Rare and Critical Metals in Pyrite, Chalcopyrite, Magnetite, and Titanite from the Vathi Porphyry Cu-Au±Mo Deposit, Northern Greece
Abstract
1. Introduction
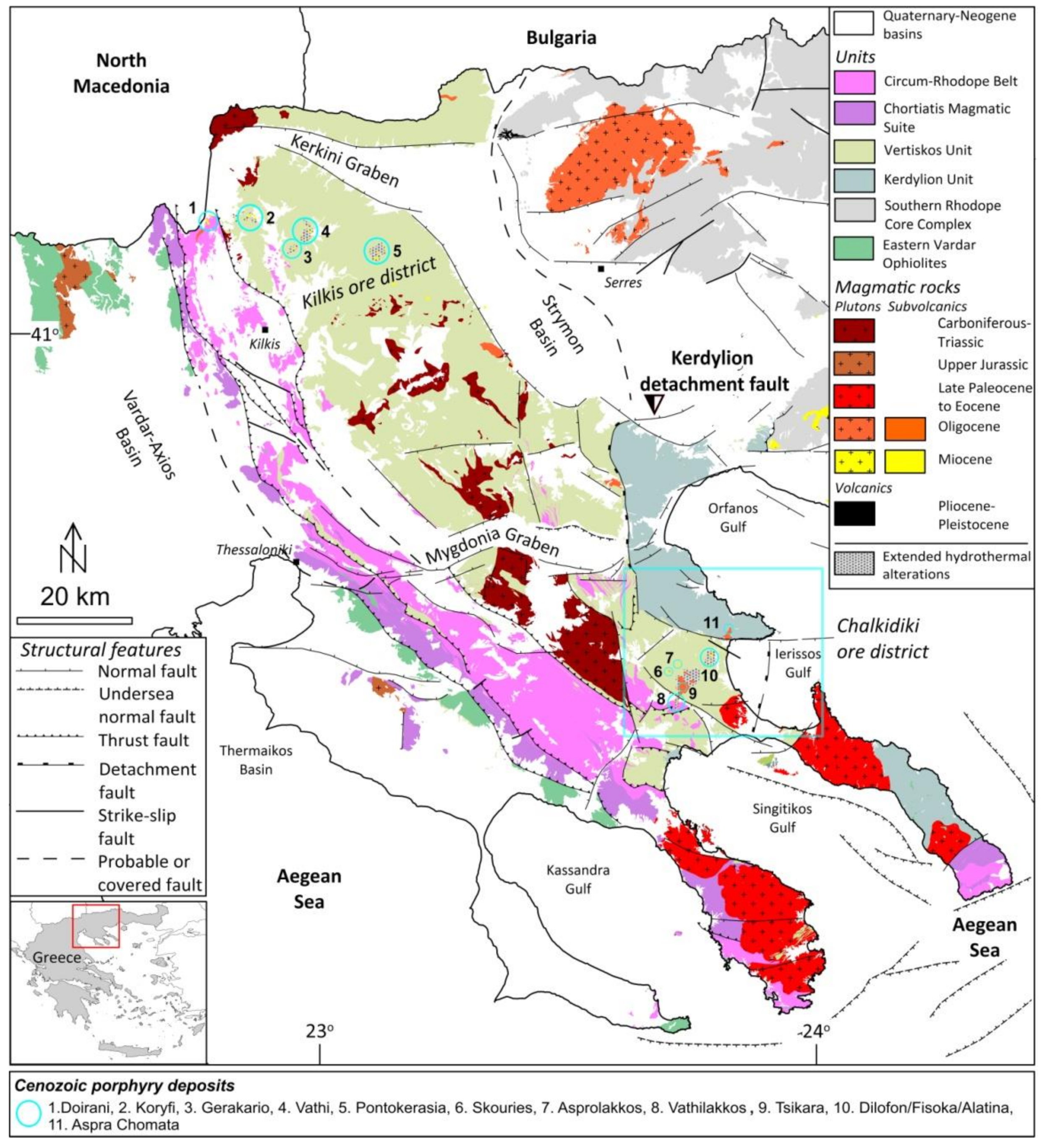
2. Geological Setting
2.1. Regional Geology
2.2. The Vathi Deposit
2.3. Mineralization Stages and Alteration Styles
3. Materials and Methods
4. Results
4.1. Bulk Geochemistry of Rare and Critical Metals in Metallic Mineralization
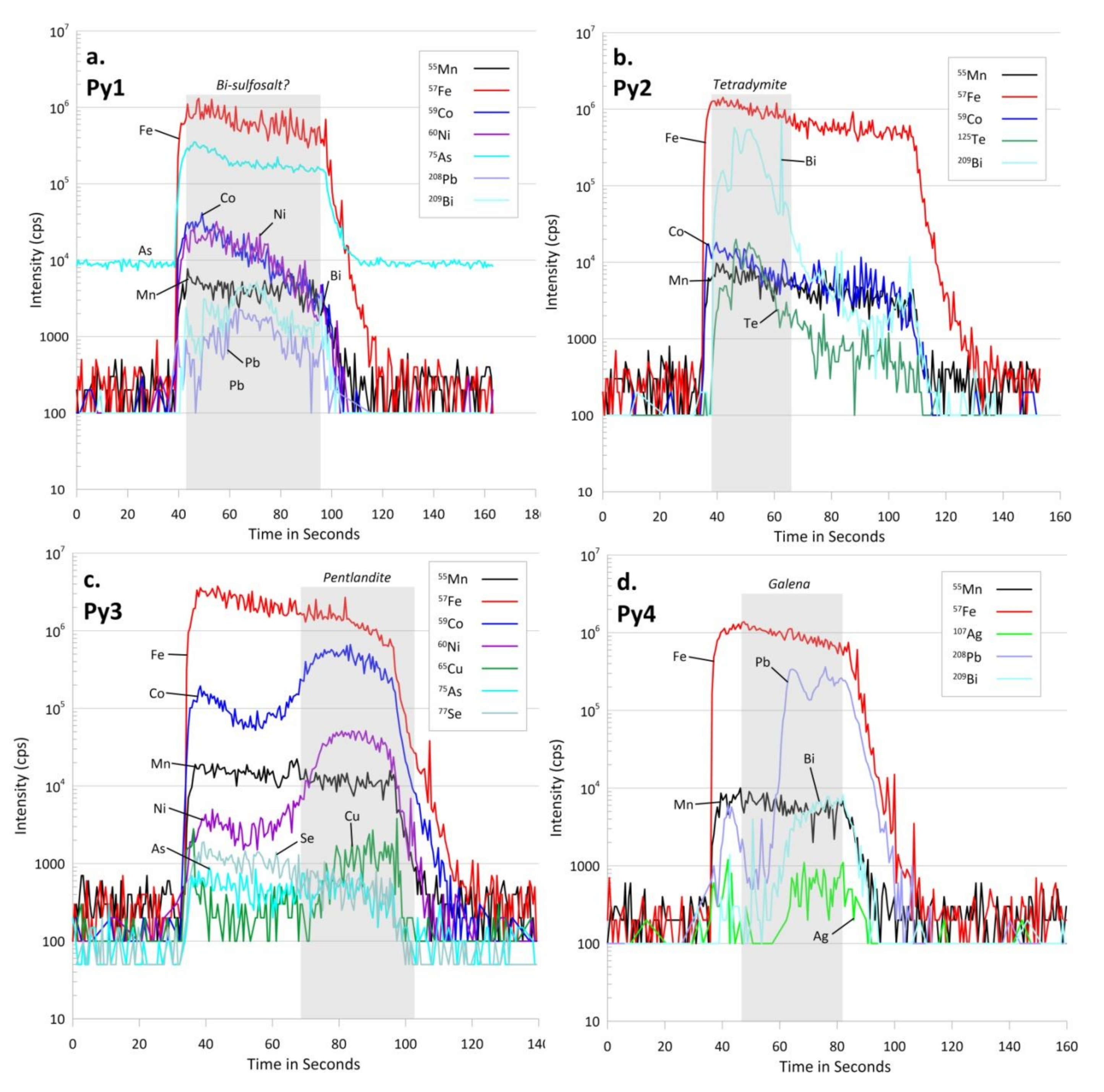
4.2. Pyrite and Chalcopyrite Mode of Occurrence
4.3. Magnetite and Titanite Mode of Occurrence
4.4. Mineral Chemistry
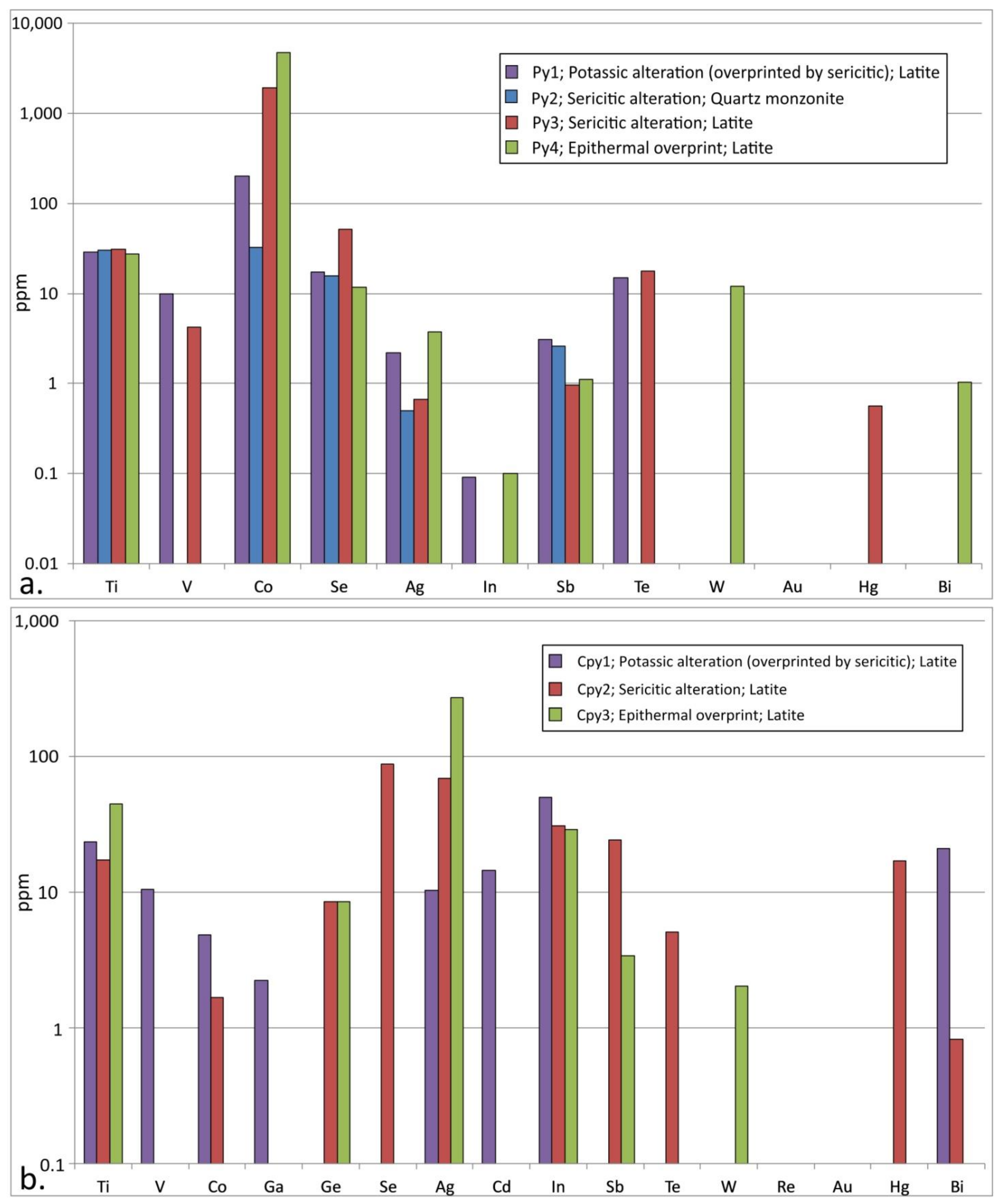
4.4.1. Trace Element Composition of Pyrite and Chalcopyrite
4.4.2. Trace Element Compositions of Magnetite and Titanite
4.4.3. Statistical Analysis of Trace Element Concentrations
5. Discussion
5.1. Hydrothermal Alterations, Mineralization Stages, and Bulk Geochemistry
5.2. Mineral Chemistry of Pyrite and Chalcopyrite and Nano-Scale Inclusions
5.3. Mineral Chemistry of Magnetite and Titanite and Nano-Scale Inclusions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verplanck, P.L.; Hitzman, M.W. Introduction: Rare Earth and Critical Elements in Ore Deposits. In Rare Earth and Critical Elements in Ore Deposits; Society of Economic Geologists Inc.: Littleton, CO, USA, 2016; pp. 1–4. [Google Scholar]
- Ayres, R.U.; Peiro, L.T. Material efficiency: Rare and critical metals. Philos. Trans. R. Soc. A 2013, 371, 20110563. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, G.; Cassard, D.; Arvanitidis, N.; Stanley, G. Map of critical raw material deposits in Europe. Energy Procedia 2016, 97, 44–50. [Google Scholar] [CrossRef]
- Watari, T.; Nansai, K.; Nakajima, K. Review of critical metal dynamics to 2050 for 48 elements. Resour. Conserv. Recycl. 2020, 155, 104669. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020; p. 24. [Google Scholar]
- Verplanck, P.L.; Mariano, A.N.; Mariano, A., Jr. Rare earth element ore geology of carbonatites. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists Inc.: Littleton, CO, USA, 2016; pp. 5–32. [Google Scholar]
- Dostal, J. Rare metal deposits associated with alkaline/peralkaline igneous rocks. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists Inc.: Littleton, CO, USA, 2016; pp. 33–54. [Google Scholar]
- Sengupta, D.; Van Gosen, B.S. Placer-type rare earth element deposits. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists Inc.: Littleton, CO, USA, 2016; pp. 81–100. [Google Scholar]
- John, D.A.; Taylor, R.D. By-products of porphyry copper and molybdenum deposits. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists Inc.: Littleton, CO, USA, 2016; pp. 137–164. [Google Scholar]
- Kelley, K.D.; Spry, P.G. Critical elements in alkaline igneous rock-related epithermal gold deposits. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists Inc.: Littleton, CO, USA, 2016; pp. 195–216. [Google Scholar]
