1. Introduction
Aluminum is a necessary technological material. Its inherent physicochemical properties, such as low density, in conjunction with properties that derive from the formation of alloys make it suitable for numerous applications in the fields of transportation, engineering, construction, building and packaging [
1,
2]. As a result of these properties, aluminum can be processed into a wide range of consumer goods and, in addition, has a high value as a recycling material, as the re-melting of aluminum scrap requires only 5% of the total energy required for the extraction of the same amount of raw material from ores. Data provided by Sverdrup [
2] and Allwood [
3] lead, with relative certainty, to two important conclusions. First of all, aluminum is projected to maintain and strengthen its position as a high-tech commodity. Secondly, according to existing data, primary production of aluminum from ores will peak much earlier than the projected peak in demand. In other words, the demand for aluminum should remain high for the next 60 years, but the possibility of producing quantities that will meet this demand with existing sources and technologies is debatable.
In the last 100 years, numerous methods have been developed for the extraction of aluminum from its ores. However, its current commercial production method is based on two energy-intensive processes developed in the late 19th century: the hydrometallurgical Bayer method, which produces pure alumina (Al
2O
3) from bauxite ores, and the electrolytic Hall–Héroult method, which produces pure aluminum metal using as a raw material metallurgical alumina [
4]. In the field of alumina production, Bayer process, contributing to 95% of alumina production, is also connected with drawbacks from environmental legislations nowadays. More specifically, the environmental impact of the method, particularly the production of the bauxite residue is of great concern.
At the same time in recent years, the availability of bauxite deposits to be processed by the Bayer method in Europe have decreased with consequent increase in the cost of their processing [
5]. Bauxite inclusion in the EU’s critical raw materials list for 2020 [
6] endorsed this point and made clear the need for the utilization of an alternative resource.
Thus, the scientific interest is focused on the production of alumina from silicate rocks with high aluminum content, such as anorthosite, kaolin, nepheline syenite and feldspar or feldspathoid minerals derived from such rocks. In addition, and following the same trend, interest is also shown to the utilization of secondary raw materials coming from the extractive mining and metallurgical industry, such as slags or mining tailings. Research on this field suggests the direct dissolution of these Al sources in strong inorganic acids, without the need for costly preparation [
3]. Chronologically, the first interesting invention in the field of acid extraction was that from Gaudernack [
7]. Gaudernack’s invention discloses a process for recovering alumina from aluminous silicate raw materials. A complete acid extraction process using hydrochloric acid solution was presented by Boudreault, Alex and Biasotto [
8]. Aluminum ores such as nepheline were used as raw materials in these processes. In nowadays data, Aranda and Mastin’s technology [
9], taking into account previous research, suggests a process for alumina and calcium carbonate production from aluminum rich materials with integrated CO
2 utilization, comprising: leaching of Al-rich materials in concentrated HCI, separating Al from solution by crystallization of AICI
3 6H
2O followed by calcination with HCI recovery.
The application of an acid treatment process is gaining attention due to the overpassing of problems of previous decades, such as the absence of available acid-proof materials and the feasibility of recycling of HCl, and some important advantages, such as the relatively simple conversion from ore to alumina and the higher tolerance to the silica content of ores [
9]. Regarding acid recovery, many patents [
10,
11,
12] consider the use of an organic extraction method, using a variety of amines. The main goal is to extract the free HCl from dilute solutions and then recover the concentrated HCl by stripping the amine.
Nepheline syenite is a plutonic igneous rock consisting mainly of nepheline, sodium, and potassium feldspars. In appearance, as a light-colored and coarse grain-sized material, it shows similarities with granite, without containing any free silica [
13,
14]. Nepheline syenite main reserves are located in Russia, Norway, Canada and Turkey. Nepheline syenite deposits are also detected as byproducts of some mining projects. An example of such a project is found in Russia where nepheline syenite is a byproduct of apatite mining at Khibiny Mountain in the Kola Peninsula [
15].
Nepheline syenite’s high Al
2O
3, Na
2O and K
2O content make it a material that can compete with feldspars in glass and ceramic industry. More specifically, Nepheline syenite is used as an active agent for the formation of a glassy phase in ceramics that enhance the physical strength of the final product. As for the glass industry, the high aluminum content of Nepheline syenite acts as a booster in resistance to breaking, as well as thermal endurance and chemical durability. [
13]. Moreover, in the last years, some studies suggest the use of nepheline syenite as a potassium source for plants, animal, and human nutrients, particularly in countries such as India where the conventional sources (such as sylvite) are not available [
13,
16].
