Hydrogen Peroxide Ammonium Citrate Extraction: Mineral Decomposition and Preliminary Waste Rock Characterization
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Mineralogical Results
3.2. Geochemical Results
4. Discussion
4.1. Mineral Decomposition in the HA Extraction
4.2. HA Extraction in the Preliminary Element Mobility Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robb, L. Introduction to Ore-Forming Processes; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Dold, B. Evolution of acid mine drainage formation in sulfidic mine tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Heikkinen, P.M.; Räisänen, M.L. Trace metal and as solid-phase speciation in sulfide mine tailings–indicators of spatial distribution of sulfide oxidation in active tailings impoundments. Appl. Geochem. 2009, 24, 1224–1237. [Google Scholar] [CrossRef]
- Acid Rock Drainage Prediction Manual. Available online: http://mend-nedem.org/wp-content/uploads/2013/01/1.16.1b.pdf (accessed on 14 October 2020).
- Price, W.A. Challenges posed by metal leaching and acid rock drainage, and approaches used to address them. In Environmental Aspects of Mine Wastes, Short Course Series; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Association of Canada: Quebec, QC, Canada, 2003; Volume 31, pp. 15–30. [Google Scholar]
- Singer, P.C.; Stumm, W. Acid mine drainage: The rate-determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J. Acid-neutralization mechanisms in inactive mine tailings. In The Environmental Geochemistry of Sulfide Mine Wastes, Short Course Series; Jambor, J.L., Blowes, D.W., Eds.; Mineralogical Association of Canada: Quebec, QC, Canada, 1994; Volume 31, pp. 95–116. [Google Scholar]
- Fletcher, W.K. (Ed.) Handbook of Exploration Geochemistry—Volume 1: Analytical Methods in Geochemical Prospecting; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Lynch, J.J. The determination of copper, nickel and cobalt in rocks by atomic absorption spectrometry using a cold leach. J. Geochem. Explor. 1971, 11, 313–315. [Google Scholar]
- Dold, B. Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J. Geochem. Explor. 2003, 80, 55–68. [Google Scholar] [CrossRef]
- Niskavaara, H. A comprehensive scheme of analysis for soils, sediments, humus and plant samples using inductively coupled plasma atomic emission spectrometry (ICP-AES). In Geological Survey of Finland, Current Research 1993–1994; Special Paper 20; Autio, S., Ed.; Geological Survey of Finland: Espoo, Finland, 1995; pp. 167–175. [Google Scholar]
- Muniruzzaman, M.; Kauppila, P.M.; Karlsson, T. Water Quality Prediction of Mining Waste Facilities Based on Predictive Models; Geological Survey of Finland: Espoo, Finland, 2018. [Google Scholar]
- Doležal, J.; Provondra, P.; Šulcek, Z. Decomposition Techniques in Inorganic Analysis; Iliffe Books Ltd.: London, UK, 1968. [Google Scholar]
- Finnish Government. Government Decree on Extractive Waste 190/2013; Finnish Government: Helsinki, Finland, 2013. Available online: https://www.finlex.fi/fi/laki/kaannokset/2013/en20130190_20150102.pdf (accessed on 14 October 2020).
- Fosso-Kankeu, E.; Waanders, F.; Mulaba-Bafubiandi, A.; Sidu, S. Leachability of suspended particles in mine water and risk of water contamination. In Proceedings of the 10th ICARD and IMWA 2015 Conference, Santiago, Chile, 21–24 April 2015; Brown, A., Bucknam, C., Burgess, J., Carballo, M., Castendyk, D., Figueroa, L., Eds.; Gecamin Publications: Santiago, Chile, 2015; pp. 788–796. [Google Scholar]
- Karlsson, T.; Kauppila, P.M. Waste rock characterization versus the actual seepage water quality. In Proceedings of the International Mine Water Association 2016 Symposium Mining Meets Water—Conflicts and Solutions, Leipzig, Germany, 11–15 July 2016; Drebenstedt, C., Paul, M., Eds.; TU Bergakademie: Freiberg, Germany, 2016; pp. 402–406. [Google Scholar]
- Karlsson, T.; Kauppila, P.M.; Lehtonen, M. Prediction of the long-term behaviour of extractive wastes based on environmental characterisation: Correspondence of laboratory prediction tests with field data. Bull. Geol. Surv. Finl. 2018, 408, 11–26. [Google Scholar]
- Karlsson, T.; Alakangas, L.; Kauppila, P.M.; Räisänen, M.L. A test of two methods for waste rock drainage quality prediction: Aqua regia extraction and single-addition net-acid generation test leachate analysis. Mine Water Environ. 2021. [Google Scholar] [CrossRef]
- Price, W.A.; Morin, K.; Hutt, N. Guidelines for prediction of acid rock drainage and metal leaching for mines in British Columbia: Part II. In Proceedings of the Recommended procedures for static and kinetic tests. In Proceedings of the 4th ICARD Conference, Vancouver, BC, Canada, 1 June 1997; Gusek, J., Wildeman, T., Eds.; Volume 1, pp. 15–30. [Google Scholar]
- Katsnelson, E.M.; Osipova, J.J. Usoveršenstvovannyi metod opredelenija sul’fidnogo nikelja. Obogaštšenie 1960, 4, 24–26, (with English abstract). [Google Scholar]
- Czamanske, G.K.; Ingamells, C.O. Selective chemical dissolution of sulfide minerals: A method of mineral separation. Am. Mineral. 1970, 55, 2131–2134. [Google Scholar]
- Penttinen, U.; Palosaari, V.; Siura, T. Selective dissolution and determination of sulfides in nickel ores by the bromine-methanol method. Bull. Geol. Soc. Finl. 1977, 49, 79–84. [Google Scholar] [CrossRef]