- Goldfarb, R.J.; Hofstra, A.H.; Simmons, S.F. Critical elements in Carlin, epithermal, and orogenic gold deposits. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists Inc.: Littleton, CO, USA, 2016; pp. 217–244. [Google Scholar]
- Velásquez, G.; Carrizo, D.; Salvi, S.; Vela, I.; Pablo, M.; Pérez, A. Tracking cobalt, REE and gold from a porphyry-type deposit by LA-ICP-MS: A geological approach towards metal-selective mining in tailings. Minerals 2020, 10, 109. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Crowe, B.B.; Ciobanu, C.L. Trace elements in hydrothermal chalcopyrite. Mineral. Mag. 2018, 82, 59–88. [Google Scholar] [CrossRef]
- Nadoll, P.; Mauk, J.L.; Leveille, R.A.; Koenig, A.E. Geochemistry of magnetite from porphyry Cu and skarn deposits in the southwestern United States. Miner. Depos. 2014, 50, 493–515. [Google Scholar] [CrossRef]
- Huang, X.W.; Sappin, A.A.; Boutroy, É.; Beaudoin, G.; Makvandi, S. Trace element composition of igneous and hydrothermal magnetite from porphyry deposits: Relationship to deposit subtypes and magmatic affinity. Econ. Geol. 2019, 114, 917–952. [Google Scholar] [CrossRef]
- Xu, L.; Bi, X.; Hu, R.; Tang, Y.; Wang, X.; Xu, Y. LA-ICP-MS mineral chemistry of titanite and the geological implications for exploration of porphyry Cu deposits in the Jinshajiang—Red River alkaline igneous belt, SW China. Miner. Petrol. 2015, 109, 181–200. [Google Scholar] [CrossRef]
- Dmitrijeva, M.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Metcalfe, A.V.; Kamenetsky, M.; Kamenetsky, V.S.; Gilbert, S. Multivariate statistical analysis of trace elements in pyrite: Prediction, bias and artefacts in defining mineral signatures. Minerals 2020, 10, 61. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Doyle, K.; Holwell, D.A.; Jenkin, G.R.T.; Barry, T.L.; Becker, J.; Rampe, J. Pyrite chemistry: A new window into Au-Te ore-forming processes in alkaline epithermal districts, Cripple Creek, Colorado. Geochim. Cosmochim. Acta 2020, 274, 172–191. [Google Scholar] [CrossRef]
- Reich, M.; Román, N.; Barra, F.; Morata, D. Silver-rich chalcopyrite from the active Cerro Pabellón geothermal system, northern Chile. Minerals 2020, 10, 113. [Google Scholar] [CrossRef]
- Marfin, A.E.; Ivanov, A.V.; Abramova, V.D.; Anziferova, T.N.; Radomskaya, T.A.; Yakich, T.Y.; Bestemianova, K.V. A trace element classification tree for chalcopyrite from Oktyabrsk deposit, Norilsk–Talnakh Ore District, Russia: LA-ICPMS Study. Minerals 2020, 10, 716. [Google Scholar] [CrossRef]
- Mavrogonatos, C.; Voudouris, P.; Zaccarini, F.; Klemme, S.; Berndt, J.; Tarantola, A.; Melfos, V.; Spry, P.G. Multi-stage introduction of precious and critical metals in pyrite: A case study from the Konos Hill and Pagoni Rachi porphyry/epithermal prospects, NE Greece. Minerals 2020, 10, 784. [Google Scholar] [CrossRef]
