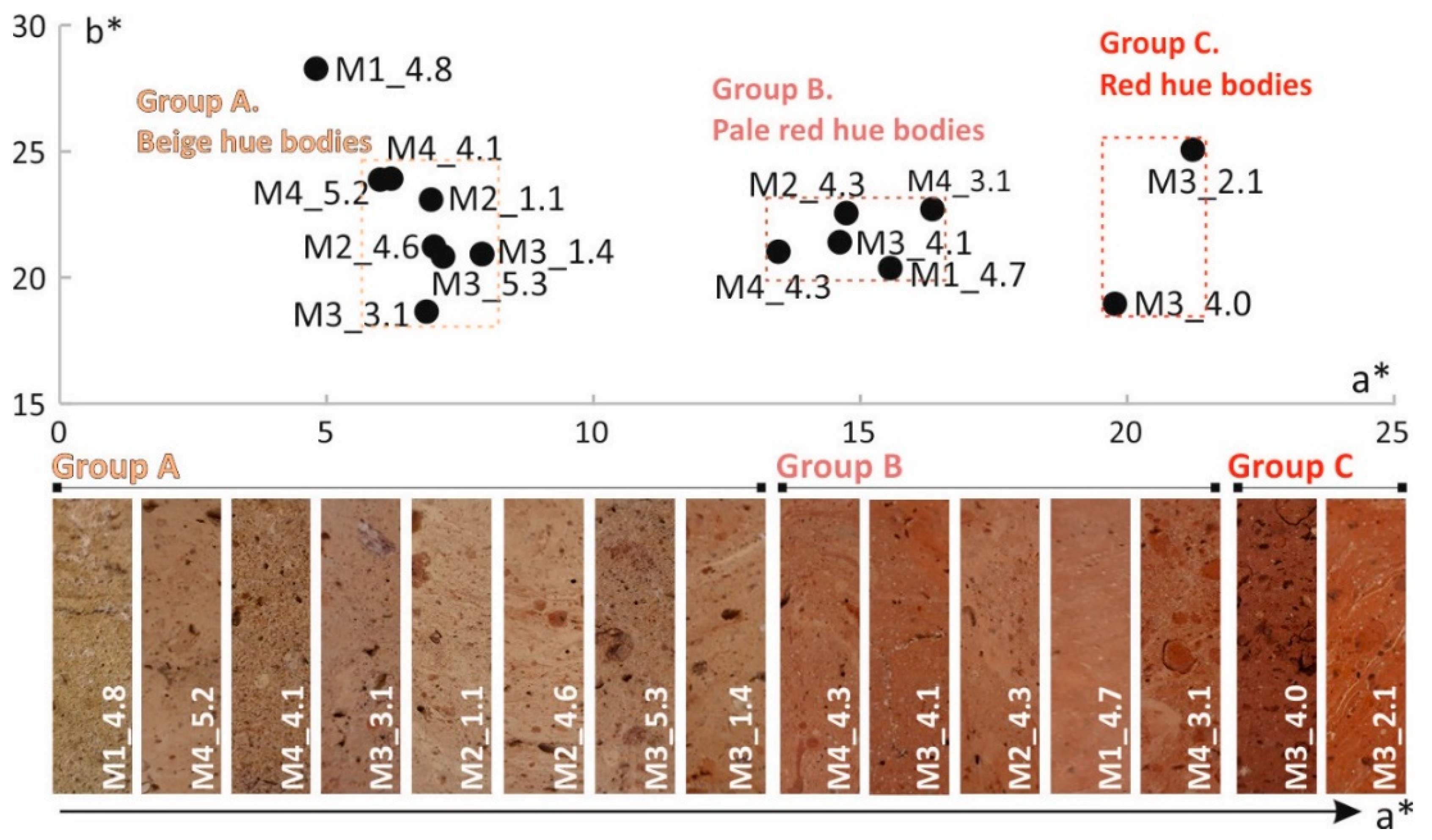
3.1.1. Bricks Color
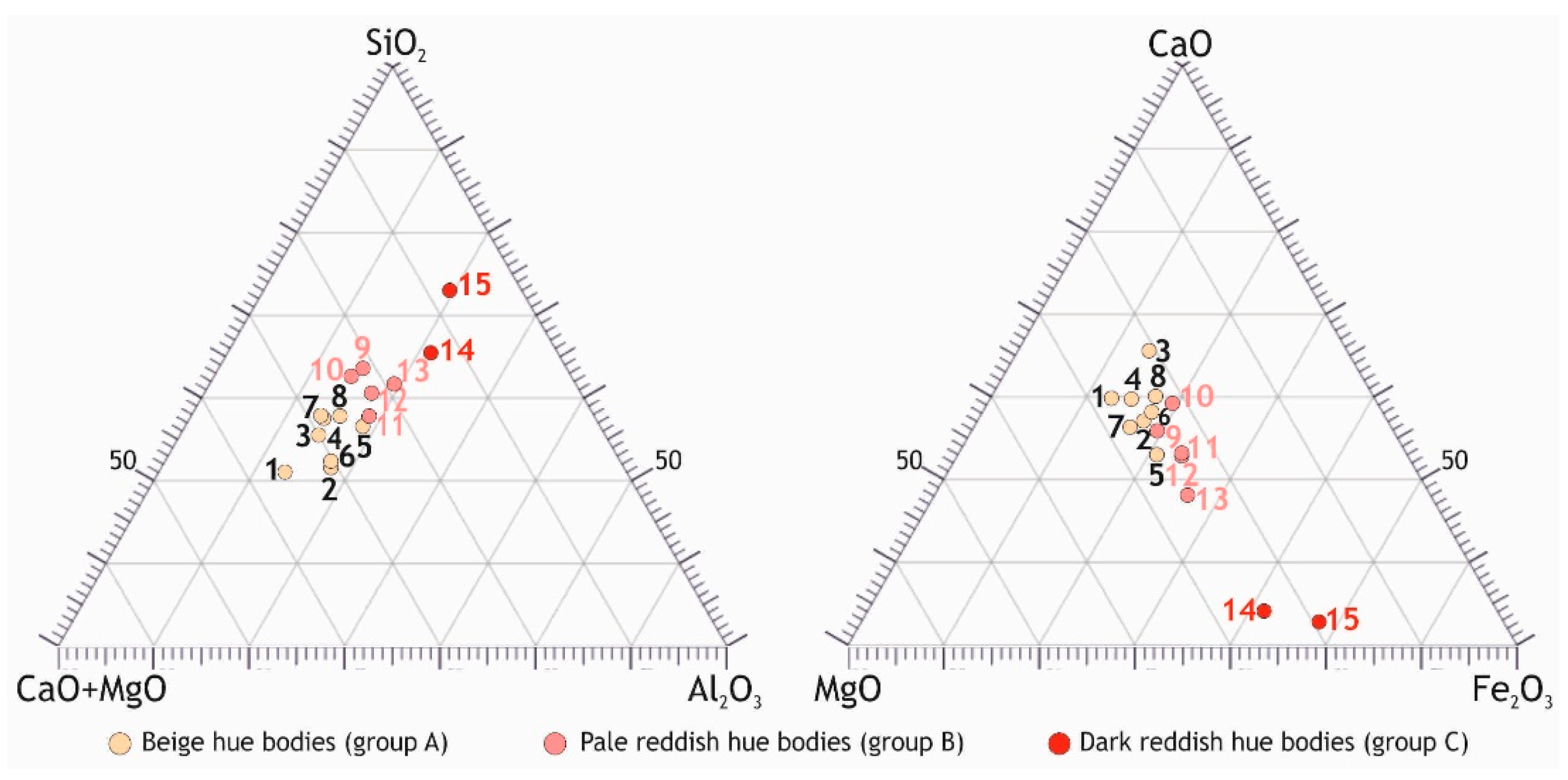
3.1.2. Chemical Composition of the Ceramic Bodies
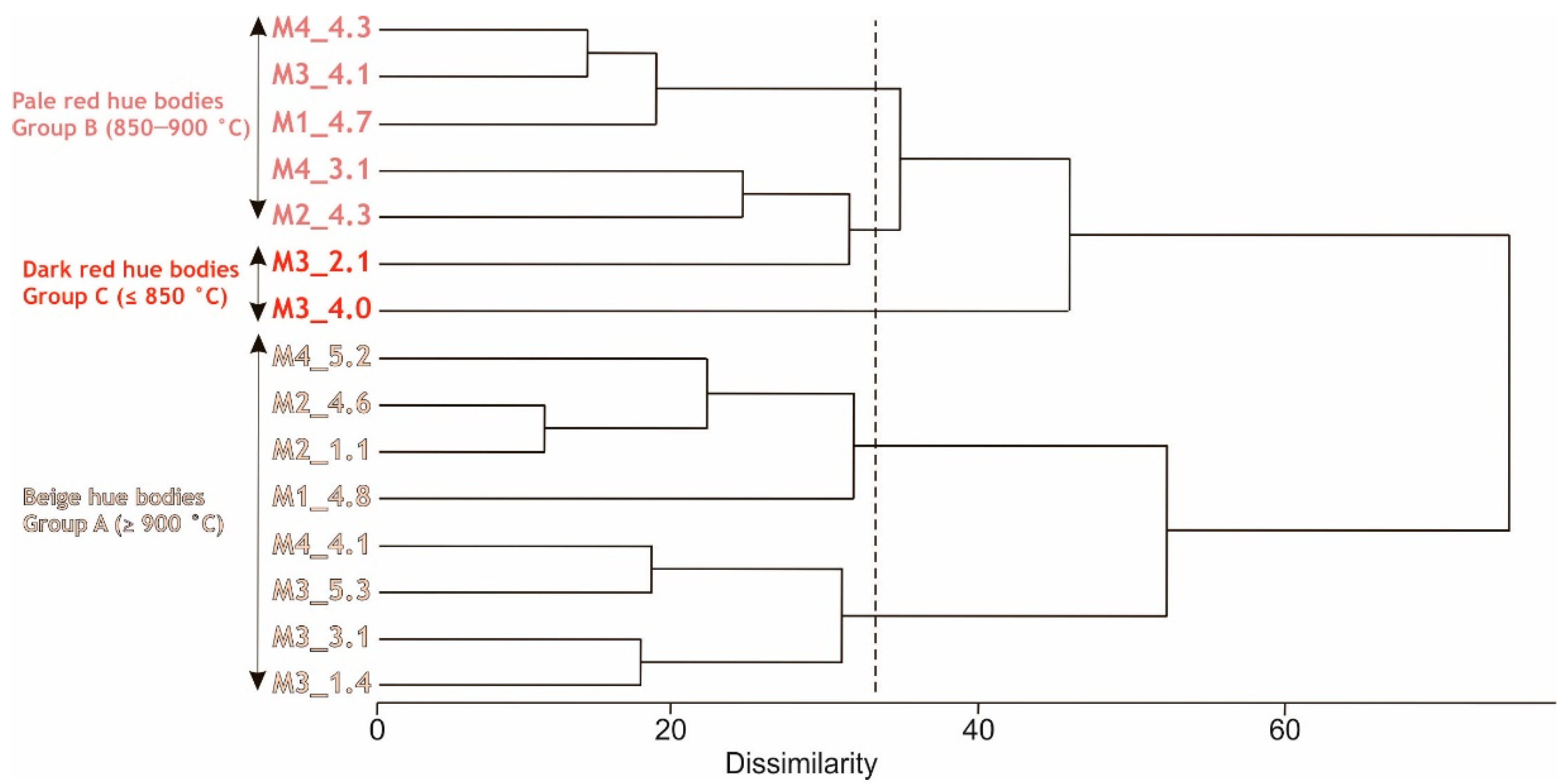
3.1.3. Mineralogical Composition of the Ceramic Bodies
muscovite dehydroxylated phase
dolomite calcite periclase
calcite lime
illite calcite gehelenite
K-feldspar calcite gehelenite
gehelenite anorthite
calcite K-feldspar anorthite
calcite anorthite
dolomite diopside
diopside periclase forsterite
periclase forsterite
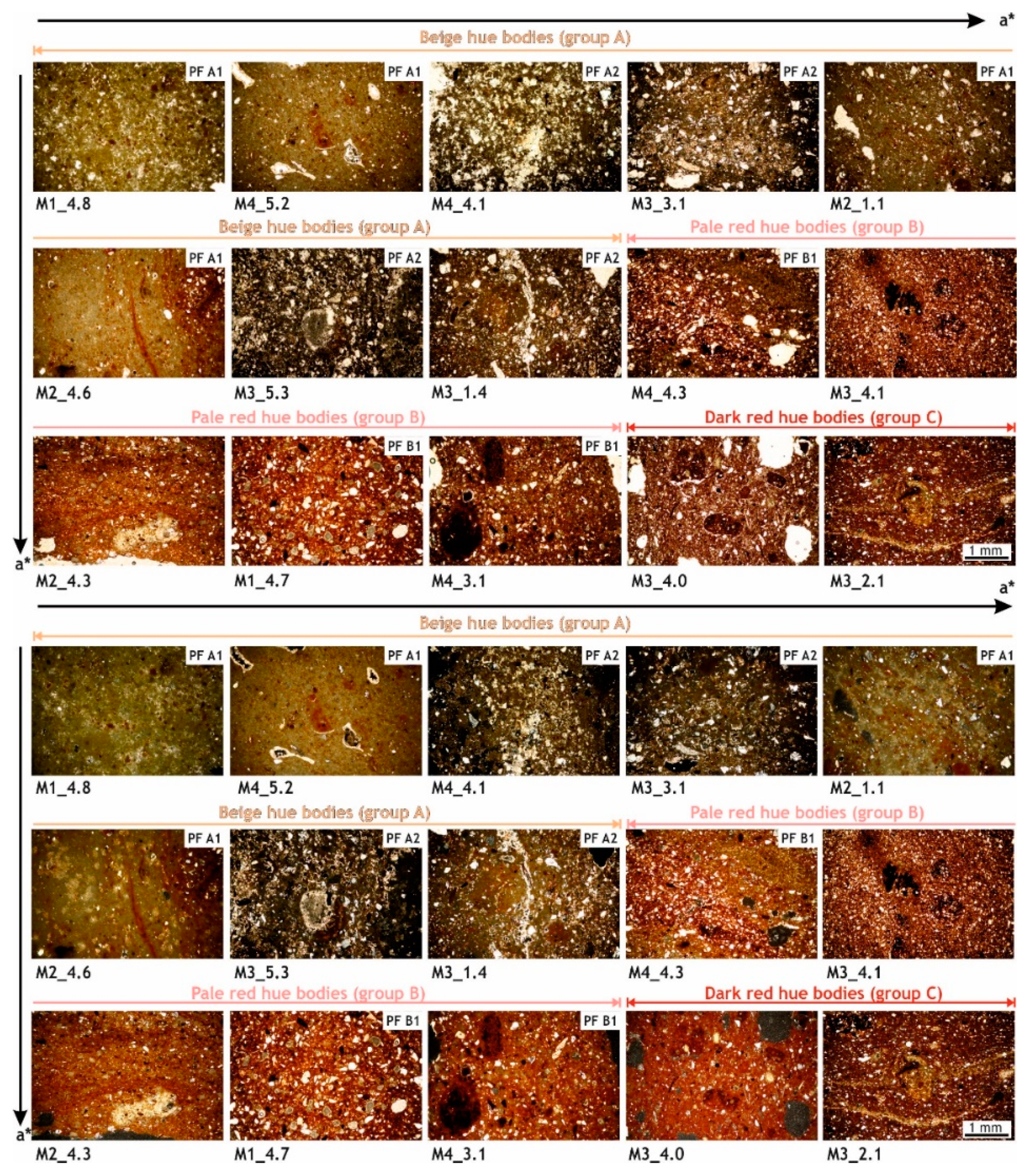
3.1.4. Petrographic Description of the Ceramic Bodies
- Beige hue bodies (group A): petro-fabrics A1 and A2
- Petro-fabric A1 (M1_4.8, M4_5.2, M2_1.1 and M2_4.6 samples) is characterized by a homogeneous, bright yellow-brown hue and optically slightly active matrix. High birefringence and clustered crystals—corresponding mainly to secondary calcite—are extended through the groundmass. A low content of inclusions (5–10%) are noted, mainly represented by greyish carbonate fragments with reaction rims, heterometric in size with sub-rounded and sub-angular shapes, which underwent decomposition during the firing process, so that they have lost their birefringence and have developed dark halos. About 5% of the mass is formed by fine-grained (silty in size) inclusions of sub-rounded monocrystalline quartz. In some bricks, quartz occurs also with angular crystals of medium sand-size, mainly due to its lower consumption during firing. Isolate K-feldspar cystals rather altered, heterometric and sub-angular in shape, and plagioclase are observed. Dark mica flakes and very fine grain-sized clay pellets with unimodal distribution are evenly distributed within the matrix. About 5% of porosity is noted, quite higher in brick M1_4.8, being represented by rounded and sub-rounded pores (vesicles), homogeneous in size and by shrinkage rims developed around inclusions, partially filled by secondary calcite.
