Iron Isotope Constraints on the Mineralization Process of Shazi Sc-Rich Laterite Deposit in Qinglong County, China
Abstract
:1. Introduction
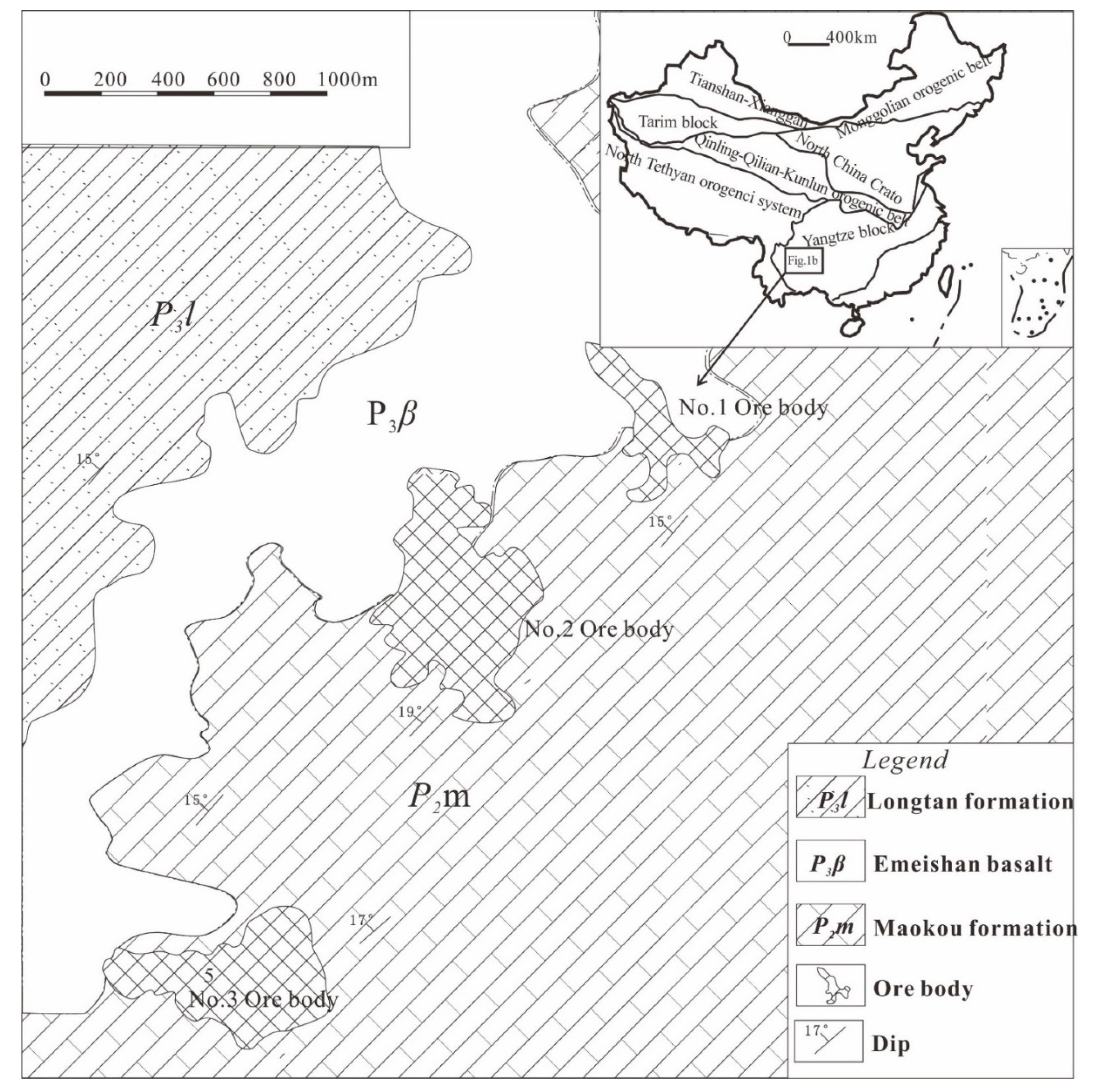
2. Geological Background
3. Samples and Analytical Methods
3.1. Samples
3.2. Analytical Methods
3.2.1. Acid Digestion of Magnetite Grains
3.2.2. Iron Extraction and Purification
3.3.3. Iron Isotope Measurement
4. Results
5. Discussion
5.1. Implications of Fe Isotope
5.2. Enrichment and Mineralization of Scandium
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, Z.-Q.; Chen, J.; Zhai, M.-G. Current status and frontiers of research on critical mineral resources. Chin. Sci. Bull. 2020, 65, 3651–3652. (In Chinese) [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474 (accessed on 15 June 2021).