- Mavrogonatos, C.; Voudouris, P.; Berndt, J.; Klemme, S.; Zaccarini, F.; Spry, P.G.; Melfos, V.; Tarantola, A.; Keith, M.; Klemd, R.; et al. Trace elements in magnetite from the Pagoni Rachi porphyry prospect, NE Greece: Implications for ore genesis and exploration. Minerals 2019, 9, 725. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of hydrothermal magnetite. Contrib. Mineral. Petrol. 2018, 173, 1–20. [Google Scholar] [CrossRef]
- Dimitrova, D.; Mladenova, V.; Hecht, L. Efflorescent sulfate crystallization on fractured and polished colloform pyrite surfaces: A migration pathway of trace elements. Minerals 2020, 10, 12. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Pudack, C.; Halter, W.E.; Heinrich, C.A.; Pettke, T. Evolution of magmatic vapor to gold-rich epithermal liquid: The porphyry to epithermal transition at Nevados de Famatina, northwest Argentina. Econ. Geol. 2009, 104, 449–477. [Google Scholar] [CrossRef]
- Canil, D.; Grondahl, C.; Lacourse, T.; Pisiak, L.K. Trace elements in magnetite from porphyry Cu–Mo–Au deposits in British Columbia, Canada. Ore Geol. Rev. 2016, 72, 1116–1128. [Google Scholar] [CrossRef]
- Arancibia, O.N.; Clark, A.H. Early magnetite-amphibole-plagioclase alteration mineralization in the Island Copper porphyry copper-gold-molybdenum deposit, British Columbia. Econ. Geol. 1996, 91, 402–438. [Google Scholar] [CrossRef]
- Tiepolo, M.; Oberti, R.; Vannucci, R. Trace-element incorporation in titanite: Constraints from experimentally determined solid/liquid partition coefficients. Chem. Geol. 2002, 191, 105–119. [Google Scholar] [CrossRef]
- Cao, M.J.; Qin, K.Z.; Li, Q.M.; Evans, N.J.; Jin, L.Y. In situ LA-(MC)-ICP-MS trace element and Nd isotopic compositions and genesis of polygenetic titanite from the Baogutu reduced porphyry Cu deposit, Western Junggar, NW China. Ore Geol. Rev. 2015, 65, 940–954. [Google Scholar] [CrossRef]
- Melfos, V.; Voudouris, P.; Melfou, M.; Sánchez, M.G.; Papadopoulou, L.; Filippidis, A.; Spry, P.G.; Schaarschmidt, A.; Klemd, R.; Haase, K.M.; et al. Mineralogical constraints on the potassic and sodic-calcic hydrothermal alteration and vein-type mineralization of the Maronia porphyry Cu-Mo ± Re ± Au deposit in NE Greece. Minerals 2020, 10, 182. [Google Scholar] [CrossRef]
- Ross, J.; Voudouris, P.; Melfos, V.; Vaxevanopoulos, M. Mines, Metals and money: Ancient world studies in science, archaeology and history. In Metallurgy in Numismatics; Sheedy, K.A., Davis, G., Eds.; Royal Numismatic Society Special Publication: London, UK, 2018; Volume 6, pp. 9–21. [Google Scholar]
- Melfos, V.; Voudouris, P.C. Geological, mineralogical and geochemical aspects for critical and rare metals in Greece. Minerals 2012, 2, 300–317. [Google Scholar] [CrossRef]
- Eldorado Gold Corporation 2020, Resources and Reserves. Available online: https://www.eldoradogold.com/assets/resources-and-reserves/default.aspx (accessed on 8 December 2020).
