The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities
Abstract
:1. Introduction
2. Organoclays and Their Properties
3. Organoclays Based on Clay Minerals with Different Structure Expansion and Surface Charge Capacities
3.1. Organoclays Based on 1:1 Structure Phyllosilicates
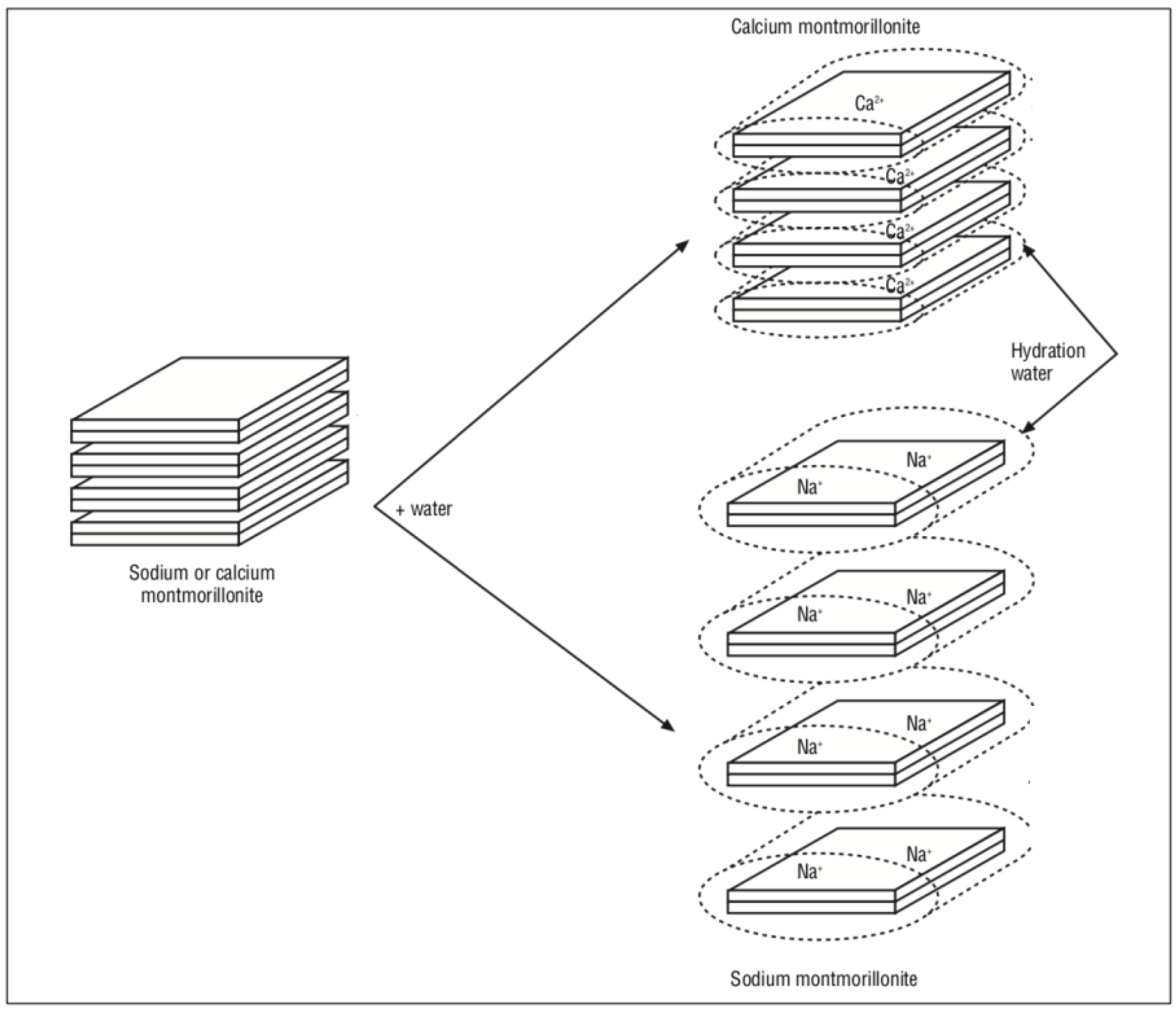
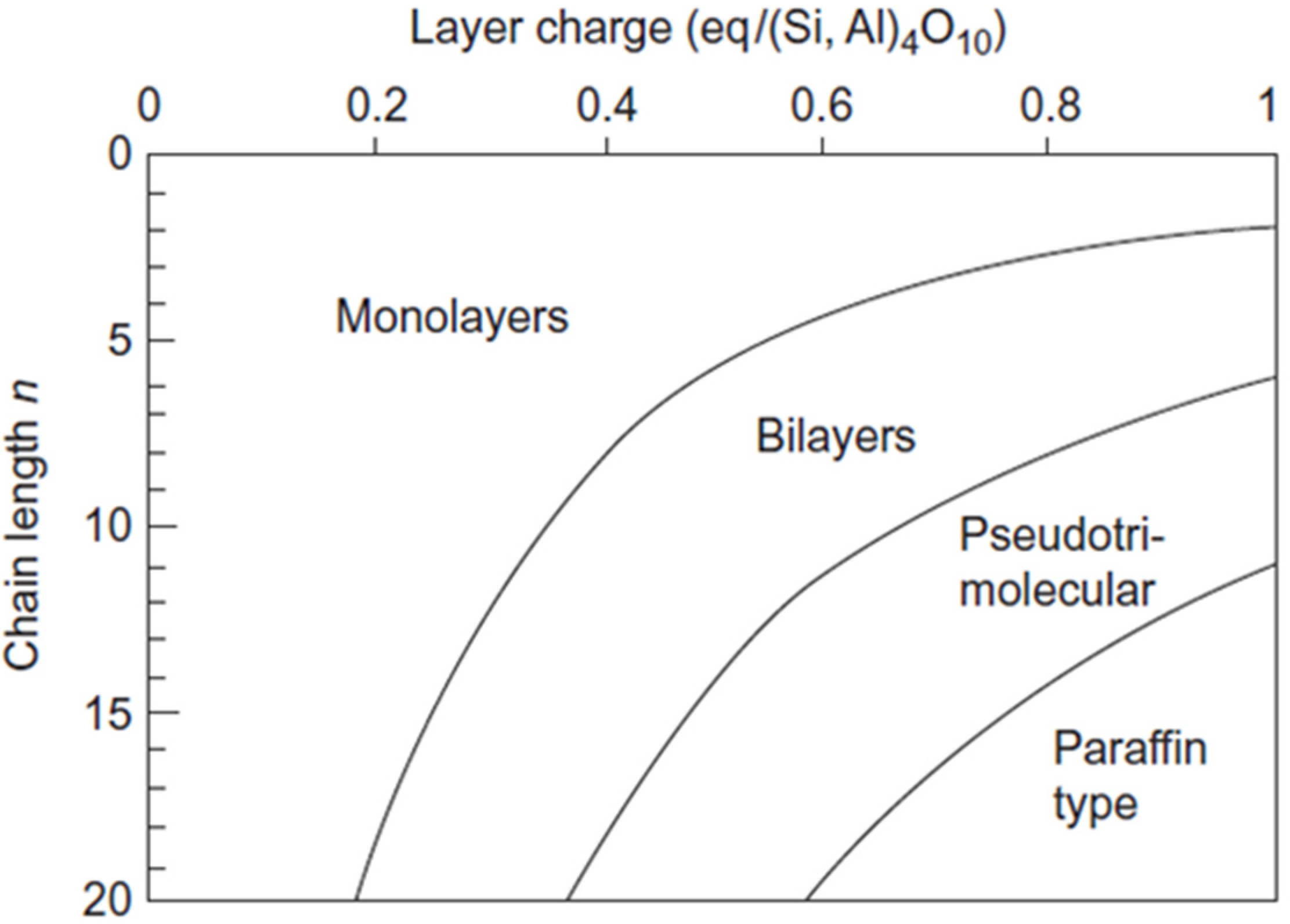
3.2. Organoclays Based on 2:1 Structure Fillosilicates
3.3. The Possibility of Using 2:1:1 Structure Phyllosilicates for the Synthesis of Organoclays
3.4. Organoclays Based on Other Silicates (Close to Inosilicates)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Churchman, G.J.; Gates, W.P.; Theng, B.K.G.; Yuan, G. Clays and clay minerals for pollution control. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 625–675. [Google Scholar]
- Srinivasan, R. Advances in Application of Natural Clay and Its Composites in Removal of Biological, Organic, and Inorganic Contaminants from Drinking Water. Adv. Mater. Sci. Eng. 2011, 2011, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Modified clay minerals for environmental applications. In Modified Clay and Zeolite Nanocomposite Materials: Environmental and Pharmaceutical Applications; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–127. [Google Scholar]
- United States Geological Survey. National Minerals Information Center. Clays Statistics and Information. Annual Publications. Mineral Commodity Summaries. 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-clays.pdf (accessed on 1 January 2021).