In the field of Al production, nepheline syenite is seen as an attractive material due to its high Al content but also challenging due to two major problems: (a) the low dissolution rate of aluminum-bearing phases, and (b) the silica gel formation that is observed in high pulp density and low treatment temperatures [
17]. Low Al extraction is connected with the existence of feldspars in nepheline syenite mass that are characterized as almost insoluble phases.
Feldspar’s insolubility drove previous research on the inclusion of a pretreatment step prior to leaching that would transform feldspars to easily dissolved phases. More specifically, two paths had been followed. Jena [
13,
16] proposed the roasting of nepheline-syenite with CaCl
2 at 900 °C. Danjun [
18] suggested sintering with an excess 5–10% of sodium carbonate at 760–880 °C for 1.0–1.5 h. Although such processes would provide high Al extraction, the high energy consumption and CO
2 footprint that derive from the thermal treatment step are of great concern.
In this work, a hydrometallurgical treatment of a mining waste characterized as nepheline syenite is presented. Leaching with an azeotropic HCl solution in the temperature range of 90–150 °C was studied in order to recover the maximum amount of aluminum. While, at the same time, an almost pure siliceous residue consisted of precipitated silica, and undissolved Na and K-feldspars were produced. The residue can be used as a raw material in the glass and ceramic industry.