- Melfos, V.; Voudouris, P. Cenozoic metallogeny of Greece and potential for precious, critical and rare metals exploration. Ore Geol. Rev. 2017, 89, 1030–1057. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Spry, P.G.; Baker, T.; Melfos, V.; Klemd, R.; Haase, K.; Repstock, A.; Djiba, A.; Bismayer, U.; et al. Porphyry and epithermal deposits in Greece: An overview, new discoveries, and mineralogical constraints on their genesis. Ore Geol. Rev. 2019, 107, 654–691. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Baker, T. Gold±copper endowment and deposit diversity in the Western Tethyan magmatic belt, southeast Europe: Implications for exploration. Econ. Geol. 2019, 114, 1237–1250. [Google Scholar] [CrossRef]
- Cassard, D.; Bertrand, G.; Billa, M.; Serrano, J.J.; Tourlière, B.; Angel, J.M.; Gaál, G. ProMine mineral databases: New tools to assess primary and secondary mineral resources in Europe. In 3D, 4D and Predictive Modelling of Major Mineral Belts in Europe. Mineral Resource Reviews; Weihed, P., Ed.; Springer: Cham, Switzerland, 2015; pp. 9–58. [Google Scholar] [CrossRef]
- Stergiou, C.L.; Melfos, V.; Voudouris, P. A review on the critical and rare metals distribution throughout the Vertiskos Unit, N. Greece. In Proceedings of the 1st International Electronic Conference on Mineral Science at Sciforum, Online. 16–31 July 2018; MDPI: Basel, Switzerland. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Tzifas, I.T.; Tsikos, H. The potential for REE and associated critical metals in coastal sand (placer) deposits of Greece: A review. Minerals 2019, 9, 469. [Google Scholar] [CrossRef]
- Stouraiti, C.; Angelatou, V.; Petushok, S.; Soukis, K.; Eliopoulos, D. Effect of Mineralogy on the Beneficiation of REE from Heavy Mineral Sands: The Case of Nea Peramos, Kavala, Northern Greece. Minerals 2020, 10, 387. [Google Scholar] [CrossRef]
- Eliopoulos, D.G.; Economou-Eliopoulos, M.; Zelyaskova-Panayiotova, M. Critical factors controlling Pd and Pt potential in porphyry Cu–Au deposits: Evidence from the Balkan Peninsula. Geosci. J. 2014, 4, 31–49. [Google Scholar] [CrossRef]
- Fornadel, A.P.; Spry, P.G.; Melfos, V.; Vavelidis, M.; Voudouris, P. Is the Palea Kavala Bi-Te-Pb-Sb±Au district, northeastern Greece, a reduced intrusionrelated system? Ore Geol. Rev. 2011, 39, 119–133. [Google Scholar] [CrossRef]
- Voudouris, P. Comparative mineralogical study of Tertiary Te-rich epithermal and porphyry systems in northeastern Greece. Miner. Petrol. 2006, 87, 24–275. [Google Scholar] [CrossRef]
- Voudouris, P.C.; Spry, P.G.; Mavrogonatos, C.; Sakellaris, G.A.; Bristol, S.K.; Melfos, V.; Fornadel, A.P. Bismuthinite derivatives, lillianite homologues and bismuth sulfotellurides as indicators of gold mineralization in the Stanos shear-zone related deposit, Chalkidiki, Northern Greece. Canad. Miner. 2013, 51, 119–142. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Kartal, T.; Schleicher, H.; Moritz, R.; Ortelli, M. The Pagoni Rachi/Kirki Cu-Mo-Re-Au-Ag-Te deposit, northern Greece: Mineralogical and fluid inclusion constraints on the evolution of a telescoped porphyry-epithermal system. Canad. Miner. 2013, 51, 411–442. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Bindi, L.; Moritz, R.; Ortelli, M.; Kartal, T. Extremely Re-rich molybdenite from porphyry Cu-Mo-Au prospects in northeastern Greece: Mode of occurrence, causes of enrichment, and implications for gold exploration. Minerals 2013, 3, 165–191. [Google Scholar] [CrossRef]
- Filippidis, A.; Kougoulis, C.; Michailidis, K. Sr-bearing stilbite in a quartz monzonite from Vathi, Kilkis, Northern Greece. Schweiz. Mineral. Petrogr. Mitt. 1988, 68, 67–76. [Google Scholar] [CrossRef]
- Frei, R. Isotope (Pb, Rb-Sr,S,O,C,U-Pb) Geochemical Investigations on Tertiary Intrusives and Related Mineralizations in the Serbomacedonian Pb-Zn, Sb+Cu-Mo Metallogenic Province in Northern Greece. Ph.D. Thesis, Swiss Federal Institute of Technology (ETH), Zurich, Switzerland, 1992. [Google Scholar]