- Bailey, S.E.; Olin, T.J.; Bricka, R.; Adrian, D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Borchardt, G. Smectites. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1989; pp. 675–727. [Google Scholar]
- Dixon, J.B. Kaolin and Serpentine Group Minerals. Methods Soil Anal. Part Mineral. Methods 2018, 467–525. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ed-Dra, A.; Zennouhi, O.; Bouymajane, A.; Rhazi Filali, F.; Nassiri, L.; Abouarnadasse, S. Chemical character-ization and adsorption of oil mill wastewater on Moroccan clay in order to be used in the agricultural field. Heliyon 2020, 6, e03164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Ma, L.; Zhu, J.; Frost, R.L.; Theng, B.K.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. Appl. Clay Sci. 2014, 100, 22–28. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Chapter 1. General introduction: Clays, clay minerals and clay science. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5A, pp. 1–19. [Google Scholar]
- Theng, B.K.G.; Churchman, G.J.; Gates, W.P.; Yuan, G. Organically modified clays for pollutant uptake and environmental protection. In Soil Mineral-Microbe-Organic Interactions: Theories and Applications; Huang, Q., Huang, P.M., Violante, A., Eds.; Springer: Berlin, Germany, 2008; pp. 145–174. [Google Scholar]
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 526p. [Google Scholar]
- Guégan, R.; Veron, E.; Le Forestier, L.; Ogawa, M.; Cadars, S. Structure and Dynamics of Nonionic Surfactant Aggregates in Layered Materials. Langmuir 2017, 33, 9759–9771. [Google Scholar] [CrossRef]
- Barrer, R.M.; MacLeod, D.M. Intercalation and sorption by montmorillonite. Trans. Faraday Soc. 1954, 50, 980–989. [Google Scholar] [CrossRef]
- Barrer, R.M.; MacLeod, D.M. Activation of montmorillonite by ion exchange and sorption complexes of tetra-alkyl ammonium montmorillonites. Trans. Faraday Soc. 1955, 51, 1290–1300. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay applications and limits in the environment. Comptes Rendus Chim. 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral–organic interactions. In Handbook of Clay Science. Developments in Clay Science; Bergaya, F., Lagaly., G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 435–505. [Google Scholar]
- Brixie, J.M.; Boyd, S.A. Treatment of Contaminated Soils with Organoclays to Reduce Leachable Pentachlorophenol. J. Environ. Qual. 1994, 23, 1283–1290. [Google Scholar] [CrossRef]
- Boeva, N.M.; Bocharnikova, Y.I.; Nasedkin, V.V.; Belousov, P.E.; Demidenok, K.V. Thermal analysis as an express method for assessing the quality and quantity of natural and synthesized organoclays. Nanotechnologies Russ. 2013, 8, 205–208. [Google Scholar] [CrossRef]
- Lagaly, G. Layer charge determination by alkylammonium ions. In Clay Minerals Society Workshop Lectures: Layer Charge Characteristics of 2:1 Silicate Clay Minerals; Mermut, A.R., Ed.; The Clay Minerals Society: Boulder, CO, USA, 1994; Volume 6, pp. 1–46. [Google Scholar]
- Lee, S.Y.; Kim, S.J. Expansion characteristics of organoclay as a precursor to nanocomposites. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 211, 19–26. [Google Scholar] [CrossRef]
- Churakov, S.V. Ab initio study of edge sites reactivity on pyrophyllite. In Proceedings of the International Meeting “Clays in Natural & Engineered Barriers for Radioactive Waste Confinement”, Tours, France, 14–18 March 2005; pp. 219–220. [Google Scholar]