2. Materials and Methods
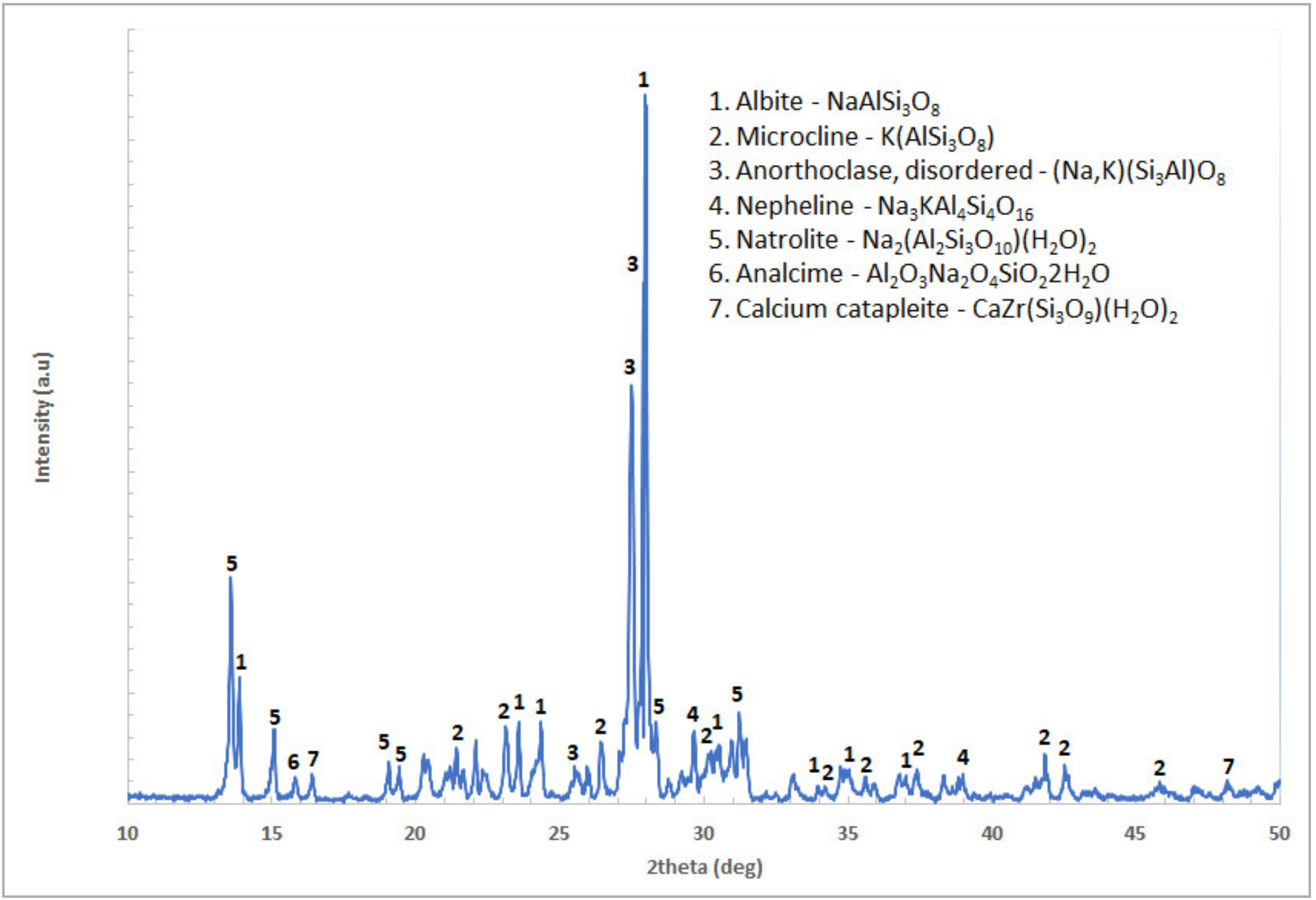
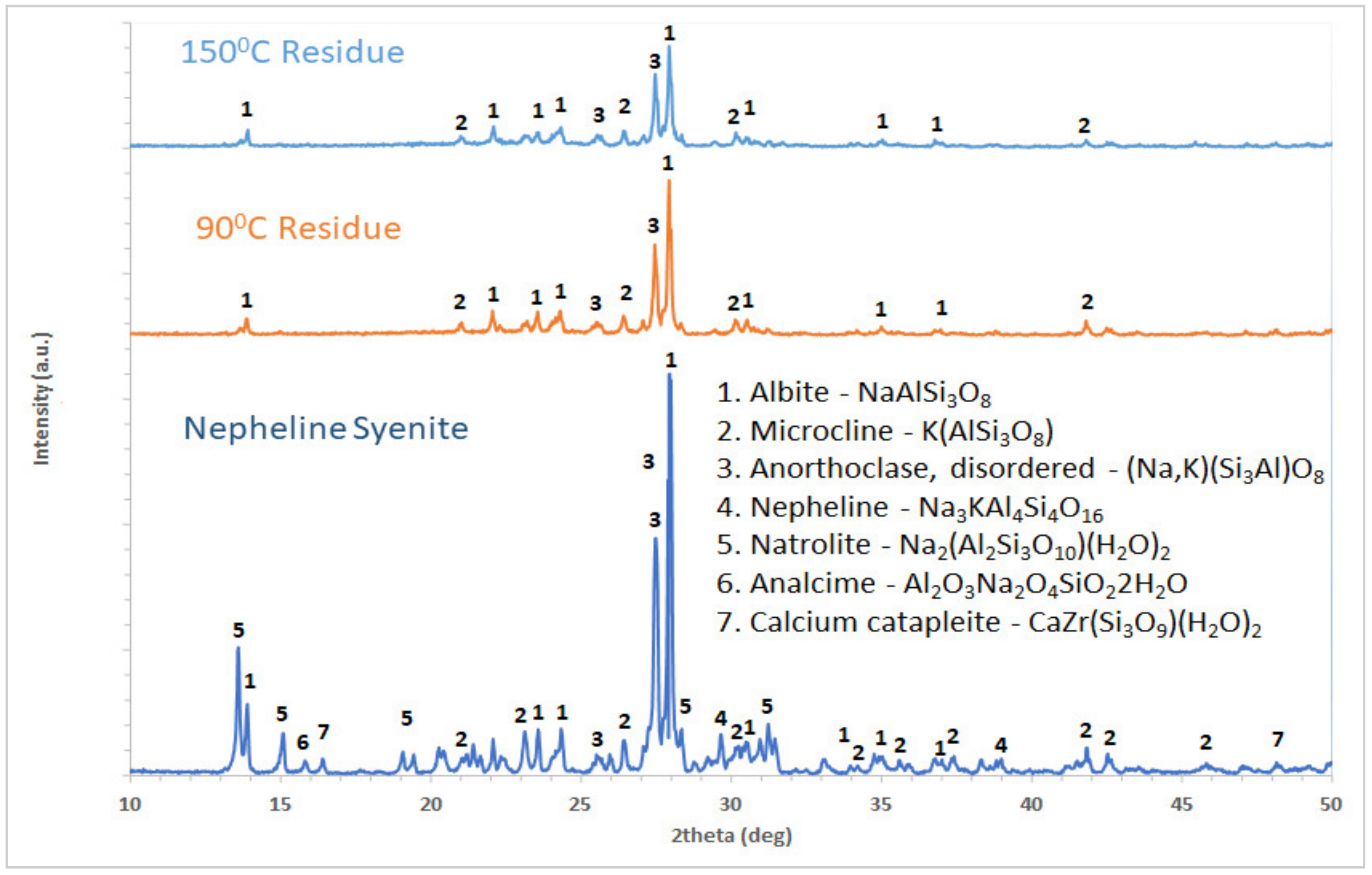
Nepheline syenite used in this study was obtained from GREENNA Mineral AB in Sweden. This sample was obtained as a byproduct from the utilization of a rare earth elements’ deposit. Nepheline syenite is characterized as a non-hazardous material, nevertheless, the significant volumes of the material produced demand a very large deposition area. Therefore, the main objective of the exploitation of this material is the reduction in land used. The sample was ground to 100%—100 μm. Then, it was dried at 100 °C for 24 h and its chemical composition was determined by wet chemical analysis techniques. The sample was solubilized via fusion method with a mixture of Li2B4O7/KNO3 at 1000 °C for 1 h and then was dissolved in a nitric acid solution. The metals in the solution were measured by Atomic Absorption Spectroscopy (AAS) with the use of PerkinElmer 2100 Atomic Absorption Spectrophotometer. Calcium content was determined with XRF. Mineralogical characterization was made by an X’Pert Pro diffractometer (PANalytical, Malvern, UK) with CuKa radiation. Diffraction patterns were recorded between 2 and 70° 2θ, in 0.02° steps and 1 s per step, while for the qualitative identification of mineralogical phases, DIFFRAC.EVA V5.1 software was used.