- Stergiou, C.L.; Melfos, V.; Voudouris, P.; Spry, P.G.; Papadopoulou, L.; Chatzipetros, A.; Giouri, K.; Mavrogonatos, C.; Filippidis, A. The geology, geochemistry, and origin of the porphyry Cu-Au-(Mo) system at Vathi, Serbo-Macedonian Massif, Greece. Appl. Sci. 2021, 11, 479. [Google Scholar] [CrossRef]
- Kelepertsis, A.E.; Reeves, R.; Andrulakis, J. Geochemical studies of porphyry type mineralization at Gerakario-Vathi of Kilkis area, northern Greece. Mineral Wealth 1986, 42, 43–48. [Google Scholar]
- Kydonakis, K.; Brun, J.P.; Poujol, M.; Monié, P.; Chatzitheodoridis, E. Inferences on the Mesozoic evolution of the North Aegean from the isotopic record of the Chalkidiki blocks. Tectonophysics 2016, 682, 65–84. [Google Scholar] [CrossRef]
- Siron, C.R.; Rhys, D.; Thompson, J.F.; Baker, T.; Veligrakis, T.; Camacho, A.; Dalampiras, L. Structural controls on porphyry Au-Cu and Au-rich polymetallic carbonate-hosted replacement deposits of the Kassandra mining district, Northern Greece. Econ. Geol. 2018, 113, 309–345. [Google Scholar] [CrossRef]
- Schmid, S.M.; Fügenschuh, B.; Kounov, A.; Matenco, L.; Nievergelt, P.; Oberhänsli, R.; Pleuger, J.; Schefer, S.; Schuster, R.; Tomljenović, B.; et al. Tectonic units of the Alpine collision zone between Eastern Alps and western Turkey. Gondwana Res. 2020, 78, 308–374. [Google Scholar] [CrossRef]
- Kilias, A.; Falalakis, G.; Mountrakis, D. Cretaceous-Tertiary structures and kinematics of the Serbomacedonian metamorphic rocks and their relation to the exhumation of the Hellenic hinterland (Macedonia, Greece). Int. J. Earth Sci. 1999, 88, 513–531. [Google Scholar] [CrossRef]
- Mposkos, E.; Krohe, A.; Baziotis, I. Deep tectonics in the Eastern Hellenides uncovered: The record of Variscan continental amalgamation, Permo-Triassic rifting, and Early Alpine collision in Pre-Variscan continental crust in the W-Rhodope (Vertiscos-Ograzden Complex, NGreece). Tectonics 2021, 40, e2019TC005557. [Google Scholar] [CrossRef]
- Brun, J.P.; Sokoutis, D. Core complex segmentation in North Aegean, a dynamic view. Tectonics 2018, 37, 1797–1830. [Google Scholar] [CrossRef]
- Tranos, M.D.; Kilias, A.A.; Mountrakis, D.M. Geometry and kinematics of the Tertiary post-metamorphic Circum Rhodope Belt Thrust System (CRBTS) Northern Greece. Bull. Geol. Soc. Greece 1999, 33, 5–16. [Google Scholar]
- Abbo, A.; Avigad, D.; Gerdes, A. Crustal evolution of peri-Gondwana crust into present day Europe: The Serbo-Macedonian and Rhodope massifs as a case study. Lithos 2019, 356, 105295. [Google Scholar] [CrossRef]
- Tsirambides, A.; Filippidis, A. Gold metallogeny of the Serbomacedonian-Rhodope metallogenic belt (SRMB). Bull. Geol. Soc. Greece 2016, 50, 2037–2046. [Google Scholar] [CrossRef]
- Guillong, M.; Meier, D.L.; Allan, M.M.; Heinrich, C.A.; Yardley, B.W.D. Appendix A6: SILLS: A MATLAB-based program for the reduction of laser ablation ICP-MS data of homogeneous materials and inclusions. In Laser Ablation ICP–MS in the Earth Sciences: Current Practices and Outstanding Issues; Sylvester, P., Ed.; Mineralogical Association of Canada Short Course 40: Vancouver, BC, Canada, 2008; pp. 328–333. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In The Crust, 1st ed.; Rudnick, R.L., Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–64. [Google Scholar]
- Voudouris, P.; Mavrogonatos, C.; Melfos, V.; Spry, P.G.; Magganas, A.; Alfieris, D.; Soukis, K.; Tarantola, A.; Periferakis, A.; Kołodziejczyk, J.; et al. The geology and mineralogy of the Stypsi porphyry Cu-Mo-Au-Re prospect, Lesvos Island, Aegean Sea, Greece. Ore Geol. Rev. 2019, 112, 103023. [Google Scholar] [CrossRef]
- Voudouris, P.; Spry, P.G.; Melfos, V.; Alfieris, D. Tellurides and bismuth sulfosalts in gold occurrences of Greece: Mineralogical and genetic considerations. In Gold Deposits in Finland; Kojonen, K.K., Cook, N.J., Ojala, V.J., Eds.; Geological Survey of Finland: Espoo, Finland, 2007; Volume 53, pp. 85–94. [Google Scholar]


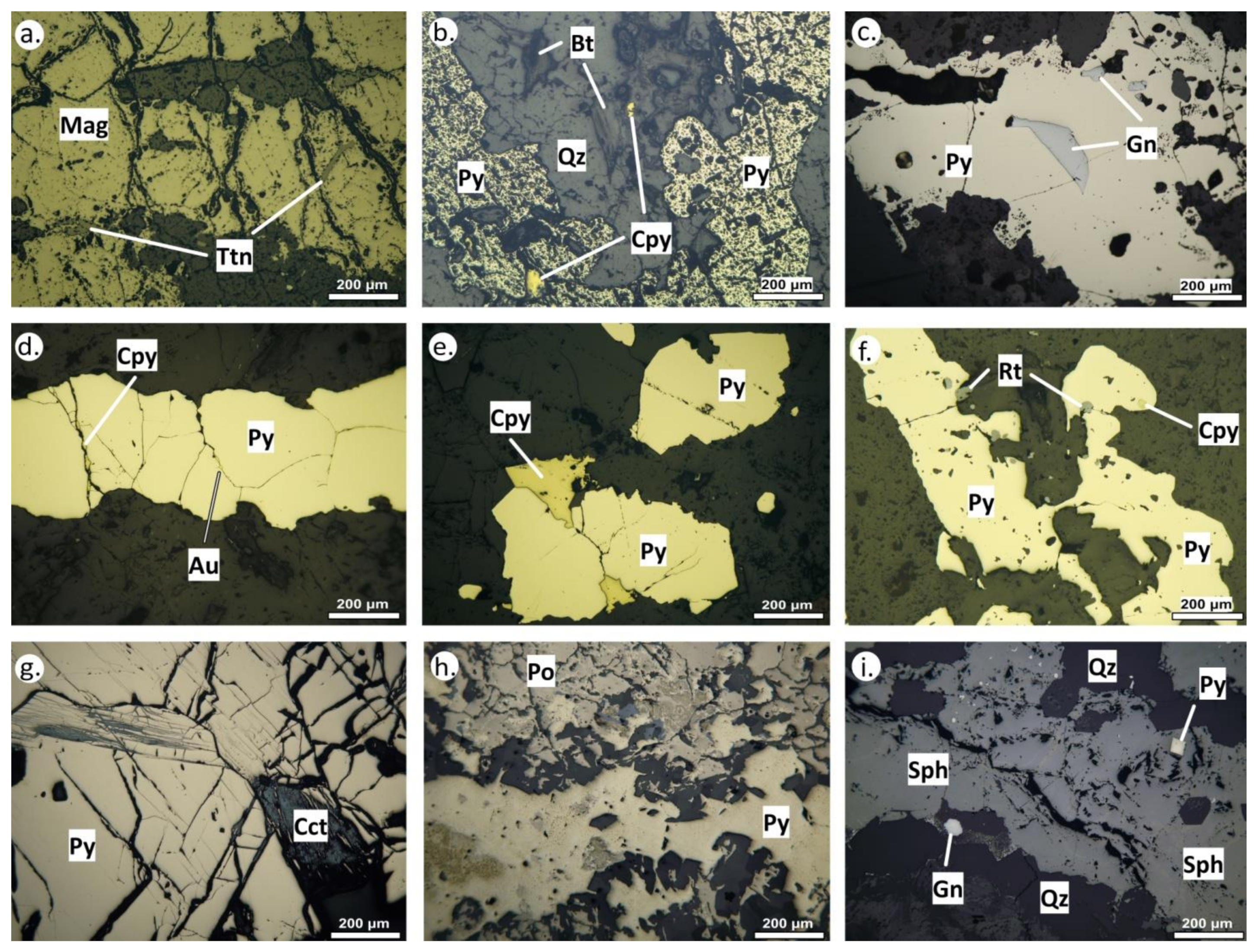


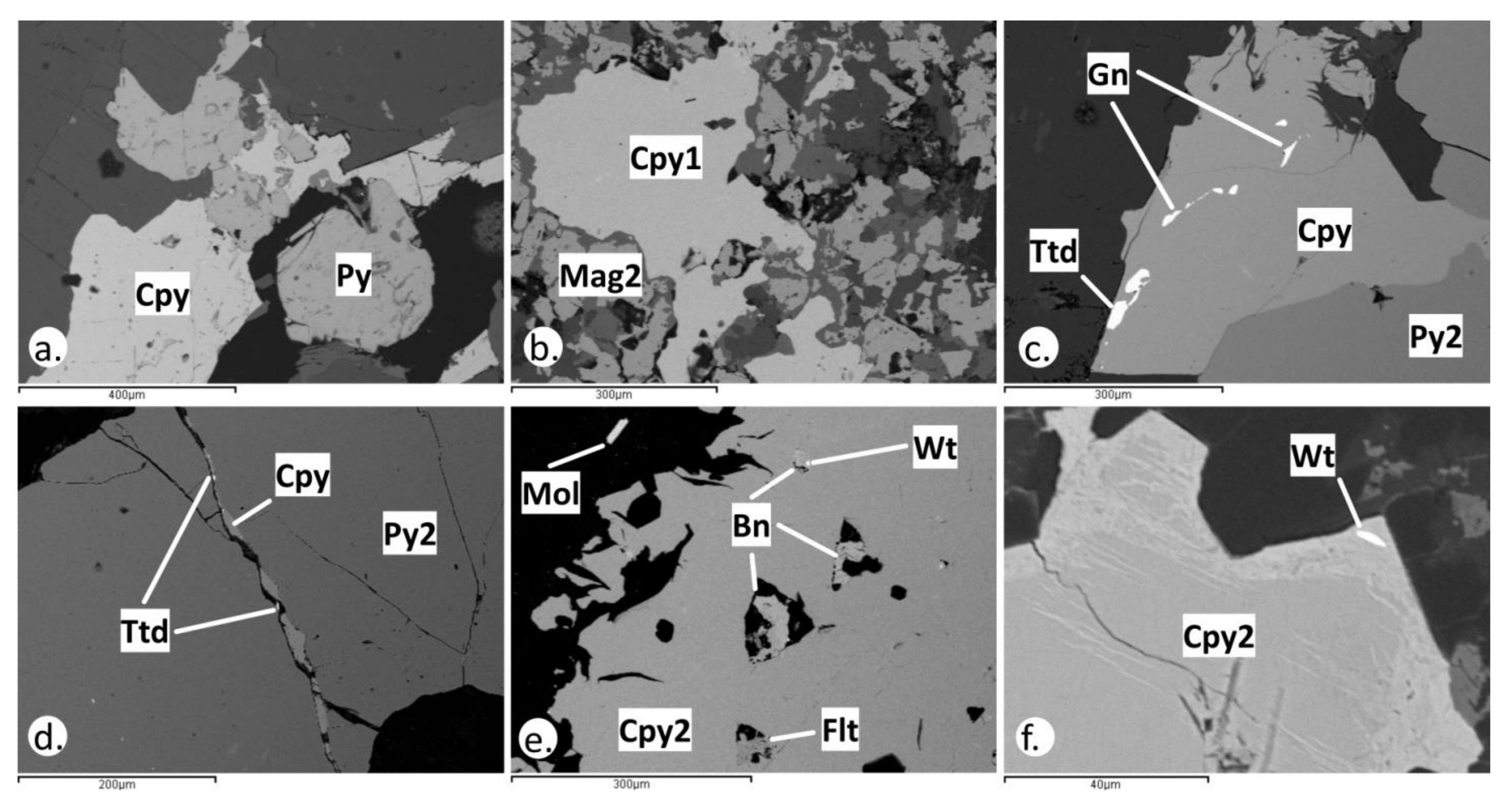



| Alteration Style | Host Rock | Mineralization Stage | Metallic Assemblage | Alteration Assemblage |
|---|---|---|---|---|