- Karapetyan, E.T. Opredelenie sul’fidnogo nikelya v sul’fidnykh rudakh I kontsentratakh. Zavod. Lab. 1968, 34, 939–940, (with English abstract). [Google Scholar]
- Klock, P.R.; Czamanske, G.K.; Foose, M.; Pesek, J. Selective chemical dissolution of sulfides: An evaluation of six methods applicable to assaying sulfide-bound nickel. Chem. Geol. 1986, 54, 157–163. [Google Scholar] [CrossRef]
- Bradley, J.; Baker, H. Mineral Resource Estimate for the Rönnbäcken Nickel Project, Sweden; SRK Consulting: Skellefteå, Sweden, 2010. [Google Scholar]
- Gray, D.; Cameron, T.; Briggs, A. Kevitsa Nickel Copper Mine, Lapland, Finland; NI 43-101 Technical Report; First Quantum Minerals: West Perth, Australia, 2016. [Google Scholar]
- Peters, L.J. Beaver and Lynx Sulfide Nickel Property (BL Property), Cariboo Mining Division, British Columbia; National Instrument 43-101 Technical Report; Inomin Mines Inc.: Vancouver, BC, Canada, 2020. [Google Scholar]
- Kuronen, E.; Tuokko, I. Horsmanaho Talc Mine. In Excursion Guidebook A4—Ore Deposits in Eastern Finland, Proceedings of the 4th Biennial SGA Meeting, Turku, Finland, 11–13 August 1997; Loukola-Ruskeeniemi, K., Sorjonen-Ward, P., Eds.; Geological Survey of Finland: Espoo, Finland, 1997; Guide 42; pp. 39–42. [Google Scholar]
- Kontinen, A.; Peltonen, P.; Huhma, H. Description and Genetic Modelling of the Outokumpu-Type Rock Assemblage and Associated Sulfide Deposits; Geological Survey of Finland: Espoo, Finland, 2006; Archive Report M 10.4/2006/1. [Google Scholar]
- Meriläinen, M.; Ekberg, M.; Lovén, P.; Koivistoinen, P.; Hakola, J.; Strauss, T. Updated Reserve and Resource Estimate of the Hitura Nickel Mine in Central Finland; National Instrument 43–101, Technical Report; Belvedere Resources: Preston, UK, 2008. [Google Scholar]
- Makkonen, H. Hitura Ni. In Metallogenic Areas in Finland; Special Paper 53; Geological Survey of Finland: Espoo, Finland, 2012; pp. 207–342. [Google Scholar]
- Hyvärinen, L. On the geology of the copper ore field in the Virtasalmi area, Eastern Finland. Bull. Comm. Géologique Finl. 1969, 240, 82. [Google Scholar]
- Lawrie, K.C. Geochemical characterisation of a polyphase deformed, altered and high grade metamorphosed volcanic terrane: Implications for the tectonic setting of the Svecofennides, South-Central Finland. Precambrian Res. 1987, 59, 171–205. [Google Scholar] [CrossRef]
- Papunen, H. Suomen metalliset malmiesiintymät. In Suomen Malmigeologia—Metalliset Malmiesiintymät; Papunen, H., Haapala, I., Rouhunkoski, P., Eds.; Suomen Geologinen Seura ry: Mänttä, Finland, 1986. (In Finnish) [Google Scholar]
- Geological Survey of Finland. Mineral Deposit Report 103: Virtasalmi; Geological Survey of Finland, Mineral Deposits-Report Database; Available online: http://tupa.gtk.fi/karttasovellus/mdae/raportti/103_Virtasalmi.pdf (accessed on 22 January 2018).
- Santaguida, F.; Luolavirta, K.; Lappalainen, M.; Ylinen, J.; Voipio, T.; Jones, S. The Kevitsa Ni-cu-PGE deposit in the Central Lapland greenstone belt in Finland. In Mineral Deposits of Finland; Maier, W., Lahtinen, R., O’Brien, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 195–210. [Google Scholar]
- ISO/IEC. General Requirements for the Competence of Testing and Calibration Laboratories; International Organization for Standardization: Geneva, Switzerland, 2005; DIN ISO/IEC 17025:2005-11. [Google Scholar]
- ISO. Soil Quality—Determination of Total Sulfur by Dry Combustion; International Organization for Standardization: Geneva, Switzerland, 2000; DIN ISO 115178:2000-11. [Google Scholar]
- ISO. Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia; International Organization for Standardization: Geneva, Switzerland, 1995; DIN ISO 11466:1995-03. [Google Scholar]
- Young, R.S. Chemical Phase Analysis; Charles Griffin and Company Ltd.: London, UK, 1974; p. 138. [Google Scholar]
- Chao, T.T.; Sanzolone, R.J. Chemical dissolution of sulfide minerals. J. Res. US Geol. Surv. 1977, 5, 409–412. [Google Scholar]
- Karlsson, T.; Räisänen, M.L.; Lehtonen, M.; Alakangas, L. Comparison of static and mineralogical ARD prediction methods in the Nordic environment. Environ. Monit. Assess 2018, 190, 719. [Google Scholar] [CrossRef] [Green Version]
- Parbhakar-Fox, A.; Fox, N.; Ferguson, T.; Hill, R.; Maynard, N. Dissection of the NAG pH test: Tracking efficacy through examining reaction products. In Proceedings of the 11th ICARD—IMWA—MWD Conference, Pretoria, South Africa, 10–14 September 2018; Wolkersdorfer, C., Sartz, L., Weber, A., Burgess, J., Tremblay, G., Eds.; International Mine Water Association: Wendelstein, Germany, 2018; pp. 949–955. [Google Scholar]
- Stewart, W.A. Development of Acid Rock Drainage Prediction Methodologies for Coal Mine Wastes. Ph.D. Thesis, University of South Australia, Adelaide, Australia, 2005. [Google Scholar]
- AMIRA. Prediction and kinetic control of acid mine drainage. In ARD Test Handbook; Project P387A; AMIRA International: Melbourne, Australia, 2002. [Google Scholar]
- Kwong, Y.T.J. Prediction and Prevention of Acid Rock Drainage from a Geological and Mineralogical Perspective; MEND Report 1.32.1; Ottawa, ON, Canada; Available online: http://mend-nedem.org/wp-content/uploads/1.32.1.pdf (accessed on 14 October 2020).