- Petro-fabric A2 (M4_4.1, M3_3.1, M3_5.3 and M3_1.4 samples) shows a slightly optically active and quite heterogenous groundmass, where dark brownish and bright yellow areas as well as micritic domains can be observed. Secondary calcite is pervasively crystallized in some areas, increasing the birefringence of the micromass. About 15–20% of inclusions are present and represented by predominant carbonate grains in orange and dark brown colors, with unimodal grain-size distrubution—occasionally up to 2 mm and with dark halos, sub-angular shapes and with the development of intense reaction rims. About 1% of the groundmass is formed by quartz inclusions, overall as single crystals with angular and sub-angular shapes and seriate grain-size distribution. K-feldspar highly altered is frequent, displays seriate grain-size distribution and sub-angular shapes. Sub-millimetric clayey pellets with unimodal distribution—occasionally up to 2 mm—and dark lamellae of biotite with very marked exfoliation—where secondary calcite is likewise formed—are common. Scattered hematite crystals and some metamorfphic rock fragments (MFR)—quartzite and gneiss—and argillaceous rock fragments (ARF) are detected. The micromass shows about 10% of porosity and secondary calcite crystals overlays the inner walls of pores, with irregular and sub-rounded morphologies (vughs and vesicles), and almost fill the porous rims.
- Pale red hue bodies (group B): three samples show analogous microscopic features (petro-fabric B1), while other two set apart (M2_4.3 and M3_4.1).