| Potassic-calcic | Quartz monzonite | M-type veins | Mag ± Ilm | Qz + Bt + Ttn+ Act + Rt ± Chl |
| Disseminated | ||||
| Potassic (overprinted by sericitic) | Quartz monzonite | A-type veins | Py + Cpy + Au ± Bn ± Gn | Qz + Bt + Kfs+ Chl + Ser ± Rt |
| Latite | Disseminated | Mag + Py + Cpy ±Bn ± Mol ± Po | ||
| Sericitic | Quartz monzonite | D-type veins | Py + Cpy + Gn + Au + Ttd | Ser + Qz + Rt± Dol ± Kln |
| Latite | D-type veins | Py ± Cpy | ||
| Disseminated | Py + Cpy + Gn + Sph + Au ± Bn ± Mol ± Po ± Pn ± Tnt ± Ttr ± Wt ± Flt ± Cup ± Sch | |||
| Propylitic | Quartz monzonite | Disseminated | Py | Chl + Qz ± Cal ± Ep |
| Latite | Py ± Cpy | |||
| Epithermal | Latite | E-type veins | Assemblage 1: Sph + Gn + Apy + Py + Cpy + Ttr ± Tnt ± Stb | Qz + Prl |
| Assemblage 2:Py + Po + Cpy ± Gn |
| Latite | Quartz Monzonite | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Propylitic alteration | Sericitic Alteration | Rel. Fresh | Potassic-Calcic alt. | Sericitic Alteration | ||||||||
| Det. limit | Vath 42 | AVG (n = 3) | Vath 34 | Vath 38 | Vath40 | Vath 45 | AVG (n = 16) | Vath 41 | Vath 44 | Vath 43 | AVG (n = 3) | |
| ppm | ||||||||||||
| Ag | 0.01 | 0.45 | 0.58 | 0.32 | 0.48 | 0.10 | 0.04 | 1.1 | 0.12 | 0.05 | 0.89 | 0.42 |
| Au | 0.00005 | 0.04 | 0.02 | 0.07 | 0.22 | 0.01 | 0.004 | 0.13 | 0.04 | 0.01 | 0.77 | 0.27 |
| Bi | 0.01 | 4.6 | 6.5 | 14.1 | 0.84 | 1.4 | 1.1 | 21 | 0.49 | 0.34 | 3.4 | 2.9 |
| Ce | 0.02 | 123 | 102 | 121 | 92 | 94 | 31 | 97 | 153 | 62 | 68 | 92 |
| Co | 0.1 | 38 | 13 | 17 | 18 | 48 | 450 | 35 | 23 | 13 | 30 | 45 |
| Ga | 0.05 | 9.4 | 16 | 2.2 | 1.7 | 1.9 | 5.1 | 15 | 6 | 7.7 | 8.5 | 15 |
| Gd | 0.05 | 6.6 | 5 | 4.5 | 4 | 4 | 2.3 | 5.1 | 7.8 | 4.1 | 3.9 | 4.7 |
| Ge | 0.05 | 0.24 | 0.24 | 0.13 | 0.11 | 0.11 | 0.09 | 0.11 | 0.18 | 0.20 | 0.19 | 0.19 |
| In | 0.005 | 0.01 | 0.11 | 0.08 | 0.08 | 0.05 | b.d.l. | 0.28 | 0.01 | 0.02 | 0.12 | 0.27 |
| La | 0.2 | 68 | 54 | 64 | 42 | 52 | 18 | 47 | 83 | 27 | 35 | 45 |
| Nb | 0.05 | 0.65 | 8.3 | 0.22 | 0.11 | 0.10 | b.d.l. | 8.6 | 1.2 | 0.6 | 1.2 | 5.3 |
| Nd | 0.1 | 46 | 38 | 47 | 35 | 33 | 12 | 40 | 85 | 27 | 28 | 39 |
| Se | 0.2 | 1.2 | 1 | b.d.l. | 3.8 | b.d.l. | 7.9 | 3.8 | 1.2 | b.d.l. | 2.8 | 1.8 |
| Sm | 0.03 | 8.1 | 6.5 | 7.7 | 5.7 | 5.4 | 2.4 | 6.9 | 10 | 5 | 4.9 | 6.8 |
| Ta | 0.01 | 1.4 | 1.1 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.84 | 1.8 | b.d.l. | b.d.l. | 0.55 |
| Te | 0.01 | 0.49 | 0.3 | 0.01 | b.d.l. | 0.27 | 0.06 | 0.17 | 0.49 | 0.03 | 1.5 | 0.73 |
| Th | 0.2 | 44 | 45 | 39 | 40 | 36 | 24 | 38 | 68 | 42 | 47 | 46 |
| U | 0.05 | 8.3 | 9.2 | 6.1 | 15 | 21 | 6.2 | 20 | 16 | 4.6 | 20 | 19 |
| W | 0.05 | 117 | 49 | 197 | 136 | 122 | 95 | 59 | 181 | 75 | 156 | 80 |
| Alteration | Potassic (Overprinted by Sericitic) | Sericitic | Epithermal Overprint | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Host rock | Latite | Quartz monzonite | Latite | |||||||||||||
| Mineralization stage | Disseminated (n = 9); Py1 | D-type veins (n = 9); Py2 | Disseminated (n = 19); Py3 | E-type veins; assemblage 2 (n = 7); Py4 | ||||||||||||
| Element | MIN | MAX | ST DEV | AVG | MIN | MAX | ST DEV | AVG | MIN | MAX | ST DEV | AVG | MIN | MAX | ST DEV | AVG |
| Ti | 20.3 | 39 | 6.7 | 29 | 26 | 34 | 2.8 | 30 | 20 | 57 | 2.6 | 31 | 21 | 41 | 7 | 28 |
| V | 9.9 | 9.9 | n.a. | 9.9 | b.d.l. | b.d.l. | n.a. | n.a. | 4.2 | 4.2 | n.a. | 4.2 | b.d.l. | b.d.l. | n.a. | n.a. |
| Cr | 27 | 47 | 7.9 | 38 | 36 | 46 | 4 | 41 | 31 | 54 | 3.8 | 42 | 30 | 42 | 3.9 | 35 |
| Mn | 48 | 91 | 16 | 61 | 53 | 59 | 2.3 | 56 | 48 | 76 | 2.7 | 55 | 48 | 53 | 2.5 | 50 |
| Co | 17 | 1341 | 409 | 201 | 11 | 93 | 33 | 33 | 0.73 | 8700 | 1391 | 1931 | 2.3 | 17,106 | 7954 | 4736 |
| Ni | 8.5 | 9448 | 3059 | 1398 | 4.1 | 59 | 22 | 20 | 2.3 | 5406 | 1411 | 602 | 6.2 | 2153 | 778 | 890 |
| Cu | b.d.l. | b.d.l. | n.a. | n.a. | 12 | 16 | 1.5 | 14 | 4.3 | 425 | 139 | 65 | 59 | 59 | n.a. | 59 |
| Zn | 4.9 | 8.3 | 1.4 | 6.6 | 6 | 6.8 | 0.56 | 6.4 | 3.1 | 9.9 | 0.24 | 6.4 | 3.7 | 11 | 4.3 | 6.4 |
| As | 339 | 7117 | 2166 | 3851 | 9.7 | 9.8 | 0.07 | 9.7 | 1.9 | 469 | 98 | 63 | 4.1 | 29 | 9.5 | 9.4 |