- Parbhakar-Fox, A.; Lottermoser, B.; Bradshaw, D. Evaluating waste rock mineralogy and microtexture during kinetic testing for improved acid rock drainage prediction. Miner. Eng. 2013, 52, 111–124. [Google Scholar] [CrossRef]
- Al, T.A.; Blowes, D.W.; Martin, C.J.; Cabri, L.J.; Jambor, J.L. Aqueous geochemistry and analysis of pyrite surfaces in sulfide-rich mine tailings. Geochim. Cosmochim. Acta 1997, 61, 2353–2366. [Google Scholar] [CrossRef]
- Koljonen, T. The Geochemical Atlas of Finland. Part 2: Till; Geological Survey of Finland: Espoo, Finland, 1992. [Google Scholar]
- Campbell, F.A.; Ethier, V.G. Nickel and cobalt in pyrrhotite and pyrite from the Faro and Sullivan orebodies. Canad. Mineral. 1984, 22, 503–506. [Google Scholar]
- Liu, Z.; Shao, Y.; Zhou, H.; Liu, N.; Huang, K.; Liu, Q.; Zhang, J.; Wang, C. Major and Trace element geochemistry of pyrite and pyrrhotite from stratiform and lamellar orebodies: Implications for the ore genesis of the dongguashan copper (gold) deposit, Eastern China. Minerals 2018, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Belzile, N.; Chen, Y.W.; Cai, M.F.; Li, Y. A review on pyrrhotite oxidation. J. Geochem. Explor. 2004, 84, 65–76. [Google Scholar] [CrossRef]
- Ding, K.; Liu, Y. Hydrolysis of Pyrite: A Possible pathway for generation of high concentrations of H2S gas in deep-buried reservoirs? Dev. Earth Sci. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Dold, B. Acid rock drainage prediction: A critical review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Plumlee, G.S.; Smith, K.S.; Mountour, M.R.; Ficklin, W.H.; Mosier, E.L. Geologic controls on the composition of natural waters and mine waters draining diverse mineral-deposit types. Rev. Econ. Geol. 1999, 6, 373–432. [Google Scholar]
- Bromine Safety Handbook; India Bromine Platform: Mumbai, India, 18 March 2019; pp. 36–65. Available online: https://www.indianchemicalcouncil.com/docs/Bromine-Safety-Handbook.pdf (accessed on 16 June 2021).
- Kauppila, P.M. Sequential Extraction Procedure. Available online: https://mineclosure.gtk.fi/sequential-extraction-procedure/ (accessed on 16 June 2021).