- Petro-fabric B1 (M1_4.7, M4_3.1 and M4_4.3 samples) is characterized by a uniform, brown-red and slightly optically active groundmass—with a Fe-rich composition—where portions with bright color and high birefringence—with a calcareous composition—were embedded. Inclusions are abundant (40–50%), mainly sub-rounded and sub-angular with bimodal grain-size distribution. Carbonate inclusions are rather altered and some display reaction rims, single crystals of quartz are angular and sub-angular in shape, K-feldspar also shows a high alteration degree and coarser grains are occasionally observed, and plagioclase crystals and mica flakes are often oriented. Sub-millimetric in size Fe-rich clay pellets—sometimes over 1 mm, ARF with unfilled porous rims, opaque and scarce MFR are also present. Porosity is quite low (less than 5%), corresponding with rounded and sub-rounded pores (vesicles) with inner rims slightly covered by secondary calcite crystals.
- Brick M2_4.3 is petrografically quite similar to petro-fabric B1, but is characterized by Fe-rich and Ca-rich domains segregated as flux textures.
- Brick M3_4.1 shows a red color groundmass with speckled birefringent areas where some bright crystallitic portions with secondary calcite are present. Inclusions are very abundant (70–80%), overall quartz and K-feldspar, in sub-angular shapes and mainly homogenous in size. Oriented plagioclase and biotite flakes, millimetric and irregular in shape carbonate grains, very dark areas that may correspond to relict of manganese nodules from the base clay and dispersed hematite crystals are also detected. Clay pellets and ARF—occasionally with porous rims, in some cases filled by secondary calcite—are also observed. Porosity is low, being represented by irregular and elongated pores (vughs and channels) with secondary calcite in the inner rims.
- Dark red bodies (group C): M3_4.0 and M3_2.1 samples
- Brick M3_4.0 displays an intense red hue and high porous groundmass with low optical activity. The inclusions content is high (40–50%) and represented by carbonate grains rather altered but without evident reaction rims, quartz and K-feldspar with unimodal grain-size distribution and angular and sub-angular shapes. Plagioclase and mica flakes are often oriented. Fe-rich clay pellets are frequent and ARFs normally display unfilled porous rims. Isolate hematite crystals, opaque—mainly oxide minerals- and very dark portions—probably also related with the high manganese content of the clayey earth—are detected. Millimetric in size empty pores with sub-rounded morphologies are abundant.