| Se | 11 | 26 | 4.4 | 18 | 14 | 19 | 2.6 | 16 | 14 | 200 | 27 | 51 | 5.8 | 18 | 4.9 | 12 |
| Mo | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 2 | 2 | n.a. | 2 | b.d.l. | b.d.l. | n.a. | n.a. |
| Ag | 2.2 | 2.2 | n.a. | 2.2 | 0.34 | 0.65 | 0.22 | 0.50 | 0.34 | 1.3 | 0.27 | 0.67 | 0.84 | 6.6 | 4.1 | 3.7 |
| In | 0.09 | 0.09 | n.a. | 0.09 | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 0.1 | 0.1 | n.a. | 0.1 |
| Sn | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 0.46 | 0.46 | n.a. | 0.46 | b.d.l. | b.d.l. | n.a. | n.a. |
| Sb | 0.34 | 10 | 4.8 | 3.1 | 2.1 | 3.23 | 0.43 | 2.6 | 0.45 | 1.6 | 0.02 | 0.96 | 0.96 | 1.2 | 0.2 | 1.1 |
| Te | 5.3 | 31 | 12 | 15 | b.d.l. | b.d.l. | n.a. | n.a. | 3 | 41 | n.a. | 18 | b.d.l. | b.d.l. | n.a. | n.a. |
| W | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 4.4 | 27 | 11 | 12 |
| Au | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Hg | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 0.46 | 0.67 | n.a. | 0.56 | b.d.l. | b.d.l. | n.a. | n.a. |
| Tl | 0.62 | 0.67 | 0.03 | 0.64 | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 1.2 | 1.2 | n.a. | 1.2 |
| Pb | b.d.l. | b.d.l. | n.a. | n.a. | 0.54 | 4.3 | 1.9 | 2.2 | b.d.l. | b.d.l. | n.a. | n.a. | 7 | 7 | n.a. | 7 |
| Bi | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 1 | 1 | n.a. | 1 |
| Alteration | Potassic (Overprinted by Sericitic) | Sericitic | Epithermal Overprint | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Host rock | Latite | |||||||||||
| Mineralization stage | Cpy 1; disseminated (n = 10) | Cpy2; disseminated (n = 12) | Cpy3; E-type veins; assemblage 2 (n = 2) | |||||||||
| Element | MIN | MAX | STDEV | AVG | MIN | MAX | STDEV | AVG | MIN | MAX | STDEV | AVG |
| Ti | 11 | 41 | 9.6 | 24 | 12 | 31 | 6.1 | 17 | 45 | 45 | n.a. | 45 |
| V | 11 | 11 | n.a. | 11 | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Cr | 20 | 38 | 6.7 | 27 | 19 | 55 | 8.8 | 29 | b.d.l. | b.d.l. | n.a. | n.a. |
| Mn | 33 | 40 | 2.8 | 36 | 30 | 42 | 1.9 | 33 | 31 | 31 | n.a. | 31 |
| Co | 4.8 | 4.9 | 0.05 | 4.9 | 0.58 | 5.3 | 2.1 | 1.7 | b.d.l. | b.d.l. | n.a. | n.a. |
| Ni | 5.1 | 6.2 | 0.8 | 5.7 | 4.1 | 7.5 | 2 | 5.3 | b.d.l. | b.d.l. | n.a. | n.a. |
| Zn | 137 | 2392 | 925 | 790 | 19 | 55 | 2.1 | 34 | 287 | 571 | 201 | 429 |
| Ga | 2.3 | 2.3 | n.a. | 2.3 | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Ge | b.d.l. | b.d.l. | n.a. | n.a. | 4.9 | 12 | 5.1 | 8.5 | 9 | 9 | n.a. | 9 |
| As | 56 | 83 | 8.9 | 67 | 4.8 | 25 | 5.6 | 12 | b.d.l. | b.d.l. | n.a. | n.a. |
| Se | b.d.l. | b.d.l. | n.a. | n.a. | 46 | 161 | 13 | 73 | b.d.l. | b.d.l. | n.a. | n.a. |
| Mo | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Ag | 6.8 | 17 | 3.4 | 10 | 8 | 519 | 103 | 47 | 134 | 407 | 193 | 271 |
| Cd | 4.4 | 45 | 15 | 14 | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| In | 32 | 62 | 11 | 50 | 3.1 | 57 | 8.1 | 25 | 1 | 57 | 39 | 29 |
| Sn | 1.8 | 9 | 2.9 | 5.3 | 18 | 144 | 9 | 119 | 48 | 91 | 31 | 70 |
| Sb | b.d.l. | b.d.l. | n.a. | n.a. | 1.1 | 48 | 26 | 24 | 3.4 | 3.4 | n.a. | 3.4 |
| Te | b.d.l. | b.d.l. | n.a. | n.a. | 3.9 | 6.8 | 1.7 | 5.1 | b.d.l. | b.d.l. | n.a. | n.a. |
| W | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 2 | 2 | n.a. | 2 |
| Au | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Hg | b.d.l. | b.d.l. | n.a. | n.a. | 0.83 | 50 | 28 | 17 | b.d.l. | b.d.l. | n.a. | n.a. |
| Tl | 0.42 | 0.42 | n.a. | 0.42 | 38 | 38 | n.a. | 38 | b.d.l. | b.d.l. | n.a. | n.a. |
| Pb | 5.4 | 14 | 6.1 | 9.7 | 4 | 4 | n.a. | 4 | b.d.l. | b.d.l. | n.a. | n.a. |
| Bi | 3 | 40 | 13 | 21 | 2.1 | 2.1 | n.a. | 2.1 | b.d.l. | b.d.l. | n.a. | n.a. |
| Mineral | Magnetite | Titanite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alteration | Potassic-calcic | Potassic (overprinted by sericitic) | Potassic-calcic | |||||||||
| Rock host | Quartz monzonite | Latite | Quartz monzonite | |||||||||
| Mineralization stage | Mag1; M-type veins (n = 7) | Mag2; disseminated (n = 9) | Ttn1; M-type veins (n = 5) | |||||||||
| Element | MIN | MAX | STDEV | AVG | MIN | MAX | STDEV | AVG | MIN | MAX | STDEV | AVG |