- Heikkinen, P.M.; Räisänen, M.L. Mineralogical and geochemical alteration of Hitura sulphide mine tailings with emphasis on nickel mobility and retention. J. Geochem. Explor. 2008, 97, 1–20. [Google Scholar] [CrossRef]
- Jambor, J.L. Mine-waste mineralogy and mineralogical perspectives of acid-base accounting. In The Environmental Geochemistry of Sulfide Mine-Wastes, Short Course Handbook; Jambor, J.L., Blowes, D.W., Eds.; Mineralogical Association of Canada: Ottawa, ON, Canada, 2002; Volume 22, pp. 59–102. [Google Scholar]
- Paktunc, A.D. Mineralogical constrains of the determination of neutralization potential and prediction of acid mine drainage. Environ. Geol. 1999, 39, 103–112. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.G. A critical review of acid rock drainage prediction methods and practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- White, W.W., III; Lapakko, K.A.; Cox, R.L. Static-test methods most commonly used to predict acid mine drainage: Practical guidelines for use and interpretation. In The Environmental Geochemistry of Mineral Deposits, Part A: Processes, Techniques, and Health Issues; Reviews in Economic Geology; Plumlee, G.S., Logsdon, M.J., Filipek, L.F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; pp. 325–328. [Google Scholar]
- Alakangas, L.; Lundberg, A.; Öhlander, B. Estimation of temporal changes in oxidation rates of sulfides in copper mine tailings at Laver, Northern Sweden. Sci. Total Environ. 2010, 408, 1386–1392. [Google Scholar] [CrossRef]
- González, R.M.; Cánovas, C.R.; Olías, M.; Macías, F. Seasonal variability of extremely metal rich acid mine drainages from the Tharsis mines (SW Spain). Environ. Pollut. 2020, 259, 113829. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, P.M.; Räisänen, M.L.; Johnson, R.H. Geochemical characterisation of seepage and drainage water quality from two sulfide mine tailings impoundments: Acid mine drainage versus neutral mine drainage. Mine Water Environ. 2009, 28, 30–49. [Google Scholar] [CrossRef]