- Brick M3_2.1 shows a red-brown hue and slightly optically active groundmass with speckled birefringent flux fabric. Carbonate grains, both fine grained within the flux textures and very coarse highly transformed, can be observed. It is suggested that decomposition of carbonate grains has provided abundant free lime that has then crystallised as secondary calcite, in the border of the coarse grains and in the adjacent micromass. Indeed, such secondary crystals are formed within the fissures extending throughout the groundmass. Quartz and K-feldspar inclusions with unimodal distribution and angular shapes are highly abundant (60–70%). Frequent plagioclase and mica flakes are often oriented to the brick walls. Dispersed clay pellets, hematite crystals, opaque, dark nodules, ARFs with no porous rims and high altered trachyte fragments with phenocrystals of biotite and hematite crystals are also present.
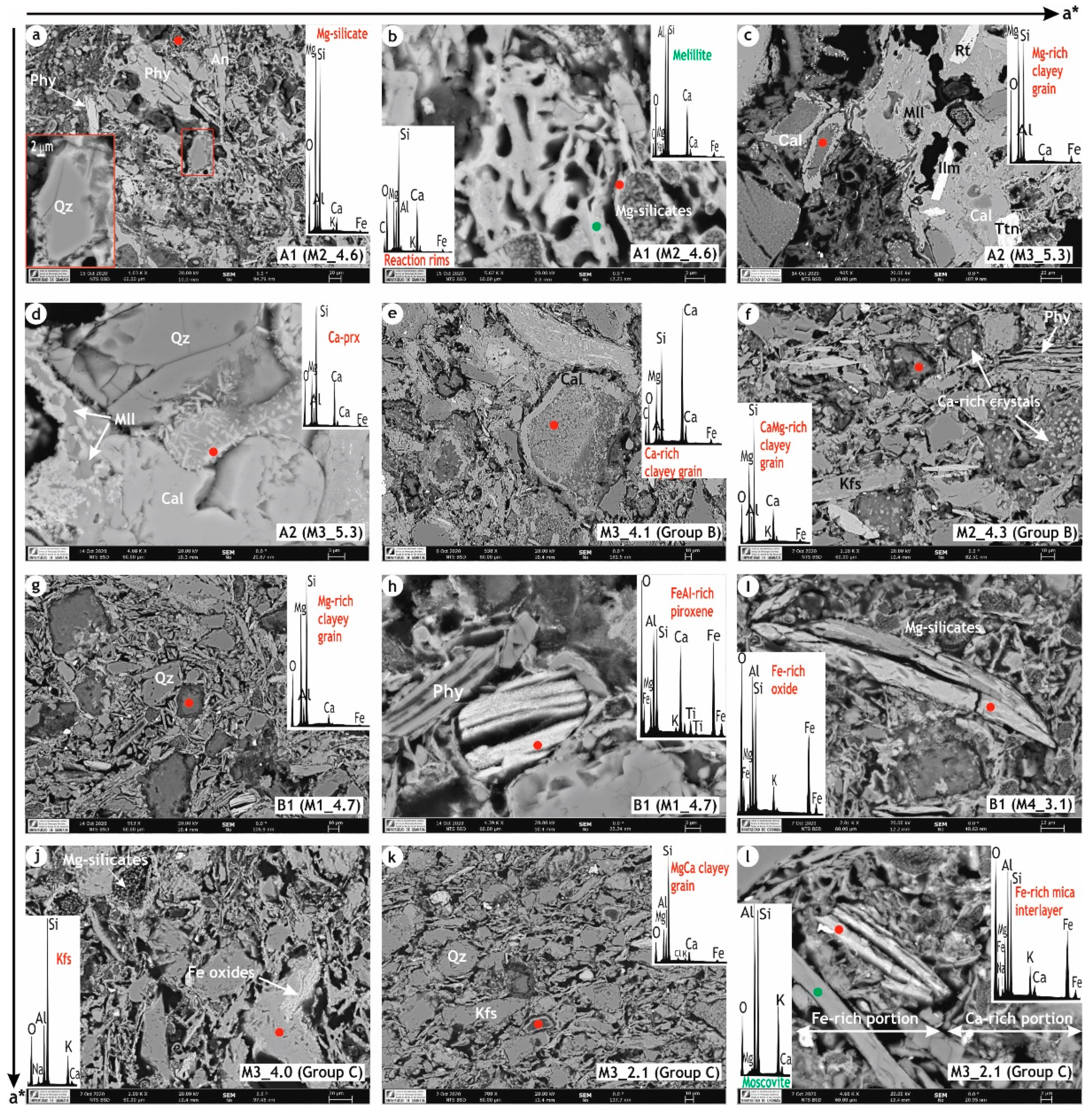
3.1.5. Microstructure of the Ceramic Bodies
3.2. Bricks Physical Performance and Durability
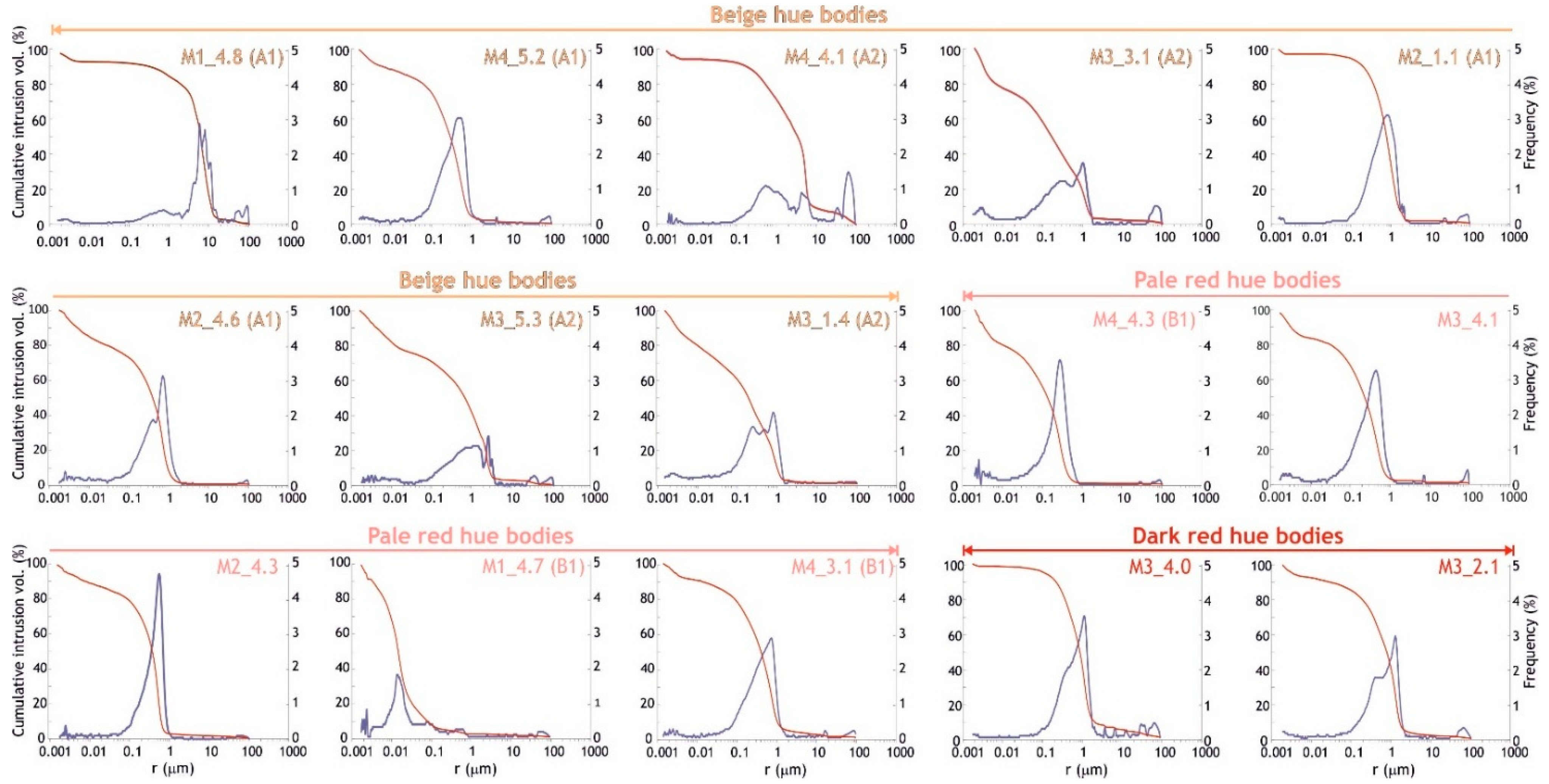
3.2.1. The Porous System
3.2.2. Compactness, Anisotropy Indices and Compressive Strength
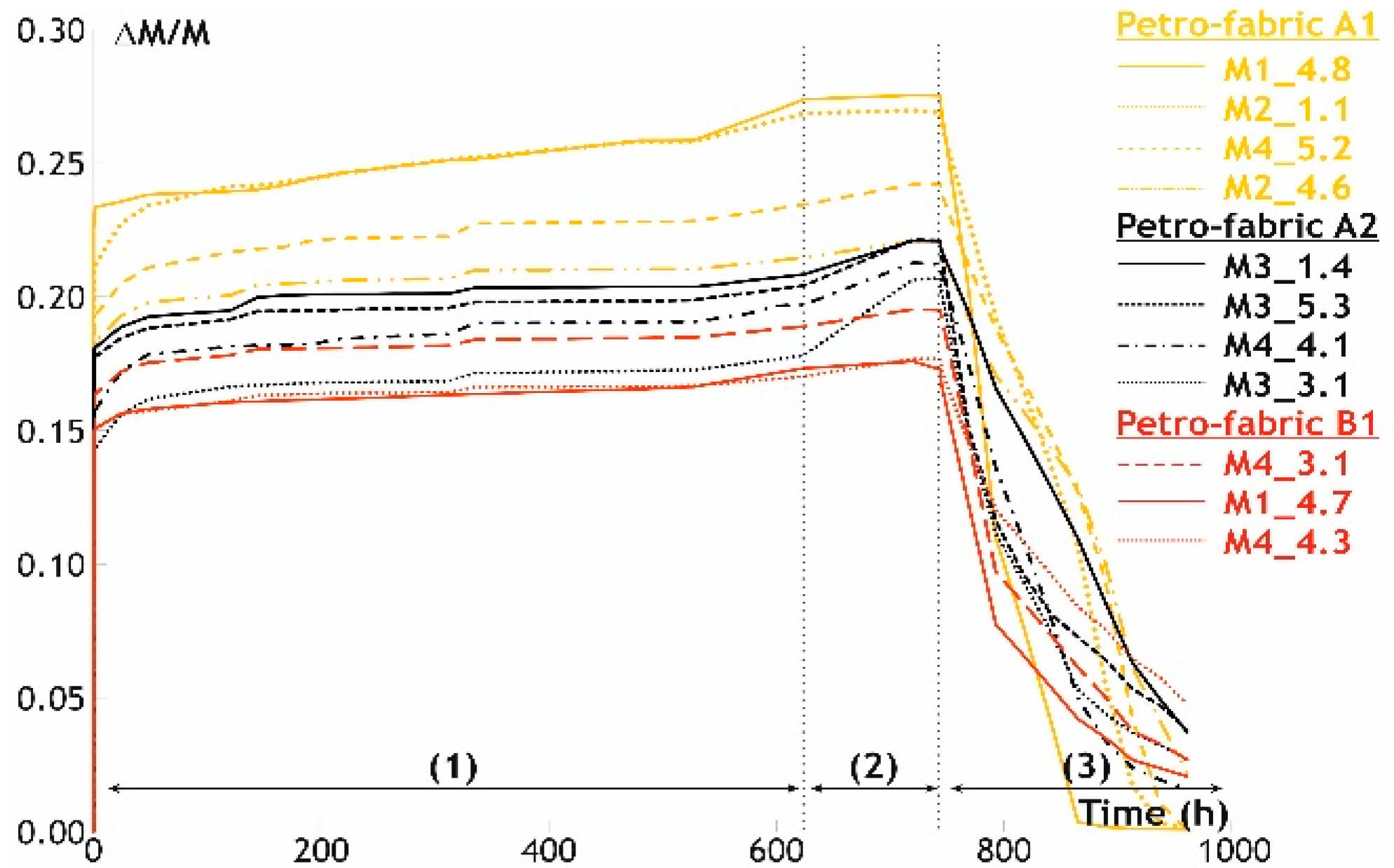
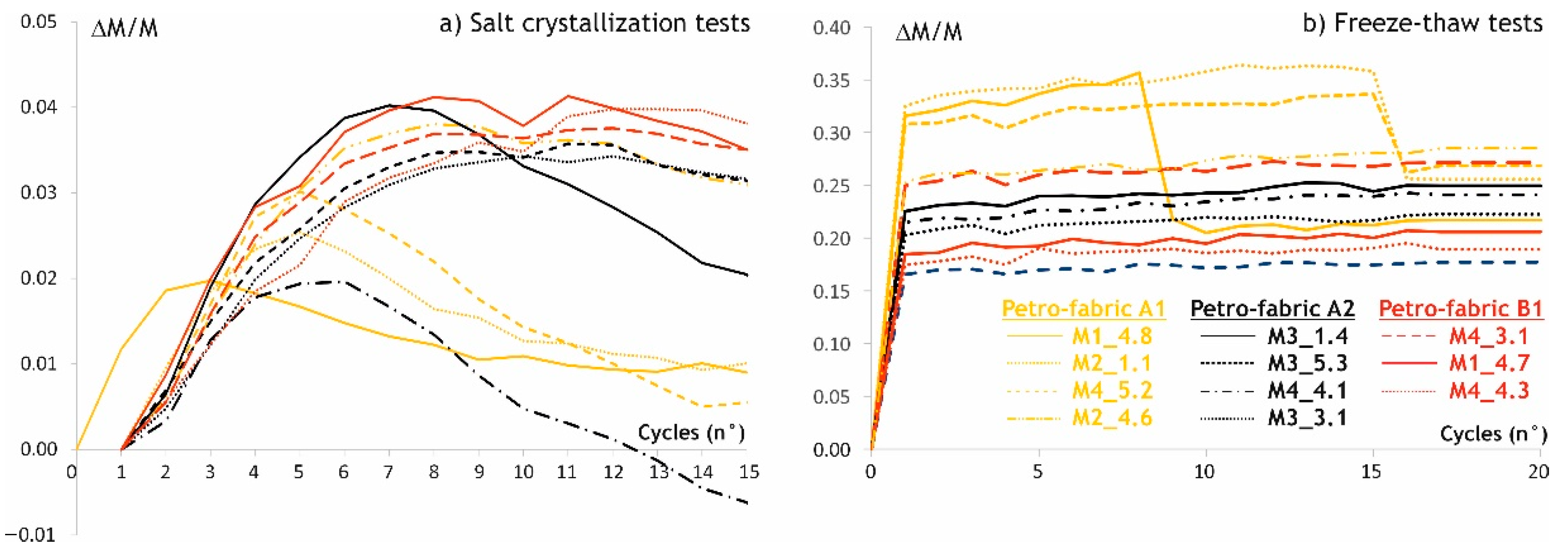
3.2.3. Durability
4. Discussion
- (i)
- Mg- and Ca- (carbonate)-rich illitic clays fired at temperatures of over 900 °C were used to manufacture the beige hue bodies, while the pale red ones were made using carbonate illitic clays rich in Fe and fired at 850–900 °C.
- (ii)
- the beige hue was partly provided by the formation of calcium-aluminosilicates and calcium/magnesium silicates (high-temperature phases), which progressive decreasing yields to red hues, as more iron remains free and may crystallize as hematite. The values of the chromatic coordinates (a* and b*) may entail markers of the mineralogical phases formed during the firing process, hence of the approximative base clays composition and firing temperatures.