| Al | 1062 | 3161 | 873 | 1966 | 943 | 4076 | 1576 | 2416 | 4505 | 5002 | 209 | 4741 |
| P | 125 | 364 | 169 | 245 | b.d.l. | b.d.l. | n.a. | n.a. | 365 | 984 | 299 | 617 |
| Ti | 1503 | 1784 | 154 | 1607 | 1074 | 2468 | 542 | 1718 | 189,900 | 203,100 | 4731 | 195,660 |
| V | 3531 | 4176 | 219 | 3763 | 1831 | 2832 | 327 | 2381 | 1164 | 2620 | 568 | 2136 |
| Cr | 39 | 282 | 96 | 112 | 63 | 2043 | 738 | 372 | b.d.l. | b.d.l. | n.a. | n.a. |
| Mn | 564 | 734 | 69 | 644 | 467 | 786 | 118 | 660 | 311 | 713 | 156 | 498 |
| Co | 9.8 | 14 | 1.6 | 12 | 5 | 13 | 3.1 | 8.4 | 4 | 4 | n.a. | 4 |
| Ni | 382 | 814 | 173 | 594 | 11 | 11 | n.a. | 11 | b.d.l. | b.d.l. | n.a. | n.a. |
| Cu | 36 | 36 | n.a. | 36 | 13 | 23 | 5.2 | 19 | 47 | 68 | 12 | 57 |
| Zn | 37 | 59 | 7.9 | 51 | 59 | 137 | 33 | 92 | 38 | 63 | 11 | 50 |
| Ga | 78 | 125 | 15 | 109 | 34 | 53 | 6.2 | 41 | 12 | 35 | 11 | 18 |
| Ge | 2.9 | 2.9 | n.a. | 2.9 | 5.5 | 16 | 6.2 | 3.7 | 24 | 40 | 7 | 35 |
| As | 43 | 60 | 12 | 52 | b.d.l. | b.d.l. | n.a. | n.a. | 39 | 146 | 40 | 104 |
| Se | 3 | 3.3 | n.a. | 3.3 | b.d.l. | b.d.l. | n.a. | n.a. | 74 | 141 | 28 | 102 |
| Nb | n.a. | n.a. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Mo | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 7.6 | 11 | 1.6 | 8.6 |
| Ag | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Cd | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| In | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 4.9 | 10 | 2.1 | 7.8 |
| Sn | 4.4 | 13 | 3.6 | 8.8 | 2.9 | 6 | 1.3 | 4.3 | 1286 | 2503 | 496 | 1988 |
| Sb | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 11 | 22 | 5.4 | 19 |
| Te | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| La | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 576 | 1369 | 343 | 1020 |
| Ce | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 1813 | 5535 | 1487 | 4047 |
| Nd | n.a. | n.a. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 872 | 4215 | 1331 | 3121 |
| Sm | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 164 | 1070 | 368 | 792 |
| Gd | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 131 | 984 | 352 | 736 |
| W | b.d.l. | b.d.l. | n.a. | n.a. | 2.7 | 13 | 4.8 | 6.8 | 11 | 81 | 34 | 31 |
| Re | n.a. | n.a. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Au | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Tl | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Pb | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Bi | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. |
| Th | b.d.l. | b.d.l. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | 32 | 309 | 103 | 206 |
| U | n.a. | n.a. | n.a. | n.a. | b.d.l. | b.d.l. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stergiou, C.L.; Melfos, V.; Voudouris, P.; Papadopoulou, L.; Spry, P.G.; Peytcheva, I.; Dimitrova, D.; Stefanova, E.; Giouri, K. Rare and Critical Metals in Pyrite, Chalcopyrite, Magnetite, and Titanite from the Vathi Porphyry Cu-Au±Mo Deposit, Northern Greece. Minerals 2021, 11, 630. https://doi.org/10.3390/min11060630
Stergiou CL, Melfos V, Voudouris P, Papadopoulou L, Spry PG, Peytcheva I, Dimitrova D, Stefanova E, Giouri K. Rare and Critical Metals in Pyrite, Chalcopyrite, Magnetite, and Titanite from the Vathi Porphyry Cu-Au±Mo Deposit, Northern Greece. Minerals. 2021; 11(6):630. https://doi.org/10.3390/min11060630
Chicago/Turabian StyleStergiou, Christos L., Vasilios Melfos, Panagiotis Voudouris, Lambrini Papadopoulou, Paul G. Spry, Irena Peytcheva, Dimitrina Dimitrova, Elitsa Stefanova, and Katerina Giouri. 2021. "Rare and Critical Metals in Pyrite, Chalcopyrite, Magnetite, and Titanite from the Vathi Porphyry Cu-Au±Mo Deposit, Northern Greece" Minerals 11, no. 6: 630. https://doi.org/10.3390/min11060630
APA StyleStergiou, C. L., Melfos, V., Voudouris, P., Papadopoulou, L., Spry, P. G., Peytcheva, I., Dimitrova, D., Stefanova, E., & Giouri, K. (2021). Rare and Critical Metals in Pyrite, Chalcopyrite, Magnetite, and Titanite from the Vathi Porphyry Cu-Au±Mo Deposit, Northern Greece. Minerals, 11(6), 630. https://doi.org/10.3390/min11060630