For the conventional leaching tests, an Amar G2360 glass autoclave was used, equipped with a 1000 mL capacity jacketed glass vessel and a polytetrafluoroethylene (PTFE) lid. The lid is designed with openings and sockets which allow (a) the recording of pressure through a manometer, (b) the release of the pressure through a release valve, (c) the immersion of a thermocouple and a 6-bladed turbine PTFE stirrer, (d) the drawing of samples and (e) the immersion of electrodes for pH measurement. Mechanical stirrer is connected to a PLC unit which allows for the control of the operating parameters while the heating was controlled by a chiller through the circulation of silicon oil in the external layer of the jacketed vessel. During leaching experiments, the leaching agent was placed in the autoclave and was heated. For the leaching agent, laboratory grade hydrochloric acid (>37%) and deionized water were mixed. When the reactor reached a predetermined temperature, the solid sample was placed inside the reactor signaling the start of the experiment. For experiments at temperatures higher than 100 °C where the reactor operated as an autoclave under saturated vapor pressure, the solid sample was inserted at 90 °C and then was very fast heated to the desired temperature together with the solution. The starting point of the experiment in this case was the moment when the system reached the desired temperature. In kinetic experiments, samples were taken at defined time intervals, and at the end of the experiment, the leachate was separated from the solid residues via vacuum filtration. The final pregnant liquid solution (PLS) and the intermediate samples were analyzed by AAS while the residue was dried, milled and its mineralogical content was analyzed by XRD.