- Wang, Z.; Li, M.Y.H.; Liu, Z.-R.R.; Zhou, M.-F. Scandium: Ore deposits, the pivotal role of magmatic enrichment and future exploration. Ore Geol. Rev. 2021, 128, 103906. [Google Scholar] [CrossRef]
- Williams-Jones, J.E.; Vasyukova, O.V. The Economic Geology of Scandium, The Runt of the Rare Earth Element Litter. Econ. Geol. 2018, 113, 973–988. [Google Scholar] [CrossRef] [Green Version]
- Halkoaho, T.; Ahven, M.; Rämö, O.T.; Hokka, J.; Huhma, H. Petrography, geochemistry, and geochronology of the Sc-enriched Kiviniemi ferrodiorite intrusion, eastern Finland. Miner. Depos. 2020, 55, 1561–1580. [Google Scholar] [CrossRef] [Green Version]
- Steffenssen, G.; Müller, A.; Munnik, F.; Friis, H.; Erambert, M.; Kristoffersen, M.; Rosing-Schow, N. Unusual scandium enrichments of the Tørdal pegmatites, south Norway. Part I: Garnet as Sc exploration pathfinder. Ore Geol. Rev. 2020, 126, 103729. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Bazai, A.V.; Sokharev, V.A.; Konopleva, N.G.; Mikhailova, J.A.; Goryainov, P.M.; Ivanyuk, G.Y. Scandium of the Kovdor baddeleyite–apatite–magnetite deposit (Murmansk Region, Russia): Mineralogy, spatial distribution, and potential resource. Ore Geol. Rev. 2016, 72, 532–537. [Google Scholar] [CrossRef]
- Klimpel, F.; Bau, M.; Graupner, T. Potential of garnet sand as an unconventional resource of the critical high-technology metals scandium and rare earth elements. Sci. Rep. 2021, 11, 5306. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Aiglsperger, T.; Proenza, J.A.; Lewis, J.F.; Labrador, M.; Svojtka, M.; Rojas-Purón, A.; Longo, F.; Ďurišová, J. Critical metals (REE, Sc, PGE) in Ni laterites from Cuba and the Dominican Republic. Ore Geol. Rev. 2016, 73, 127–147. [Google Scholar] [CrossRef]
- Chassé, M.; Griffin, W.L.; O’Reilly, S.Y.; Calas, G. Scandium speciation in a world-class lateritic deposit. Geochem. Perspect. Lett. 2017, 3, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Teitler, Y.; Cathelineau, M.; Ulrich, M.; Ambrosi, J.P.; Munoz, M.; Sevin, B. Petrology and geochemistry of scandium in New Caledonian Ni-Co laterites. J. Geochem. Explor. 2019, 196, 131–155. [Google Scholar] [CrossRef]
- Campodonico, V.A.; Pasquini, A.I.; Lecomte, K.L.; García, M.G.; Depetris, P.J. Chemical weathering in subtropical basalt-derived laterites: A mass balance interpretation (Misiones, NE Argentina). CATENA 2019, 173, 352–366. [Google Scholar] [CrossRef]
- Sun, J.; Nie, A.-G.; Cui, T. Occurrence of A Large-Scale Scandium Deposit in Guizhou, SW China. Boletín Técnico 2017, 55, 138–143. [Google Scholar]
- Qin, H.-B.; Yang, S.; Tanaka, M.; Sanematsu, K.; Arcilla, C.; Takahashi, Y. Chemical speciation of scandium and yttrium in laterites: New insights into the control of their partitioning behaviors. Chem. Geol. 2020, 552, 119771. [Google Scholar] [CrossRef]