- (iii)
- an early sintering stage was achieved for the beige hue bricks, as the melting process was blocked by the formation of the high-temperature phases, while for the red bodies it was due to the lower firing temperatures reached.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molera, J.; Pradell, T.; Vendrell-Saz, M. The colors of Ca-rich ceramic pastes: Origin and characterization. Appl. Clay Sci. 1998, 13, 187–202. [Google Scholar] [CrossRef]
- Rathossi, C.; Pontikes, Y. Effect of firing temperature and atmosphere on ceramics made of NW Peloponnese clay sediments. Part I: Reaction paths, crystalline phases, microstructure and color. J. Eur. Ceram. Soc. 2010, 30, 1841–1851. [Google Scholar] [CrossRef]
- Maritan, L.; Mazzoli, C.; Nodari, L.; Russo, U. Second Iron Age grey pottery from Este (north-eastern Italy): Study of provenance and technology. Appl. Clay Sci. 2005, 29, 31–44. [Google Scholar] [CrossRef]
- Maritan, L.; Nodari, L.; Mazzoli, C.; Milano, A.; Russo, U. Influence of firing conditions on ceramic products: Experimental study on clay rich in organic matter. Appl. Clay Sci. 2006, 31, 1–15. [Google Scholar] [CrossRef]
- Nodari, L.; Maritan, L.; Mazzoli, C.; Russo, U. Sandwich structures in the Etruscan-Padan type pottery. Appl. Clay Sci. 2004, 27, 119–128. [Google Scholar] [CrossRef]
- Valanciene, V.; Siauciunas, R.; Baltusnikaite, J. The influence of mineralogical composition on the color of clay body. J. Eur. Ceram. Soc. 2010, 30, 609–1617. [Google Scholar] [CrossRef]
- Riccardi, M.P.; Messiga, B.; Duminuco, P. An approach to the dynamics of clay firing. Appl. Clay. Sci. 1999, 15, 393–409. [Google Scholar] [CrossRef]
- Klaarenbeeck, W. The development of yellow calcareous bricks. Trans. J. Br. Ceram. Soc. 1961, 60, 738–772. [Google Scholar]
- Kreimeyer, R. Some notes on the firing color of clay bricks. Appl. Clay Sci. 1987, 2, 175–183. [Google Scholar] [CrossRef]
- Nodari, L.; Marcuz, E.; Maritan, L.; Mazzoli, C.; Russo, U. Hematite nucleation and growth in the firing of carbonate-rich clay for pottery production. J. Eur. Ceram. Soc. 2007, 27, 4665–4673. [Google Scholar] [CrossRef]
- De Bonis, A.; Cultrone, G.; Grifa, C.; Langella, A.; Leone, A.P.; Mercurio, M.; Morra, V. Different shades of red: The complexity of mineralogical and physico-chemical factors influencing the color of ceramics. Ceram. Int. 2017, 43, 8065–8074. [Google Scholar] [CrossRef]
- Mirti, P.; Davit, P. New developments in the study of ancient pottery by color measurement. J. Archaeol. Sci. 2004, 31, 741–751. [Google Scholar] [CrossRef]
- Pérez-Monserrat, E.M.; Agua, F.; Fort, R.; Alvarez de Buergo, M.; Conde, J.F.; Garcia-Heras, M. Effect of manufacturing methods on the decay of ceramic materials: A case study of bricks in modern architecture of Madrid (Spain). Appl. Clay Sci. 2017, 135, 136–149. [Google Scholar] [CrossRef]
- Segnit, E.R.; Anderson, C.A. Scanning electron microscopy of fired illite. Trans. Br. Ceram. Soc. 1972, 71, 85–88. [Google Scholar]
- Rice, P.M. Pottery Analysis. A Sourcebook; University of Chicago Press: Chicago, IL, USA, 1987. [Google Scholar]
- Cairo, A.; Messiga, B.; Riccardi, M.P. Technological features of the ‘Cotto Variegato’: A petrological approach. J. Cult. Herit. 2001, 2, 133–142. [Google Scholar] [CrossRef]
- Eramo, G. Ceramic technology. How to recognize clay processing. Archaeol. Anthropol. Sci. 2020, 12, 164. [Google Scholar] [CrossRef]
- Adell, J.M. 19th century brick architecture: Rationality and modernity. Inf. Constr. 1992, 55, 5–15. [Google Scholar]
- Galindo Diez, J. La construcción de murallas: Un aspecto del saber constructivo presente en los tratados de arquitectura militar (Siglo XV al XVIII). In Proceedings of the Actas 1er Congreso Nacional de Historia de la Construcción, Madrid, España, 19–21 September 1996; de las Casas, A., Huerta, S., Rabasa, E., Eds.; Instituto Juan de Herrera CEHOPU: Madrid, España, 1996; pp. 217–222. [Google Scholar]
- Cultrone, G.; Sebastián, E.; Elert, K.; de la Torre, M.J.; Cazalla, O.; Rodríguez Navarro, C. Influence of mineralogy and firing temperature on the porosity of bricks. J. Eur. Ceram. Soc. 2004, 24, 547–564. [Google Scholar] [CrossRef]
- Molina, E.; Cultrone, G.; Sebastián, E.; Alonso, F.J.; Carrizo, L.; Gisbert, J.; Buj, O. The pore system of sedimentary rocks as a key factor in the durability of building materials. Eng. Geol. 2011, 118, 110–121. [Google Scholar] [CrossRef]
- Coletti, C.; Maritan, L.; Cultrone, G.; Dalconi, M.C.; Hein, A.; Molina, E.; Mazzoli, C. Recycling trachyte waste from the quarry to the brick industry: Effects on physical and mechanical properties, and durability of new bricks. Constr. Build. Mater. 2018, 166, 792–807. [Google Scholar] [CrossRef]
- Charola, A.E.; Wendler, E. An overview of the water-porous building materials interactions. Restor. Build. Monum. 2015, 21, 55–65. [Google Scholar] [CrossRef]
- Mazzi, G.; Verdi, A.; Dal Piaz, V. Le mura di Padova; Il Poligrafo Editore: Padova, Italia, 2002. [Google Scholar]
- Fadini, U.; Bordignon, F.; Dal Zotto, P. Mura di Padova—Guida al Sistema Bastionato Rinascimentale; Edibus: Vicenza, Italia, 2013. [Google Scholar]
- Donvito, V.C.; Fadini, U. (Eds.) Padova è le sue Mura. Cinquecento Anni di Storia 1513–2013 Catalogo Della Mostra (Padova, Musei Civici agli Eremitani, 28 Marzo—20 Luglio 2014); Biblos: Cittadella, Padova, Italia, 2014. [Google Scholar]
- Germinario, L.; Siegesmund, S.; Maritan, L.; Simon, K.; Mazzoli, C. Trachyte weathering in the urban built environment related to air quality. Herit. Sci. 2017, 5, 44. [Google Scholar] [CrossRef]
- Piaser, S. (Ed.) Padova Sotterranea. Nel Cuore dell Mura Rinascimentali Esistenti piu Estese d’Europa; Edizioni Chartesis, Concomitato di Mure di Padova: Padova, Italia, 2018. [Google Scholar]
- Jobstraibitzer, P.; Mallesani, P. I sedimenti dei fiumi veneti. Mem. Soc. Geo. Ital. 1973, 12, 411–452. [Google Scholar]
- Maritan, L. Archaeometric study of Etruscan-Padan type pottery from the Veneto region: Petrographic, mineralogical and geochemical-physical characterization. Eu. J. Mineral. 2004, 16, 297–307. [Google Scholar] [CrossRef]
- UNE-EN 15886. Conservation of Cultural Property. Test Methods. Color Measurement of Surfaces; AENOR: Madrid, Spain, 2011. [Google Scholar]
- Govindaraju, K. Compilation of working values and sample description for 383 geostandards. Geostandard. Newsl. 1994, 18, 1–158. [Google Scholar] [CrossRef]
- De Jongh, W.K. X-ray fluorescence analysis applying theoretical matrix corrections. Stainless steel. X-ray Spectrom. 1973, 2, 151–158. [Google Scholar] [CrossRef]
- Locock, A.J.; Chesterman, D.; Caird, D.; Duke, M.J.M. Miniaturization of mechanical milling for powder X-ray diffraction. Powder Diff. 2012, 27, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Maritan, L.; Holakooei, P.; Mazzoli, C. Cluster analysis of XRPD data in ancient ceramics: What for? Appl. Clay Sci. 2015, 114, 540–549. [Google Scholar] [CrossRef]