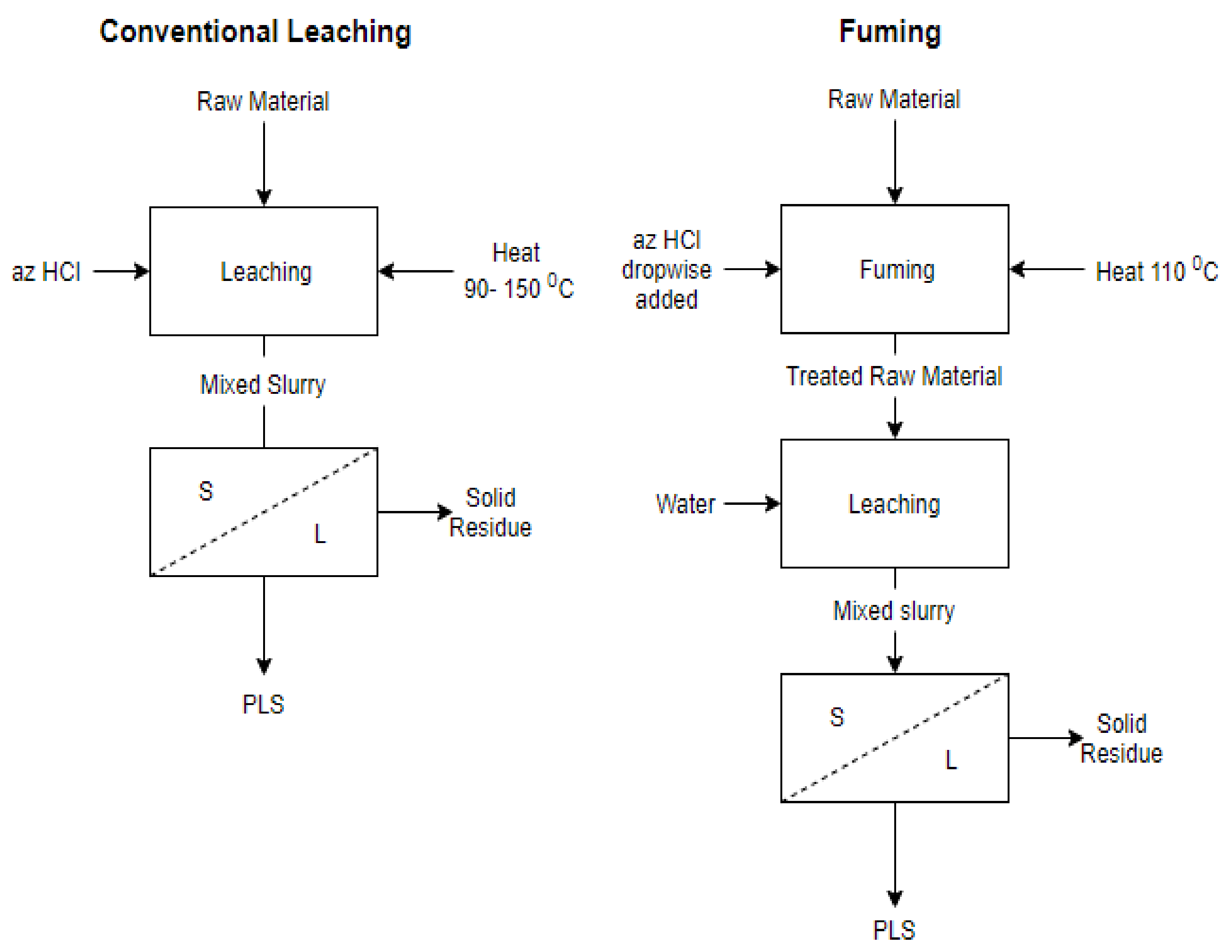
HCl fuming test was performed by adding the azeotropic HCl solution dropwise to the heated solid sample, placed in an evaporating dish, at 110 °C under continuous blending of solids. The droplets partially reacted with the solids and partially were vaporized, leaving back a dried reacted solid material which subsequently was leached with water at ambient temperature for 1 h. The solution obtained was analysed with the same techniques described above. A flowsheet of the conventional leaching and fuming technique tested in this study is depicted in
Figure 1.
In this work, the effect of temperature (90–150 °C), pulp density (2.5–10% w/v) and retention time (0–24 h) on the extraction of aluminum was studied during the leaching of the mining waste with an azeotropic HCl solution (5.89 M) under constant stirring rate of 300 rpm. In addition, the co-dissolution of Si, K and Na was monitored in all experiments. Due to silica gelation problems observed at high pulp densities, an HCl fuming test was performed instead of conventional leaching tests to investigate the avoidance of silica gel formation at higher pulp densities.
4. Discussion
Literature data collected and presented by Mase [
19] states the reactivity of most common silicates with HCl solution. Nepheline, natrolite and analcime are mentioned and characterized as phases soluble to HCl aqueous solutions. Those data are in full agreement with the presented results, as nepheline, natrolite and analcime were easily and quickly dissolved, and finally disappeared from the leaching residues. The fast dissolution mechanism of nepheline, natrolite and analcime is also connected with silica gel formation [