- Qin, H.-B.; Yang, S.; Tanaka, M.; Sanematsu, K.; Arcilla, C.; Takahashi, Y. Scandium immobilization by goethite: Surface adsorption versus structural incorporation. Geochim. Cosmochim. Acta 2021, 294, 255–272. [Google Scholar] [CrossRef]
- Fantle, M.S.; DePaolo, D.J. Iron isotopic fractionation during continental weathering. Earth Planet. Sci. Lett. 2004, 228, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Fekiacova, Z.; Pichat, S.; Cornu, S.; Balesdent, J. Inferences from the vertical distribution of Fe isotopic compositions on pedogenetic processes in soils. Geoderma 2013, 209–210, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Huang, F.; Yu, H.M.; Kang, J.T.; Yang, X.Y.; He, Y.S.; He, Z.W. Why was iron lost without significant isotope fractionation during the lateritic process in tropical environments? Geoderma 2017, 290, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-A.; Teng, F.-Z.; Li, S.; Wei, G.-J.; Ma, J.-L.; Li, D. Copper and iron isotope fractionation during weathering and pedogenesis: Insights from saprolite profiles. Geochim. Cosmochim. Acta 2014, 146, 59–75. [Google Scholar] [CrossRef]
- Poitrasson, F.; Viers, J.; Martin, F.; Braun, J.-J. Limited iron isotope variations in recent lateritic soils from Nsimi, Cameroon: Implications for the global Fe geochemical cycle. Chem. Geol. 2008, 253, 54–63. [Google Scholar] [CrossRef]
- Nie, A.; Zhang, M.; Sun, J. The first discovery of a large independent scandium deposit formed by sparse element scandium in guizhou. J. Guizhou Univ. (Nat. Sci.) 2018, 35, 8–13, (In Chinese with English Abstract). [Google Scholar]
- Zhu, X.K.; Li, Z.H.; Zhao, S.H.; Zhao, X.M.; Tang, S.H.; He, X.X.; Nick, S.B. High-precision measurements of Fe isotopics using MC-ICP-Ms and Fe isotopic compositions of reference materials. Acta Petrol. Mineral. 2008, 27, 2008. [Google Scholar]
- Taylor, P.D.P.; Maeck, R.; De Bièvre, P. Determination of the absolute isotopic composition and Atomic Weight of a reference sample of natural iron. Int. J. Mass Spectrom. Ion Process. 1992, 121, 111–125. [Google Scholar] [CrossRef]
- Chen, L.-M.; Song, X.-Y.; Zhu, X.-K.; Zhang, X.-Q.; Yu, S.-Y.; Yi, J.-N. Iron isotope fractionation during crystallization and sub-solidus re-equilibration: Constraints from the Baima mafic layered intrusion, SW China. Chem. Geol. 2014, 380, 97–109. [Google Scholar] [CrossRef]
- Frierdich, A.J.; Beard, B.L.; Scherer, M.M.; Johnson, C.M. Determination of the Fe(II)aq–magnetite equilibrium iron isotope fractionation factor using the three-isotope method and a multi-direction approach to equilibrium. Earth Planet. Sci. Lett. 2014, 391, 77–86. [Google Scholar] [CrossRef]