- Whitbread, I.K. A proposal for the systematic description of thin sections towards the study of ancient ceramic technology. In Proceedings of the 25th International Symposium on Archaeometry, Athens, Greece, 19–23 May 1986; Maniatis, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 127–138. [Google Scholar]
- Quinn, P.S. Ceramic Petrography: The Interpretation of Archaeological Pottery and Related Artefacts in Thin Sections; Archaeopress: Oxford, UK, 2013. [Google Scholar]
- UNE-EN 13755. Natural Stone Test Methods. Determination of Water Absorption at Atmospheric Pressure; AENOR: Madrid, Spain, 2008. [Google Scholar]
- NORMAL 29/88. Misura Dell’indice di Asciugamento (Drying Index); CNR-ICR: Rome, Italy, 1988. [Google Scholar]
- Cultrone, G.; de la Torre, M.J.; Sebastián, E.; Cazalla, O. Evaluation of bricks durability using destructive and non-destructive methods (DT and NDT). Mater. Constr. 2003, 53, 41–59. [Google Scholar] [CrossRef] [Green Version]
- RILEM. Recommended test to measure the deterioration of stone and to assess the differences of treatment methods. Mater. Struct. 1980, 13, 175–253. [Google Scholar]
- Benavente, D.; Linares-Fernández, L.; Cultrone, G.; Sebastián, E. Influence of microstructure on the resistance to salt crystallization damage in bricks. Mater. Struct. 2006, 39, 105–113. [Google Scholar] [CrossRef]
- ASTM D2845. Standard Test. Method for Laboratory Determination of Pulse Velocities and Ultrasonic Elastic Constant of Rock; American Society for Testing and Materials: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Guydader, J.; Denis, A. Propagation des ondes dans les roches anisotropies sous contrainte évaluation de la qualité des schistes ardoisers. Bull. Eng. Geol. 1986, 33, 49–55. [Google Scholar] [CrossRef]
- EN 772-1. Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength; European Committee for Standardization: Brussels, Belgium, 2015. [Google Scholar]
- UNI EN 12371. Natural Stone Test Methods—Determination of Frost Resistance; CNR-ICR: Rome, Italy, 2010. [Google Scholar]
- UNI EN 12370. Natural Stone Test Methods—Determination of Resistance to Salt Crystallization; CNR-ICR: Rome, Italy, 2001. [Google Scholar]
- Espinosa Marzal, R.M.; Hamilton, A.; McNall, M.; Whitaker, K.; Scherer, G.W. The chemomehanics of crystallization during rewetting of limestone impregnated with sodium sulfate. J. Mater. Res. 2011, 26, 1472–1481. [Google Scholar] [CrossRef] [Green Version]
- Kramar, S.; Lux, J.; Mladenović, A.; Pristacz, H.; Mirtič, B.; Sagadin, M.; Rogan-Šmuc, N. Mineralogical and geochemical characteristics of Roman pottery from an archaeological site near Mošnje (Slovenia). Appl. Clay Sci. 2012, 57, 39–48. [Google Scholar] [CrossRef]
- Iordanidis, A.; Garcia-Guinea, J.; Karamitrou-Mentessidic, G. Analytical study of ancient pottery from the archaeological site of Aiani, northern Greece. Mater. Charact. 2009, 60, 292–302. [Google Scholar] [CrossRef]
- Maritan, L.; Mazzoli, C. Phosphates in archaeological finds: Implications for environmental conditions of burial. Archaeometry 2004, 46, 673–683. [Google Scholar] [CrossRef]
- Maritan, L.; Angelini, I.; Artioli, G.; Mazzoli, C.; Saracino, M. Secondary phosphates in the ceramic materials from Frattesina (Rovigo, North-Eastern Italy). J. Cult. Herit. 2009, 10, 144–151. [Google Scholar] [CrossRef]
- Maritan, L. Ceramic abandonment. How to recognize post-depositional transformations. Archaeol. Anthropol. Sci. 2020, 12, 199. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Zhang. N. In situ high temperature X-ray diffraction study of illite. Appl. Clay Sci. 2017, 146, 254–263. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Hajjaji, M. A chloritic-illitic clay from Morocco: Temperature-time transformation and neoformation. Appl. Clay Sci. 2009, 45, 83–89. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Luque, A.; Rodriguez-Navarro, A.B.; Ortega-Huertas, M. Thermal decomposition of calcite: Mechanisms of formation and textural evolution of CaO nanocrystals. Am. Mineral. 2009, 94, 578–593. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Kudlacz, K.; Ruiz-Agudo, E. The mechanism of thermal decomposition of dolomite: New insights from 2D-XRD and TEM analyses. Am. Mineral. 2012, 97, 38–51. [Google Scholar] [CrossRef]
- Maritan, L.; Ganzarolli, G.; Antonelli, F.; Rigo, M.; Kapatza, A.; Bajnok, K.; Coletti, C.; Mazzoli, C.; Lazzarini, L.; Vedovetto, P.; et al. What kind of calcite? Disclosing the origin of sparry calcite temper in ancient ceramics. J. Archaeol. Sci. 2021, 129, 105358. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastian, E.; Cazalla, O.; de la Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Jordán, M.M.; Sanfeliu, T.; de la Fuente, C. Firing transformations of Tertiary clays used in the manufacturing of ceramic tile bodies. Appl. Clay Sci. 2001, 20, 87–95. [Google Scholar] [CrossRef]
- Traoré, K.; Kabre, T.S.; Blanchart, P. Low temperature sintering of a clay for pottery from Burkina Faso. Appl. Clay Sci. 2000, 17, 279–292. [Google Scholar] [CrossRef]
- Elias, M.L.; Cultrone, G. On the Use of Sodium Chloride and Calcined Diatomite Sludge as Additives to Improve the Engineering Properties of Bricks Made with a Clay Earth from Jun (Granada, Spain). Minerals 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Daghmehchi, M.; Omrani, H.; Emami, M.; Nokandeh, J. Mineralogical and thermo-chemical characteristics of the Hellenistic ceramics and raw clay from Qizlar Qal’eh (northeastern Iran). Mater. Charact. 2016, 120, 143–151. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Fabbri, B.; Mars Igli, M. An approach to the chemistry of pyroxenes formed during the firing of Ca-rich silicate ceramics. Clay Miner. 1998, 33, 443–452. [Google Scholar] [CrossRef]
- Shoval, S. Mineralogical changes upon heating calcitic and dolomitic marl rocks. Thermochim. Acta 1988, 135, 243–252. [Google Scholar] [CrossRef]
- Trindade, M.J.; Dias, M.I.; Coroado, J.; Rocha, F. Mineralogical transformations of calcareous rich clays with firing: A comparative study between calcite and dolomite rich clays from Algarve, Portugal. Appl. Clay Sci. 2009, 42, 345–355. [Google Scholar] [CrossRef]
- Tenconi, M.; Maritan, L.; Leonardi, G.; Prosdocimi, B.; Mazzoli, C. Ceramic production and distribution in north-east Italy: Study of a possible trade network between Friuli Venezia Giulia and Veneto regions during the final Bronze Age and early Iron Age through analysis of peculiar ‘flared rim and flat lip’ pottery. Appl. Clay. Sci. 2013, 82, 121–134. [Google Scholar] [CrossRef]
- Issi, A.; Kara, A. An investigation of pottery production technology for the West Slope wares from Dorylaion (Eskişehir/Turkey). Bol. Soc. Esp. Ceram. Vidr. 2013, 52, 42–47. [Google Scholar] [CrossRef]