- Søndergaard, J.; Elberling, B.; Asmund, G.; Gudrum, C.; Iversen, K.M. Temporal trends of dissolved weathering products from a high Arctic coal mine waste rock pile in Svalbard (78° N). Appl. Geochem. 2007, 22, 125–138. [Google Scholar] [CrossRef]
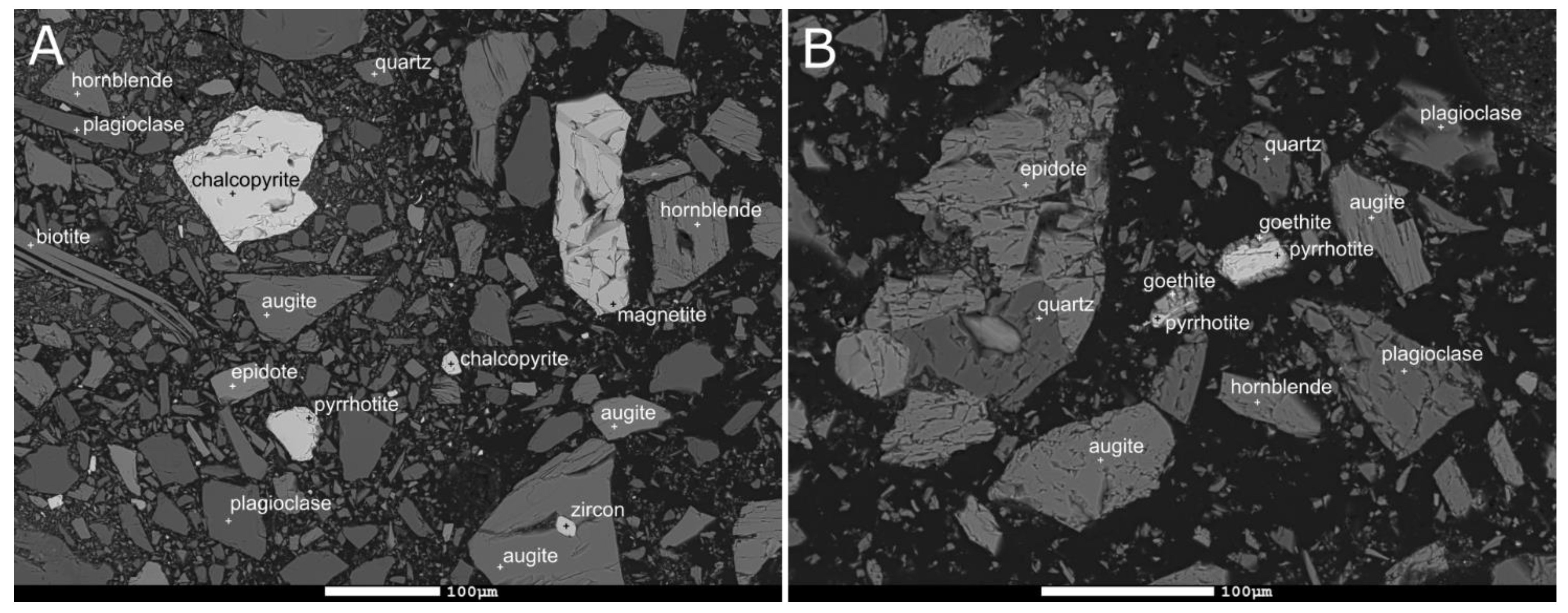
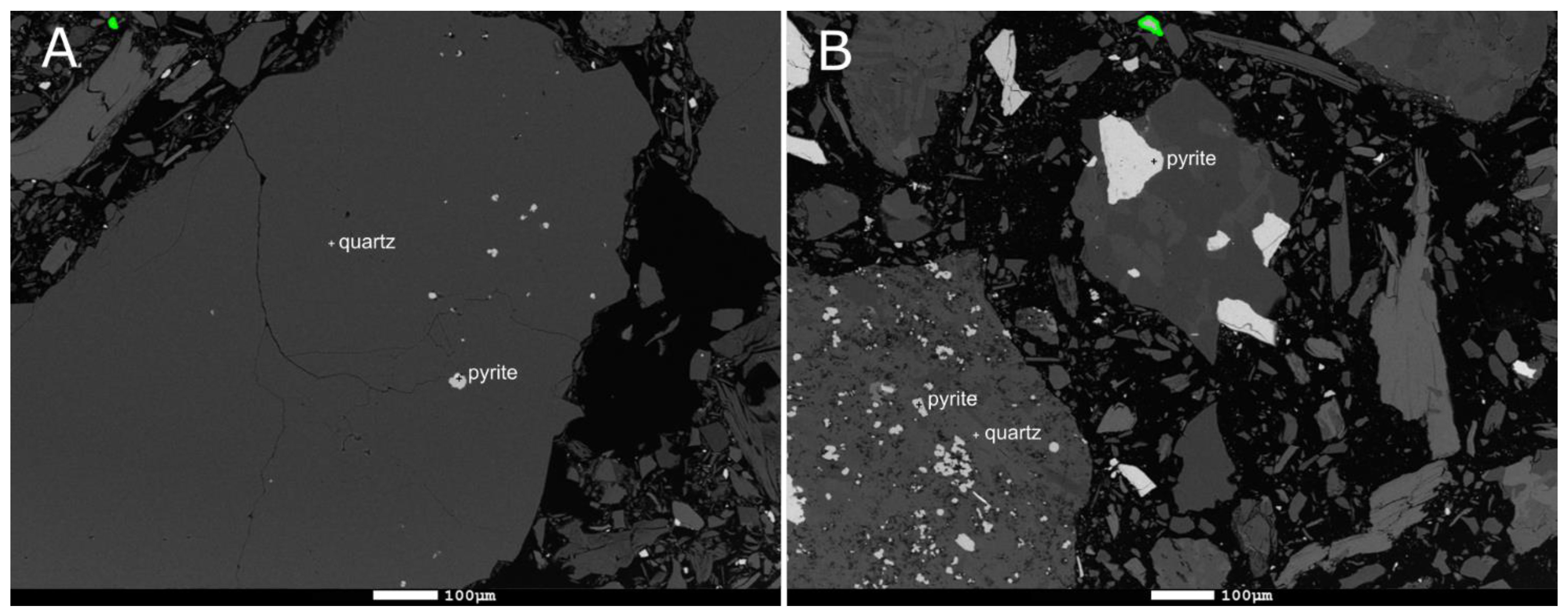
| Mine | Sample | Deposit Description | Sulfides Related to the Deposit | Waste Rock Pile Active | Reference |
|---|---|---|---|---|---|
| Mine 1 (Talc, Ni) | WR1 | Palaeoproterozoic (1.9 Ga), mixed hydrothermal deposit, closely associated with a lens of massive serpentinite, consisting of magnesite pods, lenses within talc-magnesite rock and talc- rich schistose soapstone. | Pentlandite, pyrrhotite, chalcopyrite, pyrite | 2004– | [28] |
| Mine 2 (Cu, Co, Zn, Ni, Au) | WR2 | Palaeoproterozoic (1.9 Ga) mafic–ultramafic mixed hydrothermal VMS, disseminated sulfide ore hosted by quartz rock and metacarbonate rock. | Pyrite, pyrrhotite, chalcopyrite, sphalerite | 2012– | [29] |
| Mine 3 (Ni, Co) | WR3 | Palaeoproterozoic (1.9 Ga) magmatic ultramafic intrusion-hosted nickel deposit, consisting of closely spaced serpentinite massifs surrounded by migmatised mica gneiss. | Pyrrhotite, pentlandite, chalcopyrite, vallerite, mackinawite, cubanite | 1970–1993 | [30,31] |
| Mine 4 (Cu) | WR4 | Palaeoproterozoic (1.9 Ga) mafic basinal hydrothermal SedEx (sedimentary exhalative) deposit, ore appearing as brecciated and disseminated in amphibole host rock. | Chalcopyrite, cubanite, pyrrhotite, with lesser amounts of pyrite, sphalerite, pentlandite, mackinawite, molybdenite, bornite, and other Fe- and Cu- containing sulfides. | 1966–1984 | [32,33,34,35] |
| Mine 5 (Ni, Cu, PGE) | WR5a, WR5b | Palaeoproterozoic (2.1 Ga) mafic–ultramafic magmatic deposit hosted within a composite ultramafic layered intrusion, ore appearing in olivine pyroxenite as disseminated sulfides. | Pyrrhotite, pentandite and chalcopyrite | 2012– | [36] |
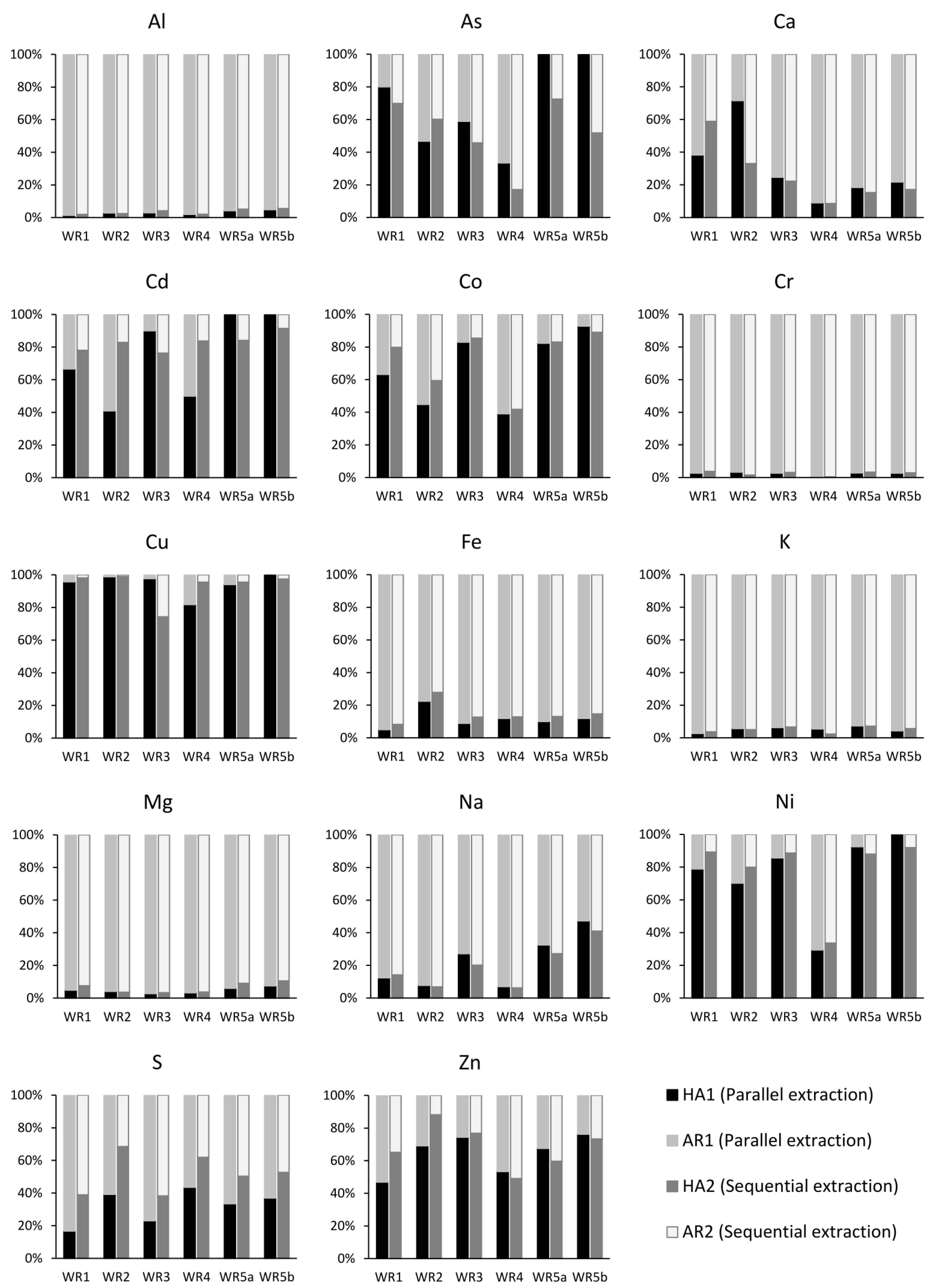
| Mineral | WR1 | WR2 | WR3 | WR4 | WR5a | WR5b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |
| Silicates | ||||||||||||
| Quartz | 20.94 | 12.17 | 8.39 | 13.16 | 11.39 | 7.89 | 2.40 | 1.69 | 0.06 | 0.14 | ||