- Chapman, J.B.; Weiss, D.J.; Shan, Y.; Lemburger, M. Iron isotope fractionation during leaching of granite and basalt by hydrochloric and oxalic acids. Geochim. Cosmochim. Acta 2009, 73, 1312–1324. [Google Scholar] [CrossRef]
- Sun, J.; Jingyu, Z.; Nie, A. Zircon U–Pb dating and whole-rock elemental geochemistry of the Shazi anatase deposit in Qinglong, Western Guizhou, SW China. Acta Geochim. 2017, 36, 329–338. [Google Scholar] [CrossRef]
- Chassé, M.; Griffin, W.L.; O’Reilly, S.Y.; Calas, G. Australian laterites reveal mechanisms governing scandium dynamics in the critical zone. Geochim. Cosmochim. Acta 2019, 260, 292–310. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Xu, Y.-G.; Chung, S.-L.; XIao, L.; Wang, Y.-M. Sedimentary evidence for a rapid, kilometer-scale crustal doming prior to the eruption of the Emeishan flood basalts. Earth Planet. Sci. Lett. 2003, 213, 391–405. [Google Scholar] [CrossRef]
- Hu, Y.-Z. Sedimentary Basin Analysis, and Antimony and Gold Mineralization in Southwest Guizhou. Ph.D. Thesis, The Kunming University of Science and Technology, Kunming, China, 2011. [Google Scholar]

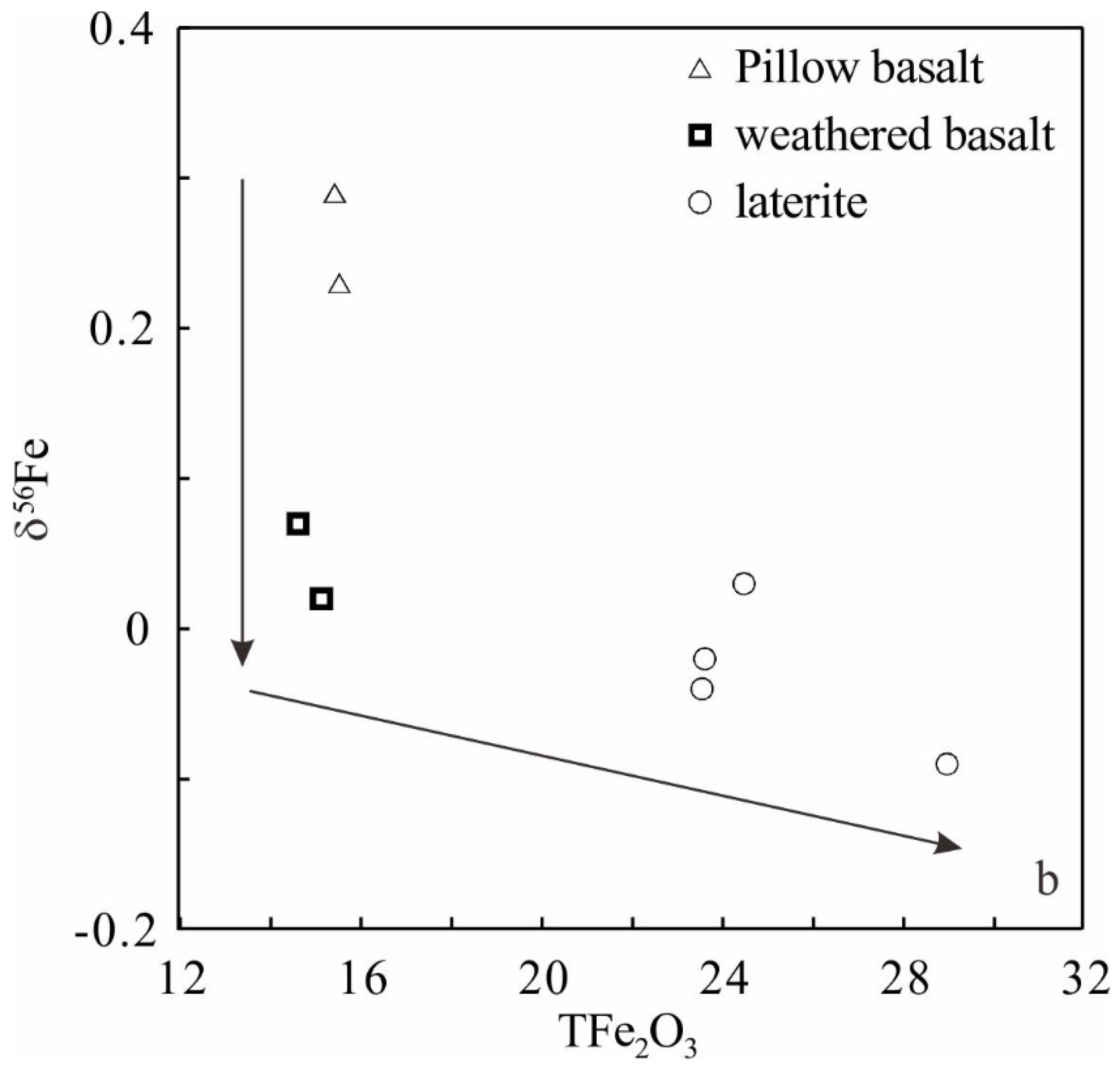
| Rock Type | No. | δ57Fe | δ56Fe | TFe2O3 * | ||||
|---|---|---|---|---|---|---|---|---|
| Ratio | 2SD | Ratio | 2SD | |||||
| Pillow basalt | T-002 | 0.32 | 0.06 | 0.23 | 0.07 | 15.51 | ||
| Pillow basalt | T-003 | 0.42 | 0.07 | 0.29 | 0.06 | 15.41 | ||
| Weathered basalt | 2-002 | 0.05 | 0.12 | 0.02 | 0.07 | 15.12 | ||
| Weathered basalt | 2-004 | 0.13 | 0.10 | 0.07 | 0.05 | 14.60 | ||
| Laterite | 2-011 | −0.07 | 0.09 | −0.04 | 0.05 | 23.53 | ||
| Laterite | 2-012 | −0.03 | 0.10 | −0.02 | 0.05 | 23.59 | ||
| Laterite | 3-003 | −0.10 | 0.12 | −0.09 | 0.06 | 28.95 | ||
| Laterite | 3-004 | 0.02 | 0.09 | 0.03 | 0.07 | 24.46 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Liu, Y.; Liu, X. Iron Isotope Constraints on the Mineralization Process of Shazi Sc-Rich Laterite Deposit in Qinglong County, China. Minerals 2021, 11, 737. https://doi.org/10.3390/min11070737
Sun J, Liu Y, Liu X. Iron Isotope Constraints on the Mineralization Process of Shazi Sc-Rich Laterite Deposit in Qinglong County, China. Minerals. 2021; 11(7):737. https://doi.org/10.3390/min11070737
Chicago/Turabian StyleSun, Jun, Yunlong Liu, and Xiqiang Liu. 2021. "Iron Isotope Constraints on the Mineralization Process of Shazi Sc-Rich Laterite Deposit in Qinglong County, China" Minerals 11, no. 7: 737. https://doi.org/10.3390/min11070737
APA StyleSun, J., Liu, Y., & Liu, X. (2021). Iron Isotope Constraints on the Mineralization Process of Shazi Sc-Rich Laterite Deposit in Qinglong County, China. Minerals, 11(7), 737. https://doi.org/10.3390/min11070737