20]. As it has been reported, one of the possible results of the action of acids on a silicate is the complete breakdown of the structure followed by the dissolution of metal cations and silica [
20]. Gelenatization of PLS would occur due to ready polymerization of silica in the aqueous solutions. Empirical rules, such as the aluminum to silicon mass ratio of framework silicates [
21] and the framework charge ratio, expressed as Al: Al + Si mass ratio [
22] are settled for the characterization of a silicate as prone to silica gel formation through an acid treatment. Based on such criteria, nepheline, natrolite and analcime are included in the list of gelatinizing minerals [
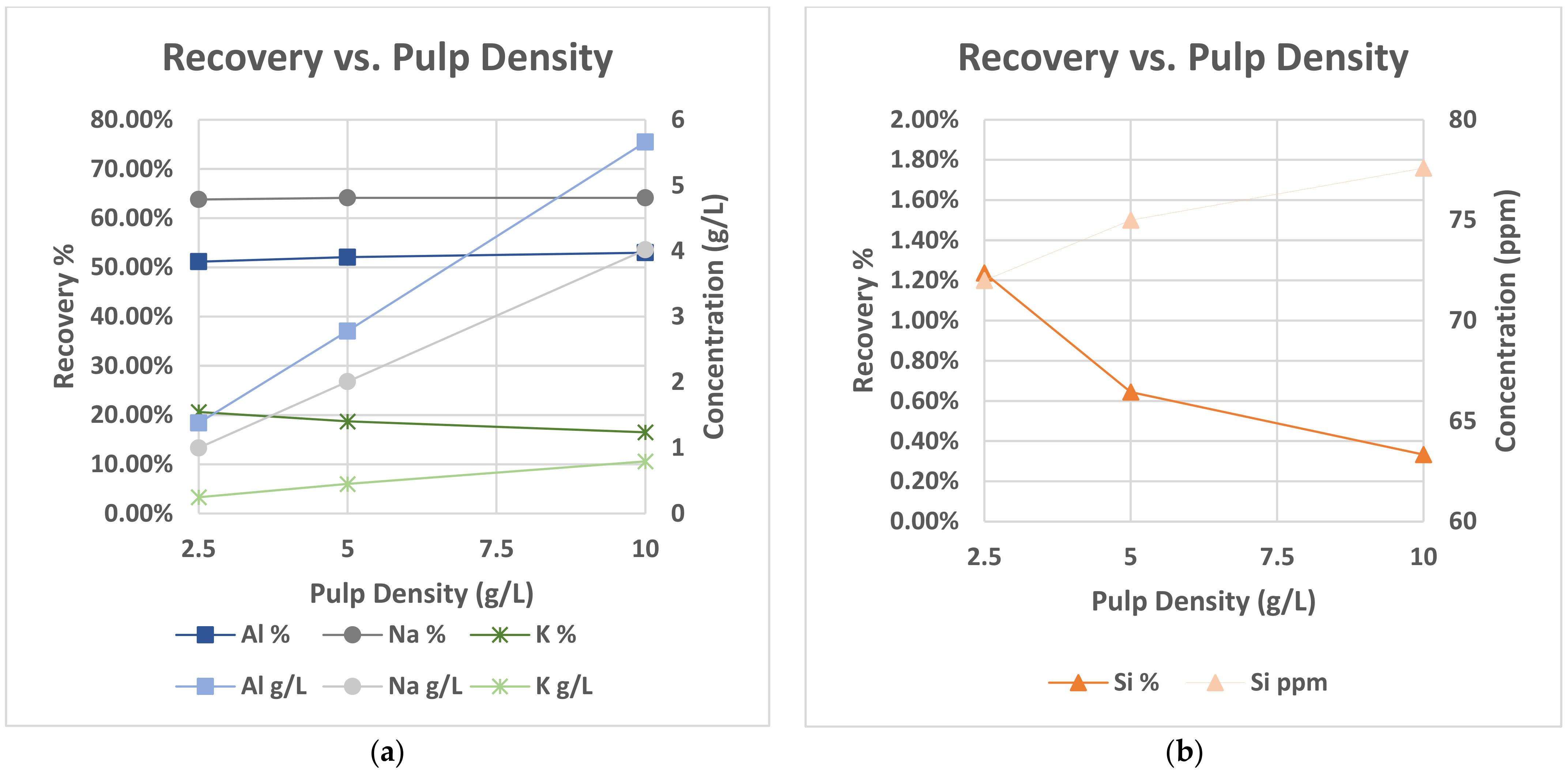
21]. Nepheline and zeolites that were detected in our raw material have a high silicon content, in comparison with the other included mineralogical phases that would reasonably lead to silica gel formation and consequently create problems to the leaching process. Silica gelation phenomena were not observed in our leaching tests at the lowest studied pulp density of 2.5%
w/
v due to extremely high excess of HCl due to azeotropic solution. At higher pulp densities, gelation was observed in PLS at retention times higher than 6 h due to a substantially higher amount of silicon dissolved in the solution under the higher pulp densities.
Silica gel formation, observed at high pulp density, would be mitigated through the application of HCl fuming [
23] approach. Indeed, the fuming test, presented in this work, led to similar extraction rates with conventional leaching without showing any indication of silica gelation.