| Biotite | 4.97 | 5.56 | 7.58 | 12.31 | 9.53 | 15.86 | 0.55 | 0.66 | 0.75 | 0.85 | ||
| Plagioclase (other than albite) | 3.01 | 2.04 | 12.31 | 5.86 | 4.35 | 3.97 | 20.72 | 16.56 | ||||
| Albite | 0.67 | 0.60 | 1.96 | 0.80 | 2.18 | 1.03 | 0.79 | 0.44 | ||||
| Diopside | 14.60 | 19.30 | 12.82 | 16.25 | ||||||||
| Tremolite | 2.85 | 2.96 | 2.52 | 1.24 | 4.71 | 5.18 | 5.19 | 6.39 | ||||
| Talc | 2.33 | 2.06 | ||||||||||
| K-feldspar | 0.68 | 0.24 | 0.69 | 0.38 | ||||||||
| Chlorite | 0.40 | 0.86 | 0.36 | 0.60 | 0.48 | 0.68 | 0.98 | 1.13 | 0.43 | 0.46 | ||
| Phlogopite | 0.23 | 0.83 | 3.69 | 5.28 | 0.58 | 0.43 | ||||||
| Muscovite | 0.16 | 0.39 | 2.07 | 1.66 | 9.55 | 6.25 | ||||||
| Hornblende | 0.09 | 0.73 | 0.41 | 0.90 | 10.70 | 15.54 | 4.76 | 3.43 | 2.25 | 2.38 | ||
| Olivine | 1.47 | 1.53 | 1.56 | 1.44 | ||||||||
| Augite | 5.16 | 3.88 | ||||||||||
| Actinolite | 1.62 | 2.04 | 5.50 | 2.52 | 7.35 | 4.32 | ||||||
| Epidote | 1.33 | 1.27 | ||||||||||
| Anthophyllite | 2.17 | 2.21 | 1.63 | 2.42 | ||||||||
| Serpentine | 8.03 | 4.72 | 10.63 | 5.81 | ||||||||
| Cordierite | 0.51 | 0.43 | ||||||||||
| Cummingtonite/Enstatite | 0.47 | 0.48 | 0.74 | 0.70 | ||||||||
| Kaolinite | 1.22 | 1.03 | ||||||||||
| Carbonates | ||||||||||||
| Magnesite | 4.61 | 1.68 | ||||||||||
| Dolomite | 1.33 | 0.14 | ||||||||||
| Calcite | 0.96 | 0.02 | 0.03 | 0.03 | ||||||||
| Sulfides | ||||||||||||
| Pyrrhotite | 1.66 | 0.31 | 0.31 | 0.22 | 3.15 | 1.15 | 0.05 | 0.00 | 0.26 | 0.20 | 0.32 | 0.41 |
| Pyrite | 0.29 | 0.05 | 4.50 | 0.08 | 0.07 | 0.00 | ||||||
| Pentlandite | 0.27 | 0.00 | 0.13 | 0.00 | 0.07 | 0.00 | 0.04 | 0.00 | ||||
| Chalcopyrite | 0.11 | 0.00 | 0.07 | 0.00 | 0.22 | 0.00 | 0.25 | 0.00 | ||||
| Sphalerite | 0.04 | 0.00 | ||||||||||
| Oxidized Fe-sulfide | 0.00 | 0.26 | 0.00 | 0.14 | 0.30 | 0.49 | 0.00 | 0.06 | 0.12 | 0.21 | 0.93 | 0.31 |
| Other | 0.59 | 1.40 | 0.21 | 1.86 | 0.37 | 1.43 | 1.58 | 1.40 | 0.83 | 0.70 | 0.87 | 0.93 |
| Total | 45.00 | 31.56 | 45.00 | 43.98 | 45.01 | 41.94 | 45.00 | 43.57 | 45.01 | 42.60 | 45.01 | 41.82 |
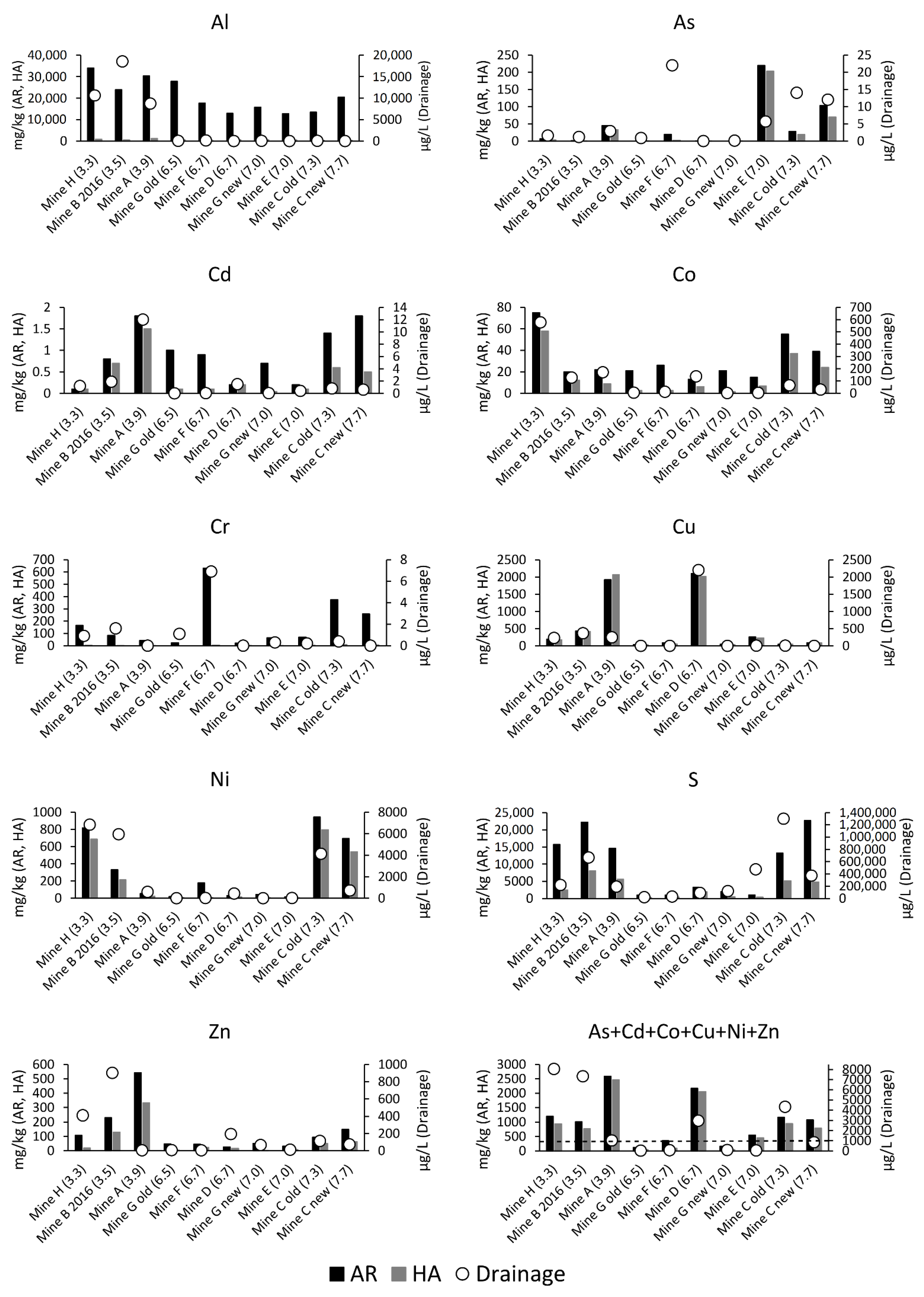