- Popov, V.G.; Abdrakhmanov, R.F. Ion Exchange Concept in Genetic Hydrogeochemistry; Gilem, Bashkir Encyclopedia: Ufa, Russia, 2013; 356p. (In Russian) [Google Scholar]
- Civan, F. Reservoir Formation Damage: Fundamentals, Modeling, Assessment, and Mitigation, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2015; 1042p. [Google Scholar]
- Sparks, D.L. Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; 352p. [Google Scholar]
- Ray, S.S. Introduction to environmentally friendly polymer nanocomposites. In Environmentally Friendly Polymer Nanocomposites, Types, Processing and Properties, 1st ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 3–24. [Google Scholar]
- Brindley, G.W. Ethylene glycol and glycerol complexes of smectites and vermiculites. Clay Miner. 1966, 6, 237–259. [Google Scholar] [CrossRef]
- Cloos, P.; Laura, R.D.; Badot, C. Adsorption of ethylene diamine on montmorillonite saturated with different cations-V: Ammonium- and triethylammonium-montmorillonite: Ion exchange, protonation and hydrogen-bonding. Clays Clay Miner. 1975, 23, 417–423. [Google Scholar] [CrossRef]
- Farmer, V.C.; Mortland, M.M. An infrared study of the co-ordination of pyridine and water to exchangeable cations in montmorillonite and saponite. J. Chem. Soc. A 1966, 344–351. [Google Scholar] [CrossRef]
- Guégan, R.; De Oliveira, T.; Le Gleuher, J.; Sugahara, Y. Tuning down the environmental interests of organoclays for emerging pollutants: Pharmaceuticals in presence of electrolytes. Chemosphere 2020, 239, 124730. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, M.; Güler, H.; Cocke, D.L. Interactions of clay minerals with organic pollutants. Sci. Total. Environ. 1994, 141, 223–240. [Google Scholar] [CrossRef]
- Gerasin, V.A.; Antipov, E.M.; Karbushev, V.V.; Kulichikhin, V.; Karpacheva, G.P.; Talroze, R.V.; Kudryavtsev, Y.V. New approaches to the development of hybrid nanocomposites: From structural materials to high-tech applications. Russ. Chem. Rev. 2013, 82, 303–332. [Google Scholar] [CrossRef]
- Brindley, G.W.; Moll, W.F. Complexes of natural and synthetic Ca-montmorillonites with fatty acids. Am. Mineral. 1965, 50, 1355–1370. [Google Scholar]
- Theng, B.K.G. Polymer–clay nanocomposites. In Developments in Clay Science; Theng, B.K.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 201–241. [Google Scholar]
- He, H.; Ma, Y.; Zhu, J.; Yuan, P.; Qing, Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl. Clay Sci. 2010, 48, 67–72. [Google Scholar] [CrossRef]
- Gougeon, R.D.; Soulard, M.; Reinholdt, M.; Miehé-Brendlé, J.; Chézeau, J.-M.; Le Dred, R.; Marchal, R.; Jeandet, P. Polypeptide Adsorption on a Synthetic Montmorillonite: A Combined Solid-State NMR Spectroscopy, X-ray Diffraction, Thermal Analysis and N2 Adsorption Study. Eur. J. Inorg. Chem. 2003, 2003, 1366–1372. [Google Scholar] [CrossRef]
- Lagaly, G. Interaction of alkylamines with different types of layered compounds. Solid State Ionics 1986, 22, 43–51. [Google Scholar] [CrossRef]
- Wang, A.; Wang, W. Introduction. In Nanomaterials from Clay Minerals. A New Approach to Green Functional Materials, 1st ed.; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–20. [Google Scholar]