- Fabbri, B.; Gualtieri, S.; Shoval, S. The presence of calcite in archaeological ceramics. J. Eur. Ceram. Soc. 2014, 34, 1899–1911. [Google Scholar] [CrossRef]
- Saenz, N.; Sebastián, E.; Cultrone, G. Analysis of tempered bricks: From raw materials and additives to fired bricks for use in construction and heritage conservation. Eur. J. Mineral. 2019, 31, 301–312. [Google Scholar] [CrossRef]
- Sondi, I.; Juracic, M. Whiting events and the formation of aragonite in the Mediterranean Karstic Marine Lakes: New evidence on its biologically induced organic origin. Sedimentology 2010, 57, 85–95. [Google Scholar] [CrossRef]
- Pérez-Monserrat, E.M.; Maritan, L.; Causarano, M.A.; Chavarría, A.; Brogiolo, G.P. Ancient bricks technologies: Improving the built heritage conservation at high humidity areas. In Proceedings of the International Conference on Metrology for Archaeology and Cultural Heritage, Trento, Italy, 22–24 October 2020; pp. 522–526. [Google Scholar]
- Buxeda i Garrigos, J.; Mommsen, H.; Tsolakidou, A. Alterations of Na, K and Rb concentrations in Mycenean pottery and a proposed explanation using X-Ray Diffraction. Archaeometry 2002, 44, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Heimann, R.B.; Maggetti, M. Experiments on simulated burial of calcareous terra sigillata: Mineralogical changes-preliminary results. In Scientific Studies in Ancient Ceramics; Hughes, M.J., Ed.; British Museum, Occasional Paper 19: London, UK, 1981; pp. 163–177. [Google Scholar]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Everhart, J.O. Use of auxiliary fluxes to improve structural clay bodies. Am. Ceram. Soc. Bull. 1957, 36, 268–271. [Google Scholar]
- Tite, M.S.; Maniatis, Y. Examination of ancient pottery using the scanning electron microscope. Nature 1975, 257, 122–123. [Google Scholar] [CrossRef]
- Cucato, M.; de Vecchi, G.P.; Mozzi, P.; Abbà, T.; Paiero, G.; Sedea, R. (Eds.) CARG Progetto. Note illustrative della Carta Geologica d’Italia alla scalla 1:50.000. Foglio 147. Padova Sud.; Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA): Regione del Veneto, Italia, 2008; p. 224. [Google Scholar]
- Cultrone, G.; Molina, E.; Arizzi, A. The combined use of petrographic, chemical and physical techniques to define the technological features of Iberian ceramics from the Canto Tortoso area (Granada, Spain). Ceram. Int. 2014, 40, 10803–10816. [Google Scholar] [CrossRef]
- Cultrone, G.; Carrillo Rosua, F.J. Growth of metastable phases during brick firing: Mineralogical and microtextural changes induced by the composition of the raw material and the presence of additives. Appl. Clay Sci. 2020, 185, 105419. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Cultrone, G.; Sanchez-Navas, A.; Sebastian, E. TEM study of mullite growth after muscovite breakdown. Am. Mineral. 2003, 88, 713–724. [Google Scholar] [CrossRef]
- Coletti, C.; Mazzoli, C.; Maritan, L.; Cultrone, G. Combined multi-analytical approach for study of pore system in bricks: How much porosity is there? Mater. Charact. 2016, 121, 82–92. [Google Scholar] [CrossRef]
- Toledo, R.; dos Santos, D.R.; Faria, R.T., Jr.; Carrio, J.G.; Auler, L.T.; Vargas, H. Gas release during clay firing and evolution of ceramic properties. Appl. Clay Sci. 2004, 27, 151–157. [Google Scholar] [CrossRef]
- Cultrone, G.; Aurrekoetxea, I.; Casado, C.; Arizzi, A. Sawdust recycling in the production of lightweight bricks: How the amount of additive and the firing temperature influence the physical properties of the bricks. Constr. Build. Mater. 2020, 235, 117436. [Google Scholar] [CrossRef]
- Karagiannis, N.; Karoglou, M.; Bakolas, A.; Krokida, M.; Moropoulou, A. Drying kinetics of building materials capillary moisture. Constr. Build. Mater. 2017, 137, 441–449. [Google Scholar] [CrossRef]
- Whiteley, P.; Russman, H.D.; Bishop, J.D. Porosity of building materials. A collection of published results. J. Oil Color. Chem. Assoc. 1977, 60, 142–150. [Google Scholar]
- Martínez-Martínez, J.; Benavente, D.; Ordóñez, S.; García del Cura, M.A. Multivariate statistical techniques for evaluating the effects of brecciated rock fabric on ultrasonic wave propagation. Int. J. Rock Mech. Min. Sci. 2008, 45, 609–620. [Google Scholar] [CrossRef]
- Elert, K.; Cultrone, G.; Rodriguez-Navarro, C.; Sebastian Pardo, E. Durability of bricks used in the conservation of historic buildings. Influence of composition and microstructure. J. Cult. Herit. 2003, 4, 91–99. [Google Scholar] [CrossRef]
- Fernandes, F.; Lourenço, P.B. Evaluation of the Compressive Strength of Ancient Clay Bricks Using Microdrilling. J. Mater. Civ. Eng. ASCE 2007, 19, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Stefanidou, M.; Papayianni, I.; Pachta, V. Analysis and characterization of Roman and Byzantine fired bricks from Greece. Mater. Struct. 2015, 48, 2251–2260. [Google Scholar] [CrossRef]
- Matysek, P.; Witkowski, M. A Comparative Study on the Compressive Strength of Bricks from Different Historical Periods. Int. J. Archit. Herit. 2016, 10, 396–405. [Google Scholar] [CrossRef]
- Garbin, E.; Panizza, M.; Valluzzi, M.R. Experimental characterization of solid clay bricks: Correlations among mechanical properties. In Structural Analysis of Historical Constructions; Aguilar, R., Torrealva, D., Moreira, S., Pando, M.A., Ramos, L.F., Eds.; RILEM Bookseries; Springer: Cham, Switzerland, 2019; Volume 18, pp. 634–643. [Google Scholar] [CrossRef]
- EN 1998-1. Eurocode 8: Design of Structures for Earthquake Resistance—Part 1: General Rules, Seismic Actions and Rules for Buildings; European Committee for Standardization: Brussels, Belgium, 2008. [Google Scholar]
- ASTM C62-05. Standard Specification for Building Brick (Solid Masonry Units Made From Clay or Shale); American Society for Testing and Materials: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Goudie, A.S. Laboratory simulation of ‘the wick effect’ in salt weathering of rock. Earth Surf. Proc. Land. 1986, 11, 275–285. [Google Scholar] [CrossRef]

| Hue | Brick | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|---|
| Beige Group A | 1. M1_4.8 1 | 62.33 ± 1.78 | 4.81 ± 0.16 | 28.29 ± 1.90 | 28.69 ± 1.86 | 80.31 ± 0.87 |
| 2. M4_5.2 | 74.18 ± 1.92 | 6.00 ± 0.24 | 23.88 ± 0.47 | 24.63 ± 0.45 | 75.88 ± 0.61 | |
| 3. M4_4.1 | 67.22 ± 1.36 | 6.20 ± 0.30 | 23.93 ± 0.51 | 24.72 ± 0.44 | 75.35 ± 0.91 | |
| 4. M3_3.1 | 70.52 ± 1.10 | 6.87 ± 0.20 | 18.66 ± 0.59 | 19.89 ± 0.58 | 69.77 ± 0.68 | |
| 5. M2_1.1 | 71.13 ± 1.61 | 6.96 ± 0.46 | 23.09 ± 0.17 | 24.12 ± 0.20 | 73.23 ± 1.10 | |
| 6. M2_4.6 | 72.27 ± 1.11 | 7.01 ± 1.00 | 21.23 ± 0.78 | 22.38 ± 0.90 | 71.72 ± 2.31 | |
| 7. M3_5.3 | 64.79 ± 1.73 | 7.18 ± 0.45 | 20.82 ± 0.73 | 22.03 ± 0.72 | 70.97 ± 1.20 | |
| 8. M3_1.4 | 69.71 ± 1.24 | 7.91 ± 0.58 | 20.93 ± 0.76 | 22.39 ± 0.82 | 69.29 ± 1.27 | |
| Pale red Group B | 9. M4_4.3 | 61.50 ± 1.66 | 13.46 ± 1.06 | 21.04 ± 0.45 | 24.99 ± 0.87 | 57.43 ± 1.71 |