| Sample | Total S | Extraction Method | Al | As | Ca | Cd | Co | Cr | Cu | Fe | K | Mg | Na | Ni | S | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WR1 | 22,500 | AR1 | 19,150 | 95 | 10,300 | 0.9 | 38 | 240 | 92 | 57,100 | 11,650 | 46,350 | 453 | 666 | 22,300 | 145 |
| HA1 | 256 ± 26 | 76 ± 9 | 3943 ± 21 | 0.6 ± 0.03 | 24 ± 0.7 | 6.5 ± 0.6 | 88 ± 0.5 | 2882 ± 946 | 319 ± 20 | 2262 ± 176 | 56 ± 28 | 525 ± 31 | 3722 ± 1520 | 68 ± 0.3 | ||
| HA2 | 281 | 68 | 3960 | 0.5 | 25 | 6.8 | 87 | 3160 | 320 | 2480 | 54 | 535 | 4140 | 68 | ||
| AR2 after HA2 | 14,164 | 29 | 2756 | 0.1 | 6.3 | 175 | 1.6 | 35,024 | 8414 | 30,431 | 327 | 65 | 6510 | 36 | ||
| WR2 | 41,800 | AR1 | 30,700 | 60 | 28,000 | 6.6 | 29 | 80 | 242 | 67,000 | 15,700 | 24,200 | 1580 | 359 | 41,200 | 929 |
| HA1 | 859 ± 67 | 28 ± 1.9 | 20,033 ± 473 | 2.7 ± 0.4 | 13 ± 0.2 | 2.6 ± 0.3 | 239 ± 10 | 15,033 ± 153 | 896 ± 67 | 979 ± 25 | 122 ± 21 | 252 ± 2 | 16,133 ± 1358 | 642 ± 9 | ||
| HA2 | 790 | 31 | 3940 | 1.9 | 13 | 1.8 | 243 | 14,700 | 842 | 1000 | 111 | 246 | 15,600 | 666 | ||
| AR2 after HA2 | 31,370 | 21 | 7984 | 0.4 | 8.9 | 112 | 2.4 | 38,015 | 15,929 | 27,168 | 1515 | 66 | 7173 | 90 | ||
| WR3 | 53,100 | AR1 | 20,900 | 1.7 | 1870 | 1.0 | 47 | 48 | 1680 | 96,000 | 11,000 | 18,200 | 469 | 2130 | 47,500 | 289 |
| HA1 | 619 ± 129 | 1.0 ± 0.2 | 460 ± 79 | 0.9 ± 0.1 | 39 ± 0.8 | 1.3 ± 0.1 | 1640 ± 53 | 8500 ± 348 | 693 ± 19 | 486 ± 53 | 127 ± 21 | 1823 ± 32 | 10,947 ± 1724 | 215 ± 6 | ||
| HA2 | 818 | 1.1 | 406 | 0.9 | 40 | 1.4 | 1670 | 10,000 | 699 | 579 | 101 | 1880 | 14,300 | 222 | ||
| AR2 after HA2 | 18,919 | 1.3 | 1417 | 0.3 | 6.8 | 44 | 580 | 68,779 | 9786 | 16,589 | 399 | 240 | 23,019 | 67 | ||
| WR4 | 3800 | AR1 | 13,200 | 0.6 | 15,100 | 0.2 | 13.3 | 20 | 2280 | 17,700 | 1120 | 3500 | 1900 | 32 | 3690 | 30 |
| HA1 | 249 ± 16 | 0.2 ± 0.04 | 1343 ± 90 | 0.1 ± 0.01 | 5.2 ± 0.3 | <1 | 1863 ± 32 | 2090 ± 26 | 61 ± 5 | 111 ± 10 | 133 ± 21 | 9.4 ± 0.6 | 1607 ± 93 | 16 ± 1.1 | ||
| HA2 | 271 | <0.2 | 1290 | 0.1 | 5.0 | <1 | 1920 | 2120 | <50 | 127 | 118 | 9.8 | 1560 | 14 | ||
| AR2 after HA2 | 12,876 | 0.5 | 13,651 | <0.04 | 7 | 21 | 89 | 14,425 | 1036 | 3301 | 1762 | 19 | 958 | 15 | ||