- Rahromostaqim, M.; Sahimi, M. Molecular Dynamics Study of the Effect of Layer Charge and Interlayer Cations on Swelling of Mixed-Layer Chlorite–Montmorillonite Clays. J. Phys. Chem. C 2020, 124, 2553–2561. [Google Scholar] [CrossRef]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Letaief, S.; Detellier, C. Functionalization of the interlayer surfaces of kaolinite by alkylammonium groups from ionic liquids. Clays Clay Miner. 2009, 57, 638–648. [Google Scholar] [CrossRef]
- Tan, D.; Yuan, P.; Annabi-Bergaya, F.; Liu, D.; He, H. Methoxy-modified kaolinite as a novel carrier for high-capacity loading and controlled-release of the herbicide amitrole. Sci. Rep. 2015, 5, srep08870. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Riela, S. Organo-clay nanomaterials based on halloysite and cyclodextrin as carriers for polyphenolic compounds. J. Funct. Biomater. 2018, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Preparation, characterization of surfactants modified clay minerals and nitrate adsorption. Appl. Clay Sci. 2010, 48, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Słomkiewicz, P.; Szczepanik, B.; Czaplicka, M. Adsorption of Phenol and Chlorophenols by HDTMA Modified Halloysite Nanotubes. Materials 2020, 13, 3309. [Google Scholar] [CrossRef]
- Hashizume, H. Adsorption of aromatic compounds in water by talc. Clay Sci. 2009, 14, 61–64. [Google Scholar]
- Desai, H.; Biswal, N.R.; Paria, S. Rheological behavior of pyrophyllite−water slurry in the presence of anionic, cationic, and nonionic surfactants. Ind. Eng. Chem. Res. 2010, 49, 5400–5406. [Google Scholar] [CrossRef]
- Erdemoğlu, M.; Sayılkan, F.; Akarsu, M.; Şener, Ş. Organo-functional modified pyrophyllite: Preparation, characterisation and Pb(II) ion adsorption property. Appl. Clay Sci. 2004, 27, 41–52. [Google Scholar] [CrossRef]
- Osipov, V.I.; Sokolov, V.N.; Rumyantseva, N.A. Microstructure of Clay Rocks; Nedra: Moscow, Russia, 1989; 211p. (In Russian) [Google Scholar]
- Pokidko, B.V.; Bukanova, E.F.; Tutorsky, I.A.; Il‘ina, M.B. Influence of Ca2+ on the adsorption of different surfactants in the bentonite-water interface. Rev. MITHT 2009, 4, 77–83. (In Russia) [Google Scholar]
- Fetter, G.; Bosch, P. Microwave effect on clay pillaring. In Pillared Clays and Related Catalysts; Gil, A., Korili, S.A., Trujillano, R., Vicente, M.A., Eds.; Springer: New York, NY, USA, 2010; pp. 1–21. [Google Scholar]
- Ammann, L.; Bergaya, F.; Lagaly, G. Determination of the cation exchange capacity of clays with copper complexes revisited. Clay Miner. 2005, 40, 441–453. [Google Scholar] [CrossRef]
- Madejová, J.; Barlog, M.; Jankovič, Ľ.; Slaný, M.; Pálková, H. Comparative study of alkylammonium- and alkylphosphonium-based analogues of organo-montmorillonites. Appl. Clay Sci. 2021, 200, 105894. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Mohamed, A.I.A.; Hussein, I.A.; Sultan, A.S.; Al-Muntasheri, G.A. Use of organoclay as a stabilizer for water-in-oil emulsions under high-temperature high-salinity conditions. J. Petrol. Sci. Eng. 2018, 160, 302–312. [Google Scholar] [CrossRef]
- Moazed, H.; Viraraghavan, T. Removal of Oil from Water by Bentonite Organoclay. J. Hazard. Toxic Radioact. Waste 2005, 9, 130–134. [Google Scholar] [CrossRef]
- Nafees, M.; Waseem, A. Organoclays as Sorbent Material for Phenolic Compounds: A Review. CLEAN Soil Air Water 2014, 42, 1500–1508. [Google Scholar] [CrossRef]
- Li, Z.; Yao, M.; Lin, J.; Yang, B.; Zhang, X.; Lei, L. Pentachlorophenol sorption in the cetyltrimethylammonium bro-mide/bentonite one-step process in single and multiple solute systems. J. Chem. Eng. Data 2013, 58, 2610–2615. [Google Scholar] [CrossRef]