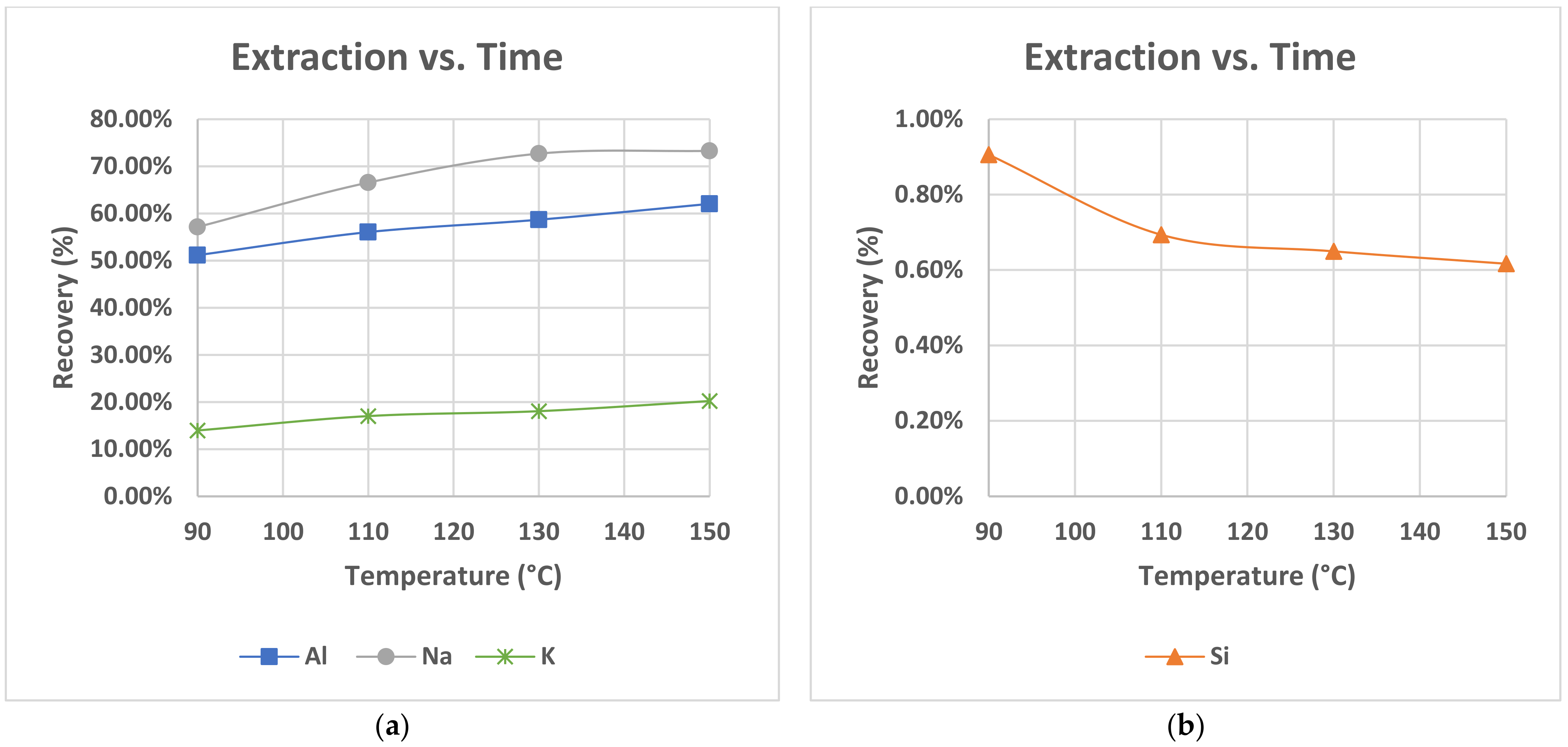
The low % extraction yield of Al, estimated to be near 50%, calculated in all leaching tests performed at a temperature of 90 °C, could be translated in that 50% of the contained Al was not dissolved. Based on the results of residue characterization, the undissolved Al is in the form of feldspars, which is in agreement with the literature [
19]. The increase on leaching temperature did not improve appreciably the Al extraction yield. Under acidic conditions, alkali feldspars’ dissolution in aqueous solutions is incongruent. Reports on the incongruent dissolution of alkali feldspars and their dissolution kinetics at a variety of examined temperatures [
24,
25] concluded that the increase in the molar concentration of alkali ions might be faster than that in the molar concentration of aluminum. This observation could be attributed to the preferable leachability of alkali ions from the feldspar matrix, or to the precipitation of an aluminum-containing phase. Different models of incongruent feldspar dissolution have been proposed and led to different routes of rate control in dissolution. Among them, two approaches proposed that dissolution rate is controlled by the inability of feldspar components to diffuse through a protective layer that is formed around feldspar grains [
26]. This layer could have the form of an amorphous silica-alumina precipitate [
27] or a crystalline precipitate whose phases are based on the chemical content of the solution [
28].