| 10. M3_4.1 | 60.62 ± 0.89 | 14.63 ± 0.65 | 20.93 ± 0.71 | 25.54 ± 0.92 | 55.04 ± 0.63 | |
| 11. M2_4.3 | 62.99 ± 1.58 | 14.73 ± 0.45 | 22.56 ± 0.38 | 26.95 ± 0.53 | 56.83 ± 0.56 | |
| 12. M1_4.7 | 59.22 ± 0.31 | 15.56 ± 0.36 | 20.37 ± 0.55 | 25.63 ± 0.63 | 52.50 ± 0.40 | |
| 13. M4_3.1 | 58.10 ± 1.77 | 16.35 ± 0.78 | 22.72 ± 0.56 | 27.99 ± 0.83 | 54.27 ± 1.20 | |
| Dark red Group C | 14. M3_4.0 | 46.23 ± 1.05 | 19.80 ± 0.47 | 18.68 ± 0.62 | 25.56 ± 0.48 | 46.97 ± 1.37 |
| 15. M3_2.1 | 52.17 ± 0.91 | 21.23 ± 0.62 | 25.06 ± 0.44 | 32.84 ± 0.71 | 49.74 ± 0.49 |
| Hue | Brick | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | Zr | LOI 1 | SUM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beige Group A | 1. M1_4.8 | 43.6 | 11.2 | 4.54 | 0.09 | 9.59 | 20.9 | 1.05 | 2.04 | 0.86 | 0.27 | 202.8 | 4.85 | 98.9 |
| 2. M4_5.2 | 43.5 | 15.0 | 5.64 | 0.10 | 7.99 | 17.8 | 1.51 | 1.73 | 0.74 | 0.18 | 153.1 | 5.35 | 99.7 | |
| 3. M4_4.1 | 45.9 | 11.8 | 3.98 | 0.07 | 5.92 | 18.5 | 1.52 | 1.77 | 0.64 | 0.24 | 165.1 | 9.52 | 99.9 | |
| 4. M3_3.1 | 47.3 | 11.7 | 4.04 | 0.07 | 7.09 | 16.6 | 0.98 | 2.00 | 0.67 | 0.15 | 155.2 | 9.29 | 99.8 | |
| 5. M2_1.1 | 48.7 | 15.9 | 5.61 | 0.10 | 7.08 | 13.9 | 1.28 | 2.94 | 0.66 | 0.17 | 162.5 | 3.24 | 99.6 | |
| 6. M2_4.6 | 42.8 | 14.4 | 5.42 | 0.10 | 7.32 | 17.3 | 1.09 | 2.58 | 0.67 | 0.19 | 146.3 | 8.04 | 99.9 | |
| 7. M3_5.3 | 46.7 | 11.3 | 4.59 | 0.08 | 7.57 | 15.5 | 1.10 | 2.23 | 0.83 | 0.27 | 174.0 | 9.81 | 99.9 | |
| 8. M3_1.4 | 49.4 | 13.2 | 4.83 | 0.09 | 6.42 | 16.5 | 1.01 | 2.11 | 0.65 | 0.25 | 161.2 | 7.39 | 101.9 | |
| Pale red Group B | 9. M4_4.3 | 52.5 | 12.5 | 4.36 | 0.07 | 5.43 | 12.1 | 0.97 | 2.39 | 0.71 | 0.14 | 188.8 | 8.81 | 99.9 |
| 10. M3_4.1 | 50.5 | 11.7 | 4.31 | 0.07 | 4.93 | 13.3 | 1.02 | 2.61 | 0.77 | 0.15 | 176.8 | 10.67 | 99.9 | |
| 11. M2_4.3 | 47.0 | 15.4 | 5.92 | 0.09 | 5.92 | 13.0 | 0.87 | 3.29 | 0.68 | 0.17 | 182.5 | 8.03 | 100.3 | |
| 12. M1_4.7 | 50.2 | 14.7 | 5.56 | 0.10 | 5.53 | 12.2 | 0.81 | 2.36 | 0.74 | 0.19 | 157.5 | 8.61 | 100.9 | |
| 13. M4_3.1 | 52.7 | 16.5 | 5.93 | 0.10 | 5.57 | 10.6 | 0.92 | 2.92 | 0.82 | 0.17 | 201.2 | 3.74 | 99.9 | |
| Dark red (group C) | 14. M3_4.0 | 55.2 | 18.3 | 8.07 | 0.10 | 4.72 | 6.6 | 1.43 | 3.17 | 1.12 | 0.30 | 190.3 | 1.01 | 99.9 |
| 15. M3_2.1 | 62.4 | 16.5 | 6.07 | 0.08 | 2.44 | 4.1 | 1.20 | 3.46 | 0.75 | 0.09 | 232.2 | 2.38 | 99.5 |
| Hygric Tests | MIP Performance | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hue | PF | Bricks | Po | ρr | ρa | Ab | Af | Ax | Di | S | PoMIP | ρrMIP | ρaMIP | SSA | 10–100 | 1–10 | 0.1–1 | <0.1 |
| Beige Group A | A1 | M1_4.8 | 51.7 | 2.8 | 1.4 | 37.7 | 38.0 | 0.8 | 1.5 | 82.3 | 49.0 | 2.9 | 1.5 | 12.6 | 17.8 | 68.7 | 7.5 | 5.9 |
| M4_5.2 | 46.4 | 2.7 | 1.5 | 30.6 | 31.9 | 4.1 | 1.6 | 83.7 | 44.1 | 2.7 | 1.5 | 23.5 | 0.8 | 4.6 | 71.4 | 23.1 | ||
| M2_1.1 | 50.3 | 2.7 | 1.4 | 36.7 | 36.9 | 0.6 | 1.6 | 82.8 | 44.4 | 2.7 | 1.5 | 8.5 | 1.4 | 42.6 | 51.5 | 4.5 | ||
| M2_4.6 | 42.9 | 2.7 | 1.5 | 27.3 | 28.3 | 3.6 | 1.6 | 87.0 | 42.4 | 2.7 | 1.6 | 25.3 | 0.2 | 6.5 | 65.7 | 27.7 | ||
| Mean | 47.8 | 2.7 | 1.4 | 33.1 | 33.8 | 2.2 | 1.6 | 83.9 | 45.0 | 2.8 | 1.5 | 17.4 | 5.1 | 30.6 | 49.0 | 15.3 | ||
| A2 | M4_4.1 | 42.1 | 2.7 | 1.6 | 24.5 | 27.0 | 9.2 | 1.6 | 80.4 | 39.9 | 2.7 | 1.6 | 9.7 | 9.2 | 62.6 | 21.8 | 6.4 | |
| M3_3.1 | 40.3 | 2.6 | 1.6 | 21.7 | 26.0 | 16.7 | 1.6 | 74.2 | 37.0 | 2.6 | 1.6 | 36.6 | 1.8 | 14.4 | 40.4 | 43.4 | ||
| M3_5.3 | 42.1 | 2.6 | 1.5 | 25.7 | 28.4 | 9.8 | 1.6 | 81.6 | 35.0 | 2.6 | 1.7 | 21.4 | 2.9 | 40.9 | 27.8 | 28.5 | ||
| M3_1.4 | 42.3 | 2.6 | 1.5 | 26.3 | 28.4 | 7.2 | 1.6 | 84.0 | 39.1 | 2.6 | 1.6 | 31.4 | 0.3 | 11.8 | 49.6 | 38.3 | ||
| Mean | 41.7 | 2.6 | 1.5 | 24.5 | 27.5 | 10.7 | 1.6 | 80.1 | 37.8 | 2.6 | 1.6 | 24.8 | 3.6 | 32.4 | 34.9 | 29.1 | ||
| Pale red Group B | B1 | M4_4.3 | 34.6 | 2.5 | 1.6 | 20.5 | 21.5 | 4.6 | 1.6 | 86.9 | 30.0 | 2.5 | 1.8 | 25.8 | 0.5 | 0.0 | 52.7 | 46.6 |
| M1_4.7 | 35.4 | 2.6 | 1.7 | 21.0 | 21.3 | 1.8 | 1.6 | 88.0 | 26.1 | 2.5 | 1.8 | 30.5 | 1.1 | 0.6 | 5.6 | 92.8 | ||
| M4_3.1 | 38.4 | 2.6 | 1.6 | 23.3 | 24.3 | 4.0 | 1.6 | 87.6 | 39.1 | 2.6 | 1.6 | 16.3 | 2.0 | 15.6 | 63.2 | 19.4 | ||
| Mean | 36.2 | 2.5 | 1.6 | 21.6 | 22.4 | 3.5 | 1.6 | 87.5 | 31.7 | 2.5 | 1.7 | 24.2 | 1.2 | 5.4 | 40.5 | 52.9 | ||
| M3_4.1 | 34.1 | 2.5 | 1.7 | 19.9 | 20.6 | 3.5 | 1.6 | 90.4 | 31.5 | 2.6 | 1.8 | 19.0 | 0.6 | 2.2 | 66.4 | 30.5 | ||
| M2_4.3 | 43.0 | 2.6 | 1.5 | 28.6 | 28.8 | 0.7 | 1.6 | 87.7 | 36.5 | 2.6 | 1.7 | 15.2 | 0.9 | 1.1 | 77.7 | 19.7 | ||
| Dark red Group C | M3_4.0 | 43.3 | 2.6 | 1.5 | 26.4 | 29.0 | 8.7 | 1.6 | 82.0 | 39.8 | 2.7 | 1.6 | 6.3 | 4.5 | 41.8 | 48.8 | 4.0 | |
| M3_2.1 | 35.4 | 2.7 | 1.7 | 19.8 | 20.6 | 3.8 | 1.6 | 84.3 | 34.6 | 2.6 | 1.7 | 12.2 | 1.6 | 36.2 | 47.1 | 14.9 | ||
| Hue | Bricks | VP1 (m/s) | VP2 (m/s) | VP3 (m/s) | Vp (m/s) | ∆M (%) | ∆m (%) | |
|---|---|---|---|---|---|---|---|---|
| Beige Group A | A1 | M1_4.8 | 1978 | 1996 | 2102 | 2025 | 3.5 | 5.2 |
| M4_5.2 | 2014 | 2178 | 2333 | 2175 | 10.7 | 6.9 | ||
| M2_1.1 | 1875 | 2088 | 2246 | 2070 | 13.5 | 7.3 | ||
| M2_4.6 | 2223 | 2441 | 2551 | 2405 | 10.9 | 4.4 | ||
| Mean 1 | 2037 | 2236 | 2377 | 2217 | 11.7 | 6.2 | ||
| A2 | M4_4.1 | 1287 | 1581 | 1779 | 1549 | 23.5 | 11.8 | |
| M3_3.1 | 2221 | 2384 | 2459 | 2355 | 8.2 | 3.1 | ||
| M3_5.3 | 2156 | 2303 | 2420 | 2293 | 8.7 | 5.0 | ||
| M3_1.4 | 2330 | 2454 | 2514 | 2433 | 6.2 | 2.4 | ||
| Mean 1 | 2236 | 2380 | 2464 | 2360 | 7.7 | 3.5 | ||
| Pale red Group B | B1 | M4_4.3 | 2326 | 2460 | 2583 | 2456 | 7.7 | 4.9 |
| M1_4.7 | 2323 | 2412 | 2541 | 2425 | 6.2 | 5.2 | ||
| M4_3.1 | 2269 | 2446 | 2548 | 2421 | 9.2 | 4.1 | ||
| Mean | 2253 | 2424 | 2547 | 2408 | 9.4 | 5.0 | ||
| M2_4.3 | 2016 | 2246 | 2387 | 2216 | 12.8 | 6.1 | ||
| M3_4.1 | 2335 | 2557 | 2676 | 2523 | 10.7 | 4.6 | ||
| Dark red Group C | M3_4.0 | 2001 | 2315 | 2420 | 2245 | 15.5 | 4.5 | |
| M3_2.1 | 2391 | 2446 | 2541 | 2459 | 4.1 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Monserrat, E.M.; Maritan, L.; Garbin, E.; Cultrone, G. Production Technologies of Ancient Bricks from Padua, Italy: Changing Colors and Resistance over Time. Minerals 2021, 11, 744. https://doi.org/10.3390/min11070744
Pérez-Monserrat EM, Maritan L, Garbin E, Cultrone G. Production Technologies of Ancient Bricks from Padua, Italy: Changing Colors and Resistance over Time. Minerals. 2021; 11(7):744. https://doi.org/10.3390/min11070744
Chicago/Turabian StylePérez-Monserrat, Elena Mercedes, Lara Maritan, Enrico Garbin, and Giuseppe Cultrone. 2021. "Production Technologies of Ancient Bricks from Padua, Italy: Changing Colors and Resistance over Time" Minerals 11, no. 7: 744. https://doi.org/10.3390/min11070744
APA StylePérez-Monserrat, E. M., Maritan, L., Garbin, E., & Cultrone, G. (2021). Production Technologies of Ancient Bricks from Padua, Italy: Changing Colors and Resistance over Time. Minerals, 11(7), 744. https://doi.org/10.3390/min11070744