- Yıldız, N.; Gönülşen, R.; Koyuncu, H.; Çalımlı, A. Adsorption of benzoic acid and hydroquinone by organically modified bentonites. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 260, 87–94. [Google Scholar] [CrossRef]
- Changchaivong, S.; Khaodhiar, S. Adsorption of naphthalene and phenanthrene on dodecylpyridinium-modified bentonite. Appl. Clay Sci. 2009, 43, 317–321. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, M.; Zhu, L. Adsorption-desorption behavior of naphthalene onto CDMBA modified bentonite: Contribution of the π-π interaction. Appl. Clay Sci 2014, 100, 29–34. [Google Scholar] [CrossRef]
- Cruz-Guzmán, M.; Celis, R.; Hermosín, M.C.; Cornejo, J. Adsorption of the Herbicide Simazine by Montmorillonite Modified with Natural Organic Cations. Environ. Sci. Technol. 2003, 38, 180–186. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, Y.; Undabeytia, T.; Polubesova, T.; Mishael, Y.G.; Nir, S.; Rubin, B. Organo-clay formulations of pesticides: Reduced leaching and photodegradation. Appl. Clay Sci. 2001, 18, 309–326. [Google Scholar] [CrossRef]
- Narayanan, N.; Gajbhiye, V.T.; Gupta, S.; Manjaiah, K.M. Novel biopolymer-nano-organoclay composites for the decontam-ination of pesticides from water. Pestic. Res. J. 2016, 28, 25–34. [Google Scholar]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Le Milbeau, C.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323 Pt A, 558–566. [Google Scholar] [CrossRef]
- França, D.B.; Trigueiro, P.; Filho, E.C.S.; Fonseca, M.G.; Jaber, M. Monitoring diclofenac adsorption by organophilic alkylpyr-idinium bentonites. Chemosphere 2020, 242, 125109. [Google Scholar] [CrossRef]
- Huang, P.; Kazlauciunas, A.; Menzel, R.; Lin, L. Determining the Mechanism and Efficiency of Industrial Dye Adsorption through Facile Structural Control of Organo-montmorillonite Adsorbents. ACS Appl. Mater. Interfaces 2017, 9, 26383–26391. [Google Scholar] [CrossRef] [Green Version]
- Huggett, J.M. Clay Minerals. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Trofimov, S.Y.; Sokolova, T.A.; Dronova, T.Y.; Tolpeshta, I.I. Mineral Components of Soils; Grif and K.: Tula, Russia, 2007; 104p. (In Russian) [Google Scholar]
- Ding, F.; Gao, M.; Shen, T.; Zeng, H.; Xiang, Y. Comparative study of organo-vermiculite, organo-montmorillonite and or-gano-silica nanosheets functionalized by an ether-spacer-containing Gemini surfactant: Congo red adsorption and wettability. Chem. Eng. J. 2018, 349, 388–396. [Google Scholar] [CrossRef]
- Tuchowska, M.; Wołowiec, M.; Solińska, A.; Kościelniak, A.; Bajda, T. Organo-Modified Vermiculite: Preparation, Characterization, and Sorption of Arsenic Compounds. Minerals 2019, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Dultz, S.; An, J.-H.; Riebe, B. Organic cation exchanged montmorillonite and vermiculite as adsorbents for Cr(VI): Effect of layer charge on adsorption properties. Appl. Clay Sci. 2012, 67-68, 125–133. [Google Scholar] [CrossRef]
- Osman, M.A.; Suter, U.W. Determination of the Cation-Exchange Capacity of Muscovite Mica. J. Colloid Interface Sci. 2000, 224, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Ravella, R. Swelling Mica-Type Clays of Variable Charge: Synthesis, Characterization and Ion Exchange Studies. Ph.D. Thesis, The Pennsylvania State University, University Park, PA, USA, 2006; 163p. [Google Scholar]