| WR5a | 15,400 | AR1 | 8440 | 0.2 | 3210 | 0.1 | 124 | 296 | 2920 | 61,200 | 1810 | 59,000 | 465 | 2170 | 13,900 | 43 |
| HA1 | 353 ± 35 | 0.2 ± 0.08 | 590 ± 81 | 0.1 ± 0.01 | 102 ± 1 | 8.3 ± 0.6 | 2747 ± 25 | 6120 ± 845 | 131 ± 5 | 3533 ± 448 | 151 ± 12 | 2007 ± 23 | 4647 ± 722 | 29 ± 1.2 | ||
| HA2 | 443 | <0.2 | 530 | 0.1 | 107 | 10.3 | 2840 | 7630 | 129 | 5160 | 140 | 2030 | 6120 | 28 | ||
| AR2 after HA2 | 8178 | <0.08 | 2934 | <0.04 | 22 | 310 | 133 | 50,547 | 1666 | 51,967 | 377 | 277 | 6039 | 19 | ||
| WR5b | 21,000 | AR1 | 4990 | 0.2 | 3040 | 0.2 | 166 | 501 | 4600 | 76,500 | 881 | 60,400 | 417 | 3210 | 21,100 | 59 |
| HA1 | 240 ± 15 | 0.2 ± 0.05 | 662 ± 104 | 0.2 ± 0.02 | 154 ± 3 | 13.4 ± 0.7 | 4643 ± 84 | 9050 ± 1518 | 38.4 ± 23 | 4510 ± 520 | 197 ± 5 | 3213 ± 25 | 7803 ± 1065 | 45 ± 2 | ||
| HA2 | 284 | <0.2 | 562 | 0.2 | 158 | 15.4 | 4620 | 11,000 | 50.5 | 6180 | 194 | 3200 | 9950 | 46 | ||
| AR2 after HA2 | 4860 | 0.1 | 2723 | <0.04 | 20 | 523 | 122 | 63,560 | 840 | 52,595 | 279 | 281 | 8902 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsson, T.; Räisänen, M.L.; Myöhänen, T.; Alakangas, L.; Lehtonen, M.; Kauppila, P. Hydrogen Peroxide Ammonium Citrate Extraction: Mineral Decomposition and Preliminary Waste Rock Characterization. Minerals 2021, 11, 706. https://doi.org/10.3390/min11070706
Karlsson T, Räisänen ML, Myöhänen T, Alakangas L, Lehtonen M, Kauppila P. Hydrogen Peroxide Ammonium Citrate Extraction: Mineral Decomposition and Preliminary Waste Rock Characterization. Minerals. 2021; 11(7):706. https://doi.org/10.3390/min11070706
Chicago/Turabian StyleKarlsson, Teemu, Marja Liisa Räisänen, Timo Myöhänen, Lena Alakangas, Marja Lehtonen, and Päivi Kauppila. 2021. "Hydrogen Peroxide Ammonium Citrate Extraction: Mineral Decomposition and Preliminary Waste Rock Characterization" Minerals 11, no. 7: 706. https://doi.org/10.3390/min11070706
APA StyleKarlsson, T., Räisänen, M. L., Myöhänen, T., Alakangas, L., Lehtonen, M., & Kauppila, P. (2021). Hydrogen Peroxide Ammonium Citrate Extraction: Mineral Decomposition and Preliminary Waste Rock Characterization. Minerals, 11(7), 706. https://doi.org/10.3390/min11070706