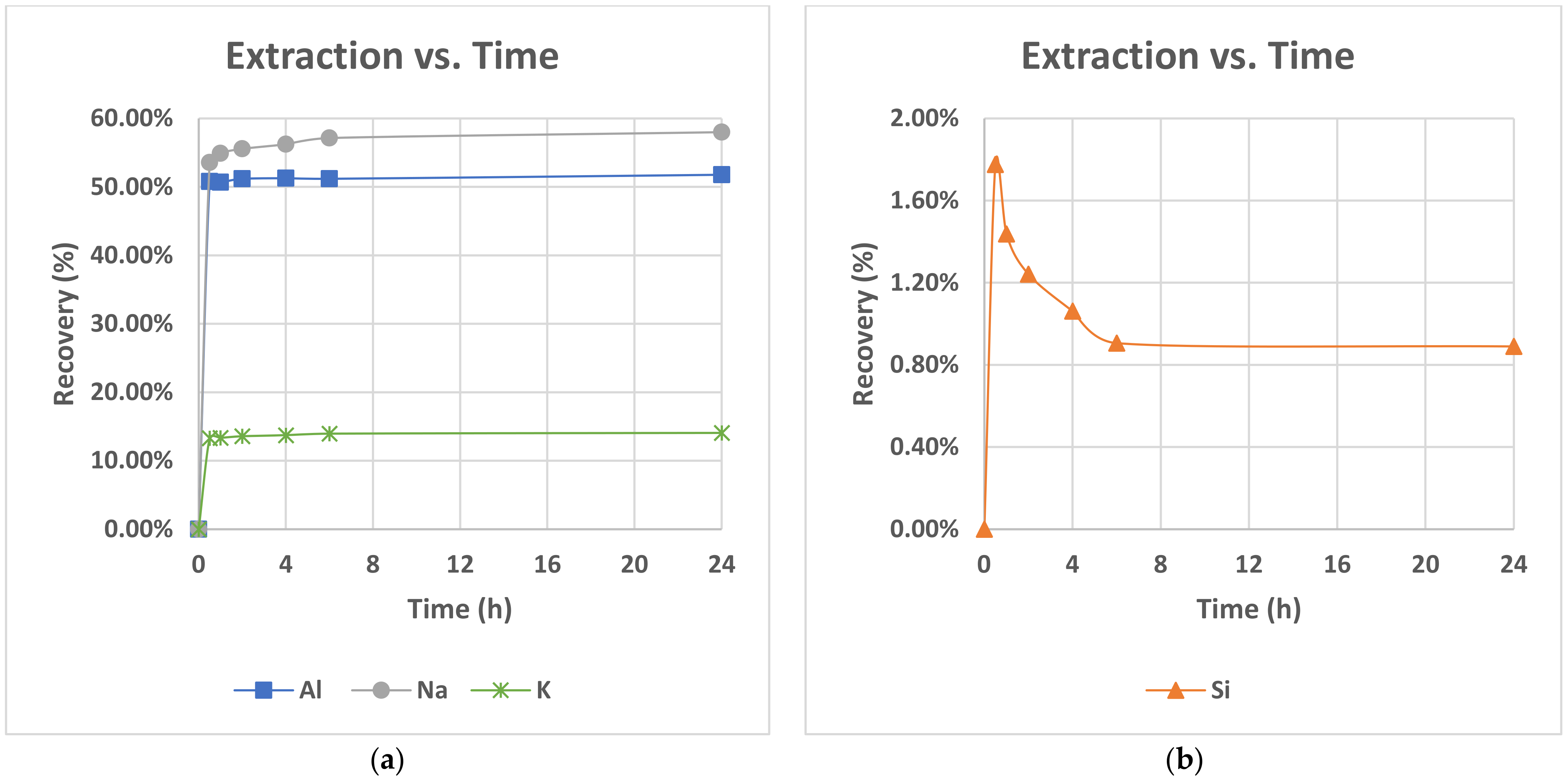
Incongruent dissolution in the matrix of nepheline syenite is also endorsed by the comparison of dissolution curves of Al, K and Na, presented in
Figure 5a. K and Na dissolution seems to follow the trend of Al dissolution as a slow increase to the extraction yields with the increase of temperature is noted. Potassium, in addition, achieved the lowest extraction yield of the order of 15–20%, as the list of soluble minerals that were dissolved during the leaching process do not have potassium content. Potassium exists only in feldspars and especially in anorthoclase which is the second most abundant mineral in the raw material and in a subordinate amount in microcline. The presence of low amounts of K in solution thus suggests low level dissolution of feldspars in agreement with the literature. Moreover, the relative high amount of dissolved sodium reached a yield of 73% at 150 °C relative to aluminium of 62% at 150 °C which (a) strengthened the above observation for initiation of the feldspars dissolution process and (b) seemed to support the incongruent dissolution mechanism of feldspars.
Finally, although the leaching process with azeotropic HCl seems to be inefficient due to (a) the difficulties which exist in dissolving the alkali feldspars and (b) the silica gelation phenomena observed at high pulp densities, the application of HCl fuming approach, that can solve the latter but not the former drawback, has the potential to produce pregnant solutions with high aluminum content proper for alumina production. Furthermore, the produced residue that contains exclusively undissolved felspars and amorphous silica is an attractive, and already used raw material for glass, ceramic and other industries [
29].