- Yu, C.J.; Choe, S.H.; Jang, Y.M.; Jang, G.H.; Pae, Y.H. First-principles study of organically modified muscovite mica with ammonium (NH4+) or methylammonium (CH3NH3+) ion. J. Mater. Sci. 2016, 51, 10806–10818. [Google Scholar] [CrossRef] [Green Version]
- Bracke, G.; Satir, M.; Krauss, P. The Cryptand [222] for exchanging cations of micas. Clays Clay Miner. 1995, 43, 732–737. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Hasegawa, K.; Nakamuro, Y.; Kodama, T.; Komarneni, S. Alkaline earth cation exchange with novel Na-3-mica: Kinetics and thermodynamic selectivities. J. Mater. Chem. 2004, 14, 1031–1035. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Wang, X.K.; Wu, N.Z.; Xie, Y.C. Cleaving of muscovite powder by molten lithium nitrate. Colloid Polym. Sci. 2005, 283, 699–702. [Google Scholar] [CrossRef]
- Weng-Lip, L.; Salleh, N.M.; Abdul Rahman, N.A.; Bakhtiar, N.S.A.A.; Akil, H.M.; Zubir, S.A. Enhanced intercalation of organo-muscovite prepared via hydrothermal reaction at low temperature. Bull. Mater. Sci. 2019, 42, 242. [Google Scholar] [CrossRef] [Green Version]
- Jaynes, W.; Boyd, S. Clay Mineral Type and Organic Compound Sorption by Hexadecyltrimethlyammonium-Exchanged Clays. Soil Sci. Soc. Am. J. 1991, 55, 43–48. [Google Scholar] [CrossRef]
- Seki, Y.; Yurdakoç, K. Paraquat adsorption onto clays and organoclays from aqueous solution. J. Colloid. Interface Sci. 2005, 287, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Boyd, S.A. Polarity effect on dichlorobenzene sorption by hexadecyltrimethylammonium-exchanged clays. Clays Clay Miner. 2000, 48, 43–50. [Google Scholar] [CrossRef]
- Peregudov, Y.S.; Mejri, R.; Gorbunova, E.M.; Niftaliev, S.I. Glauconite-based sorbents for skimming oil and oil products. Condens. Matter Interph. 2020, 22, 257–265. (In Russian) [Google Scholar]
- Scriabina, O.A. Mineralogical Composition of Soils and Parent Rocks; Publishing House of “Perm State Agricultural Academy”: Perm, Russia, 2010; 120p. (In Russian) [Google Scholar]
- Satterberg, J.; Arnarson, T.S.; Lessard, E.J.; Keil, R.G. Sorption of organic matter from four phytoplankton species to montmo-rillonite, chlorite and kaolinite in seawater. Mar. Chem. 2003, 81, 11–18. [Google Scholar] [CrossRef]
- Hanamoto, S.; Ogawa, F. Predicting the sorption of azithromycin and levofloxacin to sediments from mineral and organic components. Environ. Pollut. 2019, 255, 113180. [Google Scholar] [CrossRef] [PubMed]
- Acikyildiz, M.; Gurses, A.; Yolcu, H.H. Synthesis of super hydrophobic clay by solution intercalation method from aqueous dispersions. In Acta Physica Polonica A, Proceedings of the 4th International Congress APMAS2014, Fethiye, Turkey, 24–27 April 2014; Institute of Physics, Polish Academy of Sciences: Warsaw, Poland, 2015; Volume 127, pp. 1156–1160. [Google Scholar]
- Zaini, N.A.M.; Ismail, H.; Rusli, A. Short Review on Sepiolite-Filled Polymer Nanocomposites. Polym. Technol. Eng. 2017, 56, 1665–1679. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Adsorption of the herbicide 2,4-D on organo-palygorskite. Appl. Clay Sci. 2010, 49, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Schaumberger, S.; Ladinig, A.; Reisinger, N.; Ritzmann, M.; Schatzmayr, G. Evaluation of the endotoxin binding efficiency of clay minerals using the Limulus Amebocyte lysate test: An in vitro study. AMB Express 2014, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.C.; Batista, T.S.; Ramos, I.B.M.; Barros, T.R.B.; De Sousa, B.V. Organophilization Process and Characterization of Palygorskite Clay (Attapulgite). Mater. Sci. Forum 2016, 881, 218–223. [Google Scholar] [CrossRef]

| Surfactants | Chemical Formula | Surfactants | Chemical Formula |
|---|---|---|---|
| Methyl tallow bis-2-hydroxyethyl quaternary ammonium |  | Polyoxy propylene methyl diethyl ammonium | 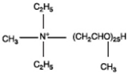 |
| Dimethyl dehydrogenated tallow quaternary ammonium |  | Octadecyl amine |  |
| Dimethyl dehydrogenated tallow 2–ethylhexyl quaternary ammonium | 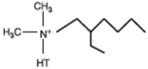 | Dimethyl octadecyl amine | 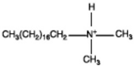 |
| Dimethyl benzyl hydrogenated tallow quaternary ammonium |  | Hexadecyl trimetyl ammonium |  |
| Dimethyl dialkyl (tallow, presented by T) ammonium |  | Dodecyl triphnyl phosphonium | 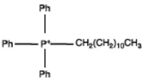 |
| Trioctyl methyl ammonium |  | Hexadecyl tributyl phosphonium | 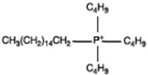 |
| Dipilyoxy ethylene alkyl (COCO) methyl ammonium |  | Dodecyl trimethyl phosphonium | 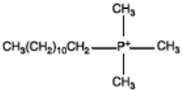 |
| Mineral Structure (Layer Types) | Charge per Formula Unit | Groups | Subgroups | Species Examples |
|---|---|---|---|---|
| 1:1 | 0 | Kaolin–Serpentine | Kaolinite | Kaolinite, dickite, nacrite |
| Serpentine | Chrysotile, lizardite, amesite | |||
| 2:1 | 0 | Talc–Pyrophyllite | Talc | Talc |
| Pyrophyllite | Pyrophyllite | |||
| 0.2–0.6 | Smectite | Montmorillonite | Montmorillonite, beidellite, nontronite | |
| Saponite | Saponite, laponite, hectorite | |||
| 0.6–0.9 | Vermiculite | Dioctahedral | Dioctahedral vermiculite | |
| Trioctahedral | Trioctahedral vermiculite | |||
| 1 | Mica | Dioctahedral | Muscovite, illite, glauconite, paragonite | |
| Trioctahedral | Phlogopite, biotite, lepidolite | |||
| 2 | Brittle mica | Dioctahedral | Margarite | |
| Trioctahedral | Clintonite, anandite | |||
| 2:1:1 | Variable | Chlorite | Dioctahedral | Donbassite |
| Trioctahedral | Chlinochlore, chamosite, nimite | |||
| Di, trioctahedral | Cookeite, sudoite | |||
| Other silicates (close to inosilicates) | 0.1 | Palygorskite–Sepiolite | Dioctahedral | Nepouite |
| Palygorskite | Palygorskite | |||
| Sepiolite | Sepiolite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelomov, L.; Mandzhieva, S.; Minkina, T.; Atroshchenko, Y.; Perelomova, I.; Bauer, T.; Pinsky, D.; Barakhov, A. The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities. Minerals 2021, 11, 707. https://doi.org/10.3390/min11070707
Perelomov L, Mandzhieva S, Minkina T, Atroshchenko Y, Perelomova I, Bauer T, Pinsky D, Barakhov A. The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities. Minerals. 2021; 11(7):707. https://doi.org/10.3390/min11070707
Chicago/Turabian StylePerelomov, Leonid, Saglara Mandzhieva, Tatiana Minkina, Yury Atroshchenko, Irina Perelomova, Tatiana Bauer, David Pinsky, and Anatoly Barakhov. 2021. "The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities" Minerals 11, no. 7: 707. https://doi.org/10.3390/min11070707
APA StylePerelomov, L., Mandzhieva, S., Minkina, T., Atroshchenko, Y., Perelomova, I., Bauer, T., Pinsky, D., & Barakhov, A. (2021). The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities. Minerals, 11(7), 707. https://doi.org/10.3390/min11070707










